Abstract
Treatment for HER2-positive breast cancer often includes trastuzumab, breast/chest wall (CW) radiation (RT), and anthracyclines, all of which have known cardiac toxicity. In 88 patients who received concurrent trastuzumab and breast/CW RT, with and without anthracyclines, we found significant acute left ventricular ejection fraction declines to be attributable to doxorubicin alone, and not to heart radiation dose.
Introduction/Background:
Treatment for HER2-postitive breast cancer often includes trastuzumab, breast/chest wall (CW) radiation (RT), and anthracyclines, all of which have cardiac toxicity. We aimed to evaluate the relationship between heart dose and acute left ventricular ejection fraction (LVEF) changes in patients who received concurrent trastuzumab and breast/CW RT with and without anthracycline use.
Patients and Methods:
We retrospectively reviewed all nonmetastatic breast cancer patients from 2008 to 2015 who received concurrent trastuzumab and breast/CW RT. Baseline LVEF was compared with the LVEF closest to treatment completion as well as with the lowest post-treatment LVEF. LVEF changes were correlated with laterality, heart dosimetric parameters, and doxorubicin use.
Results:
Eighty-eight patients were included in our analysis. The median follow-up was 45 months. Forty-one patients were right-sided and 47 left-sided. Thirty-one patients received doxorubicin, 16 right-sided and 15 left-sided. Mean heart dose was 1.10 Gy and 3.63 Gy for right- and left-sided patients, respectively (P < .001). In the entire cohort, a significant LVEF decrease of 3.0% was observed pre- and post-treatment. There was a significant effect of doxorubicin (P = .013) and a nonsignificant effect of RT laterality (P = .088) on LVEF change. The test of interaction between doxorubicin and laterality was not significant (P = .90). No significant association was found between LVEF change and heart dosimetric parameters, including percent volume of heart receiving 5 Gy (V5), 10 Gy (V10), 20 Gy (V20), and 45 Gy (V45), and maximum dose. Similar results were found when baseline LVEF was compared with the lowest post-treatment LVEF.
Conclusion:
With cardiac doses < 4 Gy, declines in LVEF were not related to tumor laterality or heart dosimetric parameters. Statistically significant LVEF decreases were mainly attributed to doxorubicin.
Keywords: Breast, Cardiac toxicity, Herceptin, Radiation, Trastuzumab
Introduction
Approximately 25% of all breast cancers overexpress the human epidermal growth factor receptor, HER2. HER2 positivity was originally considered a poor prognostic factor; however, after the development of trastuzumab, a monoclonal antibody for HER2, patients with this oncogene have had dramatic improvements in disease-free survival as well as overall survival.1
The treatment of breast cancer is often trimodal, with patients undergoing surgery, chemotherapy, and radiation (RT) therapy. For HER2-positive breast cancers, patients routinely receive an entire year of trastuzumab. Radiation is often given during trastuzumab treatment because there is evidence to support enhanced breast cancer cell radiosensitivity with concurrent administration.2
Cardiac injury is a potential side effect that might arise from RT as well as chemotherapy. Trastuzumab has been correlated with congestive heart failure (CHF),3,4 and RT has been associated with increased ischemic heart disease and heart failure in the long term.5,6 Additionally, chemotherapeutic agents such as doxorubicin, cyclophosphamide, and taxanes have been linked to angina, ischemic heart disease, and heart failure.7 There are, however, limited data regarding the effect of cardiac toxicity of RT in addition to these systemic agents.
Because trastuzumab is a relatively new treatment, follow-up is short compared with the time frame typical in the development of cardiac toxicity, which might take > 20 years to manifest. In the herceptin adjuvant trial, which evaluated the sequential addition of 1 versus 2 years of trastuzumab after chemotherapy and RT, severe cardiac toxicity after a median follow-up of 8 years was < 1% in the 1-year arm.3 These events included cardiac death, severe CHF, or > 10% decline from baseline left ventricular ejection fraction (LVEF). Other studies that examined concurrent administration of trastuzumab with RT and chemotherapy have more limited follow up, with similarly low incidence of cardiac events.8-13
In the short term, before cardiac events have time to manifest, changes in LVEF might be useful in detecting subclinical cardiotoxicity.14 Some studies suggest that changes in acute LVEF during treatment might be associated with CHF in the long term.15 LVEF is the most common method to screen for toxic effects on the heart during cancer treatment.16 Patients who receive anthracyclines and trastuzumab routinely undergo LVEF measurements during and after treatment for monitoring. Multigated acquisition (MUGA) scintigraphy is currently the gold standard for assessing LVEF and uses intravenous radionuclides to measure cardiac blood flow. This method is has been shown to have low interobserver as well as intraobserver variation.16
The question remains whether standard breast irradiation in patients who receive concurrent trastuzumab causes any additive acute changes in cardiac function. We aimed to evaluate the relationship between heart dose and short-term LVEF changes or cardiac events in patients who receive concurrent trastuzumab and breast or chest wall (CW) RT.
