Abstract
Background
Physical activity has been associated with longer chronic disease-free life expectancy, but specific cancer types have not been investigated. We examined whether leisure-time moderate-to-vigorous physical activity (LTPA) and television (TV) viewing were associated with life expectancy cancer-free.
Methods
We included 14,508 participants without a cancer history from the Atherosclerosis Risk in Communities (ARIC) study. We used multistate survival models to separately examine associations of LTPA (no LTPA, < median, ≥ median) and TV viewing (seldom/never, sometimes, often/very often) with life expectancy cancer-free at age 50 from invasive colorectal, lung, prostate, and postmenopausal breast cancer. Models were adjusted for age, gender, race, ARIC center, education, smoking, and alcohol intake.
Results
Compared to no LTPA, participants who engaged in LTPA ≥ median had a greater life expectancy cancer-free from colorectal (men-2.2 years (95% confidence interval (CI) 1.7, 2.7), women-2.3 years (95% CI 1.7, 2.8)), lung (men-2.1 years (95% CI 1.5, 2.6), women-2.1 years (95% CI 1.6, 2.7)), prostate (1.5 years (95% CI 0.8, 2.2)), and postmenopausal breast cancer (2.4 years (95% CI 1.4, 3.3)). Compared to watching TV often/very often, participants who seldom/never watched TV had a greater colorectal, lung, and postmenopausal breast cancer-free life expectancy of ~1 year.
Conclusion
Participating in LTPA was associated with longer life expectancy cancer-free from colorectal, lung, prostate, and postmenopausal breast cancer. Viewing less TV was associated with more years lived cancer-free from colorectal, lung, and postmenopausal breast cancer.
Impact
Increasing physical activity and reducing TV viewing may extend the number of years lived cancer-free.
Introduction
Cancer contributes significantly to years lived with disability and to the risk of mortality.(1) In the United States (US), 16.9 million men and women have a cancer history and an estimated 1.8 million cases are projected to occur in 2020.(2) Prostate, breast (pre- and postmenopausal), lung and bronchus, and colorectal cancer are the most frequently diagnosed cancers in the US.(2) The number of years lived without cancer can be estimated with health expectancy outcomes, a measure that combines incidence and mortality to estimate life expectancy lived with and without disease.(3) Health expectancy outcomes can complement population-based measures, such as life expectancy, by estimating years of life lived in good health and lost to poor health.(3)
Physical activity has been associated with a lower risk of many types of cancer,(4–6) longer survival after cancer diagnosis,(6–8) and longer life expectancy.(9) Recent studies on physical activity and health expectancy outcomes have observed that greater amounts of physical activity were associated with longer life expectancy disease-free from cardiovascular disease (CVD),(10–14) diabetes,(15) and chronic disease (definitions varied by study but have included composite measures of coronary heart disease, stroke, diabetes, lung disease, cancer, asthma, and arthritis)(16–20) but no studies have separately examined specific cancer types. Sedentary behavior, conversely, has been associated with an increased risk of incident cancer(21) and all-cause mortality.(22) To date, sedentary behavior has not been examined in relation to cancer health expectancy outcomes.
Given the large population burden of cancer, we sought to understand how two health behaviors, physical activity and less sedentary behavior, could extend the years lived cancer-free. Therefore, we examined how physical activity and television (TV) viewing, a common type of sedentary behavior, were associated with life expectancy cancer-free at age 50 from four leading types of cancer - colorectal, lung, prostate, and postmenopausal breast cancer, in a population-based cohort of adults from the Atherosclerosis Risk in Communities (ARIC) study.
Methods
Study population
We used data from the ARIC study, a prospective cohort of 15,792 mostly White and African-American adults. Participants, age 44 – 66 years at Visit 1, were enrolled from four geographic areas in the US (Forsyth County, North Carolina; Jackson, Mississippi; Washington County, Maryland; and Minneapolis, Minnesota). ARIC study cohort members have participated in interviews, clinical examinations, and annual (semi-annual from 2012) telephone follow-up interviews over seven examination visits (Visit 1 1987–89, Visit 2 1990–92, Visit 3 1993–95, Visit 4 1996–98, Visit 5 2011–13, Visit 6 2016–17, and Visit 7 2018–19).(23) All participants provided informed written consent at each study visit. The ARIC study was approved by the Institutional Review Boards at all participating institutions.
For this analysis, we excluded participants who did not consent to non-cardiovascular disease research (n=149), those with a history of any type of cancer at baseline (n=902), and those who experienced cancer (colorectal, lung, prostate, or breast) or death within the first year of follow-up to reduce the likelihood of reverse causation (n=130). Due to small numbers of race/ethnic groups at ARIC sites, we also excluded Asian and American Indian/Alaskan Indian participants (Forsyth County n=21, Minnesota n=15, Washington County n=12) and African-American participants in Minnesota (n=22) and Washington County (n=33). After exclusions, 14,508 (92%) participants were included in analysis for colorectal and lung models, 6,582 men for prostate models, and 7,849 postmenopausal women for postmenopausal breast models (77 women were not included in breast cancer models because they died or developed breast cancer before reaching menopause).
Physical activity and sedentary behavior
Participants reported engaging in sport or exercise activities in the past year with the Baecke questionnaire at Visits 1 and 3.(24, 25) Participants could report up to four leisure-time physical activities. For each activity, participants were asked to report the number of hours/week and months/year they engaged in the activity. A metabolic equivalent of task (MET) value was assigned to each activity based on the Compendium of Physical Activities.(26) Activities with a MET ≥ 3 were classified as moderate-to-vigorous intensity.(27) MET hours per week (MET-h/week) spent in leisure-time moderate-to-vigorous physical activity (LTPA) at Visits 1 and 3 was categorized as none, < median (0.1 – <13.2 MET-h/week), and ≥ median (13.2+ MET-h/week). The cut point was based on the Visit 1 median value of MET-h/week among those reporting any LTPA. In a supplemental analysis, to further enhance the translation of our results, we classified participants as meeting the 2018 US physical activity guidelines of participating in at least 150 minutes/week of moderate intensity activity, or 75 minutes/week of vigorous intensity activity, or an equivalent combination.(27) We classified participants as no LTPA participation, LTPA less than guidelines (0.1 - < 7.5 MET-h/week), met guidelines (7.5 – < 15.0 MET-h/week), and doubled guidelines (15.0+ MET-h/week).
