Abstract
Prostate cancer remains the most common non-skin cancer and second leading cause of death among men in the United States. Although progress has been made in diagnosis and risk assessment, many clinical questions remain regarding early identification of prostate cancer and management. The early detection of aggressive disease continues to provide high curative rates if diagnosed in a localized state. Unfortunately, prostate cancer displays significant heterogeneity within the prostate organ and between individual patients making detection and treatment strategies complex. Although prostate cancer is common among men, the majority will not die from prostate cancer, introducing the issue of over-treatment as a major concern in clinical management of the disease. The focus of the future is to identify those at highest risk for aggressive prostate cancer and to develop prevention and screening strategies, as well as, discerning the difference in malignant potential of diagnosed tumors. The Prostate Cancer Research Group of the National Cancer Institute’s Early Detection Research Network has contributed to the progress in addressing these concerns. This summary is an overview of the activities of the group.
Keywords: cancer, biomarkers, prostate
INTRODUCTION
Prostate Cancer and Early Detection
Prostate cancer remains the most commonly diagnosed non-cutaneous cancer in the United States and the second most common cause of cancer death in men (1). The 4-year survival rate for local regional disease is >99%, but if a man is diagnosed with distant disease his 4-year survival rate drops to 30% (1). Two large randomized controlled trials evaluating the effect of prostate cancer screening on mortality have demonstrated a reduction in the risk of death by 20–40% in those undergoing screening (2,3). Despite the potential benefits, considerable concern remains regarding overdiagnosis and ultimately overtreatment in the screened population (4). The concern for potential harm served as the impetus for the United States Preventive Services Task Force (USPSTF) to recommend against screening in 2012 (5) and later advise on informed decision making between physician and patients to decide on screening (6). A focus of the National Cancer Institute’s Early Detection Research Network (NCI-EDRN) is to allow data-driven discussions during the patient-provider interactions that guide individualized, informed decision making. Given the grim statistics of metastatic prostate cancer, the NCI-EDRN Prostate Cancer Research Group focuses on actionable biomarkers that can be utilized in early stage settings to prevent progression by early intervention. Important targets of the group include the identification of aggressive, potentially lethal, cancer at an early stage and providing biomarker-driven decisional support to maximize benefit and minimize harm in prostate cancer treatment. Other targets include the identification of known prostate cancer at risk for progression and metastasis or conversion to castration-resistant prostate cancer.
The EDRN Prostate Cancer Research Group
The EDRN network research activities are leveraged toward specific cancer types through organ-site collaborative teams. The Prostate Cancer Research Group is composed of the investigators that comprise major components and associate member programs, as well as prostate cancer expertise not directly funded through the NCI. In the current cycle there are three Biomarker Development Laboratories (BDL), two of these programs focus on protein-based biomarkers and one fucuses on nucleic acid-based biomarkers. There are also two Clinical Validation Centers (CVC), three Biomarker Reference Laboratories (BRL), statistical expertise from the Data Management Consulting Center (DMCC) and investigator teams supported through the EDRN Associate Membership program. Critical patient perspective and advocacy is provided through regular participation by the president of the National Association of State Prostate Cancer Coalitions. The collaborative group also includes representation from industry. All members of collaborative group participate in monthly video conference to build shared network expertise, discuss research progress and evaluate programmatic activities. The group develops core projects to support critical research needs that are then reviewed by the full steering committee of the EDRN. The core projects serve to focus the research activities across the collaborative group. For the prostate cancer research group, many of the core projects have focused on the development of unique reference sets. These references set are designed to assist in the validation of new biomarkers.
One of the most valuable aspects of the collaborative group is the establishment of biomarker development goals within the collective expertise of the team, which consists of patient advocate, clinicians, epidemiologists and basic scientists. In developing this focus, the team has considered the current clinical workflow within which decisions are made regarding clinical care and management of disease (Figure 1). Diagnosis of prostate cancer is initiated through screening and confirmed through subsequent biopsy. This is a critical junction for decision-making and an area of considerable focus of the EDRN. A positive diagnosis demands accurate risk stratification between active surveillance and treatment. A negative biopsy leads to more vigilant follow-up involving repeat biopsies and risk of associated complications. Thus, early and accurate assessment of disease is critical in the clinical care continuum for prostate cancer.
Figure 1: EDRN Prostate Group Biomarker Schema.
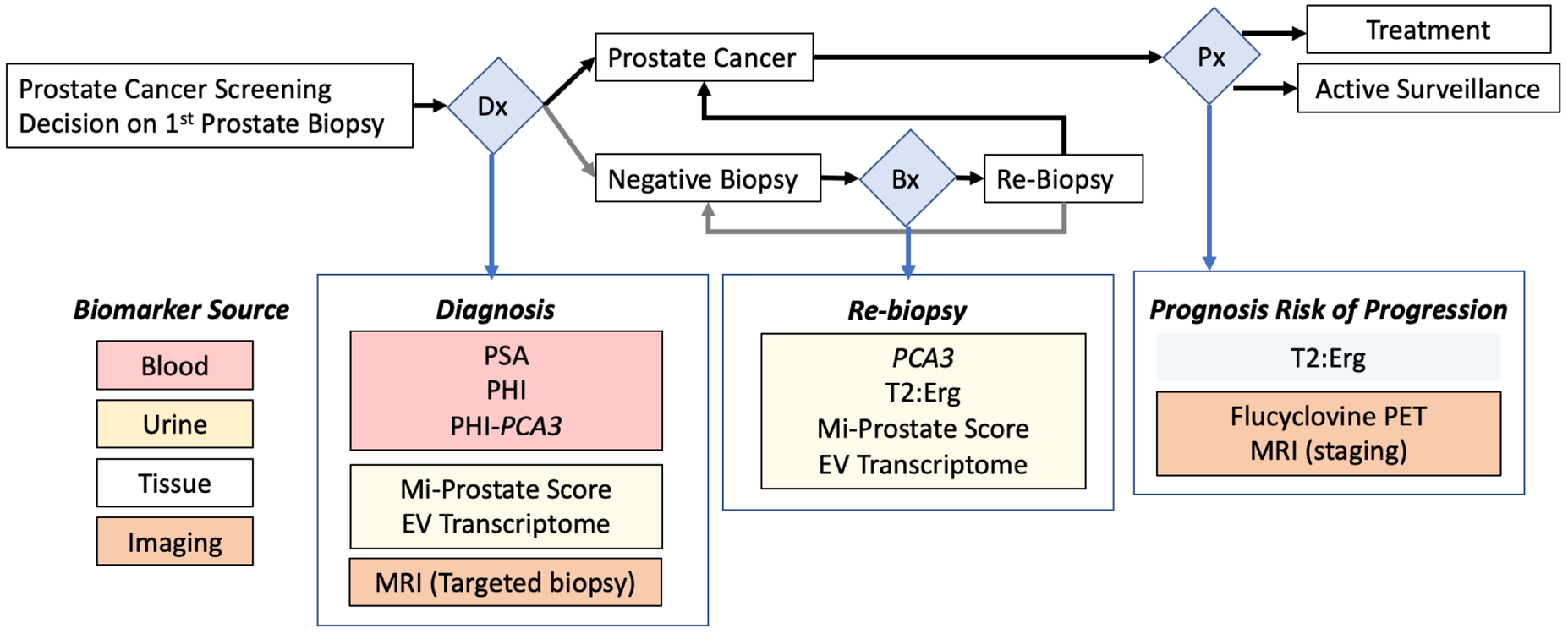
Flow-chart describing decision points (blue diamonds) leading to EDRN supported biomarkers. Shown are diagnosis (Dx), Biopsy (Bx) and Prognosis (Px). The listed biomarkers are examples of EDRN-developed tools that highlight the prostate collaborative priorities for critical clinical care decision making. The clinical specimen type from which the biomarker is derived is color coded and indicated on the lower left.
