Highlights
-
•
Anti-glutamic acid decarboxylase (GAD) antibodies cause limbic encephalitis.
-
•
Super refractory status epilepticus can be a manifestation of anti-GAD encephalitis.
-
•
Rapid treatment with tocilizumab immunological polytherapy may improve seizure control.
Abbreviations: GAD, glutamic acid decarboxylase; AED, antiepileptic drug; IVIG, intravenous immunoglobulin; GABA, gamma-aminobutyric acid; MRI, magnetic resonance imaging; EEG, electroencephalogram; CSF, cerebrospinal fluid; RNA, ribonucleic acid; PCR, polymerase chain reaction; NMDA, N-methyl-d-aspartate; MDZ, midazolam; MP, methylprednisolone; PLEX, plasma exchange
Keywords: Autoimmune encephalitis, Glutamic acid decarboxylase antibodies, Limbic encephalitis, Status epilepticus, Tocilizumab
Abstract
Antibodies against glutamic acid decarboxylase are reported in association with a number of neurological conditions including limbic encephalitis. We report a case of anti-GAD-antibody associated encephalitis presenting with super-refractory status epilepticus. We describe the clinical course, management, and the outcome. In addition, we review the presentation and outcomes of reported cases of anti-GAD encephalitis. Similar to the reported cases of anti-GAD encephalitis, our case was refractory to treatment with conventional antiseizure medication. Treatment with intravenous immune globulin (IVIG), high dose corticosteroids, and plasmapheresis had partial response, but escalation of treatment to the use of tocilizumab was associated with significant clinical improvement.
1. Introduction
Autoimmune encephalitis is increasingly recognized worldwide. Glutamic acid decarboxylase (GAD) is an enzyme implicated in the γ-aminobutyric acid inhibitory neurotransmitter modulation. Anti-GAD antibodies were reported in a variety of neurological syndromes, that include limbic encephalitis, cerebellar ataxia, and stiff person syndrome, with a highly heterogeneous clinical presentation.
Autoimmune encephalitis particularly anti-GAD positive limbic encephalitis usually presents with a prodrome of non-specific neurological and neuropsychiatric symptoms including headache, irritability, delusions, hallucinations, psychosis, and short-term memory deficits [1]. Furthermore, some reports have linked anti-GAD antibodies with epilepsy and status epilepticus including epilepsia partialis continua [2] that are usually refractory to treatment with conventional antiseizure medication (ASM) [2], [3], [4], [5].
The incidence and patient characteristics in cases of anti-GAD encephalitis is an area of interest and research worldwide given the ambiguity of clinical presentation and rarity of the disease. We present a case with anti-GAD antibodies manifesting as super-refractory status epilepticus and describe the course of her disease, with a stepwise approach to management, modalities of treatment used and the outcome of her disease.
2. Case
We report the case of an eight year old developmentally normal and previously healthy girl who presented to our institution for further management of status epilepticus. She started experiencing focal seizures with impaired awareness characterized by staring without concomitant abnormal body movements. These seizures began to occur four days following a febrile gastroenteritis. The initial brain MRI was normal. The initial EEG revealed right temporal electro-clinical seizures. Cerebrospinal fluid infectious studies were taken and the patient was started on empirical antibacterial and antiviral therapy. Her seizures progressed in frequency to multiple daily episodes of staring that failed to respond to antiepileptic drug poly-therapy including lacosamide, levetiracetam, phenytoin, clonazepam, and valproic acid. Repeated brain MRI was also unrevealing. She presented to our institution six days following her initial seizures. On presentation, she was obtunded, disoriented, unresponsive to verbal stimuli with normal power and hyperreflexia.
