Abstract
A positive fluid balance has been found to be deleterious in critically ill patients; however, the impact of early fluid balance, particularly on long-term outcomes, in critically ill patients with cancer remains unclear. We performed this retrospective study at a tertiary-care referral hospital with 1500 beds and 6 intensive care units (ICUs) in central Taiwan, and 942 patients with cancer admitted to ICUs during 2013 to 2016 were enrolled. The primary outcome was 1-year mortality. Cancer-related data were obtained from the cancer registry, and data during ICU admissions were retrieved from the electronic medical records. The association between fluid balance, which was represented by median and interquartile range, and 1-year mortality was determined by calculating the hazard ratio (HR) with 95% confidence interval (CI) using a multivariable Cox proportional hazards regression model. The in-hospital mortality rate was 22.9% (216 of 942), and the mortality within 1 year after the index ICU admission was 38.7% (365 of 942). Compared to survivors, nonsurvivors tended to have a higher Acute Physiology and Chronic Health Evaluation II score (24.1 ± 6.9 vs 20.5 ± 6.2, P < .01), a higher age (65.0 ± 14.4 vs 61.3 ± 14.3, P < .01), a higher serum creatinine (1.5 ± 1.3 vs 1.0 ± 1.0, P < .01), and a higher cumulative day 1 to 4 fluid balance (2669, 955-5005 vs 4103, 1268-7215 mL, P < .01). Multivariable Cox proportional hazards regression analysis found that cumulative day-4 fluid balance was independently associated with 1-year mortality (adj HR 1.227, 95% CI: 1.132-1.329). A positive day 1 to 4 cumulative fluid balance was associated with shorter 1-year survival in critically ill patients with cancer. Further studies are needed to validate this association.
Keywords: cancer, critical care, fluid balance, long-term outcome, mortality
Introduction
Advances in treatment for cancer have led to a marked improvement in survival rates in patients with cancer, and the number of patients with cancer admitted to the intensive care unit (ICU) has increased over the past decade.1-3 Studies have found that outcomes, mainly short-term outcomes, were associated with older age, cancer types, advanced cancer status, and the severity of illness in critically ill patients with cancer.2,4,5 However, the impacts of management during ICU admission on the long-term outcome of critically ill patients with cancer are largely unknown.
Fluid balance plays a fundamental role in the management of critically ill patients, and there is growing evidence, including our previous study on critically ill influenza patients, that a positive fluid balance is correlated with poor clinical outcomes, such as prolonged ICU stay, duration of mechanical ventilation, hospital mortality, and other short-term outcomes.6-8 However, evidence regarding the long-term impact of fluid balance in critically ill patients, particularly in those with cancer, is limited.9
In the present study, we used 2 databases, the electronic medical records at Taichung Veterans General Hospital (TCVGH) and the Taiwan Cancer Registry database, to assess the dynamic fluid balance status and to address the long-term impact of an early positive fluid balance in critically ill patients with cancer.
Patients and Methods
Ethics Approval
This study was approved by the Institutional Review Board of the TCVGH (CE16250A-1). All of the data obtained from individual patients were anonymized prior to the analysis, and informed consent was thus waived.
Study Population
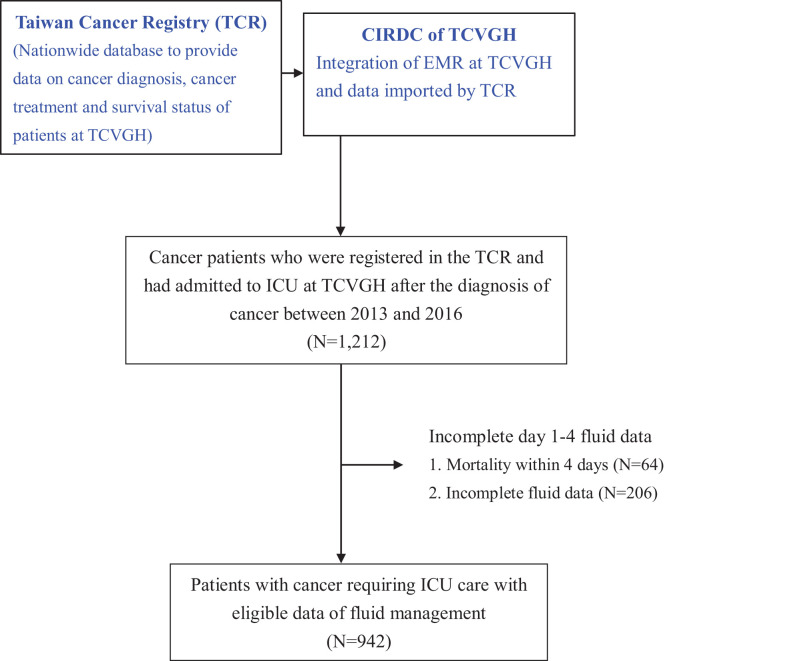
This retrospective cohort study was conducted at TCVGH, a tertiary-care referral hospital with 1500 beds and 6 ICUs, consisting of 2 medical ICUs (41 beds), surgical ICU (40 beds), cardiac ICU (17 beds), and 2 neurological ICUs (28 beds), in central Taiwan. All adult patients who were registered in the TCVGH cancer registry and had been admitted to the ICU between 2013 and 2016 following their cancer diagnosis were enrolled in the study. We only used the first ICU admission as the index ICU admission and excluded those whose discharge status was uncertain Figure 1.
Figure 1.
Flowchart of patient enrollment.
Data Source
We used 2 databases in the present study: the nationwide Taiwan Cancer Registry and the clinical data warehouse at TCVGH maintained by clinical informatics research and development center (CIRDC). Data on cancer diagnosis, cancer type, cancer treatment, and long-term survival status of patients at TCVGH were retrieved from the Taiwan Cancer Registry, a population-based cancer registry that has been organized and funded by the Ministry of Health since 1979,10 and imported into CIRDC of TCVGH. Data with regard to demographic information, ICU admission, discharge diagnoses, daily fluid input and output, Acute Physiology and Chronic Health Evaluation (APACHE) II score, surgical history, culture results, mechanical ventilation usage, renal replacement therapy, use of vasopressors, and hospital length of stay were obtained from clinical data warehouse maintained by CIRDC of TCVGH.
