Abstract
Background
Most children with atopic dermatitis(AD) suffer from sleep disturbance, but reliable and valid assessment tools are lacking.
Objectives
To test PROMIS (Patient Reported Outcomes Measurement Information System) sleep measures in pediatric AD and to develop an algorithm to screen, assess and intervene to reduce sleep disturbance.
Methods
A cross-sectional study was conducted with AD children ages 5–17 years and one parent(n=61), who completed sleep, itch, and AD-specific questionnaires; clinicians assessed disease severity. All children wore actigraphy watches for 1-week-objective sleep assessment.
Results
PROMIS sleep disturbance parent-proxy-reliability was high (Cronbach’s α=0.90) and differentiated among Patient Oriented Eczema Measure (POEM)-determined disease severity groups (mean±SD in mild vs. moderate vs. severe was 55.7±7.5 vs. 59.8±10.8 vs. 67.1±9.5, p<0.01). Sleep disturbance correlated with itch (Numerical Rating Scale/NRS, r=0.48), PROMIS sleep-related impairment (r=0.57), and worsened quality of life (Children’s Dermatology Life Quality Index/CDLQI, r=0.58), all p<0.01. Positive report on POEM sleep disturbance question has high sensitivity (95%) for PROMIS parent-proxy-reported sleep disturbance (T-score ≥60). An algorithm for screening and intervening on sleep disturbance was proposed.
Limitations
This was a local sample.
Conclusions
Sleep disturbance in pediatric AD should be screened using the POEM sleep question, with further assessment using the PROMIS sleep disturbance measure or objective sleep monitoring if needed.
Keywords: child, atopic dermatitis, eczema, sleep, quality of life, surveys and questionnaires, self-report, patient reported outcome measures, actigraphy
Capsule Summary
• The Patient Reported Outcome Measurement Information System (PROMIS) pediatric sleep measures are reliable and valid assessments of sleep disturbance in atopic dermatitis.
• Clinicians can use the algorithm reported in this manuscript to screen and treat sleep disturbance in pediatric atopic dermatitis.
Introduction
Atopic dermatitis (AD) causes sleep disturbance in most affected children,1 with about 50 minutes of sleep lost per night.2 Guidelines by the American Academy of Dermatology (AAD)3 and professional allergy societies4 recommend routine sleep assessment, but evidence-based tools to assess common pediatric sleep problems in AD are lacking.1
Sleep disturbance can be assessed objectively or by patient or parent-proxy-report. Polysomnography (PSG) is the clinical standard for objective assessment and provides extensive detail on sleep stages and timing. PSG can also evaluate other comorbid conditions, such as obstructive sleep apnea. Actigraphy is another objective measure that provides less detail than PSG but can be performed in the home environment. As our group and others have shown, actigraphy in AD provides a relevant objective assessment of rest, wake, and limb movements.1,2,5 Patient/parent-proxy sleep assessment is a different measure that has low precision for sleep timing,6 but may be a more meaningful outcome to individuals themselves as these measures capture the lived experiences of sleep.7
The NIH Patient Reported Outcome Measurement Information System (PROMIS) is a collection of freely available and well-validated assessments for multiple physical, mental, and social health domains, developed using extensive literature review, expert and stakeholder input, and both classical test theory and item response theory methodologies.8 The newly developed PROMIS Pediatric sleep disturbance (SDi) and sleep-related impairment (SRI) measures can be administered in brief short forms,9 and are suited to pediatric patients (≥ 8 years) or to a parent-proxy for children ≥ 5 years.10,11 Scoring is standardized, by definition, with a mean T-score 50 and standard deviation of 10. These assessments can be completed in just a few minutes and, as shown in children with a variety of conditions, can provide rich data about the patient/parent perspective on sleep.12 Given the availability of the PROMIS sleep measures in pediatrics and the need for improved sleep assessment in AD, our objectives were to: 1) determine the reliability and validity of the newly developed PROMIS SDi and SRI measures in AD and 2) begin to develop a sleep assessment algorithm for AD.
Methods
The Ann & Robert H. Lurie Children’s Hospital of Chicago (Lurie Children’s) Institutional Review Board (IRB) reviewed and approved study procedures. Parents provided informed consent for children, and children aged 12 years and older gave their assent.
Study sample
We recruited a convenience sample of children 5–17 years old with AD stratified by mild, moderate and severe disease via Patient Oriented Eczema Measure (POEM)13. To be eligible for the study, patients had to have an AD diagnosis by a dermatologist or allergist according to Hanifin and Rajka criteria.14 Both parent and child (if ≥8 years old) had to be able to read and understand English. Participants were recruited by phone prior to a scheduled clinic visit with materials (questionnaires and actigraphy watches) sent out and then collected at the clinic visit as previously published.2 Patients had to have controlled asthma (Asthma Control Test > 19) and report no sleep disturbance due to allergic rhinitis, to ensure SDi was related to AD, as per protocol established in our previous study.2 Although not all patients complied, patients were asked to discontinue sedating antihistamine but continue other topical and systemic therapies.
