Abstract
In our previous breast cancer case control study in Hispanics, we found 14 metabolites whose levels differed between cases and controls. To validate the results, we carried out a nested case control study of 100 incident breast cancer and 100 matched healthy women identified from the Mano-A-Mano Mexican American Cohort study. With the adjustment of parity, education, birth place, language acculturation, BMI category, smoking, drinking, physical activity, and sitting time, 4 metabolites were associated with breast cancer risk: 3-hydroxyoctanoate (Odds ratio (OR)=1.51, 95% confidence interval (CI): 1.10, 3.47), 3-hydroxybutyrate (BHBA) (OR=1.42, 95%CI: 1.01, 3.72), linoleate (18:2n6) (OR =1.39, 95% CI: 1.07, 4.04), and bilirubin (OR=0.54, 95%CI: 0.42, 0.95). Then, we used 3 non-redundant metabolites, namely 3-hydroxyoctanoate, linoleate (18:2n6), and bilirubin, to generate a metabolic risk score. Increased metabolites risk score was associated with a 1.67-fold increased risk of breast cancer (OR =1.67, 95%CI: 1.32, 3.94). And the significant association was more evident among those who were diagnosed with cancer earlier during the follow-up (≤ 5 years) than their counterparts. In conclusion, we identified four significant metabolites which may help elucidate metabolic pathways that contribute to breast carcinogenesis. Our findings warrant further replication efforts.
Keywords: metabolomics, breast cancer, Mexican Americans
Introduction
Currently, breast cancer is the most frequently diagnosed cancer among Hispanic women (1). Known risk factors account for only about 30% of breast cancers (2, 3). Therefore, a better understanding of the biological etiology and mechanisms is warranted. The metabolome reveals endogenous activities as well as ecological and lifestyle factors (4, 5). Metabolomics can identify delicate signals in metabolism and is therefore a promising means to pinpoint new etiological pathways.
In our prior two-stage analysis, we identified 14 candidate metabolites that significantly differed between breast cancer cases and healthy controls in Hispanic women (6). However, due to case-control study design, the results cannot be properly interpreted. In the current study, we attempted to validate the 14 previously identified metabolites using pre-diagnostic plasma samples obtained from 100 incident breast cancer and 100 age and gender matched healthy Mexican American (MA) women identified from the Mano-A-Mano Mexican American Cohort study (MACS).
Materials and Methods
Study population
Our study utilized self-identified Mexican or MA participants from the ongoing MACS, a large population-based prospective cohort study of MA households. Participants were individuals of Mexican descent who lived in the Houston area for at least one year. Details of the recruitment strategy and data collection procedures have been previously described (7). Our study included female participants from MACS who were followed for a median of 8.2 years until December 1, 2017. Breast cancer cases were identified during follow-up and were further verified through the Texas Cancer Registry. Of the validated cases, we randomly selected 100 index cases. We chose 1 matched control for each index case using an incidence density sampling protocol from appropriate risk sets of cohort members who were both alive and free of cancer at the time of diagnosis of the index case. Our matching criteria included age at recruitment (±2 years), date of biospecimen collection (±1 year), and gender. The study protocol was approved by MD Anderson’s Institutional Review Board.
Metabolomics Analysis
Plasma samples underwent metabolomics profiling at Metabolon Inc (Durham, NC) via ultra-high-performance liquid chromatography/mass spectroscopy and gas chromatography/mass spectroscopy. The protocol has been previously described in detail (8). After disregarding compounds that had ≥30% missing values, 526 identified metabolites were left for analysis. The missing values were deemed as the outcome of low signal strength and were substituted by half of the minimum positive values revealed in the data. The median proportion of below-limit-of-detection values was 0%. Metabolite peak intensities were run-day-normalized and log-transformed for analysis. Metabolite measurements were highly reliable in masked replicates. Over the 526 metabolites, the median intraclass correlation coefficient (ICC) was 0.93 (interquartile range (IQR)=0.86–0.97), similar to previous reports analyzed by Metabolon (9).
Statistical Analysis
Any missing values were supposed to be under the limits of detection, and these values were imputed with the metabolite minimum (minimum value imputation). Non-parametric Wilcoxon signed-rank tests were applied to assess levels of 14 candidate metabolites between breast cancer cases and healthy controls. To evaluate the risk of breast cancer, we utilized conditional logistic regression analysis. The matching variables were age at recruitment and date of biospecimen collection. The covariates included parity, education, birth place, language acculturation, and BMI category in both models 1 and 2, and smoking, drinking, physical activity, and sitting time in model 2. Bonferroni criterion was used to correct the multiple comparison. We further performed stratified analysis to assess the difference of association by social-demographics, healthy behaviors, and time duration between blood collection and cancer diagnosis. To identify the possible redundancy, we evaluated the pairwise correlations between all 14 metabolites among the controls. Next, using 3 non-redundant significant metabolites, we created a metabolic risk score. For each metabolite, we classified the study participants into high and low groups by means of the median level in the controls as the cutoff point. Next, based on the association between metabolite levels with risk of breast cancer, we counted the study subjects as either high or low risk (0 or 1) and added scores across 3 metabolites to create a risk score (range: 0–3). Conditional logistic regression analysis was used to evaluate the association between the risk score with the risk of breast cancer. Co-variates were adjusted as appropriate. In addition, stratified analysis was applied to assess the impact of time duration between blood collection and cancer diagnosis. STATA software version 14.1 (STATA, College Station, TX) were used for all analyses.
