Abstract
Moringa oleifera Lam. is an essential herb used for the treatment of inflammation, diabetes, high blood pressure, and other diseases. In this study, phenolic extracts of M. oleifera leaves were obtained and analyzed. The results showed that the main identifiable phenols were astragalin, chlorogenic acid, isoquercitrin, kaempferitrin, luteolin, quercetin, and rutin. The effects of M. oleifera polyphenol extract (MOPE) on experimental colitis induced by 3% dextran sulfate sodium (DSS) were investigated. The results showed that oral administration of MOPE significantly alleviated the symptoms of DSS-induced colitis. MOPE significantly reduced weight loss, the disease activity index, colon shortening, and mucosal damage. In addition, MOPE attenuated the infiltration of CD3+ T cells, CD177+ neutrophils, and F4/80+ macrophages and significantly inhibited the secretion of IL-6 and TNF-α. After the MOPE administration, the expression of proteins associated with the NF-κB signaling pathway changed. Specifically, compared with that of the DSS group, the protein expression of NF-κB p65 and p-IκBα was downregulated, and the expression of IκBα was upregulated. This study revealed the anti-inflammatory effects and mechanisms of MOPE in the colon, indicating its potential use in preventing inflammation-driven diseases.
1. Introduction
Moringa oleifera Lam. has been historically used as nutritious food and traditional medicine in China [1]. In 2012, M. oleifera leaves were approved by the Chinese Ministry of Health as a new resource food. M. oleifera is also an essential traditional herb used to treat inflammation, diabetes, hypertension, anemia, hypoimmunity, and other diseases [2–5]. There are some flavonoid pigments, such as alkaloids, kaempferol, rhamnose, and isoquercitrin, and various antioxidant compounds, such as ascorbic acid, flavonoids, phenols, and carotenoids, which occur naturally in M. oleifera [6] and may contribute to the anti-inflammatory activity of M. oleifera. Additionally, it has been previously reported that some active components in M. oleifera mitigate some chronic inflammation [7]. However, there have been few reports on the improvement and alleviation of inflammation by M. oleifera polyphenols.
Inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn's disease (CD) [8], is a chronic recurrent disease of the intestine [9]. IBD is exceptionally harmful and is associated with an increased risk for colon cancer [10]. IBD is affected by diets such as high-fat diets, and the incidence is high in some developed countries, such as Europe [8]. Recently, the incidence of IBD in Asia has increased rapidly due to the improvements in living conditions and the adoption of a Western lifestyle. The pathogenesis of IBD is not yet fully clear; it is generally considered to be related to the environment, infection, genetics, psychology, and immunity. In addition, the intestinal mucosal immune system plays an essential role in the pathogenesis of IBD [11]. Several studies have shown that there is a high level of activated transcription factor-kappa B in the intestinal mucosa of IBD patients, and nuclear transcription factor kappa B (NF-κB) is considered to be one of the most critical signaling pathways in IBD. High expression of NF-κB can enhance the release of proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), which leads to colonic tissue damage [12]. It has been suggested that the activation of NF-κB plays a crucial role in the regulation of immune and inflammatory responses, and thus inhibiting the NF-κB signaling pathway is a novel therapeutic strategy in IBD treatment research.
To date, there are no ideal drugs for the treatment of IBD. Existing treatments mainly include resection and relief medication, such as 5-aminosalicylic (5-ASA), corticosteroids, immunomodulatory drugs, and biological agents. However, long-term administration of these drugs has various side effects, including drug resistance and drug tolerance [13]. Therefore, it is necessary to find natural treatments with fewer side effects. Although M. oleifera has been reported to exhibit various bioactivities, knowledge about the effects of M. oleifera polyphenol extract (MOPE) on gut health is limited. The present study aimed to evaluate the effects of MOPE in an established model of acute ulcerative colitis induced with dextran sulfate sodium (DSS) and further explore its underlying mechanisms.
2. Materials and Methods
2.1. Preparation of MOPE
Dried M. oleifera leaves (Yunnan Shenbaofu Technology Development Co., Ltd., Dehong, China) were crushed and then extracted by the ultrasound-assisted method as described by Fei et al. [14]. The extraction process was performed three times under the same conditions with 70% ethanol as the solvent, a solid/solvent ratio of 1/30, and 250 W of ultrasonic power for 20 min. These three extraction supernatants were combined and passed through a D101 macroporous resin, followed by elution with an 80% ethanol solution to obtain an ethanol eluate. The eluate was vacuum freeze-dried to obtain the MOPE.
