Abstract
Purpose
MR-linacs (MRLs) have enabled the use of stereotactic magnetic resonance (MR) guided online adaptive radiotherapy (SMART) across many cancers. As data emerges to support SMART, uncertainty remains regarding optimal technical parameters, such as optimal patient positioning, immobilization, image quality, and contouring protocols. Prior to clinical implementation of SMART, we conducted a prospective study in healthy volunteers (HVs) to determine optimal technical parameters and to develop and practice a multidisciplinary SMART workflow.
Methods
HVs 18 years or older were eligible to participate in this IRB-approved study. Using a 0.35 T MRL, simulated adaptive treatments were performed by a multi-disciplinary treatment team in HVs. For each scan, image quality parameters were assessed on a 5-point scale (5 = extremely high, 1 = extremely poor). Adaptive recontouring times were compared between HVs and subsequent clinical cases with a t-test.
Results
18 simulated treatments were performed in HVs on MRL. Mean parameters for visibility of target, visibility of nearby organs, and overall image quality were 4.58, 4.62, and 4.62, respectively (range of 4–5 for all measures). In HVs, mean ART was 15.7 min (range 4–35), comparable to mean of 16.1 (range 7–33) in the clinical cases (p = 0.8963). Using HV cases, optimal simulation and contouring guidelines were developed across a range of disease sites and have since been implemented clinically.
Conclusions
Prior to clinical implementation of SMART, scans of HVs on an MRL resulted in acceptable image quality and target visibility across a range of organs with similar ARTs to clinical SMART. We continue to utilize HV scans prior to clinical implementation of SMART in new disease sites and to further optimize target tracking and immobilization. Further study is needed to determine the optimal duration of HV scanning prior to clinical implementation.
Keywords: MR-linac, Magnetic resonance imaging, Adaptive radiotherapy, Training, Workflow
Introduction
Technological developments have enabled the successful integration of a magnetic resonance imaging (MRI) scanner and linear accelerator into the same device, now commercially available as an MR-linac [1]. MR-linacs are currently manufactured by multiple vendors and present benefits due to the improved soft tissue contrast and lack of ionizing radiation provided by MRI, including: 1) motion management with MR with real-time tumor tracking, 2) MR-based setup, and 3) stereotactic MR-guided online adaptive radiotherapy (SMART) to account for daily changes in tumor and organ-at-risk (OAR) position and size. These advances have translated to safe and effective treatments for patients, with early clinical data showing promise for SMART across a range of disease cites, including prostate [2], [3], [4], lung [5], [6], [7], [8], pancreatic [9], [10], liver [11], adrenal [12], and breast [13], [14] malignancies.
The MR-linac is still considered a new technology in radiation oncology, and randomized trials are not yet available to demonstrate superiority of SMART compared to stereotactic radiotherapy delivered on a non-MR linac. Multi-institutional and single institutional prospective studies are ongoing to further define the role for SMART in the clinical care of patients with cancer.
[15], [16]. As new centers acquire MR-linacs and develop institutional programs for the delivery of SMART, a limited number of published experiences with commissioning and clinical workflows are available to guide clinical implementation [17], [18], [19], [20], [21]. However, successful delivery of SMART requires real-time collaboration between multi-disciplinary teams with input from therapy, physics, dosimetry, and clinicians. In addition, no standard best practices yet exist for optimal simulation parameters and patient positioning techniques. For example, at some centers thoracic SMART is delivered with arms up, the traditional position for linac-based treatment, and at other centers this treatment is delivered with arms down. Centers also differ in their use of immobilization during SMART. Although contouring atlases for a limited number of sites are available [22], [23], [24], these atlases do not provide instructions or consensus for contouring across MR field strengths and MR sequences and disease sites. Gaps exist in both training for SMART [25], and consensus regarding its delivery [19], [26]. In this study, a healthy volunteer imaging program was developed and implemented to establish optimal simulation parameters for SMART prior to clinical implementation and to provide a simulated clinical environment in which to develop and practice multi-disciplinary skills in the safe and effective implementation of SMART.
Materials and methods
Healthy volunteers: Healthy adults age 18 years and older were eligible to participate in this healthy volunteer study, including all races and genders. The healthy volunteer study was approved by the Institutional Review Board (IRB) and took place prior to clinical use of the MR-linac in patients at our institution. Exclusion criteria included: pregnancy, Karnofsky performance status < 90, uncontrolled intercurrent illness, refusal of hearing protection, or inability to undergo MRI due to presence of an implanted or external MRI unsafe device or MR conditional device not meeting the conditions required for the scan. The study also excluded individuals directly supervised by study investigators.
