Summary:
Although patients with early-stage cervical cancer have in general a favorable prognosis, 10% to 40% patients still recur depending on pathologic risk factors. The objective of this study was to evaluate if the presence of lymph node micrometastasis (LNmM) had an impact on patient’s survival. We performed a multi-institutional retrospective review on patients with early-stage cervical cancer, with histologically negative lymph nodes, treated with radical hysterectomy and pelvic lymphadenectomy for the study period 1994 to 2004. Tissue blocks of lymph nodes from the patient’s original surgery were recut and then evaluated for the presence of micrometastases. One hundred twenty-nine patients were identified who met inclusion criteria. LNmM were found in 26 patients (20%). In an average follow-up time of 70 mo, there were 11 recurrences (8.5%). Of the 11 recurrences, 2 (18%) patients had LNmM. Patients with LNmM were more likely to have received adjuvant radiation and chemotherapy. In stratified log-rank analysis, LNmM were not associated with any other high-risk clinical or pathologic variables. Survival data analysis did not demonstrate an association between the presence of LNmM and recurrence or overall survival. The presence of LNmM was not associated with an unfavorable prognosis nor was it associated with other high-risk clinical or pathologic variables predicting recurrence. Further study is warranted to understand the role of micrometastases in cervical cancer.
Keywords: Cervical cancer, Micrometastasis, Isolated tumor cells
Early-stage cervical cancer accounts for the majority of cervical cancers in the United States (1). Decisions regarding primary and adjuvant therapy for early-stage cervical cancer have been based on our current understanding of the tumor’s natural history, which most commonly includes local spread and lymphatic metastasis. Locally, cervical cancer spreads from the cervix to the vagina and parametria. Lymphatic spread first involves the pelvic and para-aortic nodes, and then subsequently spreads to distant lymphatics.
Patients who have undergone a radical hysterectomy and are found to have positive lymph nodes, parametrial involvement, or positive margins are at a high risk of recurrence (approximately 40%) and benefit from adjuvant chemoradiation (2). In the absence of these high-risk factors, intermediate-risk factors for recurrence have also been identified, and they include various combinations of tumor size, stromal invasion, and the presence of lymphovascular space invasion (LVSI) (3). In a GOG study from Delgado et al. (3), these intermediate-risk factors were present in 25% of all Stage IB cancers. However, in the absence of these risk factors, there is still a 10% rate of recurrence in node-negative patients. Recurrence in these lower risk patients suggests inadequate identification of patients who would benefit from adjuvant treatment.
In light of the significance of lymph node metastases, improved identification of metastases could alter adjuvant therapy choices and potential survival. Previous studies have suggested that lymph node micrometastases (LNmM) may be an occult risk factor impacting recurrence and survival (4-6). In breast cancer, LNmM have been incorporated into staging and treatment strategies due to their impact on survival (7). LNmM are clusters of cancer cells <2mm in size in the lymph nodes that are detected with or without immunohistochemistry (IHC) (8).
Although high-risk and intermediate-risk factors for recurrence have been identified and validated in patients with cervical cancer, it is still unclear whether the presence of LNmM has an association with recurrence. Previous work has shown that both the incidence of LNmM by IHC and the recurrence rate in patients with negative nodes by hematoxylin-eosin (H&E) staining is similar at 15%, but that study lacked an analysis of the association of LNmM and survival (4). The objective of this study was to determine the incidence of LNmM by IHC analysis in patients with surgically treated, early-stage cervical cancer (Stages IA2–IB2), who had previously been determined to have negative lymph nodes by routine H&E stain, and to determine if LNmM is associated with recurrence and overall survival in cervical cancer.
METHODS
After Institutional Review Board approval, cervical cancer patients were identified from the surgical database of the Division of Gynecologic Oncology at the University of Southern California Keck School of Medicine (USC) and from the tumor registry at Walter Reed Army Medical Center during the period of 1994 to 2004. Selection was restricted to patients with early-stage cervical cancer (FIGO 1988 Stage IA2 through IB2), who had undergone definitive surgical treatment with radical hysterectomy and pelvic lymphadenectomy, and who were subsequently found to be lymph node negative on H&E staining on their final pathology. Patients treated with primary chemoradiation followed by an adjuvant hysterectomy and lymph node dissection were excluded from this analysis.