2.2. Ultrahigh-Performance Liquid Chromatography Quadrupole Time-of-Flight Tandem Mass Spectrometry (UPLC-QTOF-MS/MS)
The composition of the MOPE was analyzed using a UPLC-QTOF-MS/MS system (XEVO G2-S QTOF-MS, Waters, USA). The chromatographic conditions were as follows: ACQUITY UPLC HSS T3 C18 (2.1 × 100 mm, 1.8 μm); mobile phase A (0.1% formic acid-water), mobile phase B (acetonitrile); flow rate: 0.5 mL·min−1; column temperature: 40°C; and analysis time 13 min. The sample was dissolved in 50% aqueous methanol, and the supernatant was used for analysis. The gradient elution was carried out as follows: 0 min, 10% B; 6 min, 40% B; 6.2 min, 60% B; 8.5 min, 90% B; 10.5 min, 90% B; and 10.6 min, 10% B. The mass spectrometry conditions were as follows: ESI: negative ion mode; scan range: 100–1200 Da; source temperature: 100°C; desolvation temperature: 350°C; desolvation gas flow: 700 L·h−1; LockSpray: leucine enkephalin (5-Leucine) enkephalin (LE); capillary: 2.8 kV; and sampling cone: 40 V.
2.3. Animal Treatments
The animal experiments were performed according to international guidelines. The protocol was approved by the Animal Care and Use Committee of Yunnan Agricultural University (No. YNAU-2017-011). Six-week-old male C57BL/6 mice (18–20 g, Liaoning Changsheng Biotechnology Co. Ltd., Liaoning, China) were maintained on a 12/12 h light/dark cycle at 25 ± 1°C and 55% humidity. All mice were housed 5/cage and had free access to standard mouse feed and tap water. The mice were allowed to acclimate for one week before the study began.
2.4. Induction of Colitis and MOPE Treatment
Colitis was induced with dextran sulfate sodium (DSS, 36000–50000, MP Biomedicals, USA, 2160110). The mice were randomly divided into five groups (n = 10 per group): the control group was given ultrapure (UP) water for 14 days; the DSS group was given UP water for the first seven days and then 3% DSS for the following seven days; the 5-aminosalicylic acid (5-ASA, positive control) group and the MOPE groups (50 mg/kg and 200 mg/kg, respectively) were given 5-ASA and MOPE individually for 14 days and were simultaneously given the water containing 3% DSS starting on the eighth day (Figure 1(a)).
Figure 1.
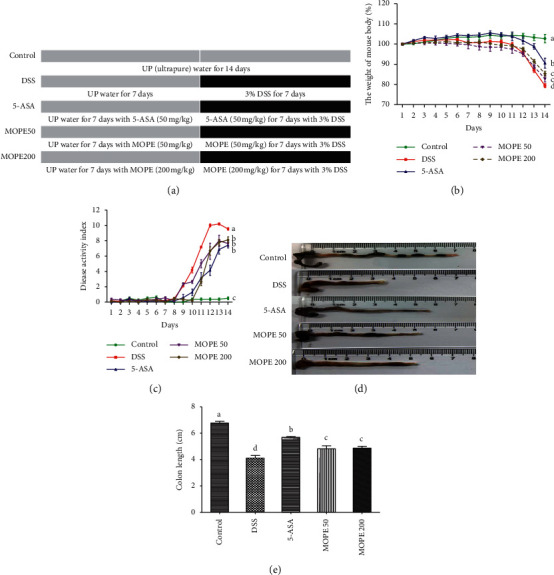
MOPE ameliorated DSS-induced colitis symptoms in mice. (a) The experimental design (n = 10 per group). (b) Body weight change in mice. (c) Disease activity index (DAI) scores. (d) Representative images of the mouse colon. (e) Colon lengths. The data are presented as the mean ± SEM. Values with different letters differ significantly (p < 0.05).
2.5. Evaluation of the Disease Activity Index (DAI)
Mice were monitored daily for the development of colitis based on body weight changes, gross rectal bleeding, and stool consistency. The disease activity index (DAI) scores were measured for each animal according to a previously described method [15]. The details of the DAI grading standards are listed in the Supplementary Materials (Table S1).
2.6. Histopathology
The colon tissues 1 cm from the distal colon were rinsed with ice-cold PBS, fixed in 10% (v/v) neutral formalin, embedded in paraffin, cut into 5 µm slices (Leica, RM2126RT, Germany), and stained with hematoxylin and eosin (H&E). Histological analysis was performed according to previously described methods [16]. A detailed description of the score standards is listed in the Supplementary Materials (Table S2).
