Abstract
Background:
Outdoor artificial light at night (ALAN) has been implicated in a growing number of adverse health outcomes. ALAN is believed to disrupt circadian rhythms and has been associated with increased inflammation, one of the hallmarks of cancer. We examined the association between outdoor ALAN and a cancer strongly associated with autoimmune and inflammatory conditions, non-Hodgkin lymphoma (NHL), in the prospective California Teachers Study cohort.
Methods:
Outdoor ALAN was assigned to participant addresses at study baseline (1995-96) through use of the New World Atlas of Artificial Night Sky Brightness. Among 105,937 women followed from 1995-2015, linkage to the California Cancer Registry identified 873 incident cases of NHL. Age-stratified Cox proportional hazards models were used to calculate hazard ratios (HR) and 95% confidence intervals (95%CI) for overall NHL and the most common NHL subtypes; diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL) and chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). Multivariate analyses adjusted for previously reported subtype specific covariates (e.g. body mass index (BMI) for DLBCL).
Results:
Compared to the lowest quintile, participants residing in the highest quintile of outdoor ALAN at baseline were more likely to develop NHL (HR=1.32, 95%CI=1.07-1.63), and, in particular, DLBCL (HR=1.87, 95%CI=1.16-3.02). The elevated risk for DLBCL remained statistically significant after adjusting for age, race/ethnicity, BMI, and socioeconomic status (DLBCL:HR=1.87, 95%CI=1.16-3.02, NHL:HR=1.32, 95%CI=1.07-1.63). There was no association between ALAN and FL or CLL/SLL.
Conclusion:
DLBCL risk was elevated among women residing in neighborhoods with greater outdoor ALAN. Future research in circadian disruption and DLBCL may clarify potential biological processes implicated in this association.
Keywords: non-Hodgkin lymphoma, light at night, circadian disruption, cohort study
Introduction
Growing scientific evidence indicates that artificial light at night (ALAN) adversely affects human health. Major contributors of outdoor ALAN include commercial lighting, industrial lighting, street lighting, and residential lights[1]. Outdoor ALAN has steadily increased for decades and at accelerating rates. Estimates range from 2-3% per year to as much as 20% in larger cities[2]. For example, the Mount Wilson Observatory in the San Gabriel Mountains of Los Angeles County experienced a rapid decline in utility for nighttime astronomical research due to increasing light pollution from the 1970s through the 2000s[2, 3]. More recent data has shown outdoor ALAN levels have begun to level off in more recent years[4].
Advances in hand-held instrumentation and wearable devices may soon lead to calibrated exposure metrics for ALAN in living environments, but currently such methods are difficult and cost-prohibitive in a large epidemiologic research setting; therefore, many studies make use of satellite measurements as a proxy measure for ground-level exposure. The most commonly used satellite products include the Defense Meteorological Satellite Program’s Optical Linescan System (DMSP-OLS) which has been previously utilized in our cohort, the California Teachers Study (CTS)[5]. This satellite detects visible and near-infrared light emissions at a 2.7 km spatial resolution; however, limitations such as the coarse resolution and oversaturation of urban areas led to the launch of its successor, the Visible Infrared Imaging Radiometer Suite (VIIRS), in 2011. VIIRS, onboard the Suomi National Polar-orbiting Partnership (SNPP) satellite obtains nighttime observations in the day-night band (DNB) at ~750 m resolution and provides improved dynamic range[1]. While VIIRS is an improvement over the DMSP-OLS, these satellite-based measures only capture upward radiating light that escapes the atmosphere and may not be as representative of light pollution at a location, which is also influenced by light scattering in the atmosphere. While no satellite metric captures these sources, researchers at the Light Pollution Science and Technology Institute, led by Dr Fabio Falchi, have developed the New World Atlas of Artificial Night Sky Brightness to address such limitations[6]. The New World Atlas represents an improvement over VIIRS DNB upward radiance through a global measure of zenith brightness (the artificial brightness of the sky directly overhead) coupled with VIIRS observations and validated against thousands of handheld sky quality measurements. Furthermore, exposure to light on the ground includes light from the entire hemisphere of the sky in addition to the zenith brightness measured by the World Atlas and, importantly, direct glare from local sources. Simons et al. have confirmed that total light exposure on the ground is more highly correlated with zenith brightness estimated by the World Atlas than it is with upward radiance from VIIRS DNB in a southern California field study[7].
Exposure to light at night before sleep has shown to suppress melatonin production and disrupt circadian rhythm[8, 9]. This desynchronization between biological time and clock time has been associated with other proinflammatory conditions such as heart disease and diabetes[10]. Melatonin has also been shown to modulate activation of the inflammatory nuclear transcription factor kappa beta (NF-κβ) pathway, which in turn modulates levels of several immune cytokines such as interleukins (IL) −2, −6, −10, tumor necrosis factor (TNF), and C-reactive protein (CRP)[11], many of which have also been implicated in NHL etiology[12]. Exposure to light at night and disruptions to hormonal and endocrine regulation has been studied more extensively in laboratory settings, where animal studies have shown the role of ALAN in foraging behavior and elevated weight[13] as well as tumor growth[14]. Xenografted rodent models have confirmed a mechanistic pathway of melatonin suppression by light and subsequent cancer growth[15].