Fluid Status
The main exposure of interest in the present study were daily fluid input, output, and balance. Input fluid included all enteral and intravenous fluids including fluids in the form of colloids and crystalloids, transfusion of blood products, and enteral as well as parenteral nutrition. Output fluid included all body fluids, including urine and output from drains, rectal, orogastric, and nasogastric tubes. Fluid status was represented as daily and cumulative fluid input, output, and balance.
Mortality
The main outcome of interest in the present study was the mortality within 1 year following ICU admission. The date of death was retrieved from the Taiwan Cancer Registry.
Statistical Analyses
Data for categorical variables were shown as frequencies and percentages, and data for continuous variables were presented as mean (standard deviation [SD]). The estimated sample size was 571 based on effective size (0.15), which was estimated based on previous studies including our study focusing on cumulative day-4 fluid balance on mortality in critically ill influenza patients, α error (0.05) and β error (0.2) using G-Power (version 3.1). The Kolmogorov-Smirnov test was used to test normality, and continuous variables that were not normally distributed were represented with median and interquartile range. Differences between the 2 groups were analyzed using the Student t test or Mann Whitney U test, while the χ2 test with Fisher exact test were used for categorical variables. Accumulating evidence, including our previous study, have shown that day 1 to 3 or 1to 4 fluid balance appeared to be the prognostic factor and potentially modifiable factor in critically ill patients7,11-13; hence, we used Kaplan-Meier analysis to analyze the association between 1-year mortality and day 1 to 4 cumulative fluid balance status. A Cox proportional hazards regression model was used to identify independent variables that predicted 1-year mortality. The adjusted hazard ratio (adjHR) and the corresponding 95% confidence interval (CI) for each variable were presented. All statistical analyses were 2-sided, and the level of significance was .05. Data cleaning and analysis were performed using SAS program version 9.4 (SAS Institute Inc, Cary, North Carolina).
Results
Patients’ Characteristics
Of the 942 patients included in the present study, 73.4% were male, and the mean age was 62.7 ± 14.5 years (Table 1; see Supplemental data set for details). Type 2 diabetes mellitus (25.6%), chronic obstructive pulmonary disease (21.9%), and cerebrovascular disease (18.6%) were the main comorbidities among the enrolled patients. With regard to cancer types, the most common cancer type was gastroesophageal cancer (21.9%), followed by head and neck cancer (14.5%), lung cancer (13.2%), and colon cancer (11.1%).
Table 1.
Characteristics of the 942 Patients With Cancer Having ICU Admission Categorized by 1-Year Survival.a
| All | Survivors | Nonsurvivors | P Value | |
|---|---|---|---|---|
| (N = 942) | (N = 577) | (N = 365) | ||
| Basic Characteristics | ||||
| Age, years | 62.7 ± 14.5 | 61.3 ± 14.3 | 65.0 ± 14.4 | <.01 |
| Male | 694 (73.4%) | 417 (72.3%) | 274 (75.1%) | .19 |
| Type II diabetes mellitus | 241 (25.6%) | 148 (25.6%) | 93 (25.5%) | .51 |
| Cerebrovascular disease | 175 (18.6%) | 107 (18.5%) | 68 (18.6%) | .52 |
| Congestive heart failure | 87 (9.2%) | 50 (8.7%) | 37 (10.1%) | .26 |
| Chronic pulmonary disease | 206 (21.9%) | 127 (22.0%) | 79 (21.6%) | .48 |
| Chronic renal disease | 43 (4.6%) | 22 (3.8%) | 43 (4.6%) | .11 |
| Types of cancer | ||||
| Gastroesophageal cancer | 206 (21.9%) | 137 (23.7%) | 69 (18.9%) | .09 |
| Head and neck cancer | 137 (14.5%) | 95 (16.5%) | 42 (11.5%) | .03 |
| Lung cancer | 124 (13.2%) | 45 (7.8%) | 79 (21.6%) | <.01 |
| Colon cancer | 105 (11.1%) | 61 (10.6%) | 44 (12.1%) | .52 |
| Brain cancer | 98 (10.4%) | 86 (14.9%) | 12 (3.3%) | <.0 1 |
| Hepatocellular carcinoma | 61 (6.5%) | 28 (4.9%) | 33 (9.0%) | .01 |
| Prostate cancer | 28 (3.0%) | 19 (3.3%) | 9 (2.5%) | .56 |
| Hematological malignancy | 26 (2.8%) | 12 (2.1%) | 14 (3.8%) | .15 |
| Other cancer types | 157 (16.7%) | 94 (16.3%) | 63 (17.3%) | .72 |
