Abstract
Purpose:
To evaluate corneal immune dendritiform cell (DC) changes in dry eye disease (DED) using in vivo confocal microscopy (IVCM) and to correlate IVCM parameters with clinical severity.
Methods:
This was a retrospective, cross-sectional study including 300 eyes of 150 DED patients and 49 eyes of 49 age-matched controls. Severity of DED was based on the Dry Eye Workshop (DEWS) classification. IVCM images of subbasal layer of the central cornea were analyzed for DC density and morphology (including number of dendrites per DC, DC size and DC field).
Results:
DC density was significantly higher in DED compared to controls (93.4 ± 6.3 vs. 25.9 ± 3.9 cells/ mm2; P < 0.001). Morphologically, number of dendrites, DC size and field were significantly larger in DED (3.3 ± 0.1, 106.9 ± 4.7 μm2, 403.8 ± 20.1 μm2 than controls (2.3 ± 0.1, 62.5 ± 5.7 μm2, 241.4 ± 24.4 μm2, P < 0.001). Significantly higher DC density compared to controls was observed as early as Level 1 DED severity (87 ± 10 cells/mm2, p < 0.001. Significant morphological changes in DC were detected for Levels 2 to 4 (p= < 0.001, and p = < 0.05) for dendrites and DC field, respectively. Similarly, DC size showed significant increase at DED level 3–4. (p < 0.05). Linear regression analysis showed that both conjunctival and corneal staining were independently associated with DC density, while corneal staining was independently associated with DC morphology.
Conclusion:
DC density and morphology correlated with clinical severity of DED. While, DC density is increased in mild DED, morphological changes are seen only in severe cases. IVCM may be a powerful tool to detect early immune changes and may complement clinical examination in DED.
Keywords: Dendritiform cells, Dry eye disease, In vivo confocal microscopy
Introduction
Dry eye disease (DED) is a global health problem that results in a significantly impaired quality of life [1,2]. Estimated to affect over 16 million adults in the United States [3,4], DED has a significant economic impact with an average annual cost of $3.84 billion for managing DED patients [5]. Initially considered to be a condition affecting primarily elderly population, an increased prevalence is now seen in the younger population as well.
Inflammation and immune activation play an integral role in the pathogenesis of DED [6,7]. This has also been reflected in the definition of DED by the TFOS Dry Eye Workshop (DEWS)– “Dry eye is a multi-factorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.” [8] Currently, the diagnostic protocol and severity grading of DED are largely based on the recommendations of DEWS [9].
Despite increased recognition of the condition, diagnosis and treatment of DED continues to remain a huge challenge. This is because most conventional tests, such as Schirmer’s test and tear break-up time (TBUT) cannot detect underlying inflammation. Some recent studies have however suggested a correlation of vital dye staining of the conjunctiva and cornea with expression of inflammatory cytokines in dry eye disease [10]. Neverthless, these tests may not be useful for the subset of dry eye disease patients who may have benign clinical exam without significant conjunctival and corneal staining. Hence, there is a pressing need for a diagnostic test that can objectively reflect the severity of the underlying immune activation and inflammation, even when the disease is clinically unapparent, and which correlates with the symptoms.
In vivo confocal microscopy (IVCM) is a powerful, non-invasive diagnostic tool to study the corneal structures at the cellular level [11]. Using IVCM, recent studies have demonstrated the role of corneal immune cells, including dendritiform cells (DC) and non-dendritiform cells, in DED [12–16]. Although in these studies increased number of corneal immune cells have been seen in patients with DED compared with the normal controls, it remains unclear whether the number and/ or morphology of immune cells, as seen by IVCM, correlate with the clinical severity of DED.
In the current study, we hypothesized that changes in DC density and morphology correlate with the clinical severity of DED and therefore could be used to stratify the severity of and monitor anti-inflammatory treatment efficacy.
Materials and methods
Study design and patients
In this retrospective study, IVCM findings were evaluated in 300 eyes of 150 patients with the clinical diagnosis of DED, as the study group, and 49 eyes of 49 age-matched normal individuals, as the control group. All subjects were recruited from the Cornea Service & Ocular Surface Imaging Center, Massachusetts Eye & Ear Infirmary, Harvard Medical School, Boston, Massachusetts. The protocol of the study was approved by the Institutional Review Board/Ethics Committee, complied with the Health Insurance Portability and Accountability Act (HIPAA) and adhered to the tenets of the Declaration of Helsinki.
DED was diagnosed clinically with the presence of typical DED symptoms, such as foreign body sensation, burning, stinging and light sensitivity, and at least one of the following signs: Schirmer’s test with anesthesia < 10 mm at 5 min, TBUT < 10 s, or positive vital staining of the cornea or conjunctiva. The subjects in the control group did not report any DED symptoms and did not have any of the above-mentioned positive DED signs. The exclusion criteria in both the DED and the control groups included use of topical anti-glaucoma or anti-inflammatory medications, active ocular allergy, a history of contact lens wear or infectious keratitis in the past three months, and a history of ocular surgery in the past six months.
The charts of all patients were reviewed and the following parameters were extracted: patients’ demographics, symptoms, score of the Ocular Surface Disease Index (OSDI), corneal fluorescein staining, conjunctival lissamine green staining, Schirmer’s test and TBUT. The clinical severity of DED for each patient was determined according to the classification proposed by DEWS 2007, stratifying the cases into four levels of severity (1 through 4) [17].