2.7. Immunohistochemistry
The tissue slices were deparaffinized by xylene for 0.5–1 h and rehydrated sequentially with 100%, 100%, 95%, and 80% ethanol and water for 5 min each. Then, the slices were immersed in methanol containing 3% hydrogen peroxide, followed by high-pressure antigen repair. The slides were incubated with primary antibodies against CD3+, CD177+, and F4/80+ (Abcam, Cambridge, MA) overnight and then incubated with the appropriate secondary antibodies, followed by diaminobenzidine (DAB) color development, hematoxylin staining, and xylene clearing. Immunostained tissue slices were visualized under a microscope (Olympus, Japan).
2.8. Determination of Serum Inflammatory Factors
At the end of the experiment, serum samples were prepared and subjected to enzyme-linked immunosorbent assay (ELISA) to determine serum levels of IL-6 and TNF-α using ELISA kits from BD Pharmingen. The procedure was performed according to the instructions.
2.9. Western Blotting
The colon tissues were homogenized and lysed in RIPA buffer (Solarbio) containing PMSF (100 : 1) for 15 min. After centrifugation (4°C, 15000 rpm, 10 min), the supernatant was collected, and the protein concentration was quantified using BCA protein assay reagent (Beyotime). The protein lysates (50 μg protein) were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to PVDF membranes (Immobilon). After being blocked with 5% bovine serum albumin (BSA) solution, the membranes were incubated with primary antibodies against TNF-α, NF-κB p65, IκBα, and phosphorylated IκBα, followed by the appropriate secondary antibodies. Then, the protein-antibody complexes were developed with ECL Luminol reagent (Santa Cruz Biotechnology) and visualized.
2.10. Statistical Analysis
GraphPad Prism software version 5.0 was used to graph the results. The data are presented as the mean ± SEM. Statistical analysis was performed by SPSS 19.0. The differences between multiple groups were analyzed by one-way analysis of variance (ANOVA), followed by Duncan's test. The results were considered statistically significant when p < 0.05.
3. Results
3.1. UPLC-QTOF-MS/MS Analysis of MOPE
Seven compounds, including chlorogenic acid, rutin, isoquercitrin, astragalin, quercetin, luteolin, and kaempferitrin, which were known phenolic substances from MOPE, were analyzed by UPLC-QTOF-MS/MS. The retention times of these seven compounds were 1.57, 2.75, 2.92, 3.37, 4.74, 5.68, and 7.70 min, respectively. The relative quantities were 4.66%, 1.88%, 3.12%, 0.93%, 5.94%, 1.30%, and 7.76%, respectively (Figure 2).
Figure 2.
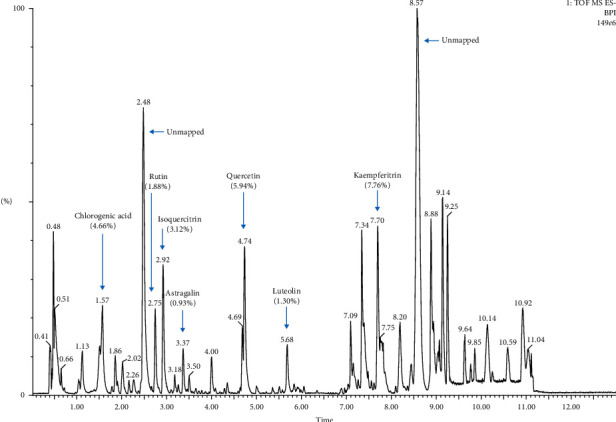
UPLC-QTOF-MS/MS chromatogram of MOPE.
3.2. MOPE Ameliorated Colitis Symptoms in Mice
The mice were treated with a low (50 mg/kg) or a high dose (200 mg/kg) of MOPE for 2 weeks (Figure 1(a)). Bodyweights and DAI scores of the mice were monitored every day throughout the experiment. The DSS-only treatment group exhibited severe bodyweight loss, while treatment with 5-ASA (positive control) and 50 mg/kg and 200 mg/kg MOPE significantly alleviated weight loss (Figure 1(b)). Additionally, mice in the DSS group exhibited high DAI scores, while 5-ASA and MOPE treatment significantly decreased the DAI scores compared to those of the DSS group (Figure 1(c)). These results indicated that 5-ASA and MOPE treatments alleviated colonic inflammatory symptoms in mice.
The length of the colon is an important indicator for indirectly assessing the severity of colitis. In this study, fecal residues in the colons of control mice were normal and granular. The DSS group was induced to develop colitis, exhibiting hyperemic colons and very severe colon shortening. Furthermore, fecal residue in the colon in the 5-ASA group and MOPE groups was loose and less hyperemic (Figure 1(d)). 5-ASA and MOPE treatment significantly prevented the colonic shortening compared to that of the DSS-treated group (Figure 1(e)).