| Presence of metastasis | 502 (53.2%) | 263 (45.6%) | 239 (65.5%) | <.01 |
| The etiology for ICU admission | <.01 | |||
| Scheduled surgery | 398 (42.3%) | 319 (55.3%) | 79 (21.6%) | <.01 |
| Emergent surgery | 106 (11.3%) | 61 (10.6%) | 45 (12.3%) | .46 |
| Acute respiratory failure | 171 (18.2%) | 58 (10.1%) | 113 (31.0%) | <.01 |
| Acute cardiac conditions | 64 (6.8%) | 36 (6.2%) | 28 (7.7%) | .43 |
| Acute neurological conditions | 37 (3.9%) | 17 (2.9%) | 20 (5.5%) | .06 |
| Pulmonary embolism | 39 (4.1%) | 12 (2.1%) | 27 (7.4%) | <.01 |
| Acute renal conditions | 21 (2.2%) | 8 (1.4%) | 13 (3.6%) | .04 |
| Others | 106 (11.3%) | 66 (11.4%) | 40 (11.0%) | .92 |
| Severity and management | ||||
| APACHE II score | 21.9 ± 6.7 | 20.5 ± 6.2 | 24.1 ± 6.9 | <.01 |
| Shock | 542 (57.5%) | 278 (48.2%) | 264 (72.3%) | <.01 |
| Mechanical ventilation | 795 (84.4%) | 473 (82.0%) | 322 (88.2%) | .01 |
| Renal replacement therapy | 38 (4.0%) | 13 (2.3%) | 25 (6.8%) | <.01 |
| Positive culturea | 447 (47.5%) | 226 (39.2%) | 221 (60.5%) | <.01 |
| White blood cell count, count/μL | 12 443.8 ± 11 641.6 | 11 563.1 ± 7921.8 | 13 836.0 ± 15 744.2 | <.01 |
| Hemoglobin, g/dL | 10.5 ± 2.2 | 10.9 ± 2.1 | 9.9 ± 2.2 | <.01 |
| Platelet, 103/μL | 181.0 ± 102.3 | 189.3 ± 97.2 | 167.8 ± 108.8 | <.01 |
| Creatinine, mg/dL | 1.2 ± 1.1 | 1.0 ± 1.0 | 1.5 ± 1.3 | <.01 |
| C-reactive protein, mg/dL | 10.9 ± 9.7 | 9.9 ± 10.2 | 11.9 ± 9.2 | .02 |
| Outcomes | ||||
| ICU stay, days | 11.0 ± 9.8 | 9.4 ± 8.7 | 13.5 ± 10.8 | .04 |
| Hospital stay, days | 23.6 ± 17.5 | 22.6 ± 16.5 | 25.1 ± 19.0 | <.01 |
| In-hospital mortality | 216 (22.9%) | NA | 216 (59.1%) | NA |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; ICU, intensive care unit; NA, not available; SD, standard deviation.
a Cultures were obtained from blood, respiratory tract, urinary tract, skin and soft tissue, or abdomen during the index admission. Data were presented as mean ± SD and N(%).
Given that TCVGH is a referral hospital in central Taiwan, and thus up to 52.3% of patients had advanced cancer status with the metastasis. The etiologies of ICU admission consisted of scheduled surgery (42.3%), acute respiratory failure (18.2%), and emergent surgery (11.3%). Importantly, the in-hospital mortality rate was 22.9% (216 of 942), and the mortality within 1 year after the index ICU admission was 38.7% (365 of 942). Therefore, the post-acute 1-year mortality rate in patients with cancer who survived the index ICU admission was 20.5% (149 of 726). Compared to survivors, nonsurvivors tended to have a higher APACHE II score (24.1 ± 6.9 vs 20.5 ± 6.2, P < .01), a higher age (65.0 ± 14.4 vs 61.3 ± 14.3, P < .01), a higher serum creatinine (1.5 ± 1.3 vs 1.0 ± 1.0, P < .01), and were more likely to have lung cancer (21.6% vs 7.8%, P < .01), hepatocellular carcinoma (9.0% vs 4.9%, P = .01), presence with metastasis (65.5% vs 45.6%, P < .01), presence with shock which was defined by the use of vasopressor (72.3% vs 48.2%, P < .01), receiving mechanical ventilation (88.2% vs 82.0%, P < .01), receiving renal replacement therapy (6.8% vs 2.3%, P < .01), and having a positive culture (60.5% vs 39.2%), whereas head and neck cancer (11.5% vs 16.5%, P < .01), brain cancer (3.3% vs 14.9%, P < .01), and admission for scheduled surgery (21.6% vs 55.3%, P < .01) were less prevalent among nonsurvivors (Table 1). Collectively, these data demonstrated that the postacute 1-year mortality rate was high, and the crucial need to determine the long-term impacts of the aforementioned variables during ICU admission.
Daily and Cumulative Fluid Status Between Day 1 and Day 7
Table 2 details the early daily and cumulative input (I), output (O), and fluid balance (I–O) data in critically ill patients with cancer categorized by 1-year survival. The fluid balance tended to be diverse among individual patients and were positive on day 1 (1465, 503-2780 mL), day 2 (1232, 327-2491 mL), and day 3 (354, −422 to 1308 mL), and were negative at day 4 (−4, −828 to 778 mL), day 5 (−105, −825 to 698 mL) and day 6 (−90, −764 to 568 mL).
Table 2.
Daily Fluid Status of the 942 Patients With Cancer Having ICU Admission Categorized by 1-Year Survival.
