Abstract
Background
Understanding variations in HIV testing preferences can help inform optimal combinations of testing services to maximize coverage. We conducted a systematic review of Discrete Choice Experiments (DCEs) eliciting HIV testing preference.
Methods
We searched the published literature for papers that conducted DCEs to assess user preferences for HIV testing.
Findings
We identified 237 publications; 14 studies conducted in 10 countries met inclusion criteria. Overall, test cost was one of the strongest drivers of preference, with participants preferring free or very low-cost testing. Confidentiality was a salient concern, particularly among key populations and persons who never tested. Participants in resource-limited settings preferred short travel distance and integration of HIV testing with other services. There was substantial heterogeneity across participant characteristics. For example, while women preferred home testing, high-risk groups (e.g. male porters, female bar workers) and men who had not tested in the last year preferred traveling a short distance for testing. HIV self-testing (HIVST) had high acceptability, particularly among those who had never HIV tested, although most users preferred blood-based sample collection over oral swabs. Participants highly valued post-test counselling availability after HIVST.
Interpretation
Overall, participants value low-cost, confidential testing with short travel distance. HIVST is a promising strategy to increase testing coverage but post-test counseling and support should be made available. Educational campaigns to increase familiarity and build confidence in results of oral testing can improve the success of HIVST. DCEs conducted within clinic settings likely have limited generalizability to those not seeking care, particularly for key populations.
Keywords: Preferences, HIV testing, Discrete choice experiment, Systematic review, HIV, Sub-Saharan Africa
Research in context.
Evidence before this study
We searched PubMed for studies published through September 8, 2020 that synthesized the literature on HIV testing preferences using discrete choice experiments (DCEs) using the terms: "discrete choice experiment" or "dce” and “review” and "HIV”. We found only one scoping review assessing the use of DCEs in HIV, which included studies up to 2017. The authors found that participants exhibited the strongest preferences for HIV testing location, testing method, accuracy, confidentiality, and cost, but do not elaborate on the details of the studies, main drivers of testing preference, or heterogeneity in preferences among different groups.
Added value of this study
The majority of studies included in our review were published on or after 2017. We find substantial heterogeneity in HIV testing preferences by sub-population assessed. For example, women preferred home testing while high-risk groups (male porters and female bar workers) and men who have not tested in the last year preferred traveling a short distance for testing. This suggests that confidentiality may be a barrier to home testing among some sub-groups. We also find that many DCEs in the literature recruited participants from HIV testing sites or HIV testing clinical trials, which may impact generalizability to populations who are not already accessing care. Household-based sampling was also frequently utilized, which may underrepresent men who travel for work. Finally, there was a lack of studies in underserved populations including men, migratory populations, never-testers, female sex workers (FSWs), and persons who inject drugs (PWIDs).
Implications of all the available evidence
Overall, we find that individuals prefer low-cost, confidential services within a short travel distance. There is substantial heterogeneity across populations, suggesting a combination of testing modalities are needed to achieve high coverage. HIVST is a promising strategy to reach underserved groups, but community sensitization and post-test support are necessary to optimize uptake. More DCEs are needed to assess HIV testing presences outside of clinic and trial settings, particularly among key populations and never testers.
Alt-text: Unlabelled box
1. Introduction
HIV testing is the first step to accessing HIV treatment and prevention. However, only 75% of persons with HIV worldwide know their status indicating that expanded testing strategies are needed to achieve the first 95 of UNAIDS’ ambitious testing, treatment and viral suppression targets by 2030 [1]. To maximize the potential of antiretroviral treatment (ART) to curb the epidemic, HIV testing services must be tailored to the preferences of those at risk, particularly underserved populations including men, adolescents and young adults (AYA), sex workers, and men who have sex with men (MSM). Sub-optimal HIV testing rates among these groups suggest that current testing services may not be well-aligned with user preferences [2,3]. Barriers and motivators to HIV testing differ by subgroup and understanding variations in preferences can help inform the optimal combination of HIV testing programs.
Discrete Choice Experiments (DCEs) are well-suited to identify individuals’ preferences for HIV testing attributes [4]. Grounded in the economic theory of utility maximization, DCEs enable researchers to describe how individuals value selected features of services by asking them to choose between different hypothetical alternatives [5]. DCEs have become an increasingly popular method of eliciting preferences for healthcare [6]. We conducted a systematic review of DCEs eliciting HIV testing preference to synthesize evidence for stakeholders charged with implementing testing strategies.
2. Methods
2.1. Search strategy
We conducted a systematic literature review following Cochrane and PRISMA guidelines [7]. A literature search was conducted with the help of a librarian on 11/9/2019. We searched PubMed, EMBASE, and Global Health Database, using MeSH and comparable terms. Studies were eligible for inclusion if they reported user preferences for HIV testing services using a discrete choice experiment/conjoint analysis. We included studies published from the year 2000 onwards to focus on the recent literature on HIV testing preferences. Full search strategy is reported in the Appendix.
2.2. Data screening/extraction
M.S. and J.O screened abstracts for inclusion and characterized eligible studies using a standardized extraction form. All included papers were reviewed by both coders; disagreements were adjudicated by reviewing full texts. Details on data extraction and quality review are provided in the Appendix.
2.3. Role of funding source
MS received support from The National Institute of Mental Health (NIMH K01MH115789). JO received support from the Australian National Health and Medical Research Council (GNT1104781). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the article.