Patients and Methods
We retrospectively reviewed all nonmetastatic HER2-positive breast cancer patients from 2008 to 2015 who received concurrent trastuzumab and breast or CW RT at our institution. This study was approved by the institutional review board.
Demographic, treatment, and follow-up data were obtained for each patient. Cardiovascular risk factors, as per the Framingham risk score, included hypertension, smoking history, diabetes, hyperlipidemia, and family history. No patients had a history of cardiovascular disease. Cardiac events included CHF, myocardial infarction, or unstable angina. Right-sided patients were used as control subjects because they received significantly less cardiac dose than left-sided patients.
Radiation was planned using computed tomography (CT) with standard tangents to treat the breast and CW. Heart blocking was used to minimize heart dose. Inclusion of supraclavicular (SCV) nodes and internal mammary nodes (IMNs) and the addition of a boost were at the discretion of the treating physician on the basis of clinical risk factors. Internal mammary nodes treated included those between the first and fourth ribs. Heart dose metrics were recorded and included mean heart dose, maximum heart dose (defined as dose to 1 cc), percent volume of heart receiving 5 Gy (V5), 10 Gy (V10), 20 Gy (V20), and 45 Gy (V45).
LVEF for each patient was measured using MUGA scans performed at a single institution. We defined baseline LVEF as the last LVEF measurement before the start of trastuzumab, RT, or chemotherapy. The follow-up LVEF was calculated in 2 ways: (1) the post-RT LVEF taken after completion of RT, closest to the trastuzumab end date (post-treatment LVEF); and (2) the lowest post-RT LVEF taken within 3 years of end of RT (lowest post-treatment LVEF). LVEFs are expressed as percentages and changes in LVEF are in absolute percentages.
Two-way analysis of variance (ANOVA) was used to examine whether there was a main laterality effect or main doxorubicin effect on LVEF change after treatment. The interaction between the 2 factors was also assessed. Linear regression models were used to examine whether there was a significant association between RT dosages on heart and LVEF percent change. Statistical analyses were performed using STATA software (version 11.0; StataCorp LP, College Station, TX).17 All P values are 2-sided.
Results
Data were collected on 124 patients. We excluded patients who did not have MUGA scans before and after treatment as well as patients who received bilateral breast/CW irradiation. Eighty-eight patients were included in our final analysis. Patient and disease characteristics are shown in Table 1.
Table 1.