A Successful Approach to Biomarker Development
Historically, potential biomarkers from independent laboratories were common and necessary to provide the initial leads on prostate biomarkers. However, in the absence of a supportive infrastructure, biomarker development would often stall at this point. The EDRN provides resource and expertise infrastructure to assist biomarker development from laboratory discovery through clinical validation. The EDRN Prostate collaborative group serves as the connection point between biomarker discovery (BDL), assay refinement (BRL) and performance validation (CVC) within statistically appropriate study design (DMCC). In an effort to facilitate the development process the Prostate Cancer Research Group has assembled critical patient cohort reference sets for biomarker validation (7). Investigators are guided to the appropriate EDRN component to assist with the development phase of biomarkers. Once the biomarker performance achieves a level of accuracy and consistency, as verified through the DMCC, a larger targeted validation study involving EDRN BRL, CVC and DMCC is developed. Sensitivity and specificity goals required for success are clearly established and common pitfalls of biomarker development such as cost, clinical utility and implementation are considered. EDRN specifically seeks to address these issues when a biomarker request is examined by the task force and steering committee. Cost prohibitive tests need to be considered carefully and strategies can be discussed to investigate the same pathway by alternative means. The EDRN’s network of clinical partners, agency collaborations and industry relationships facilitate this validation process.
DISCUSSION
Refining Existing Blood and Urine Biomarkers to Enhance Detection of Aggressive Prostate Cancer
Since the advent of serum prostate-specific antigen (PSA) screening in the early 1990s, prostate cancer mortality in the U.S. has decreased by almost 50% (1,8,9). Despite conflicting evidence, the United States Preventive Task Force currently gives prostate cancer screening a “C” grade and emphasizes the discussion of risks and benefits of screening. Nonetheless, screening, diagnosis, and treatment of early stage prostate cancer has generated intense scientific and public debate as population screening with PSA increases detection of both lethal and non-lethal cancers (10). These shortcomings promote over-detection; the diagnosis of screen-detected indolent prostate cancer that, left untreated, would otherwise not result in morbidity or mortality. Over-detection needlessly exposes patients to the risks of prostate biopsy and the anxiety of a cancer diagnosis (10). The prostate cancer early detection paradigm using PSA screening of men at risk for prostate cancer and higher serum PSA concentrations to prompt prostate biopsy has remained unchanged for decades (11,12). Traditionally about 70% of men undergoing prostate biopsy are diagnosed; approximately 30% of which are clinically significant (13–15). Over-detection of low-risk prostate cancer often leads to over-treatment resulting in significant side effects of monitoring programs, surgery and radiation therapy.
The EDRN Prostate Cancer Group has spent considerable effort over the last two decades in developing novel and clinically useful biomarkers (see Table 1). One of the earliest efforts by the collaborative was to evaluate the combination of PSA, free PSA and the [−2] form of proenzyme PSA (pro2PSA) to develop the Prostate Health Index (PHI). In European studies, in men with a PSA between 2 and 10 ng/ml, PHI performed significantly better than PSA and the ratio of free PSA and total PSA (%free PSA) in detecting prostate cancer in general (16). However, that and other pioneering work with PHI (17), did not discern whether PHI measurement could be used to reduce the over-detection of indolent prostate cancer – which is recognized as a pivotal flaw of PSA screening for prostate cancer. The collaborative group then undertook a sequence of studies evaluating how pro2PSA could enhance effectiveness of detecting aggressive prostate cancer, i.e., those cancers having histopathological Grade Group II or higher (Gleason score greater than 6), while reducing over-detection of indolent Grade Group I cancers. The EDRN collabroative group found that PHI had an AUC of 0.73 in a large cohort of community-dwelling men undergoing regular prostate cancer screening (18), and that use of PHI to select men for initial prostate biopsy significantly improved the specificity of detecting cancers having Grade Group 2 or higher (19–21).
Table 1.
EDRN Prostate Collaborative Group Biomarker Development Achievements
| Biomarker | EDRN Role | Clinical Utility | Outcome/Status |
|---|---|---|---|
| Prostate Health Index (PHI) (17) | Validation | Early Detection Prior to Initial Biopsy | FDA-approved |
| ProPSA (20) | Validation | Early Detection Prior to Initial Biopsy | FDA-approved |
| TMPRSS2:Erg Fusion (T2:Erg) (28) | Discovery, Validation | Early Detection Prior to Initial or Repeat Biopsy | CLIA-compliant*/Commercially Deployed |
| Urine PCA3, T2:Erg (e.g. MIPs) (75) | Validation | Early Detection Prior to Initial or Repeat Biopsy | FDA-approved (PCA3); CLIA (T2:Erg) |
| Tissue/Urine RNA-Seq (76) | Discovery to Clin Assay | Early Detection Biomarker Development | CLIA-compliant |
| MiCheck (77) | Validation | Early Detection Prior to Initial or Repeat Biopsy | CLIA-compliant |
| Mitochondrial Deletion (78) | Discovery, Validation | Early Detection Biomarker Development (biopsy) | CLIA-compliant |
| Decipher (SChLAP1) (79) | Discovery, Validation | Salvage Radiation Therapy After Prostatectomy | CLIA-compliant |
| GSTP1 (80) | Validation | Early Detection Biomarker Development (prostatectomy) | CLIA-compliant |
| Urine Transcriptome (32) | Discovery to Clin Assay | Early Detection Biomarker Development | CLIA-compliant |
| Flucyclovine PET (81) | Validation | Preoperative Staging | Expanded Indication |
Indicates the assay/test performance meet CLIA guidelines
In another series of studies that advanced the clinical deployment of a previously discovered prostate cancer biomarker, the collaborative group evaluated the long, non-coding transcript prostate cancer antigen 3 (PCA3), as a biomarker for prostate cancer detection. Fifteen years after its discovery as a non-coding transcript associated with prostate cancer, PCA3 had been developed as a marker for selecting which men with prior negative biopsy should undergo repeat prostate biopsy (22,23). However, the role of PCA3 in selection of men for initial prostate biopsy remained elusive. The collaborative group completed a prospective trial of collecting urine and blood prior to prostate biopsy, to refine/reduce unnecessary biopsy or overdiagnosis among men with elevated PSA or abnormal digital rectal examine (DRE). These efforts established a new paradigm for using biomarkers to predict cancers having Grade Group 2 or higher, while avoiding biopsy of men predicted to have Grade Group 1 or no cancer (24). Trial outcomes showed utility of PCA3 for this purpose, and subsequent studies showed that adding the PCA3 urine test to the Prostate Cancer Prevention Risk Calculator (25) improved the AUCs (95% confidence intervals) for predicting high-grade cancer from 69.6% (65.6% to 73.7%) to 76.3% (72.7% to 79.9%).
Biomarker Development Approaches
The prostate collaborative group has leveraged state-of-the-art omics tools to analyze clinical tissues in discovery of disease-specific clinical decision-targeted biomarkers. We provide an overview of current methodologies spanning from genomic to proteomic biomarker targets. In addition to deploying high throughput platforms for biomarker discovery, EDRN BDLs provide expertise in data analysis that is being leveraged to maximize biomarker development through a multi-omics framework to build comprehensive data models of prostate cancer from which to refine biomarker panels for streamlined validation.
