Abstract
Background:
Increased activity is beneficial during chemotherapy, but treatment-related symptoms may be a barrier. This study examines the relationship between daily fluctuations in symptoms and activity during chemotherapy.
Methods:
Women undergoing chemotherapy for breast cancer [n=67; Mage= 48.6 (SD=10.3)] wore an accelerometer 24 hours/day and received 4 text prompts/day to rate symptoms for 10 consecutive days at the beginning, middle and end of chemotherapy. Mixed-effects models were used to examine the between and within-person relationships between symptom ratings on a given day and moderate to vigorous physical activity (MVPA) and light physical activity (LPA) on that day and the following day controlling for relevant covariates and using the Bonferroni correction for multiple comparisons.
Results:
For MVPA and LPA, within-person associations were statistically significant for same day affect, fatigue, pain, walking and activities of daily living (ADL) physical function, cognitive function. Previous day anxiety was associated with next day LPA. Every one point worse symptom rating than an individual’s overall average was associated with: a) between 1.49 (pain) and 4.94 (fatigue) minutes less MVPA and between 4.48 (pain) and 24.72 (ADL physical function) minutes less LPA that day and b) 11.28 minutes less LPA the next day. : No between-person effects were significant for MVPA or LPA.
Conclusions:
Daily within-person variations in symptoms were associated with MVPA and LPA during chemotherapy for breast cancer.
Impact:
Future work should explore relationships between symptoms and activity further and identify whether tailoring to symptoms enhances efficacy of physical activity promotion interventions during chemotherapy.
Keywords: physical activity, breast cancer, chemotherapy, symptoms, ecological momentary assessment
INTRODUCTION
Approximately 1 in 8 women will be diagnosed with breast cancer during their lifetime. About two-thirds of those diagnosed receive chemotherapy(1). Chemotherapy has numerous negative side effects (e.g. nausea, vomiting, fatigue, depression) resulting in compromised quality of life [QOL;(2)]. Increased moderate to vigorous physical activity (MVPA) during chemotherapy is associated with improved treatment-related side effects, may reduce chemotherapy dose adjustments and improve disease-free survival(3,4). Higher MVPA during chemotherapy is also associated with higher MVPA post-chemotherapy(5), which is associated with improved disease-free survival and reduced recurrence and early mortality(6–9). Additionally, cumulative daily activity may be a proxy for health and functioning during cancer treatment(10). For example, a patient may demonstrate activity declines prior to hospitalization or lymphedema onset.
Existing data suggest MVPA, light physical activity (LPA) and total activity decline post-breast cancer diagnosis, generally(11,12), and during treatment(13,14), and may not return to pre-diagnosis level. Chemotherapy has been associated with even greater activity declines(11,15–19). Disease/treatment-related side effects and fatigue are also commonly reported barriers to initiating or maintaining MVPA among cancer patients(20–22) and accounted for over half of missed exercise sessions in an intervention during treatment(23). Physical activity and symptom severity likely fluctuate from day to day during chemotherapy due to chemotherapy-induced changes in biopsychosocial processes. Understanding how treatment-related side effects impact physical activity at a more granular level could help prevent activity declines and promote increased activity during chemotherapy that could persist post-treatment. However, most existing data exploring activity and treatment-related side effects during chemotherapy are based on infrequently assessed, retrospective, self-report measures. To the best of our knowledge, only two studies(14,24) to date objectively monitored activity during chemotherapy for breast cancer. However, neither study examined relationships between potential fluctuations in both physical activity and symptom burden.
Ecological momentary assessment (EMA) methodology to assess symptom burden alongside unobtrusive continuous activity measurement using accelerometry may be particularly useful for understanding relationships between physical activity and symptom burden during chemotherapy because dynamic real-time, real-world changes in activity and symptoms can be assessed. EMA analysis highlights individual differences in behavior, their distribution over time, factors affecting behavior, and mutual associations between these factors(25). Given chemotherapy has been identified as a “teachable moment” for positive behavior change(26,27), interventions during this time may be especially effective for increasing breast cancer patients’ activity. Compared to traditional intermittent questionnaire assessment approaches, an EMA approach provides a more granular understanding of activity patterns and symptom burden during chemotherapy, optimizing the ability to understand symptom burden and activity fluctuations and disentangle relationships among these factors. Ultimately, this has important implications for clinical recommendations and physical activity intervention tailoring for this population. However, a recent review of EMA studies in oncology found no EMA studies examined symptoms and physical activity during chemotherapy for breast cancer(28).
The purpose of the present study was to use EMA methodology to prospectively examine relationships relationship between daily symptom burden and MVPA and LPA in breast cancer patients at three time points during chemotherapy.
MATERIALS AND METHODS
Recruitment
Detailed recruitment methods are provided elsewhere(29). Briefly, women were recruited from a large, academic medical center using electronic medical records via the clinic, oncologist referral, or email. Potentially eligible participants were contacted up to three times. Inclusion criteria were: a) female aged ≥18 years; b) diagnosed with stage I-III breast cancer; c) scheduled to receive chemotherapy at study site; d) able to complete baseline data collection prior to/during second chemotherapy cycle; e) have/had an operable tumor; f) no history of other primary cancer except for non-melanoma skin cancer; g) own a smartphone; h) have access to computer with Internet, and i) able to read and write in English. Participants were not excluded based on current activity or body mass index (BMI). All interested individuals were screened online, in-person, or via phone for eligibility. All participants completed written online informed consent. The study was conducted in accordance with recognized ethical guidelines, and the university institutional review board approved all methods.
Study Design and Procedures
This is a prospective, longitudinal study using EMA methodology(30). Patients completed 10 days of data collection at three time points: beginning [first or second cycle; Time 1 (T1)], middle [Time 2 (T2)], and end [last cycle; Time 3 (T3)] of chemotherapy. The 10 day assessment included three days pre-, day-of, and six days post-chemotherapy. At baseline, participants completed an orientation to study procedures. At each time point, participants were emailed online questionnaires and mailed an assessment packet containing an accelerometer, study procedures instructions, and an accelerometer wear log. Participants were instructed to complete online questionnaires, wear the Actigraph 24 hours/day, and respond to 4 EMA text prompts/day on their personal smartphone for 10 consecutive days starting three days prior to their chemotherapy dose (see Figure 1). Accelerometers and accelerometer logs were mailed back to investigators in provided self-addressed, stamped envelopes upon completion. Participants were sent an email reminder the day before each data collection period started, a check-in email on day 5, and a post-collection period reminder to return materials. If a participant did not respond to any EMA prompts on a given day, they were emailed and called.
Figure 1.