Patient and Disease Characteristics
| Variable | Right RT (n = 41), n (%) | Left RT (n = 47), n (%) | Overall (n = 88), n (%) | P, Fisher Exact |
|---|---|---|---|---|
| Age at Dx, y | .3869 | |||
| 18-39 | 8 (20) | 8 (17) | 16 (18) | |
| 40-49 | 13 (32) | 12 (26) | 25 (28) | |
| 50-59 | 12 (29) | 10 (21) | 22 (25) | |
| ≥60 | 8 (20) | 17 (36) | 25 (28) | |
| Race | .3410 | |||
| Hispanic | 33 (80) | 32 (68) | 65 (74) | |
| Asian | 4 (10) | 9 (19) | 13 (15) | |
| White | 1 (2) | 0 | 1 (1) | |
| African American | 0 | 2 (4) | 2 (2) | |
| Other | 3 (7) | 4 (9) | 7 (8) | |
| Number of CV Risk Factors | .2723 | |||
| 0 | 16 (39) | 8 (17) | 24 (27) | |
| 1 | 13 (32) | 17 (36) | 30 (34) | |
| 2 | 5 (12) | 12 (26) | 17 (19) | |
| 3 | 5 (12) | 6 (13) | 11 (13) | |
| 4 | 1 (2) | 4 (9) | 5 (6) | |
| 5 | 1 (2) | 0 | 1 (1) | |
| ER Status | .1984 | |||
| Negative | 22 (54) | 18 (38) | 40 (45) | |
| Positive | 19 (46) | 29 (62) | 48 (55) | |
| PR Status | 1.0000 | |||
| Negative | 24 (59) | 27 (57) | 51 (58) | |
| Positive | 17 (41) | 20 (43) | 37 (42) | |
| Tumor Grade | .6252 | |||
| Intermediate | 9 (22) | 13 (28) | 22 (25) | |
| High | 32 (78) | 34 (72) | 66 (75) | |
| Pathologic T Stage | .1646 | |||
| T0 | 15 (37) | 9 (19) | 24 (27) | |
| T1 | 11 (27) | 18 (38) | 29 (33) | |
| T2 | 11 (27) | 9 (19) | 20 (23) | |
| T3 | 3 (7) | 9 (19) | 12 (14) | |
| T4 | 1 (2) | 2 (4) | 3 (3) | |
| Pathologic N Stage | .0273 | |||
| N0 | 24 (59) | 22 (47) | 46 (52) | |
| N1 | 4 (10) | 16 (34) | 20 (23) | |
| N2 | 9 (22) | 4 (9) | 13 (15) | |
| N3 | 4 (10) | 5 (11) | 9 (10) | |
| Extent of Surgery | .3697 | |||
| Mastectomy | 30 (73) | 30 (64) | 60 (68) | |
| Lumpectomy | 11 (27) | 17 (36) | 28 (32) | |
| Adriamycin | .5105 | |||
| Yes | 16 (39) | 15 (32) | 31 (35) | |
| No | 25 (61) | 32 (68) | 57 (65) |
Abbreviations: CV = cardiovascular; Dx = diagnosis; ER = estrogen receptor; PR = progesterone receptor; RT = radiation.
Treatment
Median follow-up was 45 months (range, 14-90 months). Of the 88 patients, all received trastuzumab concurrent with breast or CW RT. Median cumulative trastuzumab dose was 6170.5 mg and median duration of treatment was 364 days. Forty-one patients received right breast/CW RT and 47 received left breast/CW RT. Median prescribed dose was 50 Gy with a range of 40 to 52.6 Gy. Sixty-one patients received additional supraclavicular RT (30 left- and 31 right-sided). Twenty-two of those 61 also received internal mammary node RT (11 left- and 11 right-sided). Fifty-nine of 88 patients received a boost, with a median dose of 10 Gy and a range of 8 to 16 Gy. Mean dose to the heart was 1.10 Gy and 3.63 Gy for right- and left-sided patients, respectively (P < .001). All heart dose parameters are shown in Table 2.
Table 2.
Heart Dosimetry Parameters
| Variables | Right-Sided (n = 41) |
Left-Sided (n = 47) |
P |
|---|---|---|---|
| Mean Heart Dose, Gy | 1.10 | 3.63 | <.001 |
| Maximum Heart Dose, Gy | 6.23 | 40.60 | <.001 |
| V5, % | 1.83 | 13.98 | <.001 |
| V10, % | 0.24 | 6.10 | <.001 |
| V20, % | 0.06 | 3.23 | <.001 |
| V45, % | 0.00 | 0.61 | <.001 |
Abbreviation: VX = volume of heart receiving X dose.
All patients received chemotherapy. Regimens included the following agents: doxorubicin, cyclophosphamide, paclitaxel, docetaxel, and carboplatin. Chemotherapy was given neoadjuvantly in 39 patients and adjuvantly in 49 patients. A total of 31 patients received doxorubicin; 16 right-sided and 15 left-sided patients. Of the patients who received doxorubicin, median cumulative dose was 400 mg (range, 184-678 mg) and median duration of treatment was 63 days.