| All (N = 942) | Survivors (N = 577) | Non-Survivors (N = 365) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Input (I) | Output (O) | IO Balance | Input (I) | Output (O) | IO Balance | Input (I) | Output (O) | IO Balance | P a | |
| Daily fluid status | ||||||||||
| Day 1 | 3050 | 1230 | 1465 | 3049 | 1230 | 1367 | 3077 | 1230 | 1594 | <.01 |
| (1689-4717) | (610-2020) | (503-2780) | (1607-4451) | (660-2270) | (454-2590) | (1822-4873) | (540-1960) | (538-3080) | ||
| Day 2 | 3520 | 2220 | 1232 | 3472 | 2290 | 1075 | 3587 | 2080 | 1480 | <.01 |
| (2737-4709) | (1430-3110) | (327-2491) | (2780-4437) | (1690-3200) | (285-2017) | (2699-4993) | (1258-2994) | (370-2916) | ||
| Day 3 | 2982 | 2550 | 354 | 3090 | 2818 | 210 | 2862 | 2290 | 568 | <.01 |
| (2386-3628) | (1840-3390) | (−422 to 1308) | (2459-3708) | (1990-3590) | (−505 to 1100) | (2270-3547) | (1643-3189) | (−304 to 1511) | ||
| Day 4 | 2822 | 2700 | 111 | 2933 | 2850 | 40 | 2573 | 2495 | 267 | <.01 |
| (2207-3418) | (2000-3550) | (−638 to 815) | (2313-3502) | (2200-3780) | (−747 to 704) | (2113-3322) | (1835-3113) | (−540 to 942) | ||
| Day 5 | 2668 | 2725 | −4 | 2769 | 2920 | −46 | 2541 | 2355 | 79 | <.01 |
| (2191-3330) | (1940-3540) | (−828 to 778) | (2280-3404) | (2230-3820) | (−892 to 619) | (2031-3209) | (1683-3278) | (−753 to 1116) | ||
| Day 6 | 2644 | 2780 | −105 | 2740 | 2990 | −187 | 2525 | 2490 | 62 | .01 |
| (2103-3250) | (2000-3510) | (−825 to 698) | (2190-3323) | (2270-3650) | (−924 to 475) | (2008-3204) | (1753-3349) | (−782 to 944) | ||
| Day 7 | 2446 | 2530 | −90 | 2532 | 2800 | −220 | 2387 | 2323 | 58 | <.01 |
| (1987-3086) | (1870-3370) | (−764-568) | (2059-3160) | (2050-3670) | (−843 to 398) | (1940-3013) | (1663-3068) | (−564 to 829) | ||
| Cumulative fluid status | ||||||||||
| Day 1-2 | 6610 | 3580 | 2585 | 6347 | 3740 | 2299 | 7184 | 3285 | 3058.5 | <.01 |
| (5100-9139) | (2450-5060) | (1242-5089) | (5054-8993) | (2610-5380) | (1150-4257) | (5179-9578) | (2230-4852) | (1310-5721) | ||
| Day 1-3 | 9727 | 6270 | 3154 | 9563 | 6695 | 2917 | 9901 | 5752 | 3785 | <.01 |
| (8112-12 373) | (4680-8350) | (1171-5879) | (8062-12 211) | (5075-8650) | (1103-5073) | (8137-12 880) | (4275-7871) | (1487-6817) | ||
| Day 1-4 | 12 723 | 9210 | 3354 | 12 747 | 9800 | 2669 | 12 695 | 8598 | 4103 | <.01 |
| (10 679-15 583) | (6970-11 550) | (1148-6033) | (10 744-15 325) | (7530-12 410) | (955-5005) | (10472-15874) | (6385-10 853) | (1268-7215) | ||
| Day 1-5 | 15 632 | 12 170 | 3043 | 15 625 | 12 900 | 2487 | 15 722 | 11 265 | 4392 | <.01 |
| (13 089-19 050) | (9400-14 835) | (1055-6113) | (13 371-18 905) | (10 060-15 565) | (934-4960) | (12 847-19377) | (8628-13 441) | (1373-7554) | ||
| Day 1-6 | 18 444 | 15 010 | 2720 | 18 460 | 16 070 | 2081 | 18 377 | 14 005 | 4611 | <.01 |
| (15 559-21 946) | (12 120-18 280) | (615-6208) | (15 773-21 897) | (12 840-19 355) | (385-4551) | (15 073-22 205) | (10 714-16 505) | (760-7802) | ||
| Day 1-7 | 21 138 | 17 805 | 2690 | 21 230 | 18 800 | 2006 | 21 107.5 | 16 340 | 4344 | <.01 |
| (17 722-25 082) | (14 340-21 280) | (277-6199) | (18 204-25 283) | (15 400-22 720) | (−111 to 4595) | (17 209 to 24 990) | (13 158-19 574) | (974-8177) | ||
a Comparison of IO balance between the survivors and non-survivors. Data are presented as median (interquartile range).
A higher positive fluid balance was found in the nonsurvivor group compared to the survivor group on day 1 (1594, 538-3080 vs 1367, 454-2590 mL, P < .01), day 2 (1480, 370-2916 vs 1075, 285-2017 mL, P < .01), and day 3 (568, −304 to 1511 vs 210, −505 to 1100 mL, P < .01). Notably, a gradual negative fluid balance was observed in the survivor group from day 4 (day 4: 40, −747 to 704; day 5: −46, −892 to 619 mL), whereas a positive fluid balance was detected in the nonsurvivor group (day 4: 267, −540 to 1116; day 5: 79, −753 to 1116 mL). Therefore, the patients in the survivor group had a less positive fluid balance on day 1 to 3 and a negative fluid balance after day 4 than patients in the nonsurvivor group. The cumulative fluid balance data further demonstrated distinct differences in fluid balance status between the nonsurvivor and survivor groups, with a strong statistically significant difference in cumulative day 1 to 4 fluid balance (2669, 955-5005 vs 4103, 1268-7215 mL, P < .01), and these differences remained robust with respect to the day 1 to 5, day 1 to 6, and day 1 to 7 cumulative fluid balance. Collectively, these data showed distinct differences in fluid balance status with a less positive fluid balance in the survivor group.