Medical records for eligible patients were reviewed and analyzed for personal demographics to include age, race, date of surgery, date of recurrence (if applicable), as well as site of recurrence, date of death/last contact, and current disease status. In addition, pathology reports were reviewed for known pathologic risk factors: stage, tumor histology, histologic grade, tumor size, presence of LVSI, cervical stromal invasion, surgical margin status, and postoperative radiation or chemotherapy. Adjuvant therapy was prescribed at the discretion of the treating physicians. Patients with squamous cell carcinoma, adenocarcinoma, and adenosquamous carcinoma were included in the final analysis. Those with glassy cell and small cell carcinoma were excluded due to their increased risk of lymph node metastases and recurrence rates.
Histologic Techniques
The sampled lymph nodes were first reexamined for each patient to confirm the absence of metastases by conventional H&E staining. Each lymph node section was then examined using IHC staining to detect LNmM. Paraffin-embedded tissue blocks were cut into two 5-μm sections and underwent standard IHC techniques for staining to identify LNmM as previously described by Lentz et al. (4). The AE-1 and CAM 5.2 murine antibodies were used to detect the ectopic presence of cytokeratins. The slides for Walter Reed Army Medical Center patients were stained as described in the IHC protocol except a cytokeratin protocol of AE-1/AE-3 was used. All slides were labeled with arbitrary designations and examined separately by 2 pathologists at each institution who were blinded to the patients’ clinical data and outcome. Lymph nodes were considered to have occult node metastases if there were immunoreactive tumor cells within the lymph node. Lymph nodes found to have single or small groups of cells showing strong brown staining were considered to have LNmM. Any disagreement regarding the presence of LNmM was deliberated by the 2 pathologists until a final consensus was established.
Statistical Analysis
The outcomes used were time to recurrence and overall survival, calculated from the date of surgery. For each outcome, data from those who did not experience the event were censored at the date of last follow-up.
First, a univariate log-rank test was performed. The Pike estimates of relative hazard ratio were calculated with the use of observed and expected numbers of events as calculated in the log-rank test. The probability of developing recurrence or survival was estimated using the Kaplan-Meier method. To investigate the association between LNmM and the outcomes after controlling for other prognostic factors, stratified log-rank test was performed using the variables that were significant or marginally significantly associated with time to recurrence or overall survival in the univariate analysis and those that were significantly associated with LNmM as the stratifying variables 1 at a time. The probability of developing recurrence or survival was estimated either using the Kaplan-Meier method or incidence rate ratios.
RESULTS
One hundred twenty-nine early-stage cervical cancer patients were identified that met inclusion criteria. Twenty-six patients (20%) were found to have LNmM. The patient demographics are depicted in Table 1. The median age was 42 yr and the majority was Hispanic (74%). Most had squamous histology and were Stage IB1 (70%, 82%, respectively). The average lymph node count was 24 nodes per patient.
TABLE 1.
Clinicopathologic characteristics of 129 patients with early-stage cervical cancer
| n (%) |
||||
|---|---|---|---|---|
| Variables | All patients (N=129) |
LNmM (+) (N=26) |
LNmM (−) (N=103) |
P |
| Age (yr) | 0.510 | |||
| ≤40 | 56 (43.4) | 13 (50.0) | 43 (41.7) | |
| >40 | 73 (56.6) | 13 (50.0) | 60 (58.3) | |
| Race | 0.023 | |||
| Hispanic | 95 (73.6) | 14 (53.8) | 81 (78.6) | |
| Other | 34 (26.4) | 12 (46.2) | 22 (21.4) | |
| Stage | 0.318 | |||
| IA2 | 10 (7.8) | 0 (0.0) | 10 (9.7) | |
| 1B1 | 106 (82.2) | 23 (88.5) | 83 (80.6) | |
| 1B2 | 13 (10.1) | 3 (11.5) | 10 (9.7) | |
| Grade | 0.371 | |||
| 1 | 52 (40.3) | 8 (30.8) | 44 (42.7) | |
| 2 | 48 (37.2) | 13 (50.0) | 35 (34.0) | |
| 3 | 29 (22.5) | 5 (19.2) | 24 (23.3) | |
| Histologic type | 0.683 | |||
| Squamous | 90 (69.8) | 17 (65.4) | 73 (70.9) | |
| Adenocarcinoma | 26 (20.2) | 7 (26.9) | 19 (18.4) | |
| Adenosquamous | 13 (10.1) | 2 (7.7) | 11 (10.7) | |
LNmM indicates lymph node micrometastasis.