Extensive infectious and autoimmune serum and cerebrospinal fluid (CSF) evaluation was performed. Initial CSF studies showed pleocytosis, 125 WBCs and the presence of 3 RBCs. CSF protein was normal (0.29 g/L, normal range 0.1–0.5 g/L). CSF glucose was normal (70 mg/dL, normal range 40–70 mg/dL). Further studies were performed on CSF samples and included a viral panel including herpes simplex virus (HSV) PCR, culture, 16 S RNA, Tuberculosis PCR, toxoplasma PCR, Brucella culture, EBV PCR, West Nile virus PCR, anti-NMDA antibodies, anti-potassium channel antibodies, anti GABA antibodies, ammonia, lactate, amino acids, neurotransmitters and oligoclonal bands. CSF cytology was not done. Serum autoimmune workup included anti-GAD antibodies. The method of processing was through ELISARSR™ by Laboratoires Cerba® for quantitative determination of autoantibodies to glutamic acid decarboxylase (GAD65) in serum. The value of CSF anti-GAD antibodies could not be obtained as the test is not available at our institution. Serum COVID19-PCR was tested twice and was negative. Despite negative pan-cultures and a repeated CSF HSV PCR, broad spectrum antibiotics and acyclovir, (that was later switched to foscarnet to cover for the possibility of acyclovir-resistant HSV), were continued for a total of 21 days.
The patient was intubated a few hours after admission due to refractory status epilepticus and was started on Midazolam drip in addition to the ASM. Long term EEG monitoring showed frequent electrographic multifocal seizures mainly from right and left temporal areas. ASMs were adjusted with the addition of clobazam, topiramate and perampanel. Attempt to initiate ketogenic diet was unsuccessful due to an elevated lipase level, that reached a maximum of 195 U/L.
In addition, due to a high suspicion for autoimmune encephalitis, she was started on intravenous immunoglobulin at a dose of 1 gram per kg on two consecutive days, followed by a 3-day course of high dose methylprednisolone (30 mg per kg per dose). Unfortunately, her seizures remained refractory despite high-dose of midazolam (MDZ) infusion, therefore propofol IV drip was added as a continuous infusion and induced a burst-suppression pattern on EEG. An attempt to wean propofol after 72 h was unsuccessful, therefore it was replaced with an IV ketamine drip. Phenobarbital was also started, targeting a serum trough level in the high 40 s (mg/l) with a partial seizure reduction. She also received 5 sessions of plasma exchange two days after high-dose corticosteroids administration.
Ten days after admission, the ketogenic diet was initiated after normalization of lipase level. Due to inadequate response to the immunomodulatory therapies, tocilizumab was administered at a dose of 8 mg/kg/day divided in two doses one week apart. The patient became seizure-free 24 h following the first dose of tocilizumab and three days following the initiation of ketogenic diet. Ketamine and MDZ infusions were weaned and discontinued. The patient was successfully extubated a few days later.
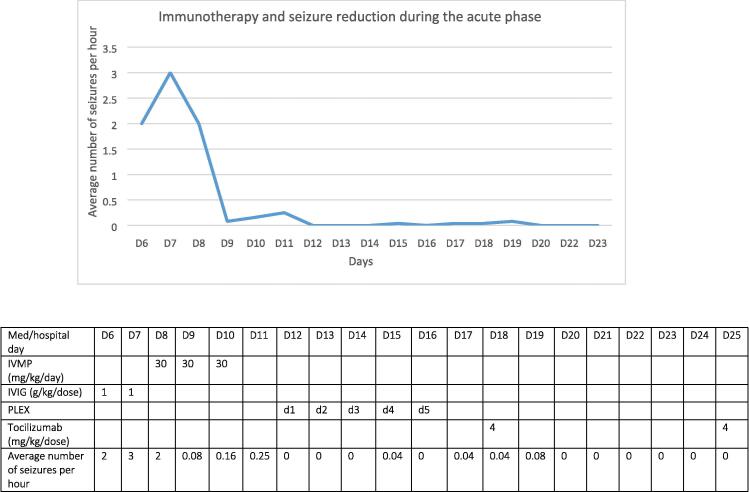
At this time, the results of infectious and autoimmune workup turned out to be unrevealing except for a positive serum anti-GAD antibody level of 303.1 U/ml (reference range <5 U/ml). The diagnosis of anti-GAD encephalitis was confirmed. Further investigations for a paraneoplastic process including a chest x-ray and a CT scan of abdomen and pelvis were unremarkable. The patient showed rapid and significant clinical improvement and was discharged home with normal cognition and a minor ataxia that is improving. A timeline describing the frequency of seizures and the various immunomodulatory therapies is illustrated in Fig. 1 (Appendix).
