Abstract
Early detection of ovarian cancer remains an important unmet medical need. Effective screening could reduce mortality by 10-30%. Used individually, neither serum CA125 nor transvaginal sonography (TVS) is sufficiently sensitive or specific. Two stage strategies have proven more effective, where a significant rise above a woman’s baseline CA125 prompts TVS and an abnormal sonogram prompts surgery. Two major screening trials have documented that this strategy has adequate specificity, but sensitivity for early stage (I-II) disease must improve to have a greater impact on mortality. To improve the first stage, different panels of protein biomarkers have detected cases missed by CA125. Autoantibodies against TP53 have detected 20% of early stage ovarian cancers 8 months prior to elevation of CA125 and 22 months prior to clinical diagnosis. Panels of autoantibodies and antigen-autoantibody complexes are being evaluated with the goal of detecting >90% of early stage ovarian cancers, alone or in combination with CA125, while maintaining 98% specificity in control subjects. Other biomarkers, including micro-RNAs, ctDNA, methylated DNA, and combinations of ctDNA alterations are being tested to provide an optimal first stage test. New technologies are also being developed with greater sensitivity than TVS to image small volumes of tumor.
Keywords: ovarian cancer, early detection, transvaginal sonography, CA125, biomarkers, pelvic mass
INTRODUCTION
There is a strong rationale for ovarian cancer screening. When limited to the ovaries (stage I), ovarian cancer can be cured in up to 90% of women with currently available surgery and chemotherapy. Even when disease has spread to the pelvis (stage II), 5 year survival can exceed 70%. Once cancer has spread throughout the abdominal cavity (stage III) or outside the abdominal cavity and/or into the parenchyma of the liver (stage IV), the cure rate slips to 20% or less. In the absence of an effective screening strategy, only 20% of ovarian cancers are diagnosed in early stage (I-II).1 Computer stimulations predict that detection of asymptomatic preclinical disease at an earlier stage could reduce mortality by 10-30%.2,3,4,5.
Given the postmenopausal prevalence of ovarian cancer (1 in 2,500), epidemiologic requirements for screening are stringent. Ultimately, ovarian cancer is generally diagnosed with an operative procedure. Gynecologic oncologists and advocates have argued that no more than 10 operations should be performed to detect each case of ovarian cancer. To achieve this positive predictive value (PPV) of 10%, a screening strategy must not only have a high sensitivity of ≥ 75%, but an even higher specificity of 99.6%.6
SCREENING WOMEN AT AVERAGE RISK
Most screening strategies have utilized the serum biomarker CA1257 and transvaginal sonography (TVS). Used alone, neither CA125 nor TVS has adequate sensitivity nor specificity to permit effective screening with a PPV >10%. This was well documented in the Prostate, Lung, Colon and Ovary (PLCO) Cancer Screening Trial that included 78,216 women between the ages of 55 and 74 who were screened with CA125 for 6 years and TVS for 4 years (n = 39,105) or were followed with conventional care (n = 39,111).8 Patients were referred to a gynecologist if CA125 was elevated or if the TVS was abnormal. In the PLCO, the PPV was 3.7% for elevated CA125 and 1% for abnormal TVS. If both tests were abnormal, the PPV rose to 23.5%, but 80% of cases would have been missed.
A similar result was obtained in the Shizuoka district of Japan, where asymptomatic postmenopausal women were randomized to annual screening with TVS and CA125 (41,688) or to conventional care (40,799).9 The fraction of stage I cases was higher in the screened group (63%) than in the control group (38%), but this difference did not achieve statistical significance (P= 0.23).
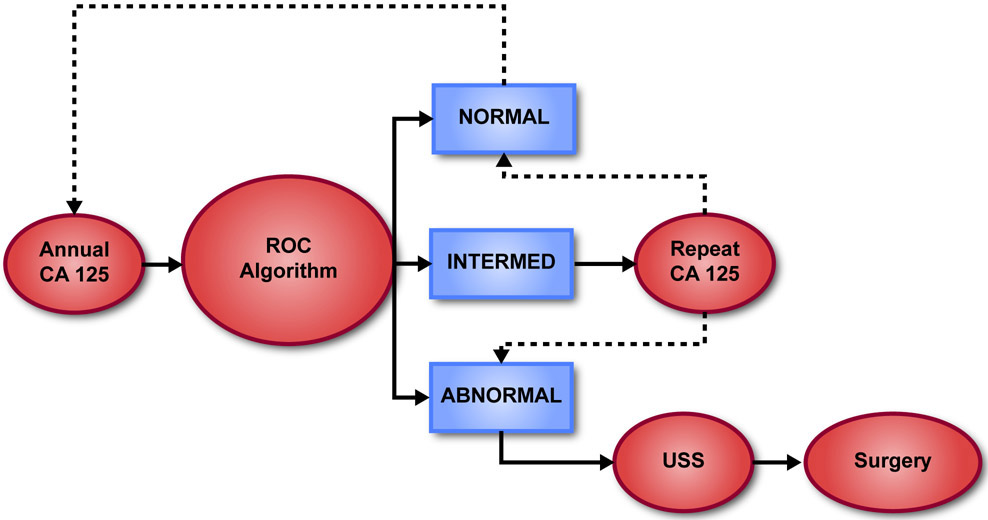
Over the last two decades, two-stage strategies have been developed using both CA125 and TVS sequentially. As cancers grow progressively, CA125 generally rises exponentially over time reflecting tumor doubling, whereas benign disease grows slowly, if at all, and CA125 levels do not change dramatically (Figure 1). In two major trials,10,11 CA125 has been measured annually in postmenopausal women at average risk for developing ovarian cancer. If CA125 rises significantly above a woman’s own baseline level of the biomarker, producing an elevated risk estimated with a Bayesian Risk of Ovarian Cancer Algorithm (ROCA), TVS is performed and if imaging suggests possible malignancy, an operation is undertaken (Figure 2). If CA125 increases more modestly, producing an intermediate risk, CA125 is repeated in three months. A further increase in CA125 usually results in an elevated risk which then triggers ultrasound and possible surgery. If the values remain stable or decline resulting in a normal risk, the participant returns in one year. This permits each woman to establish her own normal CA125 baseline, individualizing screening.
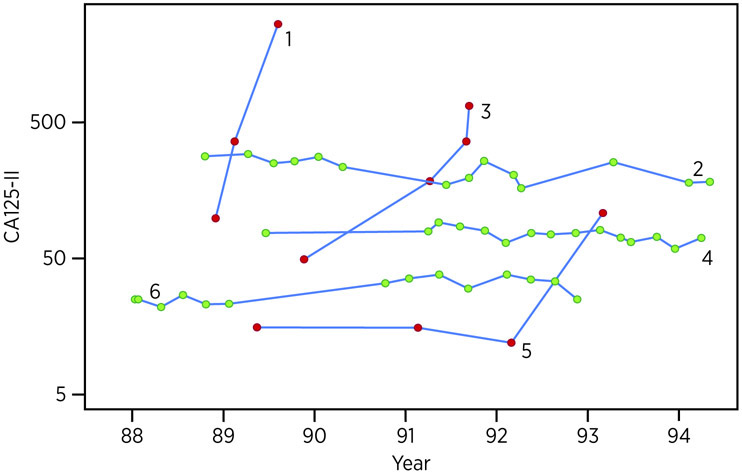
Figure 1.