Sedentary behavior, any waking behavior that expends little energy expenditure (≤ 1.5 MET) while in a sitting or reclining posture,(28) was estimated with a question on TV viewing. Participants reported at Visit 1 how often they viewed TV and were classified as never/seldom, sometimes, and often/very often, due to small numbers in some categories.
Cancer and all-cause mortality outcomes
We focused on four leading types of cancer - colorectal, lung, prostate, and postmenopausal breast, that accounted for 59% of cancer diagnoses in ARIC. First primary invasive cancer diagnoses were ascertained by linkage with statewide cancer registries from 1987 through December 31, 2012 and supplemented by abstraction of medical records and hospital discharge codes. In addition to the cancer registry linkage, participants who self-reported a cancer diagnosis at an annual/semi-annual interview or at an ARIC study visit were contacted for further information and their medical records were abstracted to confirm a cancer diagnosis and tumor characteristics. Some of the state cancer registries were not complete or established at the start of the ARIC study, and for this time period, cancer cases were identified by surveillance of hospital discharge summaries in the ARIC study regions supplemented with medical record abstraction.(29) The ARIC Cancer Coordinating Center adjudicated all potential cases of colorectal, lung, prostate, and postmenopausal breast cancers.(29) For the purpose of exclusions, participants were considered to have prevalent cancer at Visit 1 if they said a doctor told them that they had cancer of any type.
Deaths were ascertained from Visit 1 until December 31, 2012 through active surveillance of vital status and by linkage with the National Death Index.(23, 30)
Covariate assessment
Confounders were selected based on a priori knowledge of the relationship of physical activity and sedentary behavior with cancer incidence and all-cause mortality.(5, 21, 31–35) Covariates measured at Visit 1 included age (continuous), gender (male, female), race by ARIC center (African-American Forsyth county, White Forsyth County, African-American Jackson, White Minneapolis, White Washington County), education (≤high school, vocational school, some college/college degree, graduate/professional), daily servings of red meat intake (continuous), and smoking pack-years (continuous) (Supplementary Methods). Time-varying covariates that were assessed at Visits 1 – 4 included: smoking status (current smoker, past smoker, never smoker), alcohol intake (not current drinker, ≤ 100 grams, > 100 grams), body mass index (BMI) (underweight/normal <25.0 kg/m2, overweight 25.0 - < 30 kg/m2, obese ≥ 30.0 kg/m2), diabetes (yes, no), and in women, use of menopausal hormone therapy (MHT) (never users, former users of any MHT type, current users of unopposed estrogen, current users of estrogen plus progestin) and postmenopausal status (premenopausal, postmenopausal) (Supplementary Methods).
For the analysis on postmenopausal breast cancer, we included only postmenopausal women. Women were considered postmenopausal at the ARIC visit in which they reported the following: 1) having both ovaries removed, or 2) if both ovaries had not been removed, they were not taking hormones and had not had a hysterectomy, then they were considered menopausal when they reported reaching menopause or having no periods in the past two years, or 3) if both ovaries had not been removed but women reported taking hormones, or had a hysterectomy, or did not know their menopause status, then they were considered menopausal when they reached the average cohort race- and smoking status- specific age when menopause was reached (White: never-48.3, former- 47.3 current-46.6; African American: never-47.8, former-47.0, current-45.6 years).
Statistical analysis
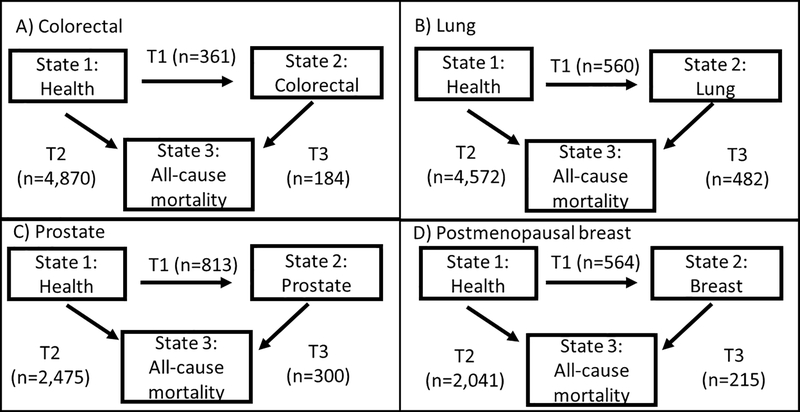
We used multistate Markov survival models to estimate how participants moved between health (state 1), disease (state 2), and all-cause mortality (state 3) states (Figure 1, Supplementary Methods and Supplementary Table S1). This model had three possible transitions – health to disease (T1), health to all-cause mortality (T2), and disease to all-cause mortality (T3). All participants started in the health state, free of cancer, and could move to the disease state (T1) or to death from any cause (T2). Participants who developed cancer could move from the disease state to the death state (T3). Separate models were specified with different disease states: colorectal, lung, prostate, and postmenopausal breast cancer. Transition-specific (T1, T2, T3) hazard ratios (HR) and 95% confidence intervals (CI) were estimated with the msm R package.(36, 37) The time scale for all models was age; participants started contributing time to the study at the age they entered at Visit 1. For breast cancer analyses, women started contributing time at the age of their first visit in which they were considered postmenopausal. Follow-up continued until death or end of study (December 31, 2012). For time-varying covariates, information from the visit closest in time preceding each type of transition was used for the specific transition. Exposures and covariates were used on all transitions (T1, T2, T3) and did not vary by type of transition.
Figure 1.
Three state multistate models used for analysis. Four sets of models were estimated separately for each cancer site – colorectal, lung, prostate, and postmenopausal breast cancer. In each type of model all participants started in the health state (free of cancer) and could move to state 2 if they developed the cancer under analysis or could move to state 3, death from any cause. Those who developed cancer could move from the cancer state to all-cause mortality from the cancer state. The arrows indicate transitions between each state and are labeled T1 (transition from state 1 to state 2), T2 (transition from state 1 to state 3), and T3 (transition from state 2 to state3). Once a participant entered a state they remained in that state until they experienced a transition or were censored at the end of study follow-up. The number of participants who experienced each type of transition for each of the four models is in parentheses.