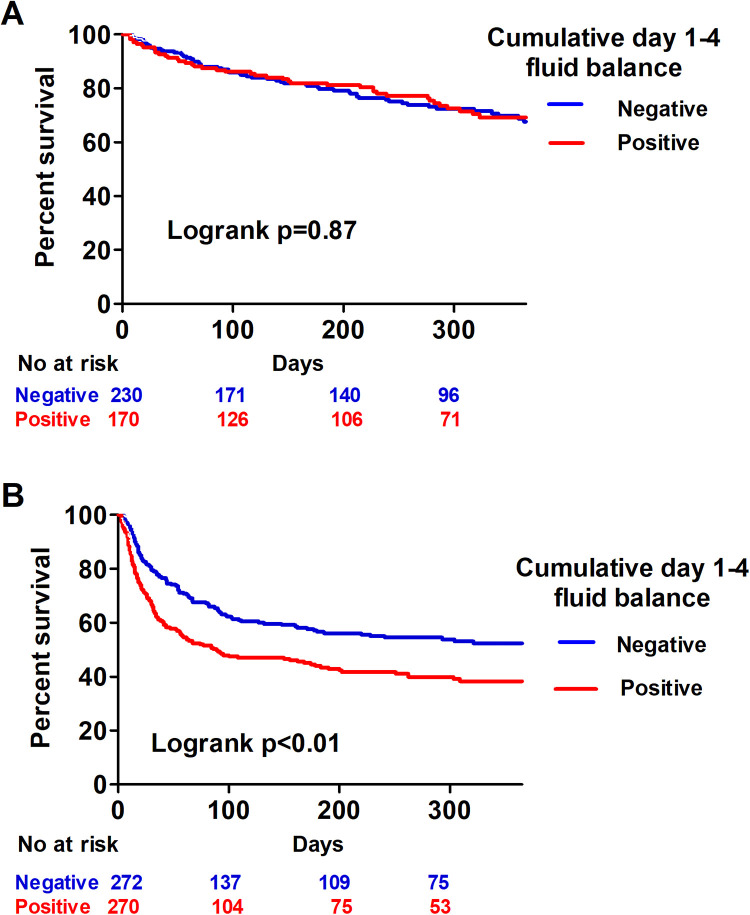
Day 1 to 4 Cumulative Fluid Balance Was Correlated With 1-Year Mortality
We used Kaplan-Meier analysis to analyze the correlation between day 1 and 4 cumulative fluid balance and 1-year mortality in critically ill patients with cancer and found that a positive day 1 to 4 cumulative fluid balance was associated with a higher risk of mortality (log-rank test, P < .01; Figure 2). Given that shock status may confound the correlation between fluid balance and mortality, we further divided the patients into shock and nonshock groups. Intriguingly, the impact of the cumulative day 1 to 4 fluid balance only affected patients with shock (Figure 3). To ascertain the potential collinearity between shock and fluid balance, we checked the variance inflation factor (VIF) and the VIF between the presence of shock and day-4 cumulative fluid balance was merely 1.0. We then investigated the independent variables to predict 1-year mortality in critically ill patients with cancer. Given that the APACHE II score is calculated using white blood cell count, platelet count, hematocrit, and serum creatinine level, we used APACHE II scores to represent severity in the multivariate regression model. In a multivariate Cox proportional hazard regression model adjusted for age, gender, cancer types, receiving mechanical ventilation, receiving renal replacement, and positive microbial culture, we found that positive cumulative day 1 to 4 fluid balance (aHR 1.227, 95% CI: 1.132-1.329), high APACHE II score (aHR 1.048, 95% CI 1.028-1.068), lung cancer (aHR 1.518, 95% CI 1.102-2.092), hepatocellular carcinoma (aHR 1.842, 95% CI 1.249-2.210), presence of metastasis (aHR 1.727, 95% CI 1.349-2.210), and shock (aHR 1.468, 95% CI 1.140-1.889) were independently associated with 1-year mortality, whereas admission to an ICU for scheduled surgery appeared to be a protective factor (aHR 0.339, 95% CI 0.251-0.458; Table 3). These findings showed the impact of day 1 to 4 cumulative fluid balance on the long-term outcome in critically ill patients with cancer.
Figure 2.
Kaplan-Meier survival curves for positive (red) and negative (blue) cumulative day 1 to 4 fluid balance in critically ill patients with cancer.
Figure 3.
Kaplan-Meier survival curves for positive (red) and negative (blue) cumulative day 1 to 4 fluid balance in the non-shock (A) and shock (B) groups.
Table 3.
Cox Proportional Hazards Regression for 1-Year Mortality.
| Characteristics | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age, per 1-year increment | 1.016 (1.009-1.024) | <.01 | 1.000 (0.992-1.008) | >.99 |
| Male gender | 1.076 (0.848-1.363) | .55 | 1.117 (0.869-1.435) | .39 |
| Gastroesophageal cancer | 0.732 (0.563-0.951) | .02 | 1.135 (0.813-1.585) | .46 |
| Head and neck cancer | 0.653 (0.474-0.901) | <.01 | 0.779 (0.533-1.137) | .20 |
| Lung cancer | 2.331 (1.817-2.992) | <.01 | 1.518 (1.102-2.092) | .01 |
| Brain cancer | 0.226 (0.127-0.402) | <.01 | 0.557 (0.299-1.036) | .06 |
| Hepatocellular carcinoma | 2.010 (1.405-2.876) | <.01 | 1.842 (1.249-2.716) | <.01 |
| Presence of metastasis | 1.842 (1.485-2.287) | <.01 | 1.727 (1.349-2.210) | <.01 |
| Scheduled surgery | 0.229 (0.178-0.294) | <.01 | 0.339 (0.251-0.458) | <.01 |
| APACHE II, per 1 increment | 1.093 (1.076 -1.111) | <.01 | 1.048 (1.028 -1.068) | <.01 |
| Shock | 2.630 (2.090-3.311) | <.01 | 1.468 (1.140 -1.889) | <.01 |
| Mechanical ventilation | 1.549 (1.127-2.130) | <.01 | 1.252 (0.887 -1.765) | .20 |
| Renal replacement therapy | 2.891 (1.922-4.350) | <.01 | 0.974 (0.633 -1.501) | .91 |
| Positive microbial culture | 1.945 (1.576-2.400) | <.01 | 1.144 (1.029 -1.068) | .25 |
| Cumulative day 1-4 fluid balance, per 1-liter increment | 1.231 (1.124 -1.348) | <.01 | 1.227 (1.132 -1.329) | <.01 |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; CI, confidence interval; HR, hazard ratio.
