Abstract
Cancer cells continuously rewire their metabolism to fulfil their need for rapid growth and survival whilst subject to changes in environmental cues. Thus, a vital component of a cancer cell lies in its metabolic adaptability. The constant demand for metabolic alterations requires flexibility, i.e. the ability to utilize different metabolic substrates, as well as plasticity, i.e. the ability to process metabolic substrates in different ways. In this review, we discuss how dynamic changes in cancer metabolism affect tumour progression and the consequential implications for cancer therapy.
Introduction
Since the original work of Otto Warburg in the 1920s that demonstrated cancer cells prefer glycolysis over mitochondrial respiration even under conditions of sufficient oxygen supply 1,2, multiple studies have shown that the metabolic reprogramming of cancer is not static, but rather a highly dynamic process. The essentiality and uniqueness of these metabolic alterations enabled new discoveries in cancer diagnosis and therapy and led to the designation of cancer metabolism as one of cancers’ hallmarks 3. Indeed, numerous metabolic alterations are co-opted by cancer cells during tumour initiation and progression, to maximize cancer fitness to the ever- changing environmental cues 4. Therefore, continuous metabolic adaptations are key for cancer cell growth and survival. These adaptations are achieved by coordinated intrinsic changes in gene expression leading to suppression and activation of enzymes, as well as by extrinsic fluctuations in the levels of metabolites, which directly induce or repress a specific metabolic pathway.
In this review we frame the nuances in the metabolic reprogramming during tumour progression as metabolic flexibility (the ability to use different nutrients) and plasticity (the ability to process the same nutrient differently) 5. In addition, we highlight the dynamic ability of metabolism during tumorigenesis as a target for improving response to cancer therapy and for overcoming resistance.
A. Metabolic flexibility and plasticity during cancer progression, an overview (Figure 1)
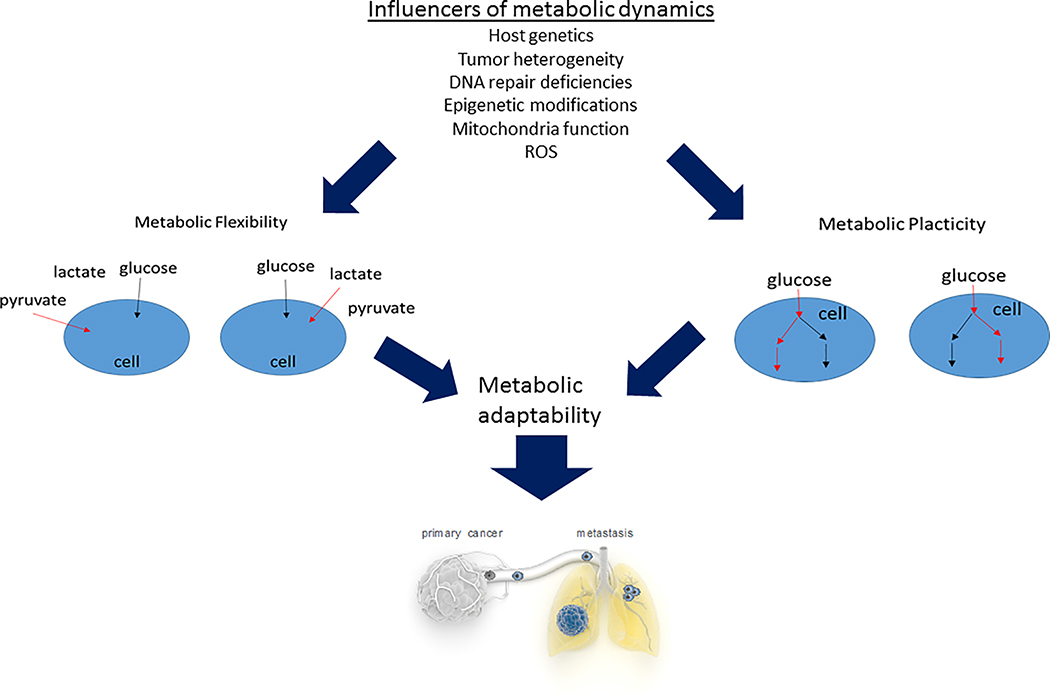
Figure 1: Metabolic flexibility and plasticity determine tumour metabolic adaptability.
Multiple features affect tumour metabolic dynamics as exemplified by its flexibility and plasticity that enable its progression via metabolic adaptability to changing environmental cues.
As tumours grow and progress, cancer cells face changing microenvironments, which are composed of different nutrients, metabolites, and cell types. This happens at first because as the tumour proliferates and grows, differently vascularized areas arise, resulting in gradients of oxygen, available nutrients and in accumulation of tumour produced metabolites. Consequently, and depending on the tumour type, metabolic intratumour heterogeneity can arise. In this respect it has been shown that within the same human lung cancer lesions, cancer cells in low perfused areas of the tumour relied on glucose for energy metabolism, while cancer cells in higher perfused areas preferred other nutrients, presumably lactate 6.
A similarly high metabolic flexibility has also been recapitulated when switching cancer cells from an in vitro to an in vivo environment 7, or when comparing the same tumour cells grown in two different organs 8. Such metabolic flexibility may be inherent to the heterogeneity of every cancer but also might be restricted to the cancer type and specific oncogenic mutations. Accordingly, it was found that in clear cell renal cell carcinoma (ccRCC), which are in 90% of the patients defined by Von Hippel-Lindau (VHL) loss and a consequent pseudo-hypoxic state, cancer cells relied mostly on glucose and low activity of the tricarboxylic acid (TCA) cycle, as compared to lung and brain tumours 9. It remains to be determined whether the low metabolic flexibility in ccRCC is caused by a decreased heterogeneity following the predominance of VHL mutations.
Nutrient availability to cancer cells is also influenced by the stroma. Accordingly, it has been observed that pancreatic ductal adenocarcinoma (PDAC) cells feed on alanine released from stroma-associated pancreatic stellate cells 10. Consequently, targeting the neutral amino acid transporter SLC38A2 impaired PDAC growth 11. Similar stroma-associated pancreatic stellate cell-derived lysophosphatidylcholines supported PDAC membrane synthesis and growth signalling 12. These and similar findings highlight that cancer cells can adapt but also trigger the release of certain nutrients from the stromal compartment.
Interestingly, it was further observed that such a conditioning of the nutrient environment by stromal cells also happens during pre-metastatic niche formation. In particular, primary breast tumour-secreted miR122 impaired glucose uptake in non-transformed cells, elevating glucose availability in the pre-metastatic niche of the lung, which ultimately increased the permissiveness of the niche for hosting metastasizing cancer cells 13. In line, there is increasing evidence that cancer cell metabolic flexibility can promote aggressiveness, ultimately leading to metastasis formation. Specifically, it was shown that lactate uptake in primary lesions correlated with aggressive oncological behaviour in human non-small cell lung cancer patients 14. Mechanistically, it was found that lactate consumption supported the anti-oxidant protection of disseminating cancer cells 15, which is important for increasing survival of metastasizing cancer cells in the circulation 16. Similarly, stimulating palmitate consumption in cancer cells, resulting in the expression of the fatty acid binding protein CD36, boosted the metastatic potential of human oral cancer orthotopic mouse models, and CD36 expression correlated with poor prognosis in multiple cancers 17. In addition, asparagine availability has been found to boost metastasis formation in experimental breast cancer mouse models 18. Hence, nutrient flexibility may contribute to cancer progression.
Interestingly, while flexibility seems widely present in the heterogeneous environment of the primary tumour, metastasizing cancer cells seem to lose this flexibility, creating a dependence on a particular nutrient. In line, it was found that inhibiting the lactate transporting protein monocarboxylate transporter 1 (MCT1) and CD36 in patient-derived mouse models of melanoma and oral squamous cell carcinomas, respectively, impaired metastasis formation but did not affect primary tumour growth 15,17. Similarly, simply restricting dietary asparagine during cancer cell dissemination also reduced metastasis formation 18. This feature of lost metabolic flexibility seems to be continued when cancer cells reach distant organs. Breast cancer cells that seed in the lung environment remodel the extracellular matrix to create a permissive niche 19. For this activity, collagen prolyl-4-hydroxylase (P4HA) is required. While this enzyme is highly transcriptionally regulated 20, it was recently discovered that pyruvate uptake from the extracellular space via MCT2 is required for P4HA-dependent collagen hydroxylation and for metastasis outgrowth in breast cancer mouse models 21. Notably, the extracellular pyruvate requirement of cancer cells for remodelling the extracellular matrix of the metastatic niche was not dependent on carbon contribution but rather on regulation via metabolite concentrations leading to alanine aminotransferase (ALT2, also known as GPT2)-dependent production of α-ketoglutarate 21. Moreover, it has been found that proline catabolism serves as an energy source in breast cancer cells colonizing the lung, but not in the corresponding primary tumours 22. Interestingly, dependency on proline catabolism was at least in vitro only found during colonization but not once colonies had formed, suggesting that the dependence on proline catabolism may be a transient event during metastasis formation.