Upon follow up 1 month later, our patient was symptom-free except for occasional breakthrough seizures, on three AEDs (phenobarbital, levetiracetam, perampanel) and on a monthly tocilizumab infusion schedule of 8 mg per kg. Her exam remained with no evidence of focal weakness and normal cognition. Serum anti-GAD antibody titer repeated three weeks later declined to 30 U/ml. A follow-up brain MRI showed mild parenchymal brain atrophy.
3. Discussion
Anti-glutamic acid decarboxylase antibodies have been discovered in a number of neurological syndromes that include limbic encephalitis [6], [7], cerebellar ataxia [3] and stiff person syndrome [8]. Anti-GAD syndromes are thought to be antibody-mediated, mainly involving a B cells activation against GAD65 leading to reduced neurotransmission at synapses [9], [10]. Given the variability in presentation of symptoms and uncertainty in independent association of anti-GAD with the disease course, it has been hard to assess basic characteristics like ethnicity, gender and incidence of anti-GAD associated syndromes. Some reports have linked anti-GAD antibodies with epilepsy and status epilepticus including epilepsia partialis continua [9] that are usually refractory to treatment with conventional ASM [2], [3], [4], [5].
We have reported in this article the case of an 8-year-old girl who presented to our institution with super-refractory status epilepticus following a prodrome of a febrile illness clinically resembling that of febrile infection-related epilepsy syndrome (FIRES). NORSE and FIRES are both cryptogenic by definition, and the outcome of both as reported by many literature sources is notoriously grim [11], [12], [13], [14]. Based on the aforementioned data, the finding of anti-GAD antibody positivity, and the decline in a repeat anti-GAD antibody titer after treatment, coinciding with clinical improvement, we came to the conclusion that we were dealing with a case of anti-GAD encephalitis rather than a case of cryptogenic NORSE or FIRES. In prior reports, GAD antibody titers were not associated with the duration, frequency, or severity of the seizures [15], [16]. The cases reported in literature reporting concomitant super refractory status epilepticus and anti-GAD antibodies are scarce and in the majority depended on serum anti GAD levels, even in modest elevation, for diagnosis. Khawaja et al reported three cases of refractory status epilepticus associated with anti-GAD antibodies based on elevated serum titer [5]. In addition, there are no fixed guidelines on numerical cutoffs of serum and CSF values for the diagnosis. This renders the diagnosis of anti-GAD encephalitis especially in the setting of super refractory status epilepticus a judgement of the expert especially in the absence of other etiologies that could explain the clinical progression. Other etiologies that could result in a high anti-GAD antibodies reported in the literature were mainly in the setting of diabetes mellitus Type 1 or following the administration of IVIG [17]. Both of the aforementioned settings were not applicable in our case. The patient’s serum glucose levels were within normal limits throughout hospitalization. The EEG in patients with GAD Ab-associated epilepsy is usually nonspecific [4]. Continuous long-term EEG monitoring in our case revealed multifocal seizures and discharges of bilateral frontal and temporal predominance. These findings were similar to those reported in the literature [4], [18].
There are no pathognomonic radiological findings for anti-GAD encephalitis, yet many reported anti-GAD limbic encephalitis cases included temporal lobe involvement [6], [19]. Normal MRI can be present in up to 25% of the patients [6]. Serial Brain MRIs done throughout our patient’s hospital course failed to define any structural abnormality, except for a mild parenchymal brain atrophy noticed in the most recent MRI one month after the onset of symptoms, changes likely resulting from the prolonged status epilepticus state effect rather than the etiology itself. A high serum lipase level was seen in our patient, that could be a side effect of valproate or propofol (although lipase was elevated before initiation of the propofol infusion), yet this has been reported in association with anti-GAD antibodies probably due to the presence of GAD receptors in pancreatic cells [20].
