Summary
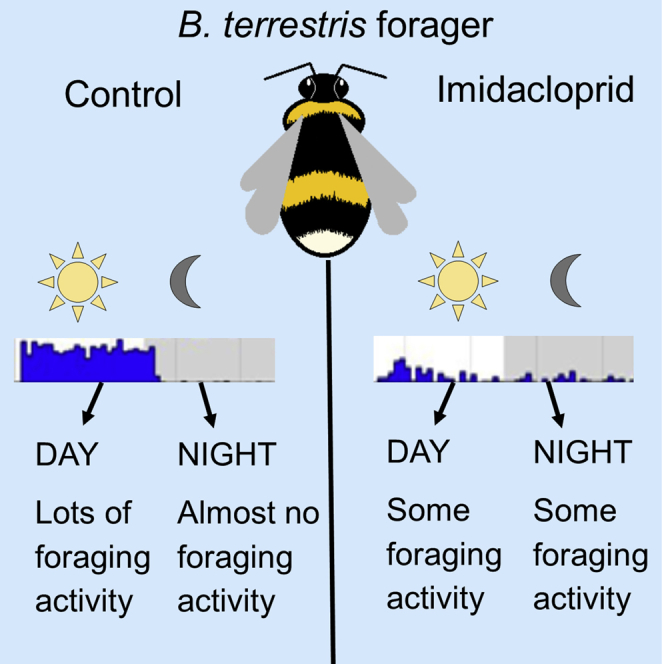
Neonicotinoids have been implicated in the large declines observed in insects such as bumblebees, an important group of pollinators. Neonicotinoids are agonists of nicotinic acetylcholine receptors that are found throughout the insect central nervous system and are the main mediators of synaptic neurotransmission. These receptors are important for the function of the insect central clock and circadian rhythms. The clock allows pollinators to coincide their activity with the availability of floral resources and favorable flight temperatures, as well as impact learning, navigation, and communication. Here we show that exposure to the field-relevant concentration of 10 μg/L imidacloprid caused a reduction in bumblebee foraging activity, locomotion, and foraging rhythmicity. Foragers showed an increase in daytime sleep and an increase in the proportion of activity occurring at night. This could reduce foraging and pollination opportunities, reducing the ability of the colony to grow and reproduce, endangering bee populations and crop yields.
Subject Areas: Ecology, Biological Sciences, Zoology, Animal Physiology, Behavioral Neuroscience
Graphical Abstract
Highlights
-
•
Field-relevant concentrations of imidacloprid disrupt bumblebee foraging rhythms
-
•
Drugged foragers are inactive for more of the day and more active at night
-
•
Neonicotinoid-treated foragers are more arrhythmic
-
•
These effects occurred in naturalistic 12 h light:12 h dark light cycles
Ecology; Biological Sciences; Zoology; Animal Physiology; Behavioral Neuroscience
Introduction
Bumblebees are a diverse and important group of pollinators and are major pollinators of both crops and wildflowers. Of the five most important crop pollinators in Europe, three are bumblebees (Nieto et al., 2014). Many crops are particularly reliant on bumblebee pollination (Willmer et al., 1994; De Luca and Vallejo-Marín, 2013), and crop pollination in Europe is worth over €22bn per annum and is essential for food security (Nieto et al., 2014). Unfortunately, despite their ecological and economic value, bumblebees face dramatic population losses, with 46% of species in Europe in decline and 24% threatened with extinction (Nieto et al., 2014). Due to crop losses to insect pests, demand for insecticides remains high (Casida and Durkin, 2013; Popp et al., 2013). The most common insecticides worldwide are neonicotinoids, global sales of which are worth US$1 billion/year (Popp et al., 2013; Casida, 2018). Neonicotinoids are agonists of nicotinic acetylcholine receptors (nAChR), the main neurotransmitter system in the insect nervous system, and they share target site cross-resistance (Matsuda et al., 2020). They were branded safe compared with their predecessors because they do not act on mammalian nAChRs (Casida, 2018; Matsuda et al., 2020). However, before their introduction to market, sublethal effects were not fully identified in beneficial insects, for which neonicotinoids have proven potent neurotoxins.
Most research on the effects of neonicotinoids on pollinators has used the honeybee, Apis mellifera (Blacquière et al., 2012). However, honeybees and bumblebees show differential responses to neonicotinoids, with bumblebees potentially experiencing a higher risk (Cresswell et al., 2012; Stoner, 2016; Gradish et al., 2018). This highlights the importance of increasing the diversity of pollinators studied to determine the ecological consequences of neonicotinoid use. Concentrations as low as 1 μg/L (or 1 part per billion [ppb]) imidacloprid can cause reduced foraging motivation in Bombus terrestris (Lämsä et al., 2018). A dose of 6 ppb imidacloprid has been shown in laboratory studies to cause lethargy within the nest (Crall et al., 2018), and cause long-term effects on nest growth and queen production in the field (Whitehorn et al., 2012). This has been replicated in a large-scale study in Sweden, which looked at the effects of neonicotinoid seed coating on wild bees in the field and found reduced colony growth and reproduction in bumblebees (Rundlöf et al., 2015). Neonicotinoids have high solubility and persistence in the environment (Wood and Goulson, 2017), meaning insects are still at risk of exposure, despite the current European Union ban on imidacloprid.
Due to the abundance and importance of nAChRs in the insect central nervous system, the potential sub-lethal effects of neonicotinoids are very broad. Their effect on many behaviors vital to pollination, such as circadian rhythms and sleep, are still unknown. The circadian clock is integral to pollinator foraging efficiency as flower opening, scent release, and nectar production are dependent on time of day (Bloch et al., 2017; Willmer and Stone, 2004). Neonicotinoids have already been shown to have a detrimental effect on pollination, with thiamethoxam exposure of caged B. terrestris resulting in reduced pollination of apple trees and fewer seeds in the fruit (Stanley et al., 2015). Circadian rhythms also affect other behaviors, such as sleep, caring for offspring, and learning, with honeybees learning novel, rewarding odors better in the morning (Lehmann et al., 2011; Bloch et al., 2017). This helps them find new foraging patches, as most flowers are nectar-rich in the morning (Bloch et al., 2017).