In vivo confocal microscopy
All patients and normal controls had undergone laser IVCM (Heidelberg Retina Tomograph 3 with the Rostock Cornea Module, Heidelberg Engineering GmbH, Heidelberg, Germany) of the central cornea in both eyes. This microscope is equipped with a 63× objective immersion lens with a numerical aperture of 0.9 (Olympus, Tokyo, Japan) and uses a 670-nm red wavelength diode laser source. Each image represents a coronal section of the cornea of 400 × 400 μm. Digital images had been recorded at the rate of 3 frames per second with the sequence mode, including 100 images per sequence, and a separation of 1 μm between adjacent images, and a lateral resolution of 1 μm/pixel. A total of six to eight sequence scans had been recorded from the full thickness of the central cornea, generating around 100 total images of the subbasal layer per eye, where epithelial DCs reside, typically at a depth of 50–80 μm for each eye. For each eye, three most-representative images of the subbasal layer, were chosen by a masked observer for analysis. The criteria for image selection were the best focused images, in a single layer, with a good contrast and without folds.
Two masked observers evaluated the IVCM images for corneal DCs in the subbasal layer of the central cornea. Bright, highly reflective dendritiform cells were recognized as corneal DCs. Although the exact histological identity of these cells cannot be ascertained due to the in vivo nature of the study, the dendritiform appearance of these cells with discrete cell bodies suggest that they are most likely to be DCs. It has been shown in previous in vitro studies and IVCM studies that epithelial immune cells are DCs [12,13,18–20]. In addition to DC density (measured by counting number of DCs per frame, which equals 160,000 μm2), the following parameters were determined for morphology of the DC in each image: DC size (the area covered by the body of the cell), number of dendrites per cell; and DC field (area bounded within the span of the dendrites). The above-mentioned parameters were analyzed using Image J software (Fig. 1) [21].
Fig. 1.
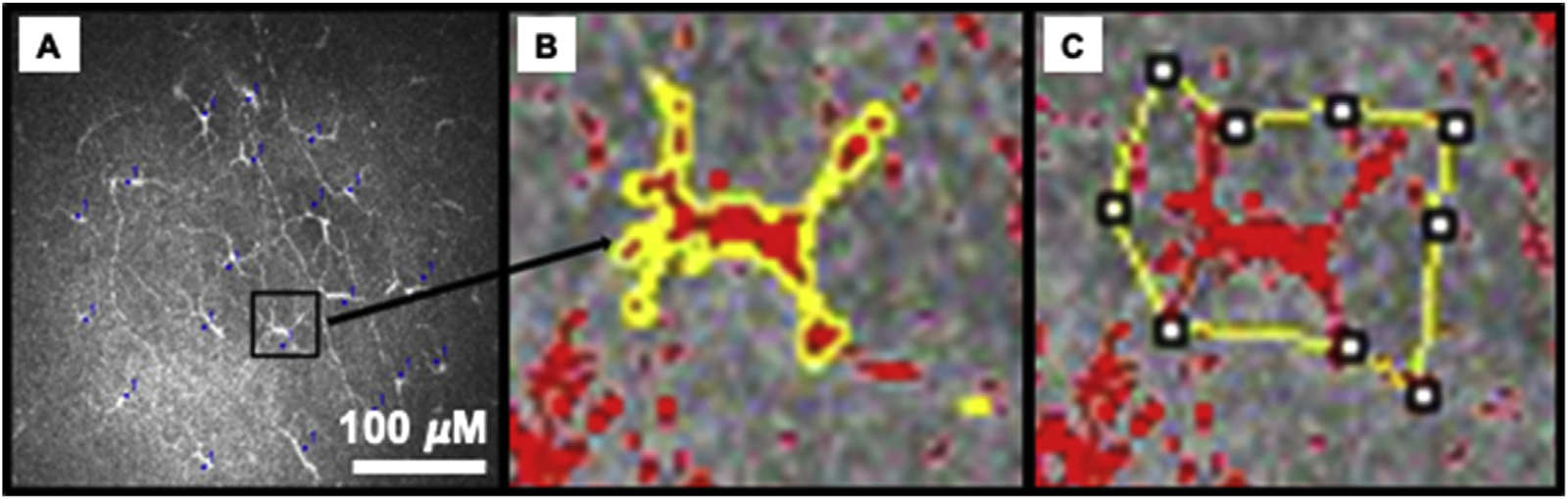
Analysis of Immune dendritiform cell (DC) density and morphology. DC density was measured using cell counter function (A). DC morphology included DC size (B), number of dendrites (C), and DC field (area bounded by the dendrites, C). The bar represents 100 μM.
Statistical analysis
Statistical analysis was performed using IBM SPSS version 19 (IBM Corp., Armonk, NY). Data were expressed as number (%) for qualitative variables or mean ± standard error of mean (SEM) for quantitative variables. Data from both eyes per person were used for the analysis for participants with DED. Intra-class coefficient (ICC) was measured to assess reliability of IVCM parameters. Qualitative variables were compared between the groups using Chi square test. The IVCM parameters between the DED and the control groups as well as between both eyes of each individual were compared using independent sample t-test. Statistical comparisons of IVCM findings among different levels of DED severity were performed using ANOVA followed by least significant difference (LSD) post-hoc analysis. General mixed model (GLM) was used to adjust the effect of examined eye on the outcome variables. GLM with LSD post-hoc was also employed to assess the differences in the clinical signs among DED subgroups. Multiple linear regression was used to evaluate the adjusted association between clinical signs and IVCM findings. Statistical significance was considered for p values of 0.05 or less.
Results
Demographics
The DED group included 300 eyes of 150 patients (98 females and 52 males) with a mean age 54.0 ± 16.3 years (range, 21–82 years); the control group included 49 eyes of 49 individuals (30 females and 19 males) with mean age 52 ± 10.9 (range, 35–74 years). There were no significant differences between the two groups in terms of age (p = 0.12) or gender (p = 0.60; Table 1).
Table 1.