Results
Table 1 illustrated the basic sociodemographic characteristics and lifestyle behaviors of the 100 breast cancer cases and 100 healthy controls. Overall, the cases and controls were matched very well. No significant difference was observed for parity, education level, place of birth, language acculturation, BMI category, smoking status, alcohol drinking status, physical activity, and sitting time. Forty-two of cases were diagnosed within 5 years after the blood was collected.
Table 1.
Distribution of characteristics among participants by case control status
| Variable | Controls, n (%) | Cases, n (%) | P value |
|---|---|---|---|
| Overall | 100 (100) | 10 (100) | |
| Age at enrollment, years | |||
| <51 years | 50 (50.00) | 46 (46.00) | |
| ≥51 years | 50 (50.00) | 54 (54.00) | 0.730 |
| Parity | |||
| Nulliparous | 9 (9.00) | 8 (8.00) | |
| 1 or 2 children | 43 (43.00) | 42 (42.00) | |
| >2 children | 48 (48.00) | 50 (50.00) | 0.964 |
| Education level | |||
| <High school | 68 (68.00) | 70 (70.00) | |
| High school | 21 (21.00) | 20 (20.00) | |
| >High school | 11 (11.00) | 10 (10.00) | 0.967 |
| Place of birth | |||
| Mexico | 70 (70.00) | 70 (70.00) | |
| United States | 30 (30.00) | 30 (30.00) | 1.000 |
| Language acculturation | |||
| Low | 69 (69.00) | 60 (60.00) | |
| High | 31 (31.00) | 40 (40.00) | 0.279 |
| BMI category | |||
| Underweight/normal weight | 15 (15.00) | 12 (12.00) | |
| Overweight | 36 (36.00) | 38 (38.00) | |
| Obese | 49 (49.00) | 50 (50.00) | 0.879 |
| Smoking status | |||
| Never | 73 (73.00) | 64 (64.00) | |
| Former | 19 (19.00) | 28 (28.00) | |
| Current | 8 (8.00) | 8 (8.00) | 0.446 |
| Alcohol drinking | |||
| Never | 67 (67.00) | 66 (66.00) | |
| Former | 21 (21.00) | 26 (26.00) | |
| Current | 12 (12.00) | 8 (8.00) | 0.646 |
| Physical activity | |||
| Low | 72 (72.00) | 80 (80.00) | |
| Medium or high | 28 (28.00) | 20 (20.00) | 0.325 |
| Sitting hours per day | |||
| <2 | 27 (27.00) | 24 (24.00) | |
| 2–4 | 28 (28.00) | 36 (36.00) | |
| 4–6 | 23 (23.00) | 24 (24.00) | |
| >6 | 22 (22.00) | 16 (16.00) | 0.700 |
| Time between blood collection and cancer diagnosis | |||
| ≤5 years | 42 (42.00) | ||
| >5 years | 48 (48.00) | ||
In the first model with the adjustment of parity, education, birth place, language acculturation, and BMI category, we observed 5 metabolites whose levels in plasma significantly differed between breast cancer cases and controls (Table 2). Higher levels of 3-hydroxyoctanoate, 3-hydroxybutyrate (BHBA), linoleate (18:2n6), and 10-nonadecenoate (19: ln9) were associated with 1.55, 1.47, 1.38, and 1.33-fold elevated risk of breast cancer. Meanwhile, higher levels of bilirubin were associated with 50% decreased risk of breast cancer. In the second model with further adjustment of smoking, drinking, physical activity, and sitting time, 4 of the 5 metabolites remained significant: 3-hydroxyoctanoate (OR=1.51, 95%CI: 1.10, 3.47), 3-hydroxybutyrate (BHBA) (OR=1.42, 95%CI: 1.01, 3.72), linoleate (18:2n6) (OR=1.39, 95%CI: 1.07, 4.04), and bilirubin (OR=0.54, 95%CI: 0.42, 0.95). However, none of the associations remained significant after Bonferroni multiple comparison adjustment.
Table 2.
