Abstract
Patients afflicted with pancreatic ductal adenocarcinoma (PDAC) face a dismal prognosis, but headway could be made if physicians could identify the disease earlier. A compelling strategy to broaden the use of surveillance for PDAC is to incorporate molecular biomarkers in combination with clinical analysis and imaging tools. This article summarizes the components involved in accomplishing biomarker validation and an analysis of the requirements of molecular biomarkers for disease surveillance. We highlight the significance of consortia for this research and highlight resources and infrastructure of the Early Detection Research Network (EDRN). The EDRN brings together the multifaceted expertise and resources needed for biomarker validation, such as study design, clinical care, biospecimen collection and handling, molecular technologies, and biostatistical analysis, and studies coming out of the EDRN have yielded biomarkers that are moving forward in validation. We close the article with an overview of the current investigational biomarkers, an analysis of their performance relative to the established benchmarks, and an outlook on the current needs in the field. The outlook for improving the early detection of PDAC looks promising, and the infrastructure of the EDRN has yielded biomarkers that are currently progressing in validation. The pace of further research should be quickened through the resources and expertise of the EDRN and other consortia.
Keywords: Pancreatic Cancer, Biomarkers, Early Detection Research Network, EDRN, Surveillance, Early Detection
Introduction
Treatments against pancreatic ductal adenocarcinoma (PDAC) are more effective when the disease is localized to the pancreas, and therefore, resectable. PDAC arises through a stepwise progression of well recognized histological precursor lesions that can be either microscopic (pancreatic intraepithelial neoplasia) or macroscopic (mucinous pancreatic cysts), culminating in invasive adenocarcinoma (1). The period of time covering this progression may be long, estimated to be over ten years in some cases (2). Despite the long development time, most patients—about 85%—discover they have the disease only after it has spread outside of the pancreas. For the ~15% who have the disease localized to the pancreas, surgical removal of the primary tumor is usually a viable option, in combination with systemic treatment using one of the first-line chemotherapeutics for pancreatic cancer. Durable responses in these conditions are possible for some patients, as about 25% are disease-free at five years and 50% survive past 4.5 years (3,4). For those who are diagnosed with metastatic disease, durable, 5-year survival is observed in only about 5% of the patients (3,4).
Underlying the late detection of PDACs is a lack of effective detection methods. Most diagnostic evaluation for PDAC occurs when a patient has symptoms suggestive of the disease, a point at which the cancer has typically progressed. Screening is not offered among people who have no symptoms because many people would be adversely affected by false-positive tests, or worse, by receiving treatments that were not necessary. Furthermore, the cost of screening to discover early-stage cancer in only about 1 of 10,000 people would not be justified. In 2019, the United States Screening and Prevention Task Force (USPSTF) reaffirmed its recommendation against screening the general population for PDAC due to the potential for overdiagnosis and possibility of harm (5).
Since screening the general population for PDAC is not feasible, current efforts have focused on identifying a subset of the population at an increased risk for PDAC development. Based on current guidelines, PDAC surveillance is recommended only in those high-risk individuals with a strong family history or a combination of family history and a germline mutation associated with risk (6). Other groups have elevated risk, but not high enough to warrant surveillance by imaging due to the high cost of identifying the relatively small proportion of subjects who would helped out of all who underwent screening (7,8).
Is there a way to broaden the surveillance for pre-invasive cancer PDAC? A viable strategy put forward recently (8) is to initially enrich the percentage of people likely to have pre-invasive disease using a lower-cost test (Fig. 1). Molecular biomarkers with high specificity and sensitivity for early-stage pancreatic cancer could meet this need. Because an image-confirmed mass or abnormality is usually required to prompt treatment, and the elevation of a biomarker alone is not sufficient to confirm a diagnosis of PDAC, the molecular test would be followed up by imaging in a two-stage strategy.
Figure 1. Overall Strategy for Surveillance of High-Risk Groups.
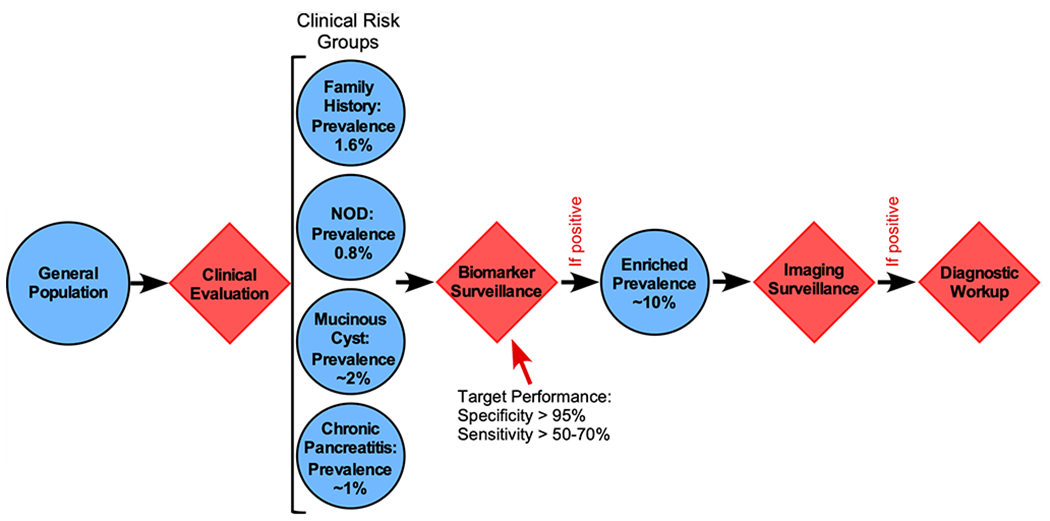
The first step is to identify individuals that fall into an elevated-risk group as defined by clinical characteristics.
Establishing the population to screen and adding a strategy for effective screening is a foundational step toward the goal of improved early detection. This article provides an analysis of the feasibility and outlook for accomplishing early detection, and it highlights the importance and contributions of collaborative networks, in particular the Early Detection Research Network, for facilitating progress. Considerable progress has been made in this area, both in the discovery of promising candidate biomarkers as well as in the development of resources and infrastructure to accelerate the pace and effectiveness of research.
Defining the Elevated-Risk Populations and the Requirements of Biomarkers
The overall strategy (Fig. 1) would be to use a combination of clinical, laboratory and molecular factors to select subjects eligible for surveillance, and then to assay a systemic biomarker at relatively low cost and in a body fluid that can be easily acquired, such as blood, saliva or urine. A positive test in the biomarker panel would lead to follow-up testing by non-invasive imaging (pancreas protocol CT or MRI scan) or tests that require more-invasive methods of sampling, such as pancreatic juice or endoscopic ultrasound combined with fine-needle aspiration (EUS-FNA). The potential benefit of this approach depends on the prevalence of early disease in the population to be monitored, as well as the assay of the biomarker used for surveillance. Below, two situations are considered: people with no symptoms, and people with pancreatic cysts.
Surveillance Among Asymptomatic Individuals
In the first case, surveillance would be performed among people without symptoms or imaging results suggestive of incipient disease, but with a clinical or genetic condition placing them at elevated risk (7,9,10).
One high-risk group that is explicitly not included in the USPSTF’s “do not screen” recommendation is individuals with familial PDAC, defined both by the frequency of PDAC cases within a family and by deleterious germline mutations in cancer-predisposition genes (11). The genes implicated in familial PDAC include BRCA2 and ATM (most commonly), as well as BRCA1, PALB2, p16/CDKN2A, and the mismatch repair genes hMLH1, hMSH2 and hPMS6. While the lifetime risk of cancer varies within this group (depending upon the specific germline mutation), the overall prevalence of either incipient cancer or high-grade dysplasia is estimated at 1.6% (12). Two studies provide evidence that surveillance could produce better outcomes among people with a family history of PDAC or risk-associated genetic mutations. In both studies, the percentage of patients diagnosed with early-stage PDAC was much higher than historical rates (12,13). Some centers already perform routine imaging surveillance on such individuals, but a molecular biomarker could enrich prevalence even more. A biomarker with specificity 95% and sensitivity 34% would enrich prevalence (i.e. the positive predictive value, PPV) to 10% for subsequent imaging with a low-cost initial test, potentially lowering costs by reducing unnecessary imaging with a negative predictive value (NPV) of 98.9%.