Serial pattern of CA125 levels in six women from a UK study prospective trial of 22,000 postmenopausal women.87 CA125 values for three women with occult ovarian cancer (red dots) and three women without ovarian cancer (green dots).
Figure 2.
Two stage screening strategy using the Risk of Ovarian Cancer Algorithm (ROCA).
The two-stage approach has improved both specificity and sensitivity. When individual cases were reviewed, some women with ovarian cancer experienced a rapid rise over 12 months, whereas others had a more gradual increase in CA125 over more than one year within a normal range, improving sensitivity (Figure 3). Improved specificity has been observed in both major screening trials.
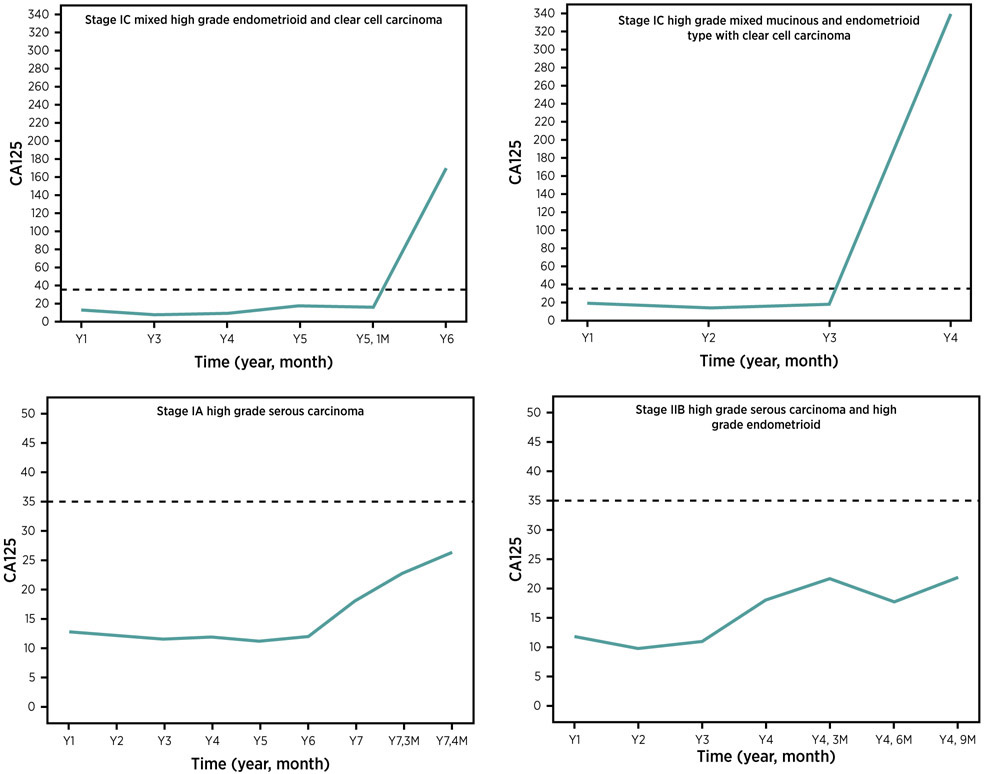
Figure 3.
Serial CA125 values for 4 patients from the NROSS10 who presented with early stage ovarian cancer.
In the Normal Risk Ovarian Screening Study (NROSS)10, some 6,872 postmenopausal women at average risk of developing ovarian cancer have been monitored over the last 19 years with 36,599 blood draws. Less than 1.5% have been referred for TVS after each annual blood test and only 2.2% of CA125 tests have undergone TVS during multiple years on study. Twenty-four operations have been indicated by the ROCA and have detected 15 cases of ovarian cancer – 2 borderline and 13 invasive high-grade cancers - with 10 of the 15 (67%) in stage I or II. In addition, two stage I endometrial cancers were detected. Using this two-stage strategy, no more than two operations were required to detect each case of ovarian cancer. In this trial, 2 borderline cancers, both in stage I, were not detected, as were three invasive cancers with one in stage I and two in stage III. To date, the sensitivity of this strategy for detecting early stage invasive disease is 13/20 (65%) with a PPV of 15/24 (62%), far above the minimum required of 10%, while maintaining a first stage test specificity of 98.5%.
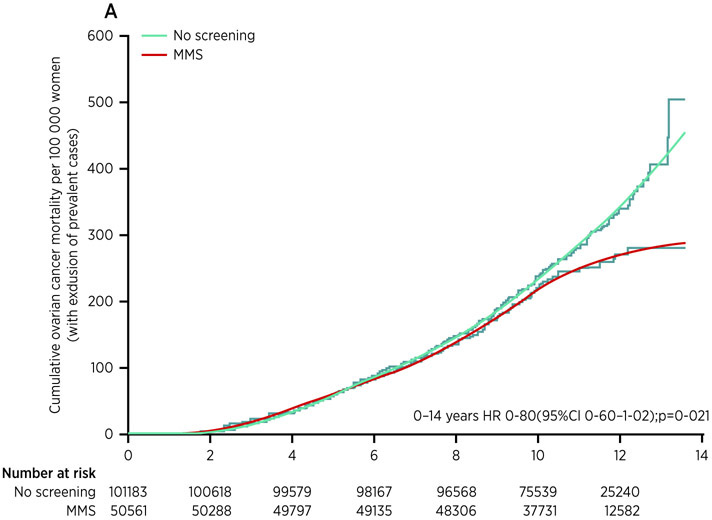
The United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) included more than 200,000 postmenopausal women at average risk who were randomized to three groups and followed initially for 14 years.11 A control group (101,359) was followed through the UK tumor registries. A second group (50,639) received TVS annually for 7 to 11 years. A third group (50,640) was screened annually from 7 to 11 years with CA125 interpreted by ROCA followed by TVS in a small fraction of cases, exactly the same protocol that was carried out in NROSS. Once again, the two-stage strategy was sufficiently specific so that there were only 3-4 operations required for each case of ovarian cancer detected. Approximately 40% of ovarian cancers were found by screening or clinically diagnosed in stage I or II, doubling the detection rate for early stage disease. Moreover, half of the ovarian cancers were detected with the algorithm before the cancers would have been detected using a single threshold of 35 U/mL, the traditional cut-point for CA125.12 ROCA is most effective when a baseline CA125 is established for each woman from which to judge whether a rise is significant. ROCA is less effective in detecting a case in early stage if the CA125 is already rising on the first test and no baseline has been established; these are prevalent ovarian cancer cases. While including all ovarian cancer cases in the analysis missed achieving statistical significance, in a pre-specified subgroup analysis that excluded prevalent cases, a 20% reduction in mortality was observed (P=0.021) for the multimodality group that was screened with annual CA125 interpreted by ROCA followed by TVS (Figure 4). As had been observed in screening for prostate cancer with PSA, mortality curves were similar for seven years and then diverged. The contrasting results between an analysis including all ovarian cancer cases and an analysis excluding prevalent cases prompted an additional five years of follow-up to June 2020, analysis of which will occur later in 2020. At present, it appears that 641 subjects must be screened to prevent one death. In a recent systematic review of the cost-effectiveness of ovarian cancer early detection, four different analyses of two-stage screening in postmenopausal women achieved incremental cost-effectiveness ratios of ~$11,564 to $96,052 / Quality Adjusted Life Year (QALY) depending on assumptions regarding extrapolation of mortality data, costs and test performance.13 As the National Institute for Health and Care Excellence (NICE) in the UK has used ~$26,200 - $39,300 / QALY as acceptable values for screening, multimodal screening could be cost-effective, particularly if mortality were reduced by 20% on updated analysis of data from the UKCTOCS.