Seizures in anti-GAD encephalitis are refractory to standard ASM. Only a few GAD-epilepsy patients become seizure-free exclusively with ASM. Prior observations support the hypothesis that GAD antibody associated neurological syndromes are mediated by a dysfunction of GABAergic inhibitory circuits [21]. This might explain why our patient responded to phenobarbital better than the many other antiepileptics that have been used.
Given the relative rarity of the disease, limited evidence exists to guide its management. As with many other antibody-mediated neurological disorders, immunomodulatory therapy should be initiated when autoimmune encephalitis is clinically suspected, even prior to anti-neuronal antibody detection, in addition to concomitant use of ASM. In cases of autoimmune encephalitis, optimal results in seizure control and prevention of long-term cognitive and motor sequelae have generally been reported with early aggressive immunotherapy [4], especially if initiated prior to any visible brain MRI changes. Anti-GAD antibodies associated epilepsy is quite resistant to immunomodulation therapy [4], [23], [24]. Intravenous methylprednisolone, IVIG and plasma exchange are the standard first line immunotherapies, however, the response to these therapies is typically only partial [4], [5], [22], [24]. Other immunomodulatory medications include rituximab, cyclophosphamide, mycophenolate mofetil, and azathioprine [4], [22], [25]. Recently, agents like tocilizumab, basiliximab, and natalizumab have been advocated as alternative therapies with variable influence in the immunological poly-pharmacotherapy [4], [24], [25], [26].
Our patient failed to show adequate response to standard therapy with methylprednisolone, IVIG, and plasma exchange. Tocilizumab was used subsequently with sustained clinical improvement and seizure control. Tocilizumab is an anti-interleukin 6 monoclonal antibody with direct impact on plasma cell survival, and therefore antibody production. Initiation of tocilizumab was followed by rapid and sustained improvement in previously reported cases with refractory autoimmune encephalitis [26], [27], [28], [29]. Earlier treatment and escalation to second-line treatments are associated with better outcome in children. Some reports recommend escalating to second-line therapy after 10–14 days if no significant improvement or if ongoing clinical deterioration is noticed following first-line therapy [24], [25]. In our patient, rapid escalation was done between different treatment modalities within 1–2 days when no adequate response was noticed. Therefore, it might be reasonable to consider rapid escalation from initial therapy with conventional immunomodulation to alternative novel immunological agents if no appropriate fast response is observed.
4. Conclusion
Anti-GAD encephalitis is rare. The clinical manifestations of this disease are heterogeneous. We report a case of anti-GAD encephalitis presenting with super-refractory status epilepticus. Despite her protracted and frightful clinical course, our patient showed dramatic clinical improvement that we relate to the early use of immunomodulatory therapy and rapid escalation to alternative treatment options. The rapid symptomatic improvement particularly after tocilizumab and the ketogenic diet renders these two therapeutic modalities as therapies of interest that could be further studied and may be considered in similar cases of super refractory status epilepticus with a presumed autoimmune etiology. Further studies are needed to better characterize the efficacy of treatment modalities and immunomodulatory therapy in such a rare disorder.
Declaration of interest
None.
Ethical Statement
We have read and have abided by the statement of ethical standards for manuscripts submitted to Epilepsy and Behavior Reports.
Appendix
Fig. 1.
Immunomodulatory therapies during hospitalization and average number of seizures per hour. IVIG: IV immunoglobulin; IVMP: IV methylprednisolone; PLEX: plasma exchange. Hospital days are reported since arrival to our institution.