Pre-treatment simulation
Healthy volunteers consenting to the study and meeting eligibility criteria were scanned on a 0.35 T MRIdian MR-linac (Viewray, Oakwood Village, OH). For pre-treatment MR simulation, participants were positioned in the scanner. The following parameters were varied during pre-treatment scanning to determine optimal study of a pre-defined anatomic region of interest: arm position (up vs. down vs. on chest), immobilization selection, coil position (varied relative to region of interest), breathing instructions (breath hold vs. free breathing), use of water as oral contrast (without or with water, and variation in timing relative to imaging acquisition). Pre-medications other than water were not utilized to minimize risks to volunteers. To determine impact of gastric filling on abdominal imaging, scans were scheduled zero to three hours from the last meal, with permission of the volunteer. Due to the nature of the MR-linac, sequence type could not be varied at the time of the study. Hearing protection was utilized for all scans.
Scan time per session did not exceed 60 min to respect safety and ethical considerations in the scanning of healthy volunteers. Images were acquired using TrueFISP sequences, which are balanced steady-state coherent sequences providing a hybrid T2/T1-weighting [27], [28]. Ability to track the simulated target with sagittal TrueFISP cine imaging was also assessed at pre-treatment simulation. For breath hold treatments, the target served as a guide for gating, and for free-breathing treatments it served as a marker for motion. If the target could not be tracked, a nearby organ was utilized for tracking and this was noted to allow for similar tracking at simulated treatment.
Although patients would also require simulation with computed tomography (CT), to calculate dose, healthy volunteers did not undergo CT simulation to avoid ionizing radiation exposure. Instead, deidentified CT scans from patients simulated for treatments at our institution on other linear accelerators were deformed to the HV MR scans. CT scans from patients treated on the MR-linac were not utilized because patients had not yet been treated on the MR-linac at our institution at the time of this healthy volunteer study.
Pre-treatment MR simulation scans acquired with parameters leading to optimal image quality were flagged for use in subsequent treatment planning and parameters were recorded to allow for replication at simulation treatment.
Treatment planning
Physicians contoured simulated targets and organs-at-risk in MIM (MIM Software, Cleveland, OH). All healthy volunteer scans were de-identified. Simulated targets were selected based on published experiences with the MR-linac, and discussions with other institutions regarding diseases most likely to benefit from treatment on an MR-linac. Organs-at-risk were selected based on our institutional practices for treatment on a conventional linac. Contours were transferred to the treatment planning software provided by the MR-linac manufacturer (Viewray), and dosimetrists and physicists created mock plans meeting organs-at-risk and target constraints. Dose constraints were determined based on TG-101 [29] and published studies [3], [6], [12], [30]. The deformed CT scans were utilized for dose calculation.
Simulated treatment
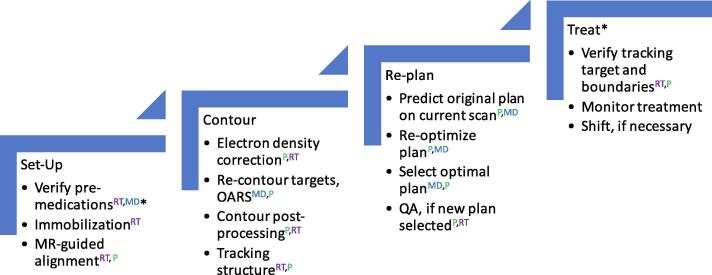
For simulated treatment, participants were positioned in the scanner in a manner similar to the optimal set-up at simulation. A SMART workflow was developed by our multi-disciplinary team and practiced during simulated treatments (Fig. 2). Although the basic components of SMART have been previously published [19], [31], our workflow provided discrete steps for the clinical implementation of SMART, utilizing safety concepts from aviation such as the model of a “pilot” and “co-pilot” [32]. The workflow assigned a “pilot” and “co-pilot” to each step in the process of SMART. The pilot was responsible for carrying out the task, and the “co-pilot” was responsible for providing oversight.
Fig. 2.
Online adaptive workflow for SMART. Caption: *step not utilized for healthy volunteers; OARS = organs-at-risk; QA = quality assurance; Rx = prescription; RT = therapist; MD = physician; P = physicist; first abbreviation in superscript refers to pilot, second abbreviation refers to co-pilot.
Simulated treatment involved acquisition of a 3D TrueFISP scan to confirm setup and perform necessary shifts, and to allow for density overrides and adaptive recontouring and re-planning. A sagittal TrueFISP cine scan was acquired to allow for target (tumor) tracking. If the tumor could not be tracked, a nearby organ was utilized for tracking. At the time of the study, sequences other than TrueFISP were not available on our scanner. Treatments were not delivered to healthy volunteers in order to prevent exposure to ionizing radiation. Simulated treatments were performed by a multi-disciplinary team consisting of at least two therapists and at least one member of the following role groups: physicist, dosimetrist, and physician.