In Drosophila, circadian entrainment (synchronicity within the clock and communication between the light-sensing organs and the central clock) is reliant upon nAChR signaling (Helfrich-Förster et al., 2002; McCarthy et al., 2011; Sheeba et al., 2008), as are the post-synaptic mushroom body (MB) output neurons that regulate sleep (Barnstedt et al., 2016; Palmer et al., 2013). Given the similarity in nAChR expression in the brains of Drosophila and the honeybee, this suggests that the clock and sleep of bees may be affected by neonicotinoid exposure (Dupuis et al., 2012). The bee central clock neurons also share similarities with those of Drosophila. In Drosophila, honeybees, and bumblebees, there are bundles of lateral and dorsal clock neurons, including a set of lateral neurons that express the neuropeptide pigment dispersing factor (PDF) (Beer et al., 2018; Weiss et al., 2009; Lin et al., 2004). Both PDF and nAChR expressing lateral pacemaker clock neurons with extensive branching patterns are also present in other insects, including crickets and cockroaches (Weiss et al., 2009; Numata et al., 2015; Baz el et al., 2013; Helfrich-Förster et al., 1998), demonstrating that clock circuitry is well conserved across insects. In the honeybee, in which the PDF neurons have been well mapped, they have been shown to project to brain regions such as the visual circuitry, pars intercerebralis and pars lateralis (Beer et al., 2018), both of which control locomotor activity and sleep in Drosophila (King and Sehgal, 2018). Furthermore, honeybee clock neurons are important for the sun-compass pathway (Beer et al., 2018), a vital navigational tool allowing the communication of resource location via the waggle dance (von Frisch, 1967). The clock also dictates the timing of sleep, which is required for many vital physiological processes including memory consolidation and synaptic homeostasis (Fisher et al., 2013; Zwaka et al., 2015; Beyaert et al., 2012; Donlea et al., 2009; Stern, 2018; Bushey et al., 2015). Furthermore, sleep timing in bumblebees is important for round-the-clock care of offspring (Nagari et al., 2019). Therefore, circadian and sleep disruption is likely to have detrimental effects to the pollination services, behavior, and fitness of beneficial insects. Due to the importance of nAChR signaling in the insect clock and sleep centers, we hypothesized that neonicotinoids would disrupt bee rhythmicity and sleep. We therefore tested the effect of field-relevant concentrations of imidacloprid on B. terrestris foragers.
Results
The activity of isolated B. terrestris foragers in individual tubes was measured using the locomotor activity monitor to assess behavior in both 12 h:12 h light:dark (LD) conditions and constant darkness (DD), (see Supplementary Information, Transparent Methods). Rhythmicity under LD conditions was studied as this provides a more naturalistic reflection of how day/night behavior may be affected in the field and also allows sleep to be investigated. The removal of light cues under constant conditions in DD reveals the endogenous circadian rhythm and clock function.
Field-Relevant Concentrations of Imidacloprid Affects Rhythmicity in Isolated Bumblebee Foragers in 12 h:12 h Light:Dark Conditions
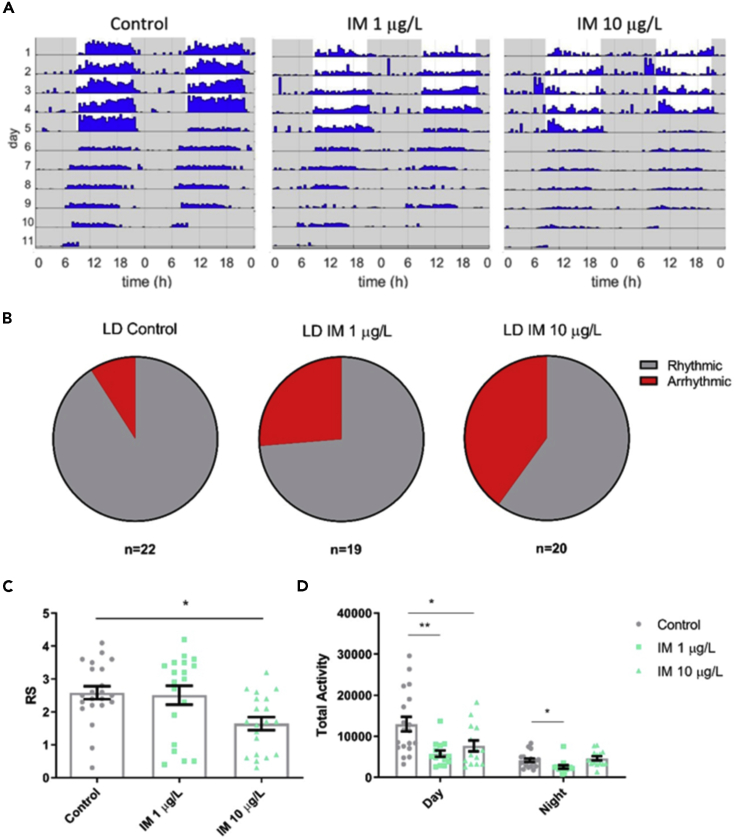
Exposure to field relevant concentrations of 1–10 μg/L imidacloprid disrupted the rhythmicity and quantity of locomotor activity in isolated foragers (Figure 1A). The rhythmicity statistic (RS) was calculated as a measure of rhythm strength (Levine et al., 2002), with RS > 1.5 by convention taken to indicate rhythmic behavior (Hodge and Stanewsky, 2008). In LD conditions, imidacloprid decreased mean rhythmicity (Figure 1C) and both 1 and 10 μg/L increased the proportion of foragers that were arrhythmic (Figure 1B), from 10% in control foragers to 36% and 67% respectively. Imidacloprid also reduced the total activity of foragers, with 1 μg/L reducing activity during both day and night and 10 μg/L reducing daytime activity (Figure 1D).
Figure 1.
Field-Relevant Concentrations of Imidacloprid Affects Rhythmicity in Isolated Bumblebee Foragers in 12 h:12 h Light:Dark Conditions
(A) Representative actograms for a Bombus terrestris forager on control food, or food containing 1 μg/L or 10 μg/L imidacloprid (IM).
(B) Proportion of foragers that were arrhythmic (RS ≤1.5) in LD for each treatment.
(C) Mean rhythmicity for either control foragers or those fed 1 or 10 μg/L imidacloprid, in LD conditions (F2,58 = 5.3, p = 0.008).
(D) Mean locomotor activity for foragers in each treatment group in LD conditions, during the day (F2,44 = 6.7, p = 0.003) and the night (F2,44 = 5.4, p = 0.008). Each data point represents a single bee, n = 19–22 bees for each treatment group.
Data are represented as mean ± SEM, ∗p ≤ 0.05, ∗∗p ≤ 0.01, tested via 1-way ANOVA with Tukey's multiple comparisons.
