Abstract
Background and aims
We investigated whether dilation modifies the association between symptoms and esophageal eosinophilia (eos/hpf) in eosinophilic esophagitis (EoE) patients enrolled into randomized trial comparing efficacy of budesonide and fluticasone.
Methods
Baseline DSQ and EEsAI were available in 102 and 73 patients, respectively, of whom 56 and 39 underwent dilation at screening endoscopy before symptom assessment. The pair-wise relationship between DSQ, EEsAI, and eos/hpf was analyzed with nonparametric correlations.
Results
In non-dilated patients, the association between baseline eos/hpf and symptoms was moderate and significant, whilst it was abolished in dilated patients.
Conclusion
Dilation modifies association between symptoms and eos/hpf. (clinicaltrials.gov NCT02019758)
Keywords: eosinophilic esophagitis, dysphagia, dysphagia symptom questionnaire, eosinophilic esophagitis activity index, esophageal eosinophilia, correlation
INTRODUCTION
Esophageal dilation is used to manage adults with eosinophilic esophagitis (EoE).1,2,3,4 Using a non-validated dysphagia measure in patients managed with dilation alone, Schoepfer et al. observed a median post-dilation dysphagia improvement lasting ≥12 months.5 To date, dilation effect on symptoms has not been evaluated by patient-reported outcomes (PROs), including Dysphagia Symptom Questionnaire (DSQ) and Eosinophilic Esophagitis Activity Index (EEsAI).
We investigated whether dilation modifies the association between symptoms assessed using validated PROs and esophageal eosinophilia in EoE adults enrolled into a randomized trial comparing budesonide and fluticasone (NCT02019758).6
METHODS
Dilation was allowed during the screening endoscopy before symptom assessment at baseline. The pair-wise relationship between DSQ (0 to 84; 24-hour recall), EEsAI (0 to 100; 7-day recall), EoE Endoscopic Reference Score (EREFS), and peak esophageal eosinophils/high-power field (eos/hpf) was analyzed with nonparametric correlations.7,8,9,10,11 We used linear regression with eos/hpf as the outcome, EEsAI and DSQ as predictors, and an interaction for dilation and symptoms (see Supplementary Materials).
RESULTS
Of the 111 trial patients, 102 patients completed DSQ ≥4 days over 7-day period at baseline and 73 patients completed EEsAI (Suppl.Figure 1). At baseline, DSQ, EREFS, and eos/hpf were similar between the two groups (Suppl.Table 1).
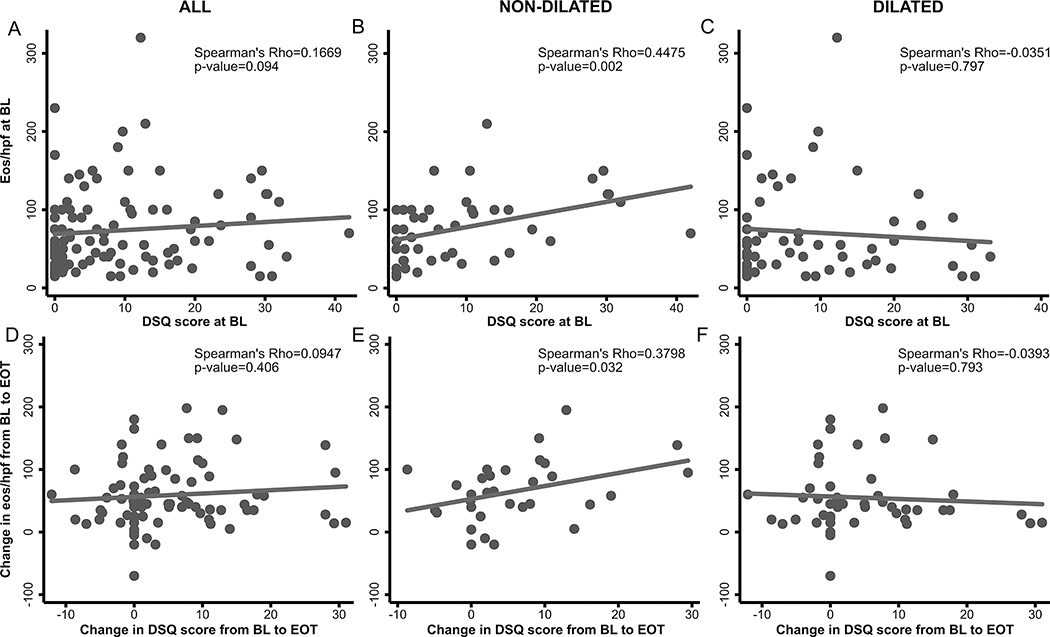
When assessing the relationship between DSQ, DSQ subscales, maximum dysphagia days/week, and eos/hpf at baseline (n=102) (Figure 1, Suppl.Table 2), we observed weak associations between eos/hpf and dysphagia symptoms. We observed moderate associations between the eos/hpf and dysphagia symptoms in non-dilated patients and no association between these in dilated patients. When examining the association between changes from baseline to end of treatment (EOT) in eos/hpf and DSQ (n=79), trends were similar.