The rationale for a network structure was to integrate discovery, assay refinement and validation. One example of streamlining the pathway from discovery to clinical translation via EDRN partnership was the targeting of the transmembrane protease serine 2:v-ets erythroblastosis virus E26 oncogene homolog (TMPRSS2:ERG or T2:Erg) fusion as a biomarker for prostate cancer detection. Leveraging the discovery of T2:Erg (26), the BDL partnered with CVC investigators to evaluate whether this gene rearrangement could be detected in urine to refine prostate cancer detection. Initial pre-validation studies showed that combing T2:Erg with PCA3 performed better than urinary PCA3 alone or serum PSA without urinary testing in predicting aggressive prostate cancer (27,28). Subsequent multi-center validation analysis following Prospective Randomized Open Blinded End-Point design (of locking predictive rule parameters before validation analysis) affirmed that combining T2:Erg measurement with PCA3 in post-DRE urine, using a Clinical Laboratory Improvements Amendments (CLIA) certified, commercially scalable platform enhanced specificity of detecting aggressive prostate cancer by one-third. This strategy provided a means of reducing unnecessary prostate biopsy and mitigating over-detection of indolent prostate cancer, while enhancing identification of men with aggressive disease for which treatment is advisable (29). This paradigm led to the development of two commercially available assays that are now in widespread clinical use (28,30). Building upon this success, EDRN investigators and international collaborators identified the extracellular vesicle fraction of urine collected after DRE was suitable for detection strategies targeting the entirety of the prostate cancer-associated transcriptome. The subsequently developed clinical assay targets the next generation of multiplex urinary transcript to further refine prostate cancer detection (31,32).
The collaborative team has also developed powerful data visualization methodology to help link independent molecular analysis as recently highlighted in a study focused on curable intermediate risk disease in which a cohort of tumors were analyzed by genomic, epigenomic and proteomic approaches (33). The study revealed a previously unrealized pivotal role of ETS-fusion events in driving numerous proteogenomic pathways. Performance analysis of study biomarker events clearly revealed the value of combined multi-omics approaches and demonstrated that multi-modal assays consistently outperform single modality measures. This same group also sought to define the relationship between genomic risk loci and epigenomic changes in prostate cancer, revealing AKT1 expression as predictive of relapse (34). Understanding the complex network of proteogenomic factors that drive disease will help focus biomarker discovery toward development of effective tools to improve clinical decision making.
Proteomics Based Biomarker Development
The Prostate Collaborative Group is well represented by expertise in the application of proteomics to biomarker development. There are two BDLs employing innovative strategies in proteomics supported by two CVCs and one BRL. Each BDL has developed approaches for discovery, verification and validation with a clear path toward clinical assay development that maximizes the network resources. Significant advances in mass spectrometry over the past 20 years have accelerated the field of clinical proteomics toward greater capacity and rigor in discovery, as well as achieving unprecedented accuracy/precision targeted quantitation (35). There has also been greater attention paid to developing analytical strategies tailored to the analysis of tumor and tumor-proximal fluids that have accelerated discovery through reduction in processing associated with protein analysis. Many of these advances in the application of proteomics technologies have been leveraged by the group with the goal of developing biomarkers for the early identification, stratification and management of disease.
Discovery Approaches
Directly assessing human prostate tissues encompassing tumor and non-tumor partitions is a clearly logical strategy for biomarker discovery. This is true whether the eventual application of the disease-specific biomarker relies on pathological assessment of tissue or measurement in body fluids as reasoned that tumor biology is a leading source of disease-specific changes. Earlier efforts at tissue-based proteomic analysis have been limited to fresh or fresh-frozen samples and were plagued with variable tissue pathology and low protein identification content (36). The bulk of these obstacles have been overcome with improved tissue lysis and proteolytic digestion methods that allow for comprehensive identification of proteins from Formalin-Fixed Paraffin-Embedded (FFPE) tissues and retain annotation by a clinical pathologist (37–40). Significant expansion in the proteome space available for interrogation has been achieved through improvements in mass spectrometry instrumentation in signal resolution, analytical speed, fragmentation and downstream data analysis. Speed in this case refers to the ability to select ions for subsequent analysis in LC-MS/MS workflows and has a direct impact on the assessable volume of a targeted proteome. Strategies to capture more physical data from a single analysis include data independent acquisition (DIA) that bypasses the data dependent selection criteria used in typical LC-MS/MS (41,42). One such strategy termed Sequential Window Acquisition of all THeoretical Mass Spectra (SWATH-MS) defines mass windows within which all data is acquired and subsequently analyzed resulting in unprecedented numbers of proteins identified in a defined proteome (43,44). A recent application of SWATH-MS to prostate cancer involved a novel modification that allowed for targeting glycoproteins derived from tissues that offered insight into potential markers of aggressive disease (45). The developers of this innovative approach co-leads one of the prostate collaborative BDLs. The biomarker development strategy from this team focusses on employing comprehensive SWATH-MS analysis of tumor tissues to discovery of protein biomarkers that stratify with disease. The most promising candidates are targets for antibody-based assays for the appropriate tissue or fluids assessment.
A separate but complimentary approach to prostate cancer biomarker discovery targets tumor-proximal fluids (46,47). In this strategy, direct expressed prostatic secretions (EPS) are tumor-proximal fluid from which enrichment of prostate tumor specific proteins has been demonstrated. An additional attractive feature to utilization of EPS in biomarker discovery is the ability to conveniently collect EPS in urine following digital rectal exam (DRE) and thus a readily available tumor-proximal clinical assay fluid. The use of post-DRE urine as a biomarker source was pioneered by researchers in the EDRN prostate collaborative and has been an area of successful transition to clinical utility (48–50). A comprehensive effort to mine EPS from disease stratified cohorts is being conducted by this same BDL following an approach this team demonstrated to be successful in identification of potential biomarkers of aggressive disease (46). This approach involves large scale discovery with Orbitrap class LC-MS/MS instrumentation coupled with a rapid 96-well based processing method (51). The combined approach allows for reproducible identification of over 3,000 high confidence proteins from 200 ul of unfractionated EPS/post-DRE urine. The second phase leverages the discovery data to build targeted Parallel Reaction Monitoring (PRM-MS) assays for hundreds of candidate proteins so as to maximize the quantitation through subsequent validation study (52,53). The result has been the realization of unprecedented surveys of large statistically powered cohorts.
Targeted Verification/Validation Approaches
The traditional path toward measurement of protein expression as a clinical biomarker is the subsequent development of targeted immunoassays. Examples of the successful implementation of clinical immunoassays include current tests for serum PSA and Promark tissue-based assay for aggressive disease in low risk groups. This tried and true approach has been adopted by the Prostate Collaborative Group and is pursued either directly or in parallel utilizing the strengths of network laboratories with experience in building such assays. A particularly innovative strategy for incorporation of immunoassay into biomarker development workflow was recently described by an EDRN BDL/BRL collaborative team (54). This team incorporated a previous discovery of increased serum levels of fucosylated PSA in patients with aggressive disease (55). Using this a priori finding they developed a tandem immune-assay, lectin-based targeting of fucosyl residues and subsequent antibody-based targeting of PSA, that resulted in better discrimination between disease aggressiveness. The strength of this discrimination was observed to be in intermediate Gleason score 7 disease.
In many instances immunoassay reagents that specifically target protein-based biomarkers are either non-existent or ineffective. This is especially true as the field explores more nuanced proteome variability that extends beyond simple protein expression levels toward post-translational modifications, isoform selection, protein cleavage/processing/degradation, and functionally associated protein interactions. A promising technical approach for quantitative analysis of these events employs a targeted mass spectrometry methodology referred to as selected reaction monitoring [SRM, for review see(56)]. A variant of SRM, PRM-MS, leverages the recent advances in high resolution mass spectrometry to allow for SRM with parallel detection of all ion transitions in a single run. A major focus of the prostate collaborative BDLs is the incorporation of PRM-MS to improve assay stability. The approach involves a two phase biomarker development strategy in which discovery is conducted on appropriately powered retrospective cohorts, multi-analyte PRM-MS assays are built using the collected empirical data, and the resulting PRM-MS tools used in all subsequent validation efforts (46).