References
- 1.Graus F., Titulaer M.J., Balu R., Benseler S., Bien C.G., Celuuci T. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016 doi: 10.1016/S1474-4422(15)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Triplett J., Vijayan S., MacDonald A., Lawn N., McLeane-Tooke A., Bynevelt M. Fulminant anti-GAD antibody encephalitis presenting with status epilepticus requiring aggressive immunosuppression. J Neuroimmunol. 2018;323:119–124. doi: 10.1016/j.jneuroim.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 3.Saiz A., Blanco Y., Sabater L., Gonzalez F., Bataller L., Cassamitjana R. Spectrum of neurological syndromes associated with glutamic acid decarboxylase antibodies: diagnostic clues for this association. Brain. 2008 doi: 10.1093/brain/awn183. [DOI] [PubMed] [Google Scholar]
- 4.Daif A., Lukas R.V., Issa N.P., Javed A., VanHaerents S., Reder A.T. Antiglutamic acid decarboxylase 65 (GAD65) antibody-associated epilepsy. Epilepsy Behav. 2018 doi: 10.1016/j.yebeh.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 5.Khawaja A.M., Vines B.L, Miller D.W., Szaflarski J.P., Amara A.W. Refractory status epilepticus and glutamic acid decarboxylase antibodies in adults: presentation, treatment and outcomes. Epileptic Disord. 2016;18(1):34–43. doi: 10.1684/epd.2016.0797. [DOI] [PubMed] [Google Scholar]
- 6.Finelli P.F. Autoimmune limbic encephalitis with GAD antibodies. Neurohospitalist. 2011;1(4):178–181. doi: 10.1177/1941875211413135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gagnon M., Savard M. Limbic encephalitis associated with GAD65 antibodies: brief review of the relevant literature. Can J Neurol Sci. 2016;43(4):486–493. doi: 10.1017/cjn.2016.13. [DOI] [PubMed] [Google Scholar]
- 8.Meinck H.M., Faber L., Morgenthaler N., Seissler J., Butler M., Solimena M. Antibodies against glutamic acid decarboxylase: prevalence in neurological diseases. J Neurol Neurosurg Psychiatry. 2001 doi: 10.1136/jnnp.71.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hampe C.S., Petrosini L., De Bartolo P., Caporali P., Cutuli D., Laricchiuta D. Monoclonal antibodies to 65kDa glutamate decarboxylase induce epitope specific effects on motor and cognitive functions in rats. Orphanet J Rare Dis. 2013 doi: 10.1186/1750-1172-8-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitoma H., Adhikari K., Aeschlimann D., Chattopadhyay P., Hadjivassiliou M., Hampe C.S. Consensus paper: Neuroimmune mechanisms of cerebellar ataxias. Cerebellum. 2016;15(2):213–232. doi: 10.1007/s12311-015-0664-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee H.F., Chi C.S. Febrile infection-related epilepsy syndrome (FIRES): therapeutic complications, long-term neurological and neuroimaging follow-up. Seizure. 2018;56:53–59. doi: 10.1016/j.seizure.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Sculier C., Gaspard N. New onset refractory status epilepticus (NORSE). Seizure: European. J Epilepsy. 2019;68:72–78. doi: 10.1016/j.seizure.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 13.Hirsch L.J., Gaspard N., Van Baalen A., Nabbout R., Demeret S., Loddenkemper T. Proposed consensus definitions for new-onset refractory status epilepticus (NORSE), febrile infection-related epilepsy syndrome (FIRES), and related conditions. Epilepsia. 2018;59:739–744. doi: 10.1111/epi.14016. [DOI] [PubMed] [Google Scholar]
- 14.Gaspard N., Hirsch L.J., Sculier C., Loddenkemper T., Van Baalen A., Lancrenon J. New-onset refractory status epilepticus (NORSE) and febrile infection-related epilepsy syndrome (FIRES): State of the art and perspectives. Epilepsia. 2018;59:745–752. doi: 10.1111/epi.14022. [DOI] [PubMed] [Google Scholar]