Objective skin assessment
At the patient’s clinic visit, the dermatologist/allergist assessed disease severity using an objective tool, the Eczema Area and Severity Index (EASI) which measures redness, thickness, scratching, lichenification and extent of disease.15,16
Questionnaire administration
The patient (if ≥8 years) and one parent completed questionnaires on sleep, itch, disease severity, and overall quality of life. Children completed the PROMIS Pediatric sleep disturbance (SDi) 8-item short form9 and PROMIS Pediatric sleep-related impairment (SRI) 8-item short form.9 SDi items capture sleep onset, sleep continuity, parasomnias, and sleep quality and SRI captures daytime sleepiness, energy, sleep offset, and the impact of sleep on cognitive function, affect, behavior and daily activities.9 See supplement for the full questions on the PROMIS Pediatric Sleep Assessment Tools.
The child also completed (with parental help if desired) the itch numerical rating scale (NRS; 0–10),17 Patient-Oriented Eczema Measure (POEM)13 and Children’s Dermatology Life Quality Index (CDLQI)18. POEM is a 7-question tool that includes one question about itch and one about sleep; “Over the last week, on how many nights has your sleep been disturbed because of the eczema?” The possible responses are: no days, 1–2 days, 3–4 days, 5–6 days or every day. A response was considered positive for sleep disturbance if ≥ 1–2 days was reported. The CDLQI question on sleep is: “Over the last week, how much has your sleep been affected by your skin problem?” On their own, parents completed PROMIS Pediatric parent-proxy short forms for SDi,9 SRI,9 Pediatric Sleep Questionnaire (PSQ),19 and the Epworth Sleepiness Scale (ESS).20
Data analysis of questionnaires and patient characteristics
PROMIS sleep measures use a 5-point Likert scale, with 8 item scores converted to a standardized T-score of 50 and standard deviation of 10 using freely available software at assessmentcenter.net. This software uses item response theory based Expected a Posteriori (EAP) scoring. Higher scores indicate higher levels of sleep disturbance for the SDi measure and higher levels of sleep-related impairment for the SRI measure.9 Key psychometric properties of the measures in AD were assessed, such as reliability (Cronbach’s alpha) and convergent validity (Pearson correlation coefficients). Other measures were scored using published scoring algorithms. The NRS,17 ESS,20 and CDLQI18 were analyzed as raw scores. A total score was calculated for the POEM and disease severity was stratified according to published standards13 as mild ≤7, moderate 8–16, and severe ≥17. The PSQ was analyzed as a yes/no variable indicating the presence/absence of sleep comorbidities.19 Demographic characteristics were analyzed using descriptive statistics stratified by POEM disease severity. All data was analyzed using IBM SPSS software version 26, a p-value <0.05 was considered significant.
Actigraphy collection and analysis
A Phillips Respironics Actiwatch device was sent home with the patient to be worn on the non-dominant wrist for 1 week (at least 5 days) to collect objective actigraphy data. Patients with parental help were asked to complete sleep diaries to track timing of sleep, wake, medications, and unusual events (e.g. late sleep time due to holiday or awakening due to illness). Analysis of actigraphy included key measures of sleep onset latency, bed time, wake time, minutes of wake after sleep onset (WASO), sleep efficiency, and total sleep time analyzed.2
Sleep disturbance screening
To determine optimal screening methods for SD, we considered clinical/research practice, such as use of the POEM measure to assess disease severity. Because the POEM has a sleep question and we previously demonstrated the potential relevance of the POEM sleep question to screen for sleep disturbance,2 we planned to evaluate the relevance of the POEM sleep question to both patient/parent report and objective assessment of sleep disturbance (Wake After Sleep Onset ≥76.4 minutes was chosen based off our previous finding of actigraphy in controls).2 Sensitivity/specificity of POEM sleep for sleep disturbance by PROMIS SDi measure and objective sleep was computed, and an ROC curve generated and interpreted according to published methods.21
RESULTS
We recruited a convenience sample of children (n=61) 5–17 years old with AD. Demographic and clinical characteristics stratified by disease severity of mild (n=17), moderate (n=23) or severe (n=21) are summarized in Table 1. Allergic and sleep disturbing comorbidities were similar between groups. Parent-proxy-reported SDi was significantly different between disease severity groups (mean±SD in mild vs. moderate vs. severe was 55.7±7.5 vs. 59.8±10.8 vs. 67.1±9.5, respectively; p<0.01, n=61), as was child reported SDi (51.2±9.3 vs. 55.5±7.3 vs. 61.1±10.3, respectively, p=0.02, n=45). The effect size for severe v. mild disease with parent-proxy and patient reported PROMIS SDi is 1.33 and 1.00, respectively. Furthermore, patients who continued on sedating antihistamine for this study (n=12) versus those who didn’t (n=49) tended to have more severe AD, and poorer sleep by parent-proxy-reported SDi (µ±SD=67.9±8.2 v. 59.5 ±10.4, p=0.01) and more minutes of wake after sleep onset (µ±SD=104.4±58.2 v. 72.4±27.8, p<0.01).