Figure 1.
Relationship between baseline DSQ and esophageal eosinophilia in all patients (n=102) (A), in patients that did not undergo dilation (n=46) (B), and in patients that were dilated (n=56) (C) at study baseline. Relationship between change from baseline to end of treatment in DSQ and esophageal eosinophilia in all patients (n=79) (D), in patients that did not undergo dilation (n=32) (E), and in patients that were dilated (n=47) (F) at screening endoscopy. Abbreviations: BL, baseline; DSQ, dysphagia symptom score; EOT (end of treatment).
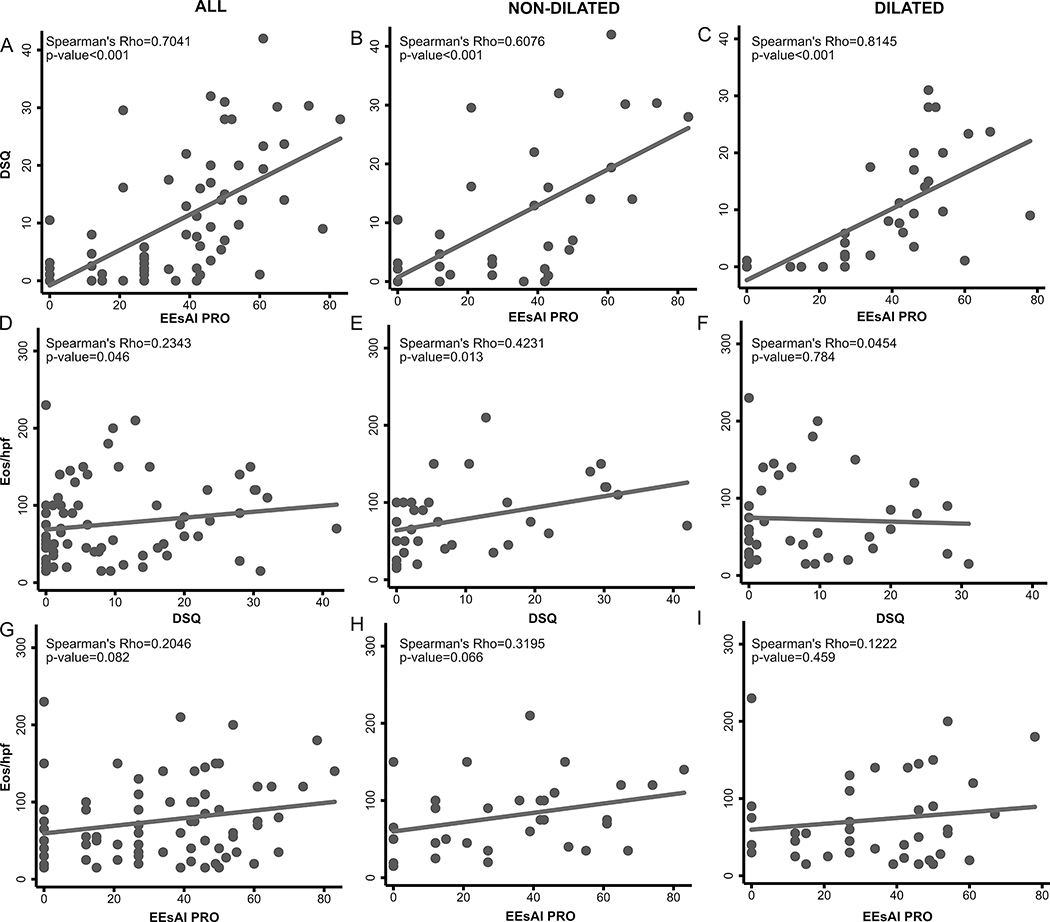
When analyzing subjects completing DSQ and EEsAI at baseline (n=73) (Figure 2, Suppl.Table 2, Suppl.Figure 2), we observed moderate to strong associations between DSQ and EEsAI scores regardless of dilation status. Irrespective of PRO used, we observed moderate correlations between symptoms and eos/hpf in non-dilated patients and no association in dilated patients.