The dependencies on certain nutrients or nutrient inflexibility during metastasis may be explained by several mechanisms. First, a reduced heterogeneity within metastasizing cancer cells may explain nutrient inflexibility, i.e. the cancer subpopulation most capable of metastasis formation may inherently not be able to use a certain nutrient. Thus, on the macroscopic level, primary tumours show flexibility due to heterogeneity while metastases may have lost it due to reduced heterogeneity. Alternatively, the phenotypic changes required for metastasis formation may result in the dependency of cancer cells on certain nutrients. In this case, nutrient flexibility in established secondary tumours should be similar to primary tumours and thus nutrient inflexibility would be transient. Lastly, nutrient inflexibility may be simply induced by the environment and thus the lack of certain nutrients in a given environment may induce nutrient inflexibility. Further studies are needed to address the nature of nutrient inflexibility in (metastatic) cancer cells. These include a further mechanistic understanding to what extent metabolic heterogeneity and microenvironment-dependent metabolic inflexibility can explain organ-specific metastasis patterns. An important additional and clinically relevant question is whether metastases regain a certain metabolic flexibility and thus to what extent metastasis prevention versus treatment can be achieved with the above-described mechanisms.
Besides metabolic flexibility, metabolic plasticity also contributes to effective cancer progression. Interestingly, the metabolic mode cancer cells use to increase their energy production during metastatic outgrowth seems to be dependent on microenvironment-induced plasticity. Specifically, it was found that breast cancer cells metastasizing to the lung show a peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α)-dependent bioenergetic plasticity in glucose metabolism 23, while the same breast cancer cells metastasizing to the liver required glycolytic energy production 24. In the latter case, silencing of pyruvate dehydrogenase kinase 1 (PDK1), which shifts cells from glycolytic to mitochondrial energy production, prevented breast cancer-derived liver but not lung metastasis 24. In addition, cancer cell origin seems to alter the preferred energy production of cancer cells colonizing a distant organ. Accordingly, it has been shown that colorectal cancer cells metastasizing to the liver have the plasticity to scavenge extracellular bioenergetics through secretion of creatine kinase, brain-type (CKB) to the extracellular space 25.
The features of the surrounding extracellular matrix also contribute to cancer plasticity. While non-transformed cells die of anoikis once they lose matrix attachment, metastasizing cancer cells can activate antioxidant pathways to evade anoikis 15,26–28. In addition, it has been found that matrix detached cancer cells of different origin can fuel glutamine carbon into reductive carboxylation 28,29, and that basal-like breast cancer cells activate the gluconeogenic enzyme fructose bisphosphatase 1 (FBP1) 30 to increase their ROS scavenging capacity. In line with these findings, metastasizing breast cancer cells can sustain α-ketoglutarate levels through activation of ALT2 21, rather than the enzyme glutamine dehydrogenase (GDH), which is frequently used by non-transformed cells and primary cancer cells. Targeting ALT2 consequently greatly impaired metastasis formation in experimental breast cancer mouse models 21.
While the examples above imply the existence of metabolic plasticity, they could also be explained by a metabolic switch creating inherently a dependency on the newly activated pathway. To further disentangle metabolic plasticity from metabolic switching during cancer progression, metabolic pathways with dual activity in primary tumours could be studied. A prominent example of metabolic plasticity in primary tumours is the use of fatty acid desaturase 2 (FADS2)-mediated sapienate production in parallel to stearoyl-CoA desaturase-1 (SCD1)-mediated palmitoleate production to generate mono-unsaturated fatty acids from palmitate 31,32. Here, neither the inhibition of FADS2 nor SCD1 blocks proliferation of certain cancer cells while the dual inhibition of both enzymes leads to impairment of proliferation. However, how this plasticity may change during metastasis formation remains to be determined. Thus, it will be important to define comprehensively to what extent true metabolic plasticity (compared to metabolic switching) contributes to metastasis formation and whether this plasticity is transient or stable and therefore can be exploited to prevent and treat metastases.
B. Mitochondrial plasticity in tumour progression
A recent single-cell assessment of gene expression of metabolic enzymes from tumour tissue revealed a high degree of heterogeneity, whereby the expression of mitochondrial enzymes exhibited the highest variability within the same tumour 33. In support of this finding, a pioneering effort to generate a new sensor for mitochondrial potential, a read-out of mitochondrial health in vivo, showed that in human and mouse lung cancer, mitochondrial function is highly heterogeneous 34 and correlates with different glycolytic subtypes. Whether this bioenergetic signature changes over time during tumour progression is currently unclear. Yet, it was shown that during cancer cell extravasation, melanoma cells expressing low levels of PGC1α, a master regulator of mitochondrial function, are selected for survival, and when they colonize the lungs, PGC1α levels are re-established 35. Of note, the PGC1α-low population showed enhanced migration in vitro and metastasis in vivo, whilst PGC1α-high population drives a proliferative phenotype in both the primary tumour and in the metastatic node 35. A similar connection between PGC1α levels, mitochondrial function and metastasis was observed in prostate and renal cancers, associating with poor outcome 36,37, (Table 1). These results are in line with the observation that mtDNA depletion in human tumours, which is generally associated with bioenergetic defects, is linked with poor patient prognosis in several human cancers 38. Yet, it should be noted that the connection between mitochondrial defects and metastasis does not apply to all cancer types. For instance, in breast cancer it has been demonstrated that metastatic cells exhibit increased mitochondrial biogenesis and respiration 39 and bioenergetic efficiency 40 (Table 1). These results indicate a requirement for PGC1a and mitochondrial activity for metastasis. Consistent with this view, very recent findings indicate that another component of mitochondrial fitness, mitochondrial morphology, is required for angiogenesis and metastasis, and the genetic ablation of OPA1, a master regulator of mitochondrial fusion, abolished metastasis in mouse models of melanoma 41.
Table 1:
Metabolic plasticity differs among different cancers during cancer progression.
| Tumour type | Primary | Circulating | Metastasis | References |
|---|---|---|---|---|
| Breast | Oxidative | OXPHOS increase | OXPHOS increase | 40,39 |
| Melanoma | Oxidative | OXPHOS suppression | Oxidative | 35 |
| Prostate, Renal | Glycolytic | OXPHOS suppression | 36,37 |
From a mechanistic point of view, the connection between mitochondrial function and cancer progression has been elusive. A seminal paper in 2008 provided the first line of evidence that replacing mitochondria with mitochondria from a metastatic cell line could transfer its aggressiveness 42. Here, they ascribed the increased aggressiveness provided by metastatic mitochondria to increased oxidative stress. However, the putative roles of ROS in metastasis have been challenged and appear more complex and dependent on tumour stage (see below). Another hypothesis is that the metabolic changes that arise from a specific mitochondrial dysfunction could support metastasis. In support of this scenario, we recently showed that the gradual increase in mtDNA heteroplasmy of a mtDNA mutation of ATP6 is directly associated with EMT and increased migration 43. We proposed that the loss of mitochondrial function caused by high levels of heteroplasmy activates glycolysis and the coupling between the glycolytic enzyme GAPDH and the enzyme malate dehydrogenase 1 (MDH1), which we found to co-localize with the cytoskeleton. Although the molecular details have yet to be defined, it is possible that mitochondrial dysfunction could prompt a metabolic rewiring that facilitates cell motility and migration. Nevertheless, it should be underscored that rather than a complete dysfunction, the extent of mitochondrial dysfunction we assessed was limited to 80% of mtDNA heteroplasmy, and cells still exhibited mitochondrial-dependent respiration. Therefore, it is possible that a stronger mitochondrial defect could be counterproductive for cancer cell growth and motility. Indeed, mutations that hamper mitochondrial function significantly could be detrimental to cancer cells, as indicated by the relatively benign nature of oncocytomas, tumours characterized by a significant mitochondrial suppression 44, and by the fact that tumours generated by cells devoid of mtDNA uptake healthy mitochondria from neighbouring cells to support pyrimidine biosynthesis 45. Consistent with this view, maintaining an appropriate turnover of mitochondria via autophagy is essential for cancer, and when autophagy is inhibited, the accumulation of unhealthy mitochondria leads to cancer cell demise (reviewed in 46).