Qualitative analysis
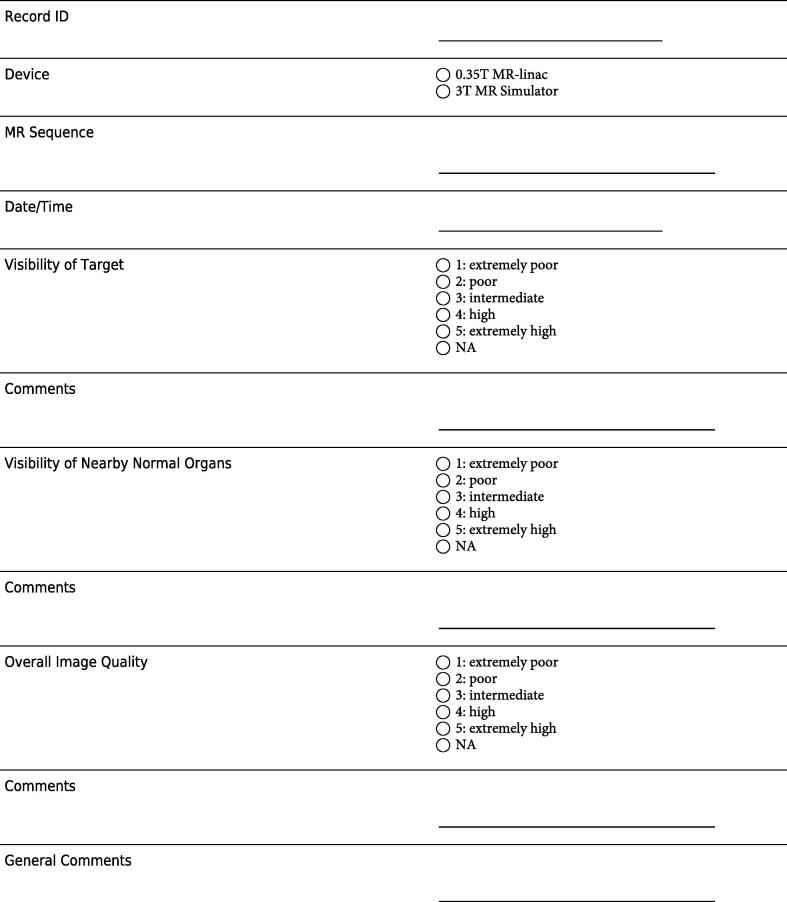
For each scan, the following qualitative parameters were assessed: 1) visibility of target, 2) visibility of nearby normal organs, 3) overall image quality, assessed on a scale from 1 to 5 (5 = extremely high, 1 = extremely poor) (Fig. 1). Study data was collected and managed using REDCap electronic data capture tools hosted at our institution [33]. Based on these qualitative results, immobilization and simulation procedures were adjusted to optimize image quality for each target. Optimal parameters were determined for each target.
Fig. 1.
Example of data entry form for healthy volunteer scans.
Initial clinical experience
Following the study in healthy volunteers, patients were treated with SMART on the MR-linac at our institution using the parameters and workflows found to be most optimal in volunteer scanning. IRB approval was obtained for retrospective review of these patient cases. The time for adaptive recontouring was identified for the first 18 clinical cases.
Quantitative analysis
In order to compare the ability of the simulated treatments to mirror the treatment environment, the time for adaptive recontouring was compared for the simulated treatments in volunteers and patients using an unpaired two-sided t-test (alpha = 0.05) in MATLAB (MathWorks, Natick, MA). This time was selected for comparison because it is the longest step in the SMART workflow and dependent on image quality and target and normal tissue visibility.