Discussion
This study investigated the long-term mortality impacts of day 1 to 4 fluid balance during ICU admissions in critically ill patients with cancer. We found that a positive cumulative day 1 to 4 fluid balance, a higher APACHE II score, presence of metastasis, and shock were independent risk factors for mortality within 1 year, whereas ICU admission for scheduled surgery appeared to be a protective factor. Notably, the long-term negative impact of an early positive fluid balance was mainly observed in patients with shock, but this phenomenon was not seen in those without shock. Our results demonstrate the deleterious effect of early fluid overload on long-term outcomes in critically ill patients with cancer, particularly those with shock.
Owing to the improved prognoses of patients with cancer that have been achieved in recent decades, the number of patients with cancer admitted to the ICU has increased worldwide.3 It is estimated that approximately 5% of patients with solid tumors and 15% of patients with hematological malignancy required an ICU admission within 2 years of cancer diagnosis.14,15 Furthermore, 1 European study reported that nearly 1 in 7 patients admitted to ICUs had a malignancy.16 These findings indicate the need to explore specific prognostic factors in critically ill patients with cancer. Indeed, a number of studies have found that outcomes, mainly short-term outcomes, in critically ill patients with cancer were associated with age, cancer status, severity of illness, use of mechanical ventilation, and the need for renal replacement therapy.2,4,5,17 However, studies on long-term outcomes in critically ill patients with cancer remain limited.
The long-term outcomes of critically ill patients with and without cancer have been the focus of an increasing number of research efforts in recent years.18,19 The long-term impacts of the management applied during ICU admission are increasingly being recognized as a crucial issue in critical care medicine due to the steady decrease in ICU mortality as well as high post-ICU mortality rates in the past 2 decades.20 Shankar-Hari et al analyzed the additional mortality at 1-year after ICU discharge in 43 studies and determined that the 1-year postacute mortality was 16.1%.21 Auclin et al found that 90-day postacute mortality was 18.3% among 262 elderly patients with a solid tumor.22 Fisher et al reported that the 180-day postacute mortality was 21% in 300 patients with solid tumors.2 The aforementioned data were largely consistent with the finding in this study that showed 1-year postacute mortality was 20.5% (149 of 726).
Our previous study as well as other investigations have found that older age, use of benzodiazepine, immunosuppression profiles, and positive culture in blood, urine, as well as blood, may have an effect on mortality, physical decline, and development of further infection in patients who are discharged from the ICU.23-25 As detailed information on fluid balance during ICU admission is rarely recorded in studies whose primary aim is to analyze cancer-related data, such as the cancer registry database, the long-term impact of fluid balance on critically ill patients with cancer remains unclear.
Indeed, early and overall fluid balance during ICU admission may have a distinct role in the long-term outcome of critically ill patients. Our previous study focusing on critically ill patients with influenza showed the impact of day 1 to 4 cumulative on 30-day overall mortality.7 Similarly, Brotfain et al reported that a positive fluid balance at discharge was associated with newly developed organ dysfunction after ICU discharge among 297 patients with sepsis.26 Notably, Brotfain et al found that day 1 to 3 fluid balance poorly correlated with overall fluid balance at the time of discharge from the ICU (r = .41, P < .001), suggesting the key role of overall fluid balance during admission. However, a number of studies have shown that the late (after day 7) fluid balance was no longer associated with outcomes in critically ill surgical patients, indicating the crucial importance of early fluid balance.27,28 Balakumar et al analyzed 18 084 patients and found that critically ill patients with a positive (≥5%) fluid balance till the end of index ICU stay or initiation of renal replacement therapy had a higher 1-year mortality risk than those with even (0%-5%) fluid balance.9 Intriguingly, Balakumar et al noted a variable correlation between a negative (<0%) fluid balance and 1-year mortality; a negative fluid balance in critically ill patients was associated with a lower mortality up to 11 days after ICU admission but with a slightly higher mortality between 88 and 365 days after ICU admission compared with patients with an even fluid balance. We postulate that early fluid balance probably reflects the net effect of fluid management of acute illness, whereas the overall fluid balance of the ICU admission likely reflects the processes involved in the restoration of fluid balance after acute illness, such as the recovery of renal function. It is possible that the overall fluid balance may somehow be affected by the duration of ICU stay, which may lead to an immortal time bias, that is, in patients with a short ICU stay, there might be insufficient time to observe the overall host responses to the alteration in fluid status. Therefore, in this study, we focused on the impact of early fluid balance on long-term outcomes.
Accumulating evidence shows that early fluid balance may affect hospital mortality in critically ill patients as a result of complex fluid-associated complications, including shearing injury to the endothelial glycocalyx, increased venous pressure with decreased perfusion pressure gradients, interstitial edema with a decrease in oxygen diffusion between capillaries and cells, and dilutional coagulopathy.29,30 For example, pulmonary edema leads to impaired oxygenation with an increased work of breathing,31 and cerebral edema weakens the autoregulation of cerebral blood flow, which in turn leads to a poor consciousness, an increased risk of pneumonia, and overall mortality.32 The aforementioned complications may further lead to organ dysfunction, including pulmonary edema, cardiac failure, neuromuscular dysfunction, delayed wound healing, and impaired bowel function.33 We think that the findings of the present study provide clinical evidence of the long-term deleterious impact of fluid overload in critically ill patients with cancer. Given that the aforementioned fluid-associated complications and organ dysfunction may also affect the general recovery of patients, particularly patients with cancer who have survived a critical illness, we speculate with caution that early fluid balance might affect short-term and long-term outcomes through similar mechanisms in critical illness. However, more studies are warranted to validate the findings in critically ill patients without cancer.
Recently, a number of approaches have been proposed to potentially reduce the amount of fluid administered during fluid resuscitation in patients with sepsis, including early use of vasopressors,34 a higher dose of vasopressors,35 using capillary refilling time as the resuscitation target,36 with 5000 cm3 as the potential limit of fluid within the first day37 and restricting fluid administration after the initial resuscitation.38 Our findings provide evidence that early positive fluid balance confers deleterious effects in critically ill patients with cancer, particularly those with shock, and suggest that treatment should involve the implementation of recently proposed approaches aimed at reducing the amount of initial resuscitated fluid, instead of merely removing the resuscitated fluid using diuretics or renal replacement therapy.