Epidemiological studies have linked satellite-measured outdoor lighting to increased breast, lung, colorectal, and prostate cancer risk[5, 16-19]; as well as proinflammatory conditions such as diabetes[20]. Of the studies that have included lymphoma, no association has been found[19]. A few studies to date have suggested potential links between disrupted circadian rhythms and non-Hodgkin lymphoma (NHL) risk. One study evaluated cancer risk based on different U.S. time zones, hypothesizing that those living in the western region of any time zone may result in altered sleep due to later onset of nighttime relative to clock time and earlier waking relative to sunrise. Gu et al reported increased risk of the NHL subtype, chronic lymphocytic leukemia (CLL) among those living closer to the western edge of a time zone[21]. Consistent with this finding is reported evidence of epigenetic silencing of the circadian clock gene, Cryptochrome 1, among CLL patients[22]. Gu et al. also saw increased risk of overall NHL among men residing in more western time zones and a link between sleep disruption from shift work and NHL has been suggested in a Finnish study, again only in men[21, 23].
Here, we sought to investigate any association between outdoor ALAN and non-Hodgkin lymphoma, a cancer of the immune response which takes years to develop. We hypothesized that outdoor ALAN may be a marker of sleep disruption and subsequent chronic inflammation. We selected ALAN as our exposure of interest because it could serve as surrogate exposure for biological processes and be measured at study baseline (1995-1996) by leveraging geospatial data available from the cohort, thus providing critical clues to whether additional research into the related pathways – sleep disruption, direct measure of circadian rhythm, inflammation – are warranted.
Materials and Methods
Study Population
The California Teachers Study (CTS) is a large prospective cohort recruited from active or retired teachers and members of the California State Teachers Retirement System in 1995. Complete details of the cohort have been published previously [24]. Briefly, 133,477 women returned the baseline questionnaire and have participated in ongoing follow-up activities. The CTS represents a broad age range (22-104 years at baseline, median age 53) in both urban and rural areas. The baseline questionnaire (1995-1996) captured detailed information on diet, alcohol use, height/weight, physical activity, smoking, residential address, environmental exposures, medication use, and personal/family history of cancer including NHL. Each participant’s address was assigned a latitude and longitude. For our analysis, participants were excluded if they lived outside of California at baseline or their address could not be geocoded (n=8,343), had prevalent cancer at baseline (n=8,115), or did not answer (n=5,904)/reported sleeping at night with a light on in the bedroom at baseline (n=5,173). The final analytic cohort of consisted of 105,937 participants. Informed consent was obtained according to the Declaration of Helsinki and this study is approved by the Institutional Review Boards of the City of Hope and University of Southern California in accordance with assurances filed and approved by the U.S. Department of Health and Human Services.
Exposure Assessment
We assessed outdoor ALAN using the New World Atlas of Artificial Night Sky Brightness[6], which provides a global measure of luminance at the zenith in millicandela per meter squared (mcd/m2) at 750 m gridded spatial resolution. The World Atlas was based on VIIRS and handheld measurements taken in 2014. The World Atlas residential outdoor ALAN was retrospectively assigned by linking geocoded residential addresses at study baseline (1995-1996) to the 750 m grid cell in which it fell using the raster package in R version 3.4.3. Cohort-wide quintiles of ALAN were computed to derive the exposure metric for each participant.
Outcome Assessment
Cases were obtained through linkage to the California Cancer Registry. Non-Hodgkin lymphoma (NHL) was defined, according to the World Health Organization algorithm[25], as having a diagnosis with the International Classification of Diseases for Oncology, third edition codes of: diffuse large B-cell lymphoma (DLBCL: 9678, 9679, 9680, 9684), follicular lymphoma (FL: 9690, 9691, 9695, 9698), mantle cell lymphoma (MCL: 9673), marginal zone lymphoma (MZL: 9699), chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL: 9670, 9823), Burkitt lymphoma (BL: ICD-O-3 codes 9687, 9826), and other B cell lymphomas (other, including not otherwise specified (NOS): 9590, 9591, 9596, 9671, 9675, 9727, 9728, 9833, 9835, 9836, 9761). We identified 873 incident cases of NHL in the cohort from baseline through December 31, 2015.
Covariates
We assessed confounding among several covariates potentially related to either NHL or ALAN, including; age, race/ethnicity (non-Hispanic White, Black, Hispanic, Asian/Pacific Islander, Other), alcohol and smoking status (current, former, never), body mass index (BMI; <18.5, 18.5-25, 25-30, or ≥30 kg/m2), United States Census block level socioeconomic status (SES; statewide quartiles), United States Census block urban/rural status[26], ultraviolet radiation (UV) level at residence, use of nonsteroidal anti-inflammatory drugs, recreational physical activity (meeting/not meeting American Heart Association recommended physical activity levels[27]), exposure to pesticides, menopausal hormone use, and family history of NHL.