Demographics and clinical data for patients with dry eye disease (DED) and the normal controls.
| Control Group | DED Group | p value | |
|---|---|---|---|
| Number of eyes (patients) | 49 (49) | 300 (150) | – |
| Age (years) | 51.0 ± 1.6 | 54.0 ± 0.9 | 0.12 |
| Gender (F/M) | 30/19 | 98/52 | 0.60 |
| Eye examined (OD/OS) | 45/51 | 150/150 | 0.59 |
| OSDI score | 0 | 44.2 ± 2.1(n = 94) | – |
| Schirmer’s score (mm) | > 10 | 10.2 ± 6.4(n = 266) | – |
| TBUT (seconds) | > 10 | 4.8 ± 0.2(n = 284) | – |
| Corneal fluorescein staining | 0 | 1.3 ± 0.1(n = 274) | – |
Data presented as mean ± standard error unless otherwise noted.
F: female; M: male; OSDI: Ocular Surface Disease Index; TBUT: tear break-up time.
Clinical signs
In the DED group, there were no significant differences between the right eye and the left eye regarding Schirmer’s score (10.2 ± 0.5 and 10.2 ± 0.6 mm, respectively, p = 0.93), TBUT (4.9 ± 0.2 vs. 4.7 ± 0.2, respectively, p = 0.66), conjunctival lissamine green staining (1.3 ± 0.1 vs. 1.4 ± 0.1, respectively, p = 0.62), or corneal fluorescein staining (1.3 ± 0.1 vs. 1.4 ± 0.1, respectively, p = 0.66). DC parameters in the right and left eye are listed in Table 2 and effect of the examined eye has been adjusted by GLM analysis. Table 3 represents the clinical information of the patients on the basis of which they were classified into the DEWS classification.
Table 2.
In vivo confocal microscopic findings in right and the left eyes of patients with dry eye disease (DED).
| Right Eye(n = 150) | Left Eye(n = 150) | p | |
|---|---|---|---|
| DC density (cells/mm2) | 84.6 ± 7.9 | 83.3 ± 7.9 | 0.90 |
| Number of dendrites (n/cell) | 3.2 ± 0.1 | 3.1 ± 0.1 | 0.59 |
| DC size (μm2) | 103.5 ± 6.2 | 97.8 ± 5.7 | 0.50 |
| DC Field (μm2) | 390.9 ± 24.4 | 370.8 ± 26.2 | 0.57 |
Data presented as mean ± standard error.
Table 3.
Clinical findings in different levels of dry eye disease (DED) clinical severity based on Dry Eye Workshop (DEWS) classification.
| DEWS Clinical Severity in DED Group | |||||
|---|---|---|---|---|---|
| Level 1(n = 97) | Level 2(n = 106) | Level 3(n = 65) | Level 4(n = 26) | p (GLM) | |
| Conjunctival Injection | 0.48 ± 0.09 | 0.42 ± 0.10 | 0.52 ± 0.11 | 1.03 ± 0.06*†‡ | 0.007 |
| Lissamine Conjunctival Staining | 0.96 ± 0.18 | 1.19 ± 0.18 | 1.58 ± 0.16* | 1.36 ± 0.10*† | 0.021 |
| Fluorescein Corneal Staining | 0.83 ± 0.11 | 1.29 ± 0.10* | 1.85 ± 0.14*† | 2.04 ± 0.07*† | < 0.001 |
| Tear BreakUp Time (TBUT) (seconds) | 6.90 ± 0.27 | 4.68 ± 0.20* | 2.99 ± 0.25*† | 2.40 ± 0.16*† | < 0.001 |
| Schirmer’s Score(mm) | 14.89 ± 0.48 | 11.13 ± 0.55* | 6.05 ± 0.57*† | 1.24 ± 0.39*†‡ | < 0.001 |
| OSDI | 38.77 ± 5.39 | 50.43 ± 5.18 | 39.01 ± 5.20 | 49.61 ± 2.93 | 0.293 |
Data presented as mean ± standard deviation.
p < 0.05 compared to level 1;
p < 0.05 compared to level 2;
p < 0.05 compared to level 3 assessed by GLM.
IVCM parameters
Intra-class co-efficient (ICC) was 0.996 at a 95% confidence interval of 0.994–0.997. The IVCM parameters in the DED group were significantly different compared with the control group (Table 4, Fig. 2 and Fig. 3). The density of DCs was significantly higher in the DED group (93.4 ± 6.3 cells/mm2) than the control group (25.9 ± 3.9 cells/mm2, p < 0.001). Morphologically, number of dendrites per cell, DC size and DC field were significantly larger in the DED group (3.3 ± 0.1, 106.9 ± 4.7 μm2 and 403.8 ± 20.1 μm2, respectively) than the control group (2.3 ± 0.1, 62.5 ± 5.7 μm2 and 241.4 ± 24.4 μm2, respectively) with the differences being statistically significant (all p < 0.001) (see Fig. 4).
Table 4.
In vivo confocal microscopic findings in patients with dry eye disease (DED) and the normal controls.
| Control Group(n = 49) | DED Group(n = 300) | P | |
|---|---|---|---|
| DC density (cells/mm2) | 25.9 ± 3.9 | 93.4 ± 6.3 | < 0.001 |
| Number of dendrites (n/cell) | 2.3 ± 0.1 | 3.3 ± 0.1 | < 0.001 |
| DC size (μm2) | 62.5 ± 5.7 | 106.9 ± 4.7 | < 0.001 |
| DC Field (μm2) | 241.4 ± 24.4 | 403.8 ± 20.1 | < 0.001 |
Data presented as mean ± standard error.
DC: Immune dendritiform cell.
Fig. 2.