A total of 3094 lymph nodes were examined with 745 lymph nodes demonstrating LNmM (24% of all nodes examined). No patients were found to have micrometastases when examining the H&E slides. Patients with micrometastases were found to have involvement in pelvic and para-aortic lymph nodes. Pelvic nodes were more commonly identified as positive compared with para-aortic lymph nodes by both H&E and IHC staining. No positive para-aortic lymph nodes were found in the absence of positive pelvic lymph nodes on both H&E and IHC staining.
The clinicopathologic variables associated with recurrence were compared among patients with and without LNmM (Table 2). There were no significant differences between these groups with the exception of postoperative radiation. Patients with LNmM were more likely to receive postoperative radiation (P = 0.037). To further characterize our patient cohort, all patients were labeled as low, intermediate, or high risk based off criteria described above (with the exception of positive lymph node involvement on H&E, as these patients were excluded from this analysis). Low-risk patients were those with neither intermediate-risk or high-risk factors. The prevalence of low-risk, intermediate-risk, and high-risk factors among the cohort was 62%, 16.3%, and 21.7%, respectively. There was no correlation, however, between the presence of LNmM and these risk factor groupings.
TABLE 2.
The association between the presence of LNmM and known risk factors for recurrence
| n (%) |
||||
|---|---|---|---|---|
| Variables | All patients (N=129) |
LNmM (+) (N=26) |
LNmM (−) (N=103) |
P |
| LVSI | 0.649 | |||
| Present | 47 (36.4) | 8 (30.8) | 39 (37.9) | |
| Absent | 82 (63.6) | 18 (69.2) | 64 (62.1) | |
| Stromal invasion | 0.201 | |||
| None | 9 (7.0) | 1 (3.8) | 8 (7.8) | |
| Inner third | 59 (45.7) | 8 (30.8) | 51 (49.5) | |
| Middle third | 21 (16.3) | 5 (19.2) | 16 (15.5) | |
| Outer third | 40 (31.0) | 12 (46.2) | 28 (27.2) | |
| Tumor size | 0.19 | |||
| <2 | 50 (38.8) | 7 (27.0) | 43 (42.0) | |
| 2.1–3.9 | 54 (41.9) | 11 (42.0) | 43 (42.0) | |
| ≥4 | 25 (19.4) | 8 (31.0) | 17 (17.0) | |
| Margins status | 0.236 | |||
| Positive/close | 20 (15.5) | 6 (23.1) | 14 (13.6) | |
| Negative | 109 (84.5) | 20 (76.9) | 89 (86.4) | |
| Parametrial involvement | ||||
| Present | 15 (11.6) | 4 (15.4) | 11 (10.7) | 0.5 |
| Absent | 114 (88.4) | 22 (84.6) | 92 (89.3) | |
| Postoperative radiation | 0.037 | |||
| Yes | 29 (22.5) | 10 (38.5) | 19 (18.4) | |
| No | 100 (77.5) | 16 (61.5) | 84 (81.6) | |
| Overall risk | 0.172 | |||
| Low | 80 (62.0) | 12 (46.2) | 68 (66.0) | |
| Intermediate | 21 (16.3) | 6 (23.1) | 15 (14.6) | |
| High | 28 (21.7) | 8 (30.8) | 20 (19.4) | |
LNmM indicates lymph node micrometastasis; LVSI, lymphovascular space invasion.
The median follow-up was 70mo for all patients. Over this time there were 11 recurrences with 3 survivors. Most recurrences were within the first 2 yr from diagnosis (62.5%). Among patients identified as having low-risk, intermediate-risk, or high-risk factors, the incidence of recurrence was 5.9%, 13.3%, and 17.9%, respectively. Of the 11 recurrences, 2 (18%) had LNmM identified, both of whom have died of disease with pelvic recurrences.