Statistical Methods
We evaluated the association between outdoor ALAN and NHL overall and among the three most common subtypes (DLBCL, FL, CLL/SLL). We calculated hazard ratios (HR) and 95% confidence intervals (95% CI) using an age-stratified Cox proportional hazards model. In the Cox regression models, the time scale (in days) was defined by age at entry into the cohort and the first of the following ages: at event (NHL diagnosis), at censoring (e.g., when a participant moved out of California for more than four months), at death, or at end of follow-up (December 31, 2015). We adjusted for subtype specific covariates previously reported; age, race, SES, BMI, and family history of NHL for DLBCL[28], age, race, SES, smoking, alcohol consumption, and family history of NHL for FL[29], and age, race SES, smoking, height, and family history of NHL for CLL/SLL[30]. In addition, we assessed the association between participant residential position (latitudinal and longitudinal degrees) and NHL risk based on the hypothesis outlined by Gu et al[21]. All analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC).
Results
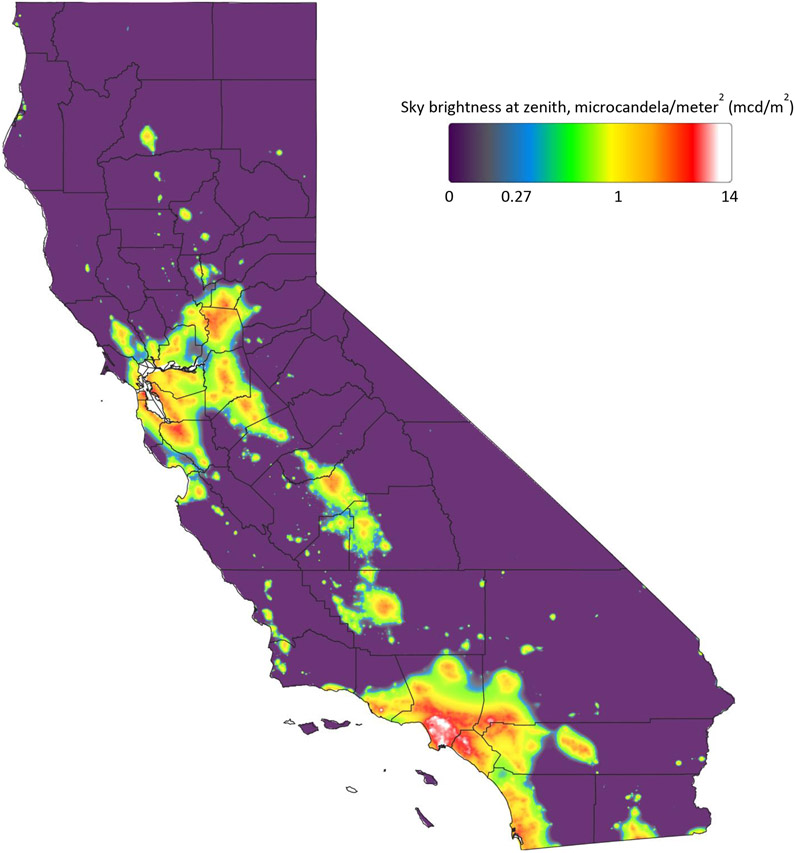
For the 105,937 study participants in the analytic sample, the most common NHL subtype was DLBCL (n=212), followed by CLL/SLL (n=216) and FL (n=171). The median level of ALAN in the cohort was 2.40 mcd/m2 (Table 1). For comparison, a natural, moonless night sky would have a brightness of 0.2 mcd/m2 and at 2 mcd/m2 the Milky Way would no longer be visible[31]. The highest levels of ALAN were for participants in Los Angeles County, especially those residing near the Port of Long Beach where levels reached 14 mcd/m2 (Figure 1). The more rural areas of California exhibited the lowest levels of ALAN.
Table 1.
Self-reported demographic characteristics and New World Atlas of Artificial Night Sky Brightness among 105,937 women in the California Teachers Study Cohort at baseline (1995-1996)
| Cohort n=105,937 |
NHL n=873 |
p-value | |||
|---|---|---|---|---|---|
| Race | |||||
| White | 92,393 | 87.2% | 792 | 90.7% | |
| Black | 2,487 | 2.3% | 16 | 1.8% | |
| Hispanic | 4,616 | 4.4% | 22 | 2.5% | |
| Asian/Pacific islander | 4,483 | 4.2% | 29 | 3.3% | |
| Other | 1,958 | 1.8% | 14 | 1.6% | 0.049 |
| Light at Night | |||||
| Median (IQR) | 2.40 | (1.22-3.95) | 2.50 | (1.27-4.29) | |
| Quintile 1 | 21,186 | 20.0% | 158 | 18.1% | |
| Quintile 2 | 21,189 | 20.0% | 181 | 20.7% | |
| Quintile 3 | 21,187 | 20.0% | 172 | 19.7% | |
| Quintile 4 | 21,188 | 20.0% | 163 | 18.7% | |
| Quintile 5 | 21,187 | 20.0% | 199 | 22.8% | 0.14 |
| UV | |||||
| Quintile 1 | 18,096 | 20.0% | 143 | 19.4% | |
| Quintile 2 | 18,095 | 20.0% | 150 | 20.4% | |
| Quintile 3 | 17,978 | 19.9% | 170 | 23.1% | |
| Quintile 4 | 18,103 | 20.0% | 163 | 22.1% | |
| Quintile 5 | 18,149 | 20.1% | 110 | 14.9% | 0.01 |
| SES | |||||
| Quartile 1 | 4,578 | 4.4% | 23 | 2.7% | |
| Quartile 2 | 18,214 | 17.5% | 131 | 15.1% | |
| Quartile 3 | 34,409 | 33.0% | 267 | 30.8% | |
| Quartile 4 | 46,944 | 45.1% | 445 | 51.4% | <0.001 |
| Rural Census Block | |||||
| Rural | 14,956 | 14.4% | 111 | 12.8% | |
| Suburban | 23,002 | 22.1% | 169 | 19.5% | |
| Urban | 66,231 | 63.6% | 586 | 67.7% | 0.016 |
| BMI | |||||
| <20 | 11,319 | 11.1% | 68 | 8.2% | |
| 20-24.9 | 51,496 | 50.6% | 405 | 48.9% | |
| 25-29.9 | 25,025 | 24.6% | 227 | 27.4% | |
| 30+ | 13,913 | 13.7% | 128 | 15.5% | 0.4 |
| Smoking | |||||
| Never | 70,547 | 67.0% | 535 | 61.7% | |
| Former | 29,541 | 28.1% | 294 | 33.9% | |
| Current | 5,185 | 4.9% | 38 | 4.4% | 0.005 |
| Alcohol | |||||
| Never | 33,606 | 33.4% | 287 | 34.0% | |
| Former | 58,546 | 58.3% | 492 | 58.3% | |
| Current | 8,316 | 8.3% | 65 | 7.7% | 0.22 |