Field-Relevant Concentrations of Imidacloprid Do Not Reduce Rhythmicity in Isolated Bumblebee Foragers in Constant Darkness
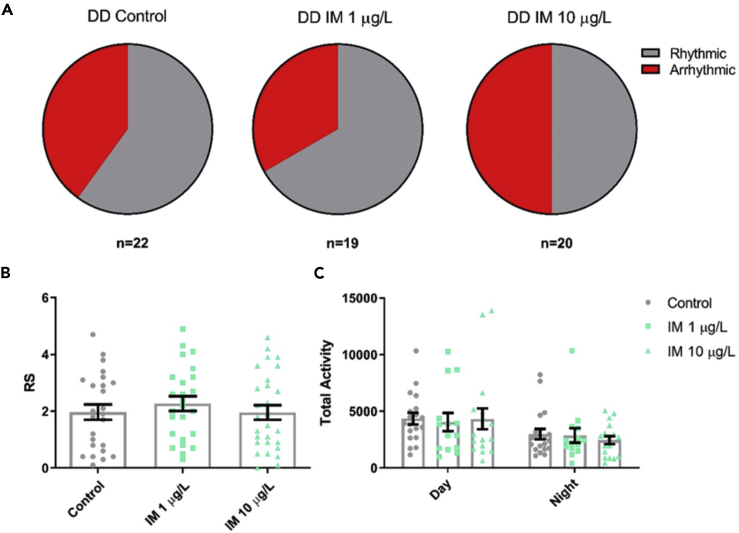
Conversely, in DD, exposure to either concentration of imidacloprid had little effect on the forager's activity or rhythmicity (Figure 2). Foragers fed 1 or 10 μg/L imidacloprid had the same mean rhythmicity and levels of activity as control foragers (Figures 2B and 2C). The proportion of each population that was arrhythmic was also similar, with 40% of control foragers arrhythmic compared with 33% at 1 μg/L and 50% at 10 μg/L imidacloprid (Figure 2A).
Figure 2.
Field-Relevant Concentrations of Imidacloprid Do Not Reduce Rhythmicity in Isolated Bumblebee Foragers in Constant Darkness
(A) Proportion of Bombus terrestris foragers that were arrhythmic (RS ≤ 1.5) in DD for foragers on control food or food containing 1 or 10 μg/L imidacloprid (IM).
(B) Mean rhythmicity (RS) for foragers in each treatment group in DD conditions (F2,47 = 0.5, p = 0.637).
(C) Mean locomotor activity for foragers in each treatment group in DD conditions, during the subjective day (F2,47 = 0.1, p = 0.947) and night (F2,47 = 0.6, p = 0.541). Each data point in the histograms represents a single bee, n = 14–22 bees for each treatment.
Data are represented as mean ± SEM, tested via one-way ANOVA with Tukey's multiple comparisons.
Field-Relevant Concentrations of Imidacloprid Increase Sleep in Isolated Bumblebee Foragers
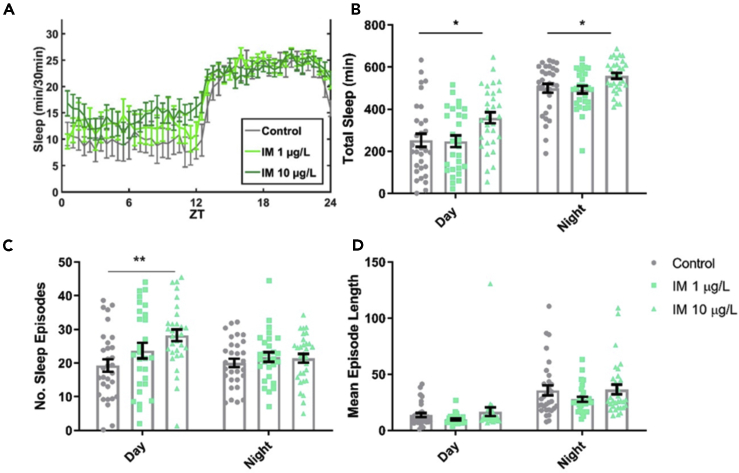
Foragers who were fed 10 μg/L imidacloprid showed an increase in inactivity lasting longer than 5 min, usually taken as a proxy for sleep (Helfrich-Förster, 2018; Eban-Rothschild and Bloch, 2008), compared with controls. This was particularly notable during the day (Figures 3A and 3B) and is likely due to the increased number of daytime sleep episodes (Figure 3C) initiated by these foragers. The length of these sleep episodes was the same as in control foragers (Figure 3D).
Figure 3.
Field-Relevant Concentrations of Imidacloprid Increase Sleep in Isolated Bumblebee Foragers
(A) Mean total sleep achieved for control Bombus terrestris foragers and those fed 1 μg/L (pale green line) or 10 μg/L (dark green line) imidacloprid (IM), per 30 min bin over the 24 h period.
(B) Mean total sleep (min) for each treatment group in the day (F2,87 = 4.9, p = 0.010) and the night (F2,87 = 4.1, p = 0.019).
(C) Mean number (No.) of sleep episodes initiated for each treatment group during the day (F2,87 = 5.4, p = 0.006) and the night (F2,87 = 0.490, p = 0.614).
(D) Mean sleep episode length for each treatment group during the day (F2,87 = 1.7, p = 0.182) and the night (F2,87 = 1.5, p = 0.238). Each data point in the histograms represents a single bee, n = 28–31 bees for each treatment.
Data are represented as mean ± SEM, ∗p ≤ 0.05, ∗∗p ≤ 0.01, tested via one-way ANOVA with Tukey's multiple comparisons.