Conditional logistic regression analysis to identify metabolites significantly associated with breast cancer
| Model 1* | Model 2# | |||||
|---|---|---|---|---|---|---|
| Metabolites | Pathways | Sub-pathways | OR (95% CI) | P value | OR (95% CI) | P value |
| 3-hydroxyoctanoate | Lipid | Fatty Acid, Monohydroxy | 1.55 (1.13, 3.32) | 0.016 | 1.51 (1.10, 3.47) | 0.022 |
| 3-hydroxybutyrate (BHBA) | Lipid | Ketone Bodies | 1.47 (1.11, 3.72) | 0.020 | 1.42 (1.01, 3.72) | 0.049 |
| stearate (18:0) | Lipid | Long Chain Fatty Acid | 1.22 (0.83, 4.01) | 0.235 | 1.26 (0.79, 4.24) | 0.332 |
| 3-hydroxydecanoate | Lipid | Fatty Acid, Monohydroxy | 1.29 (0.87, 4.09) | 0.203 | 1.35 (0.86, 4.27) | 0.194 |
| linoleate (18:2n6) | Lipid | Polyunsaturated Fatty Acid (n3 and n6) | 1.38 (1.06, 3.93) | 0.043 | 1.39 (1.07, 4.04) | 0.041 |
| 10-nonadecenoate (19:ln9) | Lipid | Long Chain Fatty Acid | 1.33 (1.05, 4.04) | 0.044 | 1.30 (0.96, 4.14) | 0.060 |
| dihomo-linoleate | Lipid | Polyunsaturated Fatty Acid (n3 and n6) | 1.20 (0.73, 3.99) | 0.589 | 1.15 (0.63, 4.02) | 0.764 |
| 3-methylxanthine | Xenobiotics | Xanthine Metabolism Hemoglobin and Porphyrin | 0.80 (0.32, 2.80) | 0.504 | 0.85 (0.33, 3.51) | 0.619 |
| bilirubin | Cofactors/Vitamins | Metabolism | 0.50 (0.47, 0.90) | 0.034 | 0.54 (0.42, 0.95) | 0.041 |
| 1-palmitoyl-GPA (16:0) glucuronate | Lipid | Lysolipid | 0.89 (0.45, 3.16) | 0.744 | 0.90 (0.40, 3.81) | 0.778 |
| Carbohydrate | Aminosugar Metabolism | 0.90 (0.32, 4.53) | 0.796 | 0.95 (0.33, 4.72) | 0.904 | |
| 1-(1-enyl-stearoyl)-2-dihomo-linolenoyl-GPE | Lipid | Plasmalogen Urea cycle; Arginine and Proline | 0.62 (0.33, 1.25) | 0.146 | 0.70 (0.35, 1.30) | 0.166 |
| urea | Amino Acid | Metabolism | 1.11 (0.59, 4.08) | 0.646 | 1.12 (0.58, 4.16) | 0.639 |
| sphingomyelin | Lipid | Sphingolipid Metabolism | 0.90 (0.41, 4.28) | 0.826 | 0.92 (0.34, 4.51) | 0.854 |
Adjusted by parity, education, birth place, language acculturation, and BMI category.
Adjusted by parity, education, birth place, language acculturation, and BMI category, smoking status, alcohol status, sitting time, and physical activity.
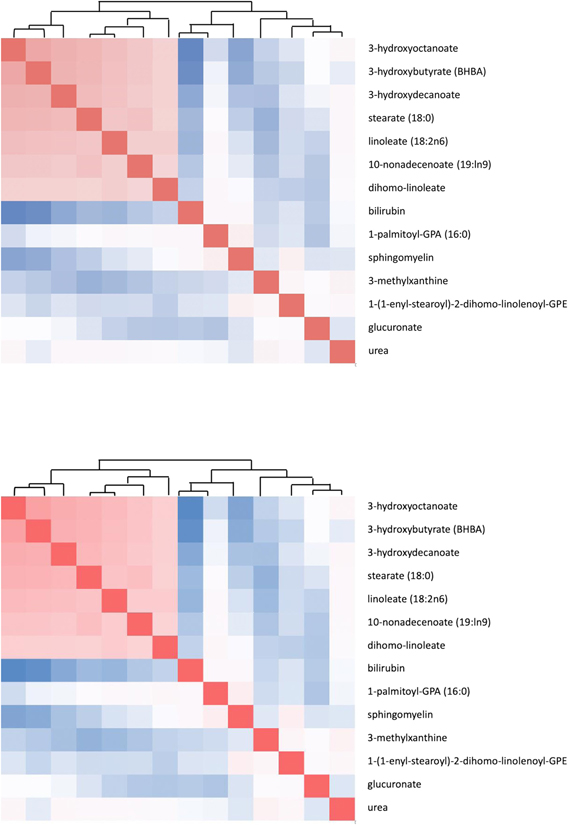
To account for potential collinearity between metabolites, we evaluated the pairwise correlations between all 14 metabolites among the controls (Figure 1). Metabolites whose pairwise correlations greater than 0.5 were considered highly correlated and to have possible redundancy. For four significant metabolites, the only significant correlation was between 3-hydroxyoctanoate and 3-hydroxybutyrate (BHBA) (Rho=0.629, P<0.001). Thus, 3-hydroxybutyrate (BHBA) was not included in further analysis.