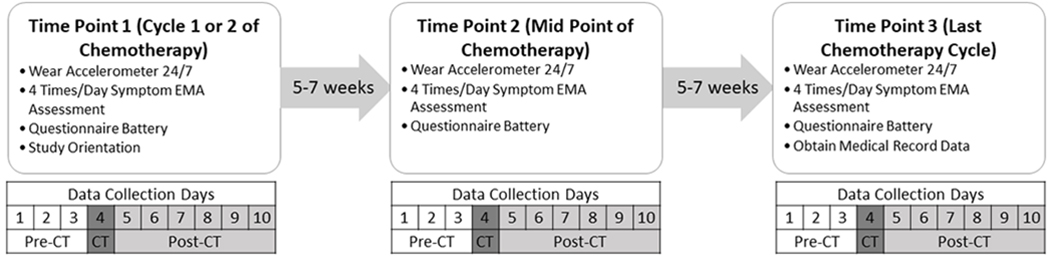
Study design and data collection scheme. The figure details what data were collected when during each of the three 10 day data collection periods during chemotherapy.
Notes: CT=Chemotherapy
Measures
Demographics.
Participants self-reported age, education, race/ethnicity, income, height and weight to calculate BMI, presence of chronic conditions and health status. Data on disease stage and each chemotherapy dose/cycle date and cycle number were extracted from medical records. BMI and age were confirmed via medical records.
EMA Symptom Prompts.
One or two items were modified from well-validated multi-item measures to reflect present moment symptom ratings [(31–33) see Table 1 for questions and response options]. Affect and cognitive functioning were reverse scored so higher scores on all items represent higher symptom burden. Four text message prompts to respond to symptom questions were sent to participants’ personal smartphones each day. Prompts were open for 60 minutes with response reminders every 15 minutes. Prompt 1 was sent at a random time within 2 hours of self-reported wake time. Prompt 2 and 3 were sent at random times ≥2 hours from the previous prompt. Prompt 4 was sent within 2 hours of self-reported bedtime and ≥2 hours since prompt 3. Average daily values for each symptom were calculated based on responses from the 4 prompts.
Table 1.
Symptom Rating Prompts
| Affect(33) |
Question: Estimate how good or bad you feel right now Responses: Likert scale from 0 (very bad) to 10 (very good)* |
| Anxiety (31,32) |
Question: My worries overwhelm me right now. Responses: 5 point Likert scale from 1 (strongly disagree) to 5 (strongly agree) |
| Depression (31,32) |
Question: How would you rate your depression right now? Responses: 5 point Likert scale from 1 (none) to 5 (very severe) |
| Fatigue(31,32) |
Question: How would you rate your fatigue right now? Responses: 5 point Likert scale from 1 (none) to 5 (very severe) |
| Physical Function(31,32) |
Question: To what extent are you able to carry out your everyday physical activities such as walking, climbing stairs, carrying groceries or moving a chair right now? Responses: 5 point Likert scale from 1 (completely ) to 5 (not at all) |
|
Question: Are you physically able to go for a walk for at least 15 minutes right now? Responses: 5 point Likert scale from 1 (without any difficulty) to 5 (unable to do) | |
| Pain(31,32) |
Question: What is your level of pain right now? Responses: 0 (no pain) to 10 (worst imaginable pain) |
| Cognitive Function(31,32) |
Question: My mind is as sharp as usual right now. Responses: 5 point Likert scale from 1 (not at all) to 5 (very much)* |
Note:
indicates items were reverse scored for analyses so higher scores indicate worse symptom ratings
Physical Activity.
The Actigraph Accelerometer (model wGT3X-BT, Actigraph Corporation, Pensacola, FL) is a valid and reliable objective activity measure(34,35). Participants were instructed to wear the monitor for 24 hours for 10 consecutive days on the non-dominant hip during waking hours, except when bathing or swimming, and the non-dominant wrist during sleeping hours. Participants were instructed to record when the monitor was switched from hip to wrist and any non-wear time on the wear log. Activity data were collected in one minute intervals (epochs). ActiLife 6.13.3 was used to derive wear time and summarize minute-by-minute data into daily variables. Non-wear time was defined as intervals of at least 90 consecutive minutes of zero counts, with allowance for ≤2 minutes of observations <100 counts/min within non-wear intervals(36). A valid day of accelerometer wear was defined as ≥10 hours of wear during waking hours(36,37). Each valid minute of waking wear time was classified according to intensity (counts/min) using well-validated activity count cut-points(38,39): sedentary (<100), LPA (100-2019), and MVPA (≥ 2020). Only daily time spent in MVPA and LPA were examined.
Statistical Power
With 67 participants, 10 days of activity data at baseline and two post-chemotherapy time points, we have adequate power to detect within-person activity changes. Assuming an intra-class correlation of 0.4 and an average of ≥8 days of accelerometer wear at all three time points(29), we have 80% power to detect effect sizes as small as 0.11.
Data Analyses
We calculated means and standard deviations for symptom and activity intensity variables at each time point (T1, T2, T3) and by treatment status (pre-chemotherapy v. day-of/post-chemotherapy dose). As a result of the nested structure of the data, such that observations were nested within-persons, multi-level linear regressions with a random intercept to account for clustering were used to conduct comparisons over time and by treatment status (40). First, we conducted exploratory analyses to examine potential symptom and activity changes across time and by treatment status. To do this, we first fit separate multi-level linear regression models regressing each symptom and activity intensity variable on the following: a) time point (T1, T2, and T3) alone, b) treatment status (pre-chemotherapy v. day-of/post-chemotherapy dose) alone, and c) time point by treatment status interaction when controlling or treatment status and time point (Model 1). Next, we repeated the above models controlling for all relevant covariates including: age, BMI, number of comorbidities, health status, disease stage, chemotherapy type (neoadjuvant v. adjuvant), weekend day, and treatment cycle number (Model 2). Finally, we examined a third model using time point (1, 2, 3) as a categorical predictor to compare outcomes between each time point to better understand symptom and physical activity time course controlling for all covariates listed above. These three pairwise comparisons for time point for each symptom and activity used a Bonferroni correction to preserve the Type 1 error rate at the .05 level.
