Abstract
Aging is the most significant risk factor for vascular cognitive impairment (VCI), and the number of individuals affected by VCI is expected to exponentially increase in the upcoming decades. Yet, there are no current preventative or therapeutic treatments available against the development and progression of VCI. Therefore, there is a pressing need to better understand the pathophysiology underlying these conditions, for the development of novel tools and interventions to improve cerebrovascular health and delay the onset of VCI. There is strong epidemiological and experimental evidence that lifestyle factors, including nutrition and dietary habits, significantly affect cerebrovascular health and thereby influence the pathogenesis of VCI. Here, recent evidence is presented discussing the effects of lifestyle interventions against age-related diseases which in turn, inspired novel research aimed at investigating the possible beneficial effects of dietary interventions for the prevention of cognitive decline in older adults.
Keywords: Time restricted feeding, Aging, Neurovascular coupling, Neurodegeneration Geroscience, Dementia, Cognitive function
1. Introduction
Declining cerebrovascular health is quickly becoming recognized as a major hallmark of age-related cognitive decline1–7. To account for this fact, the term “vascular cognitive impairment” (VCI) was coined to describe all forms of cognitive disorders associated with cerebrovascular pathologies3, 4. There is an increasing realization that the pathogenesis of Alzheimer’s disease (AD) also involves microvascular pathologies2 and as such, it may represent a special form of VCI associated with old age4.
The study of age-related VCI is a growing global health priority. The number of individuals living over the age of 60 is projected to increase from 901 million today to nearly 2.1 billion by 20508. As advanced age is the most significant risk factor for VCI, the number of individuals affected by VCI is expected to exponentially increase in the upcoming decades3, 4. Yet, there are no current preventative or therapeutic treatments available against the development and progression of VCI. There is a pressing need to better understand the pathophysiology underlying these conditions, for the development of novel tools and interventions to improve cerebrovascular health and delay the onset of VCI.
There is strong epidemiological and experimental evidence that lifestyle factors, including nutrition and dietary habits, significantly affect cerebrovascular health and thereby influence the pathogenesis of VCI3, 4. In recent years, growing interest in the effects of lifestyle interventions against age-related diseases has inspired novel research aimed at investigating the possible beneficial effects of dietary interventions for the prevention of cognitive decline in older adults.
2. Dietary interventions to promote healthy vascular aging: from calorie restriction to time-restricted feeding
Calorie restriction (CR) is a dietary regimen that reduces the daily food intake of an individual relative to its normal energy consumption, without causing malnutrition. CR exerts clear beneficial effects on healthspan and lifespan in short-lived species17–19, positively impacts health of long-lived non-human primates (Macaca mulatta)9–11, and recently was also shown to increase maximum lifespan in the gray mouse lemur (Microcebus murinus) by more than 22 percent9.
Experimental evidence suggests that CR is an effective nutritional lifestyle intervention that can improve cardiovascular health12–15 and cognitive function16–20. A published study suggests that even when implemented over a short period, CR can confer cardiovascular health benefits12. CR studies performed in the last two decades have been critical in improving our understanding of the physiological mechanisms by which CR prevents vascular aging21 and extends lifespan in many animal models10, 22–24. CR has been documented to exert multifaceted cardiovascular protective effects, including reduced oxidative damage13, 25, improved insulin sensitivity26, improved endothelial function27–31 and nitric oxide (NO) bioavailability27, 32–36, and reduced risk of atherosclerosis15. Besides the observed beneficial effects of CR on the large conduit vasculature, CR was also shown to confer persistent anti-oxidative, pro-angiogenic, and anti-inflammatory effects, and promote an anti-aging gene expression profile in cerebromicrovascular endothelial cells of aged rats37. Recent evidence from non-human primate studies suggests that circulating factors induced by CR also promote endothelial protective effects, up-regulating endothelial angiogenic processes38. This is
In the late 1980s two parallel studies10, 39 were initiated to determine the effect of CR in rhesus monkeys. While the impact on lifespan differed between these two studies, both groups observed a substantial improvement in healthspan, indicating that CR-derived benefits are conserved in monkeys40. This evidence suggests that the ability of CR to convey health benefits may also be translatable to humans40, 41. To better understand the feasibility and translatability of CR in humans, the National Institute on Aging (NIA) supported an innovative 2-year long clinical trial named Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy (CALERIE)42 where over 200 healthy young and middle-aged individuals in were assigned to a 25% CR regimen or to continue their regular diet. Amongst the results, individuals assigned to 25% CR were able to reduce their calorie intake by only 11.9%. Despite the scant adherence, CR administration in healthy individuals resulted in weight loss43, reduced whole body oxidative stress44, decreased systolic and diastolic blood pressure43, and a modest improvement in working memory45. Another study showed that long-term calorie restriction may reduce the risk for atherosclerosis in humans15.
Despite these advances, CR in humans has also shown adverse effects in some studies, which limit its clinical usefulness. These adverse effects include cold sensitivity, menstrual irregularities, and hormonal changes. CR regimens also resulted in reduced bone mineral density at clinically relevant sites of osteoporotic fractures46, diminished aerobic capacity, and episodic anemia in some participants43. Recent evidence obtained in rodents suggest that the declining body weight, and potential impaired immune functionality42 associated with strict dietary restriction may not be suitable for frail older adults at risk of malnutrition, hypothermia, and bacterial/viral infection42, 47–49. Lastly, adherence to CR regimens remains a challenge and a translational barrier as most humans are not able or willing to reduce their calorie intake by 30% over extensive periods of time. Thus, alternative nutritional strategies tailored to the needs of older adults must be developed to obtain health benefits similar to those offered by CR, while limiting the risk of undesired adverse effects. Recent excitement in favor of strategies that could recapitulate the benefits of CR has driven growing interest in a set of lifestyle interventions collectively referred to as intermittent fasting (IF) (Figure 1). Any dietary regimen that includes periods of voluntary abstinence from food falls within the definition of intermittent fasting50. Time restricted feeding (TRF) is considered to be one of the best ways to approach intermittent fasting for elderly individuals. Two other widely used paradigms are the 5:2 diet, which allows for 5 days of ad libitum feeding and 2 days of complete fasting, and alternate day feeding, which involves alternation between fast days and ad libitum feeding days. Unlike other IF approaches, TRF does not limit one’s daily calorie intake (Figure 1). In fact, some of the observed beneficial effects of CR may at least in part be attributed to the inadvertent administration of a TRF regimen. Laboratory rodents undergoing CR tend to consume the entirety of their daily food allowance in a few hours. After the food is finished they have no choice but fast for the remainder of the day (up to 20 hours) until the next feeding takes place51. This observation has generated a lot of enthusiasm and motivated a series of studies investigating the mechanistic inner workings of TRF. Indeed, recent work has indicated that the differential effects on lifespan observed in non-human primate CR studies may be largely due to the duration of fasting that occurred in the different experimental regimens52.
Figure 1. Comparison of CR-mimicking lifestyle nutritional interventions.
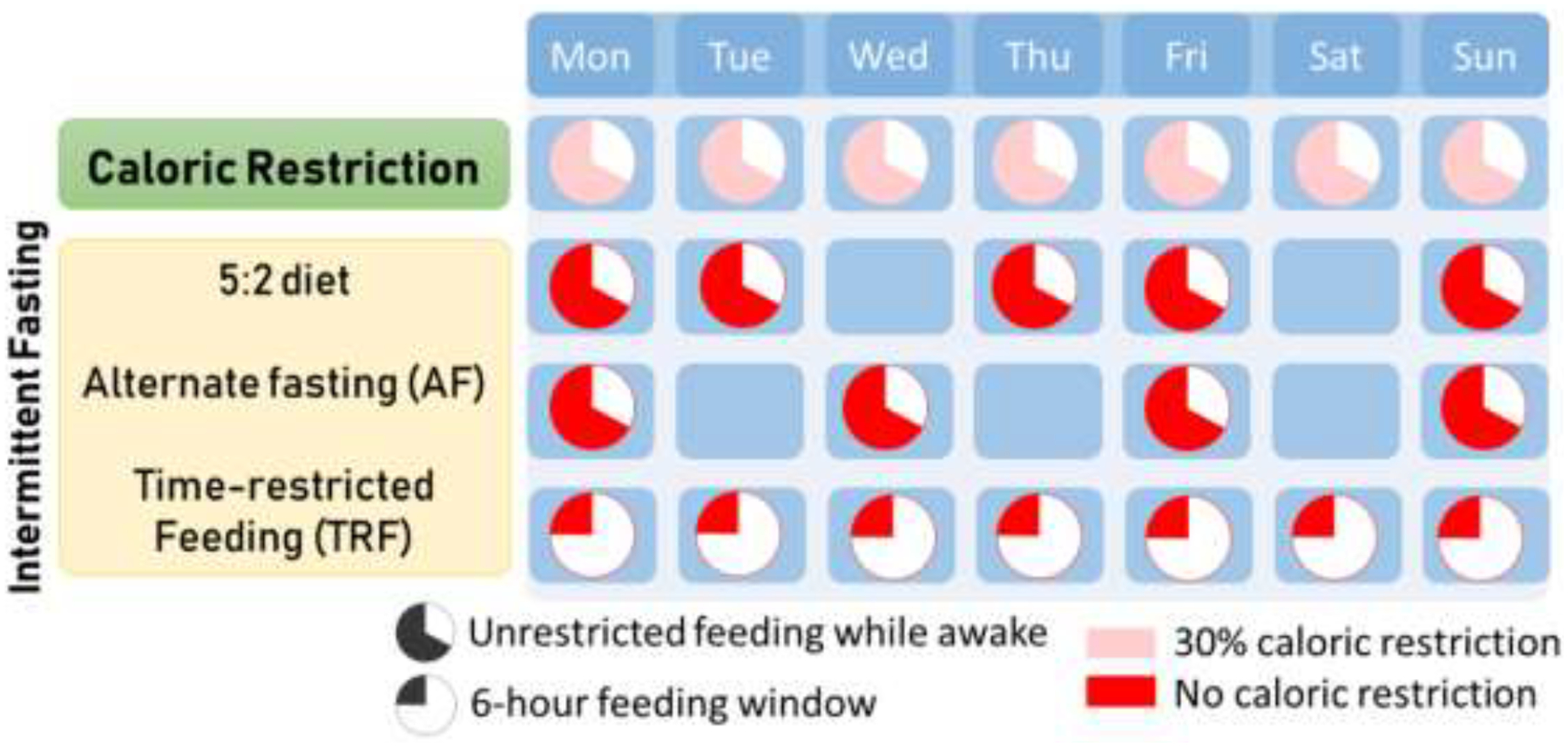
Intermittent fasting is any diet that includes regular periods of not eating or fasting. The 5:2 diet allows for ad-libitum feeding during 5 days of the week, with 2 days of complete fasting. Alternate fasting (AF) involves unrestricted eating every other day, while time-restricted feeding (TRF) consists of ad-libitum feeding every day within a specific time window that may vary from 4–8 hours.