3.3. MOPE Ameliorated Colonic Injury in Mice with DSS-Induced Colitis
The histological examination of colonic tissue from healthy mice revealed that the epithelial cells and crypts were structurally intact, the glands were arranged neatly, and there was no inflammatory cell infiltration (Figure 3(a)). In contrast, in the DSS-treated group, the lamina propria was damaged, the glands were destroyed and arranged irregularly, the epithelial cells and crypt structures were destroyed, and a large number of inflammatory cells infiltrated the muscle floor (Figure 3(b)). This damage was significantly alleviated after treatment with 5-ASA and MOPE (Figures 3(c)–3(f)).
Figure 3.
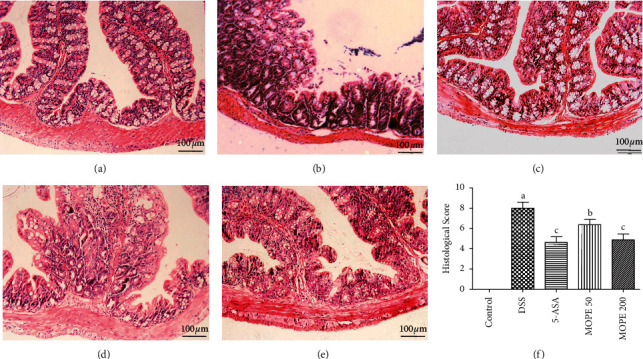
Effects of MOPE on the histopathological characterization of DSS-colitis mice. Representative HE-stained sections of the distal colonic tissues from the (a) control, (b) DSS, (c) 5-ASA (50 mg/kg), (d) MOPE (50 mg/kg), and (e) MOPE (200 mg/kg) groups. All images were acquired using 200× magnification. (f) Histological scores of colonic abnormalities. Values with different letters (a-c) differ significantly (p < 0.05).
3.4. MOPE Attenuated the Infiltration of Inflammatory Cells in Mice with DSS-Induced Colitis
The expression of CD3+, CD177+, and F4/80+ in the distal colon was measured by immunohistochemistry to detect T cell, neutrophil, and macrophage infiltration, respectively. The results indicated that T cells, neutrophils, and macrophages were increased in DSS-treated mice compared to normal mice, indicating that these inflammatory cells infiltrated the colonic injury area. 5-ASA and MOPE treatment reduced the infiltration of T cells, neutrophils, and macrophages (Figure 4).
Figure 4.
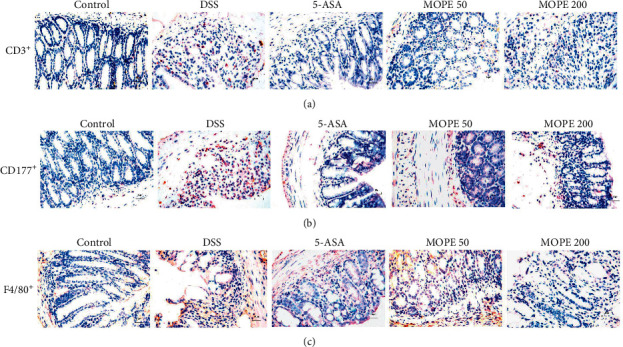
MOPE attenuated the infiltration of inflammatory cells in mice with DSS-induced colitis. Representative images of (a) CD3+, (b) CD177+, (c) and F4/80+ immunostaining in the distal colons of mice; original magnification: 400×.
3.5. MOPE Decreased the Cytokine Levels in Mice with DSS-Induced Colitis
IL-6 and TNF-α are two typical inflammatory cytokines. The serum levels of IL-6 and TNF-α were measured in the present study. Compared with those of the control group, substantial increases in IL-6 (Figure 5(a)) and TNF-α (Figure 5(b)) levels were observed in the DSS group (p < 0.001). However, MOPE treatment significantly reduced the serum levels of IL-6 (p < 0.01) and TNF-α (p < 0.05), and the effect was the same as that of 5-ASA (positive control). Moreover, MOPE treatment significantly inhibited the protein expression of TNF-α in colon tissue after DSS-induced colitis (Figures 5(c) and 5(d)).
Figure 5.