Results
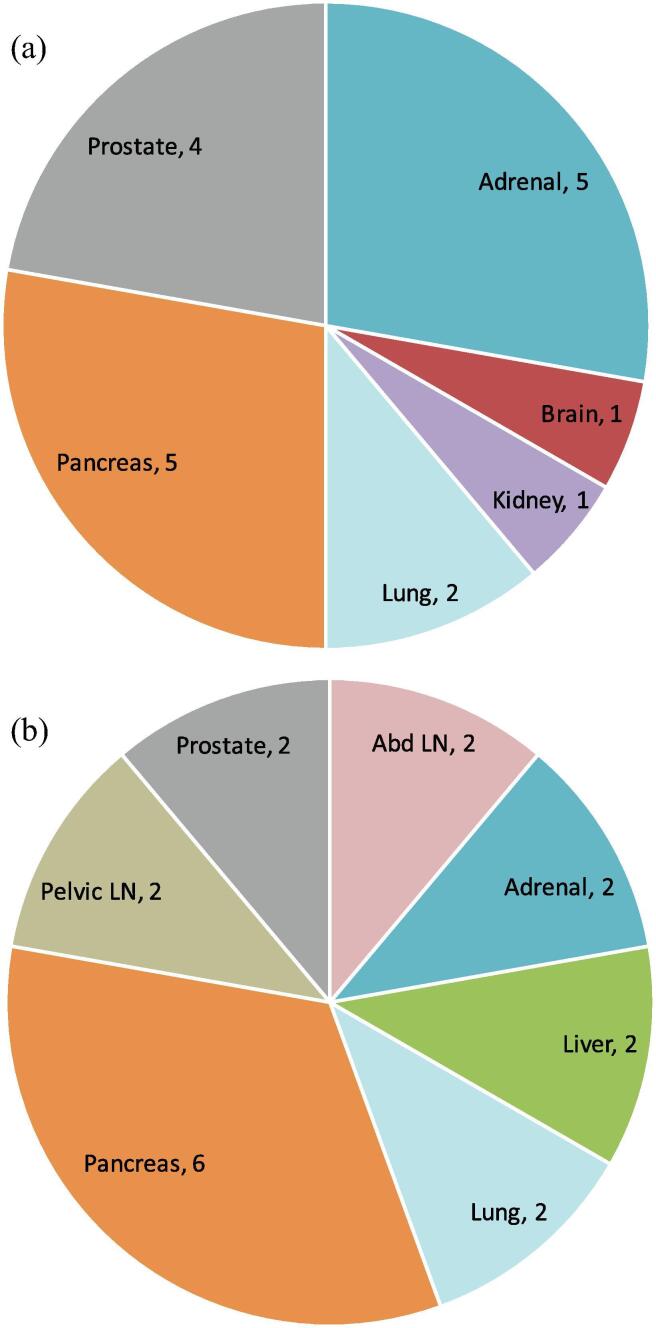
Between July 2, 2019 and September 20, 2019, 18 simulated treatments on the MR-linac took place in healthy volunteers and were included in this analysis. All volunteers provided consent. The simulated treatments targeted a variety of organs (Fig. 3a), comparing favorably with the variety of organs subsequently targeted in the first 18 clinical cases (Fig. 3b).
Fig. 3.
SMART Targets. Fig. 3a. Healthy volunteer scans. Fig. 3b. Patient scans. Caption: LN = lymph node, Abd = abdominal.
The first patient treatment took place on October 7, 2019. The mean patient age was 68.6 years old (range 52–87), compared to a mean volunteer age of 33.5 years old (range 24–49). The distribution of clinical cases was a result of referral patterns, perceived clinical benefit, and SMART eligibility (absence of MR contraindications such as presence of MR unsafe implants and ability to undergo treatment with SMART in five fractions or less). Clinical benefit was determined by the multidisciplinary MR-linac team and was based on anticipated benefit from real-time MR-guidance and online adaptive replanning due to factors such as: presence of a highly mobile tumor and/or adjacent organs-at-risk, proximity to nearby organs-at-risks, or desire to avoid fiducials. The distribution of volunteer cases was based on this anticipated clinical distribution, based on review of prior literature and discussions with institutions already practicing SMART.
Optimal MR simulation parameters were developed across a range of organs (Table 1). For body positioning, only supine positioning was attempted. The arms down position did not impact image quality but was utilized to maximize comfort during SMART, which requires a longer total time on table than non-adaptive treatment. Breath-hold improved visibility of organs-at-risk for abdominal targets, but did not impact visibility for prostate and lower pelvic targets, and thus free-breathing was utilizing for these lower pelvic targets to reduce scan time by eliminating the need for respiratory gating. Water used as an oral contrast was found to improve visualization of the duodenum during simulated pancreas treatments. Fasting was also found to reduce size of organs-at-risk for abdominal targets and was thus utilized. The parameters optimized during volunteer scanning were utilized in the subsequent clinical cases.
Table 1.
Optimal Simulation Parameters.
| Target | Immobilization | Body Position |
Arm position | Coil placement | Breathing | Other specifications |
|---|---|---|---|---|---|---|
| Pancreas | Knee cushion, mat | Supine | Arms down by side | Abdomen | Breath-hold | NPO 3 h, Water 15 & 30 min prior |
| Prostate | Footblock, mat | Supine | Arms on chest | Pelvis/lower abdomen | Free-breathing | Half full bladder, empty rectum |
| Lung | Knee cushion, mat | Supine | Arms down by side | Thorax | Breath-hold | If near stomach: NPO 3 h |
| Adrenal | Knee cushion, mat | Supine | Arms down by side | Abdomen | Breath-hold | NPO 3 h |
Image quality metrics ranged from 4 to 5 for all simulated treatments. For visibility of target, the mean score was 4.58. For visibility of nearby organs, the mean was 4.62, and for overall image quality the mean was 4.62. In the healthy volunteers, the mean time to recontour the target, or adaptive recontouring time (ART) for each case was 15.7 min (range 4–35). In the first 18 clinical SMART cases, ART was 16.1 min (range 7–33). There was no statistically significant difference in ARTs between healthy volunteer simulated treatments and clinical treatments. (p = 0.8860).