3. Results
3.1. Study characteristics
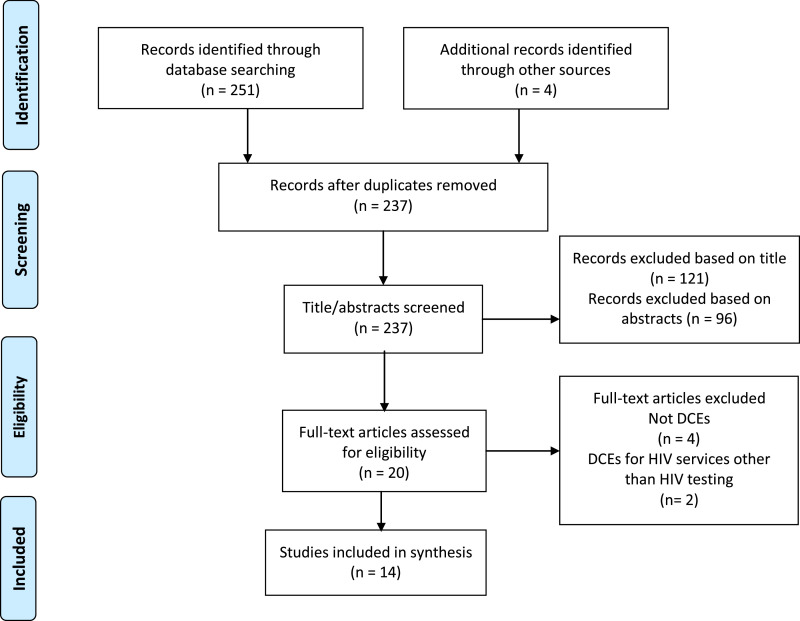
Our search resulted in 237 publications, of which 14 met eligibility criteria and were included in the review (Fig. 1). DCEs were conducted in 10 countries; eight in sub-Saharan Africa (SSA), two each in Europe, and Latin America, one in North America, and one in Asia. Populations assessed included MSM, female bar workers, male porters, truck drivers, AYA, and the general population (Table 1). Six studies reported pilot-testing DCEs before administration, which is recommended to ensure participant understanding of the survey. Only five studies reported using an opt-out (no testing) option in their design, which is necessary to assess if individuals would choose not to test over either testing option presented (Table 2).
Fig. 1.
PRISMA flow diagram.
Table 1.
Characteristics of studies included§.
| First author & year | Country | Population | Sample size | Inclusion/ exclusion criteria | HIV testing assessed | Sampling strategy | Attributes | Most important preference (s) | Heterogeneity assessed |
|---|---|---|---|---|---|---|---|---|---|
| Bristow et al., 2017 | Haiti | General population | 298 | ≥18 years, seeking HIV/STI testing or ANC at clinic | Combined HIV/syphilis testing | Clinic recruitment | Cost, accuracy, time-to-result, blood draw method, number of draws, and rapid vs laboratory test | Free testing | Non-pregnant and pregnant women, men |
| Bristow et al., 2018 | Peru | MSM and transgender women | 347 | ≥18 years, seeking testing at STI clinic | Combined HIV/syphilis testing | Clinic recruitment | Rapid vs. laboratory test, cost, potential for false positive syphilis result, time-to-result, blood draw method, number of blood draws | Free testing, no false positive result for syphilis | MSM, transgender women |
| Indravudh et al., 2017 | Malawi and Zimbabwe | Adolescents and young adults (age 15–25) | 341 | Residing in catchment area, age 16–25 years. In Malawi: no previous HIV+ diagnosis | HIVST | Random household sampling within HIVST trial | Test cost, sample collection method, HIVST or provider-administered, location, pretest support, posttest support, provider type, provider age, provider residence, location, hours, batched or individual distribution | Country, HIV testing history | |
| Free testing | |||||||||
| Llewellyn et al., 2013 | UK | University students | 233 | University students, completing online survey | HIV and STI testing | Convenience sample (internet) | Time to appointment, results waiting time, which results provided, staff type, type of STI testing included, method for reporting results | Integrated HIV testing with other STIs (syphilis, herpes) | Age, sex, testing history |
| Michaels-Igbokwe et al., 2015 | Malawi | Adolescents and young adults (age 15–24) | 537 | Age 15–24 years | Combined HIV testing/family planning | Random selection based on community mapping exercise | Age and sex of provider, confidentiality, availability of HIV services, youth friendly components, price | Confidential testing | Sex, relationship status, age |
| Miners et al., 2019 | UK | MSM | 620 | ≥16 years, no previous HIV+ diagnosis | HIVST, provider HTC | Convenience sample (paid advert on Facebook) | Testing location, sampling method, method of obtaining test, inclusion of testing for other STIs, test accuracy, test cost, infection window period, waiting time to be seen | Short waiting time | Latent class analysis |
| Ostermann et al., 2015 | Tanzania | Female bar workers, male porters | 162 female bar workers 194 male porters | ≥1 lifetime sex partner, no previous HIV+ diagnosis | home and facility HTC | Snowball sampling | Distance to testing, confidentiality of testing, weekdays vs. weekend testing, method for obtaining the sample for testing, availability of ART at testing site | Finger prick, short travel distance, | Bar workers, porters compared to general population |
| Ostermann et al., 2014 | Tanzania | General population | 486 | Residing in catchment area | Facility and home HTC | Household random sampling within HIV Testing Preferences trial | Distance to testing, confidentiality, testing days, method for obtaining sample, availability of ART at testing site. | Home testing, confidential testing | Sex, HIV testing history |
| Pan et al., 2018 | China | MSM | 803 | MSM, ≥16 years old, no previous HIV+ diagnosis | HIVST, provider HTC | Recruited from gay community organization and gay social network portals | Test location, anonymity, test administrator, disclosure of MSM status, type of test, Cost, appointment necessary | Free testing, anonymous testing | Testing history, age, sexual orientation, income |
| Phillips et al., 2002 | USA | General population | 354 | Persons attending HIV testing sites | Home and facility HTC | Clinic recruitment | Testing location, test cost, sample collection method, time to result, accuracy, privacy/anonymity, counseling method | Private/ anonymous testing | Income, sexual orientation, and education |