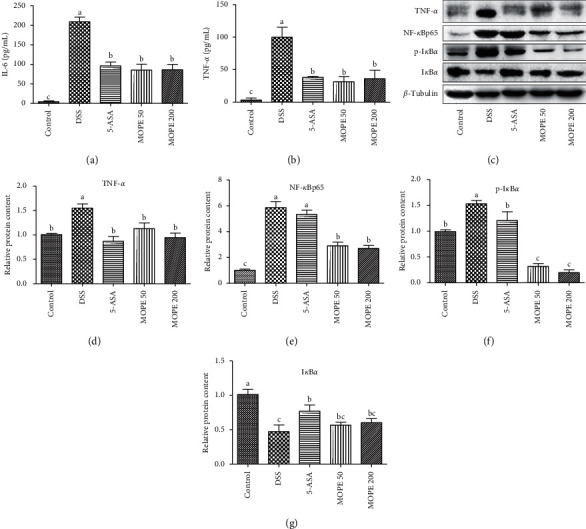
MOPE modulated cytokines and inflammation-related signaling proteins in mice with DSS-induced colitis. (a) The serum IL-6 level. (b) The serum TNF-α. (c) Western blot analysis of key signaling proteins in colonic tissue. (d) Quantitative analysis of TNF-α protein levels in colonic tissue. (e) Quantitative analysis of NF-κB p65 protein levels. (f) Quantitative analysis of (p)-IκBα protein levels. (g) Quantitative analysis of IκBα protein levels. Values with different letters (a-c) differ significantly (p < 0.05).
3.6. MOPE Modulated Inflammation-Related Signaling Proteins
The NF-κB signaling pathway strongly influences the pathogenesis of colitis. In the present study, the protein expression of NF-κB p65 in the nucleus and p-IκBα in the colon tissue of DSS model mice was higher than that of control mice, and the protein expression of IκBα was decreased (Figures 5(d)–5(g)). This result indicated that the NF-κB signaling pathway was activated in the colonic mucosal. After treatment with MOPE, the protein expression of NF-κB p65 and p-IκBα was significantly downregulated, and the expression of IκBα was upregulated. These results suggested that the anti-inflammatory effects of MOPE were related to inhibiting NF-κB pathway activation.
4. Discussion
M. oleifera has been reported to have beneficial effects on various diseases, including diabetes, hypertension, hyperlipidemia, and other chronic inflammation. There are some individual bioactive components in M. oleifera extracts that have potential preventive and therapeutic effects on inflammation [17]. Studies have shown that extracts of M. oleifera inhibit the production of NO and proinflammatory cytokines in LPS-induced macrophages [7, 18]. The pods of M. oleifera can inhibit the LPS-induced expression of IL-6 and TNF-α and inhibit LPS-induced IκB activation [19]. The ethyl acetate extract of M. oleifera can significantly inhibit LPS-induced TNF-α, IL-6, and IL-8 production in human monocyte-derived macrophages (MDMs). M. oleifera extracts effectively inhibit the expression of inflammatory mediators that may be related to the inhibition of the NF-κB signaling pathway [3, 20]. In addition, it has been reported that the hydroalcoholic extract of M. oleifera seeds can reduce acetic acid-induced colitis in rats [21]. Moreover, the isothiocyanate extracted from M. oleifera seeds can effectively alleviate DSS-induced colitis [22]. However, knowledge about the effects of MOPE on gut health is limited. Therefore, the present study aimed to investigate MOPE-mediated alleviation of DSS-induced colitis in mice.
Here, we showed that MOPE could significantly ameliorate the symptoms of DSS-induced colitis, including mitigating body weight loss, colonic tissue damage, the secretion of inflammatory cytokines, and the infiltration of inflammatory cells. IL-6 and TNF-α are proinflammatory cytokines that mediate inflammatory responses in the UC model. Excessive secretion of these inflammatory cytokines can cause colitis [15]. Many studies have reported that the levels of IL-6 and TNF-α in IBD mice are increased [23–25]. IL-6 is an interleukin produced by a variety of cells and is closely related to inflammation and the immune response. IL-6 stimulates neutrophil chemotaxis and causes tissue destruction in the colon. Some studies have found that IL-6 is elevated in many IBDs and may be associated with the NF-κB signaling pathway [26, 27]. TNF-α, a cytokine with multiple effects that is produced by activated T cells, plays an essential role in the pathogenesis of colitis by triggering the accumulation and activation of leukocytes. TNF-α overexpression is vital for the pathogenesis of the intestinal mucosa [28, 29]. In the present study, DSS induced an increase in TNF-α and IL-6 in mice, whereas MOPE treatment significantly reduced the increases in both inflammatory factors.