There are limitations in this study. First, owing to the observational nature of the present study, we were unable to make causal inferences with respect to fluid balance and outcomes. Second, this was a single-center study, and thus our findings may not be generalizable to other populations. However, the patient population included a variety of cancers typically seen in populations with cancer in academic medical centers. Third, we assumed the use of vasopressors indicated the presence of shock, and we could not further delineate subtypes of shock, such as septic shock, and cardiogenic shock. Four, the performance status and the potential palliative care could affect the strategy of fluid administration; however, we have corrected APACHE II, age, presence of metastasis, and scheduled surgery to mitigate the potential unmeasured confounders. Furthermore, we excluded those without complete day 1 to 4 fluid data. We found that these excluded patients were largely patients with less severe critical illness with a short ICU stay (2.6 ± 0.9 vs 11.0 ± 9.8 days, P < .01) as well as ventilator day (1.5 ± 1.2 vs 9.0 ± 10.7 days, P < .01), a lower APACHE II score (18.9 ± 5.3 vs 21.9 ± 6.7, P < .01), a low proportion of shock (27.2% vs 57.5%, P < .01) compared to the 942 patients with complete day 1 to 4 fluid data (Supplemental Table 1). Given that the impact of fluid balance mainly existed in those with shock and high APACHE II, the exclusion of these patients should not affect the findings of this study.
In conclusion, we linked 2 databases and found that a positive day 1 to 4 cumulative fluid balance may have long-term deleterious impacts on critically ill patients with cancer, particularly those with shock. These findings indicate the crucial need for vigilance with respect to early fluid balance and that strategies aimed at preventing over-resuscitation be implemented and more studies are warranted to elucidate the mechanisms underlying the long-term impacts of fluid overload.
Supplemental Material
Supplemental Material, Supplemental_table_1_2020_0314_(1) for Impact of Early Fluid Balance on 1-Year Mortality in Critically Ill Patients With Cancer: A Retrospective Study in Central Taiwan by Yung-Chun Chen, Zhe-Rong Zheng, Chen-Yu Wang and Wen-Cheng Chao in Cancer Control
Acknowledgment
The authors thank Clinical Informatics Research & Development Center of Taichung Veterans General Hospital for the support of the data.
Authors’ Notes: Yung-Chun Chen and Zhe-Rong Zheng contributed equally to this work. YCC, ZRZ, CYW, BJL, and WCC contributed to study concept and design. YCC, ZRZ, and WCC contributed to acquisition of data. YCC, ZRZ, and WCC contributed to analysis and interpretation of data. YCC, ZRZ, and WCC contributed to draft of the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Wen-Cheng Chao, MD, PhD  https://orcid.org/0000-0001-9631-8934
https://orcid.org/0000-0001-9631-8934
Supplemental Material: Supplemental material for this article is available online.
References
- 1. De Angelis R, Sant M, Coleman MP, et al. Cancer survival in Europe 1999-2007 by country and age: results of EUROCARE—5-a population-based study. Lancet Oncol. 2014;15(1):23–34. [DOI] [PubMed] [Google Scholar]
- 2. Fisher R, Dangoisse C, Crichton S, et al. Short-term and medium-term survival of critically ill patients with solid tumours admitted to the intensive care unit: a retrospective analysis. BMJ open. 2016;6(10):e011363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kiehl MG, Beutel G, Boll B, et al. Consensus statement for cancer patients requiring intensive care support. Ann Hematol. 2018;97(7):1271–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bos MM, Verburg IW, Dumaij I, et al. Intensive care admission of cancer patients: a comparative analysis. Cancer Med. 2015;4(7):966–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xia R, Wang D. Intensive care unit prognostic factors in critically ill patients with advanced solid tumors: a 3-year retrospective study. BMC Can. 2016;16:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sakr Y, Rubatto Birri PN, Kotfis K, et al. Higher fluid balance increases the risk of death from sepsis: results from a large international audit. Crit Care Med. 2017;45(3):386–394. [DOI] [PubMed] [Google Scholar]
- 7. Chao WC, Tseng CH, Chien YC, et al. Association of day 4 cumulative fluid balance with mortality in critically ill patients with influenza: a multicenter retrospective cohort study in Taiwan. PLoS One. 2018;13(1):e0190952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Silversides JA, Major E, Ferguson AJ, et al. Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: a systematic review and meta-analysis. Intens Care Med. 2017;43(2):155–170. [DOI] [PubMed] [Google Scholar]
- 9. Balakumar V, Murugan R, Sileanu FE, et al. Both positive and negative fluid balance may be associated with reduced long-term survival in the critically Ill. Crit Care Med. 2017;45(8):e749–e757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chiang CJ, You SL, Chen CJ, et al. Quality assessment and improvement of nationwide cancer registration system in Taiwan: a review. Jpn J Clin Oncol. 2015;45(3):291–296. [DOI] [PubMed] [Google Scholar]
- 11. Rosenberg AL, Dechert RE, Park PK, et al. Review of a large clinical series: association of cumulative fluid balance on outcome in acute lung injury: a retrospective review of the ARDSnet tidal volume study cohort. J Intensive Care Med. 2009;24(1):35–46. [DOI] [PubMed] [Google Scholar]
- 12. Acheampong A, Vincent JL. A positive fluid balance is an independent prognostic factor in patients with sepsis. Critical Care. 2015;19:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Silversides JA, Fitzgerald E, Manickavasagam US, et al. Deresuscitation of patients with iatrogenic fluid overload is associated with reduced mortality in critical illness. Crit Care Med. 2018;46(10):1600–1607. [DOI] [PubMed] [Google Scholar]