| Sibanda et al., 2019 | Zimbabwe | General population | 296 | ≥16 years, lived in community for ≥3 months | Random household sampling within HIVST trial | Distribution method, HIVST cost, pretest support, time of operation, distributor age, distributor residence, location of kit collection | Free testing | Age, sex, HIV testing history, religion | |
| Strauss et al., 2018 | Kenya | Long distance truck drivers | 305 | ≥18 years, reside in Kenya, no previous HIV+ diagnosis, attending roadside clinics | Clinic HTC and HIVST | Clinic recruitment | Sample collection method, in-person vs telephone counseling, provider-administered vs HIVST, testing location, time needed to test, cost. | Free testing | testing history, sexual behavior |
| Strauss et al., 2018 | South Africa | High school students (age 16+) at schools in CAPRISA | 248 | ≥16 years, attending school | Facility and community HTC | Researchers and teachers identified students that would represent random sample | Testing location, person conducting testing, counseling type, sample collection method, days/time testing provided, time needed to test, cost | Free or incentivized testing | Age, sex, sexual behavior, testing history, grade |
| Zanolini et al., 2018 | Zambia | General population | 1617 | age 16–49 years old | HIVST | Random household sampling using census data | Self-testing vs. facility testing, counseling available, location of HIVST pickup, cost of HIVST. | HIVST (vs. provider testing), counseling available | Regular testers and non-regular testers |
ANC: Antenatal care, STI: sexually transmitted infection, HIVST: HIV self-testing, MSM: men who have sex with men.
Table 2.
Quality indicators of studies included§.
| First author & year | Country | Population | Attribute selection | DCE pilot tested | Opt out option available | Generalizability | Acceptance rate |
|---|---|---|---|---|---|---|---|
| Bristow et al., 2017 | Haiti | General population | Not reported | Not reported | Not reported | Low: participants recruited from clinics, many were seeking STI testing | Not reported |
| Bristow et al., 2018 | Peru | MSM and transgender women | Not reported | Not reported | Not reported | Low: participants were seeking care at STI clinics | Not reported |
| Indravudh et al., 2017 | Malawi and Zimbabwe | Adolescents and young adults (age 15–25) | Literature review, IDIs, FGDs, ranking exercise | Yes | Yes | Medium: participants shown HIVST demonstration which increases familiarity compared to target population, participants in Zimbabwe received HIVST distribution before DCE | Not reported |
| Llewellyn et al., 2013 | UK | University students | Literature review and FGDs | Not reported | Yes | Medium: convenience sample recruited from university website | Not reported |
| Michaels-Igbokwe et al., 2015 | Malawi | Adolescents and young adults (age 15–24) | Literature review, IDIs, FGDs, choice mapping | Yes | No | High: participants randomly selected within villages and repeatedly contacted for enrollment | 86% |
| Miners et al., 2019 | UK | MSM | FGDs | Not reported | Yes | Medium: participants had high levels of education and were >90% White | Not reported |
| Ostermann et al., 2015 | Tanzania | Female bar workers, male porters | Literature review, IDIs, FGDs, ranking exercise | Yes | No | Medium: difficult to ascertain representativeness of snowball sampling | Not reported |
| Ostermann et al., 2014 | Tanzania | General population | Literature review, IDIs, FGDs, ranking exercise | Yes | No | Medium: Men who work long hours or travel for work were under-represented by household sampling | 79% |
| Pan et al., 2018 | China | MSM | Literature review, FGDs | Yes | Yes | Medium: sampling not likely to reach MSM not opening attending gay venues/networking portals | |
| Phillips et al., 2002 | USA | General population | Literature review, FGDs | Yes | No | Low: participants were recruited for HIV testing sites and had higher education levels than the general population | 96% |
| Sibanda et al., 2019 | Zimbabwe | General population | Literature review and FGDs | Not reported | No | Medium: households received HIVST distribution 8 weeks before DCE which could impact their testing preferences | 90% |
| Strauss et al., 2018 | Kenya | Long distance truck drivers | Not stated | Not reported | No | Low: participants were recruited from roadside clinics and were enrolled in HIV testing trial | Not reported |
| Strauss et al., 2018 | South Africa | High school students (age 16+) at schools in CAPRISA | FGDs, literature review, ranking exercise | Not reported | Not reported | Medium: recruitment from schools participating in HIV trial with high HIV testing experience and uptake | Not reported |
| Zanolini et al., 2018 | Zambia | General population | Discussions with in country stakeholders | Not reported | Yes | Medium: participants given instructional leaflet and video on HIVST which increased familiarity compared to target population. 60% of sample was female | 85% |
STI: sexually transmitted infection, FGD: focus group discussion, IDI: in-depth interview, HIVST: HIV self-testing, MSM: men who have sex with men; HTC: HIV testing and counselling.