Univariate analysis found progression-free and overall survivals to be very similar between the patients who had LNmM and those who did not (Table 3). Surgical margin was the strongest prognostic factor for recurrence with patients who had a positive margin having a much higher risk of developing recurrence or dying (Table 3, P = 0.001) than those who had negative or close margins. The other variables were not found to impact progression-free or overall survival.
TABLE 3.
Association of progression-free and overall survival with prognostic factors using univariate log-rank test
| Progression-free survival |
Overall survival |
||||
|---|---|---|---|---|---|
| Variables | Total | HR | P | HR | P |
| LNmM | |||||
| Absent | 103 | 1 | 0.93 | 1 | 0.91 |
| Present | 26 | 1.07 | 1.09 | ||
| Tumor size | |||||
| <2 | 50 | 1 | 0.59 | 1 | 0.54 |
| 2–3.9 | 54 | 0.9 | 0.79 | ||
| ≥4 | 25 | 1.85 | 1.78 | ||
| LVSI | |||||
| Absent | 82 | 1 | 0.059 | 1 | 0.16 |
| Present | 47 | 3.08 | 2.21 | ||
| Margin | |||||
| Negative/close | 116 | 1 | 0.002 | 1 | 0.001 |
| Positive | 13 | 6.11 | 6.48 | ||
| Stage | |||||
| 1A2 | 10 | 1 | 0.46 | 1 | 0.22 |
| 1B1 | 106 | 0.71 | 0.37 | ||
| 1B2 | 13 | 1.84 | 1.07 | ||
| Grade | |||||
| 1 | 52 | 1 | 0.44 | 1 | 0.81 |
| 2 | 48 | 1.6 | 1.19 | ||
| 3 | 29 | 0.47 | 0.71 | ||
| Cervical stromal invasion | |||||
| Middle/outer | 61 | 1 | 0.36 | 1 | 0.47 |
| Inner | 59 | 0.4 | 0.5 | ||
| None | 9 | 1.17 | 1.29 | ||
| Parametrial involvement | |||||
| Absent | 114 | 1 | 0.33 | 1 | 0.064 |
| Present | 15 | 2.11 | 3.21 | ||
| Postoperative radiation therapy | |||||
| No | 100 | 1 | 0.16 | 1 | 0.15 |
| Yes | 29 | 2.33 | 2.35 | ||
| Chemotherapy | |||||
| No | 17 | 1 | 0.36 | 1 | 0.28 |
| Yes | 13 | 1.28 | 1.62 | ||
| NA | 99 | 0.49 | 0.53 | ||
Adjuvant therapy was at the discretion of the treating clinician. NA were patients not meeting criteria for further therapy.
HR indicates hazard ratio; LNmM, lymph node micrometastasis; LVSI, lymphovascular space invasion.