- 15.Ceyhan Dirican A., Elibirlik S., Koksal A., Ozturk M., Altunkaynak Y., Baybas S. Evaluation of glutamic acid decarboxylase antibody levels in patients with juvenile myoclonic epilepsy and mesial temporal lobe epilepsy with hippocampal sclerosis. Noro Psikiyatr Ars. 2016 doi: 10.5152/npa.2015.9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh T.D., Fugate J.E., Hocker S.E., Rabintein A.A. Postencephalitic epilepsy: clinical characteristics and predictors. Epilepsia. 2015;56:133–138. doi: 10.1111/epi.12879. [DOI] [PubMed] [Google Scholar]
- 17.Smith T., Cunningham-Rundles C. Detection of anti glutamic acid decarboxylase antibodies in immunoglobulin products. J Allergy Clin Immunol Pract. 2018 doi: 10.1016/j.jaip.2017.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lilleker J.B., Biswas V., Mohanraj R. Glutamic acid decarboxylase (GAD) antibodies in epilepsy: diagnostic yield and therapeutic implications. Seizure. 2014;23:598–602. doi: 10.1016/j.seizure.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Kelley B.P., Patel S.C., Marin H.L., Corrigan J.J., Mitsias P.D., Griffith B. Autoimmune encephalitis: pathophysiology and imaging review of an overlooked diagnosis. Am J Neuroradiol. 2017;38(6):1070–1078. doi: 10.3174/ajnr.A5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burton A.R., Baquet Z., Eisenbarth G.S., Tisch R., Smeyne R., Workman C.J. Central nervous system destruction mediated by glutamic acid decarboxylase-specific CD4+ T cells. J Immunol. 2010;184(9) doi: 10.4049/jimmunol.0903728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graus F., Saiz A., Dalmau J. GAD antibodies in neurological disorders: insights and challenges. Nat Rev. 2020;16(7):353–365. doi: 10.1038/s41582-020-0359-x. [DOI] [PubMed] [Google Scholar]
- 22.Mäkelä K.M., Hietaharju A., Brander A., Peltola J. Clinical management of epilepsy with glutamic acid decarboxylase antibody positivity: the interplay between immunotherapy and anti-epileptic drugs. Front Neurol. 2018;9:579. doi: 10.3389/fneur.2018.00579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malter M P, Frisch C, Zeitler H, Surges R, Urbach H, Helmstaedter C. Treatment of immune-mediated temporal lobe epilepsy with GAD antibodies. Seizure. 2015;30:57–63. doi: 10.1016/j.seizure.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 24.Bien C.G., Bien C.I. Neurological Research and Practice; 2020. Autoimmune encephalitis in children and adolescents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stingl C., Cardinale K., Van Mater H. An update on the treatment of pediatric autoimmune encephalitis. Curr Treatm Opt Rheumatol. 2018 doi: 10.1007/s40674-018-0089-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Randell R.L., Adams A.V., Van Mater H. Tocilizumab in refractory autoimmune encephalitis: a series of pediatric cases. Pediatr Neurol. 2018 doi: 10.1016/j.pediatrneurol.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 27.Lee W.J., Lee S.T., Moon J., Sunwoo J.S., Byun J.I., Lim J.A. Tocilizumab in autoimmune encephalitis refractory to rituximab: an institutional cohort study. Neurotherapeutics. 2016;13(4):824–832. doi: 10.1007/s13311-016-0442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jun J.S., Lee S.T., Kim R., Chu K., Lee S.K. Tocilizumab treatment for new onset refractory status epilepticus. Ann Neurol. 2018 doi: 10.1002/ana.25374. [DOI] [PubMed] [Google Scholar]
- 29.Cantarín-Extremera V., Imenez-Legido M., Duat-Rodriguez A., Garcia-Fernandez M., Ortiz-Cabrera N.V., Ruiz-Falco-Rojas M.L. Tocilizumab in pediatric refractory status epilepticus and acute epilepsy: Experience in two patients. J Neuroimmunol. 2020;340 doi: 10.1016/j.jneuroim.2019.577142. [DOI] [PubMed] [Google Scholar]