Fig. 1.
Pair-wise correlation analysis to assess the redundancy among significant metabolites.
In further stratified analysis by sociodemographic characteristics and lifestyle behaviors, we evaluated the association between 3-hydroxyoctanoate, linoleate (18:2n6), and bilirubin with breast cancer risk in each stratified category (Table 3). In general, the association between the metabolite and breast cancer risk didn’t differ between the categories. The only exception was linoleate (18:2n6) by BMI category. The association between linoleate (18:2n6) and breast cancer risk was more evident in study participants who were obese (OR=1.48, 95%CI: 1.01, 5.14) than those who were non-obese (OR=1.32, 95%CI: 0.76, 5.48). We also assessed whether the association differed by the time duration between blood collection and cancer diagnosis. The association apparently was stronger among those who had cancer diagnosis earlier (≤ 5 years after blood collection) than those who had cancer diagnose later (> 5 years after blood collection). For example, higher 3-hydroxyoctanoate was associated with 1.65-fold increased risk of breast cancer among those who had cancer diagnosis earlier (≤ 5 years after blood collection) and 1.33-fold increased risk of breast cancer among those who had cancer diagnose later (> 5 years after blood collection) (OR=1.65, 95%CI; 1.07, 4.38; OR=1.33, 95%CI: 1.01, 5.06, respectively).
Table 3.
Stratified analysis to assess the association between candidate metabolites and breast cancer
| Variable | 3-hydroxyoctanoate* | linoleate (18:2n6)* | bilirubin* |
|---|---|---|---|
| OR (95%CI) | OR (95%CI) | OR (95%CI) | |
| Age at enrollment, years | |||
| <51 years | 1.53 (1.03, 4.46) | 1.41 (0.89, 5.98) | 0.57 (0.35, 1.06) |
| ≥51 years | 1.47 (1.05, 4.37) | 1.37 (0.82, 5.71) | 0.50 (0.31, 1.01) |
| Parity | |||
| 0–2 children | 1.49 (1.06, 4.40) | 1.44 (0.85, 5.83) | 0.51 (0.36, 1.04) |
| >2 children | 1.54 (1.05, 4.33) | 1.35 (0.80, 5.64) | 0.58 (0.39, 1.07) |
| Education level | |||
| <High school | 1.54 (1.07, 3.92) | 1.40 (0.86, 5.77) | 0.55 (0.35, 1.05) |
| ≥High school | 1.44 (0.90, 4.67) | 1.37 (0.81, 5.58) | 0.53 (0.34, 1.03) |
| Place of birth | |||
| Mexico | 1.49 (1.04, 4.04) | 1.37 (1.00, 5.36) | 0.51 (0.32, 0.99) |
| United States | 1.61 (0.72, 5.73) | 1.47 (0.65, 6.34) | 0.63 (0.26, 1.63) |
| Language acculturation | |||
| Low | 1.50 (1.03, 4.12) | 1.36 (1.01, 5.29) | 0.52 (0.34, 0.98) |
| High | 1.55 (0.82, 5.69) | 1.48 (0.62, 6.55) | 0.64 (0.24, 1.71) |
| BMI category | |||
| Non-obese | 1.46 (1.04, 4.12) | 1.32 (0.76, 5.48) | 0.53 (0.33, 0.99) |
| Obese | 1.58 (1.06, 4.30) | 1.48 (1.01, 5.14) | 0.54 (0.34, 0.98) |
| Smoking status | |||
| Never | 1.54 (1.07, 3.99) | 1.38 (1.02, 4.56) | 0.54 (0.35, 0.98) |
| Ever | 1.40 (0.76, 5.68) | 1.45 (0.65, 6.39) | 0.57 (0.21, 1.89) |
| Alcohol drinking | |||
| Never | 1.53 (1.06, 4.01) | 1.40 (1.04, 4.69) | 0.55 (0.34, 0.97) |
| Ever | 1.46 (0.86, 5.09) | 1.34 (0.60, 6.12) | 0.51 (0.22, 1.84) |
| Physical activity | |||
| Low | 1.52 (1.07, 3.86) | 1.41 (1.05, 4.71) | 0.53 (0.35, 0.97) |
| Medium or high | 1.39 (0.70, 5.38) | 1.32 (0.59, 6.03) | 0.59 (0.24, 1.91) |
| Sitting hours per day | |||
| 0–4 | 1.47 (1.03, 4.39) | 1.38 (1.02, 4.49) | 0.52 (0.29, 1.06) |
| >4 | 1.55 (1.06, 4.48) | 1.41 (1.03, 4.51) | 0.56 (0.33, 1.09) |
| Time between blood collection and cancer diagnosis | |||
| ≤5 years | 1.65 (1.07, 4.38) | 1.57 (1.06, 4.78) | 0.51 (0.30, 0.96) |
| >5 years | 1.33 (1.01, 5.06) | 1.30 (0.85, 5.43) | 0.55 (0.25, 0.99) |
Adjusted by parity, education, birth place, language acculturation, and BMI category, smoking status, alcohol status, sitting time, and physical activity, as appropriate.