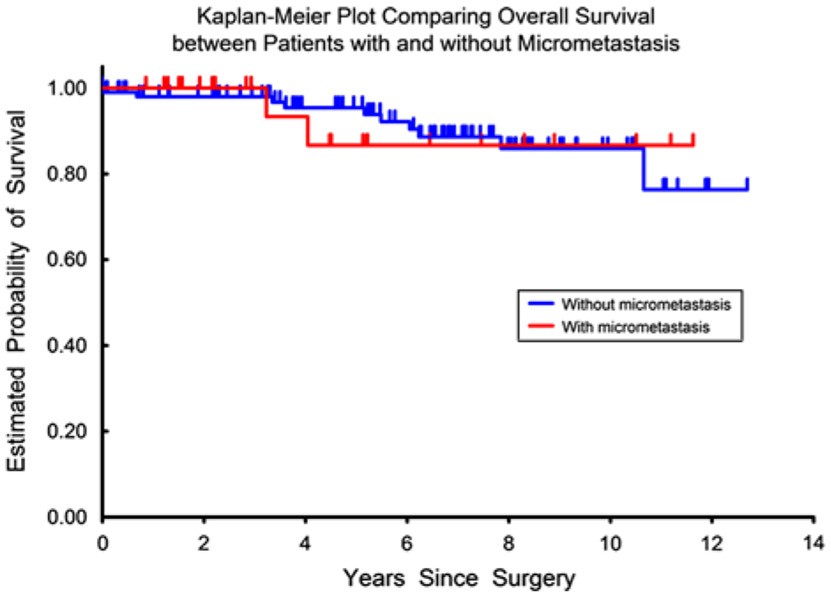
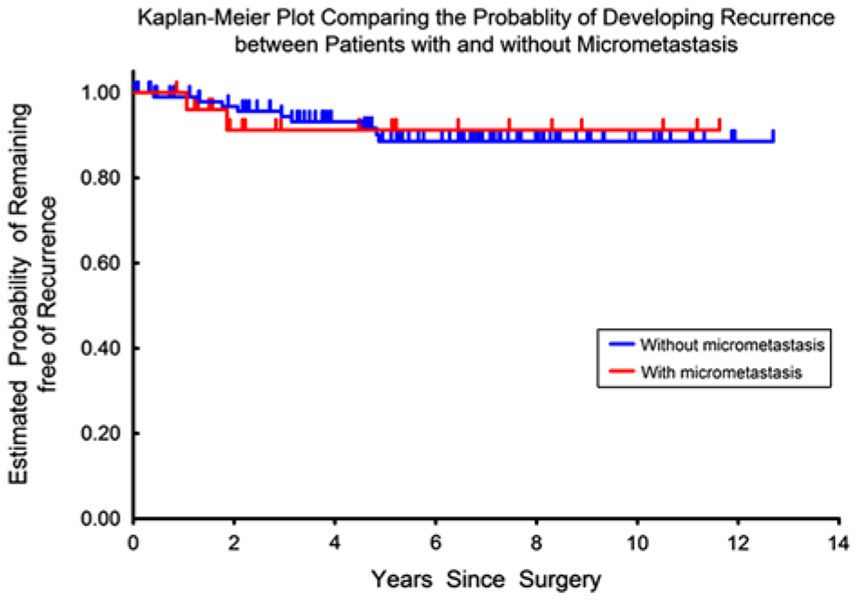
A stratified log-rank test was carried out to investigate the association between LNmM and time to recurrence and overall survival after controlling for individual prognostic factors. The relative hazards ratio and P-values for the associations between LNmM and the recurrence and survival outcomes did not change after adjusting for LVSI, margin status, depth of cervical stromal invasion, parametrial involvement, radiation therapy, and chemotherapy individually (Table 4). There was neither an increased risk of recurrence nor decreased risk of overall survival in patients with LNmM compared with patients without metastases (Figs. 1, 2).
TABLE 4.
Stratified log-rank test comparing patients with LNmM to those without the presence of LNmM
| Time to recurrence |
Overall survival |
|||
|---|---|---|---|---|
| Stratifying variables | Adjusted HR | P | Adjusted HR | P |
| LVSI | 1.18 | 0.84 | 1.23 | 0.79 |
| Margin | 1.04 | 0.96 | 0.96 | 0.97 |
| Cervical stromal invasion | 0.96 | 0.96 | 1.06 | 0.94 |
| Parametrial involvement | 0.93 | 0.93 | 0.92 | 0.91 |
| Postoperative radiation | 0.86 | 0.85 | 1.02 | 0.97 |
| Chemotherapy | 0.85 | 0.84 | 1.44 | 0.62 |
Reported outcomes include time to recurrence and overall survival.
HR indicates hazard ratio; LNmM, lymph node micrometastasis; LVSI, lymphovascular space invasion.
FIG. 1.
Overall survival comparing patients with and without presence of lymph node micrometastasis.
FIG. 2.
Recurrence risk comparing patients with and without presence of lymph node micrometastasis.
To further investigate if the presence of LNmM had an effect on recurrence in patients identified as low, intermediate, or high risk, incident rate ratios were calculated. LNmM did not represent a variable that further increased the risk of recurrence among these cohorts of patients (Table 5).
TABLE 5.