Figure 2.
Relationship between baseline DSQ and EEsAI PRO in all patients (n=73) (A), in patients that did not undergo dilation (n=34) (B), and in patients that were dilated (n=39) (C) at study baseline. Relationship between baseline DSQ and esophageal eosinophilia in all patients (D), in patients that did not undergo dilation (E), and in patients that were dilated (F) at screening endoscopy. Relationship between baseline EEsAI PRO and esophageal eosinophilia in all patients (G), in patients that did not undergo dilation (H), and in patients that were dilated (I) at study baseline. Abbreviations: DSQ, dysphagia symptom score; EEsAI PRO, eosinophilic esophagitis activity index patient-reported outcomes instrument.
For a 10-unit DSQ increase in non-dilated patients, the predicted log-transformed eos/hpf increased by 27.1% (p-value=0.016) (Suppl.Table 3). For a 10-unit DSQ increase in dilated patients, the predicted eos/hpf decreased by 7.7% (p-value=0.398). When assessing the association between change in symptoms and eos/hpf from baseline to EOT (Suppl.Table 4; positive coefficient indicates PRO improvement or inflammation reduction), we found that predicted eos/hpf improves by 21 cells per 10-point DSQ improvement in non-dilated patients (p-value=0.016). In dilated patients, predicted eos/hpf decreased by 4 cells per 10-point DSQ improvement (p-value=0.511). The trends for DSQ subcomponents were similar.
The relationship between baseline dysphagia and predicted eos/hpf, and between change from baseline to EOT in dysphagia and predicted eos/hpf, is illustrated in Figure 3. Single variable linear regression analyses for non-dilated patients (46/102) at baseline and (32/79) at EOT are in Suppl.Table 5.
Figure 3.
The margin plot of expected esophageal eosinophilia stratified on dilation (n=102) by DSQ (A), dysphagia frequency component of DSQ (B), and maximum number of dysphagia days per week (C) at study baseline. The predictive margins of change from baseline to end of treatment in esophageal eosinophilia stratified on dilation (n=79) by change in DSQ (D), by change in dysphagia frequency component of DSQ (E), and by change in maximum number of dysphagia days per week (F). Abbreviations: BL, baseline; DSQ, dysphagia symptom score EOT (end of treatment).
a (A) in non-dilated patients with the DSQ score of 10 and 30 points, predicted values of 77 eos/hpf and 110 eos/hpf, respectively, are observed (A). In dilated patients with the DSQ score of 10 and 30 points, predicted values of 70 eos/hpf and 60 eos/hpf, respectively, are observed.
b (B) in non-dilated patients with maximum dysphagia days of 2, 4, and 6, predicted values of 67, 83, and 99 eos/hpf, respectively, are observed. In dilated patients with maximum dysphagia days of 2, 4, and 6, predicted values of 69, 72, and 75 eos/hpf, respectively, are observed.
We observed no associations between PROs and EREFS at baseline and for changes in EREFS and PRO from baseline to EOT regardless of dilation status.
DISCUSSION
Dilation performed before symptom assessment modifies the associations between baseline eos/hpf and symptom severity and between the change from baseline to EOT in these parameters. In non-dilated patients, the association between esophageal eosinophilia and symptom severity is moderate, and it is abolished in dilated patients.