NF-κB is a classic signaling pathway associated with inflammation. Several studies have shown that some natural compounds, such as caffeic acid, blueberry polyphenols, and Aster glehni extract, can improve UC by inhibiting the NF-κB pathway [25, 30–32]. It was shown that the ethyl acetate extract of M. oleifera inhibited NF-κB p65 in RAW 264.7 cells, and it exerted anti-inflammatory effects by upregulating the expression of IκB inhibitor (IκBα) and blocking the nuclear translocation of NF-κB [3]. NF-κB family members are retained in the cytoplasm bound to a class of inhibitory proteins termed IκBs [33]. When cells are activated, IκB is phosphorylated by IκB kinase (IKK) and degraded, after which the released NF-κB translocates to the nucleus and activates transcription. In other words, phosphorylation of IκB (p-IκB) is a crucial step in NF-κB activation, which leads to activation of the NF-κB signaling pathway [34]. The increase in TNF-α-related indicators is an essential manifestation of NF-κB pathway activation. Many treatments target TNF-α, and some related drugs, including TNF-α blockers, have been successfully used in the treatment of patients with IBD [31, 35]. For these reasons, the proteins related to the NF-κB signaling pathways were investigated in the present study. Our results showed that the protein expression of NF-κB p65 in the nucleus and p-IκBα and TNF-α in colon tissue of DSS model mice was higher than that of control mice, and the expression of IκBα was decreased. This result indicated that the NF-κB pathway was activated in the colonic mucosa. After MOPE administration, the protein expression of NF-κB p65 and p-IκBα was downregulated, and the expression of IκBα was upregulated. Furthermore, MOPE treatment inhibited the high expression of TNF-α in colon tissue. These results indicate that the anti-inflammatory effects of MOPE may be related to the inhibition of the NF-κB signaling pathway.
In the present study, MOPE is a mixed extract. The main phenolic substances in MOPE are kaempferol, quercetin, chlorogenic acid, isoquercitrin, rutin, and luteolin, as determined by UPLC-QTOF-MS/MS analysis, which is consistent with the results of the study by Zhu et al. [36]. In the future, we will focus on the separation and purification of M. oleifera polyphenols to determine which phenolic substance has anti-inflammatory or multicomponent synergistic effects.
In conclusion, our research shows that MOPE can alleviate DSS-induced colitis, including mitigating body weight loss, colon shortening, the secretion of inflammatory cytokines, colon tissue damage, and inflammatory cell infiltration. Further study examination shows that MOPE may alleviate colitis by inhibiting NF-κB signaling pathway activation. These results indicate that M. oleifera can be developed as a potential health food for preventing colitis.
Acknowledgments
This work was supported by the Ministry of Agriculture Tropical Crop Technology Pilot Demonstration Project “M. oleifera product processing demonstration” (K2500053), “Deep Processing and Technical Demonstration of Moringa, Macadamia and Tropical Fruits” (18190026), and Personnel Training Project on Academic and Technical Leaders of Yunnan Province (2018HB040).
Contributor Information
Lingfei Li, Email: lingfeili@163.com.
Yang Tian, Email: tianyang1208@163.com.
Jun Sheng, Email: shengjun_ynau@163.com.
Data Availability
The data to support the findings of this study are included within the article. Other data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Authors' Contributions
Yunjuan Zhang and Lei Peng contributed equally to this article.
Supplementary Materials
The details of the DAI grading standards are listed in the Supplementary Materials (Table S1). A detailed description of the Histological Scores of Colon Damage is listed in the Supplementary Materials (Table S2).