Figure 4.
Cumulative ovarian cancer deaths. In the UKCTOCS trial.11 HR=hazard ratio. MMS=multimodal screening.
SCREENING WOMEN AT INCREASED GENETIC RISK
Germ-line mutations with BRCA1 or BRCA2 confer a lifetime ovarian cancer risk of 40-50% or 15-20% respectively, compared to 1.3% in the general population.14 High-grade serous cancers occur more frequently in carriers of BRCA1/2 mutations.15 In the absence of a reliable strategy for early detection in this group, risk reducing bilateral salpingo-oophorectomy is recommended as soon as women have completed their families. When surgery is delayed, TVS and CA125 are generally performed every six months, but there is no evidence that this improves survival. Judged by the presence of pre-malignant lesions, up to 70% of “ovarian” cancers that develop in women carrying BRCA1 or BRCA2 mutations may arise not from the ovary, but rather from the fimbriae of the fallopian tubes.16 While many high-grade cancers can also arise from the fallopian tube in women at conventional genetic risk, this poses an even greater challenge for early detection. As soon as malignant cells can resist “anoikis” and survive floating free from the underlying matrix, high-grade serous cancers can spread throughout the peritoneal cavity, accounting for reports of the sudden appearance of widespread peritoneal metastasis and elevated CA125 no more than three months after the last apparently normal examination.
Annual screening has not been effective in women at high risk. When the ROCA was evaluated every three months and followed by TVS to screen 4,348 women at >10% risk of developing ovarian cancer in the UK Familial Ovarian Cancer Screening Study (UKFOCSS), 10 of 19 cancers (53%) were detected in stage I or II, reflecting a significant stage shift.17 In the United States, a similar strategy used the ROCA every three months to screen 3,692 patients at increased risk in two distinct trials.18 Nineteen cancers were detected: 4 were prevalent, 6 were incident and 9 were detected at risk reducing surgery (RRS). Among incident cases, 3 of 6 (50%) were in early stage (I-II) also reflecting a significant stage shift compared to historical controls, while 6 of 9 (67%) of cancers found at RRS were in stage I. Early stage patients remained free from recurrence at 6 years. While these trials are encouraging, in that they document increased detection of early stage disease by ROCA in women at increased genetic risk, they do not provide definitive evidence of improved survival. Definitive evidence would require screening trials that randomized patients to a screened arm or to a control arm (with mortality as an endpoint) and such trials are not likely to be performed given the very high risk of developing ovarian cancer.
IMPROVING THE INITIAL STAGE OF SCREENING
Whatever the outcome of the reanalysis of the UKCTOCS, there is room for improvement in both the first and second stages of a two-stage screening strategy. Within primary tumors, only 80% of ovarian cancers express significant levels of CA125. Consequently, using CA125 alone during initial screening will miss at least 20% of ovarian cancers. Computer simulations suggest that a panel of biomarkers which improves the sensitivity of CA125 without compromising specificity could reduce overall mortality by 25% compared to 13% with CA125 alone.4
Improved sensitivity is likely to be required for effective screening. Brown and Palmer had modeled the growth of high grade serous ovarian cancers in women with BRCA1 germ line mutations and estimated that the median diameter of a serous ovarian cancer when it progresses to an advanced stage (III-IV) is approximately 3 cm.19 If their model is correct, to achieve a sensitivity of 50%, a screening strategy would need to detect cancers of 1.3 cm in diameter and a 50% reduction in mortality would require detecting cancers 0.5 cm in diameter. Hori and Gambhir have estimated, based on likely rates of shedding for CA125, that tumors must grow to 2.5 cm to raise blood levels of the antigen above the standard cutoff.20 Given the fact that early stage (I-II) disease has been detected in 40-67% of patients with ROCA through rising levels of CA125 in the UKCTOCS and NROSS trials, these estimates may be pessimistic, but do point to the need to identify biomarkers and panels with greater sensitivity and comparable specificity to ROCA using CA125 alone.
Protein antigens.
Over the last two decades, our laboratory and those of our collaborators, have evaluated 110 potential biomarkers with the goal of increasing the fraction of early stage ovarian cancers detected by CA125 alone from ~60-70% to > 90%, while retaining 98% specificity. In published studies to date, more than 35 different biomarkers have been reported to improve the sensitivity of CA125 for detecting early stage disease.21,22,23,24 Many of these biomarkers detect only a small fraction of cases missed by CA125; most studies include a relatively small number of early stage cases; and often the utility of combinations has not been confirmed with an independent validation set.
The combination of CA125 and human epididymis protein 4 (HE4) has received particular attention.26,27,28 HE4, also known as Whey acidic protein (WFDC2), is a 25 kD secreted protein that was elevated in 73% of ovarian cancers of all stages at 89% specificity in a meta-analysis of 31 reports.29 HE4 is slightly less sensitive than CA125 for detecting early stage ovarian cancer, but has better specificity for distinguishing malignant from benign pelvic masses. CA125 and HE4 have been used in combination to triage patients for specialized surgery. Significantly better outcomes have been observed when women with ovarian cancer are treated by specially trained gynecologic oncologists who can perform more aggressive cytoreductive surgery and provide intensive chemotherapy.30 At present, 30-50% of women with ovarian cancer in the United States do not receive their primary surgery from a gynecologic oncologist or high volume cancer surgeon.31 As only 20% of pelvic masses are malignant, it is often not clear who needs the care of the specially trained surgeon. A combination of CA125 and HE4 has been used in the risk of malignancy algorithm (ROMA)32,33 and has been supplemented by three other biomarkers (transferrin, apolipoprotein A1 and follicle stimulating hormone) in the OVERA test34 to distinguish malignant from benign pelvic masses. Development of both the ROMA algorithm and OVERA test were supported by the Early Detection Research Network (EDRN) and both have been cleared by the FDA. These tests can aid in referring women with pelvic masses to the physicians best qualified to manage their cancer or benign disease. In their registration trials, both the ROMA and OVERA predicted women likely to have a malignant pelvic mass with 91-94% sensitivity, 69-74% specificity and 97-99% negative predictive value. In a recent direct comparison, OVERA detected more cancers than ROMA,35 but would also have prompted substantially more referrals of patients with benign disease to gynecologic oncologists. While additional trials will be required to confirm these differences, the greatest need is to use either test more consistently to enhance referral of appropriate patients to the best qualified surgeons.
In developing panels of biomarkers for early detection, several studies have incorporated CA125 and HE4 in combination with CA72.436, CA72.4 and CA15-337 , CEA and V-CAM1,38 glycodelin22, E-cadherin and IL-639 or transthyretin.40 Addition of HE4 and CA72.4 to CA125, for example, detects 16% of cases missed by CA125, but does not provide lead-time before elevation of CA125.36 While CA125 and HE4 levels are elevated in serous41,42 and endometrioid ovarian cancers42,43, they are less frequently elevated with the mucinous histotype,41,44 whereas CA72.4 is expressed by a greater fraction of mucinous ovarian cancers.45 CA72.4 has, however, proven difficult to model statistically, but a new longitudinal ROCA algorithm that combines CA125 and HE4 is being developed with support from the EDRN.