Difference Between Baseline and Post-Treatment LVEF
In the entire cohort, a significant decrease of 3.0% was observed in LVEF pre- and post-treatment (95% confidence interval [CI], −4.5 to −1.4; Figure 1). Two-way ANOVA showed a significant doxorubicin effect (P = .013) on LVEF change. For patients who received doxorubicin, the mean LVEF change was −5.6% (95% CI, −8.6 to −2.7), compared with − 1.5% (95% CI, −3.2 to 0.19) for patients who did not receive doxorubicin. Right-sided patients had a mean LVEF change of −4.5% (95% CI, −6.5 to −2.4), compared with a mean of −1.7% (95% CI, −3.9 to 0.61) for left-sided patients. The effect of RT side on LVEF percent change was not significant (P = .088). The test of the interaction between doxorubicin and laterality was also not statistically significant (P = .90). After controlling for doxorubicin, no statistically significant association was found between RT dosages to the heart (including V5, V10, V20, V45, and maximum heart dose) and LVEF change.
Figure 1.
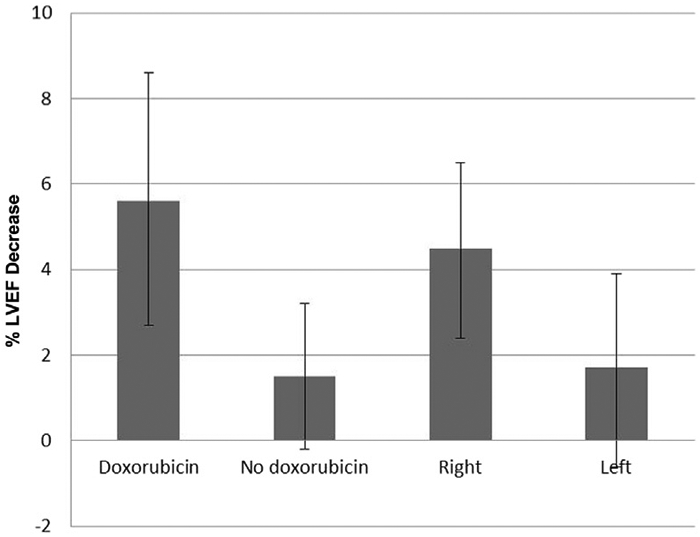
Difference Between Baseline and Post-Treatment Left Ventricular Ejection Fraction (LVEF). Error Bars Indicate 95% CIs
Difference Between Baseline and Lowest Post-Treatment LVEF
On average, a significant decrease was observed between baseline and the lowest post-treatment LVEF (Figure 2). Mean LVEF change was −4.3% (95% CI, −5.9 to −2.7). Two-way ANOVA showed that there was a significant doxorubicin main effect (P = .017) on LVEF percent change. For patients who received doxorubicin, the mean LVEF change was −6.9% (95% CI, −9.8 to −3.9), compared with −2.9% (95% CI, −4.6 to −1.1) for patients who did not receive doxorubicin. Right-sided patients had a mean LVEF change of −5.6% (95% CI, −7.5 to −3.7), compared with a mean of −3.1% (95% CI, −5.6 to −0.70) for left-sided patients. The effect of RT side on LVEF percent change was not significant (P = .15). After controlling for doxorubicin, no statistically significant association was found between RT dosages to the heart and LVEF change.
Figure 2.
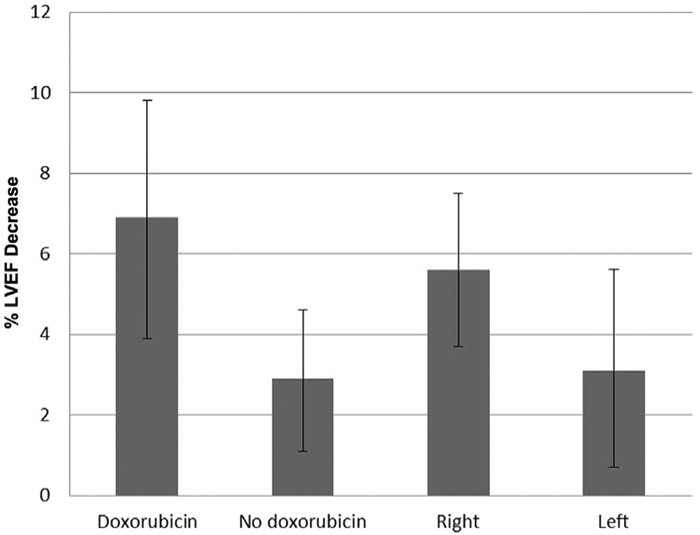
Difference Between Baseline and Lowest Post-Treatment Left Ventricular Ejection Fraction (LVEF). Error Bars Indicate 95% CIs
No cardiac events were reported. Results are shown in Table 3.