Then, we generated a risk score using those three non-redundant metabolites, namely 3-hydroxyoctanoate, linoleate (18:2n6), and bilirubin. Using the risk score as a continuous variable, we noted that increased risk score was associated with a 1.72-fold increased risk of breast cancer in model 1 and a 1.67-fold increased risk in model 2, (OR=1.72, 95%CI=1.46, 3.70; OR=1.67, 95%CI=1.32, 3.94) (Table 4). When stratified by the time duration between blood collection and cancer diagnosis (≤ 5 years vs >5 years), the association remained significant in both categories. However, it was stronger among those who had cancer diagnosis earlier (≤ 5 years after blood collection) (model 1: OR=1.90, 95%CI=1.27, 4.89; model 2: OR=1.84, 95%CI=1.15, 4.78) than those who had cancer diagnosis later (> 5 years after blood collection) (model 1: OR=1.49, 95%CI=1.04, 5.02; model 2: OR=1.43, 95%CI=1.02, 5.16). After multiple comparison adjustment, the significant association remained only for those had cancer diagnosis earlier, but not for those who had cancer diagnosis later.
Table 4.
Conditional logistic regression to assess the relationship between metabolite risk score associated with breast cancer risk
| Model 1* | Model 2# | |||
|---|---|---|---|---|
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Continuous variable | 1.72 (1.46–3.70) | <0.001 | 1.67 (1.32–3.94) | <0.001 |
| Stratified by time between blood collection and cancer diagnosis | ||||
| ≤ 5 years | 1.90 (1.27, 4.89) | 0.003 | 1.84 (1.15, 4.78) | 0.011 |
| > 5 years | 1.49 (1.04, 5.02) | 0.044 | 1.43 (1.02, 5.16) | 0.047 |
| Categorical variable | ||||
| Low (0–1) | Reference | Reference | ||
| High (2–3) | 3.91 (1.87, 10.32) | <0.001 | 3.82 (1.78–11.56) | <0.001 |
| Stratified by time between blood collection and cancer diagnosis | ||||
| ≤ 5 years | 5.55 (1.65, 13.58) | <0.001 | 5.38 (1.62, 13.57) | <0.001 |
| ≤ 5 years | 2.47 (1.08, 15.06) | 0.028 | 2.36 (1.07, 14.85) | 0.030 |
Adjusted by parity, education, birth place, language acculturation, and BMI category.
Adjusted by parity, education, birth place, language acculturation, and BMI category, smoking status, alcohol status, sitting time, and physical activity.
Next, we classified the study subjects into two strata based on the risk score among the control subjects: low (0–1) and high (2–3). When compared with study subjects with low risk scores, those with high risk scores exhibited a significantly increased risk of breast cancer in both model 1 and model 2 (OR=3.91, 95%CI=1.87, 10.32); and OR=3.82, 95%CI=1.78, 11.56). Similar stratified analysis was further applied to assess the impact of time duration between blood collection and cancer diagnosis. We found that the association was stronger among those who had cancer diagnosis earlier (model 1: OR=5.55, 95%CI=1.65, 13.58; model 2: OR=5.38, 95%CI=1.62, 13.57) than those who had cancer diagnosis later (model 1: OR=2.47, 95%CI=1.08, 15.06; model 2: OR=2.36, 95%CI=1.07, 14.85). When adjusted by Bonferroni multiple comparison, the association remained significant for those who had cancer diagnosis earlier.
Discussion
To our best knowledge, this is the first prospective study to assess the role of plasma metabolites in the development of breast cancer among MAs. In our study, we validated four metabolites, namely 3-hydroxyoctanoate, 3-hydroxybutyrate (BHBA), linoleate (18:2n6), and bilirubin, which were identified in our previous breast cancer case control study. As the associations we observe are more pronounced among cases occurring earlier during follow-up, our results suggest altered levels of circulating metabolites may be an early biomarker of subclinical cancer in Mexican Americans.