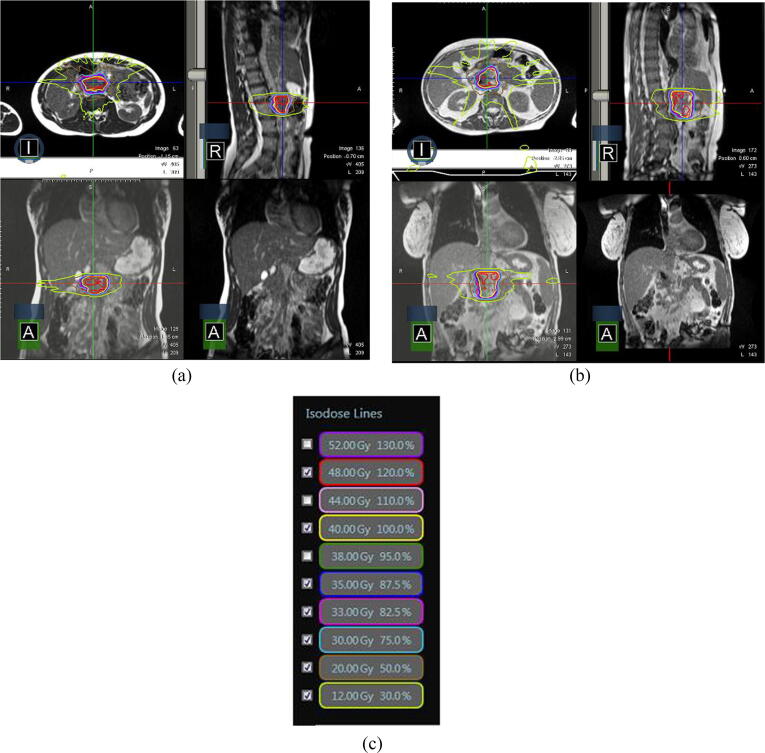
Initial difficulties with contouring during the simulated healthy volunteer pancreas treatments resulted in the multi-disciplinary treatment team meeting with a radiologist and radiation oncologist with expertise in pancreatic cancer, in order to improve in accuracy of initial and adaptive contouring for pancreas targets and organs-at-risk. Use of water as an oral contrast also led to better delineation of the duodenum on MR images, facilitating contouring. Representative SMART plans for the treatment of a mock pancreatic cancer in a simulated treatment in a healthy volunteer, and in a treatment of a pancreatic cancer in a patient are shown in Fig. 4.
Fig. 4.
Pancreas radiation treatment in a healthy volunteer versus patient. A) Images from a treatment plan of pancreatic cancer (targeting the normal pancreas) in a healthy volunteer are shown. B) Images from a treatment plan for pancreatic cancer in a patient are shown. C) Isodose levels are displayed.
Discussion
SMART requires multi-disciplinary collaboration for successful implementation and introduces new radiotherapy workflows, creating challenges for effective clinical implementation at centers new to this technology. In addition, accepted standards do not yet exist for simulation parameters, image quality and contouring across disease sites. In this study, healthy volunteer scans were utilized to optimize simulation parameters and to practice adaptive recontouring and simulated treatments across a range of disease sites. During HV scanning, similar adaptive recontouring times were achieved for HVs as were ultimately achieved for patients, suggesting that the HV study provided an effective simulated clinical environment. In addition, the variety of organs targeted in the HV scans compared favorably with the initial clinical case variety.
HV scanning enabled for end-to-end testing of all components of SMART, with the exception of actual beam-on time. Although beam-on time was not tested in HVs, this was tested in phantoms. A checklist for effective SMART delivery was developed and implemented during HV scans prior to clinical implementation. Utilizing team-based simulation, the HV scans allowed for practice of complex workflows in a non-clinical environment, removing the risk for harm and time pressures that accompany clinical care.
Simulation-based education (SBE) is an active area of research in radiation oncology [34]. SBE has been successfully applied to brachytherapy training [35], [36], [37] and training in on-call treatments [38], but to the authors’ knowledge this is a novel use of SBE, and is well-suited to the nature of SMART. With the rise of MR-guided radiotherapy, healthy volunteer scans play a critical role in sequence and workflow optimization [39], [40], as well as providing a non-clinical training environment. Similar SBE workflows could be utilized to train residents prior to involvement in patient cases.