3.2. Long distance truck drivers
Male long distance truck drivers in SSA are at increased risk for HIV partially due to risk behaviors (multiple partners, purchasing sex, and inconsistent condom use) and low healthcare access [8]. One DCE evaluated testing preferences among long distance truck drivers recruited from roadside wellness clinics who were participating in an HIV testing trial [9]. Cost was the strongest driver of preference, with participants preferring free tests. Participants preferred provider-administered HIV testing over HIV self-testing (HIVST) and a finger-prick over an oral swab. Testing at a roadside clinic was preferred over home or office testing and in-person counselling was preferred over telephone counseling. However, those who had never HIV tested preferred oral testing and telephone counselling. The authors concluded that the existing service model of roadside clinics was well-aligned with user preferences and implementing HIVST was unlikely to increase testing. However, participants recruited into the DCE were already accessing HIV testing at roadside clinics so their preferences may not be generalizable to long distance truck drivers who do not seek clinic-based testing. Further, 92% of the sample had previously HIV tested, so the study was not sufficiently powered to assess preferences in never testers.
3.3. Male Mt Kilimanjaro Porters
Similar to long distance truck drivers, male porters in Tanzania spend long periods away from home and face unstable income cycles. Porters report three-times as many sexual partners as males in the general population [10]. A DCE in Tanzania assessed the testing preferences of porters compared to males in the general population. Results showed that porters preferred testing a short distance from their home over home testing [10]. Porters preferred venipuncture over finger-prick or oral testing and placed more importance on HIV medications being immediately available. Porters most preferred their spouses knowing that they tested. Both males in the general population and porters preferred to test on weekends, although this attribute was the least important of those assessed.
3.4. Female bar workers
Female bar workers in Tanzania have a high HIV prevalence (19–26%) and twice as many lifetime sex partners as females in the general population [10]. A DCE conducted in Tanzania found that bar workers were more reluctant to test at home and more willing to travel out of town for testing compared to females in the general population [10]. They were more averse than other community members to many people knowing about their HIV test. Similar to porters, they preferred venipuncture over finger-prick or oral swab. Distance to testing was an important driver of preference.
3.5. General population
A DCE in Northern Tanzania using random household sampling found distance was the strongest driver of preference, with home testing most preferred among females and a short travel distance (~1 km) preferred among males [11]. Both sexes preferred their spouses to know about their HIV test, compared to no one knowing, and both preferred finger pricks or venipuncture to oral testing. Both males and females preferred testing in places where medications are available but this was less important than distance and confidentiality. Weekday vs. weekend testing was least important to participants. Stratification by HIV testing history revealed that males who had never tested were more likely to prefer oral swabs and less likely to prefer venipuncture compared to males who had tested previously. Males who had never tested were also more likely to prefer weekend testing. Among females, those who had never tested for HIV preferred that no one know that they tested compared to their spouse knowing. Overall, 67% of the sample consisted of females, as is common with household-based sampling. Therefore, men who travel for employment and may have higher HIV risk are less likely to be represented.
A second household-based DCE conducted in Zambia assessed preferences for HIVST testing after providing an instructional leaflet and video on HIVST [12]. Participants strongly preferred HIVST over provider-administered testing if post-test counseling was available. The strength of preference for HIVST was slightly stronger among never testers. Participants preferred lower cost self-tests, but never testers were willing to pay more for HIVST compared to previous testers ($4.60 vs $3.30USD, respectively). Similar to the DCE conducted in Tanzania, 60% of the sample was female. Generalizability to the target population may have been impacted by the instructional materials which sensitized participants to HIVST and increased their confidence in performing HIVST.
Another household-based DCE evaluated HIVST preferences among participants in the STAR HIVST distribution study in Zimbabwe [13,14]. The strongest preference was free HIVST, and participants preferred home delivery of kits by lay distributors over collection from mobile sites. Men and AYA preferred individual kit distribution rather than kits delivered to whole households. For pre-test support, participants preferred telephone hotline over in-person support or informational leaflet. Preferences for post-test support were not assessed. Only 15% of the sample had never tested for HIV and participants had been provided HIVST kits eight weeks prior to DCE administration which likely limits generalizability to populations who have not received HIVST. Further, the finding that participants preferred home HIVST delivery may have been impacted by their prior experience with this type of distribution.
One DCE conducted in a high resource setting in the USA found that participants valued timely, accurate and confidential testing and most preferred home testing over clinics [15]. This study was conducted in publicly-funded HIV testing locations, therefore may not be representative of persons not seeking testing. Similarly, a DCE conducted among a clinic population in urban Haiti evaluated preferences for dual HIV/syphilis testing [16]. Cost had the highest impact on willingness to test.
3.6. Adolescents and young adults
A DCE conducted in Malawi and Zimbabwe examining AYA's preferences for HIVST delivery strategies via household survey and qualitative assessment found high HIVST acceptability [17]. Across settings, test price was an important driver of preference, with very low or no cost HIVSTs preferred. Participants did not reveal a preference for oral vs. blood-based sampling. However, qualitative results revealed concerns about accuracy of oral testing although it was viewed as more convenient. In Malawi, AYA preferred to obtain an HIVST from lay community distributors compared to intimate partners or healthcare providers; in Zimbabwe, distributor type was not assessed but participants were indifferent to provider age and whether they came from the same community. Qualitative results in both countries indicated lack of trust of healthcare providers and preference for home HIVST distribution among AYA. In Malawi, participants preferred in-person post-test counseling to hotline or leaflet support. While this attribute was not assessed in Zimbabwe, qualitative results show the importance of in-person post-test support.