Next, we explored relationships between each symptom and MVPA and LPA using separate multi-level linear regression models. Each model specified the daily symptom rating as the main predictor variable, which was disaggregated into between-subjects (Level-2, person) and within-subjects (Level-1, daily) versions [i.e., partitioning the variance;(41)]. The person-level version represents a participant’s symptom rating average score across all 30 days of study participation (between-person variable). The within-subjects version represents the deviation of a participant’s daily mean from their overall mean [within-person variable; (42)]. This was calculated as the difference between their symptom burden that day and their average 30-day symptom rating. Two sets of models were fit for each activity and each symptom. The first set fit separate models for each symptom and each activity intensity to examine the fixed effects of between- and within-person symptom burden ratings for same day symptoms on same day MVPA and LPA, independently. A random intercept and a random time point (continuous) effect and fixed effects of time point (continuous), day of week (weekday v. weekend), treatment status (pre-chemotherapy v. day-of/post-chemotherapy dose), BMI, number of comorbidities, health status, disease stage, treatment cycle number, chemotherapy type (adjuvant v. neoadjuvant) and a time point by treatment status interaction on daily MVPA and LPA were included. The second set of models examined effects of previous day symptoms. To do this, we created a lagged symptom rating variable to reflect the previous day’s symptom rating and examined separate models for each symptom of the fixed effects of within-person previous day symptom ratings on next day MVPA and LPA, independently. We controlled for next day within-person symptom rating and variables from same day models to delineate whether previous day symptom rating was related to next day activity. To preserve the family-wise error rate at the .05 level within activity intensity analyses, we used a Bonferroni correction on all tests of significance involving symptoms. This correction corresponds to assessing significance at a p-value <.002 and calculating 99.8% confidence intervals.
All models examining physical activity controlled for accelerometer wear time. SPSS version 25 (IBM Corp, Armonk, NY, USA) was used for data analyses.
RESULTS
Participants
Of the 75 patients who consented and were eligible, 67 participated in ≥1 data collection time point, and 63 (84%) completed all 3 measurement times. Further information on recruitment and retention are detailed elsewhere(29). Table 2 presents data on demographic and disease characteristics for all patients who completed at least one time point of data collection (N=67, Mage=48.6, SD=10.3). Briefly, 76.6% of the sample was White, 12.9% were Hispanic/Latina and 78.1% had at least a college degree. The majority were diagnosed with stage 1 or 2 disease (80.0%) and were undergoing adjuvant chemotherapy (65.7%). Approximately one-third (35.8%) began study participation during cycle 1 of chemotherapy; 64.2% began during cycle 2. Average total days between time points was 36.1 for T1 and T2 and 29.8 for T2 and T3. Average days in the study was 92.3.
Table 2.
Participant Characteristics
| Characteristic | Proportion n (%) |
|---|---|
| Days of Observation Mean [Range] | 25.34 [9-30] |
| Days Between T1 and T2 (M, SD) | 36.1 (12.6) |
| Days Between T2 and T3 (M, SD) | 29.8 (11.2) |
| Average Days in Study | 92.3 (20.7) |
| Age Mean [Range] | 48.6 [31-71] |
| BMI Mean [Range] | 27.6 [17.9-52.5] |
| Race | |
| White | 51 (76.6) |
| African American | 8 (10.9) |
| Asian/Pacific Islander | 4 (6.3) |
| Other | 4 (6.3) |
| Hispanic or Latino | 9 (12.9) |
| ≥College Degree | 52 (78.1) |
| Working at Least Part-Time | 45 (67.2) |
| Annual Household Income ≥$100,000 | 31 (46.3) |
| Marital Status | |
| Married/Partnered | 45 (67.2) |
| Single | 11 (16.4) |
| Divorced/Separated | 5 (7.5) |
| Widowed | 3 (4.5) |
| Not Known | 3 (4.5) |
| Chronic Disease Diagnosis | |
| Asthma | 10 (16.9) |
| Depression | 9 (15.3) |
| Arthritis | 8 (13.6) |
| Obesity | 7 (12.1) |
| Upper Gastrointestinal Disease | 5 (8.5) |
| Osteoporosis | 4 (6.8) |
| Anxiety or Panic Disorders | 4 (6.8) |
| Visual Impairment | 4 (6.8) |
| Diabetes | 3 (5.1) |
| Degenerative Disc Disease | 3 (5.1) |
| Hearing Impairment | 2 (3.4) |
| COPD | 1 (1.7) |
| Congestive Heart Failure | 1 (1.7) |
| Overall Health Status at Baseline | |
| Excellent /Very Good | 35 (52.2) |
| Good | 25 (37.3) |
| Fair/Poor | 5 (7.5) |
| Unanswered | 2 (3.0) |
| ≥150 Minutes MVPA Pre-Cancer | 28 (44.4) |
| Disease Stage | |
| Stage I/II | 17 (80.0) |
| Stage III | 13 (20.0) |
| Chemotherapy Type | |
| Neoadjuvant | 23 (34.3) |
| Adjuvant | 44 (65.7) |
| Baseline Treatment Cycle Number | |
| Cycle 1 | 24 (35.8) |
| Cycle 2 | 43 (64.2) |
Descriptive Statistics
Table 3 details data on symptom burden and activity overall and by time point (T1, T2, and T3) and treatment status (pre-chemotherapy v. day-of/post-chemotherapy dose).
Table 3.
Physical Activity and Symptom Descriptives
| Variable | Unadjusted Means (SD) | p-value | Unadjusted Means (SD) | p-value | ||||
|---|---|---|---|---|---|---|---|---|
| Overall | Model 1 | Model 2 | T1 (a) | T2 (b) | T3 (c) | Model 1 | Model 2 | |
| Daily Symptom | ||||||||
| Affect | 2.88 (2.09) | 2.71 (2.08)c | 2.95(2.09) | 3.01 (2.10)a | 0.03 | 0.01 | ||
| Pre-CT Dose | 2.01(1.90) | <0.001 | <0.001 | 1.83( (1.96) | 1.95 (1.81) | 2.31 (1.88) | 0.34 | 0.13 |
| Day-of/Post-CT Dose | 3.24 (2.07) | 3.09 (2.01) | 3.35 (2.06) | 3.31 (2.12) | ||||
| Anxiety | 1.88 (0.94) | 1.96(0.98) | 1.84 (0.92) | 1.93 (0.95) | 0.63 | <0.01 | ||
| Pre-CT Dose | 1.79 (0.88) | <0.01 | 0.03 | 1.90 (0.93) | 1.64 (0.77) | 1.84 (0.92) | 0.01 | 0.05 |
| Day-of/Post-CT Dose | 1.91 (0.96) | 1.98 (1.00) | 1.78 (0.90) | 1.97 (0.96) | ||||
| Depression | 1.43 (0.61) | 1.43 (0.61) | 1.40 (0.59) | 1.44 (0.63) | 0.77 | <0.001 | ||