One advantage of the SRM/PRM-MS pipeline is the ability to readily incorporate biomarker discovery from outside the EDRN network to include in collaborative group validation efforts. For example, prostate collaborative group researchers employed high-Pressure high-Resolution separation with intelligent Selection and Multiplexing (PRISM)-SRM, that allows for unprecedented sensitivity without affinity enrichment (57), to evaluate over 50 candidate tissue-based gene expression markers for correlation with prostate cancer outcome. They employed PRISM-SRM to target the corresponding proteins and discovered a 5 protein panel that effectively predicted biochemical recurrence and metastasis in tumor tissue (58). This team is currently working to move this assay into body fluids and evaluate its potential utility in early detection of aggressive disease. Similarly, group members employed a PRM-MS strategy to develop a protein-based assay for the direct quantitation of genomic single nucleotide polymorphisms (SNP). One such SNP encodes a PSA variant (rs17632542) that results in a single amino acid change in the PSA protein associated with reduced relative secretion into blood and association with lower serum PSA levels (59,60). In a recent collaborative group study, investigators developed a PRM-MS assay that could accurately detect and quantify PSA wildtype and variant proteins in patient urine (61). Although genotyping is readily available, it is not routinely ordered and does not address the relationship between heterozygosity and protein expression. In addition, such protein-direct assays of the expression of genomic variants allows for research into the actual biological roles of the gene products.
Current Prostate Collaborative Group Core Studies
Upgrading of Men Diagnosed with Low-Risk Disease:
The majority of men with low-risk prostate cancer are currently being managed on active surveillance. Since prostate cancer is multi-focal, most prostate biopsies are conducted without knowledge of the location of the tumor. Although Magnetic Resonance Imaging (MRI) technologist can increase the accuracy of biopsies, patients and their families are often apprehensive that the biopsy may have missed most aggressive disease. This concern results in many men electing to undergo additional therapy, despite their low-grade cancer diagnosis. Their anxiety is clearly warranted as numerous studies find evidence of more aggressive disease in a subset of their patients (62–66). The prostate cancer can be either upgraded (i.e., the Gleason score is higher in the prostatectomy than in the biopsy) or upstaged (i.e., the TMN stage is high at time of surgery than was originally record). These studies have focused on retrospectively evaluating prostate cancer patients diagnosed with Gleason 6 disease who proceed to have a prostatectomy; the majority of the studies gathered clinical cases over a long period of time from a single institution. More than one of the studies has concluded that upgrading is associated with older age individuals (62,63,66); however, because they are retrospectively evaluated, the studies do not have matching biologics that could be used to identify biomarkers that predict upgrading/upstaging.
To assist in counseling men with low-risk prostate cancer, the EDRN prostate research group began gathering a cohort of men with low grade disease (defined as Gleason 6) who ultimately chose to have a prostatectomy. The goal of this cohort was to identify pre-therapeutic biomarkers (urine, serum and tissue) that could predict upgrading. These biological samples comprise the Upgrading Reference Set (URS) and have been recruited using EDRN core funds. The cohort enlists 10 clinical recruiting sites. In addition, we introduced a specific protocol for gathering patient urines, requiring that all of the pre-prostatectomy urines be gathered post-DRE. Another important aspect of the study was that all of the biopsies and prostatectomy specimens are centrally reviewed by a single pathology laboratory. We currently have over 80% of the URS reference set gathered with additional subjects consented but awaiting central pathology review.
In addition to having plasma, serum and urine on all of the subjects enrolled in URS, we have banked PBMCs that can be used for genetic studies. EDRN resources have been provided to isolate DNA from each subject and perform whole genome sequencing. This information will be useful for evaluating newly described polygenetic risk-score, such as those developed by the PRACTICAL consortium (67,68), and can also be used for evaluating men with DNA damage repair gene mutations such as BRCA2 and ATM, which have been shown to be associated with grade reclassification in men on active surveillance (69). We plan to bank both biopsy and prostatectomy specimens that can be evaluated with various omics technologies.
Evaluation of MRI Combined with Biomarkers Improves Detection of Aggressive Disease:
Unlike diagnostic biopsy for most other solid organ tumors, standard-of-care prostate biopsy has traditionally been performed without image guidance or selective targeting of suspected lesions. The systematically directed biopsies suffer from diagnostic inaccuracy, poor positive predictive value, and high false-negative rates. In addition, approximately 30% of men initially diagnosed with low-risk cancer on biopsy who undergo surgery are subsequently found to have aggressive tumors, indicating that standard prostate biopsy often fails to detect potentially lethal cancer (70). In the last several years, a number of commercial products have become available allowing the “fusion” of MRI images to prostate ultrasound, making it simple to biopsy MRI-detected lesions. A growing body of literature examining these ‘targeted’ fusion biopsies supports incremental value of fusion biopsy over standard template biopsy in the detection of clinically significant prostate cancer (71,72).
Modern multiparametric (mp)MRI of the prostate MRI conventionally uses three primary imaging sequences (T2 weighted, diffusion weighted, and dynamic contrast enhanced imaging). These three sequences are combined in a scoring system referred to as the Prostate Imaging Reporting And Data System (PIRADS) (73). For MRI-Ultrasound fusion targeted biopsies, the radiologist, using proprietary software, contours the suspicious lesions and records the PIRADS for each region of interest (ROI). The software links the MRI images to a live ultrasound at the time of a trans-rectal prostate biopsy. Once the images have been ‘fused’ digitally, the clinician can easily direct the needle into the MRI ROIs. Studies have identified significant diagnostic yield, upwards of 30% more high-grade prostate cancer, using this technique compared to a standard biopsy (71). The higher the PIRADs score, the greater the risk of identifying clinically significant cancer.
The commercialization of MRI fusion biopsies has resulted in a dramatic increase in the use of MRI imaging for prostate cancer. Given the ability of mpMRI to target a lesion, the role of laboratory biomarkers has been increasingly questioned. Conversely, how much MRI adds to the value of laboratory biomarkers has not been thoroughly investigated. The few studies published to date have suggested a role for a combination of blood, urine and imaging biomarkers (74).
We hypothesize that addition of prostate MRI fusion biopsy will significantly improve specificity for high-grade prostate cancer over PSA, PCA3,T2:Erg. The prostate collaborative has undertaking a systematic study of a range of biomarkers and their role given the expanding use of prostate MRI. The primary aim of this study is to see if the addition of prostate MRI to a panel including PSA, PCA3, T2:Erg will significantly improve specificity for high-grade prostate cancer. The other objectives of this cohort is to create an optimal panel of urine and blood biomarkers that will select those cases most likely to benefit from a MRI targeted biopsy. In addition, the study seeks to optimize MRI imaging to improve test performance, observe longitudinal changes, and to create a prospective reference sample set for future imaging and biomarker studies.
In order to accomplish these goals, we have initiated a multi-site, prospective, cross-discipline cohort study to investigate prostate MRI in the context of developed prostate cancer biomarkers. Subjects will have no previous biopsies and consent to prostate biopsy prior to MRI imaging (Figure 2A). The group consensus is that MRI may guide decisions regarding whether or not to obtain a prostate biopsy despite limited evidence to support this decision making. At the time of the biopsy, the 12-core systematic standard biopsy will be performed (Figure 2B), then the MRI lesion will be unmasked to the provider and patient. The provider will then obtain 2–3 targeted cores of the lesion specified by the radiologist using an MRI-Ultrasound Fusion guided technique (Figure 3). The unmasking process is important because the groups consensus is that if the target is performed first, that the target area could be avoided on the systematic biopsy. Avoiding the target area may provide a different area of tissue to examine but will not allow for robust comparisons between systematic and targeted biopsy because the systematic biopsy has been altered. The systematic biopsy may have detected the lesion without targeting if placed in the normal position. This study has begun recruitment and specimen accrual.
Figure 2: EDRN MRI Biomarker Study.