Table 1.
Socio-demographic and clinical characteristics by Patient Oriented Eczema Measure severity.
| Variable | Disease severity by Patient Oriented Eczema Measure (POEM) |
|||
|---|---|---|---|---|
| Mild (n=17) | Moderate (n=23) | Severe (n=21) | P-value | |
| Male*, n (%) | 6 (35.3) | 12 (52.2) | 13 (61.9) | 0.26 |
| Age, mean (SD) | 11.7 (3.8) | 11.1 (4.2) | 12.2 (3.7) | 0.66 |
| Race, n (%) | 0.26 | |||
| Asian | 4 (23.5) | 3 (13.0) | 1 (4.8) | |
| Black | 0 (0.0) | 5 (21.7) | 6 (28.6) | |
| White | 9 (52.9) | 9 (39.1) | 10 (47.6) | |
| Other | 4 (23.5) | 6 (26.1) | 4 (19.0) | |
| Latino, n (%) | 6 (35.3) | 5 (21.7) | 7 (33.3) | 0.58 |
|
| ||||
| POEM, mean (SD) | 3.9 (2.1) | 11.7 (2.9) | 21.8 (3.0) | <0.01 |
| CDLQI, mean (SD) (n=43) | 2.0 (1.6) | 4.2 (3.0) | 8.7 (5.7) | <0.01 |
| NRS, mean (SD) (n=59) | 2.2 (1.9) | 4.8 (2.7) | 6.2 (2.4) | <0.01 |
|
| ||||
| Asthma, n (%) | 11 (64.7) | 14 (60.9) | 14 (66.7) | 0.92 |
| Allergic Rhinitis, n (%) | 16 (94.1) | 18 (78.3) | 17 (81.0) | 0.38 |
| Food Allergy, n (%) | 11 (64.7) | 21 (91.3) | 15 (71.4) | 0.11 |
| ADHD, n (%) | 1 (5.9) | 2 (8.7) | 2 (9.5) | 0.92 |
| Other Sleep Diagnosis, n (%)** | 0 (0.0) | 0 (0.0) | 2 (9.5) | 0.14 |
|
| ||||
| Therapies during the study | ||||
| Sedating Antihistamine, n (%) | 1 (5.9) | 3 (13.0) | 8 (38.1) | 0.03 |
| Topical corticosteroid, n (%) | 12 (70.6) | 16 (69.6) | 20 (95.2) | 0.07 |
| Systemic immunosuppressant,*** n (%) | 2 (11.8) | 4 (17.4) | 5 (23.8) | 0.63 |
|
| ||||
| Objective Sleep | ||||
| Sleep efficiency %, mean (SD) | 84.2 (3.8) | 81.8 (6.1) | 80.0 (9.0) | 0.18 |
| Sleep onset latency minutes, mean (SD) | 9.4 (5.4) | 14.8 (12.1) | 17.0 (17.0) | 0.19 |
| Wake After Sleep Onset (WASO) minutes, mean (SD) | 67.6 (16.6) | 77.7 (29.1) | 88.8 (53.3) | 0.22 |
| Bed time, mean hh:mm (SD) | 23:00 (2:05) | 22:40 (1:06) | 23:08 (1:32) | 0.60 |
| Wake time, mean hh:mm (SD) | 8:00 (1:10) | 7:46 (0:45) | 8:02 (1:47) | 0.76 |
| Total Sleep Time hh:mm, mean (SD) | 7:35 (1:09) | 7:25 (0:57) | 7:02 (0:46) | 0.19 |
|
| ||||
| Parent-Proxy-Reported Sleep (%) | ||||
| Sleep Disturbance | 55.7 (7.5) | 59.8 (10.8) | 67.1 (9.5) | <0.01 |
| Sleep-Related Impairment | 52.6 (11.8) | 57.4 (7.8) | 62.1 (12.6) | 0.03 |
| Epworth Sleepiness Scale (n=59) | 6.8 (4.9) | 7.6 (3.5) | 8.1 (4.5) | 0.64 |
|
| ||||
| Patient Reported Sleep (n=45), n (%) | ||||
| Sleep Disturbance | 51.2 (9.3) | 55.5 (7.3) | 61.1 (10.3) | 0.02 |
| Sleep-Related Impairment | 49.3 (10.1) | 53.0 (8.9) | 57.8 (9.3) | 0.06 |
n=61 unless otherwise noted
Sleep walking and snoring
systemic immunosuppressants, methotrexate (1 mild, 2 moderate, 3 severe), mycophenolate mofetil (2 moderate), cyclosporine (1 mild, 1 severe), dupilumab (1 severe)
Bold P-values indicate statistically significant at P<0.05
SD=standard deviation; CDLQI=Children’s Dermatology Life Quality Index; NRS=Numerical Rating Scale
Reliability of PROMIS sleep measures in pediatric AD
High Cronbach’s alpha coefficients were found for the parent-proxy and child reported sleep disturbance measures (0.90 and 0.92, respectively) and parent and child reported sleep-related impairment (0.94 and 0.93, respectively).