A DCE of provider-administered HIV testing among adolescents in high school (≥16 years) in South Africa found cost to be the most important driver of testing preference [18]. Offering a financial incentive of $7.50 USD had the largest effect on testing, while even a small fee of $1.50 decreased preference for testing. Boys were more strongly motivated than girls by financial incentives. Participants preferred finger-prick to blood draw or oral sampling and valued in-person counseling. Test administration by a nurse from a neighboring community was preferred over a nurse from the adolescents’ community whom they know. Participants showed an aversion to group counseling, with boys showing a greater aversion. This study was conducted in schools offering HIV testing as part of the CAPRISA trial; therefore participants had more HIV testing experience than adolescents in the general population.
A DCE evaluating a combined package of family planning and HIV testing services in Malawi showed that AYA valued confidentiality and onsite availability of ART [19]. Respondents were less likely to choose a service with older providers (≥30 years old) and preferred lower cost services. Similar to the DCE among high school students in South Africa, males valued confidentiality more than females, although it was an important driver of preference for both sexes. Males were more likely to prefer the addition of youth friendly components such as sports or health talks.
One study of AYAs was conducted in a high resource setting [20]. The authors administered an online DCE to university students in the UK on preferences for sexually transmitted infection (STI) testing. Among this convenience sample, respondents reported a strong preference for integration of STI testing, including HIV, syphilis, and herpes administered by a provider with specialist STI knowledge. Participant's age, sex, or STI testing history was not associated with preference, although the sample size was not large enough to assess heterogeneity.
3.7. MSM
Our search identified three DCEs of HIV testing preferences among MSM, conducted in the UK, China, and Peru [21], [22], [23]. Notably, we did not find any studies evaluating preferences of MSM in SSA. One study administered an online DCE to MSM recruited from a social media platform in the UK [21]. The authors investigated preferences for provider-administered testing vs remote testing (defined as HIVST or self-sampling and mailing of sample to a laboratory). Remote testing preferences included higher test accuracy, shorter window period, and faster results, and oral swab (vs. finger prick). Test cost was the largest barrier to remote testing; free instead of £30 kits was the largest predictor of testing choice. Latent class analysis revealed two groups: 86% of the sample preferred provider-administered HIV testing and 14% preferred remote testing. The group favoring remote testing was more likely to be non-White and never previously HIV tested. The authors concluded MSM generally prefer face-to-face testing compared to self-testing strategies but remote testing has the potential to increase uptake in a subset of MSM. The main limitation was the use of a convenience sample which makes generalizability difficult to ascertain. Although the response rate among persons who saw the advert cannot be assessed, only 50% of those who started the survey completed all DCE questions. Those who did not complete questions were more likely to have lower education and have never HIV tested. This may indicate difficulty understanding the DCE among those with lower education. Further, the sample was 93% White and 3% Asian; only 1% of the sample was Black (seven participants).
A DCE in China recruited men from gay community-based organizations and gay networking portals [22]. Recruitment quotas were used to ensure adequate representation across income, education, and status disclosure to healthcare worker. Cost was the strongest driver of preference. Participants most strongly preferred free, anonymous testing administered by a health care provider. Testing location was of moderate importance. The relative importance of attributes was similar between men with different HIV testing histories. However, never testers most preferred home-based testing and least preferred health department testing while the opposite was true for men who tested previously. Interestingly, men most preferred testing provided by a trained healthcare worker over a lay counselor or self-testing as long as anonymity was preserved. This study was conducted in an urban city and may not generalize to rural locations or other cities in China. In addition, the sampling strategy likely did not reach MSM not openly connected with gay organizations.
Finally, a DCE in Peru evaluated preferences for dual HIV/syphilis testing among MSM and transgender women [23]. Participants were recruited from STI clinics providing services to gay men and transgender women. Participants had the strongest preference for high syphilis test accuracy and free testing. No significant differences in testing preferences were observed between MSM and transgender women. Participants were seeking testing at clinics so their preferences may not generalize to those not accessing care.
4. Discussion
Across studies, cost was one of the strongest drivers of preference, with participants preferring free or very low cost testing. This suggests that even in high-income settings, HIV test cost can be significant barrier to testing. Confidentiality was also a salient concern, particularly among key populations and never testers. HIV-related stigma can deter individuals from testing, especially in clinics where they may be seen by others in their community [24]. Further, lack of confidence in healthcare providers to maintain confidentiality is a barrier to testing. A DCE of AYA in Malawi found that participants preferred obtaining an HIVST from a lay distributor over a healthcare worker, and study among South African high school students showed that participants preferred HIV testing administered by nurses from neighboring communities instead of their own community [17,18]. Finally, a study in China showed that MSM preferred anonymous testing to providing their names to providers [22]. This is consistent with qualitative findings that show perceived health workers’ inability to maintain confidentiality and fear of HIV-related stigma are major barriers to testing [25]. Fear of losing social capital, particularly from one's spouse, family or community, can generate fear of learning one's status, especially in settings of high poverty [26]. Community awareness campaigns emphasizing the ability of ART to restore one's health and prevent onward transmission can reduce HIV-related stigma [27]. Provider training can improve attitudes towards HIV testing and confidentiality of services. Integration of HIV testing with other services including STI testing, family planning, or youth friendly activities can normalize testing and was preferred in the DCEs in our analysis.