Abbreviations: T= transition
Life expectancy, the expected average number of remaining years of life in health and disease states conditional on reaching age 50 and regardless of health status at age 50, was estimated with the R package Estimating Life Expectancies in Continuous Time (ELECT).(38) The ELECT package uses model parameters estimated from the multistate models. Total life expectancy was divided into life expectancy with and without disease. Confidence intervals were estimated using 1000 bootstrap samples. We focused on life expectancy cancer-free, which was derived from the T1 and T2 transitions from the multistate models and accounted for time from age 50 until incident cancer diagnosis, death, or end of study, whichever occurred first. We estimated life expectancy at age 50 in order to compare to studies on physical activity and life expectancy disease-free that all estimated life expectancy at age 50.(10–13, 15–18, 20) Across models, life expectancies were estimated at each level of LTPA (no LTPA, LTPA < median, and LTPA ≥ median) and TV viewing (often/very often, sometimes, seldom/never) while specifying other covariates used in analytic models to the baseline covariate distribution levels of the analytic cohort.
First, we specified models that separately examined LTPA and TV, and then specified models that included both LTPA and TV. For all models, we adjusted for age, gender, race by ARIC center, education, smoking, and alcohol intake. We conducted sensitivity analyses (Supplementary Methods) that included further adjustment for BMI (all models), MHT (breast cancer LTPA model), red meat intake (colorectal LTPA model), diabetes (colorectal LTPA model), smoking pack-years (lung LTPA model), restriction to never smokers (colorectal, prostate, and breast cancer LTPA models, lung cancer models would not converge possibly due to the small number of lung cancer cases among never smokers (n=10)), and exclusion of cases that occurred within 5 years of baseline (lung LTPA model).
Missing exposure and covariate data that occurred at each visit were imputed with Multiple Imputation by Chained Equations (Supplementary Methods).(39, 40) All analyses were carried out with SAS Version 9.4 (Cary, North Carolina) and R Version 3.3.2. Analyses were approved by the Institutional Review Board at the University of North Carolina at Chapel Hill.
Results
At baseline, the average age of the 14,508 participants was 54 years (SD 5.7), 55% were female, 28% were African-American, 56% had a high school education or less, most participants were not current smokers or current drinkers, and 89% of women were postmenopausal (Table 1). Over 60% of participants reported any LTPA and the median amount of LTPA at baseline was 13.2 MET-h/week. Close to half of participants reported watching TV sometimes.
Table 1.
Baseline characteristics of ARIC study participants, n=14,508.
| Leisure-time physical activity | TV viewing | Overall | |||||
|---|---|---|---|---|---|---|---|
| None | < median | ≥ median | Seldom/never | Sometimes | Often/ very often | ||
| N=5615 | N=4424 | N=4446 | N=2670 | N=6779 | N=5030 | N=14,508 | |
| Age at Visit 1, mean (SD) | 54.4 (5.7) | 54.4 (5.8) | 54.7 (5.8) | 54.0 (5.7) | 54.4 (5.7) | 54.9 (5.8) | 54.3 (5.7) |
| Male, n (%) | 2312 (41) | 1801 (41) | 2454 (55) | 1098 (41) | 2905 (43) | 2562 (51) | 6582 (45) |
| ARIC center, n (%) | |||||||
| Forsyth County, NC | 1175 (21) | 1207 (27) | 1297 (29) | 705 (26) | 1759 (26) | 1213 (24) | 3682 (25) |
| Jackson, MS | 2119 (38) | 784 (18) | 616 (14) | 400 (15) | 1634 (24) | 1485 (30) | 3536 (24) |
| Minneapolis, MN | 953 (17) | 1269 (29) | 1417 (32) | 845 (32) | 1644 (24) | 1150 (23) | 3640 (25) |
| Washington County, MD | 1368 (24) | 1164 (26) | 1116 (25) | 720 (27) | 1742 (26) | 1182 (24) | 3650 (25) |
| Race, n (%) | |||||||
| White | 3313 (59) | 3465 (78) | 3730 (84) | 2224 (83) | 4982 (74) | 3296 (66) | 10514 (73) |
| African-American | 2302 (41) | 959 (22) | 716 (16) | 446 (17) | 1797 (27) | 1734 (35) | 3994 (28) |
| Education, n (%) | |||||||
| high school or less | 3771 (67) | 2383 (54) | 1984 (45) | 1292 (49) | 3747 (55) | 3094 (62) | 8155 (56) |
| vocational | 414 (7) | 405 (9) | 385 (9) | 215 (8) | 566 (8) | 422 (8) | 1205 (8) |
| college | 1019 (18) | 1214 (28) | 1443 (33) | 818 (31) | 1752 (26) | 1106 (22) | 3679 (25) |
| graduate/professional | 399 (7) | 418 (10) | 627 (14) | 341 (13) | 705 (10) | 398 (8) | 1445 (10) |
| Missing | 12 | 4 | 7 | 4 | 9 | 10 | 24 |
| Leisure-time physical activity, n (%) | |||||||
| no LTPA | 849 (32) | 2603 (38) | 2161 (43) | 5615 (39) | |||
| < median | 805 (30) | 2042 (30) | 1575 (31) | 4424 (31) | |||
| ≥ median | 1016 (38) | 2134 (32) | 1294 (26) | 4446 (31) | |||
| Missing | 0 | 0 | 0 | 23 | |||
| TV viewing, n (%) | |||||||
| seldom/never | 849 (15) | 805 (18) | 1016 (23) | 2670 (18) | |||
| sometimes | 2603 (46) | 2042 (46) | 2134 (48) | 6779 (47) | |||
| often/very often | 2161 (39) | 1575 (36) | 1294 (29) | 5030 (35) | |||
| Missing | 2 | 2 | 2 | 29 | |||
| Smoking, n (%) | |||||||
| current smoker | 1803 (32) | 1058 (24) | 890 (20) | 543 (20) | 1650 (24) | 1557 (31) | 3760 (26) |