Another possible advantage that mitochondrial dysfunction provides to cancer could be induction of an anabolic/antioxidant metabolic rewiring that supports growth and survival under harsh environmental conditions by reducing cancer dependency on oxygen consumption for ATP generation. Indeed, a reduction in mitochondrial respiration together with a parallel increase in glycolysis can enable cancer cells to survive when the tumour is poorly vascularized and the supply of oxygen becomes limited. This hypothesis is supported by the observation that activating oxygen consumption in cancer cells by the expression of uncoupling protein 1 can reduce cancer cell survival 47. A corollary to this metabolic reprogramming is the accumulation of metabolites that have signalling roles and can elicit a phenotype switch that supports survival and metastasis. For instance, it was shown that the accumulation of mitochondrial metabolites such as 2HG, fumarate, and succinate, which are known to increase under conditions of poor oxygenation, could trigger EMT (reviewed in 48).
It is also possible that dysregulation of mitochondrial function during tumour progression has non-cell autonomous functions and can affect the tumour microenvironment. Indeed, tumours that are deficient for Complex I, despite their slow proliferation, induce macrophage infiltration into the tumour and increase tumour malignancy 49.
Finally, ROS have long been considered unwanted by-products of mitochondrial metabolism. More recently, ROS have been suggested to function as important signalling molecules, implicated in many diseases including cancer. However, their role in tumorigenesis is far from clear, due to both technical challenges in their detection and modulation, especially in vivo, and due to the fact that their function seems to depend on tumour stage. During the early phases of tumorigenesis, ROS appear to be mutagenic, and therefore, they support transformation. Recent evidence indicates that ROS increase upon transformation but their levels are kept in check by antioxidant programmes such as that orchestrated by NRF2 50. Independent experiments showed that mitochondrial ROS are essential for Kras-mediated tumorgenicity and anchorage-independent growth 51. Of note, cells deficient in mitochondrial DNA do not generate ROS and fail to grow in an anchorage-independent manner 51. Therefore, evidence indicates that ROS increase upon transformation and support oncogenic functions, but their lethal effects need to be counteracted by antioxidant programmes. Their role in tumour progression is even more debated. The increased ROS generation by dysfunctional mitochondria was initially linked with cancer metastasis in the above-described mitochondria swap experiment between normal and highly aggressive cancer cells 42. Of note, antioxidants appeared to reduce the metastatic potential of these cybrid cancer cells in vivo. Only a year later, it was reported that during cell detachment, one of the key steps of tumour progression that precedes metastasis, cells experience a burst of oxidative stress, which, if left unchecked, can lead to cell demise 52. Of note, antioxidants appeared to increase the chances of survival of detached cells. To sum up these two lines of evidence, on one hand, ROS increase malignancy; on the other hand, too much ROS is toxic for cancer cells detached from their matrix, with an opposite metastasis-promoting effect of antioxidants. Of note, it is still unclear why cells that detach from the matrix experience this burst in ROS, but it is possible that changes in metabolism elicited by alterations of cellular mechanics are responsible for it.
These two apparently contradicting pieces of evidence revealed that the role of ROS in cancer progression is likely context dependent. For instance, in the effort to elucidate the role of ROS in tumour progression, Porporato and colleagues found that mitochondrial-derived superoxide, caused by either the suppression of mitochondrial function or mitochondrial overload, increases aggressiveness and metastasis in vivo 53. Of note, the authors found that this increased migration involved the protein tyrosine kinases Src and Pyk2 as downstream effectors and was blunted by mitochondria-specific antioxidants. However, in a model of melanoma, it was found that metastasis activates an antioxidant programme to survive and antioxidants increase the efficiency of metastasis 54. Here, the authors concluded that oxidative stress limits the formation of distant metastasis in vivo. Consistent with this view, it was found that melanoma metastases rely on lactate not only as an energy substrate but also as a source of reducing power via the activation of the oxidative arm of the pentose phosphate shunt 15. This work is consistent with the finding that antioxidants increase rate of metastasis in melanoma in preclinical settings 27. Overall, a scenario is emerging whereby ROS affect cancer cells in a dose- and stage-dependent fashion. During the early phases of tumorigenesis, increased ROS, possibly caused by dysregulation of mitochondrial function, could activate signalling cascades that promote transformation. Of note, at this stage, ROS could further shape the fate of early tumours by causing DNA damage and genome instability 55. During this phase, the antioxidant capacity of the cells may be sufficient to avoid cell death. Yet, when the tumour grows and cells start to detach from the matrix, cells experience a burst of oxidative stress. Cells that succeed in responding to this wave of oxidative stress have the ability to extravasate and efficiently metastasize. At both stages, the presence of antioxidant programmes is essential to avoid cell death. In support of this view, it was recently shown that in pancreatic cancer, the levels of the antioxidant protein TIGAR vary during tumour progression. During the early stages, high TIGAR levels are needed to cope with the oxidative stress caused by transformation. Yet, as the tumour progresses, decreasing levels of TIGAR appear to increase the malignancy of cancer cells, consistent with the selection for cells with higher ROS and higher invasive capacity. At a later stage, though, TIGAR levels go up again to buffer the oxidative stress experienced by metastatic cells 56.
Overall, these accumulating data suggest that mitochondrial function plays a key role during tumour progression and that an important feature lies in the ability of the mitochondrial function level to be context specific. Therefore, an attractive therapeutic strategy could be to target mitochondrial potential for metabolic adaptability.
C. Host metabolism affecting metabolic adaptation modalities at varying tumour stages
Importantly, the host metabolism affects tumour metabolic adaptability potential, primarily by one’s genetics. In congenital cancer predisposition syndromes, the kinetics of cancer development is promoted by an inherent metabolic rewiring caused by constitutive activation of signalling pathways and transcriptional programs that regulate anabolic metabolism i.e., RASopathies, Li–Fraumani syndrome and Cowden syndrome. In some syndromes, such as Proteus and Beckwith–Wiedemann syndromes, the uncontrolled cellular growth manifests as overgrowth of a specific tissue or of the whole body, as well as in tumour predisposition 57. Several cancer predisposition syndromes involve mutations in metabolic genes that either cause cell toxicity leading to cancer, i.e., the development of hepatocellular carcinoma in patients diagnosed with tyrosinaemia type I 58,59, or the accumulation of metabolites with oncogenic activity. For instance, in the hereditary cancer syndromes caused by germline mutations in SDH or FH, accumulation of the oncometabolites fumarate and succinate, respectively, have been proposed to cause cancerous transformation 60, 61. To what extent the host metabolism predisposes to cancer in these tumour predisposition syndromes is currently unknown. In the case of SDH/FH-deficient tumours, patients are heterozygous for SDH/FH mutations and it will be important to determine whether the remaining wildtype allele is sufficient to maintain host physiology. It is possible that in some tissues the heterozygous loss of SDH/FH might give rise to mitochondrial dysfunction and/or mild accumulation of fumarate/succinate, affecting host metabolism and, possibly, impinging on the ability of the immune system to eradicate SDH/FH-deficient clones. Interestingly, mitochondrial dysfunction syndromes pose an increased cancer risk as can be seen in mitochondrial depletion syndromes 62.
Some congenital metabolic syndromes involve chronic changes in metabolic flux that recapitulate similar changes to those observed in cancer cells, including enhanced pentose phosphate pathway (PPP) activity, lactate production, and high synthesis of lipids and nucleotides, predisposing to tumorigenesis. For example, in glycogen storage disease type 1a, the deficiency in the glycolytic glucose‑6‑phosphatase complex leads to hypoglycaemia, lactic acidosis, hyperlipidaemia, and increased shunting via the PPP 63.
In addition, changes in host metabolic capacities due to aging, diet, health status and physical activity can affect cancer progression either directly or indirectly via the microbiome, which can generate metabolites affecting tumorigenesis 64. With age, degenerative changes in the host metabolism create an inflexible and less fertile environment, which may drive tumours to metastasize 65. Importantly, the surrounding fibroblasts in aged melanoma patients secrete high amounts of lipids that enable drug resistance; the rich lipid tumour microenvironment promotes the upregulation of the tumour fatty acid transport protein (FATP) 2, which supports mitochondrial metabolism and cancer cell survival under therapy-induced stress 66.