Comparison of patients with and without LNmM, stratifying by overall risk factors and postoperative radiation
| Total (N=129) |
Recurred (N=11) |
LNmM (+) who recurred (N=2) |
LNmM (−) who recurred (N=9) |
Incidence rate ratios (95% CI) |
|
|---|---|---|---|---|---|
| Overall risk | |||||
| Low | 80 | 4 | 0 | 4 | 0.7 (0.04–13.3) |
| Intermediate | 21 | 2 | 0 | 2 | 0.5 (0.03–10.9) |
| High | 28 | 5 | 2 | 3 | 3.0 (0.5–17.7) |
| Postoperative radiation | |||||
| Received | 29 | 4 | 0 | 4 | 0.2 (0.01–3.6) |
| None | 100 | 7 | 2 | 5 | 2.8 (0.5–14.5) |
Categories without a recurrence was assigned a value of 0.5. There were no differences between the groups as all confidence intervals (CI) crossed 1.
LNmM indicates lymph node micrometastasis.
As adjuvant radiation has been shown to decrease risk of recurrence, incident rate ratios were calculated among patients who recurred while controlling for the presence of LNmM. There was not an increased incidence of recurrence in the cohorts of patients with LNmM who were not treated with radiation (Table 5).
DISCUSSION
The incidence of immunohistochemically determined micrometastases in surgically treated, early-stage cervical cancer patients in our current study was 20%. LNmM were detected in our patients at similar rates as previous studies (4,5), and this rate of detection is similar to the rate of recurrence in early-stage cervical cancer. However, LNmM did not confer an increase in risk of recurrence. The lack of correlation between LNmM and survival persisted even when controlling for adjuvant radiation (Tables 4 and 5).
One important difference that was noted, however, was the increased likelihood of patients with LNmM to receive adjuvant radiation. Although this suggests that patients with LNmM were more likely to have an aggregate of poor prognostic factors that lead to the need for further therapy, LNmM did not correlate with individual prognostic factors, including LVSI, nor was it more frequently found in intermediate-risk or high-risk patients.
Other researchers have also investigated the role of LNmM in cervical cancer. In a smaller study of a similar patient population, Juretzka et al. (5) found an association with other poor prognostic factors such as LVSI, and tumor size of >4cm, but no difference in recurrence. In a case-control study, Marchiole et al. (9) assessed the presence of LNmM in patients with early-stage cervical cancer who recurred and compared them to a control group. This study demonstrated an increased risk of recurrence if LNmM were found; however, nearly half of the cases and controls were Stage IIB patients, a much higher risk group than our cohort.
Horn et al. (6) reported LNmM to be present in 22% of Stage IB-IIB cervical cancer patients and found that the presence of LNmM increased the relative risk of dying. This study’s identification method, however, would more correctly be called “hypersectioning” as they did not use any additional identification method other than complete processing of all lymph nodes. Expanding sectioning of node specimens has been shown to increase the number of positive nodes (4).
Part of the discrepancy in results may be due in part to the definition of micrometastases. Micrometastases have been defined as <2mm in AJCC Staging Manual (8). In our study, LNmM were typically the presence of a few cells not easily detected by H&E staining. Their presence by IHC was recorded as positive, which represented isolated tumor cells. The presence of cytokeratin-positive cellular material and individual tumor cells alone may not necessarily translate into neoplastic potential. They may represent cells cleared by the immune system or benign mesothelial cell inclusions.
A recent study by Cibula et al. (10) evaluated ultrastaging in a large number of early-stage cervical cancer in the setting of sentinel lymph node analysis. The study broke down lymph node tumor volume into the categories of macrometastasis (>2 mm), micrometastasis (<2 mm), and isolated tumor cells. The study demonstrated a negative correlation between the presence of micrometastasis and overall survival. However, almost 20% of patients were Stage IB2 or higher. They found that the presence of isolated tumor cells did not negatively impact survival.
Before studies evaluate the importance of micrometastasis in SLN mapping, it should be determined whether or not the presence of micrometastasis confers an independent risk of recurrence in patients. This question specifically applies to patients who have no other risk factors that would have been used to determine the need for adjuvant radiation, but do have LNmM. Although our numbers are small, our data suggest that LNmM do not increase the risk of recurrence in the absence of other risk factors. All other studies that have found micrometastases to correlate with worse survival have had higher stage patients (who likely needed adjuvant radiation anyway) or have not controlled for grouping patients as intermediate or high risk.