The dilation effects likely last ~12 months.5 These findings are corroborated in a multicenter observational adult cohort, in which no association between symptom and eos/hpf in dilated patients and a moderate association in non-dilated patients was found.12
These are post-hoc analyses; hence, our findings should be regarded as observational. The interaction term between EEsAI-assessed symptoms and dilation was not significant in the 73-patient subset. The study limitations are countered by sound methodology and the fact that data come from a small, rigorously conducted RCT, during which validated endpoints were used.
Dilation modifies the association between eos/hpf and symptom severity. Consideration should be given to stratified randomization on dilation status at baseline in studies assessing efficacy of anti-inflammatory therapies in EoE patients, and monitoring symptoms only as a treatment outcome should be discouraged after dilation in the clinical setting.13,14
Supplementary Material
Supplementary Figure 2. Relationship between maximum dysphagia days (based on DSQ) and the frequency of the trouble swallowing (EEsAI PRO) (A-C), as well as between dysphagia frequency score (DSQ) and the frequency of the trouble swallowing (EEsAI PRO) (D-F) features. For each distribution, the box spans the values between the quartiles 1 and 3 (interquartile range), and the median is marked by horizontal line inside the box. The whiskers extend to the maximum of 1.5× the interquartile range beyond the box boundaries. Data beyond the range of whiskers are outliers and presented as points. In the trend test for each panel, p-values ≥ 0.004 or smaller were observed. Abbreviations: DSQ, dysphagia symptom score; EEsAI PRO, eosinophilic esophagitis activity index patient-reported outcomes instrument.
Supplementary Figure 1. Flow chart of patient populations. All the patients with complete DSQ (completed for at least 4 days in a seven-day period) and EEsAI PRO subdomains data at baseline and all the patients with complete DSQ at end of treatment were analyzed for the purposes of this study.
Acknowledgments
Grant support: Work supported by NIH R01 DK101856, and used resources from UNC Center for GI Biology and Disease (NIH P30 DK034987) and the UNC Translational Pathology Lab, which is supported in part by grants from the NCI (2-P30-CA016086-40), NIEHS (2-P30ES010126-15A1), UCRF, and NCBT (2015-IDG-1007). This work is also supported by a grant given to Ekaterina Safroneeva by Swiss National Science Foundation (Project number: 32473B_185008).
Conflict of interest: Ekaterina Safroneeva received consulting fees from Aptalis Pharma, Inc., Celgene Corp., Novartis, AG, and Regeneron Pharmaceuticals Inc. Alain M. Schoepfer received consulting fees and/or speaker fees and/or research grants from Adare Pharmaceuticals, Inc., AstraZeneca, AG, Switzerland, Aptalis Pharma, Inc., Celgene Corp., Dr. Falk Pharma, GmbH, Germany, Glaxo Smith Kline, AG, Nestlé S. A., Switzerland, Novartis, AG, Switzerland, Receptos, Inc., and Regeneron Pharmaceuticals, Inc. Marcel Zwahlen has no relevant financial, professional or personal relationships to disclose. Evan S. Dellon received research funding from: Adare, Allakos, GSK, Meritage, Miraca, Nutricia, Celgene/Receptos, Regeneron, Shire/Takeda; consulting fees from: Abbott, Adare, Aimmune, Allakos, Arena, AstraZeneca, Biorasi, Calypso, Celgene/Receptos, Eli Lilly, EsoCap, GSK, Gossamer Bio, Regeneron, Robarts, Salix, Shire/Takeda; and educations grants from: Allakos, Banner, Holoclara.