Field-Relevant Concentration of Imidacloprid Reduces Foraging Rhythmicity and Activity in Bumblebee Foragers within the Colony in 12 h:12 h Light:Dark Conditions
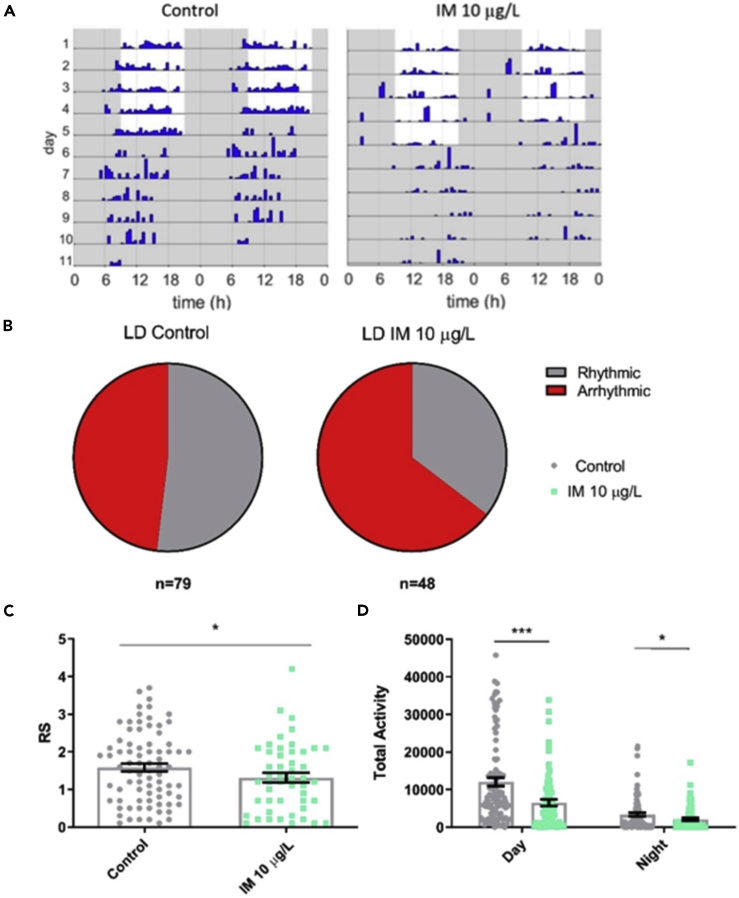
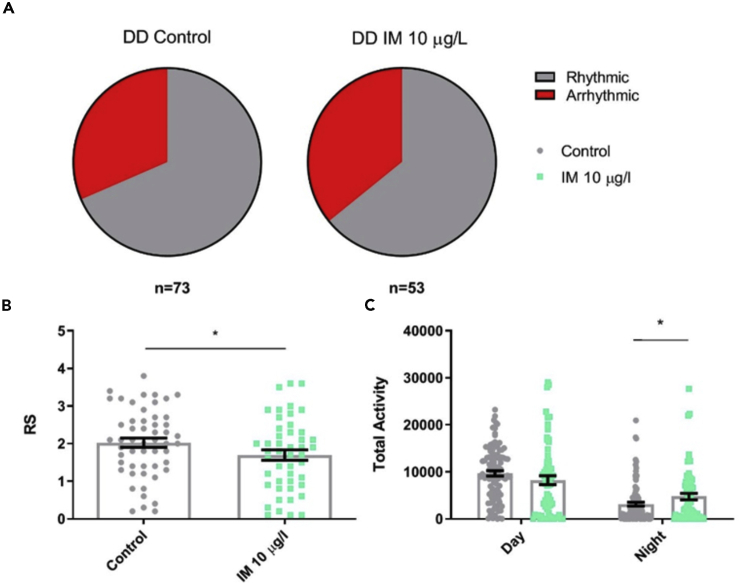
B. terrestris foragers in a full colony setting showed diurnal rhythms in foraging activity (Figures 4 and 5). This rhythmicity was decreased in foragers exposed to 10 μg/L imidacloprid in both LD (Figure 4) and constant darkness (Figure 5). In LD conditions, imidacloprid decreased mean rhythmicity of foragers (Figure 4C) and increased the proportion of foragers who were arrhythmic from 48% to 65% (Figure 4B). Imidacloprid decreased foraging activity for both daytime and night-time (Figure 4D).
Figure 4.
Field-Relevant Concentration of Imidacloprid Reduces Foraging Rhythmicity and Activity in Bumblebee Foragers within the Colony in 12 h:12 h Light:Dark Conditions
(A) Representative actograms for a Bombus terrestris forager on control food and food containing 10 μg/L imidacloprid (IM).
(B) Proportion of foragers that were arrhythmic in LD for each treatment group.
(C) Mean rhythmicity for either control foragers or foragers fed 10 μg/L IM in LD (t125 = 2.0, p = 0.048).
(D) Mean foraging activity for foragers in each treatment group in LD, during the day (t170 = 3.8, p < 0.001) and the night (t170 = 2.0, p = 0.042). Each data point in the histograms represents a single bee, n = 48–79 bees for each treatment for rhythmicity, n = 74–100 bees for each treatment for activity.
Data are represented as mean ± SEM, ∗p ≤ 0.05, ∗∗∗p ≤ 0.001, tested via one-way ANOVA with Tukey's multiple comparisons.
Figure 5.
Imidacloprid Reduces Foraging Rhythmicity and Activity in Bumblebee Foragers within the Colony in Constant Darkness
(A) Proportion of the B. terrestris foragers that were arrhythmic in DD on control food and food containing a field-relevant concentration of 10 μg/L imidacloprid (IM).
(B) Mean rhythmicity for foragers in each treatment group in DD conditions (t125 = 2.2, p = 0.029).
(C) Mean activity for foragers in each treatment group in DD conditions, during the subjective day (t169 = 1.6, p = 0.105) and night (t114 = −2.0, p = 0.043). Each data point in the histograms represents a single bee, n = 53–73 bees for each treatment for rhythmicity, n = 74–100 bees for each treatment for activity.
Data are represented as mean ± SEM, ∗p ≤ 0.05, tested via one-way ANOVA with Tukey's multiple comparisons.
Imidacloprid Reduces Foraging Rhythmicity and Activity in Bumblebee Foragers within the Colony in Constant Darkness
In DD conditions, imidacloprid reduced mean rhythmicity for foragers (Figure 5B) and increased foraging activity during the subjective night (the 12 h that were dark during the entrainment period) (Figure 5C). The proportion of foragers that were arrhythmic for control and imidacloprid-exposed bees were similar in DD, 31% and 36% respectively (Figure 5A).
Therefore, field-relevant imidacloprid concentrations disrupted circadian rhythmicity and increased mistimed daytime inactivity or sleep in isolated B. terrestris foragers and reduced foraging rhythmicity for foragers in the colony. Foragers both in isolation and the colony showed that neonicotinoids cause a profound disruption of timing of activity with reduced daytime activity.
Discussion
We report the effects of imidacloprid on pollinators, such as reduced locomotion and foraging activity, and go on to show that imidacloprid causes mistiming of these activities, increasing the proportion of foraging that occurs at night and increasing daytime inactivity. Imidacloprid has previously been shown to reduce locomotion in isolated B. terrestris (Cresswell et al., 2014), sweat bees like Melipona quadrifasciata (Tomé et al., 2012), and solitary bees like Osmia bicornis (Azpiazu et al., 2019), and to reduce in-nest activity in another bumblebee B. impatiens (Crall et al., 2018). We found that imidacloprid exposure also reduced the foraging activity within the colony, with foragers making fewer foraging trips in LD conditions. This is similar to previous laboratory studies, for example, imidacloprid exposure can cause reduced and less efficient foraging for sucrose solution in B. impatiens (Muth and Leonard, 2019) and mixed exposure to thiamethoxam and clothianidin has been shown to reduce foraging effort for both sucrose solution and pollen in B. terrestris (Fauser-Misslin et al., 2014). Other field-based studies have shown a reduction in pollen foraging efficiency in B. terrestris following exposure to imidacloprid (Gill and Raine, 2014; Gill et al., 2012), although with no reduction in the quantity of nectar collected per foraging bout. Neonicotinoid exposure appears to interfere with foraging efficiency, limiting the capacity of bees to handle flowers, carry out buzz pollination, and changing their flower preferences (Whitehorn et al., 2017; Stanley and Raine, 2016; Gill and Raine, 2014). Our study, and the other laboratory studies mentioned, focus on the foraging motivation within the colonies, which also appears to be reduced. This could in part be driven by the apparent appetite suppression that imidacloprid can cause, with 10 μg/L shown to decrease feeding by 30% in B. terrestris (Cresswell et al., 2012). Or it could be a result of reduced mobility, an effect that has been observed for imidacloprid exposure (Williamson et al., 2014).