References
- 1.Waterman C., Cheng D. M., Rojas-Silva P., et al. Stable, water extractable isothiocyanates from Moringa oleifera leaves attenuate inflammation in vitro. Phytochemistry. 2014;103:114–122. doi: 10.1016/j.phytochem.2014.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matic I., Guidi A., Kenzo M., Mattei M., Galgani A. Investigation of medicinal plants traditionally used as dietary supplements: a review on Moringa oleifera. Journal of Public Health in Africa. 2018;9(3):191–199. doi: 10.4081/jphia.2018.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arulselvan P., Tan W. S., Gothai S., et al. Anti-Inflammatory potential of ethyl acetate fraction of Moringa oleifera in downregulating the NF-kappaB signaling pathway in lipopolysaccharide-stimulated macrophages. Molecules. 2016;21(11) doi: 10.3390/molecules21111452.e1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuorkey M. J. Effects of Moringa oleiferaaqueous leaf extract in alloxan induced diabetic mice. Interventional Medicine and Applied Science. 2016;8(3):109–117. doi: 10.1556/1646.8.2016.3.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie J., Wang Y., Jiang W. W., et al. Moringa oleifera leaf petroleum ether extract inhibits lipogenesis by activating the AMPK signaling pathway. Frontiers in Pharmacology. 2018;9 doi: 10.3389/fphar.2018.01447.1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anwar F., Latif S., Ashraf M., Gilani A. H. Moringa oleifera: a food plant with multiple medicinal uses. Phytotherapy Research. 2007;21(1):17–25. doi: 10.1002/ptr.2023. [DOI] [PubMed] [Google Scholar]
- 7.Jaja-Chimedza A., Graf B. L., Simmler C., et al. Biochemical characterization and anti-inflammatory properties of an isothiocyanate-enriched moringa (Moringa oleifera) seed extract. PLoS One. 2017;12(8) doi: 10.1371/journal.pone.0182658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ni J., Wu G. D., Albenberg L., Tomov V. T. Gut microbiota and IBD: causation or correlation? Nature Reviews Gastroenterology & Hepatology. 2017;14(10):573–584. doi: 10.1038/nrgastro.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trallori G., Palli D., Saieva C., et al. A population-based study of inflammatory bowel disease in Florence over 15 years (1978-92) Scandinavian Journal of Gastroenterology. 1996;31(9):892–899. doi: 10.3109/00365529609051998. [DOI] [PubMed] [Google Scholar]
- 10.Nadeem M. S., Kumar V., Al-Abbasi F. A., Kamal M. A., Anwar F. Risk of colorectal cancer in inflammatory bowel diseases. Seminars in Cancer Biology. 2020;64:51–60. doi: 10.1016/j.semcancer.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Atreya I., Atreya R., Neurath M. F. NF-κB in inflammatory bowel disease. Journal of Internal Medicine. 2008;263(6):591–596. doi: 10.1111/j.1365-2796.2008.01953.x. [DOI] [PubMed] [Google Scholar]
- 12.Giner E., Andújar I., Recio M. C., Ríos J. L., Cerdá-Nicolás J. M., Giner R. M. Oleuropein ameliorates acute colitis in mice. Journal of Agricultural and Food Chemistry. 2011;59(24):12882–12892. doi: 10.1021/jf203715m. [DOI] [PubMed] [Google Scholar]
- 13.Sostres C., Gargallo C. J., Arroyo M. T., Lanas A. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best Practice & Research Clinical Gastroenterology. 2010;24(2):121–132. doi: 10.1016/j.bpg.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Pei F., Tao H., Cai L., et al. Optimization of polyphenols from Moringa oleifera Lam. leaves by ultrasound-assisted extraction using response surface methodology and their antioxidant activities. Food Science. 2016;37(20):24–30. in Chinese. [Google Scholar]
- 15.Kang S.-H., Jeon Y.-D., Moon K.-H., et al. Aronia berry extract ameliorates the severity of dextran sodium sulfate-induced ulcerative colitis in mice. Journal of Medicinal Food. 2017;20(7):667–675. doi: 10.1089/jmf.2016.3822. [DOI] [PubMed] [Google Scholar]
- 16.Liu W., Zhang Y., Qiu B., et al. Quinoa whole grain diet compromises the changes of gut microbiota and colonic colitis induced by dextran Sulfate sodium in C57BL/6 mice. Scientific Reports. 2018;8(1) doi: 10.1038/s41598-018-33092-9.14916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kooltheat N., Sranujit R., Chumark P., Potup P., Laytragoon-Lewin N., Usuwanthim K. An ethyl acetate fraction of Moringa oleifera Lam. inhibits human macrophage cytokine production induced by cigarette smoke. Nutrients. 2014;6(2):697–710. doi: 10.3390/nu6020697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kou X., Li B., Olayanju J. B., Drake J. M., Chen N. Nutraceutical or pharmacological potential of Moringa oleifera Lam. Nutrients. 2018;10(3):p. 343. doi: 10.3390/nu10030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muangnoi C., Chingsuwanrote P., Praengamthanachoti P., Svasti S., Tuntipopipat S. Moringa oleifera pod inhibits inflammatory mediator production by lipopolysaccharide-stimulated RAW 264.7 murine macrophage cell lines. Inflammation. 2012;35(2):445–455. doi: 10.1007/s10753-011-9334-4. [DOI] [PubMed] [Google Scholar]