Despite these advantages, this study was not without limitations. The success of our healthy volunteer program required willingness of healthy adults to undergo scanning. It may not be possible for all centers to have access to willing volunteers. In addition, prior to our clinical implementation of SMART, organs were selected for simulated treatment in HV cases based on review of organs treated by other centers [21], [41]. However, it was not possible to predict all clinical scenarios and additional organs were later identified that could have benefited from HV scanning. Additionally, the healthy volunteer study excluded individuals with chronic illness, poor performance status, or implants, characteristics which are present in a patient population; thus, the healthy volunteer study was not able to fully replicate the clinical environment. Patient factors such as presence of implants, inability to breath hold due to respiratory or other illness, or lack of abdominal fat, can impact image quality, requiring adjustment in workflows beyond what was addressed in the healthy volunteer study. In addition, only a limited number of image quality features were assessed, and the assessment was primarily qualitative. This study utilized the same noncontrast sequence for all targets; however, alternative sequences or use of intravenous contrast may improve visibility of some targets, such as liver tumors. Future work should compare imaging and simulation parameters with more quantitative measures such as signal to noise ratio. Additional work is also needed to aggregate results from experiences across centers and across vendors [26], which will better inform the most optimal parameters for SMART.
Although this study was not without limitations, our study in healthy volunteers demonstrated acceptable image quality across a variety of organs prior to clinical implementation of SMART and allowed for team-based training in complex workflows in a simulated clinical environment. Ongoing scanning in our department in healthy volunteers is aimed at further refinement of treatment protocols and expanding the variety of disease sites treated with SMART. Future work is needed to determine the optimal number of simulated cases per disease site required prior to clinical implementation of SMART.
Funding
None
Results from this study were accepted for an oral presentation at ASTRO Annual Meeting in 2020.
Acknowledgments
Acknowledgements
The authors are grateful for the assistance of Alisa Bernal, R.T. (T) in the scanning of patients and volunteers.
Data sharing statement
To protect anonymity of volunteers and comply with the details of the IRB approved protocol, volunteer-specific data will not be made publicly available.
Conflict of Interest Notification
P.B., E.H., S.B., E.N.S., F.H., I. U., J.C., and J.P. have no conflicts of interest to disclose. C.W reports funding from Viewray, outside of the submitted work.
D.C. reports grant funding from Viewray and NH Theraguix, outside of the submitted work.
R.M. reports grant funding and personal fees from Viewray, outside of the submitted work and serves on the Scientific Advisory Board for Viewray. R.M. also reports personal fees from AstraZeneca, outside of the submitted work and serves on the Scientific Advisory Board for AstraZeneca. R.M. also reports personal fees for lecture honorarium from NewRT, outside the submitted work.
L.S. reports grant funding from Brigham Research Institute and Viewray, outside of the submitted work.
References
- 1.DPhil CLE, MEH MCB. Keeping Up with the Hybrid Magnetic Resonance Linear Accelerators: How Do Radiation Therapists Stay Current in the Era of Hybrid Technologies? Journal of Medical Imaging and Radiation Sciences. 2019;50(2):195-198. doi:10.1016/j.jmir.2019.04.001. [DOI] [PubMed]
- 2.Alongi F, Rigo M, Figlia V, et al. 1.5 T MR-guided and daily adapted SBRT for prostate cancer: feasibility, preliminary clinical tolerability, quality of life and patient-reported outcomes during treatment. March 2020:1-9. doi:10.1186/s13014-020-01510-w. [DOI] [PMC free article] [PubMed]
- 3.Bruynzeel AME, Tetar SU, Oei SS, et al. A prospective single-arm phase II study of stereotactic magnetic-resonance-guided adaptive radiotherapy for prostate cancer: Early toxicity results. International Journal of Radiation Oncology*Biology*Physics. August 2019:1-2doi:10.1016/j.ijrobp.2019.08.007. [DOI] [PubMed]
- 4.Tetar SU, Bruynzeel AME, Oei SS, et al. Magnetic Resonance-guided Stereotactic Radiotherapy for Localized Prostate Cancer: Final Results on Patient-reported Outcomes of a Prospective Phase 2 Study. European Urology Oncology. June 2020:1-7. doi:10.1016/j.euo.2020.05.007. [DOI] [PubMed]