Using preclinical serum samples in the UKCTOCS biobank from women destined to develop ovarian cancer, investigators at Manchester, UK, have identified three panels of biomarkers in combination with CA125: lecithin-cholesterol acyltransferase (LCAT) and insulin-like growth factor-binding protein 2 (IGFBP2);23 phosphatidylcholine-sterol acyltransferase, vitamin K-dependent protein Z and C-reactive protein;24 and HE4, CHI3L1, PEBP4 and/or AGR2.25 Each panel detected a fraction of cases missed by CA125 and produced lead time over CA125 of 5-6 months23 to a year25 or more24. Finding an adequate number of pre-clinical serum samples to validate these panels and to identify the optimal combinations of biomarkers will be a challenge.
High-grade serous cancers could arise from the fimbriae of the fallopian tube both in women at high and normal genetic risk. While the exact fraction of these cases remains unknown, at least 70% of the 15% of BRCA I/II mutated cancers or 10% of all ovarian cancers are likely to arise from the fallopian tube.16 In addition, 20% of the “primary peritoneal” high grade serous ovarian cancers coat the ovary, rather than grow from it at the time of primary surgery and these cancers could well arise from the fallopian tube. Taken together, at least 30% of high-grade serous cancers arising from the fallopian tube could be shed into the peritoneal cavity when still quite small in volume. To obtain elevated levels of shed protein antigens in blood, a larger volume of cancer may be required.
Autoantibodies.
Autoantibodies against cancer associated proteins could be stimulated by very small ovarian cancers. Nearly all high-grade serous cancers have TP53 mutations and autoantibodies against TP53 have been reported in approximately 20-25% of cases in multiple reports. Our group, with the support of the EDRN, had studied anti-TP53 autoantibodies in sera from women who had participated in the UKCTOCS and had donated blood months to years prior to the diagnosis of ovarian cancer. Elevated levels of anti-TP53 could be detected on average 8 months prior to the elevation of CA125 and 22 months prior to clinical diagnosis in patients who did not experience an increase in CA125.46
Panels of autoantibodies have been identified that include anti-TP53. From a screen of 5177 potential autoantigens, anti-prostaglandin F receptor (anti-PTGFR) and anti-protein tyrosine phosphatase, receptor type A (anti-PTPRA) were found to complement anti-TP53.47 While each autoantibody detected 22-32% of ovarian cancers at 95% specificity, a sensitivity of 23% could be achieved at 98.3% specificity, when levels of 2 of the 3 autoantibodies were elevated. Recently, a panel of autoantibodies against TP53, TRIM-21, NY-ESO-1 (CTAG-1A) and PAX-8 achieved a sensitivity of 46-56% at a specificity of 98%.48 Interestingly, additional autoantibodies have been detected to other proteins at nodes in TP53 and MYC driven pathways that are known to underly ovarian oncogenesis.49
Other investigators have identified a number of other autoantibodies which have been well reviewed.50,51,52 Earlier results by other groups have been difficult to replicate, but IL-8 autoantibodies were increased in sera from ovarian cancer patients.53 Building on previous studies54 and supported by the EDRN, our group had found that a combination of CA125, osteopontin, macrophage inhibitory factor and anti-IL8 autoantibodies could detect 82% of early stage ovarian cancers compared to 64% with CA125 alone at 98% specificity.55 While most studies have focused on IgG autoantibodies, IGA autoantibodies against HSF-1, as well as IgG autoantibodies against CDC155, have been reported in small numbers of very early stage patients.56
Autoantibodies against HE4 and HE4 antigen-autoantibody complexes have been evaluated by our group in early stage (I-II) ovarian cancer, once again supported by the EDRN. While free autoantibodies were observed in less than 5% of cases, antigen-autoantibody complexes were found in 38% of early stage cases at 98% specificity.57 Use of CA125 and HE4 antigen-autoantibody complexes in combination, increase the fraction of cases detected from 63% with CA125 alone to 81% with both biomarkers. The levels of HE4 antigen-autoantibody complexes did not, however, rise earlier than did levels of CA125.
Noncoding RNA.
Serum miRNAs also have potential for early detection of ovarian cancer. Changes in different miRNA levels in ovarian cancer cells or tissue have been correlated with proliferation, migration, invasion and chemosensitivity.58 From a recent review, at least 15 miRNAs are upregulated and 9 downregulated in serum or plasma from ovarian cancer patients. Neural network analysis has been applied to develop an algorithm that utilizes multiple miRNAs to achieve a positive predictive value of 91.3% and a negative predictive value of 78.6% in a validation set of sera from 51 patients with ovarian disease (29 ovarian cancer cases, 22 benign controls).59 Studies with larger numbers of sera from early stage ovarian cancer patients and healthy controls will be required to determine the full potential of miRNAs for early detection.
DNA.
Circulating tumor DNA (ctDNA) in blood and cervical secretions can be detected in 55% of early stage ovarian cancers and detects cases missed by CA125 alone in preliminary studies from Johns Hopkins.60 Obtaining cervical material may be important as tumors >1 cm in diameter may be required to raise blood levels of ct DNA.61 Shed tumor cells and cell-free DNA can pass through the fallopian tube and uterine cavity to the cervical os, without the significant dilution that occurs when DNA is shed from the cancer into peripheral blood. Recent development of a repetitive element aneuploidy sequencing system (RealSeq) to detect aneuploidy in ctDNA from as little as 3 pg of DNA in relatively small amounts of plasma promises to improve sensitivity for early stage high grade ovarian cancers with copy number abnormalities.62 Using this approach, aneuploidy was detected in 62% and mutations in 73% of ovarian cancer plasma samples, although only 25% of cases were in early stage (I-II).
DNA methylation.
Methylation specific PCR (MSP) has been used to compare ctDNA in sera from early stage ovarian cancer patients and healthy individuals for seven different genes including APC, RASSF1A, CHDH1, RUNX3, TFP12, SRP5 and OPCML.63 The panel achieved 85% sensitivity at 91% specificity for early stage disease, compared to 56% sensitivity and 64% specificity for CA125. In a more recent study, a three methylated gene panel was used to evaluate sera from the control arm of the UKCTOCS trial where 57.9% of women who developed ovarian cancer within 2 years were detected at a specificity of 88.1%.64 Data are not yet available for the sensitivity of ctDNA methylation analysis at the high specificity (95-98%) required in the first stage of a two stage screening strategy.
Universal Cancer Screening.
Detection of abnormal cancer-derived DNA sequences, copy number and methylation in plasma has raised the possibility of screening for cancer at multiple sites using a single blood sample. Once abnormalities are detected in DNA, there is still the challenge of identifying the organ from which the DNA was shed, using organ specific protein biomarkers or imaging. While this approach could enhance the convenience and efficiency of early detection, universal cancer screening must attain the sensitivity, specificity and cost-effectiveness of organ-specific cancer screening. Ovarian cancer has been included in multi-site screening strategies that detect ctDNA mutations in blood and then use concomitantly elevated CA125 levels to localize the source of mutant DNA. from the ovary or fallopian tube.65 The CancerSEEK combination of DNA sequencing and protein biomarkers detected ovarian cancer with 98% sensitivity at 99% specificity among a group of 1005 patients clinically detected with different types of cancer, but 76% of patients detected were in stage III where long term survival is less than 30% and where CA125 alone would have a sensitivity of >90% at 97% specificity. In the subsequent DETECT-A study of 10,006 women not previously known to have cancer, testing for DNA mutations and protein biomarkers detected 26 cancers and 15 were confirmed by PET-CT.66 Among the 26 cancers, 6 were ovarian and only one was in early stage (17%). To assure specificity, the CA125 threshold was set at 577 U/mL, 16 times the usual threshold of CA125, that would have missed all 10 early stage cancers found in the NROSS. While universal cancer screening is an attractive concept, additional work needs to be done to improve upon strategies specific for a given cancer.