TRF is not designed as a calorie-deficit method, but instead as a pattern of eating (Figure 1) which recent work suggests may be more effective in promoting fat loss and regulating the impairment of glucose metabolism in humans, as compared with other CR regimens53. TRF is potentially the most practical strategy for normal weight older adults as it allows consumption of all required daily calories within a condensed daily feeding window (4, 6, or 8 hours), resulting in a prolonged fasting period without a net reduction in calorie intake54, 55. The potential effects of TRF against age-related VCI are currently under investigation, and based on recent evidence, TRF could be a viable and easily attainable therapeutic lifestyle-intervention for slowing the development of age-related cerebromicrovascular pathology and ameliorating age-related cognitive decline. The present review focuses on potential mechanisms of TRF-induced cerebromicrovascular protection and its possible use to prevent VCI.
3. Biological effects of time restricted feeding
Initial findings indicate that TRF could provide many of the benefits of CR, while minimizing the risks and drawbacks associated with forced calorie deficit, such as excessive weight loss, decreased bone mineral density, and poor adherence to food deprivation. Condensing food consumption within a contained time window while fasting the rest of the day recapitulates health benefits of CR, such as reduced oxidative stress and adiposity56–58. TRF has also been shown to elicit adaptive, evolutionarily conserved, systemic cellular responses across organs, resulting in decreased inflammation, increased stress resistance, improved glucose regulation59, neuroprotective effects, improved redox status, increased production of neurotrophic factors that promote neurogenesis, and improvement in mitochondrial function60 (Figure 2). Alternation between prolonged fasting and a brief feeding state, promotes an intermittent metabolic switch, as adipose–derived ketone utilization becomes preferred over liver-derived glucose as the source of fuel for the body, especially the brain61. During TRF metabolic switching is repeated every day, eliciting prolonged systemic and cellular responses that are sustained into the fed state to improve cognitive function and disease resistance62, 63. Metabolic switching may be one of the most critical factors driving the beneficial effects of CR, and even more pronounced metabolic fluctuations can be achieved using intermittent fasting regimens like TRF. Some recent evidence even suggests that in some regards the beneficial effects exerted by metabolic switching may go beyond those obtainable from CR alone64–67. The metabolic switch from glucose to ketone body utilization was found to positively affect metabolic flexibility and improve energy production efficiency57. The rationale behind the concept of TRF arose within the framework of the circadian rhythm, an adaptation evolved in humans and other animals in response to narrow windows of food availability and long fasts between meals. It is hypothesized that daytime feeding periods result in activation of mechanisms that increase cell growth (mTOR activation) and differentiation, while during night-time, fasting pathways conserving energy and activating cellular repair are preferentially activated (e.g. AMPK, SIRT-1 and autophagy)68. Reinforcing a time-restricted feeding protocol is presumed to modulate the circadian cycle and improve regulation of hypothalamic function, which controls feelings of hunger and satiety, energy metabolism, and inflammatory response69.
Figure 2. Schematic diagram illustrating the potential shared mechanisms between caloric restriction and intermittent fasting.
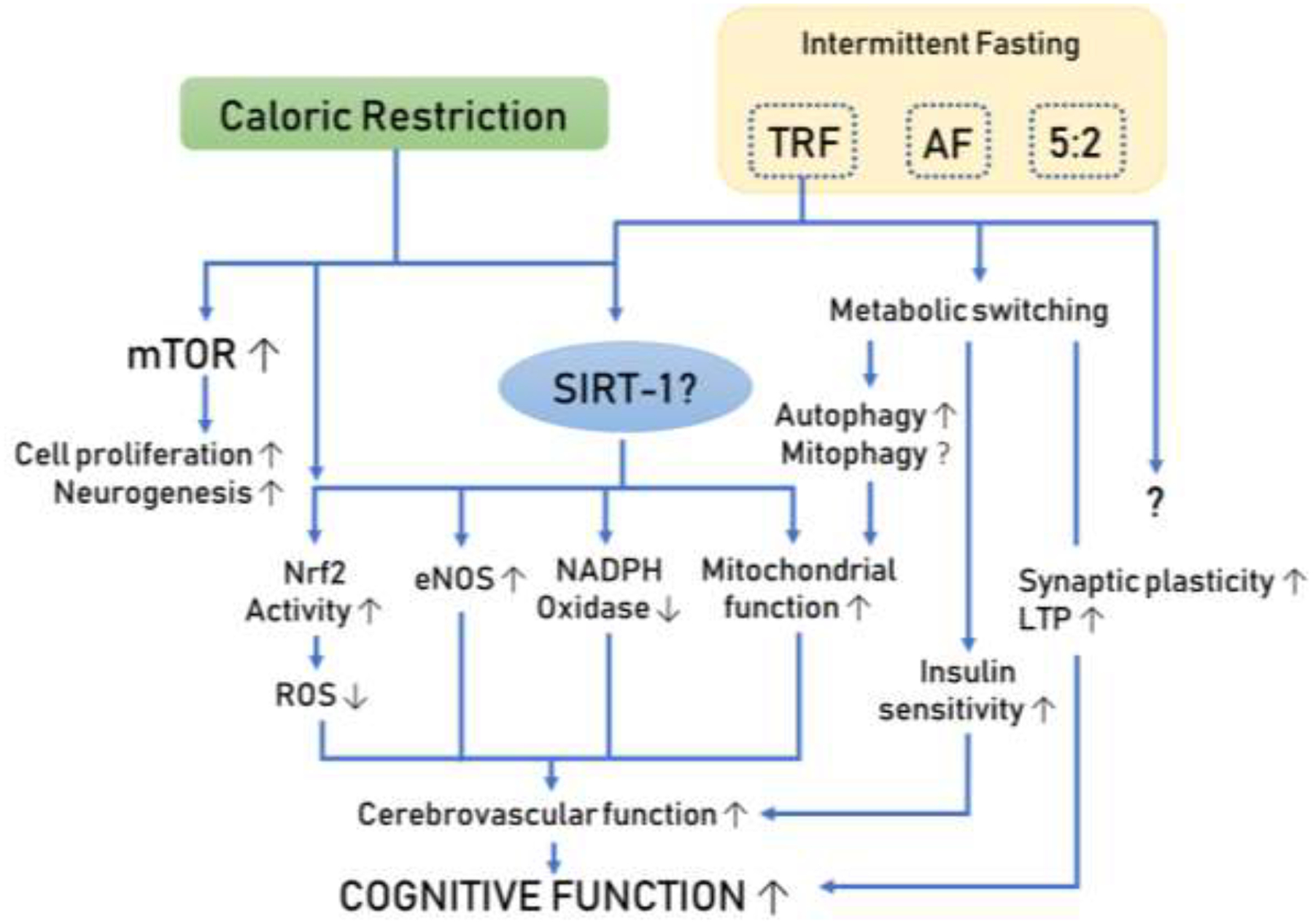
Extensive investigations of CR have demonstrated its powerful pro-longevity action and cognitive protective effects. New evidence suggests that more attainable diets (such as TRF) may confer similar beneficial effects mimicking the cerebrovascular protective effects elicited by CR.