- 5.Finazzi T, van Sörnsen de Koste JR, Palacios MA, et al. Delivery of magnetic resonance-guided single-fraction stereotactic lung radiotherapy. Physics and Imaging in Radiation Oncology. 2020;14:17-23. doi:10.1016/j.phro.2020.05.002. [DOI] [PMC free article] [PubMed]
- 6.Finazzi T., Palacios M.A., Spoelstra F.O.B. Role of On-Table Plan Adaptation in MR-Guided Ablative Radiation Therapy for Central Lung Tumors. Int J Radiat Oncol Biol Phys. 2019;104(4):933–941. doi: 10.1016/j.ijrobp.2019.03.035. [DOI] [PubMed] [Google Scholar]
- 7.Finazzi T., Palacios M.A., Haasbeek C.J.A. Stereotactic MR-guided adaptive radiation therapy for peripheral lung tumors. Radiother Oncol. 2020;144:46–52. doi: 10.1016/j.radonc.2019.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Stereotactic MR-Guided Online Adaptive Radiation Therapy (SMART) for Ultracentral Thorax Malignancies: Results of a Phase 1 Trial. 2019;4(1):201-209. doi:10.1016/j.adro.2018.10.003. [DOI] [PMC free article] [PubMed]
- 9.Bohoudi O., Bruynzeel A.M.E., Senan S. Fast and robust online adaptive planning in stereotactic MR-guided adaptive radiation therapy (SMART) for pancreatic cancer. Radiother Oncol. 2017;125(3):439–444. doi: 10.1016/j.radonc.2017.07.028. [DOI] [PubMed] [Google Scholar]
- 10.Rudra S., Jiang N., Rosenberg S.A. Using adaptive magnetic resonance image-guided radiation therapy for treatment of inoperable pancreatic cancer. Cancer Med. 2019;8(5):2123–2132. doi: 10.1002/cam4.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenberg S.A., Henke L.E., Shaverdian N. A Multi-Institutional Experience of MR-Guided Liver Stereotactic Body Radiation Therapy. Adv Radiat Oncol. 2019;4(1):142–149. doi: 10.1016/j.adro.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.PhD MAP, MSc OB, PhD AMEBM, van Sörsen de Koste PhD JR, MD PC, PhD FJLM. Role of Daily Plan Adaptation in MR-Guided Stereotactic Ablative Radiation Therapy for Adrenal Metastases. Radiation Oncology Biology. 2018;102(2):426-433. doi:10.1016/j.ijrobp.2018.06.002. [DOI] [PubMed]
- 13.Sa M.D., Bwf-V M.D., PhD T.R.M. Magnetic Resonance Image Guided Radiation Therapy for External Beam Accelerated Partial-Breast Irradiation: Evaluation of Delivered Dose and Intrafractional Cavity Motion. Radiation Oncology Biology. 2016;96(4):785–792. doi: 10.1016/j.ijrobp.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Prospective Results of Real-Time Magnetic Resonance Imaging Guided Lumpectomy Cavity Boost Treatment. Radiation Oncology Biology. 2016;96(Supplement):S62. doi:10.1016/j.ijrobp.2016.06.159.
- 15.Gani C. Feasibility of Online MR-guided Radiotherapy on a 1.5T MR-Linac. clinicaltrials.gov. https://clinicaltrials.gov/ct2/show/NCT04172753. Published June 13, 2020. Accessed June 13, 2020.
- 16.Bitterman D.S., Cagney D.N., Singer L., Nguyen P.L., Mak R.H. Master protocol trial design for efficient and rational evaluation of novel therapeutic oncology devices. J Natl Cancer Inst. 2019;5(1):40–237. doi: 10.1093/jnci/djz167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tijssen R.H.N., Philippens M.E.P., Paulson E.S. MRI commissioning of 1.5T MR-linac systems – a multi-institutional study. Radiother Oncol. 2019;132:114–120. doi: 10.1016/j.radonc.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Michael Gach H., Curcuru A.N., Wittland E.J. MRI quality control for low-field MR-IGRT systems: Lessons learned. J Appl Clin Med Phys. 2019;24(3):196–214. doi: 10.1002/acm2.12713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D OLGP, Lauren E Henke M D MCSI, D GDHP. Practical Clinical Workflows for Online and Offline Adaptive Radiation Therapy. Seminars in Radiation Oncology. 2019;29(3):219-227. doi:10.1016/j.semradonc.2019.02.004. [DOI] [PMC free article] [PubMed]
- 20.Green O., Parikh P., Roach M.C., Michalski J.M., Gach H.M.M. Practical Implications of Ferromagnetic Artifacts in Low-field MRI-guided Radiotherapy. Cureus. 2018;10(3):1–6. doi: 10.7759/cureus.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischer-Valuck B.W., Henke L., Green O. Two-and-a-half-year clinical experience with the world's first magnetic resonance image guided radiation therapy system. 2017;2(3):485–493. doi: 10.1016/j.adro.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeanine E Vasmel MD, Maureen L Groot Koerkamp M, Anna M Kirby P, et al. Consensus on contouring primary breast tumors on MRI in the setting of neoadjuvant partial breast irradiation in trials. PRRO. April 2020:1-42. doi:10.1016/j.prro.2020.03.011. [DOI] [PubMed]