| past smoker | 1510 (27) | 1409 (32) | 1739 (39) | 867 (33) | 2153 (32) | 1638 (33) | 4665 (32) |
| never smoker | 2298 (41) | 1955 (44) | 1812 (41) | 1257 (47) | 2974 (44) | 1829 (36) | 6071 (42) |
| Missing | 4 | 2 | 5 | 3 | 2 | 6 | 12 |
| Alcohol intake, n (%) | |||||||
| not current drinker | 3774 (68) | 2707 (62) | 2348 (53) | 1621 (61) | 4182 (62) | 3020 (61) | 8835 (61) |
| ≤ 100 grams | 1100 (20) | 1124 (26) | 1342 (30) | 694 (26) | 1672 (25) | 1200 (24) | 3566 (25) |
| > 100 grams | 700 (13) | 574 (13) | 731 (17) | 344 (13) | 887 (13) | 774 (16) | 2007 (14) |
| Missing | 41 | 19 | 25 | 11 | 38 | 36 | 100 |
| BMI, mean (SD) | 28.7 (6.0) | 27.4 (5.2) | 26.9 (4.6) | 26.9 (5.0) | 27.7 (5.4) | 28.3 (5.6) | 27.8 (5.4) |
| Missing | 6 | 3 | 2 | 2 | 2 | 7 | 23 |
| Menopausal Hormone Therapy, n (%) | |||||||
| current user of unopposed estrogen | 409 (13) | 335 (13) | 276 (14) | 194 (13) | 512 (14) | 314 (13) | 1020 (14) |
| current user of estrogen + progestin | 103 (3) | 154 (6) | 165 (9) | 114 (8) | 220 (6) | 88 (4) | 422 (6) |
| never used | 2194 (70) | 1650 (66) | 1175 (62) | 983 (65) | 2440 (66) | 1592 (68) | 5019 (66) |
| former user of any type | 421 (14) | 380 (15) | 296 (16) | 222 (15) | 511 (14) | 364 (15) | 1097 (15) |
| Missing | 147 | 80 | 56 | 42 | 158 | 83 | 291 |
| Postmenopausal, n (%) | 2948 (90) | 2303 (89) | 1730 (88) | 1322 (85) | 3427 (89) | 2228 (91) | 6988 (89) |
| Missing | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Daily servings of red meat intake, mean (SD) | 1.2 (1.0) | 1.1 (0.8) | 1.0 (0.8) | 1.0 (0.8) | 1.1 (0.8) | 1.2 (1.0) | 1.1 (0.9) |
| Missing | 12 | 4 | 11 | 1 | 16 | 10 | 42 |
Abbreviations: BMI= body mass index, LTPA= leisure-time moderate-to-vigorous physical activity, MET= metabolic equivalent of task
LTPA categories: < median (0.1 – < 13.2 MET-h/week), ≥ median (13.2+ MET-h/week)
Over a median 23.6 years of follow-up, 2,360 (16.3%) participants had a diagnosis of incident invasive first primary colorectal, lung, prostate, or postmenopausal breast cancer and 5,054 (34.8%) deaths from all causes occurred in the full analytic cohort. The mean age at cancer diagnosis was 68.8 years (SD 7.5) for colorectal, 69.7 years (SD 7.3) for lung, 68.6 years (SD 6.3) for prostate, and 66.7 years (SD 7.3) for postmenopausal breast cancer. HRs for LTPA and TV viewing with each transition (T1, T2, T3) are in Supplementary Tables S2–S3.
LTPA and life expectancy cancer-free
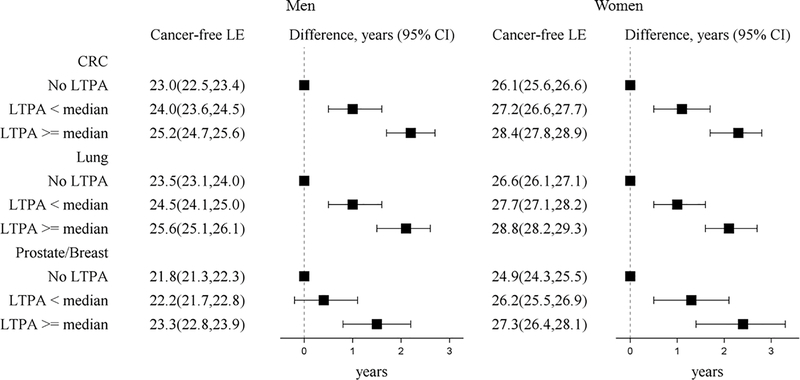
On average, participants could expect to live more than 20 years cancer-free given they were alive at age 50 and for each cancer type, life expectancy cancer-free was greater for women than men at each level of LTPA. Participation in LTPA showed a positive dose-response relationship with greater life expectancy cancer-free (Figure 2). Participants who engaged in LTPA < median had ~1 year greater life expectancy cancer-free from colorectal, lung, and breast cancer compared to participants who reported no LTPA. At LTPA ≥ median compared to none, participants had a greater life expectancy cancer-free of over 2 years from colorectal (men-2.2 years (95% CI 1.7, 2.7), women-2.3 years (95% CI 1.7, 2.8)), lung (men-2.1 years (95% CI 1.5, 2.6), women-2.1 years (95% CI 1.6, 2.7)), and breast cancer (2.4 years (95% CI 1.4, 3.3)), and over 1 year for prostate cancer (1.5 years (95% CI 0.8, 2.2)). Compared to no LTPA, life expectancy cancer-free was greater by ~1 year for LTPA less than the guidelines, ~1.4 years for meeting physical activity guidelines, and by ~2.4 years for doubling the guidelines for most cancers (Supplementary Table S4).
Figure 2. Life expectancy (years, 95% CI) cancer-free from four cancer types and life expectancy differences at age 50 by LTPA among ARIC participants (1987–2012).
LTPA categories included none, < median (0.1 - < 13.2 MET-h/week), and ≥ median (13.2+ MET-h/week). Models were specified separately for each type of cancer (colorectal cancer and lung cancer models N=14,508, prostate cancer models N=6582, and postmenopausal breast cancer models N=7849). Models were adjusted for age, gender, race by ARIC center, education, smoking status, and alcohol intake. Prostate and breast models did not include gender. Abbreviations: CI = confidence interval, CRC= colorectal cancer, LE= life expectancy, LTPA=leisure-time moderate-to-vigorous physical activity, MET= metabolic equivalent of task
TV viewing and life expectancy cancer-free
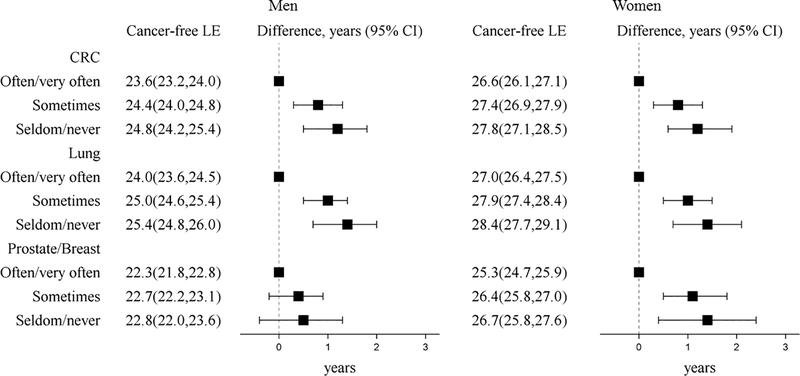
Life expectancy cancer-free was longer for viewing TV sometimes and seldom/never compared to often/very often for colorectal, lung, and breast cancer and was greater for women than men for each cancer type (Figure 3). Compared to often/very often viewing TV, viewing TV seldom/never was associated with a greater life expectancy cancer-free of ~1 year from colorectal (men-1.2 years (95% CI 0.5, 1.9), women-1.2 years (95% CI 0.6, 1.9)), lung (men-1.4 years (95% CI 0.7, 2.0), women-1.4 years (95% CI 0.7, 2.1)), and breast cancer (women-1.4 years (95% CI 0.4, 2.4)). However, for prostate cancer, seldom/never watching TV compared to often/very often was not associated with longer life expectancy cancer-free (0.5 years (95% CI −0.4, 1.3).