| Pre-CT Dose | 1.35 (0.56) | <0.001 | <0.001 | 1.31 (0.52) | 1.35 (0.55) | 1.40 (0.62) | <0.01 | 0.01 |
| Day-of/Post-CT Dose | 1.46 (0.63) | 1.48 (0.63) | 1.43 (0.61) | 1.46 (0.64) | ||||
| Fatigue | 2.15 (0.74) | 2.09 (0.69)c | 2.14 (0.73)c | 2.21 (0.78)a,b | 0.01 | 0.05 | ||
| Pre-CT Dose | 1.86 (0.66) | <0.001 | <0.001 | 1.76 (0.62) | 1.86 (0.66) | 2.00 (0.69) | <0.001 | 0.04 |
| Day-of/Post-CT Dose | 2.26 (0.74) | 2.23 (0.68) | 2.26 (0.73) | 2.30 (0.80) | ||||
| Physical Function-ADL | 1.74 (0.89) | 1.64 (0.84)c | 1.56 (0.81) | 1.81 (0.94)a | <0.001 | <0.001 | ||
| Pre-CT Dose | 1.44 (0.70) | <0.001 | <0.001 | 1.33 (0.60) | 1.46 (0.66) | 1.56 (0.82) | 0.91 | 0.40 |
| Day-of/Post-CT Dose | 1.86 (0.93) | 1.77 (0.89) | 1.90 (0.94) | 1.92 (0.97) | ||||
| Physical Function-Walk | 1.73 (0.92) | 1.57 (0.78)b,c | 1.81 (1.00)a | 1.81 (0.95)a | <0.001 | <0.001 | ||
| Pre-CT Dose | 1.39 (0.72) | <0.001 | <0.001 | 1.21 (0.47) | 1.46 (0.81) | 1.54 (0.80) | 0.48 | 0.49 |
| Day-of/Post-CT Dose | 1.86 (0.96) | 1.71 (0.83) | 1.96 (1.03) | 1.93 (0.98) | ||||
| Pain | 1.62 (2.05) | 1.34 (1.84)c | 1.71 (2.06)a | 1.86 (2.24)a | 0.02 | 0.23 | ||
| Pre-CT Dose | 1.19 (1.98) | <0.001 | <0.001 | 0.82 (1.61) | 1.24 (1.95) | 1.60 (2.31) | 0.30 | 0.88 |
| Day-of/Post-CT Dose | 1.79 (2.06) | 1.55 (1.88) | 1.90 (2.08) | 1.97 (2.21) | ||||
| Cognitive Function | 2.05 ((0.91) | 2.02 (0.88) | 2.03 (0.91) | 2.11 (0.94) | 0.35 | 0.73 | ||
| Pre-CT Dose | 1.89(0.89) | <0.001 | <0.001 | 1.81(0.85) | 1.90 (0.93) | 1.96 (0.89) | 0.04 | 0.15 |
| Day-of/Post-CT Dose | 2.11 (0.91) | 2.10 (0.89) | 2.07 (0.90) | 2.17 (0.95) | ||||
| Daily Activity Intensities (mins) | ||||||||
| MVPA | 22.74 (27.57) | 22.22 (21.87)c | 22.39 (26.85) | 23.78 (33.85)a | 0.45 | <0.001 | ||
| Pre-CT Dose | 26.28 (24.22) | <0.01 | <0.001 | 27.07 (23.68) | 27.08 (25.89) | 24.43 (22.89) | 0.01 | 0.60 |
| Day-of/Post-CT Dose | 21.25 (28.73) | 20.32 (2.39) | 20.48 (27.00) | 23.50 (37.51) | ||||
| Light Physical Activity | 228.44 (92.64) | 226.66 (81.15)b,c | 230.41 (99.45)a | 228.36 (97.62)a | <0.001 | <0.001 | ||
| Pre-CT Dose | 238.41 (89.33) | <0.01 | <0.001 | 245.50 (83.04) | 238.20 (96.36) | 229.83 (88.19) | <0.001 | <0.001 |
| Day-of/Post-CT Dose | 224.24 (93.69) | 218.48 (78.96) | 227.25 (100.54) | 227.74 (101.34) | ||||
Note: CT= Chemotherapy; Values in bold indicate significant group difference. Model 1 examined time point and treatment status independently except for the interaction model which included both time point and treatment status and the time point*treatment status interaction. Model 2 included time point, treatment status, time point*treatment status interaction and relevant covariates including age, BMI, number of comorbidities, health status, disease stage, type of chemotherapy (neoadjuvant v. adjuvant), weekend day, and treatment cycle number. All physical activity models controlled for wear time.
Comparison between time points where a= time point 1; b= time point 2; c=time point 3 and indicate significance between groups at p<0.017 when controlling for covariates, treatment status and time point*treatment status interaction.
Symptom Burden.
Overall, participants reported low symptom severity during EMA periods. Unadjusted mixed effects models regressing time point (T1, T2, and T3) on each symptom indicated affect, fatigue, activities of daily living (ADL) physical functioning, walking physical functioning, and pain all worsened over the study period. These relationships remained largely consistent in fully adjusted models, except the time point effect became significant for anxiety and depression and was no longer significant for fatigue or pain. Mixed effects models regressing treatment status (pre-chemotherapy v. day-of/post-chemotherapy dose) on each symptom variable indicated all symptoms were worse on day-of/post-chemotherapy dose days than on pre-chemotherapy days (p’s≤.01) and these relationships remained significant even when controlling for covariates. Affect, fatigue, ADL physical functioning, and walking physical functioning were all significantly (p<0.05) worse at T3 compared to T1. Additionally, average walking physical functioning was significantly worse at T2 compared to T1 and fatigue was significantly worse at T3 compared to T2. There was a time point by treatment status interaction for anxiety, depression, fatigue and cognitive function in unadjusted models. The interaction effect only remained for depression and fatigue when controlling for covariates with the difference in depression and fatigue between pre- and post-treatment days getting larger as time point increased.
Physical Activity.
On average over the entire 30-day study period, participants engaged in 22.74 (SD= 22.57) minutes of MVPA and 228.41 (SD= 89.33) minutes of LPA. Findings for initial mixed models regressing time point (continuous) on each activity intensity and controlling for wear time indicated MVPA did not change; LPA significantly (p=<0.001) declined. The time point effect remained for LPA and became significant for MVPA in fully adjusted models. When controlling for all covariates, LPA was significantly higher at T1 compared to T2 and T3; MVPA was significantly higher at T1 compared to T2, but remained stable between T2 and T3. Mixed models regressing each activity intensity on treatment status indicated MVPA and LPA were lower on day-of/post-chemotherapy days than pre-chemotherapy days (p’s<0.01) and these relationships persisted when accounting for covariates. There was also a significant time point by treatment status interaction for MVPA and LPA in unadjusted models. However, the effect only remained significant for LPA in fully adjusted models with differences in LPA between pre- and post-treatment days becoming smaller as time point increased.
Same Day Symptoms and Physical Activity
Data on mixed models examining same day symptoms and activity are presented in Figure 2 (MVPA) and 3 (LPA).
Figure 2.