There was substantial heterogeneity in preferences across populations. For example, while home HIV testing was preferred by women in the general population, high risk groups (e.g. male porters, female bar workers) and men who had not tested in the last year preferred to travel a short distance for testing [10]. Further, a DCE in Tanzania noted that while home testing was preferred on average by women, a large standard deviation around this parameter suggests that a sizeable proportion of women derived a negative utility from home testing [11]. A study of MSM in China found those who had previously tested preferred facility over home testing, while never testers preferred home testing [22]. Although, home testing is most convenient in terms of travel time, confidentiality may be a concern, particularly among individuals worried about testing HIV-positive. Fear of disclosure to others in the household may be a barrier to home testing. For example, female bar workers in Tanzania preferred that their partner does not know about their HIV test [10]. Similarly a DCE in Zimbabwe found that men and AYA preferred individual HIVST distribution to batched distribution (kits provided to the whole household). This may indicate concerns about confidentiality or coerced testing by others in the household. Travel distance to HIV testing was another source of heterogeneity. Overall, participants preferred shorter travel distances to testing services, indicating opportunity costs associated with travel time and transport costs can be a barrier to testing. However, female bar workers and male porters were more willing to travel longer distances for testing, suggesting that the convenience of closer testing may be outweighed by confidentiality concerns of testing in one's own community [10]. Together, these results suggest that while home testing has high acceptability, it is not universally preferred. To reach the first 95 of UNAIDS ambitious targets, policymakers must implement a combination of HIV testing strategies that fit the diverse preferences of different sub-populations.
HIVST is a promising strategy to overcome barriers associated with provider-administered testing including confidentiality concerns, stigma, and opportunity costs of travel and waiting. HIVST can increase testing coverage, particularly among key populations and never testers [12,21]. Overall, studies show participants prefer free or very low cost HIVST. However, a DCE conducted in Zambia found never testers had a slightly higher willingness to pay for HIVST compared to those who previously tested [12]. HIVST had high acceptability in a study of AYA in Malawi, with participants preferring distribution from lay counselors over intimate partner or healthcare workers [17]. A study of MSM in the UK found that while most men preferred provider-administered testing, a subset of participants who were more likely to be never-testers and non-White, preferred self-testing [21]. A study of MSM in China found that participants preferred HIVST, mainly due to confidentiality of services, but only in the absence of anonymous provider-administered testing [22]. Across studies, participants strongly valued availability of post-test counseling. Qualitative studies highlight participant fears about self-testing HIV-positive in the absence of counseling support, including depression, failure to link to ART, and even suicide [28]. HIVST strategies should incorporate in-person or hotline support to provide counseling and encourage individuals to link to ART or prevention [29]. Similarly, pre-test support (instructional leaflet, video, or hotline) can increase individuals’ confidence in conducting testing and interpreting results. Concerns about HIVST accuracy are frequently cited as a barrier to uptake, with individuals expressing doubt that a saliva-based test can detect a virus found in the blood [28]. DCEs among the general population, long distance truck drivers, male porters, and female bar workers show that participants prefer finger prick or venipuncture over oral testing, which may be driven by misinformation regarding reliability of oral swab testing [17]. As HIVST is scaled-up, community sensitization and awareness campaigns emphasizing test accuracy and explaining HIVST use may increase acceptability [30]. Interestingly, a DCE in Tanzania found while blood-based sampling was preferred by the general population, males who have never tested preferred oral swabs. This suggests that HIVST may overcome barriers such as fear of needles and increase HIV testing coverage among under-served groups, including men.
A significant limitation of several studies we assessed is generalizability to the population of interest. Four of the 14 DCEs recruited participants from STI clinics, most of whom were seeking HIV testing. For example, a study in Kenya that enrolled long distance truck drivers from roadside clinics found that services provided were well-aligned with participant preference and HIVST distribution was unlikely to increase testing coverage [9]. This result is not surprising given the men in the study were seeking HIV testing at a clinic. Further, their preferences are unlikely to generalize to truck drivers not accessing clinic testing likely due to differences in testing preferences. Two DCEs assessed utilized convenience samples, making generalizability difficult to determine. A study of MSM recruited from a social media platform in the UK found that most men preferred standard-of-care clinic testing [21]. However, over 90% of the sample was White, half had a college degree or higher, and 80% had tested for HIV in the past. Further only 50% of those who started the survey completed it, and those who did not complete it were more likely to be never testers and have lower education. It is likely that the preferences of highly educated, White MSM do not generalize to other sub-groups of MSM, who may be less comfortable accessing the health system. Indeed, if current testing modalities were well-aligned with the preferences of key populations, HIV testing rates would likely be higher in these groups. Although it is difficult to obtain representative samples of key populations, studies using snowball or venue-based sampling may have greater generalizability. In addition, four DCEs utilized household-based surveys; over 60% of the samples were female suggesting then men who work long hours or travel for employment may be missed. These men likely have distinct HIV testing preferences due to their mobility and may be less likely to access clinic testing. Further, several studies recruited participants from HIV testing or HIVST trials or provided instructional materials on HIVST use prior to DCE administration. Lack of familiarity with HIVST may be a barrier to acceptability so participants who receive information on HIVST may no longer be representative of the target population. Most participants recruited from HIV testing trials had already demonstrated willingness to test using existing strategies so their preferences may not be generalizable to the rest of the population. Additionally, acceptance rate is an important indicator of generalizability—low acceptance overall or among subpopulations could indicate selection bias, as those willing to complete a DCE about HIV testing may have different preferences than those who decline. Only four studies reported acceptance rates [11,13,15,19]. Further, although there was substantial heterogeneity in testing preferences, many studies were not powered to detect differences among sub-populations. Finally, there was a lack of studies in underserved populations including men, migratory populations, never-testers, female sex workers (FSWs), and persons who inject drugs (PWIDs). We found no DCEs among MSM in SSA and only one clinic-based DCE among transgender women [23]. Globally, over half of new HIV infections occur in key populations and MSM, FSWs, and PWID have >20-times the HIV risk of the general population [31]. With effective scale-up of ART, the epidemic is increasingly concentrating among key populations and assessing their HIV testing preferences is critical to informing prevention. Finally, our search yielded only 14 studies so our conclusions are limited by the small number of papers included.