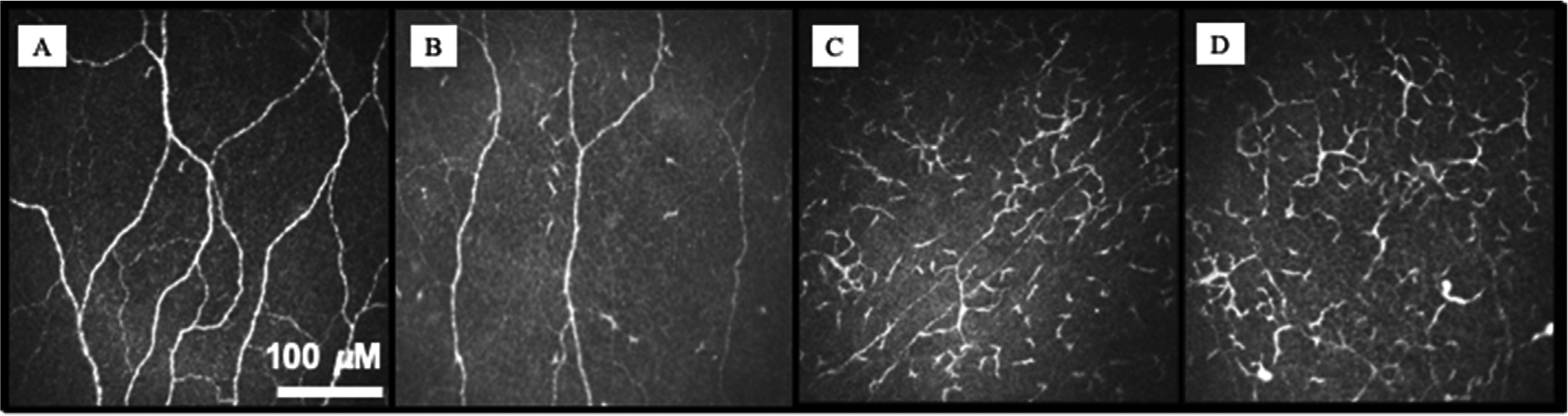
Changes in immune dendritiform cell (DC) parameters in different levels of Dry Eye Disease (DED) severity. Normal central subbasal IVCM (A) showing very few DCs. In level 1 Dry Eye Workshop (DEWS) (B), there is an increase in the number of DCs without a change in morphology. As severity level increases DEWS 2–4 (C–D), there is increase in DC density and morphologic parameters. The bar represents 100 μM.
Fig. 3.
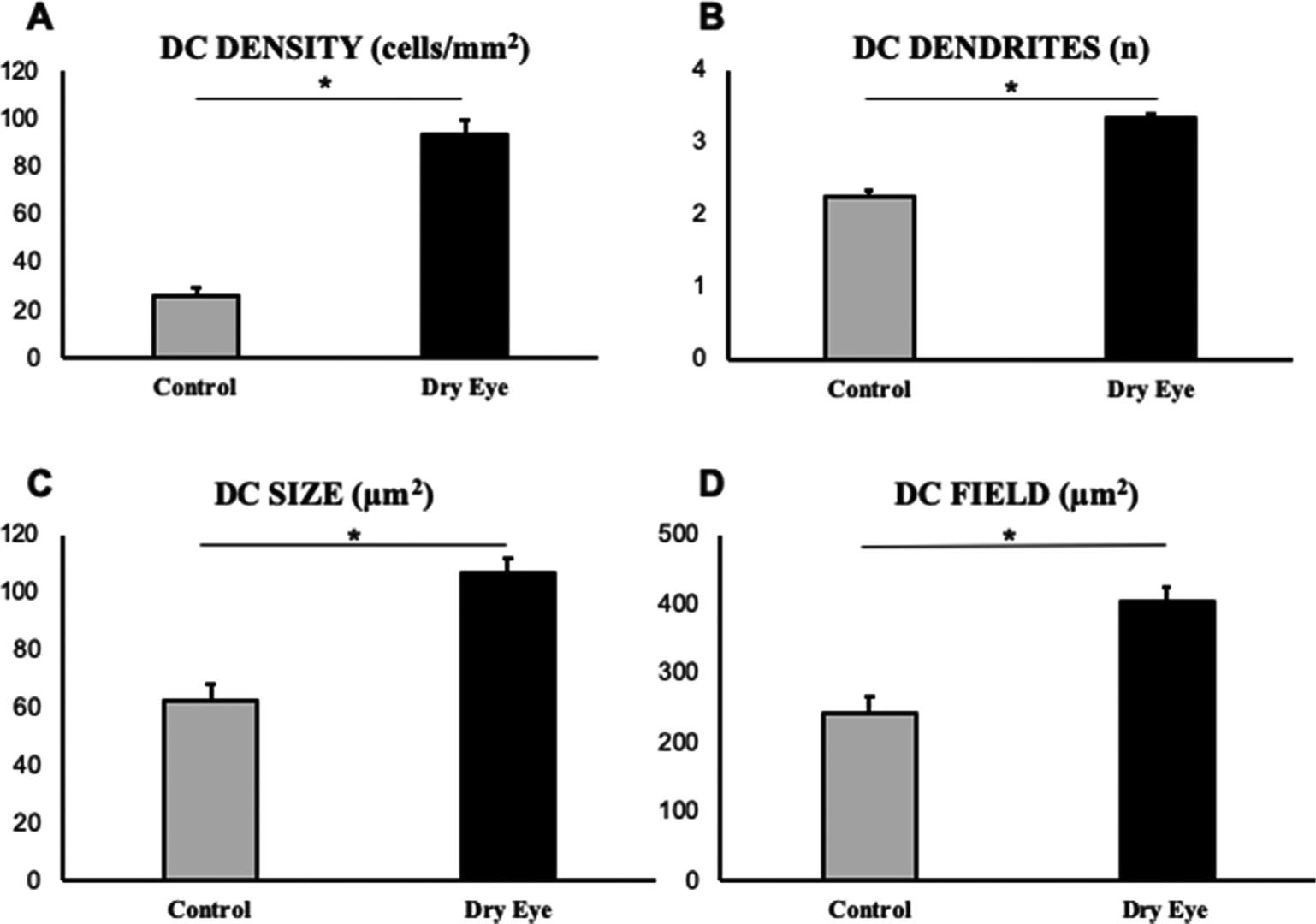
Significant increase in immune dendritiform cell (DC) density (A) and morphology (B–D) are seen in dry eye disease compared to controls. (*P < 0.05).