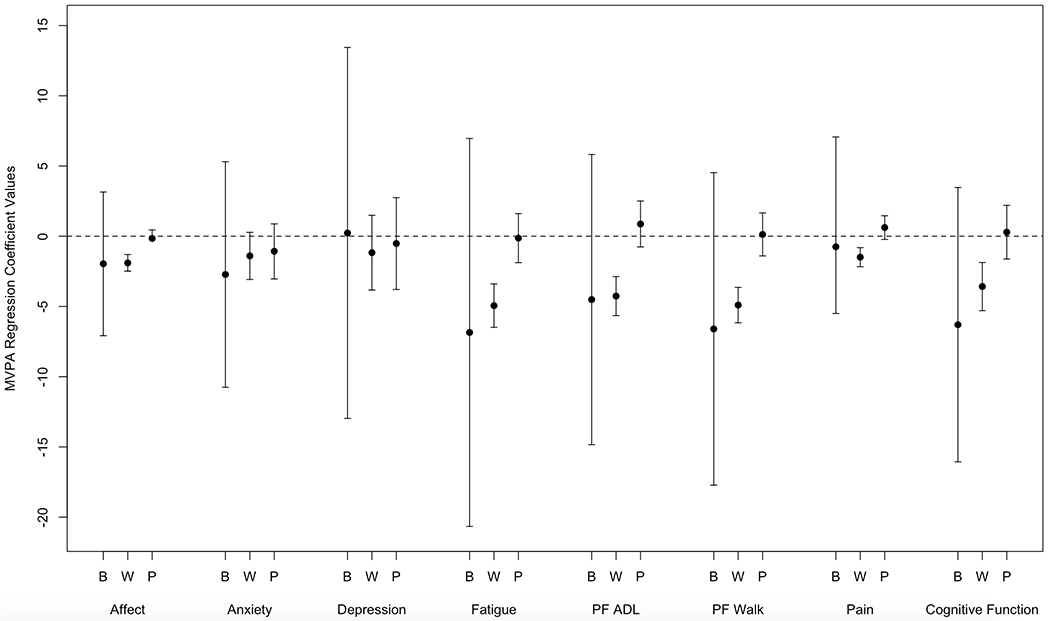
Coefficients from the regression of MVPA on Symptom Ratings. Coefficients with confidence intervals that do not include zero are statistically significant. To preserve the family-wise Type 1 error rate at .05 across all 24 tests, a Bonferroni correction was applied which corresponds to assessing significant at a p-value less than .002 and a 99.8% confidence interval. B=Between-Person Same Day Effects; W=Within-Subjects Same Day Effects; P=Previous Day Within Subjects Effects; PF= Physical Function
MVPA.
Within-person associations were significant for affect, anxiety, fatigue, walking physical function, ADL physical function, pain, and cognitive functioning and daily MVPA (p<0.001 for all). Every one point worse symptom rating than an individual’s 30-day average was associated with between 1.40 (anxiety) and 4.94 (fatigue) minutes less MVPA that day. Effects were larger (i.e. worse than average symptom ratings associated with greater MVPA decline) for pre-chemotherapy days for within-person affect (β=1.56;p=0.001), fatigue (β=3.50;p=0.003), pain (β=2.10;p=0.001), depression (β=7.86;p=<0.001), walking physical function (β=3.88;p=<0.001) and ADL physical function (β=4.75;p=<0.001). Effects were larger (i.e. worse than average symptom ratings associated with larger declines in MVPA) for within-person anxiety (β=1.50;p=0.03) and smaller (i.e. worse than average symptom rating associated with smaller decline) for within-person cognitive function (β=−3.28; p=<0.001) as time point increased. No between-person effects were significant.
LPA.
Within-person effects for affect, anxiety, fatigue, ADL physical function, walking physical function, pain, and cognitive function were significantly associated with daily LPA (p<0.01 for all). Every one point worse symptom rating than an individual’s 30-day average was associated with between 4.48 (pain) and 24.72 (ADL physical function) minutes less LPA that day. Effects were larger (i.e. worse than average symptom rating associated with greater decline in LPA) for within-person variations in anxiety (β=12.30;p=0.001) and pain (β=4.70;p=0.001) on pre-chemotherapy days and for fatigue (β=−9.37;p=0.01), ADL physical function (β=−15.72;p=<0.001), walking physical function (β=−8.57;p=0.01) and cognitive function (β=−9.49;p=0.01) on the day-of/post-chemotherapy days. Effects were smaller (i.e. worse than average symptom rating associated with smaller LPA decline) for within-person variations in anxiety (β=−4.92;p=0.03), ADL physical function (β=−15.73;p=<0.001), walking physical function (β=−4.02;p=0.03) and affect (β=−3.46;p=<0.001) as time point increased. No between-person effects were significant.
Previous Day Symptoms and Current Day Physical Activity
The relationships between previous day anxiety and next day LPA were significant (p<0.001). Every one point worse rating than an individual’s 30-day average was associated with 11.28 minutes less LPA the next day. No other lagged effects were significant (See Fig. 2 and 3).
Figure 3.
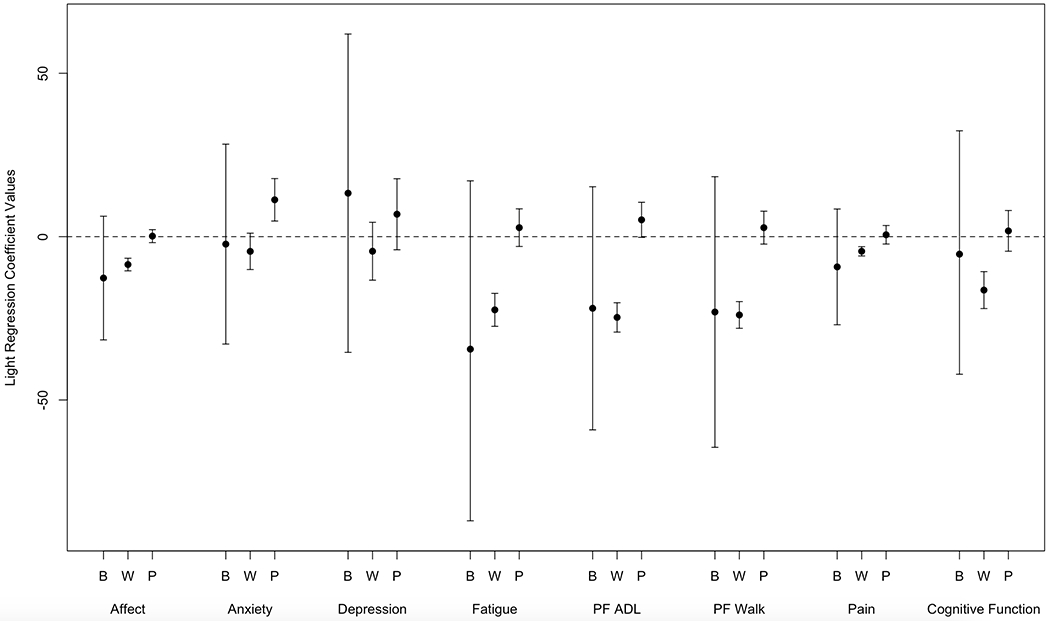
Coefficients from the regression of LPA on Symptom Ratings. Coefficients with confidence intervals that do not include zero are statistically significant. To preserve the family-wise Type 1 error rate at .05 across all 24 tests, a Bonferroni correction was applied which corresponds to assessing significant at a p-value less than .002 and a 99.8% confidence interval. B=Between-Person Same Day Effects; W=Within-Subjects Same Day Effects; P=Previous Day Within Subjects Effects; PF= Physical Function
DISCUSSION
The purpose of this study was to examine how patients’ symptom burden influences physical activity at multiple time points during chemotherapy for breast cancer in real-time in the real-world. Overall, findings indicate an individual’s deviation from their average symptom rating on a given day is associated with: a) less MVPA and LPA on that day across all symptoms explored except for depression and anxiety b) less LPA the next day for anxiety. An individual’s deviation from their average symptom rating on a given day was not associated with next day MVPA.