Among 4 validated metabolites mentioned above, 3 were lipids, including 1 monohydroxy fatty acid (3-hydroxyoctanoate), 1 ketone body (3-hydroxybutyrate (BHBA), and 1 polyunsaturated fatty acid (linoleate (18:2n6)). Higher levels of monohydroxy fatty acids may signify altered fatty acid β-oxidation in patients with breast cancer (8). Fatty acid β-oxidation has been shown to support functional mitochondria and is vital for the rapid growth of cancer cells (10, 11). In a recent study, fatty Acid β-Oxidation was found to be implicated in breast cancer stem cell self-renewal and chemoresistance (12). Therefore, altered fatty acids β-oxidation may promote breast carcinogenesis and foster the aggressive tumorigenic phenotypes (13). Similar observations were also reported in several other breast cancer case control analyses (14, 15). Ketone body 3-hydroxybutyrate (BHBA), the end product of ketogenesis and downstream of fatty acid β-oxidation, was noted associated with breast cancer risk in our study. The elevated 3-hydroxybutyrate (BHBA) level in the cases is another signal of altered fatty acid β-oxidation in breast cancer subjects. Linolenic acid has been suggested to support the tumorigenic phenotype of breast cancer (16). In our previous breast cancer study, we found that levels of linoleate (18:2n6) were significantly higher in the cases than controls (8). Interestingly, in a genome-wide association study, genetic variants in genes involved in the regulation of linolenic acid metabolism were associated with breast cancer risk (17).
We also found that increased levels of bilirubin were associated with decreased risk of breast cancer. Our results are in consistent with Bilirubin’s anti-carcinogenic property (18, 19). Endogenous antioxidant bilirubin has shown to inhibit cancer development (20). In a previous study using pre-diagnostic serum samples, bilirubin was not associated with breast cancer (20). However, serum bilirubin has been reported to associated with reduced lung cancer risk in two prospective studies (21, 22) and reduced cancer mortality in a population-based set (23). Clearly, more research is needed to clarify the role of bilirubin in breast carcinogenesis.
Interestingly, none of our validated metabolites were overlapped with significant metabolites identified in previous studies in Whites (24, 25). Metabolites are the end products of intercellular pathways and are susceptible to host and ecological stimuli. Thus, metabolite profiles can be influenced significantly by factors ranging from genetics, demographic factors, and co-morbidities to environmental exposure (26). We examined 4 breast cancer related metabolites reported in the study by Moore et al (25), namely 16a-hydroxy-DHEA-3-sulfate, 3-methylglutarylcarnitine, allo-isoleucine, and 2-methylbutyrylcarnitine. Though we did not observe any significant association for those metabolites (16a-hydroxy-DHEA-3-sulfate: OR=1.24, 95%CI=0.65–4.58; allo-isoleucine: OR=1.40, 95%CI=0.77–4.93; and 2-methylbutyrylcarnitine: OR=1.28, 95%CI=0.67–4.59), borderline association was observed for 3-methylglutarylcarnitine (OR=1.71, 95%CI=0.96–4.72).
Although our study is the largest study in MAs, relatively small sample size is a major limitation. Another limitation of our study is that we were unable to obtain repeated measures of circulating metabolites; a single measurement may not reflect circulating metabolites over a lifetime. In addition, we did not have matched tumor and normal tissues, which would have enabled us to compare metabolites in target and surrogate tissues. Nevertheless, our study is the first prospectively designed study to show the significant relationship between plasma metabolites and breast cancer risk in MAs. Larger future studies should be conducted in order to further validate our observed associations.
Highlights.
We have validated 4 plasma metabolites associated with breast cancer risk in Mexican Americans. They are 3-hydroxyoctanoate, 3-hydroxybutyrate (BHBA), linoleate (18:2n6), and bilirubin.
We have constructed a metabolic risk score. Higher risk score was associated with increased breast cancer risk.
Acknowledgements
The study was supported by U01 CA179655 from NCI/NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The study protocol was approved by MD Anderson’s Institutional Review Board.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All procedures performed in this study were approved by the Institutional Review Board at M D Anderson Cancer Center and in accordance with the ethical standards of 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from all participants.