SCREENING SYMPTOMATIC WOMEN
Ovarian cancer is not a “silent killer”, as 89% of early stage (I-II) disease is associated with new onset of symptoms, including 1) gastrointestinal symptoms of nausea, diarrhea and constipation, 2) abdominal and pelvic pain, 3) bloating, increased girth and early satiety and 4) urinary urgency and frequency. These symptoms are, however, associated with many more common conditions. A case-control study with 2,025 participants found that a symptom index detected <0.5% of early stage (I-II) disease and <1.1% of ovarian cancers overall.67 Addition of CA125 might improve detection. A recent study of 50,780 women in the U.K. found that an elevated CA125 (>35 U/mL) in symptomatic women was associated with positive predictive values of 15.2% (>50 years) and 3.4% (<50 years) for developing ovarian cancer within 12 months.68
IMPROVING THE SECOND STAGE OF SCREENING
Ovarian cancers can be imaged by multiple techniques including transvaginal sonography (TVS), computerized tomography (CT), positron emission tomography with CT (PET-CT) and magnetic resonance imaging (MRI). Aside from higher cost, CT’s ability to detect malignancy in an adnexal mass is comparable to TVS and exposes healthy individuals to ionizing radiation.69 PET-CT is also associated with radiation exposure and has relatively low spatial resolution, limiting detection of small tumors. PET/CT is also associated with physiologic uptake in normal structures which may obscure small pelvic malignancies or lead to false positives.67 Using PET or PET-CT, a wide range in sensitivity (58-100%) and specificity (67-92%) has been reported for the detection of ovarian malignancies in women with adnexal masses.70,71,72,73 MRI has reported greater accuracy and specificity in the diagnosis of malignant adnexal masses (89% and 84%, respectively, versus 64% and 40%).74 Due to high cost and more limited availability of MRI, TVS is generally the first line test for conventional diagnosis of a pelvic mass.
While the consensus of most critical reviewers is that TVS lacks both adequate sensitivity and specificity for early detection,75 one large cohort study at the University of Kentucky has monitored 46,101 asymptomatic women over age 50 as well as women greater than 25 years of age with a positive family history of ovarian cancer using TVS and Doppler flow ultrasonography.76 Among the 71 invasive epithelial ovarian cancers detected over three decades, 63% were early stage (I-II). Disease specific survival at 5, 10 and 20 years for women whose invasive ovarian cancers were detected by screening was significantly greater than that observed in unscreened patients from the same geographic area treated at the same institution (P<0.001). While the positive predictive value for ultrasound screening was 15.6%, the prevalence of ovarian cancer in the screened population was 24-fold higher than in the general population and if the observed sensitivity and specificity were applied to the general population, the PPV would drop to 0.7%.77 The high prevalence of ovarian cancer in the screened population may relate to a family history of ovarian cancer in 23% and of breast cancer in 43%. Moreover, 27% had type I cancers which are commonly diagnosed in early stage. The remarkable results from Kentucky may also relate to highly competent investigators using a technology that depends critically on experience and expertise.
While well-trained imagers can obtain concordant results,78 re-review of 1,000 archived cases with apparently normal morphology from the early years of the UKCTOCS indicated unsatisfactory visualization of the ovaries and tubes in 50% of cases.79 Imaging of the fallopian tubes, the site of origin of at least one third of high grade serous ovarian cancers, can be particularly difficult in the absence of conditions that cause thickening of the tube or accumulation of intra-luminal fluid. Even in the most expert centers, fallopian tubes could not be visualized in 23% of 549 healthy women.80
Conversely, detecting irregularities of ovarian size and shape can lead to operative intervention, accounting for the limited specificity of TVS in the PLCO trial. Similar challenges in distinguishing malignant from benign lesions, increased cost and limited resolution of small lesions, argue against use of MRI, CT or PET-CT for primary screening.75
Specificity of TVS has been improved by Doppler flow81 or use of microbubbles82 that characterize blood flow within the tumor, but sensitivity is not necessarily improved. Photoacoustic imaging can detect early tumor vascularization, but this technique is limited to a tissue depth of 5 cm with a decline in spatial resolution with increasing depth. Combination of photoacoustic tomography with ultrasound can, however, partially compensate for these limitations.83
In the UKCTOCS study, a fraction of patients was found to have a rising CA125 as judged by an elevated ROCA and normal TVS. In this setting, a method is needed to detect ovarian cancer with or without precise imaging. One technology that shows promise is Superconducting Quantum Interference Detection (SQUID), a very sensitive method to detect faint magnetic fields.84 To provide a probe to detect ovarian cancer, anti-CA125 antibodies have been conjugated with ferritin nanospheres. After injection intravenously or interperitoneally, unbound nanoparticles fail to give a signal when exposed to a magnetic pulse. When antibody conjugated nanospheres bind to ovarian cancer cells, relaxation of the magnetic field is delayed. By measuring this delay in magnetic relaxation (MRx), 106 ovarian cancer cells (0.1 mm) can be detected ex-vivo.85,86 Preclinical studies are underway to determine whether antibody-conjugated nanoparticles can localize effectively to human ovarian cancers xenografts. To compensate for intertumoral heterogeneity, a panel of four antibodies has been identified that can bind to >99% of ovarian cancers.
CONCLUSIONS
If the UKCTOCS shows a mortality advantage when an additional five years of follow-up is analyzed, the similarly designed NROSS trial has demonstrated that screening with CA125 followed by TVS is feasible in the United States. The first stage of screening can be improved by the addition of other protein antigens, autoantibodies, antigen-autoantibody complexes and possibly by miRNAs, ctDNA and methylated ctDNA. Development of more sensitive imaging and detection methods could detect small amounts of cancer on the ovary or fallopian tube missed by TVS. Finding preclinical disease at an earlier stage could reduce long-term ovarian cancer mortality by 10-30%.
ACKNOWLEDGEMENTS
This work was supported by funds from the NCI Early Detection Research Network (5 U01 CA200462-02 (RC Bast), 5 U01 CA152990-09 (SS Skates)), the MD Anderson Ovarian SPOREs (P50 CA83639; RC Bast) and P50CA217685 (RC Bast), National Cancer Institute, Department of Health and Human Services; the Cancer Prevention Research Institute of Texas (RP160145 ; RC Bast); Golfer’s Against Cancer; the Tracey Joe Wilson Foundation; National Foundation for Cancer Research; UT MD Anderson Women’s Moon Shot; and generous donations from the Ann and Henry Zarrow Foundation, the Mossy Foundation, the Roberson Endowment, Stuart and Gaye Lynn Zarrow, Barry Elson, Arthur and Sandra Williams, and from the Concord (MA) Detect Ovarian Cancer Early Fund.