Convergent Validity with Other Assessment Measures
As shown in Table 2, patient report and parent-proxy-reported PROMIS measures were compared to a long-used legacy measure of sleepiness (ESS), objective sleep, and disease-specific assessments to assess convergent validity. ESS was a poor assessment of SDi in AD. PROMIS parent-proxy measures had a relatively high correlation with patient report on SDi and SRI (r=0.67, p<0.01 and 0.55, p<0.01, respectively). Parents tended to report sleep disturbance as more severe than reported by the children (Figure 1a). PROMIS SDi measures also correlated with disease-specific assessments and itch (Table 2).
Table 2.
Correlations of the Parent- and Child-Reported Sleep Disturbance and Sleep-Related Impairment T-scores with other measures.
| Parent-Proxy-Reported Sleep | Patient Reported Sleep | Objective Sleep | POEM | Disease Specific | Itch | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sleep Disturbance | Sleep-Related Impairment | Epworth Sleepiness Scale | Sleep Disturbance | Sleep-Related Impairment | WASO | Sleep Efficiency | Onset Latency | Total Score | Sleep Question | EASI | CDLQI | NRS | ||
| Parent-Proxy-Report | Sleep Disturbance (n=61) | 1 | 0.57** | 0.20 | 0.67** | 0.60** | 0.28* | −0.25 | 0.18 | 0.51** | 0.52** | 0.41** | 0.58** | 0.48** |
| Sleep-Related Impairment (n=61) | 0.57** | 1 | 0.31* | 0.45** | 0.55** | 0.21 | −0.24 | 0.21 | 0.46** | 0.35** | 0.32* | 0.60** | 0.45** | |
| Epworth Sleepiness Scale (n=59) | 0.20 | 0.31* | 1 | 0.08 | 0.29 | −0.14 | 0.13 | −0.03 | 0.10 | 0 | 0.04 | 0.01 | 0.08 | |
| Patient Report | Sleep Disturbance (n=45) | 0.67** | 0.45** | 0.08 | 1 | 0.71** | 0.34* | −0.30* | 0.07 | 0.44** | 0.41** | 0.32* | 0.55** | 0.32* |
| Sleep-Related Impairment (n=45) | 0.60** | 0.55** | 0.29 | 0.71** | 1 | 0.22 | −0.17 | −0.08 | 0.43** | 0.29 | 0.27 | 0.37* | 0.24 | |
| Objective Sleep | WASO (n=61) | 0.28* | 0.21 | −0.14 | 0.34* | 0.22 | 1 | −0.89** | 0.34** | 0.29* | 0.40** | 0.53** | 0.17 | 0.18 |
| Sleep Efficiency (n=61) | −0.25 | −0.24 | 0.13 | −0.30* | −0.17 | −0.89** | 1 | −0.61** | −0.31* | −0.40** | −0.49** | −0.22 | −0.23 | |
| Onset Latency (n=61) | 0.18 | 0.21 | −0.03 | 0.07 | −0.08 | 0.34** | −0.61** | 1 | 0.24 | 0.21 | 0.17 | 0.15 | 0.18 | |
| POEM | Total Score (n=61) | 0.51** | 0.46** | 0.10 | 0.44** | 0.43** | 0.29* | −0.31* | 0.24 | 1 | 0.80** | 0.48** | 0.69** | 0.63** |
| Sleep Question (n=61) | 0.52** | 0.35** | 0 | 0.41** | 0.29 | 0.40** | −0.40** | 0.21 | 0.80** | 1 | 0.61** | 0.66** | 0.72** | |
| Disease Specific | EASI (n=61) | 0.41** | 0.32* | 0.04 | 0.32* | 0.27 | 0.53** | −0.49** | 0.17 | 0.48** | 0.61** | 1 | 0.42** | 0.49** |