Data sharing
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Miller KD, Goding Sauer A, Ortiz AP, Fedewa SA, Pinheiro PS, Tortolero-Luna G, Martinez-Tyson D, Jemal A, Siegel RL. Cancer Statistics for Hispanics/Latinos, 2018. CA Cancer J Clin. 2018;68(6):425–45. 10.3322/caac.21494. [DOI] [PubMed] [Google Scholar]
- 2.Islami F, Goding Sauer A, Miller KD, Siegel RL, Fedewa SA, Jacobs EJ, McCullough ML, Patel AV, Ma J, Soerjomataram I, Flanders WD, Brawley OW, Gapstur SM, Jemal A. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. 2018;68(1):31–54. 10.3322/caac.21440. [DOI] [PubMed] [Google Scholar]
- 3.Gaudet MM, Gierach GL, Carter BD, Luo J, Milne RL, Weiderpass E, Giles GG, Tamimi RM, Eliassen AH, Rosner B, Wolk A, Adami HO, Margolis KL, Gapstur SM, Garcia-Closas M, Brinton LA. Pooled Analysis of Nine Cohorts Reveals Breast Cancer Risk Factors by Tumor Molecular Subtype. Cancer Res. 2018;78(20):6011–21. 10.1158/0008-5472.CAN-18-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Roekel EH, Trijsburg L, Assi N, Carayol M, Achaintre D, Murphy N, Rinaldi S, Schmidt JA, Stepien M, Kaaks R, Kuhn T, Boeing H, Iqbal K, Palli D, Krogh V, Tumino R, Ricceri F, Panico S, Peeters PH, Bueno-de-Mesquita B, Ardanaz E, Lujan-Barroso L, Quiros JR, Huerta JM, Molina-Portillo E, Dorronsoro M, Tsilidis KK, Riboli E, Rostgaard-Hansen AL, Tjonneland A, Overvad K, Weiderpass E, Boutron-Ruault MC, Severi G, Trichopoulou A, Karakatsani A, Kotanidou A, Hakansson A, Malm J, Weijenberg MP, Gunter MJ, Jenab M, Johansson M, Travis RC, Scalbert A, Ferrari P. Circulating Metabolites Associated with Alcohol Intake in the European Prospective Investigation into Cancer and Nutrition Cohort. Nutrients. 2018;10(5). 10.3390/nu10050654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stevens VL, Wang Y, Carter BD, Gaudet MM, Gapstur SM. Serum metabolomic profiles associated with postmenopausal hormone use. Metabolomics. 2018;14(7):97 10.1007/s11306-018-1393-1. [DOI] [PubMed] [Google Scholar]
- 6.Zhao H, Shen J, Moore SC, Ye Y, Wu X, Esteva FJ, Tripathy D, Chow WH. Breast cancer risk in relation to plasma metabolites among Hispanic and African American women. Breast Cancer Res Treat. 2019;176(3):687–96. 10.1007/s10549-019-05165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow WH, Chrisman M, Daniel CR, Ye Y, Gomez H, Dong Q, Anderson CE, Chang S, Strom S, Zhao H, Wu X. Cohort Profile: The Mexican American Mano a Mano Cohort. Int J Epidemiol. 2017;46(2):e3 10.1093/ije/dyv016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen J, Yan L, Liu S, Ambrosone CB, Zhao H. Plasma Metabolomic Profiles in Breast Cancer Patients and Healthy Controls: By Race and Tumor Receptor Subtypes. Transl Oncol. 2013;6(6):757–65. 10.1593/tlo.13619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sampson JN, Boca SM, Shu XO, Stolzenberg-Solomon RZ, Matthews CE, Hsing AW, Tan YT, Ji BT, Chow WH, Cai QY, Liu DK, Yang G, Xiang YB, Zheng W, Sinha R, Cross AJ, Moore SC. Metabolomics in Epidemiology: Sources of Variability in Metabolite Measurements and Implications. Cancer Epidem Biomar. 2013;22(4):631–40. 10.1158/1055-9965.Epi-12-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez-Enriquez S, Hernandez-Esquivel L, Marin-Hernandez A, El Hafidi M, Gallardo-Perez JC, Hernandez-Resendiz I, Rodriguez-Zavala JS, Pacheco-Velazquez SC, Moreno-Sanchez R. Mitochondrial free fatty acid beta-oxidation supports oxidative phosphorylation and proliferation in cancer cells. Int J Biochem Cell B. 2015;65:209–21. 10.1016/j.biocel.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Nieman KM, Romero IL, Van Houten B, Lengyel E. Adipose tissue and adipocytes support tumorigenesis and metastasis. Bba-Mol Cell Biol L. 2013;1831(10):1533–41. 10.1016/j.bbalip.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang T, Fahrmann JF, Lee H, Li YJ, Tripathi SC, Yue C, Zhang C, Lifshitz V, Song J, Yuan Y, Somlo G, Jandial R, Ann D, Hanash S, Jove R, Yu H. JAK/STAT3-Regulated Fatty Acid beta-Oxidation Is Critical for Breast Cancer Stem Cell Self-Renewal and Chemoresistance. Cell Metab. 2018;27(1):136–50 10.1016/j.cmet.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinlaw WB, Baures PW, Lupien LE, Davis WL, Kuemmerle NB. Fatty Acids and Breast Cancer: Make Them on Site or Have Them Delivered. J Cell Physiol. 2016;231(10):2128–41. 10.1002/jcp.25332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui M, Wang QL, Chen G. Serum metabolomics analysis reveals changes in signaling lipids in breast cancer patients. Biomed Chromatogr. 2016;30(1):42–7. 10.1002/bmc.3556. [DOI] [PubMed] [Google Scholar]