| Any Daily NSAID | |||||
| no | 73,331 | 70.2% | 603 | 70.1% | |
| yes | 31,091 | 29.8% | 257 | 29.9% | 0.57 |
| Exercise | |||||
| did not meet AHA guidelines | 35,991 | 34.2% | 277 | 32.1% | |
| met AHA guidelines | 69,202 | 65.8% | 585 | 67.9% | 0.088 |
| Family History of NHL | |||||
| no | 97,154 | 95.2% | 779 | 91.8% | |
| yes | 4,894 | 4.8% | 70 | 8.2% | <0.001 |
| Any exposure to pesticides | |||||
| no | 66,555 | 80.9% | 585 | 78.8% | 0.11 |
| yes | 15,711 | 19.1% | 157 | 21.2% | |
| Histology | |||||
| DLBCL | 212 | 22.7% | |||
| FL | 171 | 18.3% | |||
| CLL/SLL | 216 | 23.2% | |||
| Marginal Zone | 84 | 9.0% | |||
| Other | 190 | 20.4% | |||
NHL: non-Hodgkin lymphoma; DLBCL: diffuse large B-cell lymphoma; FL: follicular lymphoma; CLL/SLL: chronic lymphocytic leukemia/small lymphocytic lymphoma
Figure 1.
Outdoor artificial light at night in California based on the New World Atlas of Artificial Night Sky Brightness
Compared to participants in the lowest quintile of ALAN, those in the highest quintile had 32% increased risk (HR 1.32, 95% CI 1.07-1.63) of developing NHL (Table 2). Consistent with our previous findings, covariates that were associated with NHL risk included higher SES (HR 1.80, 95% CI 1.16-2.79, Supplemental Table 1) and family history of NHL (HR 1.55, 95% CI 1.21-1.98)[32]. After adjusting for age, race/ethnicity, SES, smoking status, BMI, and family history of NHL, the highest quintile of ALAN was still associated with a 32% increased risk of NHL (95% CI 1.06-1.65). There was no association between longitude of residence and NHL risk (Supplemental Table 2).
Table 2.
Association between light at night and non-Hodgkin lymphoma and major subtypes (DLBCL, FL, CLL/SLL) among 105,937 women in the California Teachers Study Cohort followed from 1995-2015
| Overall NHL (n=873) | DLBCL (n=212) | FL (n=171) | CLL/SLL (n=216) | |||||
|---|---|---|---|---|---|---|---|---|
| Univariate age adjusted | ||||||||
| Light at Night | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI |
| Quintile 1 | 1.00 | - | 1.00 | - | 1.00 | - | 1.00 | - |
| Quintile 2 | 1.20 | (0.97-1.49) | 1.79 | (1.11-2.89) | 1.32 | (0.84-2.07) | 0.90 | (0.58-1.40) |
| Quintile 3 | 1.10 | (0.89-1.38) | 1.74 | (1.07-2.82) | 1.13 | (0.70-1.80) | 0.79 | (0.50-1.24) |
| Quintile 4 | 1.03 | (0.83-1.29) | 1.52 | (0.93-2.49) | 0.82 | (0.50-1.37) | 0.93 | (0.61-1.44) |
| Quintile 5 | 1.32 | (1.07-1.63) | 1.87 | (1.16-3.02) | 0.84 | (0.51-1.40) | 1.28 | (0.86-1.91) |
| Multivariate | ||||||||
| Light at Night | HRα | 95% CI | HRβ | 95% CI | HRγ | 95% CI | HRδ | 95% CI |
| Quintile 1 | 1.00 | - | 1.00 | - | 1.00 | - | 1.00 | - |
| Quintile 2 | 1.14 | (0.90-1.44) | 1.57 | (0.96-2.59) | 1.44 | (0.87-2.38) | 0.88 | (0.55-1.40) |
| Quintile 3 | 1.04 | (0.82-1.32) | 1.51 | (0.91-2.48) | 1.33 | (0.79-2.23) | 0.73 | (0.45-1.19) |
| Quintile 4 | 0.94 | (0.74-1.20) | 1.27 | (0.76-2.13) | 0.92 | (0.53-1.62) | 0.86 | (0.54-1.37) |
| Quintile 5 | 1.32 | (1.05-1.66) | 1.70 | (1.03-2.79) | 1.06 | (0.61-1.84) | 1.30 | (0.84-2.00) |
Age, race, SES, BMI, smoking, alcohol, family history of NHL
Age, race, SES, BMI, family history of NHL
Age, race, SES, smoking, alcohol, family history of NHL
Age, race, SES, height, family history of NHL
NHL: non-Hodgkin lymphoma; DLBCL: diffuse large B-cell lymphoma; FL: follicular lymphoma; CLL/SLL: chronic lymphocytic leukemia/small lymphocytic lymphoma
Given our hypothesis that outdoor ALAN may drive circadian disruption, we excluded women who reported sleeping with a light on in the bedroom (n=5,173). Of these participants, 39 were diagnosed with NHL, and no association was observed between sleeping with a light on and NHL (HR 0.98, 95% CI 0.71-1.37).