Table 3.
Results of LVEF Change After Radiotherapy and Concurrent Trastuzumab
| Variable | Right-Sided (n = 41) | Left-Sided (n = 47) | Overall (n = 88) |
|---|---|---|---|
| Baseline LVEF | |||
| Mean, median (range) | 69, 68 (59-82) | 66, 66 (51 to 80) | 67, 68 (51-82) |
| Follow-Up LVEF (Baseline vs. Post-Treatment LVEF) | |||
| Mean, median (range) | 64, 64 (52-78) | 64, 65 (47 to 76) | 64, 65 (47-78) |
| Change in LVEF (Baseline vs. Post-Treatment LVEF) | |||
| Mean, median (range) | −4.5, −5.0 (−17 to 10) | −1.7, −1.0 (−20 to 22) | −3.0, −3.0 (−20 to 22) |
| Follow-Up LVEF (Baseline vs. Lowest Post-Treatment LVEF) | |||
| Mean, median (range) | 63, 63 (51 to 78) | 63, 63 (47-76) | 63, 63 (47-78) |
| Change in LVEF (Baseline vs. Lowest Post-Treatment LVEF) | |||
| Mean, median (range) | −5.6, −6.0 (−17 to 10) | −3.1, −2 (−23 to 22) | −4.3, −4.0 (−23 to 22) |
Abbreviation: LVEF = left ventricular ejection fraction.
Discussion
Our results suggest that with low heart doses, concurrent trastuzumab and RT do not have a significant effect on acute changes in LVEF. The main determinant of LVEF change was attributed to doxorubicin use. Acute decreases in LVEF after administration of anthracyclines such as doxorubicin are well documented in the literature7 and consistent with our findings.
Radiation to the breast/CW can potentially cause long-term cardiac injury, especially in patients with left-sided breast cancer. Darby et al published a study of > 2000 women who underwent radiotherapy for breast cancer, and evaluated the effect of heart dose on major coronary events. The authors reported that the rate of coronary events increases linearly with mean heart dose by 7.4% per gray.5 Cardiac events can start within 5 years and continue into the third decade. However, this study did not use CT planning and estimated heart doses on the basis of virtual CT reconstructions of treatment diagrams. Additionally, before the era of CT scans, heart blocking was not routinely performed and mean heart doses were significantly higher compared with modern techniques.
Other more recent series show that low heart doses might not significantly affect acute cardiac measurements. In a prospective study of 32 women who underwent breast or CW RT, Chung et al reported no significant changes in cardiac perfusion 1 year after completion of RT with mean heart doses < 5 Gy.18
There are data regarding the additive cardiac toxicity of concurrent trastuzumab and RT, with relatively short follow-up. Studies that evaluated only cardiac events did not show any added toxicity associated with RT. Halyard et al analyzed the toxicity results of the North Central Cancer Treatment Group N9831 trial that investigated the combination of trastuzumab with standard chemotherapy regimens.11 There were no significant differences in cardiac events between patients who received RT and those who did not, as well as no difference between left- and right-sided patients after a median follow-up of 3.7 years. With short follow-up, it is unlikely to have significant numbers of cardiac events. These studies did not report on any surrogate measures of cardiac function such as LVEF.
Other retrospective studies evaluated subclinical LVEF decreases with no significant differences in left- versus right-sided RT, however, no heart dosimetry was recorded. Belkacemi et al retrospectively reviewed 146 patients who received trastuzumab and standard breast or CW RT with IMN inclusion in 71% of patients.8 Overall Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 Grade ≥ 2 LVEF decrease, which corresponds to LVEF < 50%, was 10%, with no significant difference between left- and right-sided patients, after median follow-up of 16 months. Shaffer et al reviewed 44 patients, all of whom received trastuzumab with RT.13 At a median follow-up of 15 months, the mean decrease in LVEF and cardiac events were not significantly different between left- and right-sided patients or with left-sided patients who received IMN RT.