Another indicator of elevated risk is sudden-onset type-2 diabetes (14), in which the prevalence of pancreatic cancer may be as high as 0.8% (15). While not high enough to justify screening by imaging, a molecular biomarker could reasonably be applied to this group. For example, at 0.8% prevalence, a biomarker with 96% specificity and 65% sensitivity would enrich prevalence to 11.6% (the positive predictive value, PPV) while correctly identifying 99.7% of the non-affected individuals (i.e. the NPV is 99.7%). This enrichment could be high enough to warrant further surveillance by imaging. A third condition potentially warranting an initial screen is chronic pancreatitis, but the ultimate risk for PDAC has been hard to determine with certainty.
Surveillance Among Individuals with Pancreatic Cysts
Patients with mucinous pancreatic cysts are considered separately because they have an identified precursor lesion, and because mucinous pancreatic cysts are genetically distinct from the solid precursor lesions of pancreatic intraepithelial neoplasia. The diagnosis and management of pancreatic cysts is described thoroughly elsewhere (1,8,16), but relevant to this article is that a pancreatic cyst can be a precursor to invasive cancer. Currently, over 20 types of cystic lesions of the pancreas are recognized. Many types, including serous cystadenomas and pseudocysts, are benign and can be monitored clinically. In contrast, mucinous pancreatic cysts, which include intraductal papillary mucinous neoplasms (IPMNs) and mucinous cystic neoplasms (MCNs), have the potential to progress to pancreatic ductal adenocarcinoma. Removing a pre-invasive cyst could potentially save a patient’s life, but surgery on a cyst that did not need to be removed is harmful. The operative mortality and morbidity of these procedures range from 2% to 4% and 40% to 50%, respectively (17–20). But the vast majority of patients will not need surgery, as only 3-6% of IPMNs will undergo malignant transformation within a patient’s lifetime (21,22).
The imaging tools that are used to make the initial diagnoses are not highly accurate for identifying lesions having high-grade dysplasia, considered the trigger-point for justifying surgical removal. To improve diagnostic accuracy, cyst fluid can be analyzed after endoscopic ultrasound-guided the fine-needle aspiration (EUS-FNA). Analysis usually includes assessments of the viscosity of the fluid, the carcinoembryonic antigen (CEA) concentrations, and the cytopathology of cells found in the fluid. Viscosity and CEA help to confirm the diagnosis of mucinous cysts but do not predict malignant potential (23–27). Cytopathology is highly specific for malignancy—if cancer cells are present, the diagnosis is certain—but it is often limited by the high percentage of samples with low cell counts (28–30). Several medical societies have sought to optimize the detection and treatment of high-risk mucinous cysts by developing management guidelines (31–35). However, the sensitivities and specificities of these guidelines vary widely, which is not surprising given the suboptimal quality of evidence on which the recommendations are based. Thus, there is an urgent need for additional pancreatic cyst biomarkers. Depending on the performance of a biomarker, it could be used to select patients for surgical removal or careful monitoring of the cyst, or to identify patients with suspicious-appearing but indolent cysts who do not need surgery.
Contributions of the EDRN: Moving from Discovery to Validation
Addressing the Challenge through Consortia
Carrying out the research to validate new biomarkers brings significant challenges. In the discovery phase of biomarker research, literally thousands of publications have appeared presenting potential biomarkers for pancreatic cancer. In contrast, the publications describing large-scale validation are minimal in number. Factors that contribute to the low number of validation studies include 1) poor-quality discovery research leading to false leads; 2) the difficulty of transitioning molecular discoveries to high-quality assays meeting the analytical requirements of a clinical assay; or 3) the difficulty in coordinating and executing the many components involved in validation research.
Figure 2A graphically presents the multiple components of the research to develop early-detection biomarkers. Each component is important and influences on the success of the overall project. For example, the researchers doing the molecular analysis must know how to analyze specimens from the clinic in a way that provides unbiased, accurate, and reproducible results. In order to properly carry out that work, the investigators need samples that were collected from the setting in which the test would be applied, with uniform standard operating procedures (SOPs) for handling and processing so that the molecular analyses are not negatively affected. This step requires input from biostatisticians and knowledge of appropriate study design. In particular, this expertise is paramount for selecting appropriately-matched disease controls to mitigate false-discovery rates and the “overfitting” of biomarker performance. Additional partners are needed to process and distribute samples in a blinded, randomized, and consistent way. After data are collected and analyzed, biostatisticians are again needed to provide independent analysis of the results.
Figure 2. Components of Biomarker Studies.
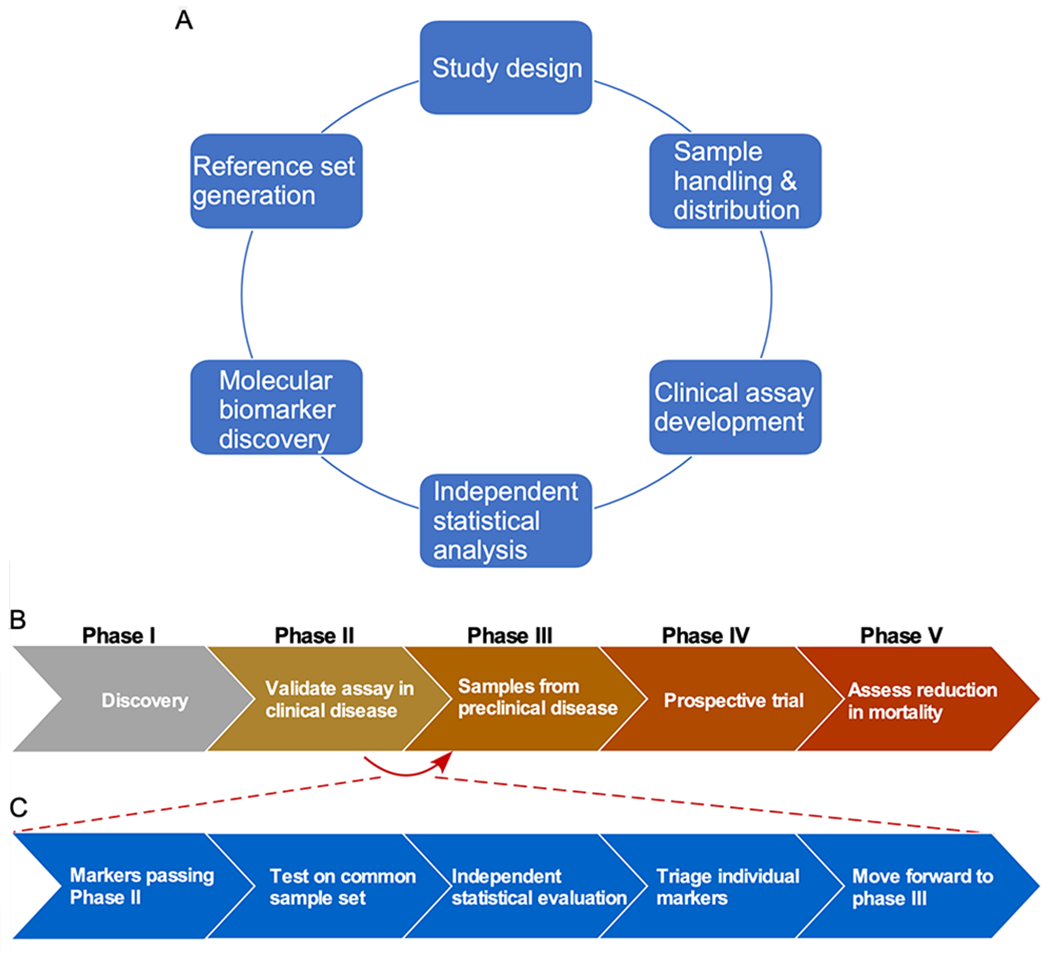
A) Various resources and areas of expertise are needed for successful biomarker projects in both the discovery and validation phases. B) The five phases of validating an early-detection biomarker are presented. C) The reference sets and comparative studies of the EDRN are valuable for determining which biomarkers should move from Phase II to Phase III. The flowchart shows the plan for the multi-laboratory studies of the EDRN.