By NHL subtype, the association with ALAN remained statistically significant for DLBCL (HR 1.87, 95% CI 1.16-3.02). There was no association between ALAN and FL (HR 0.84, 95% CI 0.51-1.40) or CLL/SLL (HR 1.28, 95% CI 0.86-1.91). Adjusting for age, race/ethnicity, BMI, family history of NHL, and SES slightly attenuated the association for DLBCL (HR 1.70, 95% CI 1.03-2.79).
Discussion
Outdoor artificial light at night was associated with an increased risk NHL for the DLBCL subtype. We did not observe statistically significant associations for either FL or CLL/SLL, but the increased risk of NHL overall was also significant on the basis of DLBCL cases. In contrast to the Gu et al study[21], we observed no association between NHL or NHL subtypes with longitude (Supplemental Table 2), but we did observe a nonsignificant increase in CLL/SLL risk for the highest quintile of ALAN. At present, it is unclear what exposure; ALAN, altered circadian rhythm, sleep disruption, or something else altogether, is the true underlying exposure that might explain the adverse health effects associated with ALAN, including increased NHL risk. Strengths of our study include the large sample size and length of followup. We were also able to examine individual-level, subtype specific covariates, including BMI, smoking, and exercise. Our linkage to the California Cancer Registry provides high quality case ascertainment with subtype information. We used the New World Atlas as a proxy for ground-level light exposure, a more accurate measure of outdoor ALAN exposure, which is both higher resolution than previous studies that were conducted with the DMSP-OLS and correlates more highly with ground-based exposure than the VIIRS DNB data [7] . Weaknesses to our study include relatively few cases to be powered to detect an association for individual subtypes. Also, our study was conducted in California, so all participants resided in the western portion of the Pacific time zone, making it difficult to assess light zone position as a contributor. We also lack additional features of the participants’-built environment such as housing and use of blinds or shades, but believe this would introduce nondifferential bias.
The New World Atlas of Artificial Night Sky Brightness does have its shortcomings when assessing exposure to ALAN. First, it only estimates brightness at the zenith and does not incorporate light from the whole hemisphere and especially on the horizon, which drives light exposures in human settlements[7]. Second, there is no distinction between the different wavelengths of light among the current satellite-based ALAN tools. Light in the blue spectrum (500-450 nm) has been shown to be especially disruptive to sleep[35], including suppression of melatonin[36]. A recently published study utilized high resolution color photography from the International Space Station to help address this limitation, though availability of such imagery is likely limited in both time and locations covered[37]. While the New World Atlas is a better measure of exposure to ALAN[7] it is based on the VIIRS data from 2014 of ALAN and was applied retrospectively to our cohort. Street lighting, the major contributor to outdoor ALAN, is fairly stable over time, therefore use of the World Atlas to retroactively assign exposure, particularly during the study follow-up period where ALAN has leveled off, is expected to be stable[2, 3, 38]. The few studies that have been conducted comparing direct satellite measured ALAN and bedroom exposure have found little to no correlation during sleep[39, 40], but this has not been examined for the New World Atlas. Disruptions to melatonin levels persist hours after exposure to a light source[41, 42], so continuous exposure to ALAN while asleep may not be necessary to contribute to disruption of circadian pathways. Finally, we do not have information on behavioral factors such as grading assignments at night, preparing for teaching the next day, television use in the bedroom, or handheld screen-based computing device usage in the cohort; the latter of which have been shown to delay onset of sleep[35], however, such exposures would likely be nondifferential as they are not expected to be associated with outdoor ALAN.
Future studies need better measures of circadian rhythm and sleep disruption. In the CTS, questions were not asked at baseline that could provide a more direct measure of circadian disruption. Although we had limited information on study participants who slept with a light on in the bedroom, the sample size was too small to detect an association with NHL risk. Additionally, this question was asked to ascertain exposure to electromagnetic fields so did not ask about other sources of light such as televisions. New technologies such as genome and epigenome wide studies could further elucidate the mechanism behind ALAN, sleep disruption, and disease risk. Both polymorphisms in circadian rhythm genes and epigenetic silencing have been associated with increased breast cancer risk[43].