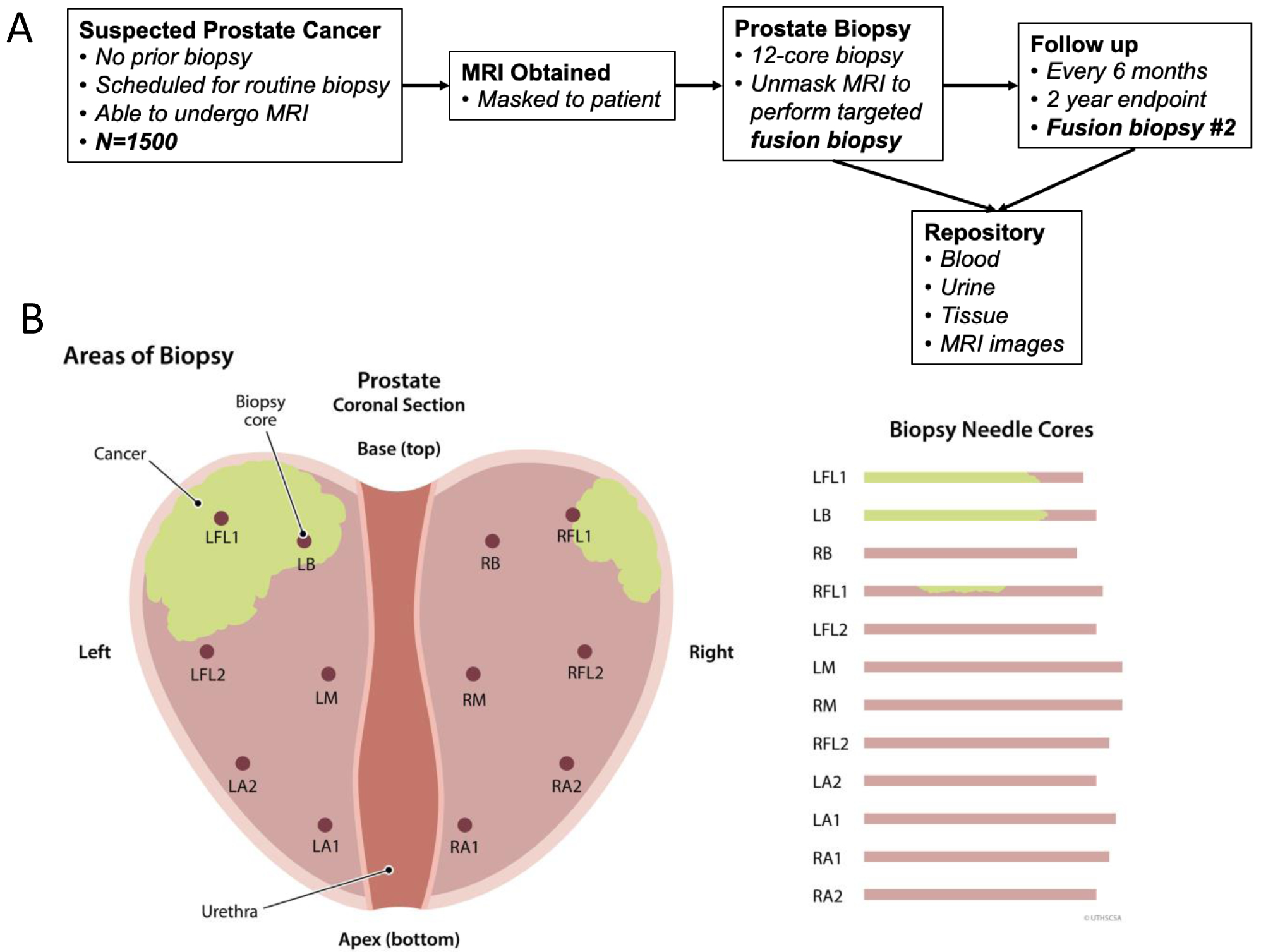
A) The most recent prospective clinical cohort developed by EDRN is the MRI Biomarker study, which is inserted at the diagnostic decision point in figure 1. Men scheduled for prostate biopsy will undergo a blinded MRI, systematic biopsy, then unblinded to MRI for a targeted biopsy. Full biomarker assessment will include blood, urine, tissue (via tissue prints), and imaging acquisition. B) A standard template systematic biopsy. The figure demonstrates the locations of a standard systematic biopsy usually directed toward the peripheral zone of the prostate. A major issue with standard systematic prostate biopsy is sampling error and allocating a cancer diagnosis in a subject that may have a false negative. Cancer (green) or more importantly high-grade can be missed by standard biopsy if located outside of the standard core template.
Figure 3: MRI-Ultrasound Fusion Targeted Prostate Biopsy.
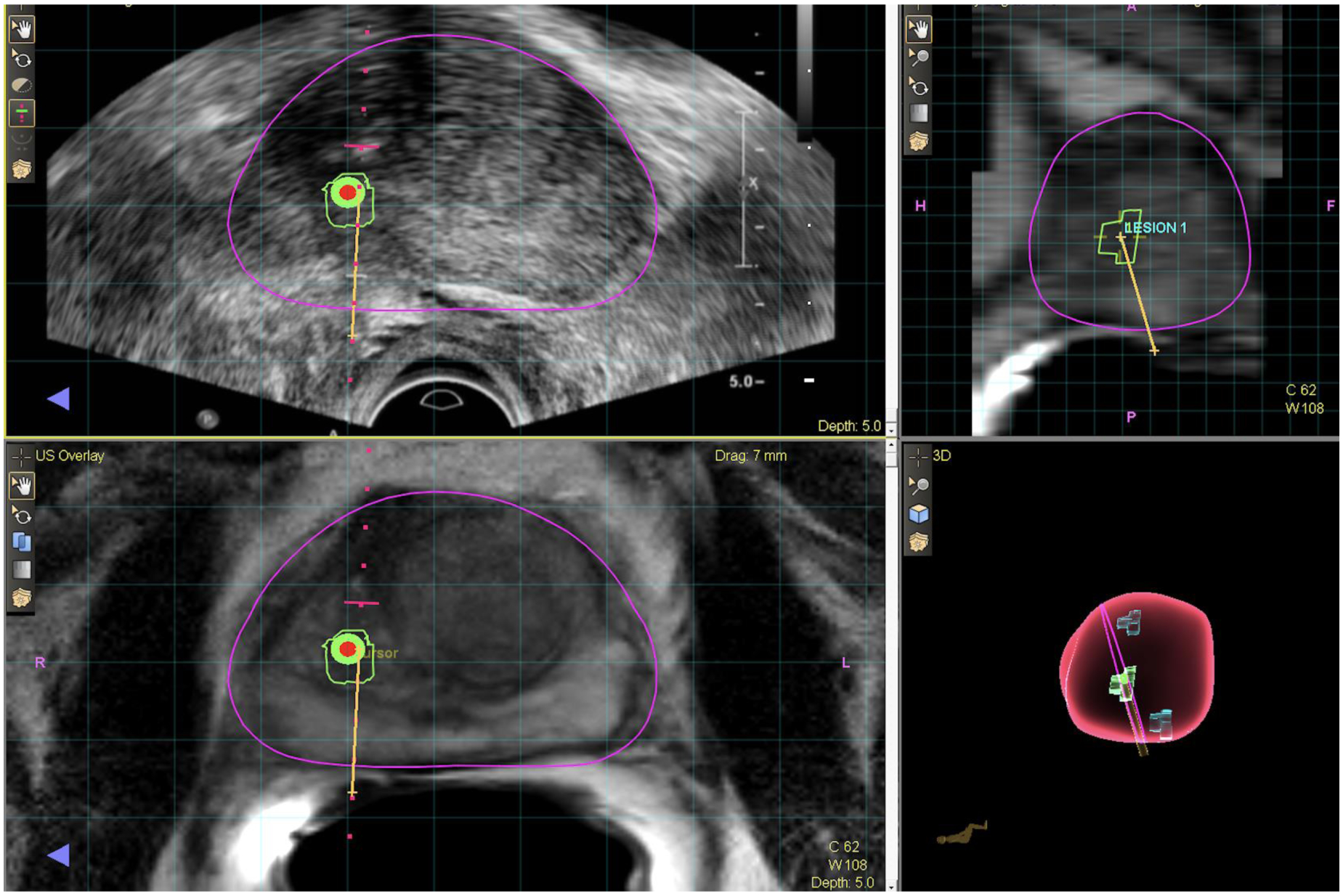
The images are snapshot pictures of the UroNav® urologist user workstation during an MRI-Ultrasound fusion prostate biopsy. MRI region of interest is outlined in a radiology workstation (DynaCAD®) and imported into an MRI-Ultrasound fusion machine. The targeting system connects the biopsy needle location in space relative to region of interest and a needle core is then obtained. The upper left panel is the real-time ultrasound image. The green outline is the region of interest and the bullseye is the center of that lesion. The yellow line represents the biopsy needle path. The lower left panel is the corresponding MRI image obtained prior to the biopsy. The ultrasound and MRI image are aligned through a serious of segmentation, alignment, and rotational adjustments. The upper right panel is the MRI in sagittal view with corresponding region of interest (blue circle) and biopsy core (yellow line). The lower right panel is a 3D representation of the prostate. The pink line represents the plane of view and in this case there were a total of three 3D target lesions (green) structures within the 3-D prostate (pink) image.