Fig. 4.

Comparison of immune dendritiform cell (DC) density (A) and morphology (B–D) between controls and different severity levels of dry eye disease (DED). Morphological changes (B–D) demonstrated no significant difference in grade 1 compared with the control group, but they increased significantly in higher grades of DED (Dry Eye Workshop (DEWS) grades 2–4).
Table 5 shows the IVCM parameters for different levels of DED clinical severity based on DEWS classification, following adjustment for the examined eye (right or left) using GLM. Significantly higher DC density was observed as early as DEWS severity Level 1 compared with the control group (p < 0.01). However, there were no significant differences in DC density among the four levels of severity (p > 0.5). The number of dendrites was significantly increased in all levels of severity of DED compared to controls (p < 0.001). Further, the number of dendrites was significantly higher in participants with more severe DED (severity Level 2–4) compared with participants with less severe Level 1 DED (p < 0.05). Similarly, we observed higher DC size in participants in all Levels of DED compared to control (p < 0.05), with further increase in patients with more severe disease (Level 3) compared to less severe stages (Level 1, p < 0.05 and Level 2; p < 0.05). Although we did not observe significant differences in the DC field between controls and participants with least severe DED (p = 0.14), participants with more severe DED (Levels 2–4) showed significantly increased DC field compared with controls (p < 0.05).
Table 5.
In vivo confocal microscopic findings in different levels of dry eye disease (DED) clinical severity based on Dry Eye Workshop (DEWS) classification.
| Control Group(n = 49) | DEWS Clinical Severity in DED Group | ||||
|---|---|---|---|---|---|
| Level 1(n = 97) | Level 2(n = 106) | Level 3(n = 65) | Level 4(n = 26) | ||
| DC Density (cells/mm2) | 25.9 ± 3.9 | 87.4 ± 11.1* | 84.6 ± 8.8* | 114.4 ± 17.1* | 100.6 ± 20.1* |
| Number of dendrites (n/cell) | 2.3 ± 0.1 | 3.0 ± 0.1* | 3.4 ± 0.1*† | 3.5 ± 0.2*† | 3.5 ± 0.2*† |
| DC size (μm2) | 62.5 ± 5.7 | 89.6 ± 4.2* | 103.7 ± 5.5* | 127.3 ± 17.3*†‡ | 115.1 ± 8.8* |
| DC field (μm2) | 241.4 ± 24.4 | 323.5 ± 23.9 | 406.9 ± 29.3* | 478.8 ± 60.8*† | 403.7 ± 42.5* |
Data presented as mean ± standard error.
DC: Immune dendritiform cell.
p < 0.05 compared to controls;
p < 0.05 compared to level 1;
p < 0.05 compared to level 2.
Adjustment for the eye examined and IVCM parameters via multiple regression demonstrated that corneal fluorescein score is independently associated with DC density (β = 0.20, p = 0.001), number of dendrites (β = −0.23, p = 0.012), and DC size (β = 0.36, p = 0.013). Conjunctival lissamine green staining was independently associated with DC density (β = 0.24, p = 0.012). TBUT was associated with number of dendrites (β = −0.18, p = 0.049) (Table 6).
Table 6.
Association between clinical signs of dry eye disease (DED) with dendritic cell density and morphological parameters.
| Conjunctival injection | Conjunctival Lissamine | Cornea fluorescein score | TBUT | Schirmer | OSDI | ||
|---|---|---|---|---|---|---|---|
| DC Density (cells/mm2) | β | 0.17 | 0.24† | 0.20† | −0.02 | −.01 | 0.22 |
| p | 0.073 | 0.012* | 0.001* | 0.777 | 0.854 | 0.156 | |
| Number of dendrites (n/cell) | β | −0.17 | 0.03 | −0.23† | −0.18† | −0.16 | 0.14 |
| p | 0.214 | 0.895 | 0.012* | 0.049* | 0.086 | 0.514 | |
| DC field (μm2) | β | −0.06 | −0.08 | −0.03 | 0.21 | −0.02 | 0.11 |
| p | 0.772 | 0.767 | 0.822 | 0.175 | 0.910 | 0.729 | |
| DC size (μm2) | β | 0.23 | −0.01 | 0.36† | −0.16 | 0.12 | −0.31 |
| p | 0.311 | 0.960 | 0.013* | 0.275 | 0.444 | 0.346 |
DC: Immune dendritiform cell.
β coeffiecint with *p < 0.05.
Discussion
This controlled study on a large number of patients with DED demonstrates increased density of corneal DCs, which are larger in size and have longer dendrites, suggestive of increased immune activation or maturation. However, the increased DC density and increase in morphological parameters were not similar across the different levels of clinical severity of DED. While the DC density increased even in patients with mild DED, significant morphological changes were seen only in moderate and severe cases. Our results suggest that corneal IVCM may be a powerful tool to detect early immune changes and potentially cellular inflammation of the ocular surface and could be used to complement clinical examination in DED.
Currently, there is no established gold standard for the diagnosis of DED [22]. Traditional diagnostic tests include Schirmer’s test, TBUT, corneal fluorescein staining and conjunctival lissamine green staining [23]. The more recent diagnostic tests recommended by DEWS II include the non-invasive tear breakup time (NIBUT), which uses automated observation of the tear film over a prolonged period and is considered superior to traditional TBUT, as tear film stability is affected by factors such as volume of fluorescein instilled, temperature and humidity changes. Further, tear osmolarity has been shown to be increased and is more variable in DED patients [9]. Although considered better than conventional tests, none of these tests meet the requirements of an ideal diagnostic test in being objective, highly reproducible and reliable [9,24]. Moreover, a recent study that evaluated the use of these clinical tools to grade severity of DED, reported a significant overlap between prospectively defined normal subjects and patients with DED [22]. These inadequacies are because the underlying pathophysiological processes that initiate and lead to the progression of DED are beyond the scope of the conventional tests.