We also show that field-relevant doses of imidacloprid impact locomotor and foraging rhythmicity. Imidacloprid reduced the rhythmicity of daily activity in foragers in LD conditions, suggesting that the neonicotinoid may be interfering with light input into the clock. Light signaling from the visual circuit and the Hofbauer-Buchner eyelet (another light sensing organ) to the clock neurons is dependent on nAChRs, so this is a potential route for disruption (Helfrich-Förster et al., 2002; Muraro and Ceriani, 2015). In Drosophila, imidacloprid appears to affect circadian rhythmicity and to prevent day/night differences in arborization and PDF accumulation in the small lateral-ventral neurons (sLNv) or pace making neurons of the clock, which receive light input from the Hofbauer-Buchner (HB) eyelet (Tasman et al., 2020). Foraging rhythmicity in DD was also reduced, possibly due to the reduction in entrainment during the LD stage of the experiments. Previous work showed that nAChR agonists can directly stimulate clock neurons in a cockroach Rhyparobia maderae causing increased calcium influx through voltage-gated calcium channels (Baz El et al., 2013). Likewise, nAChR agonists increase calcium influx of Drosophila PDF-releasing lateral ventral neurons (LNvs) (Wegener et al., 2004; Lelito and Shafer, 2012). Conversely nAChR antagonists block spike-dependent nAChR synaptic signaling required for rhythmic LNv activity (McCarthy et al., 2011). Work in another cockroach, Periplaneta americana, showed that neonicotinoids can also act on the thoracic ganglia, which control motor function in insects (Tan et al., 2007). Thus, neonicotinoids may be acting directly through multiple neurons regulating both locomotion and circadian regulation of locomotion, causing activation and/or depolarization block and hence compromising rhythmic foraging activity. In addition to direct classical pharmacological drug-receptor interactions, neonicotinoid exposure changes expression of hundreds of genes in worker bumblebees (B. impatiens), including genes that are involved in locomotion (Tan et al., 2007).
The reduction in rhythmicity observed for foragers in LD conditions and within the colony suggest that the effect of imidacloprid on rhythmicity cannot be mitigated by strong zeitgebers (i.e., time-givers or entrainment signals) such as light or social cues (Jurgen Stelzer et al., 2010; Bloch, 2010). This may imply that the reduction in foraging rhythmicity observed for bumblebee colonies exposed to imidacloprid in the laboratory are likely to reflect deleterious consequences in the field, as is the case for reductions in foraging activity (Gill and Raine, 2014; Muth and Leonard, 2019). A disruption of the clock in foragers may further reduce their foraging efficiency as they will not be able to form the time-memories required to accurately visit different flowers (van Nest et al., 2018; Bloch et al., 2017). The clock also feeds into the sun-compass navigation pathway that foragers use to navigate (Beer et al., 2018). Neonicotinoids have previously been shown to reduce homing ability in bees (Tosi et al., 2017; Fischer et al., 2014), although Stanley et al. found no effect (Stanley et al., 2016). If navigation is affected, then disruption to the clock could be a contributing factor to this. The reduced foraging motivation observed here is likely to compound the reduced pollen foraging efficiency observed in the field (Gill and Raine, 2014; Gill et al., 2012; Feltham et al., 2014) and is likely to reduce the capacity of the colony to grow and reproduce. Reduced feeding and foraging are associated with less brood production (Laycock et al., 2012) and smaller colonies, which are less resilient and less likely to produce queens (Whitehorn et al., 2012). The colony also responds to reduced foraging by producing and sending out more foragers, increasing forager mortality, and resulting in fewer workers to carry out in-nest tasks such as brood care (Gill et al., 2012).
Field-relevant concentrations of imidacloprid also increased sleep or total inactivity, with 10 μg/L increasing daytime sleep or total inactivity in foragers, hence the reduction in daytime locomotor and foraging activity observed. It is common practice to use 5 min of continuous inactivity as a proxy for sleep, and this practice has been validated (Helfrich-Förster, 2018; Eban-Rothschild and Bloch, 2008; Hendricks et al., 2020). However, as imidacloprid can cause immobilization, in this case it is not possible to differentiate between sleep and immobilization (Williamson et al., 2014). There are possible routes for either effect. The mushroom bodies, which are known to regulate the sleep/wake cycle in insects, contain groups of both wake- and sleep-promoting Kenyon neurons and signal via nAChRs to the mushroom body output neurons, which are also important for sleep regulation (Pitman et al., 2006; Helfrich-Förster, 2018; Barnstedt et al., 2016). Sleep-promoting neurons have been shown to be specifically activated by nAChRs (Aso et al., 2014), providing a possible route for imidacloprid to induce sleep. Furthermore, whole-cell patch-clamp recordings from honeybee Kenyon neurons from the mushroom body showed that they were directly stimulated by imidacloprid (Moffat et al., 2016). However, as mentioned above, the thoracic ganglia and motor neurons are also nicotinic, allowing possible stimulation or depolarizing block of these. Imidacloprid has been shown to cause immobilization, increased time spent upside down, and difficulty in motor tasks in bees (Williamson et al., 2014). Either way, increased immobility or sleep in the colony reduces opportunities for foraging and potentially for in-nest tasks such as brood care.
Neonicotinoids reduce activity and foraging motivation in pollinators (Muth and Leonard, 2019; Fauser-Misslin et al., 2014; Gill and Raine, 2014; Gill et al., 2012). Here we demonstrate that very low field-relevant concentrations of neonicotinoids disrupt rhythmicity of foraging activity as well as increasing daytime immobility, further reducing the opportunities for bees to forage and pollinate and having knock-on effects on circadian and sleep-regulated physiological and behavioral processes in the bee. This is likely to have a detrimental effect on colony fitness in the field as well as reducing the yield of crops and wild plants reliant on bee pollination. Furthermore, we establish a number of highly sensitive (down to 1 ppb neonicotinoids) high-throughput behavioral assays for measuring the detrimental sublethal effects of insecticides on pollinators.