Figure 3. Life expectancy (years, 95% CI) cancer-free from four cancer types and life expectancy differences at age 50 by TV viewing among ARIC participants (1987–2012).
Models were specified separately for each type of cancer (colorectal cancer and lung cancer models N=14,508, prostate cancer models N=6582, and postmenopausal breast cancer models N=7849). Models were adjusted for age, gender, race by ARIC center, education, smoking status, and alcohol intake. Prostate and breast models did not include gender. Abbreviations: CI = confidence interval, CRC= colorectal cancer, LE= life expectancy, TV=television
Combined LTPA and TV viewing with life expectancy cancer-free
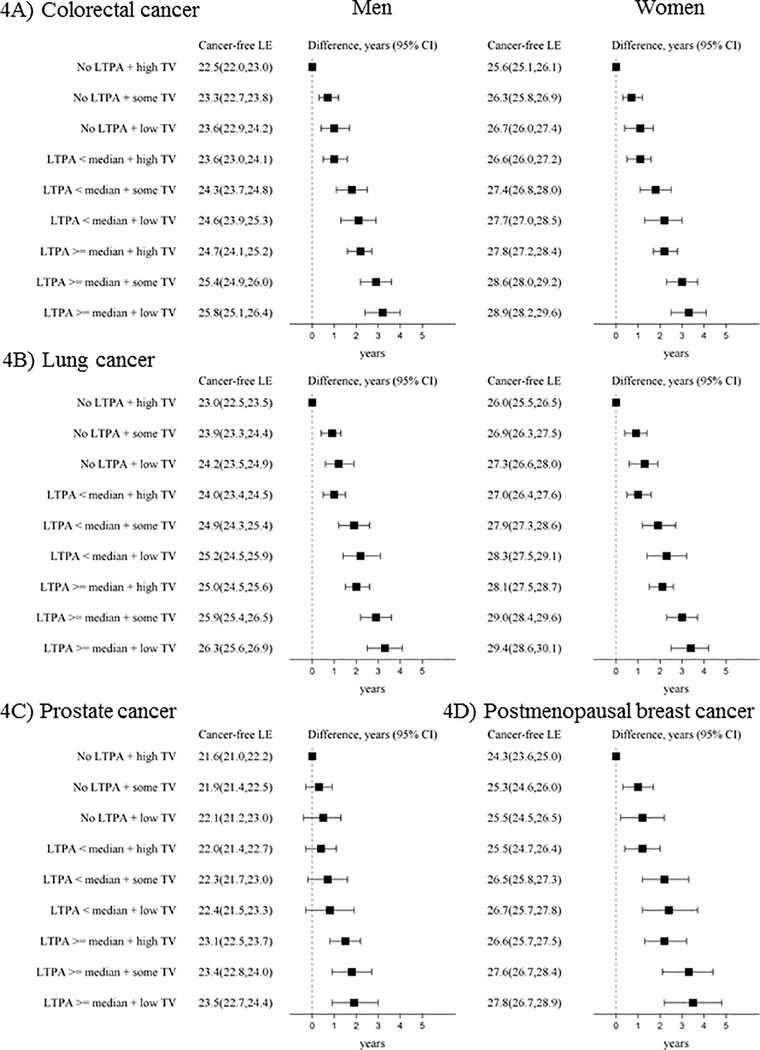
For all types of cancer, participating in more LTPA and viewing less TV were associated in a dose-response fashion with life expectancy cancer-free (Figure 4). For colorectal, lung, and breast cancer, life expectancy cancer-free was greater by ~3 years for engaging in LTPA ≥ median and seldom/never viewing TV compared to the referent group of no LTPA and often/very often viewing TV (colorectal: men-3.2 years (95% CI 2.4, 4.0), women-3.3 years (95% CI 2.4, 4.1), lung: men-3.3 years (95% CI 2.5, 4.1), women-3.4 years (95% CI 2.5, 4.2), breast: 3.5 years (95% CI 2.2, 4.8)). For prostate cancer, the life expectancy cancer-free was 2 years longer for LTPA ≥ median and seldom/never viewing TV (1.9 years (95% CI 0.9, 3.0)) compared to the referent group of no LTPA and often/very often viewing TV.
Figure 4. Life expectancy (years, 95% CI) cancer-free from A) colorectal cancer, B) lung cancer, C) prostate cancer, and D) postmenopausal breast cancer and life expectancy differences at age 50 by LTPA and TV viewing among ARIC participants (1987–2012).
LTPA categories included none, < median (0.1 - < 13.2 MET-h/week), and ≥ median (13.2+ MET-h/week). TV categories included high TV (viewing often/very often), some TV (sometimes viewing), and low TV (seldom/never viewing TV). Models were specified separately for each type of cancer (colorectal cancer and lung cancer models N=14,508, prostate cancer models N=6582, and postmenopausal breast cancer models N=7849). Models were adjusted for age, gender, race by ARIC center, education, smoking status, and alcohol intake. Prostate and breast models did not include gender. Abbreviations: CI = confidence interval, CRC= colorectal cancer, LE= life expectancy, LTPA=leisure-time moderate-to-vigorous physical activity, MET= metabolic equivalent of task, TV=television.
Sensitivity analyses
Life expectancy cancer-free was similar or modestly lower when further adjusting for BMI (all models), red meat or diabetes status (colorectal LTPA model), MHT (breast cancer LTPA model), smoking pack-years (lung cancer LTPA model), restricting to never smokers (colorectal, prostate, breast cancer LTPA models), or when excluding cases diagnosed within 5 years of baseline (lung cancer LTPA model) (Supplementary Tables S5–S8).