Jacob et al recently published results of a prospective study in which the toxicity of concurrent trastuzumab and breast or CW RT was evaluated in 308 patients, with a median follow-up of 52 months.10 Ninety-one percent of patients received anthracyclines and 83% received IMN RT. In univariate analysis, neither laterality nor IMN treatment affected LVEF. No heart dosimetry was recorded, however, maximal heart distance and central lung distances were used as surrogate measures of heart dose.
There is scarce literature on heart dosimetry as it relates to acute changes in LVEF in the setting of concurrent trastuzumab and RT. In a retrospective review of 45 patients, Cao reported that in left-sided patients, mean heart doses were higher in patients who had significant decreases in LVEF of > 10% (CTCAE version 2.0, Grade ≥ 1) compared with patients with no cardiac toxicity (10.14 Gy vs. 6.28 Gy; P < .05).19 Furthermore, minimum dose reaching at least 45% of the volume was reported to be the best predictive factor for cardiac toxicity. In this study, all patients received anthracyclines, which might raise the baseline rate of heart toxicity. Mean heart doses were 6.92 Gy in left-sided patients and 3.27 Gy in right-sided patients.
Our study is the second study, to our knowledge, to evaluate acute changes in LVEF after concurrent trastuzumab and RT taking into account heart dosimetry. This study differs from that published by Cao et al in that our average heart doses were nearly half of that in Cao’s study, with an average of 3.6 Gy and 1.1 Gy for left- and right-sided patients, respectively. Additionally, approximately half of our cohort received doxorubicin, allowing us to separate the cardiac effects of doxorubicin from that of RT. With heart-sparing RT techniques resulting in low dosages to the heart, RT likely has a minimal effect on cardiac function even when administered concurrently with trastuzumab.
Limitations of this study include its retrospective nature, which might create inequalities in the comparison groups. Additionally, the number of patients might limit the ability to draw conclusions from subgroup analyses. Although LVEF is a very reliable way to measure cardiac function, it might not be able to detect more subtle cardiac abnormalities caused by trastuzumab and RT. Additionally, changes in LVEF that are subclinical might not result in clinically significant outcomes in the long run. However, there is a lack of a better predictor for long-term cardiac toxicity. Finally, the limited follow-up is not adequate to assess for long-term cardiac events.
Conclusion
With heart-sparing techniques that decrease cardiac doses to < 4 Gy, we did not find acute declines in LVEF to be attributable to tumor laterality or heart dosimetric parameters. Statistically significant LVEF decreases were mainly attributed to doxorubicin.
Clinical Practice Points
Treatment for HER2-positive breast cancer often includes trastuzumab, breast/CW RT, and anthracyclines, all of which have known cardiac toxicity. There are a small number of reports that describe the additive cardiac toxicity of RT therapy with systemic therapy. Some retrospective studies show that combined modality therapy is safe with respect to acute changes in LVEF, which is often used as a surrogate for long-term cardiac events, however, these studies do not relate toxicity to heart dose. The only study that examined LVEF decline with concurrent trastuzumab and RT therapy as it relates to heart dosimetry showed significant acute decreases in LVEF of > 10% in patients with mean heart doses of 10 Gy (Cao et al19).
In our study, we found that there was no effect of RT on LVEF decline before and after concurrent RT and trastuzumab. We used right-sided breast patients as a control (mean heart dose of 1.1 Gy), and compared LVEF changes with that of left-sided breast patients (mean heart dose of 3.6 Gy). The entire cohort showed LVEF declines after therapy of 3% to 5%, but these were mainly attributed to the previous use of anthracyclines.
Our study showed that mean heart doses of < 4 Gy did not acutely significantly decrease LVEF. We believe this adds to the body of literature on guidelines for acceptable heart dose in the context of concurrent systemic therapy in the modern era. With mean heart doses < 4 Gy, breast and CW RT seem to be safe in terms of acute cardiac toxicity measured using LVEF.
Footnotes
Disclosure
The authors have stated that they have no conflicts of interest.