The multiple, distinct areas of expertise and resources contributing to the research are beyond what a single group or institution could routinely provide. Thus, having an infrastructure in place to provide the resources for multiple projects would be greatly helpful, both for the discovery and for the validation phases of biomarker evaluation. Meeting this need motivated development of the EDRN. By organizing the infrastructure support in all of the components of Figure 2, the EDRN consortium fosters high-quality candidates in discovery research as well as a path forward in validation. The value of this infrastructure has been displayed in the EDRN-supported research in PDAC, and as discussed throughout this special issue, in other tumor types as well. Below, we outline the resources that were developed specifically for PDAC research, and we provide an overview of the promising results coming out of projects that need use of EDRN resources.
Reference Sets
The development of reference sets of human specimens is a major contribution of the EDRN to PDAC biomarker research. Over a decade ago, most biomarkers were discovered and validated on collections of biospecimens consisting of mostly advanced-stage PDACs and healthy controls, often collected, processed and stored in a non-standardized manner by researchers. Additionally, long delays in validating a biomarker could occur, as investigators often required a second collection of biospecimen samples. To overcome these barriers to PDAC biomarker research, the EDRN created over 10 years ago a standard operating procedure (SOP) for pancreatic cancer blood-derived biomarkers (36). Successive SOPs developed by the EDRN for other sample types (cyst fluid, urine, etc.) have played a major role in determining SOPs for subsequent consortia including the Chronic Pancreatitis, Diabetes, and Pancreatic Cancer (CPDPC) consortium (37). The SOPs are available at the EDRN website.
These SOPs made it possible to collect biospecimens from multiple centers in a uniform manner, thereby limiting a potential source of bias (38) and enabling the development of sets of uniformly-collected, banked specimens, known as reference sets. A common set of banked biospecimens allows for independent, blinded evaluations of candidate biomarkers in a timely manner. In addition, it permits accurate comparisons between candidate biomarkers and facilitates the formation of biomarker panels comprising biomarkers from different laboratories.
The EDRN Pancreatic Cancer Working Group approved funding for the first of two reference sets for pancreatic cancer-related biomarker development in 2007. The aim of the first reference set was to develop well-characterized serum and plasma specimens to use in testing biomarkers for the early detection of PDAC. The PDAC samples were entirely from early-stage (stage I or II) cancer, which are the most difficult to detect yet the most important for potential positive impact. The control subjects included both healthy people and patients with benign conditions of the pancreas, as certain benign conditions can be hard to distinguish from pancreatic cancer and could cause elevations in cancer biomarkers. The set include samples from multiple institutions, so that each is not representative of only one geographical area. Sample collection followed a single, detailed SOP to prevent the introduction of bias. A major strength of the set is that the extensive inclusion criteria (detailed in reference 36) ensured the accuracy of diagnosis for each of the patient groups. For example, the diagnosis of stage I or II cancer was confirmed for all subjects by surgical pathology. A limitation is that the set the is not suitable for studying racial disparities, as 91.5% of participants were from the white population. A summary of the sample composition is in Table 1. To date this reference set has been used in several studies (36,39,40). An application for the use of the set, and the other EDRN-sponsored reference sets, is available at the EDRN website.
Table 1. Composition of the EDRN Reference Set for PDAC Studies.
The samples are available as both serum and EDTA plasma. Addition information stratified by collection site, patient group, age range, race, and tumor stage are provided in reference 36.
| Ref Set 1 - PDAC | |
|---|---|
| Total samples, N | 252 |
| Cancer, N | 98 |
| Average age, y (stdev) | 70.4 (9.7) |
| Percent male | 44 |
| Control, N | 154 |
| Average age, y (stdev) | 64.0 (10.1) |
| Percent male | 47.4 |
| Cancer stages | |
| Stage I, N (%) | 15 (15.3) |
| Stage II, N (%) | 83 (84.7) |
| Stage III, N (%) | |
| Stage IV, N (%) | |
| Control types | |
| Chronic pancreatitis, N (%) | 62 (40.3) |
| Benign biliary stricture, N (%) | 31 (20.1) |
| Healthy control, N (%) | 61 (39.6) |
We learned many notable lessons from the creation of the first reference set. First, we noted the difficulties in predicting, prior to surgery, who has early stage pancreatic cancer (defined as small tumors limited to the pancreas with negative lymph nodes). We used the rigorous standard of pathological staging (post-surgical evaluation of resected tissue) instead of just clinical staging (non-operative cases included). This standard demonstrated the rare nature of early-stage cancers, defined as small tumors limited to the pancreas with negative lymph nodes. Despite having six major sites involved with patient recruitment, we were only able to collect 25 early-stage cancers over an 18-month period and thus had to expand our eligibility criteria to include node-positive patients who underwent R0 pancreatic resections. Another lesson was the challenge of recruiting well-matched controls. Since pancreatic cancer is a disease of older individuals, it was critical to limit the accrual of younger age patients to the control population. We also learned that it was possible for multiple centers to adhere to the EDRN-created SOPs. An important part of the SOP was storage of samples in small aliquots, thereby limiting the need for a sample to undergo repeated freeze-thaws prior to distribution to multiple sites. Another part was the collection of specified information accompanying each sample, known as the EDRN common data elements. These lessons will benefit the future development of reference sets.
The second reference set supported by the EDRN was proposed in 2011 with the primary goal to be a resource for the development and evaluation of biomarkers for predicting the malignant potential of pancreatic cystic lesions (excluding main duct IPMNs). The main clinical focus is on determining those patients who would most benefit from surgical resection of their pancreatic cyst(s). This reference set also has the capability of utilizing associated demographic, radiologic and biomarker data in developing management strategies. Due to the limited number of resections at any one site, a multi-center collaborative effort under the guidance of the Early Detection Research Network was required to ensure the collection of high-quality cyst fluid and blood specimens following a standardized SOP. Cyst fluids are collected at time of EUS, surgery or both, and all blood samples are obtained prior to EUS and/or surgical resection. The inclusion and exclusion criteria are given below. Importantly, about 60% of the total 450 subjects will have surgically-verified pathology. The cystic reference set will be completed by the summer of 2020 and made available for investigators through the application process (found on the EDRN website) administered by the EDRN GI collaborative group.
Inclusion Criteria
18 years of age or older
- Meet any one of the following criteria from the international consensus guidelines for resection26:
- Symptomatic from their pancreatic cyst
- Side branch cyst along with main duct involvement (mixed main duct‐ side branch IPMN)
- Presence of mural nodules in cyst
- At least one cyst greater than 3 cm in size
- Surgeon decided to remove cyst
- At least 4ml of Serum, 2ml of Plasma AND a minimum of 1ml of Cystic Fluid obtained by EUS or by
- surgery or preferably by both options
- Blood collected w/in 12 weeks prior to Surgery
Exclusion Criteria
Prior Pancreatic Cancer
Prior Pancreatic Surgery
Prior history of any other malignancy except non‐melanoma skin cancers for five years (Disease free for 5 years)
Multi-Laboratory Studies
Another major contribution of the EDRN infrastructure in PDAC biomarker research is the coordination of multi-laboratory studies.
Characterization of CA19-9 in the PDAC reference set
An initial study using the first reference set was performed to characterize the performance of the CA19-9 assay. CA19-9 is a useful biomarker to assist diagnosis or to monitor disease burden, but it is not used to detect incipient disease because the risk of both false negative and false positive diagnoses is unacceptably high (41). The previous studies of CA19-9 were heavily weighted toward late-stage cancer, primarily due to sample availability, but with the reference set, the PDAC collaborative group of the EDRN had the opportunity to accurately define the performance of CA19-9 for distinguishing stage I-II disease from the potentially confounding conditions of benign biliary obstruction and chronic pancreatitis. The study found an average sensitivity and specificity of 70-74%, slightly lower than previous studies using all stages of cancer and mostly healthy people as controls. Another outcome related to the source of divergent results between distinct CA19-9 assays, which we traced to variation in the specificities of the antibodies for the carbohydrate structure bound by the CA19-9 antibodies (42,43). This study laid down a precise benchmark against which new biomarkers could be evaluated.