The basic pathophysiological mechanism in DED is a vicious cycle of hyperosmolarity chronic inflammation, and neurosensory abnormalities [6]. Patients with DED demonstrate increased production of pro-inflammatory cytokines, the type and concentration of which may vary with the underlying etiology of DED [25–30]. The release of pro-inflammatory mediators can subsequently activate or enhance an immune or inflammatory response [31]. Therefore, evaluation of the presence and degree of immune activation may help in judging the severity of the underlying changes in DED. To study these changes in patients with DED, IVCM has been used recently [32].
Using IVCM, immune activation in DED can be assessed by studying the corneal immune cells including assessment of corneal DC [33]. DCs are potent antigen-presenting cells and are crucial for initiation of immune responses. The presence of DCs as the immune sentinels of the cornea has been shown in various animal and human studies [34–36]. The primary location of these cells is in the epithelial subbasal layer, generally in close proximity with the nerve plexus [37]. Previous studies have shown that DC density is higher in the periphery and decreases towards the center [38,39].
Similar to previous studies [14,15,40], the present study revealed a significantly higher DC density in the central cornea of the DED group compared with the control group (Table 2). In addition to higher DC density, we also noted an increase in the morphological features of DC in the DED group compared with the control group (Table 2). There were significantly higher number of dendrites, larger DC size, and larger DC field in the DED group. Similarly, higher values of morphological parameters in the central corneal DC have also been reported in other conditions such as infectious keratitis or herpes zoster ophthalmicus [41,42]. Because the area covered by these cells (cell field) and their number of dendrites demonstrate the mature and active stage of these cells [39], the data show activation maturation of DC in DED.
It is also important clinically to define the severity of DED, as the impact on quality of life varies with the severity of disease [43]. Also, the various grades of DED severity may need different therapeutic approaches. The classification by DEWS I was used to grade DED severity into four levels based on a combination of patient symptoms and clinical signs [17]. This classification was developed by a group of leading experts in the field of DED. They modified the previously existing classification schemes such as the triple classification and the Delphi panel to make them more comprehensive. We understand that the classification scheme is not perfect as often the signs and symptoms do not correlate in DED, but out of all the available classification schemes, this clinical tool is the most objective and clinically applicable to grade DED currently.
In the current study, we demonstrated that IVCM parameters showed differential changes in different levels of DED severity (Table 5). Density of DC was significantly higher than the control group even in Level 1 of DED severity. There was also an increasing trend for DC density in higher levels of DED severity, although statistically nonsignificant. In contrast to DC density, the morphological parameters of DC showed significantly higher values compared to the control group in the higher levels of DED severity, i.e. Level 2 onwards. Therefore, it may be speculated that the early response of DC to the initiating events in DED is through increased number of these immune cells. With further activation of immune cells through increased tissue inflammation for example, which occurs in the more severe and/or chronic disease, DC may show increased size of cell body, and increased number and length of dendrites, which collectively result in larger DC field. These results of increased density but no significant change in morphology in level 1 are intriguing and warrant future prospective large studies to understand the pathophysiology in mild DED further.
Interestingly, our results show a trend towards decrease in morphological parameters in stage 4 of DED compared to stage 3. However, these changes were not statistically significant and fall within one standard deviation. Should this trend however hold true, it is possible that cornea nerves that are known to contribute to disease severity play a larger role in the latest severity stage and that active inflammation may not persist.
To elucidate whether the immune DC changes are independently associated with any clinical symptoms, clinical signs of tear production, or corneal and conjunctival epitheliopathy, as evidenced by vital dye staining, we conducted multivariate analysis of DC parameters with these factors and found that corneal staining is independently associated with DC density, DC size and number of dendrites, while conjunctival staining is independently associated with DC density. These findings indicate that vital dye staining may result in underlying immune activation or vice versa. This is in agreement with the recent study by Yang et al., where they found a correlation of vital dye staining of the conjunctiva and cornea with expression of inflammatory cytokines in DED [10].
All the microscopic changes in the ocular surface can be detected and quantified with IVCM. Therefore, IVCM may be used as a tool to detect the initial underlying immune changes of DED and also to track the progression of the disease process. A recent study by Villani et al. showed that OSDI scores and dendritic cell density decreased after treatment with topical corticosteroids [44]. Our study further elucidates morphological changes of DCs with disease severity. This is useful as the treatment regimen, particularly the type and dose of anti-inflammatory agents, can be tailored according to the stage of immune activation.
One of the limitations of this study is that it is retrospective in nature. Further, we have only analyzed the central corneal images. Although it is possible that the analysis of the peripheral cornea may reveal additional information regarding immune activation in DED, we have previously shown that there is so significant difference in mean subbasal DC density when assessing 3 representative images versus wide-field composite images of the cornea [45]. Further, routine use of IVCM in clinical practice may not always be feasible, due to time constraints, cost of equipment and dedicated personnel, and lack of automated softwares for DC analysis. However, given that IVCM can show the corneal ultrastructural details, it may be a good addition to practices focusing on ocular surface diseases. Additionally, at this time, the analysis is semi-automated but artificial intelligence softwares for automated analysis are underway [46]. Further, we have used both right and left eyes of the patients for statistical analysis independently. We used the GLM for statistical analysis to adjust the effect of examined eye on the outcome variables.
In conclusion, IVCM may prove to be a useful adjunctive diagnostic tool to help clinicians make an objective assessment of the corneal immune response in DED and could be used for tailored management based on the severity of underlying changes, even in early disease. Prospective randomized clinical trials are needed to evaluate the role of IVCM-guided management of DED in the clinical settings. Clinical trials are needed to assess changes in DC density and morphology and stratify patients based on degree of immune activation and correlate with clinical severity. This image-guided approach to diagnosis and treatment provides objective parameters for evaluating disease severity and potentially monitoring treatment efficacy.