Limitations of the Study
A key limitation of the study is the inability to differentiate between sleep and inactivity. Another is that the dose consumed by each bee was not quantified exactly, although bees were allowed to feed freely from sugar syrup with a field-realistic concentration of imidacloprid and the dose estimated from the average quantity consumed.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Dr. James Hodge (james.hodge@bristol.ac.uk).
Materials Availability
No novel materials or reagents were used for these experiments.
Data and Code Availability
Original data have been deposited to Mendeley Data: https://doi.org/10.17632/m8pykxzkyb.1.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Drs. Stephen Montgomery and Herman Wijnen for providing useful comments on the manuscript. This work was supported by a BBSRC studentship BB/J014400/1 and Leverhulme project grant RPG-2016-318 awarded to J.J.L.H.
Author Contributions
All experiments were carried out by K.T. K.T. wrote the first draft of the paper. The project was supervised by J.J.L.H. and S.A.R., who secured funding and edited the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: December 18, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101827.
Contributor Information
Sean A. Rands, Email: sean.rands@bristol.ac.uk.
James J.L. Hodge, Email: james.hodge@bristol.ac.uk.
Supplemental Information
References
- Aso Y., Sitaraman D., Ichinose T., Kaun K.R., Vogt K., Belliart-Guerin G., Placais P.Y., Robie A.A., Yamagata N., Schnaitmann C. Mushroom body output neurons encode valence and guide memory-based action selection in Drosophila. eLife. 2014;3:e04580. doi: 10.7554/eLife.04580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azpiazu C., Bosch J., Vinuela E., Medrzycki P., Teper D., Sgolastra F. Chronic oral exposure to field-realistic pesticide combinations via pollen and nectar: effects on feeding and thermal performance in a solitary bee. Scientific Rep. 2019;9:13770. doi: 10.1038/s41598-019-50255-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnstedt O., Owald D., Felsenberg J., Brain R., Moszynski J.P., Talbot C.B., Perrat P.N., Waddell S. Memory-relevant mushroom body output synapses are cholinergic. Neuron. 2016;89:1237–1247. doi: 10.1016/j.neuron.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baz El S., Wei H., Grosshans J., Stengl M. Calcium responses of circadian pacemaker neurons of the cockroach Rhyparobia maderae to acetylcholine and histamine. J. Comp. Physiol. A. 2013;199:365–374. doi: 10.1007/s00359-013-0800-3. [DOI] [PubMed] [Google Scholar]
- Beer K., Kolbe E., Kahana N.B., Yayon N., Weiss R., Menegazzi P., Bloch G., Helfrich-Förster C. Pigment-Dispersing Factor-expressing neurons convey circadian information in the honey bee brain. Open Biol. 2018;8:170224. doi: 10.1098/rsob.170224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyaert L., Greggers U., Menzel R. Honeybees consolidate navigation memory during sleep. J. Exp. Biol. 2012;215:3981–3988. doi: 10.1242/jeb.075499. [DOI] [PubMed] [Google Scholar]
- Blacquière T., Smagghe G., Van Gestel C.A., Mommaerts V. Neonicotinoids in bees: a review on concentrations, side-effects and risk assessment. Ecotoxicology. 2012;21:973–992. doi: 10.1007/s10646-012-0863-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch G. The social clock of the honeybee. J. Biol. Rhythms. 2010;25:307–317. doi: 10.1177/0748730410380149. [DOI] [PubMed] [Google Scholar]
- Bloch G., Bar-Shai N., Cytter Y., Green R. Time is honey: circadian clocks of bees and flowers and how their interactions may influence ecological communities. Philos. Trans. R. Soc. B. 2017;372:20160256. doi: 10.1098/rstb.2016.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushey D., Tononi G., Cirelli C. Sleep- and wake-dependent changes in neuronal activity and reactivity demonstrated in fly neurons using in vivo calcium imaging. Proc. Natl. Acad. Sci. U S A. 2015;112:4785–4790. doi: 10.1073/pnas.1419603112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casida J.E. Neonicotinoids and other insect nicotinic receptor competitive modulators: progress and prospects. Annu. Rev. Entomol. 2018;63:125–144. doi: 10.1146/annurev-ento-020117-043042. [DOI] [PubMed] [Google Scholar]
- Casida J.E., Durkin K.A. Neuroactive insecticides: targets, selectivity, resistance, and secondary effects. Annu. Rev. Entomol. 2013;58:99–117. doi: 10.1146/annurev-ento-120811-153645. [DOI] [PubMed] [Google Scholar]
- Crall J.D., Switzer C.M., Oppenheimer R.L., Ford Versypt A.N., Dey B., Brown A., Eyster M., Guerin C., Pierce N.E., Combes S.A., De Bivort B.L. Neonicotinoid exposure disrupts bumblebee nest behavior, social networks, and thermoregulation. Science. 2018;362:683–686. doi: 10.1126/science.aat1598. [DOI] [PubMed] [Google Scholar]
- Cresswell J.E., Page C.J., Uygun M.B., Holmbergh M., Li Y., Wheeler J.G., Laycock I., Pook C.J., De Ibarra N.H., Smirnoff N., Tyler C.R. Differential sensitivity of honey bees and bumble bees to a dietary insecticide (imidacloprid) Zoology. 2012;115:365–371. doi: 10.1016/j.zool.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Cresswell J.E., Robert F.-X.L., Florance H., Smirnoff N. Clearance of ingested neonicotinoid pesticide (imidacloprid) in honey bees (Apis mellifera) and bumblebees (Bombus terrestris) Pest Manage. Sci. 2014;70:332–337. doi: 10.1002/ps.3569. [DOI] [PubMed] [Google Scholar]
- de Luca P.A., Vallejo-Marín M. What's the ‘buzz’ about? The ecology and evolutionary significance of buzz-pollination. Curr. Opin. Plant Biol. 2013;16:429–435. doi: 10.1016/j.pbi.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Donlea J.M., Ramanan N., Shaw P.J. Use-dependent plasticity in clock neurons regulates sleep need in Drosophila. Science. 2009;324:105–108. doi: 10.1126/science.1166657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis J., Louis T., Gauthier M., Raymond V. Insights from honeybee (Apis mellifera) and fly (Drosophila melanogaster) nicotinic acetylcholine receptors: from genes to behavioral functions. Neurosci. Biobehav. Rev. 2012;36:1553–1564. doi: 10.1016/j.neubiorev.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Eban-Rothschild A., Bloch G. Differences in sleep architecture of forager and young honeybees (Apis mellifera) J. Exp. Biol. 2008;211:2408–2416. doi: 10.1242/jeb.016915. [DOI] [PubMed] [Google Scholar]