The host health status is a crucial determinant of its cancer risk 67. In patients with obesity, cancer development is facilitated by metabolic reprogramming caused by the prevalence of diabetes and insulin resistance, leading to IGF-1 overproduction and to enhanced shunting of glycolytic intermediates via the PPP 68,69. Oppositely, a caloric restriction diet which likely constrains metabolic adaptability, affects the signalling of IGF-1, phosphoinositide 3-kinase (PI3K), phosphatase and tensin homolog (PTEN) and mammalian target of rapamycin (mTOR), resulting in inhibition of cancer growth. Interestingly, alterations in the circadian clock, which centrally regulates daily rhythms of cellular metabolism, have been shown to predict poor survival in cancer patients and to associate with increased incidence of several cancers in shift workers 70. Furthermore, perturbations of circadian clock components led to increased c-Myc expression and to metabolic dysregulations, consequently promoting lung tumour progression and decreasing survival 71.
Thus, host fitness determines ones capacity to accommodate cancer demands for nutrients, thus affecting cancer metabolic adaptability.
D. A framework for the metabolic evolution of cancer (Figure 2)
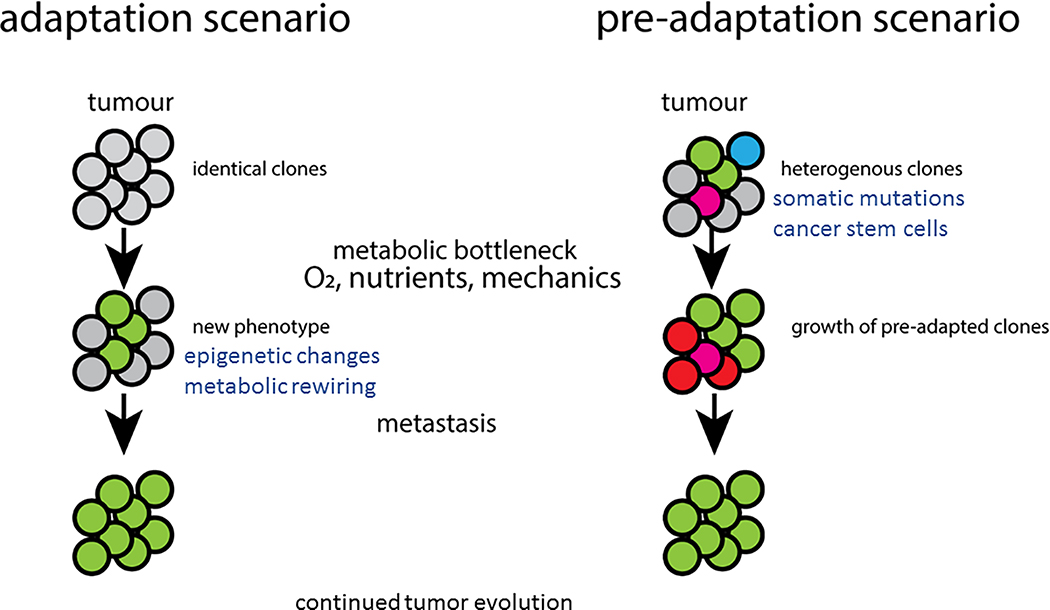
Figure 2: Two potential scenarios that promote tumour evolution.
Primary tumour heterogeneity can evolve through changes induced by different metabolic deprivations (adaptation), or exist and proliferate following selection for fitness (pre-adaptation).
In the previous sections, we have highlighted some of most investigated metabolic pathways and how they contribute to tumour initiation and progression towards metastasis. However, the determinants of these metabolic changes are only poorly understood. Are they intrinsically present in the original tumour, or are they acquired during tumour evolution? We propose the following two scenarios to explain the adaptability of cancer. In the first scenario, an initial tumour mass is composed of phenotypically identical clones. Depending on nutrient and oxygen availability, and likely external forces, some cells will undergo a phenotypic switch that prepares them to face the new environment. Only cells that have sufficient metabolic flexibility and plasticity will survive, extravasate, and contribute to metastasis. In this scenario, the adaptability can be driven by (epi)genetic changes that modulate the expression of metabolic genes. In addition, external cues, such as nutrients or growth factors, can further regulate these metabolic enzymes. Interestingly, chromatin structure and function depend on the availability of ATP, methyl donors, and other metabolites (aKG, succinate, fumarate, etc) that regulate chromatin modifiers. Therefore, it is possible that when a group of cancer cells experience changes in nutrient availability, the resulting epigenetic and transcriptional response is what drives their adaptation phase. In this scenario, the resulting metabolic phenotype is plastic and can be reverted or further changed depending on the new metabolic niche the cancer cell will experience. Such a scenario is supported by experiments showing that consecutive reimplantations of secondary lesions into the primary site re-establishes the original metastatic phenotype 26.
The second scenario relies on the intrinsic phenotypical heterogeneity of the tumour mass. Here, we postulate that within a tumour mass, genetically identical cancer cells exhibit an intrinsic variability in their metabolic phenotype. While this intrinsic metabolic heterogeneity can be initially “neutral”, i.e. it does not enhance growth or survival in the primary tumour, it may give rise to clones that can adapt to a new, harsher metabolic environment. It is possible that only a few cells within the tumour tissue will have the appropriate metabolic configuration that enables survival, but these will be sufficient to either invade the tissue or extravasate. This scenario is supported by some evidence. For instance, it has been demonstrated in silico that metabolic changes during evolution may be indirect consequence of the inherent structure of a given metabolic network, which provides flexibility to use other energy sources. For instance, the metabolic network used for growth on glucose could enable the use many more carbon sources when glucose becomes scarce 72. In addition, almost a decade ago it was demonstrated that mutant Ras could generate sub-clones that express high levels of the glucose transporter Glut1. This condition, which does not provide a growth advantage when glucose is available, provides unique advantages to the mutant cells when glucose becomes scarce 73. Although it is unclear how this heterogeneity in the expression of Glut1 is maintained, it is possible that epigenetic mechanisms govern it. We speculate that additional mutations that “fix” this metabolic configuration might arise in the clonal population, allowing for the emergence of a stable clone. It is possible that the genetic heterogeneity observed in tumours arises as a combination of non-genetic pre-adaptations and subsequent fixation of the phenotype by mutations in the genome.
Additionally, recent papers suggest that it is the existence of cancer stem cells with inherently high metabolic plasticity that generates resistance to therapy by allowing dynamic transitioning between different metabolic phenotypes, enabling tumours to regain growth following therapy 74. Indeed, it has been reported that cancer stem cells are able to switch to a glycolytic metabolism when OXPHOS is blocked 75. Interestingly, a combined analysis of biological, biochemical, pharmacological, and genetic studies revealed that cancer stem cells’ stemness may arise from metabolic events occurring in non- cancer stem cells. Specific metabolic hits are thought to affect chromatin organization and activate epigenetic programs involved in the metabolic-driven reprogramming of cancer stem cells 76. According to this, the identification of key metabolic processes involved in this reprogramming might be useful to identify and target cancer stem cell survival.
E. Tumor metabolic reprograming contributes to therapeutic resistance
Tumour metabolic plasticity and flexibility contribute to resistance in most types of anti-cancer therapy. Undoubtedly, one of the main contributors to this metabolic adaptability potential is genetic heterogeneity. Here, resistance evolves by clonal selection of a specific signaling pathway that promotes the required metabolic rewiring that can meet the stress imposed by the drug. Since most anti-cancer therapies target the uncontrolled proliferation of cancer cells, the purpose of the compensatory metabolic reprograming is to restore cancer cell survival and growth. This is best exemplified in chemo-resistance caused by plasticity in glucose metabolism. For example, following cisplatin chemotherapy, several key glycolytic enzymes as HK2, PFK, and PKM2, and glucose transporters as GLUT-1, are induced in cervical cancer by activating adenosine monophosphate-activated protein kinase (AMPK) signalling 77,78. This augmentation in glycolysis results in high levels of glycolytic intermediates for branching pathways such as the PPP that supports nucleotide synthesis and redox homeostasis. Importantly, the resultant high lactate secretion generates a hypoxic microenvironment that limits drug entry into the cells. In addition, increased glucose consumption by upregulation of glucose transporters cues the cell to glucose deprivation and activates the stress machinery to induce autophagy and escape apoptosis 79. In parallel, AMPK also promotes glutamine metabolism, which by itself contributes to chemo-resistance by supplying substrates to the TCA cycle to preserve mitochondrial function and support cancer cell survival 80. Indeed, combining the chemotherapy cisplatin with metabolic inhibitors of glycolysis, such as 3-BrPA (3-bromopyruvate) - a specific inhibitor of HK-2 kinase, increase chemotherapy efficacy 81,82. Increasing mitochondrial metabolism can also provide an escape route for cancer cell survival from the effects of therapy; more than half of melanoma patients with BRAF mutations and a consequent mitogen-activated protein kinase (MAPK) activation develop resistance to MAPK inhibitors that is dependent on mitochondrial OXPHOS 83. Indeed, therapy combining a MAPK inhibitor with a Gamitrinib, a small molecule targeting the mitochondria, augmented the efficacy of MAPK inhibitor treatment in melanoma cells by inducing mitochondrial dysfunction and inhibiting tumour bioenergetics 83.