In conclusion, we observed an increased risk for DLBCL, and NHL overall, among those living in the highest quintile of outdoor ALAN exposure compared to the lowest. As we are continuing to better understand the effects of our environment on sleep disruption, further studies assessing the risk associated with NHL and its subtypes are warranted.
Supplementary Material
Highlights.
The New World Atlas of Artificial Night Sky Brightness was used to assess exposure to light at night
There was increased risk of NHL for those in highest quintile of light at night
The risk association was strongest for the DLBCL subtype
The World Atlas is a unique resource for studying health effects associated with light at night
Acknowledgements
The California Teachers Study and the research reported in this publication were supported by the National Cancer Institute of the National Institutes of Health under award number U01 CA199277; P30 CA033572; P30 CA023100; UM1 CA164917; and R01 CA077398.
The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries, under cooperative agreement 5NU58DP006344; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201800032I awarded to the University of California, San Francisco, contract HHSN261201800015I awarded to the University of Southern California, and contract HHSN261201800009I awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and do not necessarily reflect the opinions of the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors.
Footnotes
Conflict of interest: The authors declare no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Elvidge CD, Baugh K, Zhizhin M, Hsu FC, Ghosh T, VIIRS night-time lights, International Journal of Remote Sensing 38(21) (2017) 5860–5879. [Google Scholar]
- [2].Hölker F, Moss T, Griefahn B, Kloas W, Voigt CC, Henckel D, Hänel A, Kappeler PM, Völker S, Schwope A, Franke S, Uhrlandt D, Fischer J, Klenke R, Wolter C, Tockner K, The Dark Side of Light: A Transdisciplinary Research Agenda for Light Pollution Policy, 15(4) (2010). [Google Scholar]
- [3].Cinzano P, The Growth of the Artificial Night Sky Brightness over North America in the Period 1947–2000: a Preliminary Picture, in: Schwarz HE (Ed.), Light Pollution: The Global View: Proceedings of the International Conference on Light Pollution, La Serena, Chile, held 5-7 March 2002, Springer; Netherlands, Dordrecht, 2003, pp. 39–47. [Google Scholar]
- [4].Stare J, Light pollution map, 2019. https://www.lightpollutionmap.info/. (Accessed 11/15/2019 2019). [Google Scholar]
- [5].Hurley S, Goldberg D, Nelson D, Hertz A, Horn-Ross PL, Bernstein L, Reynolds P, Light at Night and Breast Cancer Risk Among California Teachers, Epidemiology 25(5) (2014) 697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Falchi F, Cinzano P, Duriscoe D, Kyba CCM, Elvidge CD, Baugh K, Portnov BA, Rybnikova NA, Furgoni R, The new world atlas of artificial night sky brightness, Science Advances 2(6) (2016) e1600377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Simons AL, Yin X, Longcore T, High correlation but high scale-dependent variance between satellite measured night lights and terrestrial exposure, Environmental Research Communications (2020). [Google Scholar]
- [8].Reiter RJ, Tan D-X, Korkmaz A, Erren TC, Piekarski C, Tamura H, Manchester LC, Light at Night, Chronodisruption, Melatonin Suppression, and Cancer Risk: A Review, 13(4) (2007) 303–328. [DOI] [PubMed] [Google Scholar]
- [9].Lewy A, Wehr T, Goodwin F, Newsome D, Markey S, Light suppresses melatonin secretion in humans, Science (New York, N.Y.) 210(4475) (1980) 1267–1269. [DOI] [PubMed] [Google Scholar]
- [10].Touitou Y, Reinberg A, Touitou D, Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: Health impacts and mechanisms of circadian disruption, Life Sciences 173 (2017) 94–106. [DOI] [PubMed] [Google Scholar]
- [11].Favero G, Franceschetti L, Bonomini F, Rodella LF, Rezzani R, Melatonin as an Anti-Inflammatory Agent Modulating Inflammasome Activation, International Journal of Endocrinology 2017 (2017) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Makgoeng SB, Bolanos RS, Jeon CY, Weiss RE, Arah OA, Breen EC, Martínez-Maza O, Hussain SK, Markers of Immune Activation and Inflammation, and Non-Hodgkin Lymphoma: A Meta- Analysis of Prospective Studies, JNCI Cancer Spectr 2(4) (2018) pky082–pky082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Russart KLG, Nelson RJ, Light at night as an environmental endocrine disruptor, Physiology & Behavior 190 (2018) 82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Agbaria S, Haim A, Fares F, Zubidat AE, Epigenetic modification in 4T1 mouse breast cancer model by artificial light at night and melatonin – the role of DNA-methyltransferase, Chronobiology International 36(5) (2019) 629–643. [DOI] [PubMed] [Google Scholar]