- Fauser-Misslin A., Sadd B.M., Neumann P., Sandrock C. Influence of combined pesticide and parasite exposure on bumblebee colony traits in the laboratory. J. Appl. Ecol. 2014;51:450–459. [Google Scholar]
- Feltham H., Park K., Goulson D. Field realistic doses of pesticide imidacloprid reduce bumblebee pollen foraging efficiency. Ecotoxicology. 2014;23:317–323. doi: 10.1007/s10646-014-1189-7. [DOI] [PubMed] [Google Scholar]
- Fischer J., Müller T., Spatz A.K., Greggers U., Grünewald B., Menzel R. Neonicotinoids interfere with specific components of navigation in honeybees. PLoS One. 2014;9:e91364. doi: 10.1371/journal.pone.0091364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher S.P., Foster R.G., Peirson S.N. The circadian control of sleep. Handbook Exp. Pharmacol. 2013;217:157–183. doi: 10.1007/978-3-642-25950-0_7. [DOI] [PubMed] [Google Scholar]
- Gill R.J., Raine N.E. Chronic impairment of bumblebee natural foraging behaviour induced by sublethal pesticide exposure. Funct. Ecol. 2014;28:1459–1471. [Google Scholar]
- Gill R.J., Ramos-Rodriguez O., Raine N.E. Combined pesticide exposure severely affects individual- and colony-level traits in bees. Nature. 2012;491:105–108. doi: 10.1038/nature11585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradish A.E., Van Der Steen J., Scott-Dupree C.D., Cabrera A., Cutler G.C., Goulson D., Klein O., Lehmann D.M., Lückmann J., O'neill B. Comparison of pesticide exposure in honey bees (hymenoptera: apidae) and bumble bees (hymenoptera: apidae): implications for risk assessments. Environ. Entomol. 2018;48:12–21. doi: 10.1093/ee/nvy168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich-Förster C. Sleep in insects. Annu. Rev. Entomol. 2018;63:69–86. doi: 10.1146/annurev-ento-020117-043201. [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster C., Edwards T., Yasuyama K., Wisotzki B., Schneuwly S., Stanewsky R., Meinertzhagen I.A., Hofbauer A. The extraretinal eyelet of Drosophila: development, ultrastructure, and putative circadian function. J. Neurosci. 2002;22:9255–9266. doi: 10.1523/JNEUROSCI.22-21-09255.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich-Förster C., Stengl M., Homberg U. Organization of the circadian system in insects. Chronobiol. Int. 1998;15:567–594. doi: 10.3109/07420529808993195. [DOI] [PubMed] [Google Scholar]
- Hendricks J.C., Finn S.M., Panckeri K.A., Chavkin J., Williams J.A., Seghal A., Pack A.I. Rest in Drosophila is a sleep-like state. Neuron. 2000;25:129–138. doi: 10.1016/s0896-6273(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Hodge J.J., Stanewsky R. Function of the Shaw potassium channel within the Drosophila circadian clock. PLoS One. 2008;3:e2274. doi: 10.1371/journal.pone.0002274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgen Stelzer R., Stanewsky R., Chittka L. Circadian foraging rhythms of bumblebees monitored by radio-frequency identification. J. Biol. Rhythms. 2010;25:257–267. doi: 10.1177/0748730410371750. [DOI] [PubMed] [Google Scholar]
- King A.N., Sehgal A. Molecular and circuit mechanisms mediating circadian clock output in the Drosophila brain. Eur. J. Neurosci. 2018;51:268–281. doi: 10.1111/ejn.14092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lämsä J., Kuusela E., Tuomi J., Juntunen S., Watts P.C. Low dose of neonicotinoid insecticide reduces foraging motivation of bumblebees. Proc. R. Soc. B. 2018;285:20180506. doi: 10.1098/rspb.2018.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laycock I., Lenthall K.M., Barratt A.T., Cresswell J.E. Effects of imidacloprid, a neonicotinoid pesticide, on reproduction in worker bumble bees (Bombus terrestris) Ecotoxicology. 2012;21:1937–1945. doi: 10.1007/s10646-012-0927-y. [DOI] [PubMed] [Google Scholar]
- Lehmann M., Gustav D., Galizia C.G. The early bee catches the flower - circadian rhythmicity influences learning performance in honey bees, Apis mellifera. Behav. Ecol. Sociobiol. 2011;65:205–215. doi: 10.1007/s00265-010-1026-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelito K., Shafer O. Reciprocal cholinergic and GABAergic modulation of the small ventrolateral pacemaker neurons of Drosophila's circadian clock neuron network. J. Neurophysiol. 2012;8:2096–2108. doi: 10.1152/jn.00931.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J.D., Funes P., Dowes H.B., Hall J.C. Signal analysis of behavioural and molecular cycles. BMC Neurosci. 2002;3:1. doi: 10.1186/1471-2202-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Stormo G.D., Taghert P.H. The neuropeptide Pigment-Dispersing Factor coordinates pacemaker interactions in the Drosophila circadian system. J. Neurosci. 2004;24:7951–7957. doi: 10.1523/JNEUROSCI.2370-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda K., Ihara M., Sattelle D.B. Neonicotinoid insecticides: molecular targets, resistance, and toxicity. Annu. Rev. Pharmacol. Toxicol. 2020;60:241–255. doi: 10.1146/annurev-pharmtox-010818-021747. [DOI] [PubMed] [Google Scholar]
- Mccarthy E.V., Wu Y., Decarvalho T., Brandt C., Cao G., Nitabach M.N. Synchronized bilateral synaptic inputs to Drosophila melanogaster neuropeptidergic rest/arousal neurons. J. Neurosci. 2011;31:8181–8193. doi: 10.1523/JNEUROSCI.2017-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat C., Buckland S.T., Samson A.J., Mcarthur R., Chamosa Pino V., Bollan K.A., Huang J.T.J., Connolly C.N. Neonicotinoids target distinct nicotinic acetylcholine receptors and neurons, leading to differential risks to bumblebees. Scientific Rep. 2016;6:24764. doi: 10.1038/srep24764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraro N.I., Ceriani M.F. Acetylcholine from visual circuits modulates the activity of arousal neurons in Drosophila. J. Neurosci. 2015;50:16315–16327. doi: 10.1523/JNEUROSCI.1571-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muth F., Leonard A.S. A neonicotinoid pesticide impairs foraging, but not learning, in free-flying bumblebees. Scientific Rep. 2019;9:4764. doi: 10.1038/s41598-019-39701-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagari M., Gera A., Jonsson S., Bloch G. Bumble bee workers give up sleep to care for offspring that are not their own. Curr. Biol. 2019;29:3488–3493.e4. doi: 10.1016/j.cub.2019.07.091. [DOI] [PubMed] [Google Scholar]