- 15.O’Flaherty JT, Wooten RE, Samuel MP, Thomas MJ, Levine EA, Case LD, Akman SA, Edwards IJ. Fatty Acid Metabolites in Rapidly Proliferating Breast Cancer. Plos One. 2013;8(5). 10.1371/journal.pone.0063076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, Zhou L, Shi W, Song N, Yu K, Gu Y. A mechanism underlying the effects of polyunsaturated fatty acids on breast cancer. Int J Mol Med. 2012;30(3):487–94. 10.3892/ijmm.2012.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, Humphreys K, Heikkinen T, Aittomaki K, Blomqvist C, Pharoah PD, Dunning AM, Ahmed S, Hooning MJ, Martens JW, van den Ouweland AM, Alfredsson L, Palotie A, Peltonen-Palotie L, Irwanto A, Low HQ, Teoh GH, Thalamuthu A, Easton DF, Nevanlinna H, Liu J, Czene K, Hall P. A combined analysis of genome-wide association studies in breast cancer. Breast Cancer Res Treat. 2011;126(3):717–27. 10.1007/s10549-010-1172-9. [DOI] [PubMed] [Google Scholar]
- 18.Liu XA, Meng QH, Ye YQ, Hildebrandt MAT, Gu J, Wu XF. Prognostic significance of pretreatment serum levels of albumin, LDH and total bilirubin in patients with non-metastatic breast cancer. Carcinogenesis. 2015;36(2):243–8. 10.1093/carcin/bgu247. [DOI] [PubMed] [Google Scholar]
- 19.Srivastava P, Hira SK, Srivastava DN, Gupta U, Sen P, Singh RA, Manna PP. Protease-Responsive Targeted Delivery of Doxorubicin from Bilirubin-BSA-Capped Mesoporous Silica Nanoparticles against Colon Cancer. Acs Biomater Sci Eng. 2017;3(12):3376–85. 10.1021/acsbiomaterials.7b00635. [DOI] [PubMed] [Google Scholar]
- 20.Kuhn T, Sookthai D, Graf ME, Schubel R, Freisling H, Johnson T, Katzke V, Kaaks R. Albumin, bilirubin, uric acid and cancer risk: results from a prospective population-based study. Br J Cancer. 2017;117(10):1572–9. 10.1038/bjc.2017.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horsfall LJ, Rait G, Walters K, Swallow DM, Pereira SP, Nazareth I, Petersen I. Serum Bilirubin and Risk of Respiratory Disease and Death. Jama-J Am Med Assoc. 2011;305(7):691–7. doi: DOI 10.1001/jama.2011.124. [DOI] [PubMed] [Google Scholar]
- 22.Wen CP, Zhang FM, Liang D, Wen C, Gu J, Skinner H, Chow WH, Ye YQ, Pu X, Hildebrandt MAT, Huang MS, Chen CH, Hsiung CA, Tsai MK, Tsao CK, Lippman SM, Wu XF. The Ability of Bilirubin in Identifying Smokers with Higher Risk of Lung Cancer: A Large Cohort Study in Conjunction with Global Metabolomic Profiling. Clin Cancer Res. 2015;21(1):193–200. 10.1158/1078-0432.Ccr-14-0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Temme EHM, Zhang JJ, Schouten EG, Kesteloot H. Serum bilirubin and 10-year mortality risk in a Belgian population. Cancer Cause Control. 2001;12(10):887–94. doi: Doi 10.1023/A:1013794407325. [DOI] [PubMed] [Google Scholar]
- 24.Playdon MC, Ziegler RG, Sampson JN, Stolzenberg-Solomon R, Thompson HJ, Irwin ML, Mayne ST, Hoover RN, Moore SC. Nutritional metabolomics and breast cancer risk in a prospective study. Am J Clin Nutr. 2017;106(2):637–49. 10.3945/ajcn.116.150912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore SC, Playdon MC, Sampson JN, Hoover RN, Trabert B, Matthews CE, Ziegler RG. A Metabolomics Analysis of Body Mass Index and Postmenopausal Breast Cancer Risk. J Natl Cancer Inst. 2018;110(6):588–97. 10.1093/jnci/djx244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Claudino WM, Goncalves PH, di Leo A, Philip PA, Sarkar FH. Metabolomics in cancer: a bench-to-bedside intersection. Crit Rev Oncol Hematol. 2012;84(1):1–7. 10.1016/j.critrevonc.2012.02.009. [DOI] [PubMed] [Google Scholar]