The anticancer mechanism of antiangiogenic therapy is to reduce tumour vascularity and cause tissue hypoxia. Yet, inducing hypoxia triggers upregulation of HIF1 that can lead to worse outcomes in terms of resistance 84. HIF1 induces the expression of glycolysis-related genes such as GLUT1, GLUT3, PDK1, PKM2, PFKFB3, GYS1, ENO1, LDHA, HK2 and GAPDH, again enhancing glycolysis and its branching pathways 85. In addition, hypoxia in the tumour microenvironment leads to lipolysis and release of free fatty acids, while in parallel, hypoxia increases fatty acid uptake by the tumour via upregulation of the fatty acid importer CD36 expression. Within tumour cells, hypoxia increases glutamine uptake, providing substrates for the TCA cycle for the synthesis of citrate and for ATP synthesis by OXPHOS. The resultant increase in ATP and metabolite levels supports tumour proliferation and contributes to cancer resistance 86. Here again, combined therapy of nintedanib and 3PO, a selective glycolytic inhibitor of 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFKFB3), was synergistic in inhibiting tumour growth in a breast cancer model 87. While the increase in glycolysis may be a general metabolic strategy for chemo resistance, other tumours including melanoma and haematological malignancies appear to increase OXPHOS in resistant clones (reviewed in: 88). For instance, in melanoma, the increase in OXPHOS in resistant clones is supported by PGC1α and is needed to buffer oxidative stress 89. In line with this finding, in chronic myeloid leukaemia, targeting mitochondrial function can eradicate therapy-resistant cells 90.
In some cancer therapy that target a selected metabolic vulnerability, resistance may occur when the nutrient circumstances change following therapy. Under the new conditions, cancer cells benefit from selecting for clones with re-programming of the targeted metabolic pathway. For example, in multiple human malignancies including melanoma, hepatocellular carcinoma, prostate cancers, osteosarcoma and mesothelioma, argininosuccinate synthetase 1 (ASS1) is silenced by epigenetic methylation. ASS1 is a urea cycle enzyme that outside the liver participates in the citrulline-arginine cycle for the generation of arginine and its downstream metabolites 91. Silencing of ASS1 increases the availability of its substrate, aspartate, for the synthesis of pyrimidines that are utilized for DNA and RNA synthesis that support cell proliferation 92. Tumours with ASS1 silencing are hence proliferative but in parallel, become arginine auxotrophic, meaning they cannot synthesize arginine and require extracellular arginine supplementation for survival. This metabolic vulnerability is taken advantage of for therapy; arginine starvation agents such as PEGylated arginine deiminase (ADI-PEG20) and human arginase 1, which degrade extracellular arginine, are in various stages of clinical trials 93. Yet, resistance to arginine-degrading treatment develops and involves re-expression of ASS1 caused by binding of MYC to its promoter 94. Such evolving resistance exemplifies the advantage of epigenetic flexibility over genetic rigidity determined by mutations.
Importantly, the tumour microenvironment can provide tumours with metabolites that enable metabolic plasticity and flexibility, leading to cancer resistance. Increasing autophagy and exosome secretion in the microenvironment can provide essential metabolites as amino acids, fatty acids and nucleic acids to support the metabolic flexibility required for cancer cell survival and growth under nutrient deprivation 95. The exosome cargo can also provide metabolic plasticity which affects tumour progression by carrying exosome-associated miRNAs to the tumour cell. For example, in malignant mesothelioma cells, miR-126, an angiogenesis inducer, can regulate cancer metabolism by decreasing the levels of its downstream target insulin receptor substrate-1, causing upregulation in the expression of oxidative stress defence and gluconeogenesis genes 96. The resultant increase in glucose yields a glycolytic shift, which supports a less malignant mesothelioma phenotype 97.
Thus, metabolic adaptability is an important component in cancer resistance to therapy and targeting it directly will likely be therapeutically beneficial. An attractive therapeutic approach could be to exploit these metabolic features to enhance therapy by targeting cancer adaptive mechanisms in particular, cancer heterogeneity and the specific signaling molecules that enable metabolic rewiring.
F. Future therapeutic directions to potentially target tumor metabolic adaptability features
A reasonable strategy to target tumour heterogeneity is based on identifying and targeting driver mutations (i.e.,BRAF) or a signaling pathway shared by multiple mutations in the same cancer (i.e., mTOR). Here, although a single drug can target multiple clones, the majority of patients eventually develop resistance and hence combining these signaling inhibitor drugs with metabolic inhibitors will likely be more efficient 98,99. Indeed, concurrent inhibition of BRAF and glycolysis, or a combination of MAPK and mitochondrial inhibitors, induces cell death in BRAF inhibitor-resistant melanoma cells 100,83.
Another approach to diminish tumour heterogeneity is to stress tumours to develop a metabolic dependency that sensitizes tumour cells to specific therapies. For example, methionine is essential for protein synthesis, one carbon metabolism and nucleotide synthesis, gene regulation by DNA methylation, as well as for redox metabolism, which are all essential for carcinogenesis 101. Restricting methionine by diet has been shown to sensitize PDX models of colorectal cancer to chemotherapy with 5-fluorouracil (5-FU) (31367041). To identify drugs that are relevant for implementing this approach, a metabolic sensitivity assay can be added to high-throughput drug screens.
Another strategy for targeting cancer adaptability potential is to target cancer stem cells by for example, using antibodies against cancer stem cell-specific cell surface markers as CD20, CD52 etc., 102. Similarly, attempts at targeting specific proteins that enable the switch between different metabolic states may also prove to be an attractive strategy. For example, the heat shock protein 90 (HSP90) molecular chaperone controls metabolic rewiring through either direct binding to chromatin or via control of transcription factors and epigenetic effectors 103. Indeed, HSP90 interacts with and modulates several signaling pathways involved in metabolic plasticity including c-Myc, HIF1α and AKT/PKB. HSP90 can also influence cancer metabolism by directly binding glycolytic enzymes as GAPDH and PKM2. Specifically, HSP90 mitochondrial isoform TRAP1 stabilizes the binding of HK2 to the mitochondrial voltage-dependent anion channel (VDAC), maximizing its activity, and also binds and inhibits the activity of the respiratory chain complex II (succinate dehydrogenase—SDH) 104. Likewise, reintroducing wild-type p53 or inhibiting c-MET might decrease the metabolic flexibility potential 105.