4. Potential effects of TRF on cerebromicrovascular pathophysiological alterations relevant for the genesis of VCI
Age-related structural and functional cerebrovascular alterations thought to contribute to the pathogenesis of VCI are illustrated in Figure 370. While available data on the direct effects of TRF on cerebromicrovascular mechanisms contributing to the development of VCI are scarce, potential beneficial cerebromicrovascular effects of TRF can be predicted based on its effects on the peripheral vasculature and/or circulating biomarkers in preclinical and clinical studies. By expanding on those findings that drive vasculo-protection in the peripheral circulation, novel studies may be performed to identify potential understudied mechanisms that may be protective of the cerebral microcirculation as this could be beneficial against age-related vascular cognitive impairment and related dementias.
Figure 3. Scheme of cerebromicrovascular mechanisms contributing to the pathogenesis of age-related vascular cognitive impairment.
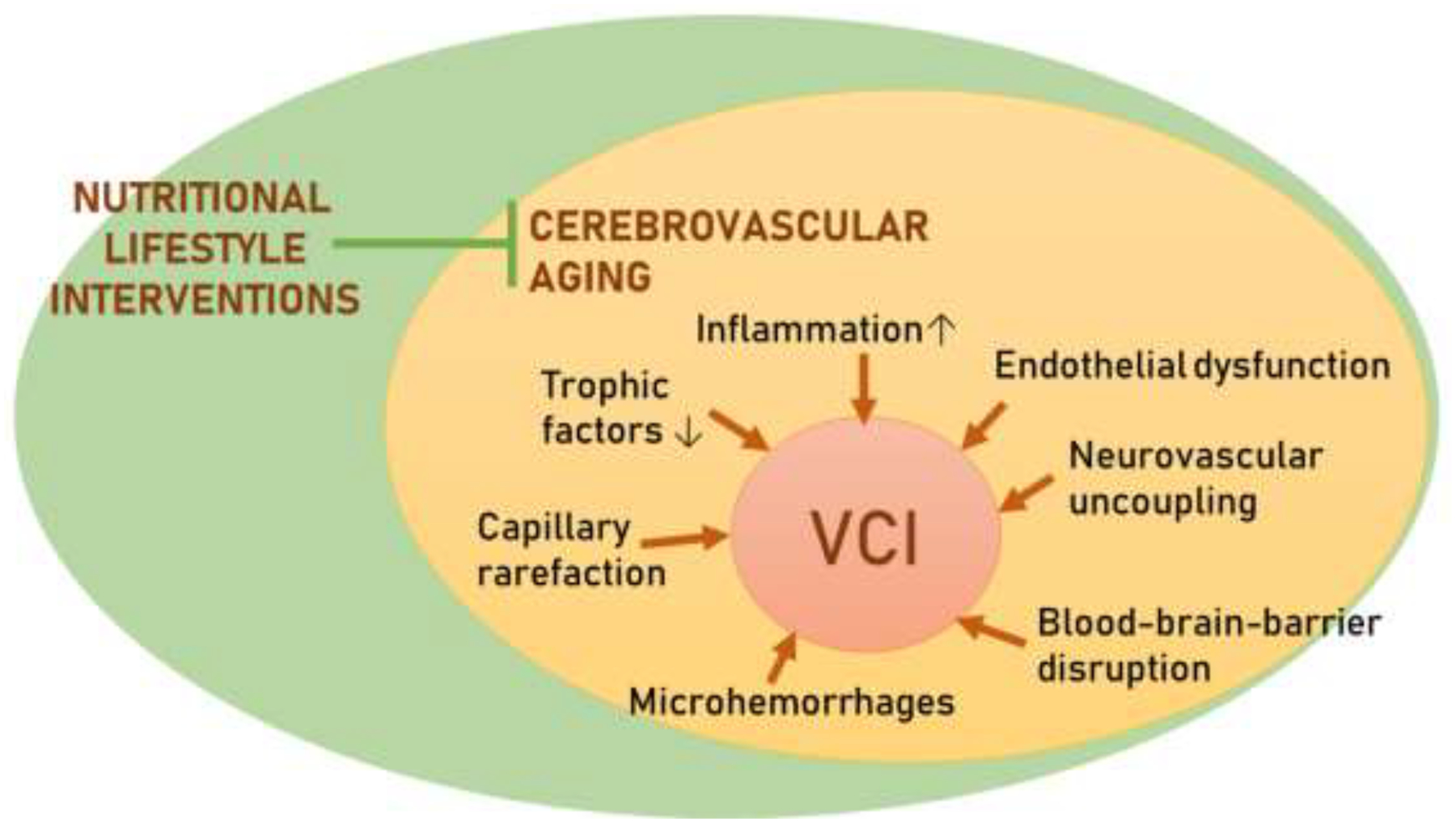
Nutritional lifestyle interventions, such as CR, have proved to be efficacious in preventing and, at least partially, reversing the downstream consequence of vascular aging. In humans, adherence to CR regimen remains a challenge and a translational barrier. Alternative nutritional strategies tailored to the needs of older adults must be developed to reap health benefits similar to that offered by CR while limiting the risk of undesired adverse effects.
4.1. Endothelial dysfunction
Aging-induced cerebromicrovascular dysfunction plays a central role in impaired blood supply of the aging brain71–76. Age-related increases in the production of reactive oxygen species (ROS) by NADPH oxidases75, 77–79 and mitochondria7, 37, 71, 72, 76, 80–82 are believed to contribute to the development of endothelial dysfunction in both pre-clinical83 and clinical studies84. Excess production of superoxide reacts with endothelium-derived nitric oxide (NO) to produce the highly reactive oxidant peroxynitrite (ONOO−)85, which mediates many of the detrimental effects of vascular oxidative stress. This includes cytotoxic effects, mitochondria dysfunction, and upregulation of pro-inflammatory pathways. Excess free radical production exacerbates vascular inflammation and contributes to the pathogenesis of age-related cognitive impairment86, 87. CR31, 37 and fasting88 were shown to attenuate endothelial oxidative stress and restore endothelial function. Interestingly, administration of TRF was also reported to diminish ROS production and improve endothelial function89, which may be predictive of a cerebrovascular protective role against the age-related dysregulation of cerebral blood flow and genesis of VCI. This possibility should be investigated in clinical and experimental studies.
In this regard, observational studies performed on individuals practicing TRF for a month during Ramadan, when eating is not allowed during hours of daylight, creating ~12 hour fasting window90, 91, are particularly interesting. After the Ramadan fasting period, participants exhibit lower circulating markers of oxidative stress and inflammation92, 93. TRF practiced during Ramadan also improves plasma cholesterol and lipid profiles, and lowers blood pressure in older adults with at least one risk factor for CVD94. Although, the calorie intake and exact fasting time in studies on Ramadan practitioners is difficult to estimate, the aforementioned data warrant further studies to assess relevant cerebromicrovascular and cognitive endpoints.
4.2. Impaired neurovascular coupling
In humans, as well as in laboratory animals, oxygen and nutrient storage capacity in the central nervous system is limited, and even momentary interruptions in oxygen supply and nutrient delivery rapidly impair neuronal function1, 95. To maintain intact cognitive abilities, the actively-firing brain neurons require a constant provision of oxygen and nutrients as well as effective wash-out of metabolic waste. These are achieved through a homeostatic feed-forward mechanism present in the brain, known as neurovascular coupling, which adjusts cerebral blood flow to match neural activity and prevents neural ischemic damage, neurodegeneration and cognitive impairment1. Clinical and experimental studies suggest that age-related functional impairments of cerebral microcirculation compromise neurovascular coupling responses, which likely contributes to age-related cognitive decline1, 5, 7, 71, 96–102. Pre-clinical studies show that treatments designed to restore endothelial function and cellular energetics71, and improve endothelium-dependent NO bioavailability7, 71, were successful in reversing the age-related impairment in neurovascular coupling responses.
Recent pre-clinical work in rodents has shown that TRF can potentially confer vasoprotective effects through attenuation of pro-inflammatory processes54, 65, 103, 104. As such, it is plausible that TRF-induced improvement in endothelial function105 may reverse the age-associated decrease in endothelial-dependent NO bioavailability, improving cerebromicrovascular function and brain perfusion. Future studies should test this possibility by assessing the effects on endothelium-dependent neurovascular coupling responses.
4.3. Cerebral microhemorrhages
Cerebral microhemorrhages or microbleeds are small chronic intracerebral hemorrhages which are caused by rupture of small arterioles or capillaries106–108. Aging and hypertension are major risk factors for the genesis of cerebral microhemorrhages106. The prevalence of cerebral microhemorrhages reaches over 50% in older adults at risk106. Cerebral microhemorrhages are clinically significant as they were shown to contribute to cognitive impairment, geriatric psychiatric syndromes, and gait disorders106, 108, 109. Studies have shown that increased vascular oxidative stress is closely linked to increased matrix metalloproteinase activity in the vascular wall108, 110, resulting in impaired structural integrity of the microvasculature and increased risk cerebral microhemorrhages.