In general, average daily symptom burden increased during chemotherapy while average daily MVPA and LPA declined when controlling for relevant covariates. Although, the trajectory of change across time differed by variable examined whereby some got worse between T1 and T2 and were then stable between T2 and T3 or were only different between T1 and T3. Additionally, daily symptom ratings were worse and MVPA and LPA lower on day-of/post-treatment days than pre-treatment days when accounting for relevant covariates. However, it is important to note no differences between time points met the 0.5 standard deviation difference, a common threshold to determine clinical significance(43); only differences for affect, fatigue and walking physical function met this threshold when examining treatment status. Although not clinically significant, overall trends are consistent with existing literature exploring symptom burden and activity trends during chemotherapy. Chemotherapy can cause substantial side effects, which increases symptom burden, and may make it more difficult to perform both exercise and ADLs. Symptom burden was generally rated as low across symptoms assessed in the present study and was similar to other studies that examined pain and fatigue in post-treatment survivors(44,45) and fatigue in breast cancer patients undergoing chemotherapy(46). No other EMA studies examined other symptoms from the present study on comparable scales. MVPA levels we observed are consistent with a study that used a research grade monitor during the first 14 days of chemotherapy(24) (22.7 v. 21.9 to 23.3), but we observed lower LPA (228.4 v. 310.7 to 313.4). However, MVPA (22.7 v. 10.5) and LPA (228.4 v. 179.1) levels in our study were higher compared to a study using Fitbits continuously during treatment(14). This could be due to our study’s shorter observation period or device measurement discrepancies. Future work should use research grade monitors or combine research grade and commercially available monitors for longer duration during chemotherapy to better characterize activity patterns.
Relationships between symptom ratings and both MPVA and LPA were most robust for same day within-person physical function and fatigue. For every one point worse than one’s average rating of fatigue, physical function ADL or physical function walking, participants engaged in 4 to 5 minutes less MVPA and 22 to 25 minutes less LPA that day, which could equate to up to 35 minutes less MVPA and 175 minutes less LPA over the course of a week. If these relationships and activity deficits were to persist long-term, this could have substantial detrimental effects on QOL, future activity, and potentially even cancer mortality as each 1.0 metabolic equivalent (MET) hour/week of physical activity post-diagnosis has been associated with a 2% decrease in mortality among cancer survivors(7). Future research should examine the best way to target these symptoms and explore intervention effects on activity behavior and important health outcomes across the survivorship continuum in highly controlled trials.
Interestingly, same-day within-person effects for MVPA were larger for pre- than post/day-of chemotherapy and as time point in chemotherapy increase. Same day relationships were less consistent for LPA; within-person effects varied for pre-/post chemotherapy day and across time point depending on symptom whereby some effects were larger and some smaller. There were no significant relationships between previous day symptom and current day MVPA; only previous day anxiety was significant for LPA. Overall, findings indicate individual fluctuations from “usual” symptom ratings may be important predictors of physical activity on that day during chemotherapy for breast cancer and should be considered in intervention development for this population.
Our study is not without limitations. First, our sample size was relatively small, although appropriately powered for within-person analyses, and was recruited from a large academic medical setting. Selection bias may have also occurred whereby individuals who agreed to participate in our study: a) were not as “sick” or were more motivated than those choosing not to participate and b) may be higher income due to smartphone ownership. Thus, our sample may not represent the general breast cancer patient population. Additionally, descriptive findings and between-person findings were exploratory and should be interpreted with caution given the small sample size. Future studies should use larger more diverse samples to be fully powered to examine both within and between-person differences. Second, while the three ten day timeframes we assessed go beyond traditional “snap shot” assessments and represent the longest objective activity assessment during chemotherapy using a research grade monitor to date, these discrete 10 day time points assessing limited symptoms may still not fully capture how relationships change throughout chemotherapy. Third, while the present study examines relationships at the day level, it is plausible relationships are even more granular. Future work should explore micro-temporal relationships between activity and symptoms (i.e. 30 or 60 mins pre-/post-prompt). Further, although single items used in this study were adapted from well-validated measures, they are not well-validated single items. This is a limitation of this study and EMA research more generally. Future work should evaluate the validity of these items. Finally, while the accelerometer does not provide feedback on activity, reactivity to study procedures could have caused participants to increase their activity. Similarly, answering symptom prompts could raise awareness of symptoms which could impact ratings and behavior.
Despite these limitations, this is one of the first studies to combine self-reported EMA symptom ratings with objective activity data using research-grade accelerometers in cancer patients throughout chemotherapy. Additionally, no in-person study visits were required, participants used their own smartphones and our sample represents a range of demographic and disease characteristics which may increase generalizability despite potential limitations noted above. Future work is warranted to further explore activity patterns and symptoms among cancer patients and survivors in individuals diagnosed with other cancer types and undergoing alternative cancer treatments (i.e. radiation, surgery) including longer assessment time frames from diagnosis to long-term survivorship and examining other potential symptoms (e.g., nausea, neuropathy). Future studies should consider integrating additional data streams from passive sensors (e.g. GPS, glucose, heart rate), medical records (e.g. hospitalizations, adverse events, treatment response, treatment adherence), biomarkers (e.g. cortisol, glucose) and self-reports (e.g. activities being performed, social context). This will improve the understanding of multi-level changes in determinants and outcomes of post-diagnosis activity, and more accurately characterize activity trajectories(47) and profiles(48,49) and potentially predict health and disease outcomes among cancer survivors. Additionally, as wearable technology becomes more ubiquitous and continues to improve, using commercially available devices to monitor activity or integrating activity monitoring and EMA prompting into one device (e.g. smartwatch), may increase compliance and acceptability to monitoring for longer periods of time (i.e. every day-of chemotherapy). Further, technological advances provide unique opportunities to create highly tailored interventions that could account for daily fluctuations in activity and symptoms, as well as other contextual factors (weather, symptoms, time of day, motivation, activity level) in real time in cancer patients undergoing chemotherapy. Future work should explore how to best tailor activity interventions and the feasibility and acceptability of such an approach.
