Abstract
Sex-specific mortality is frequent in animals although the causes of different male versus female mortalities remain poorly understood. Parasitism is ubiquitous in nature with widespread detrimental effects to hosts, making parasitism a likely cause of sex-specific mortalities. Using sex-specific blood and gastrointestinal parasite prevalence from 96 and 54 avian host species, respectively, we test the implications of parasites for annual mortality in wild bird populations using phylogenetic comparative methods. First, we show that parasite prevalence is not different between adult males and females, although Nematodes showed a statistically significant but small male-biased parasite prevalence. Second, we found no correlation between sex-biased host mortalities and sex-biased parasite prevalence. These results were consistent in both blood and gastrointestinal parasites. Taken together, our results show little evidence for sex-dependent parasite prevalence in adults in wild bird populations, and suggest that parasite prevalence is an unlikely predictor of sex difference in adult mortalities, not withstanding sampling limitations. We propose that to understand causes of sex-biased mortalities, more complex analyses are needed that incorporate various ecological and life history components of animals life that may include sex differences in exposure to predators, immune capacity and cost of reproduction.
Subject terms: Sexual selection, Animal behaviour
Introduction
Although sex ratio at birth is often close to 1:1 in wild populations, adult (or tertiary) sex ratios (the proportion of males to females in a population) are highly variable suggesting that sex differences in post-birth maturation, mortalities and/or population movements drive skewed adult sex ratios (ASR)1–4.
Mortality is a complex process, influenced by many factors that in simple terms could be classified as intrinsic and extrinsic to the individual (for example, immune capacity and ambient environment, respectively5). Predation, disease and starvation are important causes of mortality in wild animal populations, whereas body size and sexual selection are general predictors of mortality according to life history theory6,7, with larger animals often dying at lower rates than smaller ones8–11. Furthermore, social activities such as competition for food and/or mates may increase mortality of one sex more than the other12–14.
One important cause of mortality are pathogens or infectious agents. For instance, the history of the modern human has been marked by diseases of epidemic scale that resulted in millions of deaths that were caused by bacteria, viruses and parasites15. Most recently, the COVID-19 pandemic, although with relatively low mortality, showed to be more lethal for men than for women16. In wild animals, examples of elevated mortality due to pathogen infection often include native species exposed to exotic pathogens, driving populations to critically low numbers (e.g. Darwin’s finches17, Serengeti’s wild dogs and lions18) or even to the edge of extinction19. Moreover, pathogens have shown to also provoke mortality not by directly killing the host but debilitating and deteriorating their overall condition, increasing the chances of predation20–23.
Interestingly, despite the presumed relationship between pathogens (i.e. biological agent that causes disease or illness) and mortality in animals, information on the relationship between sex-biased infections and biased sex ratio is scarce. A notable exception occurred in mammals, where Moore and Wilson1 found a positive correlation across 106 mammal species for the bias in sexual size dimorphism (SSD) and the sex bias in parasitism, and that sex bias in parasitism predicted the sex bias in mortality, concluding that sexual selection for the larger sex (i.e. males) implicated a mortality cost through parasitism (see also24). Also, male mammals have a weaker immune competence, which correlates with higher presence of pathogens and mortality compared to females25,26. In birds, sex-biased infections and its implications on survival have not been assessed across a broad range of taxa, although indirect evidence would suggest so since a previous across-species meta-analysis found a male-biased prevalence of gastrointestinal parasites27. In addition, more recent complementary evidence shows that larger avian species are more affected by parasites, possibly because in larger hosts, parasites have greater space and niches to colonize and are likely to accumulate through life as larger species tend to live longer than smaller ones9,28,29. Therefore, from this perspective, and considering that in birds males are in general larger than females30, we could expect parasitism in birds to be male-biased. Although the difference in body size of birds is modest compared to mammals, it is unknown at what extent this could influence parasite load between the sexes. From a hormonal perspective, the sex hormones influence the reproductive behavior e.g. courting, territoriality, aggression, competition and nesting31,32, which could translate into sex-different rates of parasite acquisition due to immunosuppression mediated by testosterone or stress-released corticosterone (cost for males25,33,34), or sex-differences in behavior such as nesting time or breeding dispersal (cost for females35,36). However, current studies disregard the effect of sex hormones in immunity, as well as challenge the idea of sex-different immune response in birds37,38, finding little evidence supporting a significant effect of sex hormones in immunity in studies using physiological concentrations of hormones. Moreover, another study26 showed a lack of sex-differences in immunity across 241 immune estimates in birds, while recent evidence showed that, in general, immunosenescence also lacked sexual dimorphism across animals, including birds39.