In pre-clinical and clinical studies, administration of TRF results in attenuated risk for cardiovascular diseases89, restores endothelial vasorelaxation111 and substantially decreases blood pressure94. CR was shown to down-regulate matrix metalloproteinases and protect against pathologic remodeling of the extracellular matrix in aged arteries and prevent aneurysm formation112, 113. These findings, taken together with the demonstrated CR-like anti-oxidative effects of intermittent fasting51, 114, raise the possibility that TRF might confer protection against the development of microhemorrhages. Additional experimental studies are warranted to test this hypothesis.
4.4. Blood-brain barrier disruption
Strong evidence in murine models supports the concept that age-related cerebrovascular dysfunction is associated with blood-brain barrier disruption and the resulting neuroinflammation101, 115. There is data showing that CR exerts protective effects on the blood brain barrier116, which may contribute to its beneficial effects on cognitive function. Further preclinical studies are needed to investigate whether the TRF-induced reduction in oxidative stress and changes in endothelial vasodilation also lead to improved blood-brain barrier function in aging. Importantly, a recent study reported no effect of an every-other-day feeding regimen on blood-brain barrier permeability in a mouse model of Alzheimer’s disease117. However, the study reported that this type of treatment enhanced neuronal deficits and inflammation
4.5. Age-related changes in the synthesis of paracrine mediators: cytokines, chemokines and growth factors
The microvascular endothelium is an important source of paracrine mediators, including cytokines, chemokines37 and growth factors (e.g. brain-derived neurotrophic factor [BDNF], insulin-like growth factor-1 [IGF-1], pituitary adenylate cyclase-activating peptide [PACAP]118, 119) that play an important role in autocrine regulation of microvascular functions as well as in regulating the function of neurons, astrocytes and glial cells. In aging the synthesis/release of these paracrine mediators is significantly altered, which contributes to age-related neuronal and glial dysfunction, increased neuroinflammation, and disruption of neurogenic niches70.
CR was shown to ‘rejuvenate’ the trophic function of cerebromicrovascular endothelial cells37. Additionally, chronic intermittent fasting has been shown to up-regulate microvascular BDNF and VEGF signaling120. Recent evidence suggests that β-hydroxybutyrate, a ketone generated in response to fasting, can upregulate the expression of BDNF, which confers mitochondrial protection, and promotes synaptic plasticity and cellular stress resistance59. Further pre-clinical studies should be conducted to investigate the effect of TRF on the modulation on vascular trophic factors that could affect cognitive outcomes.
4.6. Cerebromicrovascular rarefaction
Cerebromicrovascular density typically declines with advanced age73, 121–123, which contributes to decreased cerebral blood flow and promotes cognitive decline124. The mechanisms underlying age-related cerebromicrovascular rarefaction include increased endothelial apoptosis and impaired angiogenic processes37, 118, 122, 124–126. Important in that regard is that CR confers significant anti-apoptotic and pro-angiogenic endothelial effects and results in increased capillarization in the aged rodent brain37, 38, 127. It would be of interest to explore if TRF also exerts anti-apoptotic and pro-angiogenic endothelial effects, reversing age-related cerebromicrovascular rarefaction. Age-related decline in circulating IGF-1 has been causally linked to capillary rarefaction128–130 as well as other functional aspects of cerebromicrovascular aging (e.g, neurovascular dysfunction131, 132, impaired autoregulation133, pathological remodeling and increased microvascular fragility134, 135). In rodents CR decreases serum IGF- 1 concentration by 20–40%, whereas 2 year of CR in the CALERIE study had no effect on circulating IGF-1 in humans136. Previous studies have reported mixed results concerning the effect of TRF on circulating IGF- 1. No changes in circulating IGF-1 were observed during Ramadan intermittent fasting137. In contrast, eight weeks of a TRF regimen (16/8) was shown to result in a significant decline in IGF-1 levels in study participants138. Because of the significant cerebromicrovascular protective effects of IGF-1 future studies should compare the effects of different TRF regimens and explore the impact of TRF-induced changes in IGF-1 on cerebromicrovascular physiology.
5. Conclusions
The pre-clinical and clinical studies discussed in this review provide prima facie evidence for the potential vasoprotective effects of metabolic lifestyle interventions such as CR, intermittent fasting and TRF. TRF interventions are an innovative approach, despite it is possible that CR and TRF may share some commons mechanisms, there may be other mechanisms involved that could exert CR-independent beneficial effects139. Future studies should address the effects of TRF on cerebromicrovascular health in both experimental studies on pre-clinical models of aging, and clinical investigations. In particular, investigating the effects of TRF on endothelial-dependent vasodilation and neurovascular coupling response would be of critical importance to understand the translational implications of TRF and other similar nutritional lifestyle interventions. Assessment of cognitive outcomes in response to administration of TRF, both in pre-clinical and clinical studies, will establish its usefulness for prevention of VCI. New TRF regimens combined with dietary advice tailored to the needs of older adults should be developed to reap the full cerebrovascular and cognitive health benefits while limiting the risk of undesired adverse effects. Although TRF seems to be protective against different unhealthy diets in mice54, 55, in older adults a combination of a healthy diets and an effective modified eating pattern is desirable. Ideally, effective TRF regimens should be adapted to different lifestyles. The cerebrovascular effects of the occasional deviation from TRF (e.g. a change in eating pattern between weekdays and weekends) should be elucidated. Future studies should also determine the legacy effect on cerebrovascular and cognitive endpoint after cessation of TRF. Finally, the therapeutic effect of TRF on vascular/cerebrovascular impairment associated with pre-existing diet-induced obesity88, 126, 140–145 has to be explored. Addressing these questions both in preclinical studies and the clinical setting will be critical to elucidate the effectiveness and limitations of TRF for prevention of VCI.
Highlights.
Caloric restriction has powerful pro-longevity and cognitive protective effects
Evidence suggests TRF may be more easily attainable diet
TRF may mimic the cerebrovascular protective effects elicited by CR
Potential role of TRF against age-related vascular cognitive impairment
Acknowledgement
This work was supported by grants from the American Heart Association (to ST), the NIA-supported Geroscience Training Program in Oklahoma (T32AG052363), the NIA-supported Oklahoma Nathan Shock Center, and the NIGMS supported Center of Biomedical Research Excellence (CoBRE) (1P20GM125528-01A1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Tarantini S, Tran CHT, Gordon GR, Ungvari Z and Csiszar A. Impaired neurovascular coupling in aging and Alzheimer’s disease: Contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp Gerontol. 2017;94:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sweeney MD, Kisler K, Montagne A, Toga AW and Zlokovic BV. The role of brain vasculature in neurodegenerative disorders. Nat Neurosci. 2018;21:1318–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Flier WM, Skoog I, Schneider JA, Pantoni L, Mok V, Chen CLH and Scheltens P. Vascular cognitive impairment. Nat Rev Dis Primers. 2018;4:18003. [DOI] [PubMed] [Google Scholar]
- 4.Iadecola C, Duering M, Hachinski V, Joutel A, Pendlebury ST, Schneider JA and Dichgans M. Vascular Cognitive Impairment and Dementia: JACC Scientific Expert Panel. J Am Coll Cardiol. 2019;73:3326–3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Csipo T, Mukli P, Lipecz A, Tarantini S, Bahadli D, Abdulhussein O, Owens C, Kiss T, Balasubramanian P, Nyul-Toth A, Hand RA, Yabluchanska V, Sorond FA, Csiszar A, Ungvari Z and Yabluchanskiy A. Assessment of age-related decline of neurovascular coupling responses by functional near-infrared spectroscopy (fNIRS) in humans. Geroscience. 2019;41:495–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Csipo T, Lipecz A, Fulop GA, Hand RA, Ngo BN, Dzialendzik M, Tarantini S, Balasubramanian P, Kiss T, Yabluchanska V, Silva-Palacios F, Courtney DL, Dasari TW, Sorond F, Sonntag WE, Csiszar A, Ungvari Z and Yabluchanskiy A. Age-related decline in peripheral vascular health predicts cognitive impairment. Geroscience. 2019;41:125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tarantini S, Valcarcel-Ares MN, Toth P, Yabluchanskiy A, Tucsek Z, Kiss T, Hertelendy P, Kinter M, Ballabh P, Sule Z, Farkas E, Baur JA, Sinclair DA, Csiszar A and Ungvari Z. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol. 2019;24:101192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wortmann M. Dementia: a global health priority - highlights from an ADI and World Health Organization report. Alzheimers Res Ther. 2012;4:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pifferi F, Terrien J, Perret M, Epelbaum J, Blanc S, Picq JL, Dhenain M and Aujard F. Promoting healthspan and lifespan with caloric restriction in primates. Commun Biol. 2019;2:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, Barnard D, Ward WF, Qi W, Ingram DK and de Cabo R. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW and Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dolinsky VW and Dyck JR. Calorie restriction and resveratrol in cardiovascular health and disease. Biochim Biophys Acta. 2011;1812:1477–89. [DOI] [PubMed] [Google Scholar]