The strengths of our study include the relatively long median follow-up, and controlling for other clinical factors that have been used to identify a patient’s overall risk of recurrence. Weaknesses of our study include its retrospective nature and limited number of patients. Despite our study’s limitations, our research suggests that in the absence of other previously identified risk factors, the presence of LNmM is not associated with an increased risk of recurrence in early-stage cervical cancer. We therefore do not support the use of adjuvant therapy in these patients.
Footnotes
The authors declare no conflict of interest.
Contributor Information
Michael P. Stany, Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Bethesda, Maryland
Pamela J. B. Stone, SCL Physicians, Denver, Colorado
Juan C. Felix, Department of Obstetrics and Gynecology, Division of Gynecologic Oncology; Department of Pathology, Los Angeles, California
Charles A. Amezcua, Department of Pathology, Los Angeles, California
Susan Groshen, Department of Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, California
Wei Ye, Department of Preventive Medicine, University of Southern California Keck School of Medicine, Los Angeles, California
Kathy L. Kyser, Department of Obstetrics and Gynecology, Bethesda, Maryland
Robin S. Howard, Department of Research Programs, Walter Reed National Military Medical Center, Bethesda, Maryland
Chris M. Zahn, Department of Uniformed Services University of the Health Sciences, Bethesda, Maryland
Laila I. Muderspach, Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Los Angeles, California
Scott E. Lentz, Kaiser Permanente Medical Center, Los Angeles, California
Mildred R. Chernofsky, Sibley Memorial Hospital, Washington, District of Columbia
REFERENCES
- 1.Horner MJ, Ries LAG, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2006. Bethesda, MD: National Cancer Institute; http://seer.cancer.gov/csr/1975_2006/. [Google Scholar]
- 2.Peters WA, Liu PY III, Barrett RJ II, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol 2000;18:1606–13. [DOI] [PubMed] [Google Scholar]
- 3.Delgado G, Bundy B, Zaino R, et al. Prospective surgical-pathologic study of disease-free interval in patients with stage 1B squamous carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol 1990;38:352–7. [DOI] [PubMed] [Google Scholar]
- 4.Lentz S, Muderspach L, Felix J, et al. Identification of micrometastases in histologically negative lymph nodes of early-stage cervical cancer patients. Gynecol Oncol 2004;103:1204–10. [DOI] [PubMed] [Google Scholar]
- 5.Juretzka M, Jensen K, Longacre T, et al. Detection of pelvic lymph node micrometastases in stage IA2-IB2 cervical cancer by immunohistochemical analysis. Gynecol Oncol 2004;93:100–11. [DOI] [PubMed] [Google Scholar]
- 6.Horn L, Hentschel B, Fischer U, et al. Detection of micrometastases in pelvic lymph nodes in patients with carcinoma of cervix uteri using step sectioning: frequency, topographic distribution and prognostic impact. Gynecol Oncol 2008;111:276–81. [DOI] [PubMed] [Google Scholar]
- 7.Maibenco D, Dombi G, Kau T, et al. Significance of micrometastases on the survival of women with T1 breast cancer. Cancer 2006;107:1234–9. [DOI] [PubMed] [Google Scholar]
- 8.Edge S, Byrd D, Compton C, eds. Fritz A. AJCC Cancer Staging Manual.; 7th ed Philadelphia: Lippincott Raven; 2010. [Google Scholar]
- 9.Marchiole P, Buenerd A, Benchaib M, et al. Clinical significance of lymphovascular space involvement and lymph node micrometastases in early-stage cervical cancer: a retrospective case-control surgico-pathological study. Gynecol Oncol 2005;97:727–32. [DOI] [PubMed] [Google Scholar]
- 10.Cibula D, Abu-Rustum NR, Duske L, et al. Prognostic significance of low volume sentinel lymph node disease in early-stage cervical cancer. Gynecol Oncol 2012;124:496–501. [DOI] [PubMed] [Google Scholar]