Comparative studies of PDAC biomarkers
Another multi-laboratory study using the resources of the EDRN addressed the transition of biomarkers through successive phases of validation. The phases of validation required for a biomarker for early detection of cancer were outlined in a landmark analysis in 2001 (44), depicted in Figure 2B. Phase I is for discovery, Phase II is for validation of an assay using samples from clinically-identified disease, and Phase III is for validation using samples collected prior to clinically-identified disease or symptoms. Phases III and IV deal with large clinical trials. With each phase, sample resources become more costly. The bottlenecks for validating biomarkers are determining which candidates are worthy of advancing and then gaining access to the resources needed. More often than not, promising results in early studies are not substantiated in follow up studies (45,46). A consistent and systematic approach to evaluating candidate biomarkers is required.
The infrastructure of the EDRN is ideally suited for validating candidates that could move from Phase II to Phase III (Fig. 2C). In a recent study, the collaborators within the EDRN performed a comparative analysis of the best biomarkers from EDRN laboratories using a common set of samples collected through the EDRN clinical validation centers. A set of plasma specimens was relabeled by personnel from the EDRN Data Management and Coordinating Center (DMCC) and distributed to multiple sites. The investigators at each site ran their own assays (listed in Table 2), made case/control calls on the samples, and sent the calls to a DMCC biostatistician, who then provided an objective assessment of the performance of the biomarkers by comparing the case/control calls to a gold-standard, “true” case/control status. This is the most rigorous and objective test of a biomarker, and the most accurate comparison between biomarkers. Most reports of candidate biomarkers do not include such a blinded validation test. Instead, they generally report an evaluation of the performance after adjustments of the thresholds and rules used for classifying cases and controls.
Table 2. Markers Tested in the Comparative Study.
VAI, Van Andel Institute; MDACC, MD Anderson Cancer Center; UNMC, University of Nebraska Medical Center; UPMC, University of Pittsburgh Medical Center; FHCRC, Fred Hutchinson Cancer Research Center. All studies used blood plasma.
Aside from assessing individual biomarkers, an additional opportunity provided by this study design is to assess the complementarity of disparate biomarkers. Two or more biomarkers might work well together in combination, but to find such a relationship, the markers must be run together on the same sample set. Below we describe the outcomes from such an analysis.
Cross-laboratory study of cyst-fluid biomarkers
While many cyst-fluid biomarkers have been retrospectively on various patient cohorts, a comprehensive and rigorous comparison of the top candidates has not been performed. Hence, the EDRN is sponsoring a large validation study with the intention to advance pancreatic cyst biomarker development to clinically available assays. This study makes use of a reference set of cyst fluid specimens that was assembled using specimens from four different centers. All centers had followed an EDRN SOP and contributed the samples to a central site, from which aliquots were distributed blinded to six laboratories that each ran their own biomarker assays. This study will provide invaluable information about the performance of the various cyst-fluid biomarkers that have shown promise in previous studies.
Markers Developed Through the EDRN
Results from the multi-laboratory comparative study
The EDRN’s multi-laboratory study described above resulted in the validation of a biomarker that achieved a significant improvement over CA19-9. The biomarker panel is a simple combination between CA19-9 and another marker called sTRA, so named because it detects the sialylated version of the glycan bound by the TRA-1-60 (tumor-related antigen) antibody. The sTRA glycan is structurally related to the CA19-9 antigen—it also is a glycan—and it is produced by a different subtype of cancer cells than make CA19-9 (47,48). As a result, elevations in the new biomarker are complementary to elevations in CA19-9 (Figs. 3A and 3B). The combination test, in which patients who are elevated in either sTRA or CA19-9 were classified as a ‘case,’ performed significantly better than CA19-9 in the blinded analysis with preset thresholds and classification rules (49) (Fig. 3C), which is the most rigorous evaluation of a biomarker. The collaborative group is now moving this marker panel forward to the next round of blinded validation.
Figure 3. Validated Biomarker from the EDRN Comparative Study.
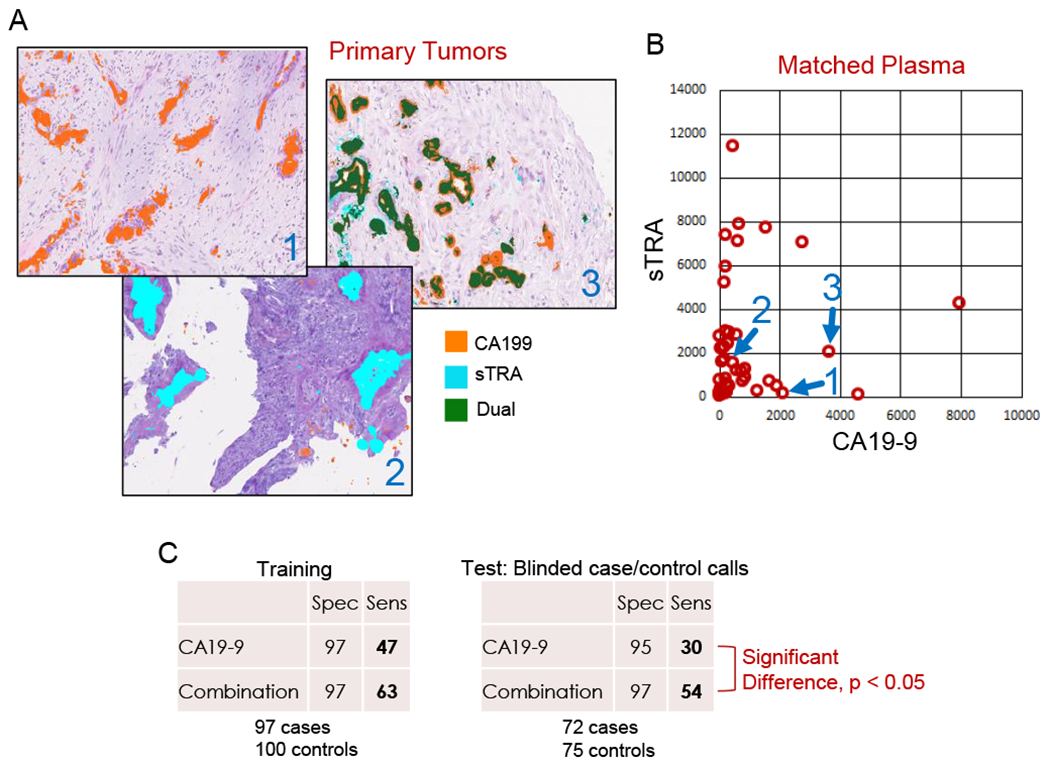
A) The new sTRA biomarker is complementary to CA19-9 in immunofluorescence staining of primary tumors. Tumors frequently have predominantly one or the other marker, or both. B) In plasma specimens from the same patients as in panel A, the relative plasma levels match the tumor levels. C) In a simple combination test in which patients that have elevated plasma levels of either CA19-9 or sTRA, the sensitivity at 95% specificity is enhanced relative to CA19-9. Using case/control calls made on blinded specimens and independently evaluated, the improvement was statistically significant.
Other markers showed promise in the re-analysis phase. One such marker was MUC5AC in combination with CA19-9 (50), which was particularly valuable for differentiating early-stage pancreatic cancer (up to stage 2B) from chronic pancreatitis. The combination gave a sensitivity and specificity of 86% and 78%, respectively, compared to 69.8% sensitivity 31.7 % specificity for CA19-9. Another marker panel used CA19-9 combined with TIMP1 and LRG1, which in previous research performed better than CA19-9 alone in several independent sample sets (51,52). Further improvement was possible by combining with a five-metabolite panel (53). In the comparative study, both TIMP1 and LRG1 had an AUC > 0.63 as individual markers, good enough for further investigation as members of biomarker panels. TIMP1 in particular contributed to the novel cross-laboratory panel described below. For each of the markers, we explored the effects of the demographic covariates of age, race, and smoking status by fitting logistic regression models with and without the covariates. The effects of those demographic variables were not statistically significant.
Combining disparate markers across laboratories revealed potential benefit. From the 13 candidate markers that were run across five different laboratories on the common set, a panel selected by lasso analysis included the following markers: CA19-9, angiostatin, thrombospondin, sTRA, DCD, PMS2, TIMP1, and MUC4 (Table 2). The naïve panel performance was AUC = 0.91 and sensitivity 0.79 at 95% specificity, which was superior to the performance of any individual biomarker. Further validation must be done, but this result warrants further testing and suggests the value of including markers of different molecular types within a panel. The panel shown in Table 2 includes proteins, glycans, and protein-autoantibody complexes.