- 13.Weiss EP and Fontana L. Caloric restriction: powerful protection for the aging heart and vasculature. Am J Physiol Heart Circ Physiol. 2011;301:H1205–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Prieto CF and Fernandez-Alfonso MS. Caloric Restriction as a Strategy to Improve Vascular Dysfunction in Metabolic Disorders. Nutrients. 2016;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fontana L, Meyer TE, Klein S and Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A. 2004;101:6659–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin CK, Anton SD, Han H, York-Crowe E, Redman LM, Ravussin E and Williamson DA. Examination of cognitive function during six months of calorie restriction: results of a randomized controlled trial. Rejuvenation Res. 2007;10:179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Witte AV, Fobker M, Gellner R, Knecht S and Floel A. Caloric restriction improves memory in elderly humans. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prehn K, Jumpertz von Schwartzenberg R, Mai K, Zeitz U, Witte AV, Hampel D, Szela AM, Fabian S, Grittner U, Spranger J and Floel A. Caloric Restriction in Older Adults-Differential Effects of Weight Loss and Reduced Weight on Brain Structure and Function. Cereb Cortex. 2017;27:1765–1778. [DOI] [PubMed] [Google Scholar]
- 19.Pitsikas N and Algeri S. Deterioration of spatial and nonspatial reference and working memory in aged rats: protective effect of life-long calorie restriction. Neurobiology of aging. 1992;13:369–73. [DOI] [PubMed] [Google Scholar]
- 20.Kuhla A, Lange S, Holzmann C, Maass F, Petersen J, Vollmar B and Wree A. Lifelong caloric restriction increases working memory in mice. PLoS One. 2013;8:e68778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cruzen C and Colman RJ. Effects of caloric restriction on cardiovascular aging in non-human primates and humans. Clin Geriatr Med. 2009;25:733–43, ix–x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sohal RS and Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez-Lluch G and Navas P. Calorie restriction as an intervention in ageing. J Physiol. 2016;594:2043–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ungvari Z, Parrado-Fernandez C, Csiszar A and de Cabo R. Mechanisms underlying caloric restriction and lifespan regulation: implications for vascular aging. Circ Res. 2008;102:519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E and Pennington CT. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295:1539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, Klein S, Holloszy JO and Washington University School of Medicine CG. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr. 2006;84:1033–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donato AJ, Walker AE, Magerko KA, Bramwell RC, Black AD, Henson GD, Lawson BR, Lesniewski LA and Seals DR. Life-long caloric restriction reduces oxidative stress and preserves nitric oxide bioavailability and function in arteries of old mice. Aging Cell. 2013;12:772–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fornieri C, Taparelli F, Quaglino D Jr., Contri MB, Davidson JM, Algeri S and Ronchetti IP. The effect of caloric restriction on the aortic tissue of aging rats. Connect Tissue Res. 1999;40:131–43. [DOI] [PubMed] [Google Scholar]
- 29.Ahmet I, Tae HJ, de Cabo R, Lakatta EG and Talan MI. Effects of calorie restriction on cardioprotection and cardiovascular health. J Mol Cell Cardiol. 2011;51:263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castello L, Froio T, Cavallini G, Biasi F, Sapino A, Leonarduzzi G, Bergamini E, Poli G and Chiarpotto E. Calorie restriction protects against age-related rat aorta sclerosis. FASEB J. 2005;19:1863–5. [DOI] [PubMed] [Google Scholar]
- 31.Csiszar A, Labinskyy N, Jimenez R, Pinto JT, Ballabh P, Losonczy G, Pearson KJ, de Cabo R and Ungvari Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev. 2009;130:518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chou SH, Lee YC, Huang CF, Wang YR, Yu HP and Lau YT. Gender-specific effects of caloric restriction on the balance of vascular nitric oxide and superoxide radical. Cardiovasc Res. 2010;87:751–9. [DOI] [PubMed] [Google Scholar]
- 33.Zanetti M, Gortan Cappellari G, Burekovic I, Barazzoni R, Stebel M and Guarnieri G. Caloric restriction improves endothelial dysfunction during vascular aging: Effects on nitric oxide synthase isoforms and oxidative stress in rat aorta. Exp Gerontol. 2010;45:848–55. [DOI] [PubMed] [Google Scholar]
- 34.Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K and Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:14855–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zanetti M, Barazzoni R, Vadori M, Stebel M, Biolo G and Guarnieri G. Lack of direct effect of moderate hyperleptinemia to improve endothelial function in lean rat aorta: role of calorie restriction. Atherosclerosis. 2004;175:253–9. [DOI] [PubMed] [Google Scholar]
- 36.Ozbek E, Simsek A, Ozbek M and Somay A. Caloric restriction increases internal iliac artery and penil nitric oxide synthase expression in rat: comparison of aged and adult rats. Arch Ital Urol Androl. 2013;85:113–7. [DOI] [PubMed] [Google Scholar]
- 37.Csiszar A, Gautam T, Sosnowska D, Tarantini S, Banki E, Tucsek Z, Toth P, Losonczy G, Koller A, Reglodi D, Giles CB, Wren JD, Sonntag WE and Ungvari Z. Caloric restriction confers persistent anti-oxidative, pro-angiogenic, and anti-inflammatory effects and promotes anti-aging miRNA expression profile in cerebromicrovascular endothelial cells of aged rats. Am J Physiol Heart Circ Physiol. 2014;307:H292–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Csiszar A, Sosnowska D, Tucsek Z, Gautam T, Toth P, Losonczy G, Colman RJ, Weindruch R, Anderson RM, Sonntag WE and Ungvari Z. Circulating factors induced by caloric restriction in the nonhuman primate Macaca mulatta activate angiogenic processes in endothelial cells. J Gerontol A Biol Sci Med Sci. 2013;68:235–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ingram DK, Cutler RG, Weindruch R, Renquist DM, Knapka JJ, April M, Belcher CT, Clark MA, Hatcherson CD, Marriott BM and et al. Dietary restriction and aging: the initiation of a primate study. J Gerontol. 1990;45:B148–63. [DOI] [PubMed] [Google Scholar]
- 40.Mattison JA, Colman RJ, Beasley TM, Allison DB, Kemnitz JW, Roth GS, Ingram DK, Weindruch R, de Cabo R and Anderson RM. Caloric restriction improves health and survival of rhesus monkeys. Nat Commun. 2017;8:14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balasubramanian P, Howell PR and Anderson RM. Aging and Caloric Restriction Research: A Biological Perspective With Translational Potential. EBioMedicine. 2017;21:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang D, Tao S, Chen Z, Koliesnik IO, Calmes PG, Hoerr V, Han B, Gebert N, Zornig M, Loffler B, Morita Y and Rudolph KL. Dietary restriction improves repopulation but impairs lymphoid differentiation capacity of hematopoietic stem cells in early aging. J Exp Med. 2016;213:535–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kraus WE, Bhapkar M, Huffman KM, Pieper CF, Krupa Das S, Redman LM, Villareal DT, Rochon J, Roberts SB, Ravussin E, Holloszy JO, Fontana L and Investigators C. 2 years of calorie restriction and cardiometabolic risk (CALERIE): exploratory outcomes of a multicentre, phase 2, randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7:673–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Il’yasova D, Fontana L, Bhapkar M, Pieper CF, Spasojevic I, Redman LM, Das SK, Huffman KM, Kraus WE and Investigators CS. Effects of 2 years of caloric restriction on oxidative status assessed by urinary F2-isoprostanes: The CALERIE 2 randomized clinical trial. Aging Cell. 2018;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leclerc E, Trevizol AP, Grigolon RB, Subramaniapillai M, McIntyre RS, Brietzke E and Mansur RB. The effect of caloric restriction on working memory in healthy non-obese adults. CNS Spectr. 2019:1–7. [DOI] [PubMed] [Google Scholar]
- 46.Villareal DT, Fontana L, Das SK, Redman L, Smith SR, Saltzman E, Bales C, Rochon J, Pieper C, Huang M, Lewis M, Schwartz AV and Group CS. Effect of Two-Year Caloric Restriction on Bone Metabolism and Bone Mineral Density in Non-Obese Younger Adults: A Randomized Clinical Trial. J Bone Miner Res. 2016;31:40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wasselin T, Zahn S, Maho YL, Dorsselaer AV, Raclot T and Bertile F. Exacerbated oxidative stress in the fasting liver according to fuel partitioning. Proteomics. 2014;14:1905–21. [DOI] [PubMed] [Google Scholar]