Financial support
Funding was provided through grants from Allergan, Inc., Irvine, CA, USA, NIH K08-EY020575 (PH), and Falk Medical Research Foundation (PH).
Footnotes
Meeting presentations
This study was presented, in part, at the Tear Film and Ocular Surface Society Meeting, Sicily, Italy, 2013 and the Biennial Cornea Conference, Harvard Medical School, Boston 2013.
Declaration of competing interest
PH and BMC are patent inventors. Other authors have no conflicts of interest.
References
- [1].Friedman NJ. Impact of dry eye disease and treatment on quality of life. Curr Opin Ophthalmol 2010;21:310–6. [DOI] [PubMed] [Google Scholar]
- [2].Miljanovic B, Dana R, Sullivan DA, Schaumberg DA. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol 2007;143:409–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Farrand KF, Fridman M, Stillman IO, Schaumberg DA. Prevalence of diagnosed dry eye disease in the United States among adults aged 18 Years and older. Am J Ophthalmol 2017;182:90–8. [DOI] [PubMed] [Google Scholar]
- [4].Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, et al. TFOS DEWS II epidemiology report. Ocul Surf 2017;15:334–65. [DOI] [PubMed] [Google Scholar]
- [5].Yu J, Asche CV, Fairchild CJ. The economic burden of dry eye disease in the United States: a decision tree analysis. Cornea 2011;30:379–87. [DOI] [PubMed] [Google Scholar]
- [6].Bron AJ, de Paiva CS, Chauhan SK, Bonini S, Gabison EE, Jain S, et al. TFOS DEWS II pathophysiology report. Ocul Surf 2017;15:438–510. [DOI] [PubMed] [Google Scholar]
- [7].Stevenson W, Chauhan SK, Dana R. Dry eye disease: an immune-mediated ocular surface disorder. Arch Ophthalmol 2012;130:90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, et al. TFOS DEWS II definition and classification report. Ocul Surf 2017;15:276–83. [DOI] [PubMed] [Google Scholar]
- [9].Wolffsohn JS, Arita R, Chalmers R, Djalilian A, Dogru M, Dumbleton K, et al. TFOS DEWS II diagnostic methodology report. Ocul Surf 2017;15:539–74. [DOI] [PubMed] [Google Scholar]
- [10].Yang S, Lee HJ, Kim DY, Shin S, Barabino S, Chung SH. The use of conjunctival staining to measure ocular surface inflammation in patients with dry eye disease. Cornea 2019;38:698–705. [DOI] [PubMed] [Google Scholar]
- [11].Guthoff RF, Zhivov A, Stachs O. In vivo confocal microscopy, an inner vision of the cornea -a major review. Clin Exp Ophthalmol 2009;37:100–17. [DOI] [PubMed] [Google Scholar]
- [12].He J, Ogawa Y, Mukai S, Saijo-Ban Y, Kamoi M, Uchino M, et al. In vivo confocal microscopy evaluation of ocular surface with graft-versus-host disease-related dry eye disease. Sci Rep 2017;7:10720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kheirkhah A, Rahimi Darabad R, Cruzat A, Hajrasouliha AR, Witkin D, Wong N, et al. Corneal epithelial immune dendritic cell alterations in subtypes of dry eye disease: a pilot in vivo confocal microscopic study. Invest Ophthalmol Vis Sci 2015;56:7179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lin H, Li W, Dong N, Chen W, Liu J, Chen L, et al. Changes in corneal epithelial layer inflammatory cells in aqueous tear-deficient dry eye. Invest Ophthalmol Vis Sci 2010;51:122–8. [DOI] [PubMed] [Google Scholar]
- [15].Marsovszky L, Resch MD, Nemeth J, Toldi G, Legány N, Kovács L, et al. In vivo confocal microscopic evaluation of corneal Langerhans cell density, and distribution and evaluation of dry eye in rheumatoid arthritis. Innate Immun 2013;19:348–54. [DOI] [PubMed] [Google Scholar]
- [16].Tepelus TC, Chiu GB, Huang J, Huang P, Sadda SR, Irvine J, et al. Correlation between corneal innervation and inflammation evaluated with confocal microscopy and symptomatology in patients with dry eye syndromes: a preliminary study. Graefes Arch Clin Exp Ophthalmol 2017;255:1771–8. [DOI] [PubMed] [Google Scholar]
- [17].The definition and classification of dry eye disease: report of the definition and classification subcommittee of the international dry eye WorkShop. Ocul Surf 2007 2007;5:75–92. [DOI] [PubMed] [Google Scholar]
- [18].Knickelbein JE1, Buela KA, Hendricks RL. Antigen-presenting cells are stratified within normal human corneas and are rapidly mobilized during ex vivo viral infection. Invest Ophthalmol Vis Sci 2014;55:1118–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Knickelbein JE1, Watkins SC, McMenamin PG, Hendricks RL. Stratification of antigen-presenting cells within the normal cornea. Ophthalmol Eye Dis 2009;1:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hamrah P, Zhang Q, Liu Y, Dana MR. Novel characterization of MHC class II-negative population of resident corneal Langerhans cell-type dendritic cells. Invest Ophthalmol Vis Sci 2002;43:639–46. [PubMed] [Google Scholar]
- [21].Developed by wayne rasband National Institutes of Health, Bethesda, MD, available at: http://rsb.info.nih.gov/ij/http://rsb.info.nih.gov/ij/. [Google Scholar]
- [22].Sullivan DA, Hammitt KM, Schaumberg DA, Sullivan BD, Begley CG, Gjorstrup P, et al. Report of the TFOS/ARVO Symposium on global treatments for dry eye disease: an unmet need. Ocul Surf 2012;10:108–16. [DOI] [PubMed] [Google Scholar]
- [23].Methodologies to diagnose and monitor dry eye disease: report of the diagnostic methodology subcommittee of the international dry eye WorkShop (2007). Ocul Surf 2007;5:108–52. [DOI] [PubMed] [Google Scholar]