Footnotes
Conflicts of interest: Dr. Bast receives royalties from Fujirebio Diagnostics Inc. for the discovery of CA125. Massachusetts General Hospital co-licensed the Risk of Ovarian Cancer Algorithm to Abcodia Inc. and Dr. Skates is a consultant to Abcodia.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017; 67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Skates SJ, Singer DE. Quantifying the potential benefit of CA 125 screening for ovarian cancer. J Clin Epidemiol 1991;44(4–5):365–80. [DOI] [PubMed] [Google Scholar]
- 3.Moss HA, Berchuck A, Neely ML, Myers ER, Havrilesky LJ. Estimating cost-effectiveness of a multimodal ovarian cancer screening program in the United States: Secondary analysis of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). JAMA Oncol 2018; 4:190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drescher CW, Hawley S, Thorpe JD, Martike S, McIntosh M, Gambhir SS, et al. Impact of Screening Test Performance and Cost on Mortality Reduction and Cost-effectiveness of Multimodal Ovarian Cancer Screening. Cancer Prev Res 2012; 5; 1015–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Havrilesky LJ, Sanders GD, Kulasingam S, Chino JP, Berchuck A, Marks JR, et al. Development of and ovarian cancer screening decision model that incorporates disease heterogeneity: Implications for potential morality reduction. Cancer 2011; 117: 545–553. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs I, Bast RC Jr. The CA 125 tumour-associated antigen: a review of the literature. Hum Reprod 1989; 4 :1–12. [DOI] [PubMed] [Google Scholar]
- 7.Bast RC Jr, Klug TL, St. John E, Jenison E, Niloff JM, Lazarus H, et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. New Engl J Med 1983; 309:883–887. [DOI] [PubMed] [Google Scholar]
- 8.Buys SS, Partridge E, Greene MH, Prorok PC, Reding D, Riley TL, et al. Ovarian cancer screening in the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial: findings from the initial screen of a randomized trial. Am J Obstet Gynecol 2005;193(5):1630–9. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi H, Yamada Y, Sado T, Sakata M, Yoshida S, Kawaguchi R, et al. A randomized study of screening for ovarian cancer: a multicenter study in Japan. Int J Gynecol Cancer 2008; 18:414–420. [DOI] [PubMed] [Google Scholar]
- 10.Lu KH, Skates S, Hernandez MA, Bedi D, Bevers T, Leeds L, et al. A 2-stage ovarian cancer screening strategy using the risk of ovarian cancer algorithm (ROCA) identifies early-stage incident cancers and demonstrates high positive predictive value. Cancer 2013; 119:3454–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobs IJ, Menon U, Ryan A, Gentry-Maharaj A, Burnell M, Kalsi JK, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet 2016; 387:945–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menon U, Ryan A, Kalsi J, Gentry-Maharaj A, Dawnay A, Habib M, et al. Risk algorithm using serial biomarker measurements doubles the number of screen-detected cancers compared with a single-threshold rule in the United Kingdom Collaborative Trial of Ovarian Cancer Screening. J Clin Oncol 2015; 33:2062–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sroczynski G, Gogollari A, Kuehne F, Hallsson DR, Widschwendter M, Pashayan NA. Systematic review on cost-effectiveness studies evaluating ovarian cancer early detection and prevention strategies. Cancer Prev Res 2020; 13:429–42. [DOI] [PubMed] [Google Scholar]
- 14.Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin 2018; 68:284–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lakhani SR, Manek S, Penault-Llorca F, Flanagan A, Arnout L, Merrett S, et al. Pathology of Ovarian Cancers in BRCA1 and BRCA2 Carriers. Clin Cancer Res 2004; 10: 2473–2481. [DOI] [PubMed] [Google Scholar]
- 16.Mehra KK, Chang MC.; Folkins AK, Raho CJ, Lima JF, Yuan L, et al. The impact of tissue block sampling on the detection of p53 signatures in fallopian tubes from women with BRCA 1 or 2 mutations (BRCA+) and controls. Mod. Pathol 2011; 24:152–156. [DOI] [PubMed] [Google Scholar]
- 17.Rosenthal AN, Fraser LSM, Philpott S, Manchanda R, Burnell M, Badman P, et al. Evidence of stage shift in women diagnosed with ovarian cancer during phase II of the United Kingdom Familial Ovarian Cancer Screening Study. J Clin Oncol 2017; 35:1411–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skates SJ, Greene MH, Buys SS, Mai PL, Brown PL, Piedmonte M, et al. Early detection of ovarian cancer using the risk of ovarian cancer algorithm with frequent CA125 testing in women at increased familial risk – combined results from two screening trials. Clin Cancer Res 2017; 23: 3628–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown PO, Palmer C. The preclinical natural history of serous ovarian cancer: defining the target for early detection. PlosMedicine 2009; 6:e1000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hori SS, Gambhir SS. Mathematical model identifies blood biomarker strategies and limitations. Sci Translational Medicine 2011; 3: 109ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muinao T, Boruah HPD, Pal M. Multi-biomarker panel signature as the key to diagnosis of ovarian cancer. Heliyon 2019; 5, e02826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blyuss O, Gentry-Maharaj G, Fourkala E-O, Ryan A, Zaikin A, et al. Serial Patterns of Ovarian Cancer Biomarkers in a Prediagnosis Longitudinal Dataset. Biomed Res Intl. 2015; 2015: 681416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russell MR, Graham C, D’Amato A, Gentry-Maharaj A, Ryan A, Kalsey JK, et al. A combined biomarker panel shows improved sensitivity for the early detection of ovarian cancer allowing the identification of the most aggressive type II tumours. British Journal of Cancer 2017; 117: 666–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russell MR, Graham C, D’Amato A, Gentry Maharaj A, Ryan A, Kalsi JK, et al. Diagnosis of epithelial ovarian cancer using a combined protein biomarker panel. British Journal of Cancer 2019; 121:483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitwell HJ, Worthington J, Blyuss O, Gentry-Maharaj A, Ryan A, Gunu R, et al. Improved early detection of ovarian cancer using longitudinal multimarker models. British Journal of Cancer 2020; 122:847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helstrom I, Raycraft J, Hayden-Ledbetter M, Ledbetter J, Schummer M, et al. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Clin Cancer Res 2003; 63:3695–3700. [PubMed] [Google Scholar]
- 27.Simmons AR, Baggerly K, Bast RC. The emerging role of HE4 in the evaluation of advanced epithelial ovarian and endometrial carcinomas. Oncology 2013; 27:548–556. [PMC free article] [PubMed] [Google Scholar]
- 28.Haque R, Skates SJ, Armstrong MA, Lentz SE, Anderson M, Jiang W, et al. Feasibility, patient compliance and acceptability of ovarian cancer surveillance using two serum biomarkers and Risk of Ovarian Cancer Algorithm compared to standard ultrasound and CA 125 among women with BRCA mutations. Gynecol Oncol 2020; 157:521–528. [DOI] [PubMed] [Google Scholar]