- [15].Blask DE, Dauchy RT, Dauchy EM, Mao L, Hill SM, Greene MW, Belancio VP, Sauer LA, Davidson L, Light Exposure at Night Disrupts Host/Cancer Circadian Regulatory Dynamics: Impact on the Warburg Effect, Lipid Signaling and Tumor Growth Prevention, PLOS ONE 9(8) (2014) e102776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kloog I, Haim A, Stevens RG, Barchana M, Portnov BA, Light at Night Co-distributes with Incident Breast but not Lung Cancer in the Female Population of Israel, Chronobiology International 25(1) (2008) 65–81. [DOI] [PubMed] [Google Scholar]
- [17].James P, Bertrand KA, Hart JE, Schernhammer ES, Tamimi RM, Laden F, Outdoor Light at Night and Breast Cancer Incidence in the Nurses' Health Study II, Environmental health perspectives 125(8) (2017) 087010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Al-Naggar RA, Anil S, Artificial Light at Night and Cancer: Global Study, Asian Pacific journal of cancer prevention : APJCP 17(10) (2016) 4661–4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kim KY, Lee E, Kim YJ, Kim J, The association between artificial light at night and prostate cancer in Gwangju City and South Jeolla Province of South Korea, Chronobiology International 34(2) (2017) 203–211. [DOI] [PubMed] [Google Scholar]
- [20].Koo YS, Song J-Y, Joo E-Y, Lee H-J, Lee E, Lee S.-k., Jung K-Y , Outdoor artificial light at night, obesity, and sleep health: Cross-sectional analysis in the KoGES study, Chronobiology International 33(3) (2016) 301–314. [DOI] [PubMed] [Google Scholar]
- [21].Gu F, Xu S, Devesa SS, Zhang F, Klerman EB, Graubard BI, Caporaso NE, Longitude Position in a Time Zone and Cancer Risk in the United States, Cancer Epidemiology Biomarkers & Prevention 26(8) (2017) 1306–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hanoun M, Eisele L, Suzuki M, Greally JM, Hüttmann A, Aydin S, Scholtysik R, Klein-Hitpass L, Dührsen U, Dürig J, Epigenetic Silencing of the Circadian Clock Gene CRY1 is Associated with an Indolent Clinical Course in Chronic Lymphocytic Leukemia, PLoS ONE 7(3) (2012) e34347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lahti TA, Partonen T, Kyyrönen P, Kauppinen T, Pukkala E, Night-time work predisposes to non- Hodgkin lymphoma, International Journal of Cancer 123(9) (2008) 2148–2151. [DOI] [PubMed] [Google Scholar]
- [24].Bernstein L, Allen M, Anton-Culver H, Deapen D, Horn-Ross PL, Peel D, Pinder R, Reynolds P, Sullivan-Halley J, West D, Wright W, Ziogas A, Ross RK, High breast cancer incidence rates among California teachers: results from the California Teachers Study (United States), Cancer Causes & Control 13(7) (2002) 625–635. [DOI] [PubMed] [Google Scholar]
- [25].Turner JJ, Morton LM, Linet MS, Clarke CA, Kadin ME, Vajdic CM, Monnereau A, Maynadié M, Chiu BCH, Marcos-Gragera R, Costantini AS, Cerhan JR, Weisenburger DD, InterLymph hierarchical classification of lymphoid neoplasms for epidemiologic research based on the WHO classification (2008): update and future directions, Blood 116(20) (2010) e90–e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ratcliffe M, Burd C, Holder K, Fields A, Defining Rural at the U.S. Census Bureau, 2016. https://www2.census.gov/geo/pdfs/reference/ua/Defining_Rural.pdf. (Accessed 5/17/2019 2019). [Google Scholar]
- [27].Troiano RP, Physical Activity Guidelines for Americans From the US Department of Health and Human Services, (2018). [DOI] [PubMed] [Google Scholar]
- [28].Cerhan JR, Kricker A, Paltiel O, Flowers CR, Wang SS, Monnereau A, Blair A, Maso LD, Kane EV, Nieters A, Foran JM, Miligi L, Clavel J, Bernstein L, Rothman N, Slager SL, Sampson JN, Morton LM, Skibola CF, Medical History, Lifestyle, Family History, and Occupational Risk Factors for Diffuse Large B-Cell Lymphoma: The InterLymph Non-Hodgkin Lymphoma Subtypes Project, JNCI Monographs 2014(48) (2014) 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Linet MS, Vajdic CM, Morton LM, de Roos AJ, Skibola CF, Boffetta P, Cerhan JR, Flowers CR, de Sanjosé S, Monnereau A, Cocco P, Kelly JL, Smith AG, Weisenburger DD, Clarke CA, Blair A, Bernstein L, Zheng T, Miligi L, Clavel J, Benavente Y, Chiu BCH, Medical History, Lifestyle, Family History, and Occupational Risk Factors for Follicular Lymphoma: The InterLymph Non-Hodgkin Lymphoma Subtypes Project, JNCI Monographs 2014(48) (2014) 26–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Slager SL, Benavente Y, Blair A, Vermeulen R, Cerhan JR, Costantini AS, Monnereau A, Nieters A, Clavel J, Call TG, Maynadié M, Lan Q, Clarke CA, Lightfoot T, Norman AD, Sampson JN, Casabonne D, Cocco P, de Sanjosé S, Medical History, Lifestyle, Family History, and Occupational Risk Factors for Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma: The InterLymph Non-Hodgkin Lymphoma Subtypes Project, JNCI Monographs 2014(48) (2014) 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bortle JE, Introducing the Bortle dark-sky scale, Sky and Telescope 101(2) (2001). [Google Scholar]