We synthesized the literature on HIV testing preferences to provide an overview of attributes influencing HIV testing acceptability. Overall, individuals prefer low-cost, confidential services within a short travel distance. There is substantial heterogeneity across populations, suggesting a combination of testing modalities are needed to achieve high coverage. HIVST is a promising strategy to reach underserved groups, but community sensitization and post-test support are necessary. More DCEs are needed to access HIV testing presences outside of clinic and trial settings, particularly in key populations and never testers.
Declaration of Competing Interest
Dr. Sharma reports grants from The National Institute of Mental Health during the conduct of the study. Dr. Celum reports grants from The National Institutes of Mental Health during the conduct of the study and personal fees from Merck and Gilead Sciences outside the submitted work. The other authors declare that they have nothing to disclose.
Acknowledgments
Acknowledgements
We thank the University of Washington librarians for their assistance in designing search terms for this analysis.
Author's contributions
MS and FTP conceived of the analysis and conducted the literature search. MS and JO completed data abstraction. MS wrote the first draft of the paper. All authors critically reviewed the manuscript and approved the final version.
Funding sources
MS received support from The National Institute of Mental Health (NIMH K01MH115789). JO received support from the Australian National Health and Medical Research Council (GNT1104781). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the article.
Data sharing statement
All data utilized in this systematic review are published and publically available.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100653.
Appendix. Supplementary materials
References
- 1.UNAIDS: Fast-track, Ending the AIDS epidemic by 2030. 2020. https://www.unaids.org/sites/default/files/media_asset/JC2686_WAD2014report_en.pdf. Accessed on 1/16/2020.
- 2.Joint United Nations Programme on HIV/AIDS (UNAIDS). Ending AIDS: progress towards the 90-90-90 targets. Geneva, Switzerland: UNAIDS. 2020. https://www.unaids.org/en/resources/documents/2017/20170720_Global_AIDS_update_2017. Accessed on 9/8/2020.
- 3.WHO Consolidated guidelines on HIV prevention, diagnosis, treatment, and care for key populations. 2020. https://apps.who.int/iris/bitstream/handle/10665/246200/9789241511124-eng.pdf?sequence=8. Accessed on 9/8/2020. [PubMed]
- 4.Phillips K.A., Johnson F.R., Maddala T. Measuring what people value: a comparison of "attitude" and "preference" surveys. Health Serv Res. 2002;37(6):1659–1679. doi: 10.1111/1475-6773.01116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lancsar E., Louviere J. Conducting discrete choice experiments to inform healthcare decision making: a user's guide. Pharmacoeconomics. 2008;26(8):661–677. doi: 10.2165/00019053-200826080-00004. [DOI] [PubMed] [Google Scholar]
- 6.Soekhai V., de Bekker-Grob E.W., Ellis A.R., Vass C.M. Discrete choice experiments in health economics: past, present and future. Pharmacoeconomics. 2019;37(2):201–226. doi: 10.1007/s40273-018-0734-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clin Res ed) 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kohli A., Kerrigan D., Brahmbhatt H., Likindikoki S., Beckham J., Mwampashi A. Social and structural factors related to HIV risk among truck drivers passing through the Iringa region of Tanzania. AIDS Care. 2017;29(8):957–960. doi: 10.1080/09540121.2017.1280127. [DOI] [PubMed] [Google Scholar]
- 9.Strauss M., George G., Lansdell E., Mantell J., Govender K., Romo M. HIV testing preferences among long distance truck drivers in Kenya: a discrete choice experiment. Sex Transm Infect. 2017;93:A214. doi: 10.1080/09540121.2017.1367086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ostermann J., Njau B., Mtuy T., Brown D.S., Muhlbacher A., Thielman N. One size does not fit all: HIV testing preferences differ among high-risk groups in Northern Tanzania. AIDS Care. 2015;27(5):595–603. doi: 10.1080/09540121.2014.998612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ostermann J., Njau B., Brown D.S., Muhlbacher A., Thielman N. Heterogeneous HIV testing preferences in an urban setting in Tanzania: results from a discrete choice experiment. PLoS ONE. 2014;9(3):e92100. doi: 10.1371/journal.pone.0092100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zanolini A., Sikombe K., Sikazwe I., Eshun-Wilson I., Somwe P., Bolton Moore C. Understanding preferences for HIV care and treatment in Zambia: evidence from a discrete choice experiment among patients who have been lost to follow-up. PLoS Med. 2018;15(8) doi: 10.1371/journal.pmed.1002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sibanda E.L., d'Elbee M., Maringwa G., Ruhode N., Tumushime M., Madanhire C. Applying user preferences to optimize the contribution of HIV self-testing to reaching the "first 90" target of UNAIDS fast-track strategy: results from discrete choice experiments in Zimbabwe. J Int AIDS Soc. 2019;22(Suppl 1):e25245. doi: 10.1002/jia2.25245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.STAR Project. HIV self-testing Africa project. 2020. http://hivstar.lshtm.ac.uk/. Accessed on 1/24/2020.