- [24].Johnson ME. The association between symptoms of discomfort and signs in dry eye. Ocul Surf 2009;7:199–211. [DOI] [PubMed] [Google Scholar]
- [25].Sullivan BD, Whitmer D, Nichols KK, Tomlinson A, Foulks GN, Geerling G, et al. An objective approach to dry eye disease severity. Invest Ophthalmol Vis Sci 2010;51:6125–30. [DOI] [PubMed] [Google Scholar]
- [26].Zheng X, de Paiva CS, Li DQ, Farley WJ, Pflugfelder SC. Desiccating stress promotion of Th17 differentiation by ocular surface tissues through a dendritic cell-mediated pathway. Invest Ophthalmol Vis Sci 2010;51:3083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Li S, Sack R, Vijmasi T, Sathe S, Beaton A, Quigley D, et al. Antibody protein array analysis of the tear film cytokines. Optom Vis Sci 2008;85:653–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Solomon A, Dursun D, Liu Z, Xie Y, Macri A, Pflugfelder SC. Pro-and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci 2001;42:2283–92. [PubMed] [Google Scholar]
- [29].Katsifis GE, Rekka S, Moutsopoulos NM, Pillemer S, Wahl SM. Systemic and local interleukin-17 and linked cytokines associated with Sjogren’s syndrome immunopathogenesis. Am J Pathol 2009;175:1167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kang MH, Kim MK, Lee HJ, Lee HI, Wee WR, Lee JH. Interleukin-17 in various ocular surface inflammatory diseases. J Kor Med Sci 2011;26:938–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Massingale ML, Li X, Vallabhajosyula M, Chen D, Wei Y, Asbell PA. Analysis of inflammatory cytokines in the tears of dry eye patients. Cornea 2009;28:1023–7. [DOI] [PubMed] [Google Scholar]
- [32].Pflugfelder SC, de Paiva CS, Li DQ, Stern ME. Epithelial-immune cell interaction in dry eye. Cornea 2008;27(Suppl 1):S9–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Alhatem A, Cavalcanti B, Hamrah P. In vivo confocal microscopy in dry eye disease and related conditions. Semin Ophthalmol 2012;27:138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Villani E, Mantelli F, Nucci P. In-vivo confocal microscopy of the ocular surface: ocular allergy and dry eye. Curr Opin Allergy Clin Immunol 2013;13:569–76. [DOI] [PubMed] [Google Scholar]
- [35].Hamrah P, Liu Y, Zhang Q, Dana MR. Alterations in corneal stromal dendritic cell phenotype and distribution in inflammation. Arch Ophthalmol 2003;121:1132–40. [DOI] [PubMed] [Google Scholar]
- [36a].Hamrah P, Zhang Q, Liu Y, Dana MR. Novel characterization of MHC class II-negative population of resident corneal Langerhans cell-type dendritic cells. Invest Ophthalmol Vis Sci 2002;43:639–46. [PubMed] [Google Scholar]
- [37].Villanova F, Di Meglio P, Inokuma M, Aghaeepour N, Perucha E, Mollon J, et al. Integration of lyoplate based flow cytometry and computational analysis for standardized immunological biomarker discovery. PLoS One 2013;8:e65485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Tuisku IS, Konttinen YT, Konttinen LM, Tervo TM. Alterations in corneal sensitivity and nerve morphology in patients with primary Sjogren’s syndrome. Exp Eye Res 2008;86:879–85. [DOI] [PubMed] [Google Scholar]
- [39].Zhivov A, Stave J, Vollmar B, Guthoff R. In vivo confocal microscopic evaluation of Langerhans cell density and distribution in the normal human corneal epithelium. Graefes Arch Clin Exp Ophthalmol 2005;243:1056–61. [DOI] [PubMed] [Google Scholar]
- [36b].Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 1998;392:245–52. [DOI] [PubMed] [Google Scholar]
- [40].Villani E, Magnani F, Viola F, Santaniello A, Scorza R, Nucci P, et al. In vivo confocal evaluation of the ocular surface morpho-functional unit in dry eye. Optom Vis Sci 2013;90:576–86. [DOI] [PubMed] [Google Scholar]
- [41].Cavalcanti BM, Cruzat A, Sahin A, Pavan-Langston D, Samayoa E, Hamrah P. In vivo confocal microscopy detects bilateral changes of corneal immune cells and nerves in unilateral herpes zoster ophthalmicus. Ocul Surf 2018;16:101–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Cruzat A, Witkin D, Baniasadi N, Zheng L, Ciolino JB, Jurkunas UV, et al. Inflammation and the nervous system: the connection in the cornea in patients with infectious keratitis. Invest Ophthalmol Vis Sci 2011;52:5136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Patel VD, Watanabe JH, Strauss JA, Dubey AT. Work productivity loss in patients with dry eye disease: an online survey. Curr Med Res Opin 2011;27:1041–8. [DOI] [PubMed] [Google Scholar]
- [44].Villani E, Garoli E, Termine V, Pichi F, Ratiglia R, Nucci P. Corneal confocal microscopy in dry eye treated with corticosteroids. Optom Vis Sci 2015;92:e290–5. [DOI] [PubMed] [Google Scholar]
- [45].Kheirkhah A, Muller R, Mikolajczak J, Ren A, Kadas EM, Zimmermann H, et al. Comparison of standard versus wide-field composite images of the corneal subbasal layer by in vivo confocal microscopy. Invest Ophthalmol Vis Sci 2015;56:5801–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Koseglu ND, Beam A, Hamrah P. The utilization of artificial intelligence for corneal nerve analyses of in vivo confocal microscopy images for the diagnosis of neuropathic corneal pain. ARVO abstract. Invest Ophthalmol Vis Sci 2018;59(9):3440.30025089 [Google Scholar]