Discussion
In this population-based cohort of White and African-American adults, participation in any LTPA and viewing less TV were associated with extended life expectancy cancer-free from major common cancers. Participation in LTPA showed a positive dose-response relationship with life expectancy cancer-free, such that at each level of LTPA participation life expectancy cancer-free was greater by approximately one year. Compared to no LTPA participation, at LTPA < median and ≥ median life expectancy cancer-free was approximately one and two years greater, respectively. Viewing TV sometimes or seldom/never compared to often/very often was associated with a longer life expectancy cancer-free, but the magnitude of associations was more modest than those observed for LTPA. Life expectancy cancer-free findings were similar in magnitude for colorectal, lung, and breast cancer but the years lived disease-free were lower for prostate cancer compared to the other cancers.
Our findings are consistent with those of other studies on physical activity and chronic disease-free life expectancy. Higher levels of physical activity compared to lower levels or none have been associated with a greater life expectancy disease-free of 2–3 years for CVD,(10–12) 4 years for diabetes,(15) and 1 to 6 years for chronic disease.(16–18, 20) Similar to other reports,(10, 11, 13–16, 18, 20) life expectancy disease-free by level of physical activity was higher for women than men. Our work complements these studies by examining four leading cancer types. For colorectal, lung, and breast cancer, at LTPA < median, life expectancy cancer-free was approximately 1 year greater, compared to no LTPA participation. Also, for the same cancers, at LTPA ≥ median, the life expectancy estimates were 2 years greater compared to no LTPA. Physical activity is hypothesized to lower cancer risk by reducing adiposity, enhancing immune function, lowering levels of sex hormones, improving insulin sensitivity, and for colorectal cancer, by reducing bowel transit time.(41, 42)
We specified LTPA as MET-h/week to enhance the translation of our findings. One can reach 13.2 MET-h/week (the median level of LTPA in our sample) by taking a brisk walk (at 3.3 METs) for 48 minutes/day for 5 days of the week. Additionally, life expectancy cancer-free was greater by ~1.4 years for meeting the 2018 US physical activity guidelines and by ~2.4 years for doubling the guidelines for most cancers compared to no LTPA. One can reach the guidelines (7.5 MET-h/week) by taking a brisk walk for 28 minutes/day for 5 days of the week. Even at activity levels less than the guidelines, we observed greater life expectancy cancer-free of ~1 year, suggesting that physical activity levels lower than the recommendations were associated with more years lived cancer-free. According to a recent analysis with 2003–2006 NHANES data, the average MET-h/week of self-reported LTPA was 14.2 MET-h/week, but only 64% of adults age 40 and older reported participating in any LTPA.(43) Similar to the NHANES prevalence of engaging in any LTPA, 61% of our analytic sample reported participating in any LTPA. Based on our observational findings, it is likely that if population levels of physical activity increased, that more years of life would be spent in health rather than managing the morbidity related to certain cancer diagnoses.
For colorectal, lung, and post-menopausal breast cancer, viewing TV sometimes or seldom/never was associated with ~1 year gain in life expectancy cancer-free compared to often/very often watching TV. To our knowledge, no other studies on life expectancy disease-free have examined sedentary behavior, but the direction of our associations of TV viewing with cancer incidence and all-cause mortality are consistent with existing findings.(21, 44–50) Additionally, we found that life expectancy cancer-free was increased by ~3.5 years for seldom/never viewing TV and engaging in LTPA greater than or at the median compared to those who did no LTPA and often/very often viewed TV for most cancers. This finding suggests that engagement in multiple health promoting behaviors can further extend the years lived cancer-free.
Our study has a number of strengths, most importantly we used health expectancy metrics to examine how health behaviors influenced years lived cancer-free.(3) Presenting the number of years one is expected to live healthy, free of cancer, is likely to be of interest to many people and may be an effective motivational tool for behavior change. To estimate health expectancies, we used multistate survival models, which allow participants to move between multiple health states and handle the competing risk of disease events and death. Additionally, we observed the results from multiple sensitivity analyses to be robust to adjustment for many factors. We used time-varying exposures and covariates to update behaviors as they changed throughout follow-up and addressed missing exposure and covariate data with multiple imputation techniques. Although we used a time-varying measure of physical activity and a baseline measure of TV viewing, it is possible these behaviors changed after the last measurement of each.
Despite the strengths of our study, limitations should be considered. We did not account for physical activity from travel, occupation, and housework and it is possible that the strength of observed associations would be greater if activity from multiple domains were included. Additionally, our measure of sedentary behavior, TV viewing, does not include time spent sitting for work or commuting. Moreover, the TV question assessed frequency of viewing but not the number of hours. Both LTPA and TV viewing were self-reported; LTPA may be overestimated by self-report, however, the Baecke questionnaire has good to excellent reliability and acceptable validity.(51, 52) We may have selected a healthy cohort because all participants had to be free of prevalent cancer to be included in analysis. Cancer often develops at older ages and the older participants who were cancer-free and included in our analysis may have been healthier than participants who were excluded.
We have presented one of the few analyses on how physical activity and sedentary behavior are associated with years lived cancer-free, and future research in this area could extend our approach. We included self-reported physical activity and sedentary behavior from one domain each (leisure-time physical activity and TV viewing), but future research could be enhanced by including physical activity and sedentary behavior that occurs from multiple domains (transport, housework, and occupation). Furthermore, use of accelerometers would capture total time spent over the course of a day in physical activity and sedentary behavior. Although we examined the leading types of cancer, more cancer sites could be examined by pooling multiple cohort studies or conducting analysis among cancer consortiums in effort to increase the number of rare cancers. Our analysis was conducted with the ARIC cohort, which is one of the few studies on life expectancy disease-free that included a diverse population from four regions in the US. Prior to our analysis, the majority of findings on lifestyle behaviors with life expectancy disease-free were mostly from the Framingham Heart Study(11, 13, 15) and from European cohorts.(10, 14, 16, 17) Recent findings have included more cohorts from the US(18, 20) but future analysis should be conducted in diverse cohorts according to race/ethnicity, socioeconomic status, and geography to improve generalizability of findings. Finally, many prospective cohort studies could contribute to research on how lifestyle behaviors influence life expectancy cancer-free, as many studies have the necessary data (cancer incidence, mortality, and risk factors) and statistical methods to estimate life expectancy cancer-free are widely available.(18, 20, 36, 38)
Conclusion
Participating in LTPA and viewing less TV were associated with longer life expectancy cancer-free. Findings were similar for colorectal, lung, and postmenopausal breast cancer, but weaker for prostate cancer. Our results suggest that participating in more LTPA and viewing less TV may contribute to living more years free of colorectal, lung, prostate, and postmenopausal breast cancer.