| CDLQI (n=43) | 0.58** | 0.60** | 0.01 | 0.55** | 0.37* | 0.17 | −0.22 | 0.15 | 0.69** | 0.66** | 0.42** | 1 | 0.71** | |
| Itch | NRS (n=59) | 0.48** | 0.45** | 0.08 | 0.32* | 0.24 | 0.18 | −0.23 | 0.18 | 0.63** | 0.72** | 0.49** | 0.71** | 1 |
WASO=Wake After Sleep Onset; EASI=Eczema Area and Severity Index; NRS=Numerical Rating Scale; CDLQI=Children’s Dermatology Life Quality Index
p≤0.05
p≤0.01
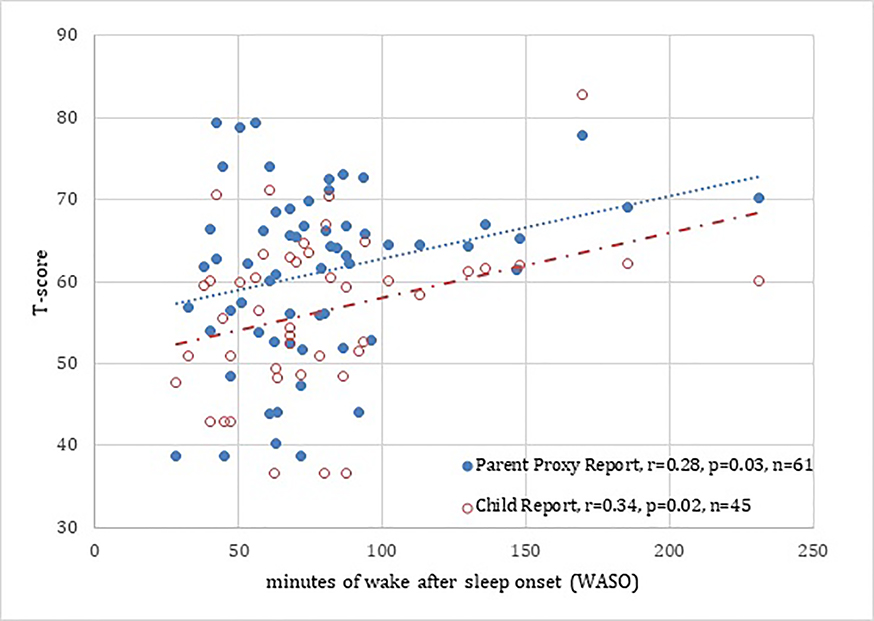
Figure 1.
Correlation of Parent Proxy/Child reported Sleep Disturbance with objective actigraphy, minutes of Wake After Sleep Onset (WASO) in children with atopic dermatitis.
There is a mild/moderate correlation between parent proxy/child reported sleep disturbance versus objective sleep disturbance. Parents tended to report sleep disturbance as more severe than children. The parent/patient-reported measures of sleep disturbance capture a distinct domain of the sleep experience in children with atopic dermatitis.
Convergent Validity with Objective Sleep
Figure 1 depicts the relationship between objective actigraphy (WASO and sleep efficiency) and PROMIS sleep disturbance. We found a mild/moderate correlation between parent-proxy and child reported SDi with objective sleep disturbance (r=0.28, p=0.03 and 0.34, p=0.02, respectively). Interestingly, objective sleep (WASO) had the largest correlation with clinician-assessed disease severity by EASI, specifically with WASO (r= 0.53, p< 0.01) (Table 2).
Screening for Sleep Disturbance Clinically
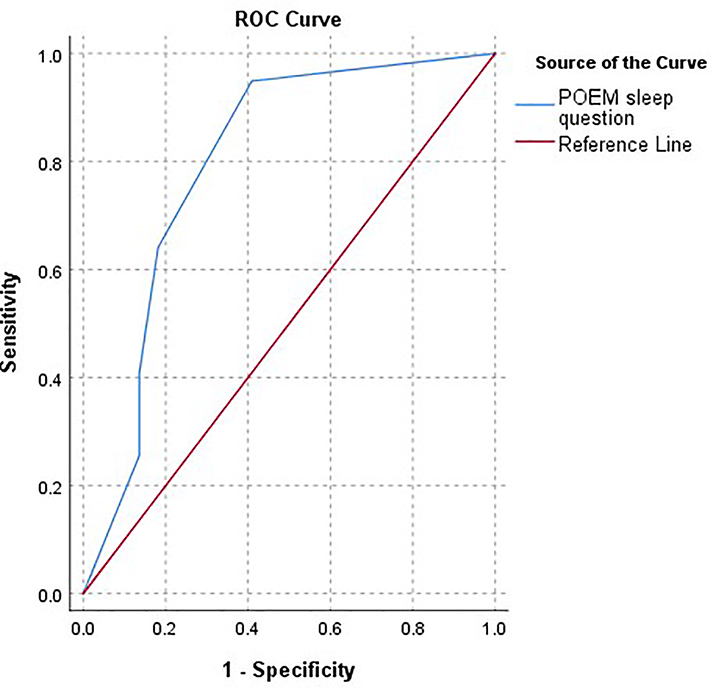
Given that POEM can be used to define severity and includes a sleep question, we chose to evaluate its assessment capability as a screen for significant sleep disturbance. Indeed, the POEM sleep question performs well as a screen for significant sleep disturbance on PROMIS parent-proxy SDi measure (T-score ≥ 60), with high sensitivity (94.9%) but low specificity (37.2%). In fact, POEM was a better screen than the CDLQI sleep question (sensitivity 87.9% and specificity 65.0%). Although the POEM sleep question only had a moderate correlation with WASO (r=0.40, p<0.01), it was a sensitive screen for objective sleep disturbance (measured by WASO of ≥76.4 minutes) (sensitivity 92.3% and specificity 37.1%). An ROC curve was also computed and depicts the overall sensitivity/specificity of the POEM sleep question in predicting PROMIS SD scores. Accuracy of the POEM sleep questions for PROMIS-assessed SD is fair to good (AUC 0.798) (Figure 2). In Figure 3, we propose a schematic for sleep screening in children with AD.