Abbreviations
- Adj.
adjusted
- CI
confidence interval
- DSQ
dysphagia symptom score
- EEsAI
eosinophilic esophagitis activity index
- eos/hpf
peak esophageal eosinophil counts per high-power filed
- EREFS
endoscopic reference score
- IQR
interquartile range
- PRO
patient-reported outcomes
Footnotes
Guarantor of the article: Evan S. Dellon, MD, MPH
REFERENCES
- 1.Lucendo AJ, Molina-Infante J, Arias Á, et al. Guidelines on eosinophilic esophagitis: evidence-based statements and recommendations for diagnosis and management in children and adults. United European Gastroenterol J. 2017;5:335–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dellon ES, Gonsalves N, Hirano I, et al. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol. 2013;108:679–92. [DOI] [PubMed] [Google Scholar]
- 3.Runge TM, Eluri S, Cotton CC, et al. Outcomes of Esophageal Dilation in Eosinophilic Esophagitis: Safety, Efficacy, and Persistence of the Fibrostenotic Phenotype. Am J Gastroenterol 2016;111:206–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dougherty M, Runge TM, Eluri S, Dellon ES. Esophageal dilation with either bougie or balloon technique as a treatment for eosinophilic esophagitis: a systematic review and meta-analysis. Gastrointest Endosc 2017;86:581–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schoepfer AM, Gonsalves N, Bussmann C, et al. Esophageal dilation in eosinophilic esophagitis: effectiveness, safety, and impact on the underlying inflammation. Am J Gastroenterol. 2010;105:1062–70. [DOI] [PubMed] [Google Scholar]
- 6.Dellon ES, Woosley JT, Arrington A, et al. Efficacy of Budesonide vs Fluticasone for Initial Treatment of Eosinophilic Esophagitis in a Randomized Controlled Trial. Gastroenterology. 2019;157:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schoepfer AM, Straumann A, Panczak R, et al. Development and validation of a symptom-based activity index for adults with eosinophilic esophagitis. Gastroenterology. 2014;147:1255–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dellon ES, Irani AM, Hill MR, Hirano I. Development and field testing of a novel patient-reported outcome measure of dysphagia in patients with eosinophilic esophagitis. Aliment Pharmacol Ther. 2013;38:634–42. [DOI] [PubMed] [Google Scholar]
- 9.Hirano I, Moy N, Heckman MG, et al. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut 2013;62:489–95. [DOI] [PubMed] [Google Scholar]
- 10.Dellon ES, Cotton CC, Gebhart JH, et al. Accuracy of the Eosinophilic Esophagitis Endoscopic Reference Score in Diagnosis and Determining Response to Treatment. Clin Gastroenterol Hepatol 2016;14:31–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dellon ES, Fritchie KJ, Rubinas TC, et al. Inter- and intraobserver reliability and validation of a new method for determination of eosinophil counts in patients with esophageal eosinophilia. Dig Dis Sci 2010;55:1940–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Safroneeva E, Aceves SS, King E, et al. Mo1187 Dilation reduces positive correlation between symptoms and histologic findings in adults with eosinophilic esophagitis. Gastroenterology 2020;158:S–819. [Google Scholar]
- 13.Dellon ES, Gupta SK. A conceptual approach to understanding treatment response in eosinophilic esophagitis. Clin Gastroenterol Hepatol 2019;17:2149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Runge TM, Eluri S, Woosley JT, et al. Control of inflammation decreases the need for subsequent esophageal dilation in patients with eosinophilic esophagitis. Dis Esophagus. 2017;30:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 2. Relationship between maximum dysphagia days (based on DSQ) and the frequency of the trouble swallowing (EEsAI PRO) (A-C), as well as between dysphagia frequency score (DSQ) and the frequency of the trouble swallowing (EEsAI PRO) (D-F) features. For each distribution, the box spans the values between the quartiles 1 and 3 (interquartile range), and the median is marked by horizontal line inside the box. The whiskers extend to the maximum of 1.5× the interquartile range beyond the box boundaries. Data beyond the range of whiskers are outliers and presented as points. In the trend test for each panel, p-values ≥ 0.004 or smaller were observed. Abbreviations: DSQ, dysphagia symptom score; EEsAI PRO, eosinophilic esophagitis activity index patient-reported outcomes instrument.
Supplementary Figure 1. Flow chart of patient populations. All the patients with complete DSQ (completed for at least 4 days in a seven-day period) and EEsAI PRO subdomains data at baseline and all the patients with complete DSQ at end of treatment were analyzed for the purposes of this study.