CONCLUSIONS
The research activities of the Prostate Collaborative Group are focused toward providing tools to improve the clinical management of men with prostate cancer. The EDRN supported infrastructure of both resources and expertise are leveraged by the group to facilitate discovery, guide progress through biomarker development, provide unbiased evaluation of progress and design and implement appropriate validation studies. State-of-the-art omics technologies provide comprehensive data-driven discovery with an eye toward combined multi-omics assays that can be integrated into current clinical decision making. The strong focus on biomarker application optimizes the development of biomarkers with clinical utility as well as the early adoption of disruptive technologies, such as MRI imaging, into biomarker development workflows. Likewise, efforts to validate findings from laboratories outside of the EDRN, such as polygenic risk scores, and capture of in-depth data from clinical cohorts, provides unique resources to the biomarker community.
ACKNOWLEDGEMENTS
This work was supported by funds from the NCI Early Detection Research Network (U01 CA 113913 (MGS), NIH U01 CA214194 (OJS), NIH U01 CA086402 (MAL & RJL).
Footnotes
The authors declare no potential conflicts of interests.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Hugosson J, Carlsson S, Aus G, Bergdahl S, Khatami A, Lodding P, et al. Mortality results from the Göteborg randomised population-based prostate-cancer screening trial. Lancet Oncol. 2010;11:725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schröder FH, Hugosson J, Roobol MJ, Tammela TLJ, Ciatto S, Nelen V, et al. Screening and prostate-cancer mortality in a randomized european study. N Engl J Med. 2009;360:1320–8. [DOI] [PubMed] [Google Scholar]
- 4.Loeb S, Bjurlin MA, Nicholson J, Tammela TL, Penson DF, Carter HB, et al. Overdiagnosis and overtreatment of prostate cancer. Eur. Urol 2014;65:1046–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moyer VA. Screening for prostate cancer: U.S. preventive services task force recommendation statement. Ann. Intern. Med 2012;157:120–34. [DOI] [PubMed] [Google Scholar]
- 6.Grossman DC, Curry SJ, Owens DK, Bibbins-Domingo K, Caughey AB, Davidson KW, et al. Screening for prostate cancer USPreventive servicestaskforcerecommendation statement. JAMA. 2018;319:1901–13. [DOI] [PubMed] [Google Scholar]
- 7.Feng Z, Kagan J, Pepe M, Thornquist M, Rinaudo JA, Dahlgren J, et al. The early detection research network’s specimen reference sets: Paving the way for rapid evaluation of potential biomarkers. Clin. Chem 2013;59:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jhaveri FM, Klein EA, Kupelian PA, Zippe C, Levin HS. Declining rates of extracapsular extension after radical prostatectomy: Evidence for continued stage migration. J Clin Oncol. 1999;17:3167–72. [DOI] [PubMed] [Google Scholar]
- 9.Etzioni R, Gulati R, Tsodikov A, Wever EM, Penson DF, Heijnsdijk EAM, et al. The prostate cancer conundrum revisited: Treatment changes and prostate cancer mortality declines. Cancer 2012;118:5955–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carroll PR, Parsons JK, Andriole G, Bahnson RR, Castle EP, Catalona WJ, et al. NCCN Guidelines Insights: Prostate Cancer Early Detection, Version 2.2016. J Natl Compr Cancer Netw. 2016;14:509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berg CD, Andriole GL, Crawford ED, Grubb RL, Buys SS, Chia D, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johansson JE, Andrén O, Andersson SO, Dickman PW, Holmberg L, Magnuson A, et al. Natural history of early, localized prostate cancer. J Am Med Assoc. 2004;291:2713–9. [DOI] [PubMed] [Google Scholar]
- 13.Djavan B, Fong YK, Ravery V, Remzi M, Horninger W, Susani M, et al. Are repeat biopsies required in men with PSA levels ≤4 ng/ml? a multiinstitutional prospective European study. Eur Urol. 2005;47:38–44. [DOI] [PubMed] [Google Scholar]
- 14.Scattoni V, Zlotta A, Montironi R, Schulman C, Rigatti P, Montorsi F. Extended and Saturation Prostatic Biopsy in the Diagnosis and Characterisation of Prostate Cancer: A Critical Analysis of the Literature. Eur. Urol 2007;53:1309–22. [DOI] [PubMed] [Google Scholar]
- 15.Loeb S, Carter HB, Berndt SI, Ricker W, Schaeffer EM. Complications after prostate biopsy: Data from SEER-Medicare. J Urol. 2011;186:1830–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jansen FH, van Schaik RHN, Kurstjens J, Horninger W, Klocker H, Bektic J, et al. Prostate-Specific Antigen (PSA) Isoform p2PSA in Combination with Total PSA and Free PSA Improves Diagnostic Accuracy in Prostate Cancer Detection. Eur Urol. 2010;57:921–7. [DOI] [PubMed] [Google Scholar]
- 17.Catalona WJ, Partin AW, Sanda MG, Wei JT, Klee GG, Bangma CH, et al. A multicenter study of [−2]pro-prostate specific antigen combined with prostate specific antigen and free prostate specific antigen for prostate cancer detection in the 2.0 to 10.0 ng/ml prostate specific antigen range. J Urol. 2011;185:1650–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang Y, Ankerst DP, Ketchum NS, Ercole B, Shah G, Shaughnessy JD, et al. Prospective evaluation of operating characteristics of prostate cancer detection biomarkers. J Urol. 2011;185:104–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loeb S, Sanda MG, Broyles DL, Shin SS, Bangma CH, Wei JT, et al. The prostate health index selectively identifies clinically significant prostate cancer. J Urol. 2015;193:1163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sokoll LJ, Sanda MG, Feng Z, Kagan J, Mizrahi IA, Broyles DL, et al. A prospective, multicenter, National Cancer Institute Early Detection Research Network study of [−2]proPSA: improving prostate cancer detection and correlating with cancer aggressiveness. Cancer Epidemiol Biomarkers Prev. 2010;19:1193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De La Calle C, Patil D, Wei JT, Scherr DS, Sokoll L, Chan DW, et al. Multicenter evaluation of the prostate health index to detect aggressive prostate cancer in biopsy Naïve men. J Urol. 2015;194:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bussemakers MJG, Van Bokhoven A, Verhaegh GW, Smit FP, Karthaus HFM, Schalken JA, et al. DD3: A new prostate-specific gene, highly overexpressed in prostate cancer. Cancer Res. 1999;59:5975–9. [PubMed] [Google Scholar]
- 23.Hessels D, Klein Gunnewiek JMT, Van Oort I, Karthaus HFM, Van Leenders GJL, Van Balken B, et al. DD3PCA3-based molecular urine analysis for the diagnosis of prostate cancer. Eur Urol. 2003;44:8–16. [DOI] [PubMed] [Google Scholar]
- 24.Wei JT, Feng Z, Partin AW, Brown E, Thompson I, Sokoll L, et al. Can urinary PCA3 Supplement PSA in the early detection of prostate cancer? J Clin Oncol. 2014;32:4066–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ankerst DP, Goros M, Tomlins SA, Patil D, Feng Z, Wei JT, et al. Incorporation of Urinary Prostate Cancer Antigen 3 and TMPRSS2:ERG into Prostate Cancer Prevention Trial Risk Calculator. Eur Urol Focus. 2019;5:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science; 2005;310:644–8. [DOI] [PubMed] [Google Scholar]