- 15.Phillips K.A., Maddala T., Johnson F.R. Measuring preferences for health care interventions using conjoint analysis: an application to HIV testing. Health Serv Res. 2002;37(6):1681–1705. doi: 10.1111/1475-6773.01115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bristow C.C., Lee S.J., Severe L., William Pape J., Javanbakht M., Scott Comulada W. Attributes of diagnostic tests to increase uptake of dual testing for syphilis and HIV in Port-au-Prince, Haiti. Int J STD AIDS. 2017;28(3):259–264. doi: 10.1177/0956462416642340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Indravudh P.P., Sibanda E.L., d'Elbee M., Kumwenda M.K., Ringwald B., Maringwa G. 'I will choose when to test, where I want to test': investigating young people's preferences for HIV self-testing in Malawi and Zimbabwe. AIDS. 2017;31(Suppl 3):S203–SS12. doi: 10.1097/QAD.0000000000001516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strauss M., George G.L., Rhodes B.D. Determining preferences related to HIV counselling and testing services among high school learners in KwaZulu-Natal: a discrete choice experiment. AIDS Behav. 2018;22(1):64–76. doi: 10.1007/s10461-016-1602-8. [DOI] [PubMed] [Google Scholar]
- 19.Michaels-Igbokwe C., Lagarde M., Cairns J., Terris-Prestholt F. Designing a package of sexual and reproductive health and HIV outreach services to meet the heterogeneous preferences of young people in Malawi: results from a discrete choice experiment. Health Econ Rev. 2015;5:9. doi: 10.1186/s13561-015-0046-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Llewellyn C.D., Sakal C., Lagarde M., Pollard A., Miners A.H. Testing for sexually transmitted infections among students: a discrete choice experiment of service preferences. BMJ Open. 2013;3(10) doi: 10.1136/bmjopen-2013-003240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miners A., Nadarzynski T., Witzel C., Phillips A.N., Cambiano V., Rodger A.J. Preferences for HIV testing services among men who have sex with men in the UK: a discrete choice experiment. PLoS Med. 2019;16(4) doi: 10.1371/journal.pmed.1002779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan S.W., Durvasula M., Ong J.J., Liu C., Tang W., Fu H. No place like home? disentangling preferences for HIV testing locations and services among men who have sex with men in China. AIDS Behav. 2019;23(4):847–859. doi: 10.1007/s10461-018-2366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bristow C.C., Kojima N., Lee S.J., Leon S.R., Ramos L.B., Konda K.A. HIV and syphilis testing preferences among men who have sex with men and among transgender women in Lima, Peru. PLoS ONE. 2018;13(10) doi: 10.1371/journal.pone.0206204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Treves-Kagan S., El Ayadi A.M., Pettifor A., MacPhail C., Twine R., Maman S. Gender, HIV testing and stigma: the association of HIV testing behaviors and community-level and individual-level stigma in rural South Africa differ for men and women. AIDS Behav. 2017 doi: 10.1007/s10461-016-1671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Musheke M., Ntalasha H., Gari S., McKenzie O., Bond V., Martin-Hilber A. A systematic review of qualitative findings on factors enabling and deterring uptake of HIV testing in Sub-Saharan Africa. BMC Public Health. 2013;13:220. doi: 10.1186/1471-2458-13-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan B.T., Tsai A.C., Siedner M.J. HIV treatment scale-up and HIV-related stigma in Sub-Saharan Africa: a longitudinal cross-country analysis. Am J Public Health. 2015;105(8):1581–1587. doi: 10.2105/AJPH.2015.302716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma M., Barnabas R.V., Celum C. Community-based strategies to strengthen men's engagement in the HIV care cascade in sub-Saharan Africa. PLoS Med. 2017;14(4) doi: 10.1371/journal.pmed.1002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Njau B., Covin C., Lisasi E., Damian D., Mushi D., Boulle A. A systematic review of qualitative evidence on factors enabling and deterring uptake of HIV self-testing in Africa. BMC Public Health. 2019;19(1):1289. doi: 10.1186/s12889-019-7685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.d'Elbee M., Indravudh P.P., Mwenge L., Kumwenda M.M., Simwinga M., Choko A.T. Preferences for linkage to HIV care services following a reactive self-test: discrete choice experiments in Malawi and Zambia. AIDS. 2018;32(14):2043–2049. doi: 10.1097/QAD.0000000000001918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bulterys M.A., Mujugira A., Ware N.C., Fairbanks J., Celum C., Sharma M. Men's perspectives on HIV self-testing strategies in Uganda: a qualitative study. Poster, 23rd International AIDS Conference (AIDS 2020: Virtual).
- 31.UNAIDS Fact Sheet: Wold AIDS Day 2019. https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf. Accessed on 1/15/2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.