Thus, the current evidence highlights males but not females as the sex more likely to be affected by parasites in birds27,28,34, although it is nevertheless unknown whether this variable could relate to the overall lower male mortality compared to females found in birds13, and suggested by their overall male-biased ASR3,40.
Nevertheless, studies using unsexed birds suggest an association between mortality and both blood and gastrointestinal parasitism, supported by evidence established through direct analysis of carcasses of mortality events or through capture-recapture survival analyses41–44. Moreover, blood (protozoan and microphilaria) and gastrointestinal parasites (helminths and coccidia) have different means of transmission that in turn could also influence patterns of sex-specific infection and thus mortality. For instance, nest type (open versus close) is often considered a risk factor for malaria infection because open-nesting offers increase exposure to dipteran vectors such as mosquitoes45.
To examine the relationship between sex-specific parasite prevalence and mortality, we obtained data from a total of 138 bird species (across 96 species from 13 avian orders for blood parasites and 54 species from 9 orders for gastrointestinal parasites) from published literature to test two hypotheses using phylogenetic comparative analyses. We use parasite prevalence because it gives an estimation of the infection status of a population, thus providing hints of their susceptibility to parasite infection (although not without limitations46). Also, determinants of parasite prevalence depend on a number of ecological and behavioral variables47 that could differ between the sexes48, as well as being one of the most commonly available parasite estimates in parasitology and ecology. First, we investigate whether males had higher parasite prevalence compared to females, as predicted by male’s modest but significantly larger body size30, male’s frequent stress-inducing behavior (corticosterone mediated immunosuppression32,34), and as previously shown in across-species studies in mammals1 and in birds (particularly gastrointestinal parasites27). Second, we evaluate whether sex-specific parasite prevalence predicted sex-specific adult annual mortality. Specifically, we (i) test the effect of parasite prevalence on mortality in males and females separately as they present variation in their physiology and life histories49 that could influence the degree of exposure and/or infection to parasites and subsequent mortality48,50. Finally, we (ii) evaluate whether sex-specific adult annual mortality is predicted by sex-specific adult parasite prevalence, including SSD and mating competition in the analysis as potential confounding variables24,51,52.
Material and methods
Literature search
We collected data on sex-specific prevalence of parasitism in birds using ISI Web of Science and Google Scholar. The use of Google Scholar in systematic reviews has been recently criticized53, however, in our study we used Google Scholar because it expands searches to include grey literature, such as technical reports and theses. The searches were conducted by using the following keyword combinations: “scientific name of host species” + parasit*, prevale*, helmint*, blood, malar*, haemoparasit*, mite* or lice. Because our aim was to evaluate the effect of parasitism on sex-specific mortality, the list of names searched initially corresponded to 369 bird species included in the dataset of sex-specific annual mortality data provided by Székely et al.52. If the bird species name had synonyms, the search was repeated with every name. The references of previous reviews and meta-analyses were also checked (see supplementary material). The inclusion criteria required the parasite prevalence to be: (i) determined from adult birds with known sex, (ii) obtained from wild birds (not captive), and (iii) from infection naturally acquired (not experimentally infected). We only included studies reporting results for both males and females to avoid difficulties comparing prevalences within species generated by different sampling/diagnostic methods or different populations. We included studies with haemoparasite detection through molecular and optic microscopy methods because both bring comparable results and to date there is not consensus about which technique is better over the other54,55. All studies available for gastrointestinal and external parasites used exclusively taxonomic keys diagnosis through microscopic examination. Studies based on parasite’s egg counts were not considered to minimize the chances of including studies containing false negative results originated by the variation in egg shedding rhythms seen in some gastrointestinal parasites56. In order to obtain a robust estimate of parasite prevalence for a given host species, all publications that met the inclusion criteria were included in our dataset. Further details of the literature search as well as the full list of studies consulted are given in the supplementary material (Tables S1 and S2).
Body mass, adult mortality and sexual competition
Data on sex-specific body mass, annual adult mortality and sexual competition were obtained from Székely et al.52. Data were augmented following the method provided by Székely et al.52 and Liker et al.57, consisting of searching the name of the additional bird species in scientific citation indexes, books, species monographs and electronic databases (see supplementary material). We included mortality estimates obtained from field studies in which the estimates for both males and females were determined in the same population and with the same method. Three main methods were used to determine mortality rates: capture-recapture, ringing recoveries and local return rates. Mating system was determined as a five-point score by the frequency of polygamy for each sex, with “0” corresponding to very rare or no polygamy, “1” to rare polygamy, “2” to uncommon polygamy, “3” to moderate polygamy and “4” to common polygamy (for more details see57).