Supplementary Material
Acknowledgements
The authors thank the staff and participants of the ARIC study for their important contributions.
We would like to thank Dr. Chirayath M. Suchindran for his guidance on multistate models and multistate life expectancies.
C. Cuthbertson was supported by a National Heart, Lung, and Blood Institute National Research Service Award (NRSA: T32-HL007055).
The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, and HHSN268201700004I. Studies on cancer in ARIC are also supported by the National Cancer Institute (U01 CA164975).
Cancer incidence data have been provided by the Maryland Cancer Registry, Center for Cancer Surveillance and Control, Maryland Department of Health, 201 W. Preston Street, Room 400, Baltimore, MD 21201. We acknowledge the State of Maryland, the Maryland Cigarette Restitution Fund, and the National Program of Cancer Registries (NPCR) of the Centers for Disease Control and Prevention (CDC) for the funds that helped support the availability of the cancer registry data.
The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest Disclosures
The authors declare no potential conflicts of interest.
References
- 1.Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T, Alizadeh-Navaei R, Allen C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: A systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2018;4(11):1553–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer facts & figures 2020. Published 2020. Retrieved from https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf. Accessed March 31, 2020.
- 3.Salomon JA, Wang H, Freeman MK, Vos T, Flaxman AD, Lopez AD, et al. Healthy life expectancy for 187 countries, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2144–62. [DOI] [PubMed] [Google Scholar]
- 4.Moore SC, Lee IM, Weiderpass E, Campbell PT, Sampson JN, Kitahara CM, et al. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med. 2016;176(6):816–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity and cancer: A global perspective. Continuous update project expert report 2018. Published 2018. Retrieved from https://www.wcrf.org/sites/default/files/Summary-of-Third-Expert-Report-2018.pdf. Accessed May 1, 2019.
- 6.McTiernan A, Friedenreich CM, Katzmarzyk PT, Powell KE, Macko R, Buchner D, et al. Physical activity in cancer prevention and survival: A systematic review. Med Sci Sports Exerc. 2019;51(6):1252–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedenreich CM, Neilson HK, Farris MS, Courneya KS. Physical activity and cancer outcomes: A precision medicine approach. Clin Cancer Res. 2016;22(19):4766–75. [DOI] [PubMed] [Google Scholar]
- 8.Friedenreich CM, Stone CR, Cheung WY, Hayes SC. Physical activity and mortality in cancer survivors: A systematic review and meta-analysis. JNCI Cancer Spectr. 2020;4(1):pkz080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore SC, Patel AV, Matthews CE, Berrington de Gonzalez A, Park Y, Katki HA, et al. Leisure time physical activity of moderate to vigorous intensity and mortality: A large pooled cohort analysis. PLoS Med. 2012;9(11):e1001335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Doherty MG, Cairns K, O’Neill V, Lamrock F, Jorgensen T, Brenner H, et al. Effect of major lifestyle risk factors, independent and jointly, on life expectancy with and without cardiovascular disease: Results from the Consortium on Health and Ageing Network of Cohorts in Europe and the United States (CHANCES). Eur J Epidemiol. 2016;31(5):455–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franco OH, de Laet C, Peeters A, Jonker J, Mackenbach J, Nusselder W Effects of physical activity on life expectancy with cardiovascular disease. Arch Intern Med. 2005;165(20):2355–60. [DOI] [PubMed] [Google Scholar]
- 12.Cuthbertson CC, Tan X, Heiss G, Kucharska-Newton A, Nichols HB, Kubota Y, et al. Associations of leisure-time physical activity and television viewing with life expectancy free of nonfatal cardiovascular disease: The ARIC study. J Am Heart Assoc. 2019;8(18):e012657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nusselder WJ, Franco OH, Peeters A, Mackenbach JP. Living healthier for longer: Comparative effects of three heart-healthy behaviors on life expectancy with and without cardiovascular disease. BMC Public Health. 2009;9:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhana K, Koolhaas CM, Berghout MA, Peeters A, Ikram MA, Tiemeier H, et al. Physical activity types and life expectancy with and without cardiovascular disease: The rotterdam study. Journal of Public Health. 2016;39(4):e209–e18. [DOI] [PubMed] [Google Scholar]
- 15.Jonker JT, De Laet C, Franco OH, Peeters A, Mackenbach J, Nusselder WJ. Physical activity and life expectancy with and without diabetes: Life table analysis of the Framingham Heart Study. Diabetes Care. 2006;29(1):38–43. [DOI] [PubMed] [Google Scholar]
- 16.Leskinen T, Stenholm S, Aalto V, Head J, Kivimaki M, Vahtera J. Physical activity level as a predictor of healthy and chronic disease-free life expectancy between ages 50 and 75. Age Ageing. 2018;47(3):423–9. [DOI] [PubMed] [Google Scholar]
- 17.Stenholm S, Head J, Kivimaki M, Kawachi I, Aalto V, Zins M, et al. Smoking, physical inactivity and obesity as predictors of healthy and disease-free life expectancy between ages 50 and 75: A multicohort study. Int J Epidemiol. 2016;45(4):1260–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Schoufour J, Wang DD, Dhana K, Pan A, Liu X, et al. Healthy lifestyle and life expectancy free of cancer, cardiovascular disease, and type 2 diabetes: Prospective cohort study. BMJ. 2020;368:l6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nyberg ST, Singh-Manoux A, Pentti J, Madsen IEH, Sabia S, Alfredsson L, et al. Association of healthy lifestyle with years lived without major chronic diseases. JAMA Intern Med. 2020;180(5):760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaninotto P, Head J, Steptoe A. Behavioural risk factors and healthy life expectancy: Evidence from two longitudinal studies of ageing in england and the US. Sci Rep. 2020;10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmid D, Leitzmann MF. Television viewing and time spent sedentary in relation to cancer risk: A meta-analysis. J Natl Cancer Inst. 2014;106(7). [DOI] [PubMed] [Google Scholar]
- 22.Chau JY, Grunseit AC, Chey T, Stamatakis E, Brown WJ, Matthews CE, et al. Daily sitting time and all-cause mortality: A meta-analysis. Plos One. 2013;8(11):e80000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The Atherosclerosis Risk in Communities (ARIC) study: Design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 24.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936–42. [DOI] [PubMed] [Google Scholar]
- 25.Folsom AR, Arnett DK, Hutchinson RG, Liao F, Clegg LX, Cooper LS. Physical activity and incidence of coronary heart disease in middle-aged women and men. Med Sci Sports Exerc. 1997;29(7):901–9. [DOI] [PubMed] [Google Scholar]
- 26.Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR Jr., Tudor-Locke C, et al. 2011 compendium of physical activities: A second update of codes and MET values. Med Sci Sports Exerc. 2011;43(8):1575–81. [DOI] [PubMed] [Google Scholar]
- 27.U.S. Department of Health and Human Services. Physical activity guidelines for Americans, 2nd edition. Published 2018. Retrieved from https://health.gov/paguidelines/second-edition/pdf/Physical_Activity_Guidelines_2nd_edition.pdf. Accessed July 24, 2019.