G. Conclusions (Figure 3)
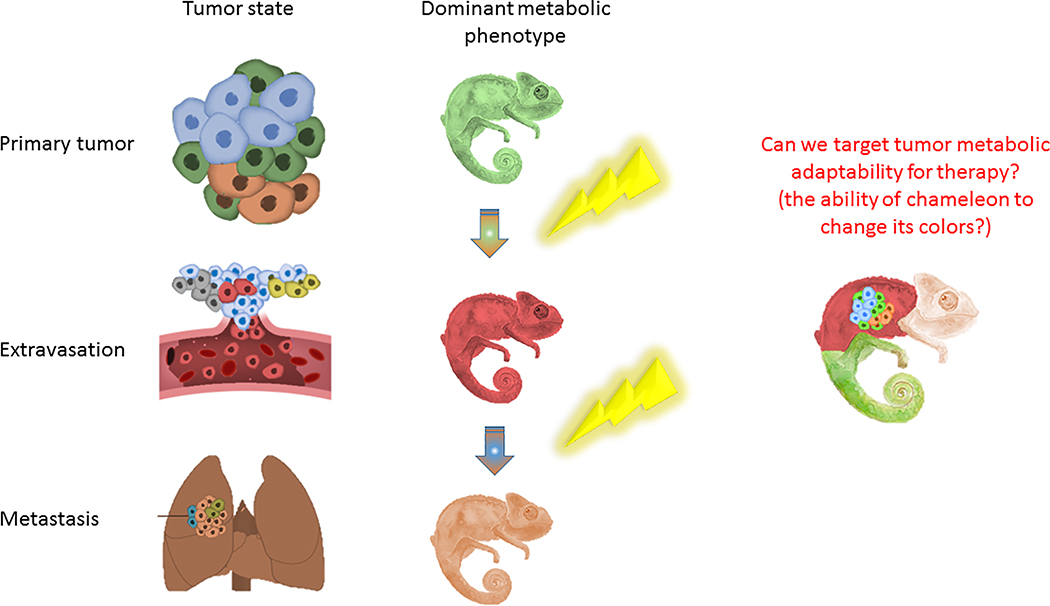
Figure 3: Schematic demonstration of the metabolic changes that accompany tumour progression.
Targeting tumour potential for metabolic adaptability can be a therapeutic strategy against tumour resistance.
There is no doubt that tumour evolution is determined mostly by the pressure to supply its metabolic needs under changing environmental metabolic stresses. This ability for metabolic adaptation to fluctuating stresses should hence be regarded as tumour’s metabolic Achilles Heel and therapies targeting this should be included in the future arsenal against cancer.
Notably, cancer cells’ survival mechanisms such as metabolic adaptability are hijacked from normal cells. The signalling and metabolic pathways providing this metabolic adaptability are all used by normal cells, and especially by proliferating cells, under physiological conditions. Hence, in targeting cancers’ metabolic plasticity and flexibility features, it may be challenging to balance between causing side effects and developing therapy resistance. Along these lines, accumulating papers suggest changing the goal of cancer management from cure to chronic disease. Here, the idea of treatment is to avoid inducing extreme stress on cancer cells that presumably select for more aggressive clones, and rather aim to restrict and control cancer growth. For this, one would need to continuously identify targetable molecular changes and tailor treatments that are predicted to be most effective against resistance and relapse. This may be achieved by monitoring cancer burden with imaging together with following the precise molecular signature of the evolving cancer using liquid biopsies and repeated sequencing 106.
Undoubtedly, more preclinical research and clinical trials must be completed before such interventions become common practice in cancer therapy. In the optimal scenario, combinatory drugs will abolish cancer adaptability potential, while in the more realistic scenario, we should aim to restrict the ability of cancer to adapt as much as possible.
Significance.
Recognizing cancer dynamic metabolic adaptability as an entity can lead to targeted therapy that is expected to decrease drug resistance.
ACKNOWLEDGMENTS
We acknowledge and thank the Weizmann Institute for providing financial and infrastructural support. AE is incumbent of the Leah Omenn Career Development Chair and is supported by research grants from the European research program (ERC818943), and from the Israel Science Foundation (860/18). AE received additional support from The Moross Integrated Cancer Center, Sagol Institute for Longevity Research, Adelis Foundation, Rising Tide Foundation, and from Manya and Adolph Zarovinsky. CF work is funded by the MRC Core award grant MRC_MC_UU_12022/6, the ERC (ERC819920), and CRUK Programme Foundation award C51061/A27453. S.-M.F. acknowledges funding from the ERC Consolidator Grant Agreement n. (ERC771486), FWO – Research Projects (G098120N and G088318N), KU Leuven – Methusalem Co-Funding and Fonds Baillet Latour.
Authors declare no conflict of interest. SMF has received funding from Bayer, Merck BlackBelt Therapeutics and has consulted for Funds +.
REFERENCES
- 1.Warburg O On the origin of cancer cells. Science 123, 309–314 (1956). [DOI] [PubMed] [Google Scholar]
- 2.Warburg O, Wind F & Negelein E The Metabolism of Tumors in the Body. J Gen Physiol 8, 519–530 (1927). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanahan D & Weinberg RA Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Faubert B, Solmonson A & DeBerardinis RJ Metabolic reprogramming and cancer progression. Science 368(6487), doi: 10.1126/science.aaw5473 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelley DE & Mandarino LJ Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes 49, 677–683, doi: 10.2337/diabetes.49.5.677 (2000). [DOI] [PubMed] [Google Scholar]
- 6.Hensley, Christopher T. et al. Metabolic Heterogeneity in Human Lung Tumors. Cell 164, 681–694, doi: 10.1016/j.cell.2015.12.034 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidson, Shawn M. et al. Environment Impacts the Metabolic Dependencies of Ras-Driven Non-Small Cell Lung Cancer. Cell metabolism 23, 517–528, doi: 10.1016/j.cmet.2016.01.007 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christen S et al. Breast cancer-derived lung metastasis show increased pyruvate carboxylase-dependent anaplerosis. Cell Reports 17, 837–848 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Courtney KD et al. Isotope Tracing of Human Clear Cell Renal Cell Carcinomas Demonstrates Suppressed Glucose Oxidation In Vivo. Cell metabolism 28, 793–800.e792, doi: 10.1016/j.cmet.2018.07.020 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sousa CM et al. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature 536, 479–483, doi: 10.1038/nature19084 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parker SJ et al. Selective Alanine Transporter Utilization Creates a Targetable Metabolic Niche in Pancreatic Cancer. Cancer discovery 10, 1018–1037, doi: 10.1158/2159-8290.Cd-19-0959 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Auciello FR et al. A Stromal Lysolipid–Autotaxin Signaling Axis Promotes Pancreatic Tumor Progression. Cancer discovery 9, 617, doi: 10.1158/2159-8290.CD-18-1212 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fong MY et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat Cell Biol 17, 183–194, doi: 10.1038/ncb3094 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faubert B et al. Lactate Metabolism in Human Lung Tumors. Cell 171, 358–371.e359, doi: 10.1016/j.cell.2017.09.019 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tasdogan A et al. Metabolic heterogeneity confers differences in melanoma metastatic potential. Nature 577, 115–120, doi: 10.1038/s41586-019-1847-2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elia I, Doglioni G & Fendt S-M Metabolic Hallmarks of Metastasis Formation. Trends in Cell Biology 28, 673–687, doi: 10.1016/j.tcb.2018.04.002 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Pascual G et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature 541, 41–45, doi:10.1038/nature2079110.1038/nature20791http://www.nature.com/nature/journal/v541/n7635/abs/nature20791.html#supplementary-informationhttp://www.nature.com/nature/journal/v541/n7635/abs/nature20791.html#supplementary-information (2017). [DOI] [PubMed] [Google Scholar]
- 18.Knott SRV et al. Asparagine bioavailability governs metastasis in a model of breast cancer. Nature 554, 378, doi:10.1038/nature2546510.1038/nature25465https://www.nature.com/articles/nature25465#supplementary-informationhttps://www.nature.com/articles/nature25465#supplementary-information (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilkes DM et al. Collagen Prolyl Hydroxylases are Essential for Breast Cancer Metastasis. Cancer research 73, 3285–3296, doi: 10.1158/0008-5472.CAN-12-3963 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Gilkes DM, Semenza GL & Wirtz D Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nature reviews. Cancer 14, 430–439, doi: 10.1038/nrc3726 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elia I et al. Breast cancer cells rely on environmental pyruvate to shape the metastatic niche. Nature 568, 117–121, doi: 10.1038/s41586-019-0977-x (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elia I et al. Proline metabolism supports metastasis formation and could be inhibited to selectively target metastasizing cancer cells. Nature communications 8, 15267 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andrzejewski S et al. PGC-1α Promotes Breast Cancer Metastasis and Confers Bioenergetic Flexibility against Metabolic Drugs. Cell metabolism, S1550–4131(1517)30557–30550 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Dupuy F et al. PDK1-Dependent Metabolic Reprogramming Dictates Metastatic Potential in Breast Cancer. Cell metabolism 22, 577–589, doi: 10.1016/j.cmet.2015.08.007 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Loo, Jia M. et al. Extracellular Metabolic Energetics Can Promote Cancer Progression. Cell 160, 393–406, doi: 10.1016/j.cell.2014.12.018 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piskounova E et al. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 12, 186–191, doi:10.1038/nature1572610.1038/nature15726http://www.nature.com/nature/journal/vaop/ncurrent/abs/nature15726.html#supplementary-informationhttp://www.nature.com/nature/journal/vaop/ncurrent/abs/nature15726.html#supplementary-information (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Gal K et al. Antioxidants can increase melanoma metastasis in mice. Sci Transl Med 7, 308re308, doi: 10.1126/scitranslmed.aad3740 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Labuschagne CF, Cheung EC, Blagih J, Domart M-C & Vousden KH Cell Clustering Promotes a Metabolic Switch that Supports Metastatic Colonization. Cell metabolism 30, 720–734.e725, doi: 10.1016/j.cmet.2019.07.014 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang L et al. Reductive carboxylation supports redox homeostasis during anchorage-independent growth. Nature 532, 255–258, doi:10.1038/nature1739310.1038/nature17393http://www.nature.com/nature/journal/v532/n7598/abs/nature17393.html#supplementary-informationhttp://www.nature.com/nature/journal/v532/n7598/abs/nature17393.html#supplementary-information (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong C et al. Loss of FBP1 by Snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer cell 23, 316–331, doi: 10.1016/j.ccr.2013.01.022 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vriens K et al. Evidence for an alternative fatty acid desaturation pathway increasing cancer plasticity. Nature 566, 403–406, doi: 10.1038/s41586-019-0904-1 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Triki M et al. mTOR Signaling and SREBP Activity Increase FADS2 Expression and Can Activate Sapienate Biosynthesis. Cell Reports 31, 107806, doi: 10.1016/j.celrep.2020.107806 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao Z, Dai Z & Locasale JW Metabolic landscape of the tumor microenvironment at single cell resolution. Nat Commun 10, 3763, doi: 10.1038/s41467-019-11738-0 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Momcilovic M et al. In vivo imaging of mitochondrial membrane potential in non-small-cell lung cancer. Nature 575, 380–384, doi: 10.1038/s41586-019-1715-0 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo C et al. A PGC1alpha-mediated transcriptional axis suppresses melanoma metastasis. Nature 537, 422–426, doi: 10.1038/nature19347 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torrano V et al. The metabolic co-regulator PGC1alpha suppresses prostate cancer metastasis. Nat Cell Biol 18, 645–656, doi: 10.1038/ncb3357 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.LaGory EL et al. Suppression of PGC-1alpha Is Critical for Reprogramming Oxidative Metabolism in Renal Cell Carcinoma. Cell Rep 12, 116–127, doi: 10.1016/j.celrep.2015.06.006 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reznik E, Wang Q, La K, Schultz N & Sander C Mitochondrial respiratory gene expression is suppressed in many cancers. Elife 6, 6:e21592, doi: 10.7554/eLife.21592 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LeBleu VS et al. PGC-1alpha mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol 16, 992–1003, 1001–1015, doi: 10.1038/ncb3039 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andrzejewski S et al. PGC-1alpha Promotes Breast Cancer Metastasis and Confers Bioenergetic Flexibility against Metabolic Drugs. Cell Metab 26, 778–787 e775, doi: 10.1016/j.cmet.2017.09.006 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Herkenne S et al. Developmental and Tumor Angiogenesis Requires the Mitochondria-Shaping Protein Opa1. Cell Metab 31, 987–1003 e1008, doi: 10.1016/j.cmet.2020.04.007 (2020). [DOI] [PubMed] [Google Scholar]
- 42.Ishikawa K et al. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science 320, 661–664, doi: 10.1126/science.1156906 (2008). [DOI] [PubMed] [Google Scholar]
- 43.Gaude E et al. NADH Shuttling Couples Cytosolic Reductive Carboxylation of Glutamine with Glycolysis in Cells with Mitochondrial Dysfunction. Molecular cell 69, 581–593 e587, doi: 10.1016/j.molcel.2018.01.034 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Luise M et al. Molecular and metabolic features of oncocytomas: Seeking the blueprints of indolent cancers. Biochim Biophys Acta Bioenerg 1858, 591–601, doi: 10.1016/j.bbabio.2017.01.009 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Bajzikova M et al. Reactivation of Dihydroorotate Dehydrogenase-Driven Pyrimidine Biosynthesis Restores Tumor Growth of Respiration-Deficient Cancer Cells. Cell Metab 29, 399–416 e310, doi: 10.1016/j.cmet.2018.10.014 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kimmelman AC & White E Autophagy and Tumor Metabolism. Cell Metab 25, 1037–1043, doi: 10.1016/j.cmet.2017.04.004 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Y, Cairns R, Papandreou I, Koong A & Denko NC Oxygen consumption can regulate the growth of tumors, a new perspective on the Warburg effect. PLoS One 4, e7033, doi: 10.1371/journal.pone.0007033 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sciacovelli M & Frezza C Metabolic reprogramming and epithelial-to-mesenchymal transition in cancer. The FEBS journal 284, 3132–3144, doi: 10.1111/febs.14090 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kurelac I et al. Inducing cancer indolence by targeting mitochondrial Complex I is potentiated by blocking macrophage-mediated adaptive responses. Nat Commun 10, 903, doi: 10.1038/s41467-019-08839-1 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DeNicola GM et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 475, 106–109, doi: 10.1038/nature10189 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weinberg F et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci U S A 107, 8788–8793, doi: 10.1073/pnas.1003428107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schafer ZT et al. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature 461, 109–113, doi: 10.1038/nature08268 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Porporato PE et al. A mitochondrial switch promotes tumor metastasis. Cell Rep 8, 754–766, doi: 10.1016/j.celrep.2014.06.043 (2014). [DOI] [PubMed] [Google Scholar]
- 54.Piskounova E et al. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 527, 186–191, doi: 10.1038/nature15726 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Srinivas US, Tan BWQ, Vellayappan BA & Jeyasekharan AD ROS and the DNA damage response in cancer. Redox Biol 25, 101084, doi: 10.1016/j.redox.2018.101084 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheung EC et al. Dynamic ROS Control by TIGAR Regulates the Initiation and Progression of Pancreatic Cancer. Cancer Cell 37, 168–182 e164, doi: 10.1016/j.ccell.2019.12.012 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kamien B et al. A Clinical Review of Generalized Overgrowth Syndromes in the Era of Massively Parallel Sequencing. Mol Syndromol 9, 70–82, doi: 10.1159/000484532 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Erez A & DeBerardinis RJ Metabolic dysregulation in monogenic disorders and cancer - finding method in madness. Nat Rev Cancer 15, 440–448, doi: 10.1038/nrc3949 (2015). [DOI] [PubMed] [Google Scholar]
- 59.Erez A, Shchelochkov OA, Plon SE, Scaglia F & Lee B Insights into the pathogenesis and treatment of cancer from inborn errors of metabolism. Am J Hum Genet 88, 402–421 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sciacovelli M & Frezza C Oncometabolites: Unconventional triggers of oncogenic signalling cascades. Free Radic Biol Med 100, 175–181, doi: 10.1016/j.freeradbiomed.2016.04.025 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sciacovelli M, Schmidt C, Maher ER & Frezza C Metabolic Drivers in Hereditary Cancer Syndromes. Annual Review of Cancer Biology 4, 77–97, doi: 10.1146/annurev-cancerbio-030419-033612 (2020). [DOI] [Google Scholar]
- 62.Alston CL, Rocha MC, Lax NZ, Turnbull DM & Taylor RW The genetics and pathology of mitochondrial disease. J Pathol 241, 236–250, doi: 10.1002/path.4809 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zois CE & Harris AL Glycogen metabolism has a key role in the cancer microenvironment and provides new targets for cancer therapy. J Mol Med (Berl) 94, 137–154, doi: 10.1007/s00109-015-1377-9 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bhatt AP, Redinbo MR & Bultman SJ The role of the microbiome in cancer development and therapy. CA Cancer J Clin 67, 326–344, doi: 10.3322/caac.21398 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.DePinho RA The age of cancer. Nature 408, 248–254, doi: 10.1038/35041694 (2000). [DOI] [PubMed] [Google Scholar]