The current study advances the understanding of relationships between activity and symptoms by moving beyond a “snap shot” perspective to demonstrate: a) symptom burden increases and activity declines during one treatment cycle and throughout chemotherapy and b) higher than average symptom ratings on a given day are associated with both lower MVPA and LPA that day, and in some cases, the following day. Future work is warranted to better understand relationships between activity and symptoms across the cancer care continuum, explore other factors that may be related to these relationships, and investigate how to effectively target these factors in activity promotion interventions to improve health and disease outcomes among cancer patients and survivors.
ACKNOWLEDGMENTS
This study was supported by the Northwestern Memorial Hospital Lynn Sage Cancer Research Foundation Grants Initiative and the National Cancer Institute K07CA196840 awarded to Siobhan Phillips. Lisa Auster-Gussman is supported by T32CA193193.
Footnotes
Conflict of Interest: The authors have no conflicts of interest to disclose. CS has research funding from Pfizer, Astrazeneca, and Tesaro; and has served on advisory boards for BMS, Genomic Health, Polyphor, Halozyme, and Athenex.
REFERENCES
- 1.2011 based on November 2010 SEER data submission, posted to the SEER web site, 2011. SEER Cancer Statistics Review, 1975-2008. National Cancer Institute; <http://seer.cancer.gov/csr/1975_2009_pops09/>. based on November 2010 SEER data submission, posted to the SEER web site, 2011. [Google Scholar]
- 2.Medicine Io. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, D.C.: National Academies Press; 2005. [Google Scholar]
- 3.Courneya KS, Segal RJ, McKenzie DC, Dong H, Gelmon K, Friedenreich CM, et al. Effects of exercise during adjuvant chemotherapy on breast cancer outcomes. Med Sci Sports Exerc 2014;46(9):1744–51. [DOI] [PubMed] [Google Scholar]
- 4.van Waart H, Stuiver MM, van Harten WH, Geleijn E, Kieffer JM, Buffart LM, et al. Effect of low-intensity physical activity and moderate-to high-intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: results of the PACES randomized clinical trial. J Clin Oncol 2015;33(17):1918–27. [DOI] [PubMed] [Google Scholar]
- 5.Courneya KS, Segal RJ, Gelmon K, Reid RD, Mackey JR, Friedenreich CM, et al. Six-month follow-up of patient-rated outcomes in a randomized controlled trial of exercise training during breast cancer chemotherapy. Cancer Epidemiology Biomarkers & Prevention 2007;16(12):2572–8. [DOI] [PubMed] [Google Scholar]
- 6.Ibrahim EM, Al-Homaidh A. Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol 2011;28(3):753–65 doi 10.1007/s12032-010-9536-x. [DOI] [PubMed] [Google Scholar]
- 7.Li T, Wei S, Shi Y, Pang S, Qin Q, Yin J, et al. The dose–response effect of physical activity on cancer mortality: findings from 71 prospective cohort studies. Br J Sports Med 2016;50(6):339–45. [DOI] [PubMed] [Google Scholar]
- 8.Lahart IM, Metsios GS, Nevill AM, Carmichael AR. Physical activity, risk of death and recurrence in breast cancer survivors: A systematic review and meta-analysis of epidemiological studies. Acta Oncol 2015;54(5):635–54. [DOI] [PubMed] [Google Scholar]
- 9.Furmaniak AC, Menig M, Markes MH. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst Rev 2016(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maddocks M, Byrne A, Johnson CD, Wilson RH, Fearon KC, Wilcock A. Physical activity level as an outcome measure for use in cancer cachexia trials: a feasibility study. Support Care Cancer 2010;18(12):1539–44. [DOI] [PubMed] [Google Scholar]
- 11.Irwin ML, Crumley D, McTiernan A, Bernstein L, Baumgartner R, Gilliland FD, et al. Physical activity levels before and after a diagnosis of breast carcinoma. Cancer 2003;97(7):1746–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Littman AJ, Tang M-T, Rossing MA. Longitudinal study of recreational physical activity in breast cancer survivors. J Cancer Surviv 2010;4(2):119–27. [DOI] [PubMed] [Google Scholar]
- 13.Andrykowski MA, Beacham AO, Jacobsen PB. Prospective, longitudinal study of leisure-time exercise in women with early-stage breast cancer. Cancer Epidemiology Biomarkers & Prevention 2007;16(3):430–8. [DOI] [PubMed] [Google Scholar]
- 14.Nelson SH, Weiner LS, Natarajan L, Parker BA, Patterson RE, Hartman SJ. Continuous, objective measurement of physical activity during chemotherapy for breast cancer: the Activity in Treatment pilot study. Translational behavioral medicine 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devoogdt N, Van Kampen M, Geraerts I, Coremans T, Fieuws S, Lefevre J, et al. Physical activity levels after treatment for breast cancer: one-year follow-up. Breast Cancer Res Treat 2010;123(2):417–25. [DOI] [PubMed] [Google Scholar]
- 16.De Groef A, Geraerts I, Demeyer H, Van der Gucht E, Dams L, de Kinkelder C, et al. Physical activity levels after treatment for breast cancer: two-year follow-up. The Breast 2018;40:23–8. [DOI] [PubMed] [Google Scholar]
- 17.Emery CF, Yang HC, Frierson GM, Peterson LJ, Suh S. Determinants of physical activity among women treated for breast cancer in a 5‐year longitudinal follow‐up investigation. Psycho-Oncology: Journal of the Psychological, Social and Behavioral Dimensions of Cancer 2009;18(4):377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwan ML, Sternfeld B, Ergas IJ, Timperi AW, Roh JM, Hong C-C, et al. Change in physical activity during active treatment in a prospective study of breast cancer survivors. Breast Cancer Res Treat 2012;131(2):679–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huy C, Schmidt ME, Vrieling A, Chang-Claude J, Steindorf K. Physical activity in a German breast cancer patient cohort: one-year trends and characteristics associated with change in activity level. Eur J Cancer 2012;48(3):297–304. [DOI] [PubMed] [Google Scholar]
- 20.Henriksson A, Arving C, Johansson B, Igelström H, Nordin K. Perceived barriers to and facilitators of being physically active during adjuvant cancer treatment. Patient Educ Couns 2016;99(7):1220–6. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez S, Franklin J, Amlani N, DeMilleVille C, Lawson D, Smith J. Physical activity and cancer: A cross-sectional study on the barriers and facilitators to exercise during cancer treatment. Canadian Oncology Nursing Journal/Revue canadienne de soins infirmiers en oncologie 2015;25(1):37–42. [DOI] [PubMed] [Google Scholar]