- 27.Salami SS, Schmidt F, Laxman B, Regan MM, Rickman DS, Scherr D, et al. Combining urinary detection of TMPRSS2: ERG and PCA3 with serum PSA to predict diagnosis of prostate cancer. Urol Oncol Semin Orig Investig. 2013;31:566–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomlins SA, Aubin SMJ, Siddiqui J, Lonigro RJ, Sefton-Miller L, Miick S, et al. Urine TMPRSS2:ERG fusion transcript stratifies prostate cancer risk in men with elevated serum PSA. Sci Transl Med. 2011;3:94ra72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanda MG, Feng Z, Howard DH, Tomlins SA, Sokoll LJ, Chan DW, et al. Association between combined TMPRSS2:ERG and PCA3 RNA urinary testing and detection of aggressive prostate cancer. JAMA Oncol. 2017;3:1085–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKiernan J, Donovan MJ, O’Neill V, Bentink S, Noerholm M, Belzer S, et al. A novel urine exosome gene expression assay to predict high-grade prostate cancer at initial biopsy. JAMA Oncol. 2016;2:882–9. [DOI] [PubMed] [Google Scholar]
- 31.Connell SP, Yazbek-Hanna M, McCarthy F, Hurst R, Webb M, Curley H, et al. A four-group urine risk classifier for predicting outcomes in patients with prostate cancer. BJU Int. 2019;124:609–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pellegrini KL, Patil D, Douglas KJS, Lee G, Wehrmeyer K, Torlak M, et al. Detection of prostate cancer-specific transcripts in extracellular vesicles isolated from post-DRE urine. Prostate. 2017;77:990–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sinha A, Huang V, Livingstone J, Wang J, Fox NS, Kurganovs N, et al. The Proteogenomic Landscape of Curable Prostate Cancer. Cancer Cell. 2019;35:414–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Houlahan KE, Shiah Y-JJ, Gusev A, Yuan J, Ahmed M, Shetty A, et al. Genome-wide germline correlates of the epigenetic landscape of prostate cancer. Nat Med. 2019;25:1615–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Macklin A, Khan S, Kislinger T. Recent advances in mass spectrometry based clinical proteomics: Applications to cancer research. Clin. Proteomics 2020;17:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hood BL, Conrads TP, Veenstra TD. Mass spectrometric analysis of formalin-fixed paraffin-embedded tissue: unlocking the proteome within. Proteomics. 2006;14:4106–14. [DOI] [PubMed] [Google Scholar]
- 37.Sprung RW, Brock JWC, Tanksley JP, Li M, Washington MK, Slebos RJC, et al. Equivalence of protein inventories obtained from formalin-fixed paraffin-embedded and frozen tissue in multidimensional liquid chromatography-tandem mass spectrometry shotgun proteomic analysis. Mol Cell Proteomics. 2009;8:1988–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao H, Zhang F, Liang S, Zhang Q, Lyu M, Qian L, et al. Accelerated Lysis and Proteolytic Digestion of Biopsy-Level Fresh-Frozen and FFPE Tissue Samples Using Pressure Cycling Technology. J Proteome Res. 2020;19:1982–90. [DOI] [PubMed] [Google Scholar]
- 39.Zhu Y, Weiss T, Zhang Q, Sun R, Wang B, Yi X, et al. High-throughput proteomic analysis of FFPE tissue samples facilitates tumor stratification. Mol Oncol. 2019;13:2305–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uchida Y, Sasaki H, Terasaki T. Establishment and validation of highly accurate formalin-fixed paraffin-embedded quantitative proteomics by heat-compatible pressure cycling technology using phase-transfer surfactant and SWATH-MS. Sci Rep. 2020;10:11271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Venable JD, Dong MQ, Wohlschlegel J, Dillin A, Yates JR. Automated approach for quantitative analysis of complex peptide mixtures from tandem mass spectra. Nat Methods. 2004;1:39–45. [DOI] [PubMed] [Google Scholar]
- 42.Purvine S, Eppel JT, Yi EC, Goodlett DR. Shotgun collision-induced dissociation of peptides using a time of flight mass analyzer. Proteomics. 2003;6:847–50. [DOI] [PubMed] [Google Scholar]
- 43.Meyer JG, Schilling B. Clinical applications of quantitative proteomics using targeted and untargeted data-independent acquisition techniques. Expert Rev. Proteomics. 2017;14:419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gillet LC, Navarro P, Tate S, Röst H, Selevsek N, Reiter L, et al. Targeted data extraction of the MS/MS spectra generated by data-independent acquisition: A new concept for consistent and accurate proteome analysis. Mol Cell Proteomics. 2012;11:O111.016717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Y, Chen J, Sethi A, Li QK, Chen L, Collins B, et al. Glycoproteomic analysis of prostate cancer tissues by SWATH mass spectrometry discovers N-acylethanolamine acid amidase and protein tyrosine kinase 7 as signatures for tumor aggressiveness. Mol Cell Proteomics. 2014;13:1753–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim Y, Jeon J, Mejia S, Yao CQ, Ignatchenko V, Nyalwidhe JO, et al. Targeted proteomics identifies liquid-biopsy signatures for extracapsular prostate cancer. Nat Commun. 2016;7:11906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim Y, Ignatchenko V, Yao CQ, Kalatskaya I, Nyalwidhe JO, Lance RS, et al. Identification of differentially expressed proteins in direct expressed prostatic secretions of men with organ-confined versus extracapsular prostate cancer. Mol Cell Proteomics. 2012;11:1870–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Principe S, Jones EE, Kim Y, Sinha A, Nyalwidhe JO, Brooks J, et al. In-depth proteomic analyses of exosomes isolated from expressed prostatic secretions in urine. Proteomics. 2013;13:1667–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Principe S, Kim Y, Fontana S, Ignatchenko V, Nyalwidhe JOJO, Lance RSRS, et al. Identification of prostate-enriched proteins by in-depth proteomic analyses of expressed prostatic secretions in urine. J Proteome Res. 2012;11:2386–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drake RR, Elschenbroich S, Lopez-Perez O, Kim Y, Ignatchenko V, Ignatchenko A, et al. In-depth proteomic analyses of direct expressed prostatic secretions. J Proteome Res. 2010;9:2109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berger ST, Ahmed S, Muntel J, Polo NC, Bachur R, Kentsis A, et al. MStern blotting-high throughput polyvinylidene fluoride (PVDF) membrane-based proteomic sample preparation for 96-well plates. Mol Cell Proteomics. 2015;14:2814–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gallien S, Duriez E, Crone C, Kellmann M, Moehring T, Domon B. Targeted proteomic quantification on quadrupole-orbitrap mass spectrometer. Mol Cell Proteomics. 2012;11:1709–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peterson AC, Russell JD, Bailey DJ, Westphall MS, Coon JJ. Parallel reaction monitoring for high resolution and high mass accuracy quantitative, targeted proteomics. Mol Cell Proteomics. 