- 14. Puxty K, McLoone P, Quasim T, et al. Survival in solid cancer patients following intensive care unit admission. Intens Care Med. 2014;40(10):1409–1428. [DOI] [PubMed] [Google Scholar]
- 15. Azoulay E, Pene F, Darmon M, et al. Managing critically Ill hematology patients: time to think differently. Blood Rev. 2015;29(6):359–367. [DOI] [PubMed] [Google Scholar]
- 16. Taccone FS, Artigas AA, Sprung CL, et al. Characteristics and outcomes of cancer patients in European ICUs. Critical Care. 2009;13(1): R15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Azevedo LC, Caruso P, Silva UV, et al. Outcomes for patients with cancer admitted to the ICU requiring ventilatory support: results from a prospective multicenter study. Chest. 2014;146(2):257–266. [DOI] [PubMed] [Google Scholar]
- 18. Bein T, Bienvenu OJ, Hopkins RO. Focus on long-term cognitive, psychological and physical impairments after critical illness. Intens Care Med 2019;45(10):1466–1468. [DOI] [PubMed] [Google Scholar]
- 19. Camou F, Didier M, Leguay T, et al. Long-term prognosis of septic shock in cancer patients. Supp Care Cancer. 2020;28(3):1325–1333. [DOI] [PubMed] [Google Scholar]
- 20. Kaukonen KM, Bailey M, Suzuki S, et al. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. Jama. 2014;311(13):1308–1316. [DOI] [PubMed] [Google Scholar]
- 21. Shankar-Hari M, Rubenfeld GD. Understanding long-term outcomes following sepsis: implications and challenges. Curr Infect Disease Rep. 2016;18(11):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Auclin E, Charles-Nelson A, Abbar B, et al. Outcomes in elderly patients admitted to the intensive care unit with solid tumors. Ann Intensive Care. 2017;7(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pfoh ER, Wozniak AW, Colantuoni E, et al. Physical declines occurring after hospital discharge in ARDS survivors: a 5-year longitudinal study. Intens Care Med. 2016;42(10):1557–1566. [DOI] [PubMed] [Google Scholar]
- 24. van Vught LA, Klein Klouwenberg PM, Spitoni C, et al. Incidence, risk factors, and attributable mortality of secondary infections in the intensive care unit after admission for sepsis. Jama. 2016;315(14):1469–1479. [DOI] [PubMed] [Google Scholar]
- 25. Chiang HY, Wu TH, Hsu CY, et al. Association between positive cultures during admission and 1-year mortality in patients with cancer receiving perioperative intensive care. Cancer Control. 2018;25(1):1073274818794162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brotfain E, Koyfman L, Toledano R, et al. Positive fluid balance as a major predictor of clinical outcome of patients with sepsis/septic shock after ICU discharge. Am J Emerg Med. 2016;34(11):2122–2126. [DOI] [PubMed] [Google Scholar]
- 27. Yeh DD, Tang JF, Chang Y. The use of furosemide in critically ill trauma patients: a retrospective review. J Emerg Trauma Shock. 2014;7(2):83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Elofson KA, Eiferman DS, Porter K, et al. Impact of late fluid balance on clinical outcomes in the critically ill surgical and trauma population. J Crit Care. 2015;30(6):1338–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hippensteel JA, Uchimido R, Tyler PD, et al. Intravenous fluid resuscitation is associated with septic endothelial glycocalyx degradation. Critical care. 2019;23(1):259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Silversides JA, Perner A, Malbrain M. Liberal versus restrictive fluid therapy in critically ill patients. Intens Care Med. 2019;45(10):1440–1442. [DOI] [PubMed] [Google Scholar]
- 31. Semler MW, Wheeler AP, Thompson BT, et al. Impact of initial central venous pressure on outcomes of conservative versus liberal fluid management in acute respiratory distress syndrome. Crit Care Med. 2016;44(4):782–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goodson CM, Rosenblatt K, Rivera-Lara L, et al. Cerebral blood flow autoregulation in sepsis for the intensivist: why its monitoring may be the future of individualized care. J Intensive Care Med. 2018;33(2):63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reuter DA, Chappell D, Perel A. The dark sides of fluid administration in the critically ill patient. Intens Care Med. 2018;44(7):1138–1140. [DOI] [PubMed] [Google Scholar]
- 34. Permpikul C, Tongyoo S, Viarasilpa T, et al. Early use of norepinephrine in septic shock resuscitation (CENSER). a randomized trial. Am J Respir Crit Care Med. 2019;199(9):1097–1105. [DOI] [PubMed] [Google Scholar]
- 35. Komorowski M, Celi LA, Badawi O, et al. The artificial intelligence clinician learns optimal treatment strategies for sepsis in intensive care. Nat Med. 2018;24(11):1716–1720. [DOI] [PubMed] [Google Scholar]
- 36. Hernandez G, Ospina-Tascon GA, Damiani LP, et al. Effect of a resuscitation strategy targeting peripheral perfusion status vs serum lactate levels on 28-day mortality among patients with septic shock: the andromeda-shock randomized clinical trial. JAMA 2019;321(7):654–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marik PE, Linde-Zwirble WT, Bittner EA, et al. Fluid administration in severe sepsis and septic shock, patterns and outcomes: an analysis of a large national database. Intens Care Med. 2017;43(5):625–632. [DOI] [PubMed] [Google Scholar]
- 38. Hjortrup PB, Haase N, Bundgaard H, et al. Restricting volumes of resuscitation fluid in adults with septic shock after initial management: the CLASSIC randomised, parallel-group, multicentre feasibility trial. Intens Care Med. 2016;42(11):1695–1705. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Supplemental_table_1_2020_0314_(1) for Impact of Early Fluid Balance on 1-Year Mortality in Critically Ill Patients With Cancer: A Retrospective Study in Central Taiwan by Yung-Chun Chen, Zhe-Rong Zheng, Chen-Yu Wang and Wen-Cheng Chao in Cancer Control