Overall, the success of this complex study, involving multiple sample sources, several experimental sites, and independent statistical analyses, demonstrates the effectiveness of the collaborative group structure operated by the EDRN. This initial multi-laboratory study had the statistical relevance to: estimate the performance of individual markers; demonstrate the potential of an individual marker to improve over CA19-9; and provide an estimated biomarker panel across labs that can be validated in future study. The sensitivities and specificities of markers estimated in this study will provide parameters to guide the design of future epidemiological studies for validating marker performance.
Cyst-fluid biomarkers
The EDRN has supported the development of a wide range of pancreatic cyst fluid biomarkers, including genetic, epigenetic, proteomic, and carbohydrate-based markers, that are currently being validated. An approach that has recently translated into clinically-available assays is next-generation sequencing (NGS) in the evaluation of pancreatic cyst fluid. NGS studies previously identified distinct mutational profiles for the major pancreatic cysts and those that have progressed to pancreatic cancer, and the adaptation of the technology to detect KRAS and/or GNAS mutations in cyst fluid is highly accurate for mucinous cysts with sensitivities of 76-89% and specificities of 96-100% (24,54,55). Further, mucinous cyst-associated cancers are reported to harbor alterations in TP53, CDKN2A, SMAD4 and genes within the mTOR pathway with sensitivities and specificities that range between 71% and 79% and 96% and 100%, respectively (24,54–60). While NGS assays of pancreatic cyst fluid are available at a few major academic centers, its widespread use has been impeded by high costs. However, with increasing availability of NGS, decreasing prices in reagents, and the ability to batch specimens, the costs of NGS are declining rapidly and are currently one-third of the cost of an MRI scan.
Other biomarkers could be a good alternative to NGS-based biomarkers, potentially by providing simpler alternatives at locations where NGS is not available, or by providing complementary data that adds value to NGS. The abundant expression of mucin proteins, such as MUC2, MUC4 and MUC5AC, within pancreatic cyst fluid is frequently associated with mucinous differentiation (61,62). Further, glycan variants of MUC5AC are particularly sensitive (87-89%) and specific (80-100%) for diagnosing mucinous cysts (63). Studies have also shown an accumulation of aberrant DNA methylation patterns in mucinous cyst-associated cancers. These alterations can be detected within pancreatic cyst fluid and have demonstrated sensitivities of 88-90% and specificities of 86-92% for high-grade dysplasia and invasive adenocarcinoma (64,65). High levels of a marker detected by the Das-1 monoclonal antibody in pancreatic cyst fluid was discovered to be a promising biomarker for high-grade dysplasia and invasive adenocarcinoma in mucinous cysts and cross validated in a large cohort of pancreatic cystic lesions (66,67). Similarly, telomerase activity is enhanced in mucinous cysts with high-grade dysplasia and/or invasive carcinoma and could be used as a predictor of neoplastic grade (68). Since various biomarkers are showing good results so far, the ability to evaluate directly and compare performance in a common sample set, as described above, will be critical to assessing which biomarkers are best suited for further validation. This is the goal of the ongoing EDRN-sponsored Pancreatic Cyst Biomarker Validation Study, which is a blinded analysis with the intent to improve the classification and prognostication of pancreatic cysts, and ultimately to advance the investigational biomarkers to clinically-available assays.
Assessing the Current Investigational Biomarkers
Specifying a benchmark for early-detection biomarkers allows us to ask the critical questions, how are the biomarkers performing, and how likely are they to make an impact? The answers that are the most accurate will come from well-designed, blinded comparative studies such as those undertaken by the EDRN. In the recent study by the EDRN, the validated panel performance (Fig. 3) approaches the benchmark with specificity 95% and sensitivity 54% with the blinded case/control calls and sensitivity 63% using post-hoc adjustments. In light of this level of validation, the panel could be considered the most promising panel from the currently available information. If further validated, it may already be good enough for application to patients with inherited risk, where prevalence is highest, and potentially for other groups.
Assessments of other biomarkers may not be accurate until a study is performed like the one undertaken by the EDRN, but it is still useful to perform general comparisons based on published results. One of the most promising developments has been the detection of mutated, cell-free DNA in the circulation of cancer patients. The majority of patients with pancreatic cancer harbor oncogenic mutations in the KRAS genes in their tumors. A PCR-based assay to detect such mutated DNA in the circulation identified about 30% of pancreatic cancer patients with near-perfect specificity relative to healthy controls, and the combination with CA19-9 and other markers could increase sensitivity to 64% at 99.5% specificity (69). These studies did not characterize specificity among benign conditions, and they did not validate using case/control calls made on blinded specimens.
Several other markers or marker panels show sufficient performance in the initial reports to warrant further study but await rigorous validation. A combination protein-metabolite panel developed through the EDRN has reported sensitivities >60% at specificity >95% (53); thrombospondin-2 in combination with CA19-9 gave 87% sensitivity and 98% specificity for distinguishing PDAC from healthy controls (70); and a drop in specific isoforms of apolipoprotein AII in combination with elevated CA19-9 using optimized thresholds gave >50% sensitivity and >95% specificity for discriminative early-stage PDAC healthy controls (39). These selected highlights of a robust field of research show that it may be possible to meet or exceed the standards specified for surveillance among high-risk populations.
The discovery pipeline is also generating multiple candidate biomarkers for further validation. For example, high-content molecular analysis technologies could yield dozens or even hundreds of candidates, which in turn could be examined in combination with previously validated biomarkers. The discovery technologies span proteins, glycans, metabolites, nucleic acids, epigenetics, lipids, microbiota—in short, every known type of cellular and molecular feature. The reports are more numerous than can be covered here, but some examples of novel approaches are the following. Genomic and proteomic analyses of peripheral blood mononuclear cells yielded 114 proteins deregulated in pancreatic cancer (71). Autoantibodies targeting mutated, misfolded, de novo/over-expressed and differentially modified tumor and tumor-associated antigens could constitute biomarkers for early diagnosis and improved prognosis of multiple malignancies including pancreatic cancer (reviewed in (72)). Ginesta et al, assessed the methylation status of HRH2, EN1, SPARC, CDH13 and APC across 61 fine needle aspirates of 43 PC and 18 CP cases. Whenever two or more promoters were hypermethylated, 73% SN (95% CI 56-86%) and absolute specificity was observed. Combination of KRAS mutation improved the SN to 84% (95% CI 69-93%), maintaining a 100% specificity (73).
Challenges and Outlook
The data summarized here boosts optimism that improved detection of pre-invasive pancreatic cancer will be achieved. The importance of early detection is highlighted by a recent study showing 5-year survival of around 85% among patients diagnosed with stage 1a PDAC (74). Establishing the goals, requirements, and roadmap for implementation is a major foundational step, and through the EDRN and other consortia, the resources and infrastructure are in place to conduct the complex, multifaceted research required. Another encouraging fact is that the new biomarkers in validation are finally showing improvements over CA19-9. Some significant challenges remain to be addressed, as described below.
Phase III Validation: Samples Collected Prior to Diagnosis from Asymptomatic People
The biomarkers described above are still relatively early in the complete validation process. Most of the validation work has been in Phase II studies (Figure 2B), but the transition to stage 3 is particularly important for biomarkers for surveillance. The development of sample resources for Phase III, involving subjects not showing symptoms of the disease, continues to be a challenge. For practical purposes, most of the discovery and validation studies use samples collected from patients who have presented with symptoms suspicious for an underlying cancer that prompted further evaluation. Such a patient population is enriched in PDAC cases, permitting the collection of a statistically-meaningful number of cases in a relatively short time-span. To collect samples from asymptomatic patients, a prospective study with long-term follow-up is needed. In order to accrue enough cases of patients who subsequently developed cancer, a prospective trial needs to enroll a very large cohort, especially for a disease like PDAC that is relatively uncommon at an incidence rate of ~13 cases per 100,000 adults. Therefore, the samples are rare and valuable and normally reserved only for biomarkers that have shown significant promise in previous studies.