- 48.Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, Navarrete G, Dubeau L, Yap LP, Park R, Vinciguerra M, Di Biase S, Mirzaei H, Mirisola MG, Childress P, Ji L, Groshen S, Penna F, Odetti P, Perin L, Conti PS, Ikeno Y, Kennedy BK, Cohen P, Morgan TE, Dorff TB and Longo VD. A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell Metab. 2015;22:86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martens CR and Seals DR. Practical alternatives to chronic caloric restriction for optimizing vascular function with ageing. J Physiol. 2016;594:7177–7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patterson RE, Laughlin GA, LaCroix AZ, Hartman SJ, Natarajan L, Senger CM, Martinez ME, Villasenor A, Sears DD, Marinac CR and Gallo LC. Intermittent Fasting and Human Metabolic Health. J Acad Nutr Diet. 2015;115:1203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Cabo R and Mattson MP. Effects of Intermittent Fasting on Health, Aging, and Disease. N Engl J Med. 2019;381:2541–2551. [DOI] [PubMed] [Google Scholar]
- 52.Mitchell SJ, Bernier M, Mattison JA, Aon MA, Kaiser TA, Anson RM, Ikeno Y, Anderson RM, Ingram DK and de Cabo R. Daily Fasting Improves Health and Survival in Male Mice Independent of Diet Composition and Calories. Cell Metab. 2019;29:221–228 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rynders CA, Thomas EA, Zaman A, Pan Z, Catenacci VA and Melanson EL. Effectiveness of Intermittent Fasting and Time-Restricted Feeding Compared to Continuous Energy Restriction for Weight Loss. Nutrients. 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chaix A, Zarrinpar A, Miu P and Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014;20:991–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davis CS, Clarke RE, Coulter SN, Rounsefell KN, Walker RE, Rauch CE, Huggins CE and Ryan L. Intermittent energy restriction and weight loss: a systematic review. Eur J Clin Nutr. 2016;70:292–9. [DOI] [PubMed] [Google Scholar]
- 56.Panda S. Circadian physiology of metabolism. Science. 2016;354:1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Di Francesco A, Di Germanio C, Bernier M and de Cabo R. A time to fast. Science. 2018;362:770–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Longo VD and Mattson MP. Fasting: molecular mechanisms and clinical applications. Cell Metab. 2014;19:181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mattson MP, Moehl K, Ghena N, Schmaedick M and Cheng A. Intermittent metabolic switching, neuroplasticity and brain health. Nat Rev Neurosci. 2018;19:63–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schafer MJ, Dolgalev I, Alldred MJ, Heguy A and Ginsberg SD. Calorie Restriction Suppresses Age-Dependent Hippocampal Transcriptional Signatures. PLoS One. 2015;10:e0133923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anton SD, Moehl K, Donahoo WT, Marosi K, Lee SA, Mainous AG 3rd, Leeuwenburgh C and Mattson MP. Flipping the Metabolic Switch: Understanding and Applying the Health Benefits of Fasting. Obesity (Silver Spring). 2018;26:254–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mattson MP and Arumugam TV. Hallmarks of Brain Aging: Adaptive and Pathological Modification by Metabolic States. Cell Metab. 2018;27:1176–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilhelmi de Toledo F, Grundler F, Bergouignan A, Drinda S and Michalsen A. Safety, health improvement and well-being during a 4 to 21-day fasting period in an observational study including 1422 subjects. PLoS One. 2019;14:e0209353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anson RM, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, Ingram DK, Lane MA and Mattson MP. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:6216–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, Ellisman MH and Panda S. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:848–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, Cuzick J, Jebb SA, Martin B, Cutler RG, Son TG, Maudsley S, Carlson OD, Egan JM, Flyvbjerg A and Howell A. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond). 2011;35:714–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harvie M, Wright C, Pegington M, McMullan D, Mitchell E, Martin B, Cutler RG, Evans G, Whiteside S, Maudsley S, Camandola S, Wang R, Carlson OD, Egan JM, Mattson MP and Howell A. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br J Nutr. 2013;110:1534–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Longo VD and Panda S. Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell Metab. 2016;23:1048–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Puttonen S, Viitasalo K and Harma M. Effect of shiftwork on systemic markers of inflammation. Chronobiol Int. 2011;28:528–35. [DOI] [PubMed] [Google Scholar]
- 70.Ungvari Z, Tarantini S, Donato AJ, Galvan V and Csiszar A. Mechanisms of Vascular Aging. Circ Res. 2018;123:849–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Fulop GA, Hertelendy P, Gautam T, Farkas E, Perz A, Rabinovitch PS, Sonntag WE, Csiszar A and Ungvari Z. Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell. 2018;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tarantini S, Yabluchanskiy A, Csipo T, Fulop G, Kiss T, Balasubramanian P, DelFavero J, Ahire C, Ungvari A, Nyul-Toth A, Farkas E, Benyo Z, Toth A, Csiszar A and Ungvari Z. Treatment with the poly(ADP-ribose) polymerase inhibitor PJ-34 improves cerebromicrovascular endothelial function, neurovascular coupling responses and cognitive performance in aged mice, supporting the NAD+ depletion hypothesis of neurovascular aging. Geroscience. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Toth P, Tarantini S, Csiszar A and Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol. 2017;312:H1–H20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ungvari Z, Tarantini S, Nyul-Toth A, Kiss T, Yabluchanskiy A, Csipo T, Balasubramanian P, Lipecz A, Benyo Z and Csiszar A. Nrf2 dysfunction and impaired cellular resilience to oxidative stressors in the aged vasculature: from increased cellular senescence to the pathogenesis of age-related vascular diseases. Geroscience. 2019;41:727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wiedenhoeft T, Tarantini S, Nyul-Toth A, Yabluchanskiy A, Csipo T, Balasubramanian P, Lipecz A, Kiss T, Csiszar A, Csiszar A and Ungvari Z. Fusogenic liposomes effectively deliver resveratrol to the cerebral microcirculation and improve endothelium-dependent neurovascular coupling responses in aged mice. Geroscience. 2019;41:711–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Csiszar A, Yabluchanskiy A, Ungvari A, Ungvari Z and Tarantini S. Overexpression of catalase targeted to mitochondria improves neurovascular coupling responses in aged mice. Geroscience. 2019;41:609–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Toth P, Tarantini S, Tucsek Z, Ashpole NM, Sosnowska D, Gautam T, Ballabh P, Koller A, Sonntag WE, Csiszar A and Ungvari Z. Resveratrol treatment rescues neurovascular coupling in aged mice: role of improved cerebromicrovascular endothelial function and downregulation of NADPH oxidase. Am J Physiol Heart Circ Physiol. 2014;306:H299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A and Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circulation research. 2002;90:1159–66. [DOI] [PubMed] [Google Scholar]
- 79.Toth P, Tarantini S, Tucsek Z, Ashpole NM, Sosnowska D, Gautam T, Ballabh P, Koller A, Sonntag WE, Csiszar A and Ungvari ZI. Resveratrol treatment rescues neurovascular coupling in aged mice:role of improved cerebromicrovascular endothelial function and down-regulation of NADPH oxidas. Am J Physiol Heart Circ Physiol. 2014;306:H299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jacobson A, Yan C, Gao Q, Rincon-Skinner T, Rivera A, Edwards J, Huang A, Kaley G and Sun D. Aging enhances pressure-induced arterial superoxide formation. Am J Physiol Heart Circ Physiol. 2007;293:H1344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kiss T, Nyul-Toth A, Balasubramanian P, Tarantini S, Ahire C, Yabluchanskiy A, Csipo T, Farkas E, Wren JD, Garman L, Csiszar A and Ungvari Z. Nicotinamide mononucleotide (NMN) supplementation promotes neurovascular rejuvenation in aged mice: transcriptional footprint of SIRT1 activation, mitochondrial protection, anti-inflammatory, and anti-apoptotic effects. Geroscience. 2020;42:527–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Springo Z, Tarantini S, Toth P, Tucsek Z, Koller A, Sonntag WE, Csiszar A and Ungvari Z. Aging Exacerbates Pressure-Induced Mitochondrial Oxidative Stress in Mouse Cerebral Arteries. J Gerontol A Biol Sci Med Sci. 2015;70:1355–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ungvari Z, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith K and Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-kappaB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293:H37–47. [DOI] [PubMed] [Google Scholar]
- 84.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE and Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circulation research. 2007;100:1659–66. [DOI] [PubMed] [Google Scholar]