- [32].Lu Y, Sullivan-Halley J, Cozen W, Chang ET, Henderson K, Ma H, Deapen D, Clarke C, Reynolds P, Neuhausen SL, Anton-Culver H, Ursin G, West D, Bernstein L, Family history of haematopoietic malignancies and non-Hodgkin's lymphoma risk in the California Teachers Study, British Journal Of Cancer 100 (2009) 524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Morton LM, Slager SL, Cerhan JR, Wang SS, Vajdic CM, Skibola CF, Bracci PM, de Sanjosé S, Smedby KE, Chiu BCH, Zhang Y, Mbulaiteye SM, Monnereau A, Turner JJ, Clavel J, Adami H-O, Chang ET, Glimelius B, Hjalgrim H, Melbye M, Crosignani P, di Lollo S, Miligi L, Nanni O, Ramazzotti V, Rodella S, Costantini AS, Stagnaro E, Tumino R, Vindigni C, Vineis P, Becker N, Benavente Y, Boffetta P, Brennan P, Cocco P, Foretova L, Maynadié M, Nieters A, Staines A, Colt JS, Cozen W, Davis S, de Roos AJ, Hartge P, Rothman N, Severson RK, Holly EA, Call TG, Feldman AL, Habermann TM, Liebow M, Blair A, Cantor KP, Kane EV, Lightfoot T, Roman E, Smith A, Brooks- Wilson A, Connors JM, Gascoyne RD, Spinelli JJ, Armstrong BK, Kricker A, Holford TR, Lan Q, Zheng T, Orsi L, Dal Maso L, Franceschi S, La Vecchia C, Negri E, Serraino D, Bernstein L, Levine A, Friedberg JW, Kelly JL, Berndt SI, Birmann BM, Clarke CA, Flowers CR, Foran JM, Kadin ME, Paltiel O, Weisenburger DD, Linet MS, Sampson JN, Etiologic Heterogeneity Among Non-Hodgkin Lymphoma Subtypes: The InterLymph Non-Hodgkin Lymphoma Subtypes Project, JNCI Monographs 2014(48) (2014) 130–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Batista JL, Birmann BM, Epstein MM, Epidemiology of Hematologic Malignancies, in: Loda M, Mucci LA, Mittelstadt ML, Van Hemelrijck M, Cotter MB (Eds.), Pathology and Epidemiology of Cancer, Springer International Publishing, Cham, 2017, pp. 543–569. [Google Scholar]
- [35].Mortazavi SAR, Parhoodeh S, Hosseini MA, Arabi H, Malakooti H, Nematollahi S, Mortazavi G, Darvish L, Mortazavi SMJ, Blocking Short-Wavelength Component of the Visible Light Emitted by Smartphones' Screens Improves Human Sleep Quality, Journal of biomedical physics & engineering 8(4) (2018) 375–380. [PMC free article] [PubMed] [Google Scholar]
- [36].Tähkämö L, Partonen T, Pesonen A-K, Systematic review of light exposure impact on human circadian rhythm, Chronobiology International 36(2) (2019) 151–170. [DOI] [PubMed] [Google Scholar]
- [37].Garcia-Saenz A, Miguel A.S.d., Espinosa A, Valentin A, Aragonés N, Llorca J, Amiano P, Sánchez VM, Guevara M, Capelo R, Tardón A, Peiró-Perez R, Jiménez-Moleón JJ, Roca-Barceló A, Pérez-Gómez B, Dierssen-Sotos T, Fernández-Villa T, Moreno-Iribas C, Moreno V, García-Pírez J, Castaño-Vinyals G, Pollán M, Aubé M, Kogevinas M, Evaluating the Association between Artificial Light- at-Night Exposure and Breast and Prostate Cancer Risk in Spain (MCC-Spain Study), Environmental health perspectives 126(4) (2018) 047011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kyba CCM, Aronson KJ, Assessing Exposure to Outdoor Lighting and Health Risks, Epidemiology 26(4) (2015) e50. [DOI] [PubMed] [Google Scholar]
- [39].Huss A, Wel L.v., Bogaards L, Vrijkotte T, Wolf L, Hoek G, Vermeulen R, Shedding Some Light in the Dark—A Comparison of Personal Measurements with Satellite-Based Estimates of Exposure to Light at Night among Children in the Netherlands, Environmental health perspectives 127(6) (2019) 067001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Rea MS, Brons JA, Figueiro MG, Measurements of Light at Night (LAN) for a Sample of Female School Teachers, Chronobiology International 28(8) (2011) 673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gooley JJ, Chamberlain K, Smith KA, Khalsa SBS, Rajaratnam SMW, Van Reen E, Zeitzer JM, Czeisler CA, Lockley SW, Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans, J Clin Endocrinol Metab 96(3) (2011) E463–E472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Akacem LD, Wright KP Jr., LeBourgeois MK, Sensitivity of the circadian system to evening bright light in preschool-age children, Physiol Rep 6(5) (2018) e13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Reszka E, Przybek M, Chapter Three - Circadian Genes in Breast Cancer, in: Makowski GS (Ed.), Advances in Clinical Chemistry, Elsevier; 2016, pp. 53–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.