References
- 1.Perez EA, Romond EH, Suman VJ, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol 2011; 29:3366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pietras RJ, Poen JC, Gallardo D, et al. Monoclonal antibody to HER2/neureceptor modulates repair of radiation-induced DNA damage and enhances radiosensitivity of human breast cancer cells overexpressing this oncogene. Cancer Res 1999; 59:1347–55. [PubMed] [Google Scholar]
- 3.De Azambuja E, Procter MJ, Van Veldhuisen DJ, et al. Trastuzumab-associated cardiac events at 8 years of median follow-up in the herceptin adjuvant trial (BIG 1-01). J Clin Oncol 2014; 32:2159–65. [DOI] [PubMed] [Google Scholar]
- 4.Tan-Chiu E, Yothers G, Romond E, et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast can. J Clin Oncol 2005; 23:7811–9. [DOI] [PubMed] [Google Scholar]
- 5.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013; 368:987–98. [DOI] [PubMed] [Google Scholar]
- 6.Harris EE, Correa C, Hwang WT, et al. Late cardiac mortality and morbidity in early-stage breast cancer patients after breast-conservation treatment. J Clin Oncol 2006; 24:4100–6. [DOI] [PubMed] [Google Scholar]
- 7.Bovelli D, Plataniotis G, Roila F. Cardiotoxicity of chemotherapeutic agents and radiotherapy-related heart disease:ESMO clinical practice guidelines. Ann Oncol 2010; 21:277–82. [DOI] [PubMed] [Google Scholar]
- 8.Belkacemi Y, Gligorov J, Ozsahin M, et al. Concurrent trastuzumab with adjuvant radiotherapy in HER2-positive breast cancer patients: acute toxicity analyses from the French multicentric study. Ann Oncol 2008; 19:1110–6. [DOI] [PubMed] [Google Scholar]
- 9.Caussa L, Kirova YM, Gault N, et al. The acute skin and heart toxicity of a concurrent association of trastuzumab and locoregional breast radiotherapy including internal mammary chain: a single-institution study. Eur J Cancer 2011; 47:65–73. [DOI] [PubMed] [Google Scholar]
- 10.Jacob J, Belin L, Pierga JY, et al. Concurrent administration of trastuzumab with locoregional breast radiotherapy: long-term results of a prospective study. Breast Cancer Res Treat 2014; 148:345–53. [DOI] [PubMed] [Google Scholar]
- 11.Halyard MY, Pisansky TM, Dueck AC, et al. Radiotherapy and adjuvant trastuzumab in operable breast cancer: tolerability and adverse event data from the NCCTG phase III trial N9831. J Clin Oncol 2009; 27:2638–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meattini I, Cecchini S, Muntoni C, et al. Cutaneous and cardiac toxicity of concurrent trastuzumab and adjuvant breast radiotherapy: a single institution series. Med Oncol 2014; 31:891. [DOI] [PubMed] [Google Scholar]
- 13.Shaffer R, Tyldesley S, Rolles M, et al. Acute cardiotoxicity with concurrent trastuzumab and radiotherapy including internal mammary chain nodes: a retrospective single-institution study. Radiother Oncol 2009; 90:122–6. [DOI] [PubMed] [Google Scholar]
- 14.Chung WB, Yi JE, Jin JY, et al. Early cardiac function monitoring for detection of subclinical doxorubicin cardiotoxicity in young adult patients with breast cancer. J Breast Cancer 2013; 16:178–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nousiainen T, Jantunen E, Vanninen E, et al. Early decline in left ventricular ejection fraction predicts doxorubicin cardiotoxicity in lymphoma patients. Br J Cancer 2002; 86:1697–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altena R, Perik PJ, van Veldhuisen DJ, et al. Cardiovascular toxicity caused by cancer treatment: strategies for early detection. Lancet Oncol 2009; 10: 391–9. [DOI] [PubMed] [Google Scholar]
- 17.StataCorp. Stata Statistical Software: Release 11. College Station, TX: StataCorp LP; 2009. [Google Scholar]
- 18.Chung E, Corbett JR, Moran JM, et al. Is there a dose-response relationship for heart disease with low-dose radiation therapy? Int J Radiat Oncol Biol Phys 2013; 85:959–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao L, Hu WG, Kirova YM, et al. Potential impact of cardiac dose-volume on acute cardiac toxicity following concurrent trastuzumab and radiotherapy. Cancer Radiother 2014; 18:119–24. [DOI] [PubMed] [Google Scholar]