Samples from screening trials have been used in early-phase research, as demonstrated in two studies supported by the EDRN. One of the studies used samples from the Prostate, Lung, Colon, and Ovarian (PLCO) cohort study performed by the NCI (75). The PLCO screening trial was designed to study the effects of various screening methods on mortality from prostate, lung, colon, or ovarian cancers. A retrospective analysis uncovered 248 cases of PDAC (or non-neuroendocrine cancer) out of 62,581 subjects (76). Samples from 175 of the PDAC cases, collected 1-34 months before diagnosis, were used in a multiplexed-immunoassay profiling study. The investigators found that a panel consisting of CA19-9, CEA, and Cyfra 21-1 provided a significantly elevated sensitivity of 32.4% and 29.7% in samples collected <12 and >12 months prior to diagnosis, respectively, compared to 25.7% and 17.2% for CA19-9 alone (77). Another study used pre-diagnostic samples from the Women’s Health Initiative (WHI), which focused on the prevention of heart disease, breast cancer, and osteoporosis in post-menopausal women (78). A three-marker panel had an AUC of 0.64 and in combination with CA19-9 improved the specificity of CA19-9 alone from 0.46 to 0.52 (79). The panel is being further trained and tested in collaboration with the EDRN, using samples from PLCO.
Another prospectively-assembled cohort that has become a “goldmine” for discovery of disease-associated biomarkers (both genetic and laboratory based) is the UK Biobank (80). This open access resource collected genotypic and phenotypic data from 500,000 British adults between 2006-2010. The UKBB set has over 700 PDAC cases with pre-diagnostic blood samples, ~235 of which were obtained within three years preceding diagnosis. Pre-diagnostic biospecimens can be requested for biomarker studies and have already yielded promising data for various cancers. A pair of studies out of the UK used samples from the biobank that were collected >2 years before a PDAC diagnosis (81,82). The authors found that a low level of TSP1 in combination with CA19-9 gave a modest improvement over CA19-9 for identifying subjects who go on to develop pancreatic cancer. The studies confirmed the elevation of CA19-9 prior to diagnosis in a subset of patients—about 20-30% with a 5-10% false-positive rate—and provided hints that additional markers could positively add to CA19-9. Studies with blinded case/control calls have not been performed.
One of the goals of the EDRN PDAC working group is to test its “optimal” biomarker panel in these invaluable sets of samples and to integrate the panels with laboratory and/or genetic variables in order to identify the individuals most likely to harbor an asymptomatic PDAC. Similarly, the European Prospective Investigation into Cancer and Nutrition (EPIC) is a prospective study led by the International Agency for Research on Cancer (IARC) that has collected longitudinal biospecimens and information on lifestyle and environmental factors from all participants. This cohort could be another valuable source of pre-diagnostic specimens in future trans-Atlantic collaborative endeavors with the EDRN.
Table 3. Development of a Cross-Laboratory Biomarker Panel.
Estimates and 95% Confidence interval of AUC (area-under-the-curve) and sensitivity at 95% specificity for individual markers and a marker panel. See Table 2 for site abbreviations.
| Site | Biomarker | AUC | Sensitivity at 95% specificity |
|---|---|---|---|
| - | CA19-9 | 0.85 (0.79, 0.91) | 0.5 (0.23, 0.67) |
| VAI | sTRA | 0.85 (0.77, 0.91) | 0.57 (0.28, 0.77) |
| UPMC | Thrombospondin | 0.72 (0.62, 0.81) | 0.25 (0.09, 0.44) |
| FHCRC | DCD | 0.62 (0.49, 0.71) | 0.1 (0.02, 0.27) |
| MDACC | TIMP1 | 0.62 (0.49, 0.72) | 0.15 (0.03, 0.32) |
| UNMC | MUC4 | 0.58 (0.48, 0.67) | 0.09 (0.03, 0.18) |
| UPMC | Angiostatin | 0.55 (0.47, 0.63) | 0.06 (0.01, 0.13) |
| FHCRC | PMS2 | 0.51 (0.46, 0.62) | 0.07 (0.01, 0.22) |
| All | Panel | 0.91 (0.88, 0.98) | 0.79 (0.55, 0.93) |
Acknowledgements
Financial support: This work was supported by the National Cancer Institute (Early Detection Research Network, U01 CA152653 to R.E.B. and B.B.H; Alliance of Glycobiologists for Cancer Detection, U01 CA226158 to B.B.H. and R.E.B.; U01 CA200468, U01 CA196403, R01 CA218004 and P50 CA221707 to A.M; U01 CA200466 to R.E.B and S.K.B.; U01 CA210240 to S.K.B.)
Footnotes
Conflict of interest statement: The authors declare no potential conflicts of interest.
References
- 1.Pittman ME, Rao R, Hruban RH. Classification, Morphology, Molecular Pathogenesis, and Outcome of Premalignant Lesions of the Pancreas. Arch Pathol Lab Med 2017;141:1606–14. [DOI] [PubMed] [Google Scholar]
- 2.Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 2010;467:1114–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 2007;297:267–77. [DOI] [PubMed] [Google Scholar]
- 4.Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. The New England journal of medicine 2018;379:2395–406. [DOI] [PubMed] [Google Scholar]
- 5.Force UPST. Screening for Pancreatic Cancer: US Preventive Services Task Force Reaffirmation Recommendation Statement. JAMA 2019;322:438–44. [DOI] [PubMed] [Google Scholar]
- 6.Goggins M, Overbeek KA, Brand R, Syngal S, Del Chiaro M, Bartsch DK, et al. Management of patients with increased risk for familial pancreatic cancer: updated recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut 2020;69:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lennon AM, Wolfgang CL, Canto MI, Klein AP, Herman JM, Goggins M, et al. The Early Detection of Pancreatic Cancer: What Will It Take to Diagnose and Treat Curable Pancreatic Neoplasia? Cancer research 2014;74:3381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singhi AD, Koay EJ, Chari ST, Maitra A. Early Detection of Pancreatic Cancer: Opportunities and Challenges. Gastroenterology 2019;156:2024–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly KA, Hollingsworth MA, Brand RE, Liu CH, Singh VK, Srivastava S, et al. Advances in Biomedical Imaging, Bioengineering, and Related Technologies for the Development of Biomarkers of Pancreatic Disease: Summary of a National Institute of Diabetes and Digestive and Kidney Diseases and National Institute of Biomedical Imaging and Bioengineering Workshop. Pancreas 2015;44:1185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young MR, Wagner PD, Ghosh S, Rinaudo JA, Baker SG, Zaret KS, et al. Validation of Biomarkers for Early Detection of Pancreatic Cancer: Summary of The Alliance of Pancreatic Cancer Consortia for Biomarkers for Early Detection Workshop. Pancreas 2018;47:135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudley B, Brand RE. Pancreatic Cancer Surveillance: Who, When, and How. Current treatment options in gastroenterology 2019;17:681–91. [DOI] [PubMed] [Google Scholar]
- 12.Canto MI, Almario JA, Schulick RD, Yeo CJ, Klein A, Blackford A, et al. Risk of Neoplastic Progression in Individuals at High Risk for Pancreatic Cancer Undergoing Long-term Surveillance. Gastroenterology 2018;155:740–51.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vasen H, Ibrahim I, Ponce CG, Slater EP, Matthai E, Carrato A, et al. Benefit of Surveillance for Pancreatic Cancer in High-Risk Individuals: Outcome of Long-Term Prospective Follow-Up Studies From Three European Expert Centers. J Clin Oncol 2016;34:2010–9. [DOI] [PubMed] [Google Scholar]
- 14.Sah RP, Nagpal SJ, Mukhopadhyay D, Chari ST. New insights into pancreatic cancer-induced paraneoplastic diabetes. Nat Rev Gastroenterol Hepatol 2013;10:423–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chari ST, Leibson CL, Rabe KG, Ransom J, de Andrade M, Petersen GM. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology 2005;129:504–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raman A, Lennon AM. Cyst Fluid Biomarkers - Diagnosis and Prediction of Malignancy for Cystic Lesions of the Pancreas. Visceral medicine 2018;34:178–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimura W, Miyata H, Gotoh M, Hirai I, Kenjo A, Kitagawa Y, et al. A pancreaticoduodenectomy risk model derived from 8575 cases from a national single-race population (Japanese) using a web-based data entry system: the 30-day and in-hospital mortality rates for pancreaticoduodenectomy. Ann Surg 2014;259:773–80. [DOI] [PubMed] [Google Scholar]