Figure 2.
Receiver Operating Characteristic (ROC) Curve for POEM sleep question to predict PROMIS SD score ≥60
The blue line depicts the sensitivity/specificity of the 5 possible responses on the POEM sleep question to detect a PROMIS SD score ≥60. Patients/parents reporting sleep has been disturbed 5–6 days per week or every day, had high sensitivity for a PROMIS SD score ≥60. High specificity was only obtained once patients reported to have nightly sleep disturbance on POEM.
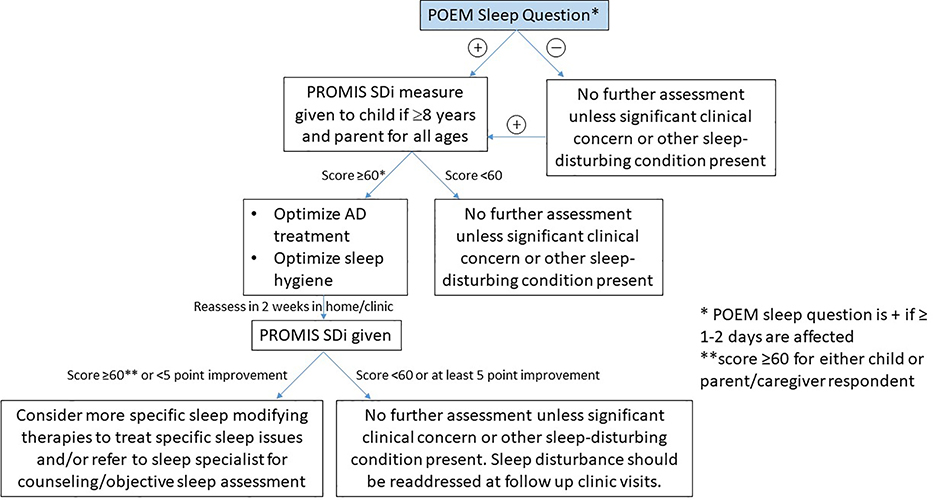
Figure 3.
Clinical screening algorithm for sleep disturbance in pediatric AD.
Score of 60 refers to 1 SD greater than a T-score of 50 on the PROMIS SD measure; POEM=Patient Oriented Eczema Measure; SD= Sleep Disturbance; PROMIS= Patient Reported Outcome Measurement Information System. POEM sleep questions: Over the last week, on how many nights has your sleep been disturbed because of the eczema?” with possible responses of, no days, 1–2 days, 3–4 days, 5–6 days or every day. A response was considered positive for sleep disturbance if ≥ 1–2 days was reported.
Discussion
Sleep disturbance in children with AD can be accurately measured via patient/parent-proxy-report using the new PROMIS Pediatric or Parent-Proxy Short Form v.1.0 Sleep Disturbance 8-item tool, or by objective assessment measures, such as actigraphy and polysomnography. SDi by patient or parent-proxy report has only a mild/moderate-sized correlation with actigraphy-assessed minutes of WASO (r=0.34 and r=0.28, respectively, p<0.05 for both). This is consistent with other patient/parent-reported sleep assessments that often find no or mild-sized correlation with objective polysomnography or actigraphy.9,22 This is because patient or parent-proxy report of sleep reflects the lived experience of sleep. Objective sleep assessment provides detail on sleep timing, minutes of wake onset, sleep efficiency, sleep fragmentation; assessments that are not accurately obtained via self-reported questionnaires. On the other hand, patient-reported outcomes capture the individual’s evaluation of the quality of their sleep and how sleep affects their daily functioning. Importantly patient/parent report of sleep disturbance, in contrast to objective assessment, more accurately reflects the key symptom of itch and overall quality of life.
Overall, we found that sleep disturbance worsens with increasing disease severity and the PROMIS SDi measure by both patient and parent report had high reliability and convergent validity with various disease specific measures, such as itch NRS, CLDQI, POEM and EASI. The PROMIS SRI measure also had high reliability and validity and can be used clinically to obtain more information on sleep-related impairment in children with AD.
As far as screening for sleep disturbance, both the POEM sleep question or CDLQI sleep question can serve as sensitive screening tools for sleep disturbance in AD as assessed by a PROMIS SDi T-score >1 standard deviation (≥60) from the mean (sensitivity 94.9% and 87.9%, respectively). As shown in Figure 2, changing score cut-offs could also increase specificity. Given POEM correlates better with physician assessed disease severity than CDLQI, and is shorter,23 our screening algorithm recommends the POEM sleep question to serve as a screen for SD. The low specificity of the POEM sleep question for significant PROMIS assessed SDi (T-score ≥ 60) and objective sleep disturbance (WASO≥76.4 minutes) suggests that the 8-question PROMIS Pediatric or Parent-Proxy Sleep Disturbance Short Form is needed to gather more detailed information on patient/parent-proxy report of sleep disturbance, or potentially objective sleep assessment depending on the clinician’s concerns.