- 66.Alicea GM et al. Changes in Aged Fibroblast Lipid Metabolism Induce Age-dependent Melanoma Cell Resistance to Targeted Therapy Via the Fatty Acid Transporter FATP2. Cancer Discov, doi: 10.1158/2159-8290.CD-20-0329 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Luo G & Liu N An integrative theory for cancer (Review). Int J Mol Med 43, 647–656, doi: 10.3892/ijmm.2018.4004 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Slawson C, Copeland RJ & Hart GW O-GlcNAc signaling: a metabolic link between diabetes and cancer? Trends Biochem Sci 35, 547–555, doi: 10.1016/j.tibs.2010.04.005 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Calle EE & Kaaks R Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 4, 579–591, doi: 10.1038/nrc1408 (2004). [DOI] [PubMed] [Google Scholar]
- 70.Filipski E & Levi F Circadian disruption in experimental cancer processes. Integr Cancer Ther 8, 298–302, doi: 10.1177/1534735409352085 (2009). [DOI] [PubMed] [Google Scholar]
- 71.Papagiannakopoulos T et al. Circadian Rhythm Disruption Promotes Lung Tumorigenesis. Cell Metab 24, 324–331, doi: 10.1016/j.cmet.2016.07.001 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barve A & Wagner A A latent capacity for evolutionary innovation through exaptation in metabolic systems. Nature 500, 203–206, doi: 10.1038/nature12301 (2013). [DOI] [PubMed] [Google Scholar]
- 73.Yun J et al. Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science 325, 1555–1559, doi: 10.1126/science.1174229 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Snyder V, Reed-Newman TC, Arnold L, Thomas SM & Anant S Cancer Stem Cell Metabolism and Potential Therapeutic Targets. Front Oncol 8, 203, doi: 10.3389/fonc.2018.00203 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chae YC & Kim JH Cancer stem cell metabolism: target for cancer therapy. BMB Rep 51, 319–326, doi: 10.5483/bmbrep.2018.51.7.112 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.De Francesco EM, Sotgia F & Lisanti MP Cancer stem cells (CSCs): metabolic strategies for their identification and eradication. The Biochemical journal 475, 1611–1634, doi: 10.1042/BCJ20170164 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin J et al. The roles of glucose metabolic reprogramming in chemo- and radio-resistance. J Exp Clin Cancer Res 38, 218, doi: 10.1186/s13046-019-1214-z (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Botzer LE et al. Hexokinase 2 is a determinant of neuroblastoma metastasis. Br J Cancer 114, 759–766, doi: 10.1038/bjc.2016.26 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ma L & Zong X Metabolic Symbiosis in Chemoresistance: Refocusing the Role of Aerobic Glycolysis. Front Oncol 10, 5, doi: 10.3389/fonc.2020.00005 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu L et al. Deregulated MYC expression induces dependence upon AMPK-related kinase 5. Nature 483, 608–612, doi: 10.1038/nature10927 (2012). [DOI] [PubMed] [Google Scholar]
- 81.Ihrlund LS, Hernlund E, Khan O & Shoshan MC 3-Bromopyruvate as inhibitor of tumour cell energy metabolism and chemopotentiator of platinum drugs. Mol Oncol 2, 94–101, doi: 10.1016/j.molonc.2008.01.003 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fan T, Sun G, Sun X, Zhao L, Zhong R, Peng Y. Tumor Energy Metabolism and Potential of 3-Bromopyruvate as an Inhibitor of Aerobic Glycolysis: Implications in Tumor Treatment. Cancers (Basel) 11 (3):317, doi: 10.3390/cancers11030317 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang G et al. Targeting mitochondrial biogenesis to overcome drug resistance to MAPK inhibitors. J Clin Invest 126, 1834–1856, doi: 10.1172/JCI82661 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abdalla AME et al. Current Challenges of Cancer Anti-angiogenic Therapy and the Promise of Nanotherapeutics. Theranostics 8, 533–548, doi: 10.7150/thno.21674 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McIntyre A & Harris AL Metabolic and hypoxic adaptation to anti-angiogenic therapy: a target for induced essentiality. EMBO Mol Med 7, 368–379, doi: 10.15252/emmm.201404271 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cao Y Adipocyte and lipid metabolism in cancer drug resistance. J Clin Invest 129, 3006–3017, doi: 10.1172/JCI127201 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pisarsky L et al. Targeting Metabolic Symbiosis to Overcome Resistance to Anti-angiogenic Therapy. Cell Rep 15, 1161–1174, doi: 10.1016/j.celrep.2016.04.028 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ashton TM, McKenna WG, Kunz-Schughart LA & Higgins GS Oxidative Phosphorylation as an Emerging Target in Cancer Therapy. Clin Cancer Res 24, 2482–2490, doi: 10.1158/1078-0432.CCR-17-3070 (2018). [DOI] [PubMed] [Google Scholar]
- 89.Vazquez F et al. PGC1alpha expression defines a subset of human melanoma tumors with increased mitochondrial capacity and resistance to oxidative stress. Cancer Cell 23, 287–301, doi: 10.1016/j.ccr.2012.11.020 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kuntz EM et al. Targeting mitochondrial oxidative phosphorylation eradicates therapy-resistant chronic myeloid leukemia stem cells. Nature medicine 23, 1234–1240, doi: 10.1038/nm.4399 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Keshet R, Szlosarek P, Carracedo A & Erez A Rewiring urea cycle metabolism in cancer to support anabolism. Nat Rev Cancer 18, 634–645, doi: 10.1038/s41568-018-0054-z (2018). [DOI] [PubMed] [Google Scholar]
- 92.Rabinovich S et al. Diversion of aspartate in ASS1-deficient tumours fosters de novo pyrimidine synthesis. Nature 527, 379–383, doi: 10.1038/nature15529 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Long Y et al. Cisplatin-induced synthetic lethality to arginine-starvation therapy by transcriptional suppression of ASS1 is regulated by DEC1, HIF-1alpha, and c-Myc transcription network and is independent of ASS1 promoter DNA methylation. Oncotarget 7, 82658–82670, doi: 10.18632/oncotarget.12308 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Long Y et al. Arginine deiminase resistance in melanoma cells is associated with metabolic reprogramming, glucose dependence, and glutamine addiction. Molecular cancer therapeutics 12, 2581–2590, doi: 10.1158/1535-7163.MCT-13-0302 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mowers EE, Sharifi MN & Macleod KF Autophagy in cancer metastasis. Oncogene 36, 1619–1630, doi: 10.1038/onc.2016.333 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Saber SH, Hamdy HEA, Gaballa R, Gaballah M, Ali HI, Zerfaoui M, et al. Exosomes are the Driving Force in Preparing the Soil for the Metastatic Seeds: Lessons from the Prostate Cancer. Cells 9(3):564, doi: 10.3390/cells9030564 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tomasetti M et al. MicroRNA-126 suppresses mesothelioma malignancy by targeting IRS1 and interfering with the mitochondrial function. Antioxid Redox Signal 21, 2109–2125, doi: 10.1089/ars.2013.5215 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Karachaliou N et al. Melanoma: oncogenic drivers and the immune system. Ann Transl Med 3, 265, doi: 10.3978/j.issn.2305-5839.2015.08.06 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Magaway C, Kim E & Jacinto E Targeting mTOR and Metabolism in Cancer: Lessons and Innovations. Cells 8(12):1584, doi: 10.3390/cells8121584 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Parmenter TJ et al. Response of BRAF-mutant melanoma to BRAF inhibition is mediated by a network of transcriptional regulators of glycolysis. Cancer Discov 4, 423–433, doi: 10.1158/2159-8290.CD-13-0440 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kanarek N, Petrova B & Sabatini DM Dietary modifications for enhanced cancer therapy. Nature 579, 507–517, doi: 10.1038/s41586-020-2124-0 (2020). [DOI] [PubMed] [Google Scholar]
- 102.Yang L et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther 5, 8, doi: 10.1038/s41392-020-0110-5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Condelli V, Crispo F, Pietrafesa M, Lettini G, Matassa DS, Esposito F, et al. HSP90 Molecular Chaperones, Metabolic Rewiring, and Epigenetics: Impact on Tumor Progression and Perspective for Anticancer Therapy. Cells 8(6):532, doi: 10.3390/cells8060532 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Masgras I, Sanchez-Martin C, Colombo G & Rasola A The Chaperone TRAP1 As a Modulator of the Mitochondrial Adaptations in Cancer Cells. Front Oncol 7, 58, doi: 10.3389/fonc.2017.00058 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Desbats MA, Giacomini I, Prayer-Galetti T & Montopoli M Metabolic Plasticity in Chemotherapy Resistance. Front Oncol 10, 281, doi: 10.3389/fonc.2020.00281 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Beck S & Ng T C2c: turning cancer into chronic disease. Genome Med 6, 38, doi: 10.1186/gm555 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]