2012;11:1475–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang C, Höti N, Lih T-SM, Sokoll LJ, Zhang R, Zhang Z, et al. Development of a glycoproteomic strategy to detect more aggressive prostate cancer using lectin-immunoassays for serum fucosylated PSA. Clin. Proteomics 2019;16:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li QK, Chen L, Ao MH, Chiu JH, Zhang Z, Zhang H, et al. Serum fucosylated prostate-specific antigen (PSA) improves the differentiation of aggressive from non-aggressive prostate cancers. Theranostics. 2015;5:267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi T, Song E, Nie S, Rodland KD, Liu T, Qian WJ, et al. Advances in targeted proteomics and applications to biomedical research. Proteomics. 2016;16:2160–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi T, Fillmore TL, Sun X, Zhao R, Schepmoes AA, Hossain M, et al. Antibody-free, targeted mass-spectrometric approach for quantification of proteins at low picogram per milliliter levels in human plasma/serum. Proc Natl Acad Sci U S A. 2012;109:15395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gao Y, Wang Y-T, Chen Y, Wang H, Young D, Shi T, et al. Proteomic Tissue-Based Classifier for Early Prediction of Prostate Cancer Progression. Cancers. 2020;12:1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gudmundsson J, Besenbacher S, Sulem P, Gudbjartsson DF, Olafsson I, Arinbjarnarson S, et al. Genetic correction of PSA values using sequence variants associated with PSA levels. Sci Transl Med. 2010;2:62ra92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kote-Jarai Z, Amin Al Olama A, Leongamornlert D, Tymrakiewicz M, Saunders E, Guy M, et al. Identification of a novel prostate cancer susceptibility variant in the KLK3 gene transcript. Hum Genet. 2011;129:687–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Otto JJ, Correll VL, Engstroem HA, Hitefield NL, Main BP, Albracht B, et al. Targeted Mass Spectrometry of a Clinically Relevant PSA Variant from Post-DRE Urines for Quantitation and Genotype Determination. Proteomics - Clin Appl. 2020;2000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Busch J, Magheli A, Leva N, Ferrari M, Kramer J, Klopf C, et al. Higher rates of upgrading and upstaging in older patients undergoing radical prostatectomy and qualifying for active surveillance. BJU Int. 2014;114:517–21. [DOI] [PubMed] [Google Scholar]
- 63.Kim DW, Chen MH, Huland H, Graefen M, Tilki D, D’Amico AV. Association of Age With Risk of Adverse Pathological Findings at Radical Prostatectomy in Men With Gleason Score 6 Prostate Cancer. JAMA. 2020;3:e202041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pham DM, Kim JK, Lee S, Hong SK, Byun S-S, Lee SE. Prediction of pathologic upgrading in Gleason score 3+4 prostate cancer: Who is a candidate for active surveillance? Investig Clin Urol. 2020;61:405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Soenens C, Dekuyper P, De Coster G, Van Damme N, Van Eycken E, Quackels T, et al. Concordance Between Biopsy and Radical Prostatectomy Gleason Scores: Evaluation of Determinants in a Large-Scale Study of Patients Undergoing RARP in Belgium. Pathol Oncol Res. 2020;26:2605–12. [DOI] [PubMed] [Google Scholar]
- 66.Leeman JE, Chen MH, Huland H, Graefen M, D’Amico AV., Tilki D Advancing Age and the Odds of Upgrading and Upstaging at Radical Prostatectomy in Men with Gleason Score 6 Prostate Cancer. Clin Genitourin Cancer. 2019;17:e1116–21. [DOI] [PubMed] [Google Scholar]
- 67.Schumacher FR, Al Olama AA, Berndt SI, Benlloch S, Ahmed M, Saunders EJ, et al. Association analyses of more than 140,000 men identify 63 new prostate cancer susceptibility loci. Nat Genet. 2018;50:928–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seibert TM, Fan CC, Wang Y, Zuber V, Karunamuni R, Parsons JK, et al. Polygenic hazard score to guide screening for aggressive prostate cancer: Development and validation in large scale cohorts. BMJ. 2018;360:j5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carter HB, Helfand B, Mamawala M, Wu Y, Landis P, Yu H, et al. Germline Mutations in ATM and BRCA1/2 Are Associated with Grade Reclassification in Men on Active Surveillance for Prostate Cancer. Eur Urol. 2019;75:743–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beauval JB, Ploussard G, Soulié M, Pfister C, Van Agt S, Vincendeau S, et al. Pathologic findings in radical prostatectomy specimens from patients eligible for active surveillance with highly selective criteria: A multicenter study. Urology. 2012;80:656–60. [DOI] [PubMed] [Google Scholar]
- 71.Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA. 2015;313:390–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ahdoot M, Wilbur AR, Reese SE, Lebastchi AH, Mehralivand S, Gomella PT, et al. MRI-Targeted, Systematic, and Combined Biopsy for Prostate Cancer Diagnosis. N Engl J Med. 2020;382:917–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baldisserotto M, Neto EJD, Carvalhal G, de Toledo AF, de Almeida CM, Cairoli CED, et al. Validation of PI-RADS v.2 for prostate cancer diagnosis with MRI at 3T using an external phased-array coil. J Magn Reson Imaging. 2016;44:1354–9. [DOI] [PubMed] [Google Scholar]
- 74.Fenstermaker M, Mendhiratta N, Bjurlin MA, Meng X, Rosenkrantz AB, Huang R, et al. Risk Stratification by Urinary Prostate Cancer Gene 3 Testing Before Magnetic Resonance Imaging-Ultrasound Fusion-targeted Prostate Biopsy Among Men With No History of Biopsy. Urology. 2017;99:174–9. [DOI] [PubMed] [Google Scholar]
- 75.Tomlins SA, Day JR, Lonigro RJ, Hovelson DH, Siddiqui J, Kunju LP, et al. Urine TMPRSS2:ERG Plus PCA3 for Individualized Prostate Cancer Risk Assessment. Eur Urol. 2016;70:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shukla S, Evans JR, Malik R, Feng FY, Dhanasekaran SM, Cao X, et al. Development of a RNA-seq based prognostic signature in lung adenocarcinoma. J Natl Cancer Inst. 2017;109:djw200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shore ND, Pieczonka CM, Henderson RJ, Bailen JL, Saltzstein DR, Concepcion RS, et al. Development and evaluation of the MiCheck test for aggressive prostate cancer. Urol Oncol Semin Orig Investig. 2020;38:e11–e18. [DOI] [PubMed] [Google Scholar]
- 78.Maragh S, Veltri RW, Lund SP, Mangold L, Isharwal S, Christudass CS, et al. Evaluation of two mitochondrial DNA biomarkers for prostate cancer detection. Cancer Biomarkers. 2015;15:763–73. [DOI] [PubMed] [Google Scholar]
- 79.Freedland SJ, Choeurng V, Howard L, De Hoedt A, du Plessis M, Yousefi K, et al. Utilization of a Genomic Classifier for Prediction of Metastasis Following Salvage Radiation Therapy after Radical Prostatectomy. Eur Urol. 2016;70:588–96. [DOI] [PubMed] [Google Scholar]
- 80.Cairns P, Esteller M, Herman JG, Schoenberg M, Jeronimo C, Sanchez-Cespedes M, et al. Molecular Detection of Prostate Cancer in Urine by GSTP1 Hypermethylation. Clin Cancer Res. 2001;7:2727–30. [PubMed] [Google Scholar]
- 81.Alemozaffar M, Akintayo AA, Abiodun-Ojo OA, Patil D, Saeed F, Huang Y, et al. [18 F]fluciclovine PET/CT for Preoperative Staging in Patients with Intermediate to High Risk Primary Prostate Cancer. J Urol. 2020;204:734–40. [DOI] [PMC free article] [PubMed] [Google Scholar]