- 85.van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V and Lüscher TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192:1731–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tangestani Fard M and Stough C. A Review and Hypothesized Model of the Mechanisms That Underpin the Relationship Between Inflammation and Cognition in the Elderly. Front Aging Neurosci. 2019;11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gauthier S, Zhang H, Ng KP, Pascoal TA and Rosa-Neto P. Impact of the biological definition of Alzheimer’s disease using amyloid, tau and neurodegeneration (ATN): what about the role of vascular changes, inflammation, Lewy body pathology? Transl Neurodegener. 2018;7:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Csipo T, Fulop GA, Lipecz A, Tarantini S, Kiss T, Balasubramanian P, Csiszar A, Ungvari Z and Yabluchanskiy A. Short-term weight loss reverses obesity-induced microvascular endothelial dysfunction. Geroscience. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Headland ML, Clifton PM and Keogh JB. Effect of Intermittent Energy Restriction on Flow Mediated Dilatation, a Measure of Endothelial Function: A Short Report. Int J Environ Res Public Health. 2018;15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Trepanowski JF and Bloomer RJ. The impact of religious fasting on human health. Nutr J. 2010;9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mazidi M, Rezaie P, Chaudhri O, Karimi E and Nematy M. The effect of Ramadan fasting on cardiometabolic risk factors and anthropometrics parameters: A systematic review. Pak J Med Sci. 2015;31:1250–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Faris MA, Hussein RN, Al-Kurd RA, Al-Fararjeh MA, Bustanji YK and Mohammad MK. Impact of ramadan intermittent fasting on oxidative stress measured by urinary 15-f(2t)-isoprostane. J Nutr Metab. 2012;2012:802924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Faris MA, Kacimi S, Al-Kurd RA, Fararjeh MA, Bustanji YK, Mohammad MK and Salem ML. Intermittent fasting during Ramadan attenuates proinflammatory cytokines and immune cells in healthy subjects. Nutr Res. 2012;32:947–55. [DOI] [PubMed] [Google Scholar]
- 94.Nematy M, Alinezhad-Namaghi M, Rashed MM, Mozhdehifard M, Sajjadi SS, Akhlaghi S, Sabery M, Mohajeri SA, Shalaey N, Moohebati M and Norouzy A. Effects of Ramadan fasting on cardiovascular risk factors: a prospective observational study. Nutr J. 2012;11:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Watts ME, Pocock R and Claudianos C. Brain Energy and Oxygen Metabolism: Emerging Role in Normal Function and Disease. Front Mol Neurosci. 2018;11:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gao YZ, Zhang JJ, Liu H, Wu GY, Xiong L and Shu M. Regional cerebral blood flow and cerebrovascular reactivity in Alzheimer’s disease and vascular dementia assessed by arterial spinlabeling magnetic resonance imaging. Curr Neurovasc Res. 2013;10:49–53. [DOI] [PubMed] [Google Scholar]
- 97.Tarantini S, Hertelendy P, Tucsek Z, Valcarcel-Ares MN, Smith N, Menyhart A, Farkas E, Hodges EL, Towner R, Deak F, Sonntag WE, Csiszar A, Ungvari Z and Toth P. Pharmacologically-induced neurovascular uncoupling is associated with cognitive impairment in mice. J Cereb Blood Flow Metab. 2015;35:1871–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tarantini S, Yabluchanskiy A, Csipo T, Fulop G, Kiss T, Balasubramanian P, DelFavero J, Ahire C, Ungvari A, Nyul-Toth A, Farkas E, Benyo Z, Toth A, Csiszar A and Ungvari Z. Treatment with the poly(ADP-ribose) polymerase inhibitor PJ-34 improves cerebromicrovascular endothelial function, neurovascular coupling responses and cognitive performance in aged mice, supporting the NAD+ depletion hypothesis of neurovascular aging. Geroscience. 2019;41:533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lipecz A, Csipo T, Tarantini S, Hand RA, Ngo BN, Conley S, Nemeth G, Tsorbatzoglou A, Courtney DL, Yabluchanska V, Csiszar A, Ungvari ZI and Yabluchanskiy A. Age-related impairment of neurovascular coupling responses: a dynamic vessel analysis (DVA)-based approach to measure decreased flicker light stimulus-induced retinal arteriolar dilation in healthy older adults. Geroscience. 2019;41:341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tarantini S, Fulop GA, Kiss T, Farkas E, Zolei-Szenasi D, Galvan V, Toth P, Csiszar A, Ungvari Z and Yabluchanskiy A. Demonstration of impaired neurovascular coupling responses in TG2576 mouse model of Alzheimer’s disease using functional laser speckle contrast imaging. Geroscience. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tarantini S, Valcarcel-Ares MN, Yabluchanskiy A, Tucsek Z, Hertelendy P, Kiss T, Gautam T, Zhang XA, Sonntag WE, de Cabo R, Farkas E, Elliott MH, Kinter MT, Deak F, Ungvari Z and Csiszar A. Nrf2 Deficiency Exacerbates Obesity-Induced Oxidative Stress, Neurovascular Dysfunction, Blood-Brain Barrier Disruption, Neuroinflammation, Amyloidogenic Gene Expression, and Cognitive Decline in Mice, Mimicking the Aging Phenotype. J Gerontol A Biol Sci Med Sci. 2018;73:853–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tarantini S, Yabluchanskiy A, Fulop GA, Kiss T, Perz A, O’Connor D, Johnson E, Sorond F, Ungvari Z and Csiszar A. Age-related alterations in gait function in freely moving male C57BL/6 mice: translational relevance of decreased cadence and increased gait variability. J Gerontol A Biol Sci Med Sci. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sherman H, Frumin I, Gutman R, Chapnik N, Lorentz A, Meylan J, le Coutre J and Froy O. Long-term restricted feeding alters circadian expression and reduces the level of inflammatory and disease markers. J Cell Mol Med. 2011;15:2745–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E and Peterson CM. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018;27:1212–1221 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Antoni R, Johnston KL, Collins AL and Robertson MD. Investigation into the acute effects of total and partial energy restriction on postprandial metabolism among overweight/obese participants. Br J Nutr. 2016;115:951–9. [DOI] [PubMed] [Google Scholar]
- 106.Ungvari Z, Tarantini S, Kirkpatrick AC, Csiszar A and Prodan CI. Cerebral microhemorrhages: mechanisms, consequences, and prevention. Am J Physiol Heart Circ Physiol. 2017;312:H1128–H1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ungvari Z, Yabluchanskiy A, Tarantini S, Toth P, Kirkpatrick AC, Csiszar A and Prodan CI. Repeated Valsalva maneuvers promote symptomatic manifestations of cerebral microhemorrhages: implications for the pathogenesis of vascular cognitive impairment in older adults. Geroscience. 2018;40:485–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Toth P, Tarantini S, Springo Z, Tucsek Z, Gautam T, Giles CB, Wren JD, Koller A, Sonntag WE, Csiszar A and Ungvari Z. Aging exacerbates hypertension-induced cerebral microhemorrhages in mice: role of resveratrol treatment in vasoprotection. Aging Cell. 2015;14:400–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ungvari Z, Tarantini S, Kirkpatrick AC, Csiszar A and Prodan CI. Cerebral microhemorrhages: mechanisms, consequences and prevention. Am J Physiol Heart Circ Physiol. 2017:in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Amin M, Pushpakumar S, Muradashvili N, Kundu S, Tyagi SC and Sen U. Regulation and involvement of matrix metalloproteinases in vascular diseases. Front Biosci (Landmark Ed). 2016;21:89–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Erdem Y, Ozkan G, Ulusoy S, Arici M, Derici U, Sengul S, Sindel S, Erturk S, Turkish Society of H and Renal D. The effect of intermittent fasting on blood pressure variability in patients with newly diagnosed hypertension or prehypertension. J Am Soc Hypertens. 2018;12:42–49. [DOI] [PubMed] [Google Scholar]
- 112.Liu Y, Wang TT, Zhang R, Fu WY, Wang X, Wang F, Gao P, Ding YN, Xie Y, Hao DL, Chen HZ and Liu DP. Calorie restriction protects against experimental abdominal aortic aneurysms in mice. J Exp Med. 2016;213:2473–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang M, Zhang L, Zhu W, Zhang J, Kim SH, Wang Y, Ni L, Telljohann R, Monticone RE, McGraw K, Liu L, de Cabo R and Lakatta EG. Calorie Restriction Curbs Proinflammation That Accompanies Arterial Aging, Preserving a Youthful Phenotype. J Am Heart Assoc. 2018;7:e009112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.de Cabo R, Carmona-Gutierrez D, Bernier M, Hall MN and Madeo F. The search for antiaging interventions: from elixirs to fasting regimens. Cell. 2014;157:1515–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fulop GA, Ahire C, Csipo T, Tarantini S, Kiss T, Balasubramanian P, Yabluchanskiy A, Farkas E, Toth A, Nyul-Toth A, Toth P, Csiszar A and Ungvari Z. Cerebral venous congestion promotes blood-brain barrier disruption and neuroinflammation, impairing cognitive function in mice. Geroscience. 2019;41:575–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Parikh I, Guo J, Chuang KH, Zhong Y, Rempe RG, Hoffman JD, Armstrong R, Bauer B, Hartz AM and Lin AL. Caloric restriction preserves memory and reduces anxiety of aging mice with early enhancement of neurovascular functions. Aging (Albany NY). 2016;8:2814–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lazic D, Tesic V, Jovanovic M, Brkic M, Milanovic D, Zlokovic BV, Kanazir S and Perovic M. Every-other-day feeding exacerbates inflammation and neuronal deficits in 5XFAD mouse model of Alzheimer’s disease. Neurobiol Dis. 2020;136:104745. [DOI] [PubMed] [Google Scholar]