Our group and others have published on treatment options and algorithms for sleep disturbance in AD.1,24 Here we propose a new clinical screening algorithm. Given the significant cost associated with objective sleep disturbance assessment by polysomnography or actigraphy and the importance of patient/parent-proxy assessment,25 our screening algorithm starts with a clinical screen using the POEM sleep question. If positive, we recommend that the PROMIS Pediatric/Parent-Proxy Sleep Disturbance measure be administered to quantify the burden of sleep disturbance. PROMIS Pediatric/Parent-Proxy Sleep-Related Impairment and CDLQI instruments can also be administered if more detail is desired about the impact of sleep disturbance on daytime functioning (sleep-related impairment) or quality of life (CDLQI). Given that parents tend to report more severe sleep disturbance than patients, we recommend administering the PROMIS sleep disturbance measure to both parent and child (if ≥8 years old) and just parent if 5–7 years old. PROMIS sleep measures specific to children <5 years old are under development. If either parent/child-assessment provides a T-score ≥60, optimization of AD therapy and sleep hygiene should be addressed.1 Close follow-up is recommended (in ~2–4 weeks) to assess for improvement in sleep disturbance via repeat administration on the PROMIS sleep disturbance measure which can be completed in clinic or at home (score should be below 60 and/or improve by at least 5 points) (personal communication with A. Carle, December 2019). Additionally, 5 points is considered a meaningful effect size across many PROMIS measures in disease conditions, including the adult PROMIS sleep measure.26 If adequate improvement in SDi is not noted, more tailored sleep interventions or referral to a sleep specialist is recommended. Objective sleep assessment should be considered at any time if sleep does not improve despite good disease control and sleep hygiene interventions, or if specific concerns for comorbidities, such as severe OSA, remain.
Limitations
Future work will determine the relevance of the PROMIS Pediatric sleep measures in a broader geographic sample. To ensure our findings represented sleep disturbance related to AD, patients in our cohort had controlled asthma and allergic rhinitis. However, this limits our ability to develop a more complex screening algorithm for sleep disturbance in patients with comorbid and uncontrolled asthma/AR. Furthermore, the proposed sleep disturbance screening algorithm has not been prospectively tested. The algorithm proposed requires further testing for validation. We also did not assess change over time or test-retest reliability of the PROMIS Pediatric sleep measures.
Conclusion
PROMIS Pediatric/Parent-Proxy short forms for Sleep Disturbance and Sleep-Related Impairment had high levels of reliability and validity to assess sleep in AD. The PROMIS Pediatric SDi measure administered by patient or parent-proxy can be used in conjunction with the POEM sleep question to screen for sleep disturbance in AD. Objective sleep assessment is a distinct domain from patient/parent report of sleep disturbance and objective sleep assessment should be considered in those not responding to treatment for sleep disturbance in AD or in whom other sleep comorbidities are being considered.
Supplementary Material
Funding sources
Supported by Ann & Robert H. Lurie Children’s Hospital of Chicago, Northwestern University Practice Based Research Award; and the Agency for Healthcare Research and Quality (grant number K12HS023011 to AF). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
Conflicts of Interest: AF has investigator-initiated grants from Pfizer, served as a consultant to National Eczema Association, Regeneron/Sanofi, and helped produce atopic dermatitis CME for the France Foundation.