- 28.Sedentary Behaviour Research Network. Letter to the editor: Standardized use of the terms “sedentary” and “sedentary behaviours”. Appl Physiol Nutr Metab. 2012;37(3):540–2. [DOI] [PubMed] [Google Scholar]
- 29.Joshu CE, Barber JR, Coresh J, Couper DJ, Mosley TH, Vitolins MZ, et al. Enhancing the infrastructure of the Atherosclerosis Risk in Communities (ARIC) study for cancer epidemiology research: ARIC cancer. Cancer Epidemiol Biomarkers Prev. 2017;27(3):295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) study: Methods and initial two years’ experience. J Clin Epidemiol. 1996;49(2):223–33. [DOI] [PubMed] [Google Scholar]
- 31.Wu Y, Zhang D, Kang S. Physical activity and risk of breast cancer: A meta-analysis of prospective studies. Breast Cancer Res Treat. 2013;137(3):869–82. [DOI] [PubMed] [Google Scholar]
- 32.Wolin KY, Yan Y, Colditz GA, Lee IM. Physical activity and colon cancer prevention: A meta-analysis. Br J Cancer. 2009;100(4):611–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Hu F, Li D, Wang F, Zhu L, Chen W, et al. Does physical activity reduce the risk of prostate cancer? A systematic review and meta-analysis. Eur Urol. 2011;60(5):1029–44. [DOI] [PubMed] [Google Scholar]
- 34.Zhong S, Ma T, Chen L, Chen W, Lv M, Zhang X, et al. Physical activity and risk of lung cancer: A meta-analysis. Clin J Sport Med. 2016;26(3):173–81. [DOI] [PubMed] [Google Scholar]
- 35.2018 Physical Activity Guidelines Advisory Committee. 2018. Physical Activity Guidelines Advisory Committee scientific report. Published 2018. Retrieved from https://health.gov/paguidelines/second-edition/report.aspx. Accessed July 24, 2019.
- 36.Jackson CH. Multi-state models for panel data: The msm package for R. Journal of Statistical Software. 2011;38(8):1–28. [Google Scholar]
- 37.Jackson CH. Multi-state modelling with R: The msm package. Retrieved from https://cran.r-project.org/web/packages/msm/vignettes/msm-manual.pdf. Published 2016. Accessed July 24, 2019.
- 38.van den Hout A ELECT: Estimation of life expectancies using continuous-time multi-state survival models. Retrieved from http://www.ucl.ac.uk/~ucakadl/ELECT/ELECTManual_version0_1_2.pdf. Published 2014. Accessed February 20, 2017.
- 39.Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: What is it and how does it work? Int J Methods Psychiatr Res. 2011;20(1):40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30(4):377–99. [DOI] [PubMed] [Google Scholar]
- 41.McTiernan A Mechanisms linking physical activity with cancer. Nat Rev Cancer. 2008;8(3):205–11. [DOI] [PubMed] [Google Scholar]
- 42.Friedenreich CM, Neilson HK, Lynch BM. State of epidemiological evidence on physical activity and cancer prevention. European Journal of Cancer. 2010;46(2593–2604). [DOI] [PubMed] [Google Scholar]
- 43.Evenson KR, Wen F, Herring AH. Associations of accelerometry-assessed and self-reported physical activity and sedentary behavior with all-cause and cardiovascular mortality among US adults. Am J Epidemiol. 2016;184(9):621–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou Y, Zhao H, Peng C. Association of sedentary behavior with the risk of breast cancer in women: Update meta-analysis of observational studies. Ann Epidemiol. 2015;25(9):687–97. [DOI] [PubMed] [Google Scholar]
- 45.Lynch BM, Friedenreich CM, Kopciuk KA, Hollenbeck AR, Moore SC, Matthews CE. Sedentary behavior and prostate cancer risk in the NIH-AARP Diet and Health Study. Cancer Epidemiol Biomarkers Prev. 2014;23(5):882–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ukawa S, Tamakoshi A, Wakai K, Noda H, Ando M, Iso H. Prospective cohort study on television viewing time and incidence of lung cancer: Findings from the Japan Collaborative Cohort Study. Cancer Causes Control. 2013;24(8):1547–53. [DOI] [PubMed] [Google Scholar]
- 47.Lam TK, Moore SC, Brinton LA, Smith L, Hollenbeck AR, Gierach GL, et al. Anthropometric measures and physical activity and the risk of lung cancer in never-smokers: A prospective cohort study. Plos One. 2013;8(8):e70672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Howard RA, Freedman DM, Park Y, Hollenbeck A, Schatzkin A, Leitzmann MF. Physical activity, sedentary behavior, and the risk of colon and rectal cancer in the NIH-AARP Diet and Health Study. Cancer Causes Control. 2008;19(9):939–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morris JS, Bradbury KE, Cross AJ, Gunter MJ, Murphy N. Physical activity, sedentary behaviour and colorectal cancer risk in the UK Biobank. Br J Cancer. 2018;118(6):920–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun JW, Zhao LG, Yang Y, Ma X, Wang YY, Xiang YB. Association between television viewing time and all-cause mortality: A meta-analysis of cohort studies. Am J Epidemiol. 2015;182(11):908–16. [DOI] [PubMed] [Google Scholar]
- 51.Richardson MT, Ainsworth BE, Wu HC, Jacobs DR Jr., Leon AS. Ability of the Atherosclerosis Risk in Communities (ARIC)/Baecke questionnaire to assess leisure-time physical activity. Int J Epidemiol. 1995;24(4):685–93. [DOI] [PubMed] [Google Scholar]
- 52.Jacobs DR Jr., Ainsworth BE, Hartman TJ, Leon AS. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med Sci Sports Exerc. 1993;25(1):81–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.