- 29.Yang z, Wei C, Luo Z, Li L. Clinical value of serum human epididymis protein 4 assay in the diagnosis of ovarian cancer: a meta-analysis. OncoTargets and Therapy 2013; 6:957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mercado C, Zingmond D, Karlan BY, Sekaris E, Gross J, Maggard-Gibbons M, et al. Quality of care in advanced ovarian cancer: The importance of provider specialty. Gynecol Oncol 2010; 117:18–22. [DOI] [PubMed] [Google Scholar]
- 31.Goff B, Miller JW, Matthews B, Trivers KF, Andrilla HA, Lishner DM, et al. Involvement of gynecologic oncologists in the treatment of patients with a suspicious ovarian mass. Obstet Gynecol 2011; 118:854–62. [DOI] [PubMed] [Google Scholar]
- 32.Moore RG, McMeekin SD, Brown AK, DiSilvestro P, Miller C, Allard WJ, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol 2009; 112: 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore RG, Miller MC, Disilvestro P, Landrum LM, Gajewski W, Ball JJ, et al. Evaluation of the Diagnostic Accuracy of the Risk of Ovarian Malignancy Algorithm in Women with a Pelvic Mass. Obstet Gynecol 2011;118:280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coleman R, Herzog TJ, Chan DW, Munroe DG, Pappas TC, Smith A, et al. Validation of a second-generation multivariate index assay for malignancy risk of adnexal masses. Am J Obstet Gynecol 2016; 215: 82.e1–11. [DOI] [PubMed] [Google Scholar]
- 35.Shulman LP, Francis M, Bullock, Pappas T. Clinical performance comparison of two in-vitro diagnostic multivariate index assays (IVDMIAs) for presurgical assessment for ovarian cancer risk. Adv Ther 2019; 36:2402–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simmons AR, Fourkala EO, Gentry-Maharaj A, Ryan A, Sutton MN, Baggerly K, et al. Complementary Longitudinal Serum Biomarkers to CA125 for Early Detection of Ovarian Cancer. Cancer Prev Res 2019; 12:391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terry KL, Schock H, Fortner R, Husing A, Fichorova, et al. A prospective evaluation of early detection biomarkers for ovarian cancer in the European EPIC cohort. Clin Cancer Res. 2016; 22:4664–4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yurkovetsky Z, Skates S, Lomakin A, Nolen B, Pulsipher T, Modugno F, et al. Development of a multimarker assay for early detection of ovarian cancer. J Clin Oncol 2010; 28:2159–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han C, Bellone S, Siegel ER, Altwerger G, Menderes G, Bonazzoli E, et al. A Novel Multiple Biomarker Panel for the Early Detection of High-grade Serous Ovarian Carcinoma. Gynecol Oncol 2018; 149: 585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng X, Chen S, Li L, Liu X, Liu X, Dai S, et al. Evaluation of HE4 and TTR for diagnosis of ovarian cancer: Comparison with CA-125. J Gynecol Obstet Hum Reprod 2018; 47:227–230 [DOI] [PubMed] [Google Scholar]
- 41.Zanaboni F, Vercadoro F, Presti M, Gallo P, Lombardi F, Bo Li G. Tumor antigen CA125 in preclinical invasive epithelial ovarian cancer. Gynecol Oncol 1987; 28:61–67. [DOI] [PubMed] [Google Scholar]
- 42.Blackman A, Mitchell J, Turner R, Singh R, Kim K, Eklund E, et al. Analysis of serum HE4 levels in various histologic subtypes of epithelial ovarian cancer and other malignant tumors. Submitted for publication. [DOI] [PubMed] [Google Scholar]
- 43.Grandi G, Perrone AM, Toss A, Vitagliano A, Friso A, Facchinetti F, et al. The generally low sensitivity of CA 125 for FIGO stage I ovarian cancer diagnosis increases for endometrioid histotype. Minerva Med 2020; 111:133–140. [DOI] [PubMed] [Google Scholar]
- 44.Kabawat SE, Bast RC Jr, Bhan AK, Welch WR, Knapp RC, Colvin RB. Tissue distribution of a coelomic-epithelium-related antigen recognized by the monoclonal antibody OC125. Int J Gynecol Pathol 1983; 2:275–285. [DOI] [PubMed] [Google Scholar]
- 45.Negishi Y, Iwabuchi H, Sakunaga H, Sakamoto M, Okabe K, Sato H, et al. Serum and tissue measurement of CA72–4 in ovarian cancer patients. Gynecol Oncol 1993; 48:148–154. [DOI] [PubMed] [Google Scholar]
- 46.Yang WL, Gentry-Maharaj A, Simmons A, Ryan A, Fourkala EO, Lu Z, et al. Elevation of TP53 autoantibody before CA125 in preclinical invasive epithelial ovarian cancer. Clin Cancer Res 2017; 23:5912–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anderson KS, Cramer DW, Sibani S, Wallstrom GW, Wong J and Park J, et al. Autoantibody signature for the serologic detection of ovarian cancer. J Proteome Res 2015; 14:578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hurley LC, Levin NK, Chatterjee M, Coles J, Muszkat S, Howarth Z, et al. Evaluation of paraneoplastic antigens reveals TRIM21 autoantibodies as biomarker for early detection of ovarian cancer in combination with autoantibodies to NY-ESO-1 and TP53. Cancer Biomark 2020; 27:407–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kobayashi M, Katayama H, Irajizad E, Vykoukal JV, Fahrmann JF, Kundnani DL, et al. Proteome profiling uncovers an autoimmune response signature that reflects ovarian cancer pathogenesis. Cancers 2020; 12: 485, 2/2020. e-Pub 2/2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi J-X, Qin J-J, Ye U, Wang P, Wang K-J, Zhang JY. Tumor associated antigens or anti-TAA autoantibodies as biomarkers in the diagnosis of ovarian cancer: a systematic review with meta-analysis. Expert Review of Molecular Diagnostics 2015; 15: 829–852, [DOI] [PubMed] [Google Scholar]
- 51.Chatterjee M, Hurley LC, Tainsky MA. Paraneoplastic antigens as biomarkers for early diagnosis of ovarian cancer. Gynecol Oncol Reports 2017; 21:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fortner RT, Damms-Machado A, Kaaks R. Systematic review: tumor-associated antigen autoantibodies and ovarian cancer early detection. Gynecol Oncol 2017; 147:465–80. [DOI] [PubMed] [Google Scholar]
- 53.Lokshin AE, Winans M, Landsittel D, Marrangoni AM, Velikokhatnaya L, Modugno F, et al. Circulating IL-8 and anti-IL-8 autoantibody in patients with ovarian cancer. Gynecol Oncol 2006;102: 244–51. [DOI] [PubMed] [Google Scholar]
- 54.Mor G, Visintin I, Lai Y, Zhao H, Schwartz P, Rutherford T, et al. Serum protein markers for early detection of ovarian cancer. Proc. Natl. Acad. Sci. USA 2005; 102:7677–7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guo J, Yang WL, Pak D, Celestino J, Lu KH, Ning J, et al. Osteopontin, macrophage migration inhibitory factor and anti-interleukin-8 autoantibodies complement CA125 for detection of early stage ovarian cancer. Cancers 2019; 11:596. doi: 10.3390/cancers11050596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilson AL, Moffitt LR, Duffield N, Rainczuk A, Jobling TW, Plebanski M, et al. Autoantibodies against HSF1 and CCDC155 as biomarkers of early-stage, high-grade serous ovarian cancer. Cancer Epidemiol Biomarkers Prev 2017; 27:183–92. [DOI] [PubMed] [Google Scholar]