- 20.Fard M. T., Arulselvan P., Karthivashan G., Adam S. K., Fakurazi S. Bioactive extract from Moringa oleifera inhibits the pro-inflammatory mediators in lipopolysaccharide stimulated macrophages. Pharmacognosy Magazine. 2015;11(4):556–563. doi: 10.4103/0973-1296.172961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Minaiyan M., Asghari G., Taheri D., Saeidi M., Nasr-Esfahani S. Anti-inflammatory effect of Moringa oleifera Lam. seeds on acetic acid-induced acute colitis in rats. Avicenna Journal of Phytomedicine. 2014;4(2):127–136. [PMC free article] [PubMed] [Google Scholar]
- 22.Kim Y., Wu A. G., Jaja-Chimedza A., et al. Isothiocyanate-enriched Moringa seed extract alleviates ulcerative colitis symptoms in mice. PLoS One. 2017;12(9) doi: 10.1371/journal.pone.0184709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Z., Wu X., Cao S., et al. Chlorogenic acid ameliorates experimental colitis by promoting growth of Akkermansia in mice. Nutrients. 2017;9(7):p. 677. doi: 10.3390/nu9070677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu B., Yang T., Zeng L., et al. Crude extract of Fuzhuan brick tea ameliorates DSS-induced colitis in mice. International Journal of Food Science & Technology. 2016;51(12):2574–2582. doi: 10.1111/ijfs.13241. [DOI] [Google Scholar]
- 25.Zhang Z., Wu X., Cao S., et al. Caffeic acid ameliorates colitis in association with increased Akkermansia population in the gut microbiota of mice. Oncotarget. 2016;7(22):31790–31799. doi: 10.18632/oncotarget.9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tong L.-C., Wang Y., Wang Z.-B., et al. Propionate ameliorates dextran sodium sulfate-induced colitis by improving intestinal barrier function and reducing inflammation and oxidative stress. Frontiers in Pharmacology. 2016;7:p. 253. doi: 10.3389/fphar.2016.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jang S.-E., Lim S.-M., Jeong J.-J., et al. Gastrointestinal inflammation by gut microbiota disturbance induces memory impairment in mice. Mucosal Immunology. 2018;11(2):369–379. doi: 10.1038/mi.2017.49. [DOI] [PubMed] [Google Scholar]
- 28.van Deventer S. J. Immunology in medical practice. IV. Inflammatory bowel diseases: pathogenic starting points for specific therapy. Nederlands Tijdschrift Voor Geneeskunde. 1997;141(41):1956–1959. [PubMed] [Google Scholar]
- 29.Biasi F., Leonarduzzi G., Oteiza P. I., Poli G. Inflammatory bowel disease: mechanisms, redox considerations, and therapeutic targets. Antioxidants & Redox Signaling. 2013;19(14):1711–1747. doi: 10.1089/ars.2012.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pervin M., Hasnat M. A., Lim J.-H., et al. Preventive and therapeutic effects of blueberry (Vaccinium corymbosum) extract against DSS-induced ulcerative colitis by regulation of antioxidant and inflammatory mediators. The Journal of Nutritional Biochemistry. 2016;28:103–113. doi: 10.1016/j.jnutbio.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z.-L., Fan H.-Y., Yang M.-Y., Zhang Z.-K., Liu K. Therapeutic effect of a hydroxynaphthoquinone fraction on dextran sulfate sodium-induced ulcerative colitis. World Journal of Gastroenterology. 2014;20(41):15310–15318. doi: 10.3748/wjg.v20.i41.15310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi J.-H., Chung K.-S., Jin B.-R., et al. Anti-inflammatory effects of an ethanol extract of Aster glehni via inhibition of NF-κB activation in mice with DSS-induced colitis. Food & Function. 2017;8(7):2611–2620. doi: 10.1039/c7fo00369b. [DOI] [PubMed] [Google Scholar]
- 33.Liu X., Togo S., Al-Mugotir M., et al. NF-kappaB mediates the survival of human bronchial epithelial cells exposed to cigarette smoke extract. Respiratory Research. 2008;9(1):p. 66. doi: 10.1186/1465-9921-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tak P. P., Firestein G. S. NF-κB: a key role in inflammatory diseases. Journal of Clinical Investigation. 2001;107(1):7–11. doi: 10.1172/jci11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hyams J. S., Lerer T., Griffiths A., et al. Long-term outcome of maintenance infliximab therapy in children with Crohnʼs disease. Inflammatory Bowel Diseases. 2009;15(6):816–822. doi: 10.1002/ibd.20845. [DOI] [PubMed] [Google Scholar]
- 36.Zhu Y., Yin Q., Yang Y. Comprehensive investigation of Moringa oleifera from different regions by simultaneous determination of 11 polyphenols using UPLC-ESI-MS/MS. Molecules. 2020;25(3) doi: 10.3390/molecules25030676.e676 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The details of the DAI grading standards are listed in the Supplementary Materials (Table S1). A detailed description of the Histological Scores of Colon Damage is listed in the Supplementary Materials (Table S2).
Data Availability Statement
The data to support the findings of this study are included within the article. Other data used to support the findings of this study are available from the corresponding author upon request.