Parasite prevalence
The final dataset included 96 bird species (sample size range of 4–1045) with sex-specific blood parasite prevalence data, 54 species (5–9729) with gastrointestinal parasite prevalence data and only 3 species (13–131) with ectoparasite prevalence data. Ectoparasites were excluded from further analyses due to the low sample size. Blood parasites were divided into five categories: Haemoproteus, Leucocytozoon, Plasmodium, Trypanosoma and Microfilaria. Whereas gastrointestinal parasites were categorized as Cestoda, Acanthocephala, Nematoda, Trematoda and Protozoa. Finally, one last category received data presented as the combination of two or more parasite categories (for example, we often found blood parasite studies reporting the overall prevalence of Haemoproteus, Leucocytozoon and Plasmodium, three parasites categories combined in one single datum). Some studies of blood parasitism included avian species that presented 0% prevalence in both sexes. These studies were included in the dataset, although it was uncertain whether birds never got parasitized due to vector absence in their habitats58, were able to reduce parasitemia under detection limits, or because the parasites were unable to complete their life-cycle in the host59. Along with parasite prevalence data we also recorded the period of the year when parasites were samples, which was divided in three categories: breeding (sampling took place mostly during the hosts' breeding period), nonbreeding (sampling took place outside the breeding period) and year-round (sampling included both breeding and nonbreeding periods).
Phylogenetic meta-analysis
To investigate sex difference in parasite prevalence, a phylogenetic multilevel meta-analysis was performed using the R package metafor60. Because all studies only provided prevalence and sample size values, we opted to group the birds as infected and not infected males and females in 2 × 2 contingency tables and then calculate the effect size as log odds ratio60. We conducted the meta-analyses including period of sample (breeding, nonbreeding and year-round) and method of parasite detection (only for blood parasites, consisting of three categories: molecular detection, optic microscopy detection, and both) as moderators (i.e. fixed-effect), and study and phylogeny (a variance–covariance matrix) as random-effect variables.
Publication bias (due to missing studies that were not published because of negative or null results61) was evaluated using Egger’s regression test62,63 by including the standard error of the effect sizes as an additional moderator within the model. If the intercept significantly deviated from zero (significance of P < 0.1062) the overall relationship between the precision and size of studies included in the dataset was considered asymmetrical, or in other words, biased63. Of the twelve models conducted, two suggested presence of publication bias, corresponding to the gastrointestinal parasites Nematoda (P = 0.035) and Trematoda (P = 0.043). Diagnostic tests for identifying influential data points and outliers, and rules for excluding these types of cases are still evolving, particularly for multivariate/multilevel meta-analytical models64. To address this, our approach consisted of identifying the influential outliers causing the bias and running the models after excluding these values [see65].
Statistical power in random-effects meta-analysis can be difficult to determine. It has been suggested that, in general, meta-analyses with at least five studies offer more power than the individual studies alone66. Therefore, outcomes below this five-studies threshold should be taken carefully.
Phylogenetic comparative analysis
We used phylogenetic generalized least squares (PGLS) to test whether parasite prevalence was related to annual mortality, adult body mass and sexual competition. This approach allows controlling for the non-independence among species by incorporating a variance–covariance matrix that represents their phylogenetic relatedness67. In all models we used Pagel’s lambda (λ) as measure of phylogenetic signal68 and it was set to the maximum-likelihood value69. Prior to the analyses, prevalence and mortality were logit-transformed70. Mortality bias was expressed as log(male mortality/female mortality). Average body mass (in grams) of male and female adults was log-transformed, whereas SSD in adult body mass was expressed as log(male body mass (g)/female body mass (g)). The sex bias in mating system was calculated as the difference between male and female polygamy scores57. Because often each host species had several estimates of prevalence (i.e. studies reporting estimates for more than one parasite group), the sex bias in parasite prevalence of each bird species was incorporated into this analysis as the weighted average effect size of all comparisons. Instances where multiple studies reported prevalence estimates for the same host species were handled by adding sister tip labels (of the same branch length) to the phylogeny. The effect size per species was calculated using the function escalc of the R package metafor with log odds ratio as measure. We fitted both single-predictor and multi-predictor models to blood parasites and gastrointestinal parasites and each model was run separately for females, males and sex bias. To account for phylogeny, we used the avian phylogeny from Jetz et al.71. The analyses were run using consensus trees (one for each type of parasitism, Fig. S1) obtained through the method 50% majority-rule72,73 from 1,000 randomly selected trees from a pool of 10,000 available (https://birdtree.org), using the methodology described by Rubolini et al.74. These phylogenetic trees were not fully resolved, and polytomies were arbitrarily resolved by adding a branch distance of 10–08 to one randomly chosen branch in the polytomy using the function multi2di from the R package ape75. All PGLS analyses were conducted in R using the package caper76.