- 118.Banki E, Sosnowska D, Tucsek Z, Gautam T, Toth P, Tarantini S, Tamas A, Helyes Z, Reglodi D, Sonntag WE, Csiszar A and Ungvari Z. Age-related decline of autocrine pituitary adenylate cyclase-activating polypeptide impairs angiogenic capacity of rat cerebromicrovascular endothelial cells. J Gerontol A Biol Sci Med Sci. 2015;70:665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Reglodi D, Atlasz T, Szabo E, Jungling A, Tamas A, Juhasz T, Fulop BD and Bardosi A. PACAP deficiency as a model of aging. Geroscience. 2018;40:437–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Katare RG, Kakinuma Y, Arikawa M, Yamasaki F and Sato T. Chronic intermittent fasting improves the survival following large myocardial ischemia by activation of BDNF/VEGF/PI3K signaling pathway. J Mol Cell Cardiol. 2009;46:405–12. [DOI] [PubMed] [Google Scholar]
- 121.Ingraham JP, Forbes ME, Riddle DR and Sonntag WE. Aging reduces hypoxia-induced microvascular growth in the rodent hippocampus. J Gerontol A Biol Sci Med Sci. 2008;63:12–20. [DOI] [PubMed] [Google Scholar]
- 122.Tarantini S, Tucsek Z, Valcarcel-Ares MN, Toth P, Gautam T, Giles CB, Ballabh P, Wei JY, Wren JD, Ashpole NM, Sonntag WE, Ungvari Z and Csiszar A. Circulating IGF-1 deficiency exacerbates hypertension-induced microvascular rarefaction in the mouse hippocampus and retrosplenial cortex: implications for cerebromicrovascular and brain aging. Age (Dordr). 2016;38:273–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Toth P, Tucsek Z, Sosnowska D, Gautam T, Mitschelen M, Tarantini S, Deak F, Koller A, Sonntag WE, Csiszar A and Ungvari Z. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J Cereb Blood Flow Metab. 2013;33:1732–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ungvari Z, Tarantini S, Kiss T, Wren JD, Giles CB, Griffin CT, Murfee WL, Pacher P and Csiszar A. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat Rev Cardiol. 2018;15:555–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kiss T, Balasubramanian P, Valcarcel-Ares MN, Tarantini S, Yabluchanskiy A, Csipo T, Lipecz A, Reglodi D, Zhang XA, Bari F, Farkas E, Csiszar A and Ungvari Z. Nicotinamide mononucleotide (NMN) treatment attenuates oxidative stress and rescues angiogenic capacity in aged cerebromicrovascular endothelial cells: a potential mechanism for prevention of vascular cognitive impairment GeroScience. 2019:in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tucsek Z, Toth P, Tarantini S, Sosnowska D, Gautam T, Warrington JP, Giles CB, Wren JD, Koller A, Ballabh P, Sonntag WE, Ungvari Z and Csiszar A. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J Gerontol A Biol Sci Med Sci. 2014;69:1339–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lynch CD, Cooney PT, Bennett SA, Thornton PL, Khan AS, Ingram RL and Sonntag WE. Effects of moderate caloric restriction on cortical microvascular density and local cerebral blood flow in aged rats. Neurobiol Aging. 1999;20:191–200. [DOI] [PubMed] [Google Scholar]
- 128.Sonntag WE, Lynch CD, Cooney PT and Hutchins PM. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology. 1997;138:3515–20. [DOI] [PubMed] [Google Scholar]
- 129.Tarantini S, Tucsek Z, Valcarcel-Ares M, Toth P, Gautam T, Giles C, Ballabh P, Wei Y, Wren J, Ashpole N, Sonntag W, Ungvari Z and Csiszar A. Circulating IGF-1 deficiency exacerbates hypertension-induced microvascular rarefaction in the mouse hippocampus and retrosplenial cortex: implications for cerebromicrovascular and brain aging. Age (Dordr). 2016;38:273–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sonntag WE, Deak F, Ashpole N, Toth P, Csiszar A, Freeman W and Ungvari Z. Insulin-like growth factor-1 in CNS and cerebrovascular aging. Front Aging Neurosci. 2013;5:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Farias Quipildor GE, Mao K, Hu Z, Novaj A, Cui MH, Gulinello M, Branch CA, Gubbi S, Patel K, Moellering DR, Tarantini S, Kiss T, Yabluchanskiy A, Ungvari Z, Sonntag WE and Huffman DM. Central IGF-1 protects against features of cognitive and sensorimotor decline with aging in male mice. Geroscience. 2019;41:185–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Toth P, Tarantini S, Ashpole NM, Tucsek Z, Milne GL, Valcarcel-Ares NM, Menyhart A, Farkas E, Sonntag WE, Csiszar A and Ungvari Z. IGF-1 deficiency impairs neurovascular coupling in mice: implications for cerebromicrovascular aging. Aging Cell. 2015;14:1034–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Toth P, Tucsek Z, Tarantini S, Sosnowska D, Gautam T, Mitschelen M, Koller A, Sonntag WE, Csiszar A and Ungvari Z. IGF-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J Cereb Blood Flow Metab. 2014;34:1887–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fulop GA, Ramirez-Perez FI, Kiss T, Tarantini S, Valcarcel Ares MN, Toth P, Yabluchanskiy A, Conley SM, Ballabh P, Martinez-Lemus LA, Ungvari Z and Csiszar A. IGF-1 deficiency Promotes Pathological Remodeling of Cerebral Arteries: A Potential Mechanism Contributing to the Pathogenesis of Intracerebral Hemorrhages in Aging. J Gerontol A Biol Sci Med Sci. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Springo Z, Fulop GA, Ashpole N, Gautam T, Giles CB, Wren JD, Sonntag WE, Csiszar A and Ungvari Z. Insulin-like growth factor 1 deficiency exacerbates hypertension-induced cerebral microhemorrhages in mice, mimicking the aging phenotype. Aging Cell. 2017;16:469–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fontana L, Villareal DT, Das SK, Smith SR, Meydani SN, Pittas AG, Klein S, Bhapkar M, Rochon J, Ravussin E, Holloszy JO and Group CS. Effects of 2-year calorie restriction on circulating levels of IGF-1, IGF-binding proteins and cortisol in nonobese men and women: a randomized clinical trial. Aging Cell. 2016;15:22–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bouhlel E, Zaouali M, Miled A, Tabka Z, Bigard X and Shephard R. Ramadan fasting and the GH/IGF-1 axis of trained men during submaximal exercise. Ann Nutr Metab. 2008;52:261–6. [DOI] [PubMed] [Google Scholar]
- 138.Moro T, Tinsley G, Bianco A, Marcolin G, Pacelli QF, Battaglia G, Palma A, Gentil P, Neri M and Paoli A. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J Transl Med. 2016;14:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hwangbo DS, Lee HY, Abozaid LS and Min KJ. Mechanisms of Lifespan Regulation by Calorie Restriction and Intermittent Fasting in Model Organisms. Nutrients. 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bailey-Downs LC, Sosnowska D, Toth P, Mitschelen M, Gautam T, Henthorn JC, Ballabh P, Koller A, Farley JA, Sonntag WE, Csiszar A and Ungvari Z. Growth Hormone and IGF-1 Deficiency Exacerbate High-Fat Diet-Induced Endothelial Impairment in Obese Lewis Dwarf Rats: Implications for Vascular Aging. J Gerontol A Biol Sci Med Sci. 2012;67:553–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bailey-Downs LC, Tucsek Z, Toth P, Sosnowska D, Gautam T, Sonntag WE, Csiszar A and Ungvari Z. Aging exacerbates obesity-induced oxidative stress and inflammation in perivascular adipose tissue in mice: a paracrine mechanism contributing to vascular redox dysregulation and inflammation. J Gerontol A Biol Sci Med Sci. 2013;68:780–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mattison JA, Wang M, Bernier M, Zhang J, Park SS, Maudsley S, An SS, Santhanam L, Martin B, Faulkner S, Morrell C, Baur JA, Peshkin L, Sosnowska D, Csiszar A, Herbert RL, Tilmont EM, Ungvari Z, Pearson KJ, Lakatta EG and de Cabo R. Resveratrol prevents high fat/sucrose diet-induced central arterial wall inflammation and stiffening in nonhuman primates. Cell Metab. 2014;20:183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tarantini S, Valcarcel-Ares MN, Yabluchanskiy A, Tucsek Z, Hertelendy P, Kiss T, Gautam T, Zhang XA, Sonntag WE, de Cabo R, Farkas E, Elliott ME, Kinter MT, Deak F, Ungvari Z and Csiszar A. Nrf2 deficiency exacerbates obesity-induced oxidative stress, neurovascular dysfunction, blood brain barrier disruption, neuroinflammation, amyloidogenic gene expression and cognitive decline in mice, mimicking the aging phenotype. J Gerontol A Biol Sci Med Sci. 2018:in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tucsek Z, Toth P, Sosnowsk D, Gautam T, Mitschelen M, Koller A, Szalai G, Sonntag WE, Ungvari Z and Csiszar A. Obesity in aging exacerbates blood brain barrier disruption, neuroinflammation and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease J Gerontol A Biol Sci Med Sci. 2014;69:1212–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Valcarcel-Ares MN, Tucsek Z, Kiss T, Giles CB, Tarantini S, Yabluchanskiy A, Balasubramanian P, Gautam T, Galvan V, Ballabh P, Richardson A, Freeman WM, Wren JD, Deak F, Ungvari Z and Csiszar A. Obesity in Aging Exacerbates Neuroinflammation, Dysregulating Synaptic Function-related Genes and Altering Eicosanoid Synthesis in the Mouse Hippocampus: Potential Role in Impaired Synaptic Plasticity and Cognitive Decline. J Gerontol A Biol Sci Med Sci. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]