- 18.Hsu CC, Wolfgang CL, Laheru DA, Pawlik TM, Swartz MJ, Winter JM, et al. Early mortality risk score: identification of poor outcomes following upfront surgery for resectable pancreatic cancer. J Gastrointest Surg 2012;16:753–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenblatt DY, Kelly KJ, Rajamanickam V, Wan Y, Hanson T, Rettammel R, et al. Preoperative factors predict perioperative morbidity and mortality after pancreaticoduodenectomy. Ann Surg Oncol 2011;18:2126–35. [DOI] [PubMed] [Google Scholar]
- 20.Beltrame V, Gruppo M, Pastorelli D, Pedrazzoli S, Merigliano S, Sperti C. Outcome of pancreaticoduodenectomy in octogenarians: Single institution’s experience and review of the literature. J Visc Surg 2015;152:279–84. [DOI] [PubMed] [Google Scholar]
- 21.Pergolini I, Sahora K, Ferrone CR, Morales-Oyarvide V, Wolpin BM, Mucci LA, et al. Long-term Risk of Pancreatic Malignancy in Patients With Branch Duct Intraductal Papillary Mucinous Neoplasm in a Referral Center. Gastroenterology 2017;153:1284–94 e1. [DOI] [PubMed] [Google Scholar]
- 22.Oyama H, Tada M, Takagi K, Tateishi K, Hamada T, Nakai Y, et al. Long-term Risk of Malignancy in Branch-Duct Intraductal Papillary Mucinous Neoplasms. Gastroenterology 2020;158:226–37 e5. [DOI] [PubMed] [Google Scholar]
- 23.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology 2004;126:1330–6. [DOI] [PubMed] [Google Scholar]
- 24.Singhi AD, McGrath K, Brand RE, Khalid A, Zeh HJ, Chennat JS, et al. Preoperative next-generation sequencing of pancreatic cyst fluid is highly accurate in cyst classification and detection of advanced neoplasia. Gut 2018;67:2131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maire F, Voitot H, Aubert A, Palazzo L, O’Toole D, Couvelard A, et al. Intraductal papillary mucinous neoplasms of the pancreas: performance of pancreatic fluid analysis for positive diagnosis and the prediction of malignancy. The American journal of gastroenterology 2008;103:2871–7. [DOI] [PubMed] [Google Scholar]
- 26.Linder JD, Geenen JE, Catalano MF. Cyst fluid analysis obtained by EUS-guided FNA in the evaluation of discrete cystic neoplasms of the pancreas: a prospective single-center experience. Gastrointest Endosc 2006;64:697–702. [DOI] [PubMed] [Google Scholar]
- 27.Shami VM, Sundaram V, Stelow EB, Conaway M, Moskaluk CA, White GE, et al. The level of carcinoembryonic antigen and the presence of mucin as predictors of cystic pancreatic mucinous neoplasia. Pancreas 2007;34:466–9. [DOI] [PubMed] [Google Scholar]
- 28.Scheiman JM, Hwang JH, Moayyedi P. American gastroenterological association technical review on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 2015;148:824–48 e22. [DOI] [PubMed] [Google Scholar]
- 29.Maker AV, Lee LS, Raut CP, Clancy TE, Swanson RS. Cytology from pancreatic cysts has marginal utility in surgical decision-making. Ann Surg Oncol 2008;15:3187–92. [DOI] [PubMed] [Google Scholar]
- 30.Khalid A, Brugge W. ACG practice guidelines for the diagnosis and management of neoplastic pancreatic cysts. Am J Gastroenterol 2007;102:2339–49. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka M, Fernandez-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017;17:738–53. [DOI] [PubMed] [Google Scholar]
- 32.Vege SS, Ziring B, Jain R, Moayyedi P, Clinical Guidelines C, American Gastroenterology A. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 2015;148:819–22; quize 12–3. [DOI] [PubMed] [Google Scholar]
- 33.Elta GH, Enestvedt BK, Sauer BG, Lennon AM. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am J Gastroenterol 2018;113:464–79. [DOI] [PubMed] [Google Scholar]
- 34.Megibow AJ, Baker ME, Morgan DE, Kamel IR, Sahani DV, Newman E, et al. Management of Incidental Pancreatic Cysts: A White Paper of the ACR Incidental Findings Committee. J Am Coll Radiol 2017;14:911–23. [DOI] [PubMed] [Google Scholar]
- 35.European Study Group on Cystic Tumours of the P. European evidence-based guidelines on pancreatic cystic neoplasms. Gut 2018;67:789–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haab BB, Huang Y, Balasenthil S, Partyka K, Tang H, Anderson M, et al. Definitive Characterization of CA 19–9 in Resectable Pancreatic Cancer Using a Reference Set of Serum and Plasma Specimens. PLoS One 2015;10:e0139049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fisher WE, Cruz-Monserrate Z, McElhany AL, Lesinski GB, Hart PA, Ghosh R, et al. Standard Operating Procedures for Biospecimen Collection, Processing, and Storage: From the Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer. Pancreas 2018;47:1213–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng Z, Kagan J, Pepe M, Thornquist M, Ann Rinaudo J, Dahlgren J, et al. The Early Detection Research Network’s Specimen reference sets: paving the way for rapid evaluation of potential biomarkers. Clinical chemistry 2013;59:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Honda K, Kobayashi M, Okusaka T, Rinaudo JA, Huang Y, Marsh T, et al. Plasma biomarker for detection of early stage pancreatic cancer and risk factors for pancreatic malignancy using antibodies for apolipoprotein-AII isoforms. Scientific reports 2015;5:15921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balasenthil S, Huang Y, Liu S, Marsh T, Chen J, Stass SA, et al. A Plasma Biomarker Panel to Identify Surgically Resectable Early-Stage Pancreatic Cancer. Journal of the National Cancer Institute 2017;109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19–9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol 2007;33:266–70. [DOI] [PubMed] [Google Scholar]
- 42.Partyka K, Maupin KA, Brand RE, Haab BB. Diverse monoclonal antibodies against the CA 19–9 antigen show variation in binding specificity with consequences for clinical interpretation. Proteomics 2012;12:2212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Magnani JL, Nilsson B, Brockhaus M, Zopf D, Steplewski Z, Koprowski H, et al. A monoclonal antibody-defined antigen associated with gastrointestinal cancer is a ganglioside containing sialylated lacto-N-fucopentaose II. The Journal of biological chemistry 1982;257:14365–9. [PubMed] [Google Scholar]
- 44.Sullivan Pepe M, Etzioni R, Feng Z, Potter JD, Thompson ML, Thornquist M, et al. Phases of biomarker development for early detection of cancer. Journal of the National Cancer Institute 2001;93:1054–61. [DOI] [PubMed] [Google Scholar]
- 45.Ransohoff DF. Bias as a threat to the validity of cancer molecular-marker research. Nat Rev Cancer 2005;5:142–9. [DOI] [PubMed] [Google Scholar]
- 46.Ransohoff DF. How to improve reliability and efficiency of research about molecular markers: roles of phases, guidelines, and study design. J Clin Epidemiol 2007;60:1205–19. [DOI] [PubMed] [Google Scholar]