- 57.Yang WL, Lu Z, Guo J, Fellman BM, Ning J, Lu KH, et al. Human epididymis protein 4 antigen-autoantibody complexes complement cancer antigen 125 for detecting early-stage ovarian cancer. Cancer 2019; 126:725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Staicu CE, Predescu DV, Rusu CM, Radu BM, Cretoiu D, Suciu N, et al. Role of microRNAs as clinical cancer biomarkers for ovarian cancer: A short overview. Cells 2020; 9: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Elias KM, Fendler W, Stawiski K, Fiascone SJ, Vitonis AF, Berkowitz RS, et al. Diagnostic potential for a serum miRNA neural network for detection of ovarian cancer. eLife 2017; 6: e28932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bast RC Jr, Matulonis UA, Sood AK, Ahmed AA, Amobi AE, Balkwill FR, et al. Critical questions in ovarian cancer research and treatment: Report of an American Association for Cancer Research Special Conference. Cancer 2019; 125:1963–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ren A, Fiala C, Diamandis E, Kulasingam V. Pitfalls in cancer biomarker discovery and and validation with emphasis on circulating tumor DNA. Cancer Epidemiology Biomarkers Prev 2020; (this issue). [DOI] [PubMed] [Google Scholar]
- 62.Douvillea C, Cohen JD, Ptak J, Popolia M, Schaefer J, Silliman J, et al. Assessing aneuploidy with repetitive element sequencing. Proc Natl Acad Sci USA 2020; 117:4858–4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Q, Hu G, Yang Q, Dong R, Xie X, Ma D, et al. A multiplex methylation-specific PCR for the detection of early stage ovarian cancer using cell-free serum DNA. Gynecol Oncol 2013; 130: 132–139. [DOI] [PubMed] [Google Scholar]
- 64.Widschwendter M, Zikan M, Wahl B, Lempiäinen H, Paprotka T, Evans I, et al. The potential of circulating tumor DNA methylation analysis for the early detection and management of ovarian cancer. Genome Medicine 2017; 9:116 DOI 10.1186/s13073-017-0500-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cohen JD, Li L, Wang Y, Thoburn C, Afsari B, Danilova L, et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 2018; 359: 926–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lennon AM, Buchanan AH, Kinde I, Warren A, Ashley Honushefsky A, Cohain AT, et al. Feasibility of blood testing combined with PET-CT to screen for cancer and guide intervention. Science 2020; 10.1126/science.abb9601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rossing MA, Wicklund KG, Cushing-Haugen KL, Weiss NL. Predictive value of symptoms for early detection of ovarian cancer. J Natl Cancer Inst 2010; 102:222–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Funston G, Hamilton W, Abel G, Crosbie E, Rous B, Walter F. The diagnostic performance of CA125 for the detection of ovarian and non-ovarian cancer in primary care: a population-based cohort study. Plos Medicine 2020, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iyer VR and Lee SI. MRI, CT, and PET/CT for ovarian cancer detection and adnexal lesion characterization. AJR American J Roentgenol 2010; 194:311–321. [DOI] [PubMed] [Google Scholar]
- 70.Rieber A, Nussle K, Stohr I, Grab D, Fenchel S, Kreienberg R, et al. Preoperative diagnosis of ovarian tumors with MR imaging: comparison with transvaginal sonography, positron emission tomography, and histologic findings. AJR Amer J Roentgenol 2001; 177:123–129. [DOI] [PubMed] [Google Scholar]
- 71.Fenchel S, Grab D, Nuessle K, Kotzerke J, Rieber A, Kreienberg R, et al. Asymptomatic adnexal masses: correlation of FDG PET and histopathologic findings. Radiology 2002; 223: 780–788. [DOI] [PubMed] [Google Scholar]
- 72.Kubik-Huch RA, Dorffler W, von Schulthess GK, Marincek B, Kochli OR, Seifert B, et al. Value of (18F)-FDG positron emission tomography, computed tomography, and magnetic resonance imaging in diagnosing primary and recurrent ovarian carcinoma. Eur Radiol 2000; 10:761–767. [DOI] [PubMed] [Google Scholar]
- 73.Risum S, Hogdall C, Loft A, Berthelsen AK, Hogdall E, Nedergaard L, et al. The diagnostic value of PET/CT for primary ovarian cancer--a prospective study. Gynecol Oncol 2007; 105:145–149. [DOI] [PubMed] [Google Scholar]
- 74.Sohaib SA, Mills TD, Sahdev A, Webb JA, Vantrappen PO, Jacobs IJ, et al. The role of magnetic resonance imaging and ultrasound in patients with adnexal masses. Clinical Radiology 2005; 60: 340–348. [DOI] [PubMed] [Google Scholar]
- 75.Mathieu KB, Bedi DG, Thrower SL, Qayyum A, Bast RC Jr. Screening for ovarian cancer: imaging challenges and opportunities for improvement. Ultrasound Obstet Gynecol 2018; 51:293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van Nagell JR Jr, Burgess BT, Miller RW, Baldwin L, DeSimone CP, Ueland FR, et al. Survival of women with type I and II epithelial ovarian cancer detected by ultrasound screening. Obstet Gynecol 2018; 132:1091–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Robertson SE, Peipert JF. Ultrasound screening for cancer: Are we there yet? Obstet Gynecol 2018; 132:1089–1090. [DOI] [PubMed] [Google Scholar]
- 78.Sayasneh A, Kaijser J, Preisler J, Smith AA, Raslan F, Johnson S, et al. Accuracy of ultrasonography performed by examiners with varied training and experience in predicting specific pathology of adnexal masses. Ultrasound Obstet Gynecol 2015; 45:605–612. [DOI] [PubMed] [Google Scholar]
- 79.Stott W, Campbell S, Franchini A, Blyuss O, Zaikin A, Ryan A, et al. Sonographers’ self‐reported visualization of normal postmenopausal ovaries on transvaginal ultrasound is not reliable: results of expert review of archived images in UKCTOCS. Ultrasound Obstet Gynecol 2018; 51:401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lefringhaus JR, Neward E, Ueland FR, Baldwin LA, Miller RW, DeSimone CP, et al. Probability of fallopian tube and ovarian detection with transvaginal ultrasonography in normal women. Women’s Health 2016; 12:303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Medeiros LR, Rosa DD, da Rosa MI, Bozzetti MC. Accuracy of ultrasonography with color Doppler in ovarian tumor: a systematic quantitative review. Int J Gynecol Cancer 2009; 19: 230–236. [DOI] [PubMed] [Google Scholar]
- 82.Szymanski M, Socha MW, Kowalkowska ME, Zielinska IB, Eljaszewicz A, Szymanski W. Differentiating between benign and malignant adnexal lesions with contrast-enhanced transvaginal ultrasonography. Int J Gynaecol Obstet 2015; 131:147–151 [DOI] [PubMed] [Google Scholar]
- 83.Zackrisson S, van de Ven SM, Gambhir SS. Light in and sound out: emerging translational strategies for photoacoustic imaging. Cancer Res 2014; 74:979–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Flynn ER, Bryant HC. A biomagnetic system for in vivo cancer imaging. Phys Med Biol 2005; 50:1273–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.De Haro LP, Karaulanov T, Vreeland EC, Anderson B, Hathaway HJ, Huber DL, et al. Magnetic relaxometry as applied to sensitive cancer detection and localization. Biomed Tech (Berl) 2015; 60:445–455. [DOI] [PubMed] [Google Scholar]
- 86.Mathieu K, Lu Z, Yang H, Pang L, Kulp A, Hazle J, et al. Feasibility of magnetic relaxometry for early ovarian cancer detection: preliminary evaluation of sensitvity and specificity in cell culture and in mice. AACR Proceedings 2017; Abstract 1864. [Google Scholar]
- 87.Skates SJ, Menon U, MacDonald N, Rosenthal AN, Oram DH, Knapp RC, et al. , Calculation of the risk of ovarian cancer from serial CA-125 values for preclinical detection in postmenopausal women J Clin Oncol 2003; 21:2006s–210s DOI: 10.1200/JCO.2003.02.955. [DOI] [PubMed] [Google Scholar]