- 22.Nielsen AM, Welch WA, Gavin KL, Cottrell AM, Solk P, Torre EA, et al. Preferences for mHealth physical activity interventions during chemotherapy for breast cancer: a qualitative evaluation. Support Care Cancer 2019:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Courneya KS, Segal RJ, Gelmon K, Reid RD, Mackey JR, Friedenreich CM, et al. Predictors of supervised exercise adherence during breast cancer chemotherapy. Med Sci Sports Exerc 2008;40(6):1180–7. [DOI] [PubMed] [Google Scholar]
- 24.Tonosaki A, Ishikawa M. Physical activity intensity and health status perception of breast cancer patients undergoing adjuvant chemotherapy. European Journal of Oncology Nursing 2014;18(2):132–9. [DOI] [PubMed] [Google Scholar]
- 25.Marszalek J, Morgulec-Adamowicz N, Rutkowska I, Kosmol A. Using Ecological Momentary Assessment to Evaluate Current Physical Activity. BioMed research international 2014;2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Demark-Wahnefried W, Aziz NM, Rowland JH, Pinto BM. Riding the crest of the teachable moment: promoting long-term health after the diagnosis of cancer. J Clin Oncol 2005;23(24):5814–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sabiston CM, Brunet J, Vallance JK, Meterissian S. Prospective examination of objectively-assessed physical activity and sedentary time after breast cancer treatment: Sitting on the crest of the teachable moment. Cancer Epidemiology Biomarkers & Prevention 2014:cebp.1179.2013. [DOI] [PubMed] [Google Scholar]
- 28.Kampshoff CS, Verdonck‐de Leeuw IM, van Oijen MG, Sprangers MA, Buffart LM. Ecological momentary assessments among patients with cancer: A scoping review. Eur J Cancer Care (Engl) 2019:e13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solk P, Gavin K, Fanning J, Welch W, Lloyd G, Cottrell A, et al. Feasibility and acceptability of intensive longitudinal data collection of activity and patient-reported outcomes during chemotherapy for breast cancer. Qual Life Res 2019:1–14. [DOI] [PubMed] [Google Scholar]
- 30.Shiffman S, Stone AA. Ecological momentary assessment: A new tool for behavioral medicine research. Technology and methods in behavioral medicine 1998:117–31. [Google Scholar]
- 31.Garcia SF, Cella D, Clauser SB, Flynn KE, Lad T, Lai J-S, et al. Standardizing patient-reported outcomes assessment in cancer clinical trials: a patient-reported outcomes measurement information system initiative. J Clin Oncol 2007;25(32):5106–12. [DOI] [PubMed] [Google Scholar]
- 32.Pilkonis PA, Choi SW, Reise SP, Stover AM, Riley WT, Cella D, et al. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS®): depression, anxiety, and anger. Assessment 2011;18(3):263–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hardy CJ, Rejeski WJ. Not what, but how one feels: The measurement of affect during exercise. J Sport Exerc Psychol 1989;11(3):304–17. [Google Scholar]
- 34.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc 1998;30(5):777–81. [DOI] [PubMed] [Google Scholar]
- 35.Welk GJ, McClain JJ, Eisenmann JC, Wickel EE. Field validation of the MTI Actigraph and BodyMedia armband monitor using the IDEEA monitor. Obesity 2007;15(4):918–28. [DOI] [PubMed] [Google Scholar]
- 36.Bassett DR. Validity of four motion sensors in measuring moderate intensity physical activity. Med Sci Sports Exerc 2000;32(9):S471. [DOI] [PubMed] [Google Scholar]
- 37.Tudor-Locke C, Swift DL, Schuna JM, Dragg AT, Davis AB, Martin CK, et al. WalkMore: a randomized controlled trial of pedometer-based interventions differing on intensity messages. BMC Public Health 2014;14(1):168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kozey-Keadle S, Libertine A, Lyden K, Staudenmayer J, Freedson PS. Validation of wearable monitors for assessing sedentary behavior. Med Sci Sports Exerc 2011;43(8):1561–7. [DOI] [PubMed] [Google Scholar]
- 39.Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc 2008;40(1):181. [DOI] [PubMed] [Google Scholar]
- 40.DR H RDG. Longitudinal data analysis. Hoboken, N.J.: Wiley-Interscience; 2006. [Google Scholar]
- 41.Hedeker D, Mermelstein RJ, Demirtas H. Modeling between‐subject and within‐subject variances in ecological momentary assessment data using mixed‐effects location scale models. Stat Med 2012;31(27):3328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Curran PJ, Bauer DJ. The disaggregation of within-person and between-person effects in longitudinal models of change. Annu Rev Psychol 2011;62:583–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care 2003;41(5):582–92. [DOI] [PubMed] [Google Scholar]
- 44.Curran SL, Beacham AO, Andrykowski MA. Ecological momentary assessment of fatigue following breast cancer treatment. J Behav Med 2004;27(5):425–44. [DOI] [PubMed] [Google Scholar]
- 45.Badr H, Basen-Engquist K, Taylor CLC, De Moor C. Mood states associated with transitory physical symptoms among breast and ovarian cancer survivors. J Behav Med 2006;29(5):461–75. [DOI] [PubMed] [Google Scholar]
- 46.Ratcliff CG, Lam CY, Arun B, Valero V, Cohen L. Ecological momentary assessment of sleep, symptoms, and mood during chemotherapy for breast cancer. Psycho-Oncology 2014;23(11):1220–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brunet J, Amireault S, Chaiton M, Sabiston CM. Identification and prediction of physical activity trajectories in women treated for breast cancer. Ann Epidemiol 2014;24(11):837–42. [DOI] [PubMed] [Google Scholar]
- 48.Sweegers M, Boyle T, Vallance J, Chinapaw M, Brug J, Aaronson N, et al. Which cancer survivors are at risk for a physically inactive and sedentary lifestyle? Results from pooled accelerometer data of 1447 cancer survivors. Int J Behav Nutr Phys Act 2019;16(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lucas AR, Levine BJ, Avis NE. Posttreatment trajectories of physical activity in breast cancer survivors. Cancer 2017;123(14):2773–80. [DOI] [PMC free article] [PubMed] [Google Scholar]