- 47.Tang H, Partyka K, Hsueh P, Sinha JY, Kletter D, Zeh H, et al. Glycans related to the CA19-9 antigen are elevated in distinct subsets of pancreatic cancers and improve diagnostic accuracy over CA19-9. Cell Mol Gastroenterol Hepatol 2016;2:201–21 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barnett D, Liu Y, Partyka K, Huang Y, Tang H, Hostetter G, et al. The CA19-9 and Sialyl-TRA Antigens Define Separate Subpopulations of Pancreatic Cancer Cells. Scientific reports 2017;7:4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Staal B, Liu Y, Barnett D, Hsueh P, He Z, Gao CF, et al. The sTRA Plasma Biomarker: Blinded Validation of Improved Accuracy over CA19-9 in Pancreatic Cancer Diagnosis. Clin Cancer Res 2019;29:2745–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaur S, Smith LM, Patel A, Menning M, Watley DC, Malik SS, et al. A Combination of MUC5AC and CA19-9 Improves the Diagnosis of Pancreatic Cancer: A Multicenter Study. The American journal of gastroenterology 2017;112:172–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Capello M, Bantis LE, Scelo G, Zhao Y, Li P, Dhillon DS, et al. Sequential Validation of Blood-Based Protein Biomarker Candidates for Early-Stage Pancreatic Cancer. Journal of the National Cancer Institute 2017;109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Faca VM, Song KS, Wang H, Zhang Q, Krasnoselsky AL, Newcomb LF, et al. A mouse to human search for plasma proteome changes associated with pancreatic tumor development. PLoS medicine 2008;5:e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fahrmann JF, Bantis LE, Capello M, Scelo G, Dennison JB, Patel N, et al. A Plasma-Derived Protein-Metabolite Multiplexed Panel for Early-Stage Pancreatic Cancer. Journal of the National Cancer Institute 2019;111:372–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Springer S, Wang Y, Dal Molin M, Masica DL, Jiao Y, Kinde I, et al. A Combination of Molecular Markers and Clinical Features Improve the Classification of Pancreatic Cysts. Gastroenterology 2015;149:1501–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Springer S, Masica DL, Dal Molin M, Douville C, Thoburn CJ, Afsari B, et al. A multimodality test to guide the management of patients with a pancreatic cyst. Sci Transl Med 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosenbaum MW, Jones M, Dudley JC, Le LP, Iafrate AJ, Pitman MB. Next-generation sequencing adds value to the preoperative diagnosis of pancreatic cysts. Cancer Cytopathol 2017;125:41–47. [DOI] [PubMed] [Google Scholar]
- 57.Jones M, Zheng Z, Wang J, Dudley J, Albanese E, Kadayifci A, et al. Impact of next-generation sequencing on the clinical diagnosis of pancreatic cysts. Gastrointest Endosc 2016;83:140–8. [DOI] [PubMed] [Google Scholar]
- 58.Garcia-Carracedo D, Chen ZM, Qiu W, Huang AS, Tang SM, Hruban RH, et al. PIK3CA mutations in mucinous cystic neoplasms of the pancreas. Pancreas 2014;43:245–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schonleben F, Qiu W, Ciau NT, Ho DJ, Li X, Allendorf JD, et al. PIK3CA mutations in intraductal papillary mucinous neoplasm/carcinoma of the pancreas. Clin Cancer Res 2006;12:3851–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schonleben F, Qiu W, Remotti HE, Hohenberger W, Su GH. PIK3CA, KRAS, and BRAF mutations in intraductal papillary mucinous neoplasm/carcinoma (IPMN/C) of the pancreas. Langenbecks Arch Surg 2008;393:289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nagata K, Horinouchi M, Saitou M, Higashi M, Nomoto M, Goto M, et al. Mucin expression profile in pancreatic cancer and the precursor lesions. J Hepatobiliary Pancreat Surg 2007;14:243–54. [DOI] [PubMed] [Google Scholar]
- 62.Moniaux N, Chaturvedi P, Varshney GC, Meza JL, Rodriguez-Sierra JF, Aubert JP, et al. Human MUC4 mucin induces ultra-structural changes and tumorigenicity in pancreatic cancer cells. Br J Cancer 2007;97:345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sinha J, Cao Z, Dai J, Tang H, Partyka K, Hostetter G, et al. A Gastric Glycoform of MUC5AC Is a Biomarker of Mucinous Cysts of the Pancreas. PLoS One 2016;11:e0167070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hata T, Dal Molin M, Hong SM, Tamura K, Suenaga M, Yu J, et al. Predicting the Grade of Dysplasia of Pancreatic Cystic Neoplasms Using Cyst Fluid DNA Methylation Markers. Clin Cancer Res 2017;23:3935–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Majumder S, Taylor WR, Yab TC, Berger CK, Dukek BA, Cao X, et al. Novel Methylated DNA Markers Discriminate Advanced Neoplasia in Pancreatic Cysts: Marker Discovery, Tissue Validation, and Cyst Fluid Testing. Am J Gastroenterol 2019;114:1539–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Das KK, Geng X, Brown JW, Morales-Oyarvide V, Huynh T, Pergolini I, et al. Cross Validation of the Monoclonal Antibody Das-1 in Identification of High-Risk Mucinous Pancreatic Cystic Lesions. Gastroenterology 2019;157:720–30 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Das KK, Xiao H, Geng X, Fernandez-Del-Castillo C, Morales-Oyarvide V, Daglilar E, et al. mAb Das-1 is specific for high-risk and malignant intraductal papillary mucinous neoplasm (IPMN). Gut 2014;63:1626–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hata T, Dal Molin M, Suenaga M, Yu J, Pittman M, Weiss M, et al. Cyst Fluid Telomerase Activity Predicts the Histologic Grade of Cystic Neoplasms of the Pancreas. Clin Cancer Res 2016;22:5141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cohen JD, Javed AA, Thoburn C, Wong F, Tie J, Gibbs P, et al. Combined circulating tumor DNA and protein biomarker-based liquid biopsy for the earlier detection of pancreatic cancers. Proceedings of the National Academy of Sciences of the United States of America 2017;114:10202–07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim J, Bamlet WR, Oberg AL, Chaffee KG, Donahue G, Cao XJ, et al. Detection of early pancreatic ductal adenocarcinoma with thrombospondin-2 and CA19-9 blood markers. Sci Transl Med 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li H, Mao Y, Xiong Y, Zhao HH, Shen F, Gao X, et al. A Comprehensive Proteome Analysis of Peripheral Blood Mononuclear Cells (PBMCs) to Identify Candidate Biomarkers of Pancreatic Cancer. Cancer Genomics Proteomics 2019;16:81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dumstrei K, Chen H, Brenner H. A systematic review of serum autoantibodies as biomarkers for pancreatic cancer detection. Oncotarget 2016;7:11151–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ginesta MM, Mora J, Mayor R, Farre A, Peinado MA, Busquets J, et al. Genetic and epigenetic markers in the evaluation of pancreatic masses. J Clin Pathol 2013;66:192–7. [DOI] [PubMed] [Google Scholar]
- 74.Blackford AL, Canto MI, Klein AP, Hruban RH, Goggins M. Recent trends in the incidence and survival of Stage 1A Pancreatic Cancer: A Surveillance, Epidemiology, and End Results analysis. Journal of the National Cancer Institute 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gohagan JK, Prorok PC, Hayes RB, Kramer BS. The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial of the National Cancer Institute: history, organization, and status. Controlled clinical trials 2000;21:251s–72s. [DOI] [PubMed] [Google Scholar]
- 76.Anderson KE, Mongin SJ, Sinha R, Stolzenberg-Solomon R, Gross MD, Ziegler RG, et al. Pancreatic cancer risk: associations with meat-derived carcinogen intake in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) cohort. Mol Carcinog 2012;51:128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nolen BM, Brand RE, Prosser D, Velikokhatnaya L, Allen PJ, Zeh HJ, et al. Prediagnostic serum biomarkers as early detection tools for pancreatic cancer in a large prospective cohort study. PLoS One 2014;9:e94928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Controlled clinical trials 1998;19:61–109. [DOI] [PubMed] [Google Scholar]
- 79.Mirus JE, Zhang Y, Li CI, Lokshin AE, Prentice RL, Hingorani SR, et al. Cross-species antibody microarray interrogation identifies a 3-protein panel of plasma biomarkers for early diagnosis of pancreas cancer. Clin Cancer Res 2015;21:1764–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS medicine 2015;12:e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jenkinson C, Elliott VL, Evans A, Oldfield L, Jenkins RE, O’Brien DP, et al. Decreased Serum Thrombospondin-1 Levels in Pancreatic Cancer Patients Up to 24 Months Prior to Clinical Diagnosis: Association with Diabetes Mellitus. Clin Cancer Res 2016;22:1734–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.O’Brien DP, Sandanayake NS, Jenkinson C, Gentry-Maharaj A, Apostolidou S, Fourkala EO, et al. Serum CA19-9 Is Significantly Upregulated up to 2 Years before Diagnosis with Pancreatic Cancer: Implications for Early Disease Detection. Clin Cancer Res 2015;21:622–31. [DOI] [PMC free article] [PubMed] [Google Scholar]