- Nieto A., Roberts S.P.M., Kemp J., Rasmont P., Kuhlmann M., García Criado M., Biesmeijer J.C., Bogusch P., Dathe H.H., De La Rúa P. Luxembourg: Publication Office of the European Union; 2014. European Red List of Bees. [Google Scholar]
- Numata H., Miyazaki Y., Ikeno T. Common features in diverse insect clocks. Zool. Lett. 2015;1:10. doi: 10.1186/s40851-014-0003-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer M.J., Moffat C., Saranzewa N., Harvey J., Wright G.A., Connolly C.N. Cholinergic pesticides cause mushroom body neuronal inactivation in honeybees. Nat. Commun. 2013;4:1634. doi: 10.1038/ncomms2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman J.L., Mcgill J.J., Keegan K.P., Allada R. A dynamic role for the mushroom bodies in promoting sleep in Drosophila. Nature. 2006;441:753–756. doi: 10.1038/nature04739. [DOI] [PubMed] [Google Scholar]
- Popp J., Peto K., Nagy J. Pesticide productivity and food security, a review. Agron. Sustain. Dev. 2013;33:243–255. [Google Scholar]
- Rundlöf M., Andersson G., Bommarco R., Fries I., Hederström V., Herbertsson L., Jonsson O., Klatt B., Pedersen T., Yourstone J., Smith H. Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature. 2015;521:77–80. doi: 10.1038/nature14420. [DOI] [PubMed] [Google Scholar]
- Sheeba V., Fogle K.J., Kaneko M., Rashid S., Chou Y.-T., Sharma V.K., Holmes T.C. Large ventral lateral neurons modulate arousal and sleep in Drosophila. Curr. Biol. 2008;18:1537–1545. doi: 10.1016/j.cub.2008.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley D.A., Garratt M.P.D., Wickens J.B., Wickens V.J., Potts S.G., Raine N.E. Neonicotinoid pesticide exposure impairs crop pollination services provided by bumblebees. Nature. 2015;528:548–550. doi: 10.1038/nature16167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley D.A., Raine N.E. Chronic exposure to a neonicotinoid pesticide alters the interactions between bumblebees and wild plants. Funct. Ecol. 2016;30:1132–1139. doi: 10.1111/1365-2435.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley D.A., Russell A.L., Morrison S.J., Rogers C., Raine N.E. Investigating the impacts of field-realistic exposure to a neonicotinoid pesticide on bumblebee foraging, homing ability and colony growth. J. Appl. Ecol. 2016;53:1440–1449. doi: 10.1111/1365-2664.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern P. Synaptic downscaling during “up” states. Science. 2018;360:504. [Google Scholar]
- Stoner K.A. Current pesticide risk assessment protocols do not adequately address differences between honey bees (Apis mellifera) and bumble bees (Bombus spp.) Front. Environ. Sci. 2016;4:79. [Google Scholar]
- Tan J., Galligan J.J., Hollingworth R.M. Agonist actions of neonicotinoids on nicotinic acetylcholine receptors expressed by cockroach neurons. Neurotoxicology. 2007;28:829–842. doi: 10.1016/j.neuro.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Tasman Kiah, Hidalgo Sergio, Zhu Bangfu, Rands Sean, A, Hodge James., J L Neonicotinoids disrupt memory, circadian behaviour and sleep. Under consideration. 2020 doi: 10.1101/2020.04.07.030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomé H.V.V., Martins G.F., Lima M.A.P., Campos L.A.O., Guedes R.N.C. Imidacloprid-induced impairment of mushroom bodies and behavior of the native stingless bee Melipona quadrifasciata anthidioides. PLoS One. 2012;7:e38406. doi: 10.1371/journal.pone.0038406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosi S., Burgio G., Nieh J.C. A common neonicotinoid pesticide, thiamethoxam, impairs honey bee flight ability. Sci. Rep. 2017;7:1201. doi: 10.1038/s41598-017-01361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nest B.N., Otto M.W., Moore D. High experience levels delay recruitment but promote simultaneous time-memories in honey bee foragers. J. Exp. Biol. 2018;221:jeb187336. doi: 10.1242/jeb.187336. [DOI] [PubMed] [Google Scholar]
- Von Frisch K. Belknap Press; 1967. The Dance Language and Orientation of Bees. [Google Scholar]
- Wegener C., Hamasaka Y., Nassel D.R. Acetylcholine increases intracellular Ca2+ via nicotinic receptors in cultured PDF-containing clock neurons of Drosophila. J. Neurophysiol. 2004;91:912–923. doi: 10.1152/jn.00678.2003. [DOI] [PubMed] [Google Scholar]
- Weiss R., Dov A., Fahrbach S.E., Bloch G. Body size-related variation in pigment dispersing factor-immunoreactivity in the brain of the bumblebee Bombus terrestris (hymenoptera, apidae) J. Insect Physiol. 2009;55:479–487. doi: 10.1016/j.jinsphys.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Whitehorn P.R., O’connor S., Wackers F.L., Goulson D. Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science. 2012;336:351–352. doi: 10.1126/science.1215025. [DOI] [PubMed] [Google Scholar]
- Whitehorn P.R., Wallace C., Vallejo-Marin M. Neonicotinoid pesticide limits improvement in buzz pollination by bumblebees. Sci. Rep. 2017;7:15562. doi: 10.1038/s41598-017-14660-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson S.M., Willis S.J., Wright G.A. Exposure to neonicotinoids influences the motor function of adult worker honeybees. Ecotoxicology. 2014;23:1409–1418. doi: 10.1007/s10646-014-1283-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmer P.G., Bataw A.A.M., Hughes J.P. The superiority of bumblebees to honeybees as pollinators: insect visits to raspberry flowers. Ecol. Entomol. 1994;19:271–284. [Google Scholar]
- Willmer P.G., Stone G.N. Behavioral, ecological, and physiological determinants of the activity patterns of bees. Adv. Study Behav. 2004;34:347–446. [Google Scholar]
- Wood T.J., Goulson D. The environmental risks of neonicotinoid pesticides: a review of the evidence post 2013. Environ. Sci. Pollut. Res. Int. 2017;24:17285–17325. doi: 10.1007/s11356-017-9240-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaka H., Bartels R., Gora J., Franck V., Culo A., Gotsch M., Menzel R. Context odor presentation during sleep enhances memory in honeybees. Curr. Biol. 2015;21:2869–2874. doi: 10.1016/j.cub.2015.09.069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Original data have been deposited to Mendeley Data: https://doi.org/10.17632/m8pykxzkyb.1.