Abbreviations and Acronyms
- AD
atopic dermatitis
- CDLQI
Children’s Dermatology Life Quality Index
- EASI
Eczema Area and Severity Index
- ESS
Epworth Sleepiness Scale
- IRB
Institutional Review Board
- POEM
Patient-Oriented Eczema Measure
- PROMIS
Patient Reported Outcomes Measurement Information Systems
- PSQ
Pediatric Sleep Questionnaire
- SCORAD
SCORing Atopic Dermatitis
- SDi
PROMIS Pediatric Sleep Disturbance Tool
- SRI
PROMIS Pediatric Sleep-Related Impairment Tool
- NRS
Numerical Rating Scale
- WASO
Wake After Sleep Onset
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fishbein AB, Vitaterna O, Haugh IM, et al. Nocturnal eczema: Review of sleep and circadian rhythms in children with atopic dermatitis and future research directions. The Journal of allergy and clinical immunology. 2015;136(5):1170–1177. [DOI] [PubMed] [Google Scholar]
- 2.Fishbein AB, Mueller K, Kruse L, et al. Sleep disturbance in children with moderate/severe atopic dermatitis: A case-control study. Journal of the American Academy of Dermatology. 2018;78(2):336–341. [DOI] [PubMed] [Google Scholar]
- 3.Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. Journal of the American Academy of Dermatology. 2014;70(2):338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schneider L, Tilles S, Lio P, et al. Atopic dermatitis: a practice parameter update 2012. The Journal of allergy and clinical immunology. 2013;131(2):295–299.e291–227. [DOI] [PubMed] [Google Scholar]
- 5.Fishbein AB, Lin B, Beaumont J, Paller AS, Zee P. Nocturnal Movements in Children with Atopic Dermatitis have a Timing Pattern: A Case Control Study. Journal of the American Academy of Dermatology. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bender BG, Leung SB, Leung DY. Actigraphy assessment of sleep disturbance in patients with atopic dermatitis: an objective life quality measure. The Journal of allergy and clinical immunology. 2003;111(3):598–602. [DOI] [PubMed] [Google Scholar]
- 7.Buysse DJ. Sleep health: can we define it? Does it matter? Sleep. 2014;37(1):9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fries JF, Bruce B, Cella D. The promise of PROMIS: using item response theory to improve assessment of patient-reported outcomes. Clinical and experimental rheumatology. 2005;23(5 Suppl 39):S53–57. [PubMed] [Google Scholar]
- 9.Forrest CB, Meltzer LJ, Marcus CL, et al. Development and validation of the PROMIS Pediatric Sleep Disturbance and Sleep-Related Impairment item banks. Sleep. 2018;41(6). [DOI] [PubMed] [Google Scholar]
- 10.Forrest CB, Bevans KB, Tucker C, et al. Commentary: the patient-reported outcome measurement information system (PROMIS(R)) for children and youth: application to pediatric psychology. Journal of pediatric psychology. 2012;37(6):614–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Broderick JE, DeWitt EM, Rothrock N, Crane PK, Forrest CB. Advances in Patient-Reported Outcomes: The NIH PROMIS((R)) Measures. EGEMS (Washington, DC). 2013;1(1):1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meltzer LJ, Forrest CB, de la Motte A, Bevans KB. Clinical Validity of the PROMIS Pediatric Sleep Measures across Populations of Children with Chronic Illnesses and Neurodevelopment Disorders. Journal of pediatric psychology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charman CR, Venn AJ, Williams HC. The patient-oriented eczema measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Archives of dermatology. 2004;140(12):1513–1519. [DOI] [PubMed] [Google Scholar]
- 14.Hanifin JM. Diagnostic features of atopic dermatitis. Acta Derm Venereol (Suppl). 1980;92:44–47. [Google Scholar]
- 15.Grinich EE, Schmitt J, Kuster D, et al. Standardized reporting of the Eczema Area and Severity Index (EASI) and the Patient-Oriented Eczema Measure (POEM): a recommendation by the Harmonising Outcome Measures for Eczema (HOME) Initiative. The British journal of dermatology. 2018;179(2):540–541. [DOI] [PubMed] [Google Scholar]
- 16.Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Experimental dermatology. 2001;10(1):11–18. [DOI] [PubMed] [Google Scholar]
- 17.Li JC, Fishbein A, Singam V, et al. Sleep Disturbance and Sleep-Related Impairment in Adults With Atopic Dermatitis: A Cross-sectional Study. Dermatitis : contact, atopic, occupational, drug. 2018;29(5):270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. The British journal of dermatology. 1995;132(6):942–949. [DOI] [PubMed] [Google Scholar]
- 19.Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep medicine. 2000;1(1):21–32. [DOI] [PubMed] [Google Scholar]
- 20.MW J The assessment of sleepiness in children and adolescents. Sleep Biol Rhythms. 2015;13(Suppl 1): 97. [Google Scholar]
- 21.Carter JV, Pan J, Rai SN, Galandiuk S. ROC-ing along: Evaluation and interpretation of receiver operating characteristic curves. Surgery. 2016;159(6):1638–1645. [DOI] [PubMed] [Google Scholar]
- 22.Erwin AM, Bashore L. Subjective Sleep Measures in Children: Self-Report. Frontiers in pediatrics. 2017;5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suh TP, Ramachandran D, Patel V, et al. Product of Investigator Global Assessment and body surface area (IGAxBSA): a practice-friendly alternative to the Eczema Area and Severity Index (EASI) to assess atopic dermatitis severity in children. Journal of the American Academy of Dermatology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang YS, Chiang BL. Sleep disorders and atopic dermatitis: A 2-way street? The Journal of allergy and clinical immunology. 2018;142(4):1033–1040. [DOI] [PubMed] [Google Scholar]
- 25.Silverberg JI, Yu S. Measuring Sleep Disturbance in Atopic Dermatitis: Patient-Reported Versus Objective Outcomes. Dermatitis : contact, atopic, occupational, drug. 2017;28(5):328–329. [DOI] [PubMed] [Google Scholar]
- 26.Jensen RE, Moinpour CM, Potosky AL, et al. Responsiveness of 8 Patient-Reported Outcomes Measurement Information System (PROMIS) measures in a large, community-based cancer study cohort. Cancer. 2017;123(2):327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.