- 23.Lukovic J., Henke L., Gani C. MRI-Based Upper Abdominal Organs-at-Risk Atlas for Radiation Oncology. Int J Radiat Oncol Biol Phys. 2020;106(4):743–753. doi: 10.1016/j.ijrobp.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Heerkens H.D., Hall W.A., Li X.A. Recommendations for MRI-based contouring of gross tumor volume and organs at risk for radiation therapy of pancreatic cancer. Pract Radiat Oncol. 2017;7(2):126–136. doi: 10.1016/j.prro.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Singer L., Rosenberg S.A. The Impact of MRI on Radiation Oncology Graduate Medical Education. J Am Coll Radiol. 2019;16(6):859–863. doi: 10.1016/j.jacr.2018.11.030. [DOI] [PubMed] [Google Scholar]
- 26.Kiser K.J., Smith B.D., Wang J., Fuller C.D. “Après Mois, Le Déluge”: Preparing for the Coming Data Flood in the MRI-Guided Radiotherapy Era. Front Oncol. 2019;9:1468–1510. doi: 10.3389/fonc.2019.00983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bieri O., Scheffler K. Fundamentals of balanced steady state free precession MRI. J Magn Reson Imaging. 2013;38(1):2–11. doi: 10.1002/jmri.24163. [DOI] [PubMed] [Google Scholar]
- 28.Chavhan G.B., Babyn P.S., Jankharia B.G., Cheng H.-L.M., Steady-State Shroff MM., Imaging M.R. RadioGraphics. 2008;28(4):1147–1160. doi: 10.1148/rg.284075031. [DOI] [PubMed] [Google Scholar]
- 29.Benedict S.H., Yenice K.M., Followill D. Stereotactic body radiation therapy: The report of AAPM Task Group 101. Med Phys. 2010;37(8):4078–4124. doi: 10.1118/1.3438081. [DOI] [PubMed] [Google Scholar]
- 30.Tetar S.U., Bruynzeel A.M.E., Lagerwaard F.J., Slotman Ben J, Bohoudi O., Palacios M.A. Clinical implementation of magnetic resonance imaging guided adaptive radiotherapy for localized prostate cancer. Physics and Imaging. Radiation Oncology. 2019;9:69–76. doi: 10.1016/j.phro.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hill P, Bayouth JE, Geurts MW, et al. A New Era of Image Guidance with Magnetic Resonance-guided Radiation Therapy for Abdominal and Thoracic Malignancies. Cureus. May 2018:1-12. doi:10.7759/cureus.2422. [DOI] [PMC free article] [PubMed]
- 32.Kapur N., Parand A., Soukup T., Reader T., Sevdalis N. Aviation and healthcare: a comparative review with implications for patient safety. JRSM Open. 2016;7(1) doi: 10.1177/2054270415616548. 205427041561654–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ba M.K.R., Md F.Z., Md E.F.G. Simulation as More Than a Treatment-Planning Tool: A Systematic Review of the Literature on Radiation Oncology Simulation-Based Medical Education. Radiation Oncology Biology. 2018;102(2):257–283. doi: 10.1016/j.ijrobp.2018.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao S., Francis L., Todor D., Fields E.C. Proficiency-based cervical cancer brachytherapy training. Brachytherapy. 2018;17(4):653–659. doi: 10.1016/j.brachy.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 36.Singer L., Braunstein S., Klopp A., Joyner M. Development and Implementation of a Simulation-Based Educational Workshop on Gynecological Brachytherapy: Pilot Study at a National Meeting. Pract Radiat Oncol. 2019;9(5):e465–e472. doi: 10.1016/j.prro.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 37.Thaker N.G., Kudchadker R.J., Swanson D.A. Establishing high-quality prostate brachytherapy using a phantom simulator training program. Int J Radiat Oncol Biol Phys. 2014;90(3):579–586. doi: 10.1016/j.ijrobp.2014.06.036. [DOI] [PubMed] [Google Scholar]
- 38.Brown L.C., Laack T.A., Ma D.J., Olivier K.R., Laack N.N. Multidisciplinary medical simulation: a novel educational approach to preparing radiation oncology residents for oncologic emergent on-call treatments. Int J Radiat Oncol Biol Phys. 2014;90(3):705–706. doi: 10.1016/j.ijrobp.2014.06.053. [DOI] [PubMed] [Google Scholar]
- 39.Eccles C.L., Smith G.A., Bower L. Magnetic resonance imaging sequence evaluation of an MR Linac system; early clinical experience. Tech Innov Patient Support Radiat Oncol. 2019;12:56–63. doi: 10.1016/j.tipsro.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keiper TD, Tai A, Chen X, et al. Feasibility of real‐time motion tracking using cine MRI during MR‐guided radiation therapy for abdominal targets. Med Phys. June 2020:mp.14230–13. doi:10.1002/mp.14230. [DOI] [PubMed]
- 41.van Sörnsen de Koste PhD JR, PhD MAP, PhD AMEBM, PhD FJLM. MR-guided Gated Stereotactic Radiation Therapy Delivery for Lung, Adrenal, and Pancreatic Tumors: A Geometric Analysis. Radiation Oncology Biology. 2018;102(4):858-866. doi:10.1016/j.ijrobp.2018.05.048. [DOI] [PubMed]