Results
Phylogenetic meta-analysis
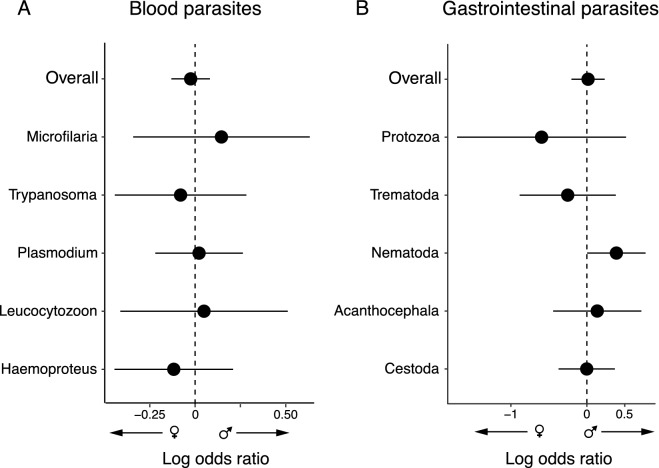
Overall, males and females did not exhibit different prevalence of blood parasites nor gastrointestinal parasites (Fig. 1 and Table 1). In the analysis broken down for parasite category (five categories of blood parasites and five of gastrointestinal parasites; Table 1), only Nematodes showed a weak male-biased prevalence (Fig. 1b; k = 33, estimate = 0.388, Z statistic = 1.979, P = 0.048, 95% CI = 0.004, 0.773).
Figure 1.
Sex bias in prevalence of (A) blood parasites and (B) gastrointestinal parasites in birds. Weighted average effect size estimates, showing lower and upper 95% confidence intervals in overall meta-analyses and broken down results according to parasite category (see Material and methods). The dashed vertical line indicates no sex difference, positive values represent male bias prevalence and negative values female bias. See Table 1 for statistics.
Table 1.
Phylogenetic meta-analysis of sex difference in prevalence of blood parasites and gastrointestinal parasites. The estimate represents the weighted average effect size as log odds ratio and its positive or negative value represents the sex bias directionality (see Fig. 1).
| QREML (P-value) | k | n | Studies | Estimate (95% CI) | Z statistic (P-value) | |
|---|---|---|---|---|---|---|
| Prevalence of blood parasite (overall) | 265.994 (0.043) | 229 | 96 | 78 | − 0.024 (− 0.130, 0.082) | − 0.451 (0.652) |
| Haemoproteus | 61.240 (0.575) | 69 | 60 | 51 | − 0.117 (− 0.444, 0.210) | − 0.704 (0.481) |
| Leucocytozoon | 39.710 (0.6559) | 49 | 43 | 33 | 0.049 (− 0.413, 0.511) | 0.209 (0.835) |
| Plasmodium | 30.820 (0.822) | 44 | 39 | 29 | 0.022 (− 0.220, 0.263) | 0.178 (0.859) |
| Trypanosoma | 17.257 (0.8375) | 28 | 23 | 21 | − 0.080 (− 0.443, 0.283) | 0.186 (0.666) |
| Microfilaria | 5.186 (0.878) | 13 | 10 | 10 | 0.145 (− 0.341, 0.632) | 0.591 (0.555) |
| Prevalence of gastrointestinal parasites (overall) | 226.818 (< 0.001) | 116 | 49 | 37 | 0.016 (− 0.203, 0.234) | 0.140 (0.889) |
| Cestoda | 68.354 (< 0.001) | 27 | 23 | 22 | − 0.002 (− 0.372, 0.368) | − 0.011 (0.991) |
| Acanthocephala | 6.141 (0.726) | 12 | 10 | 10 | 0.137 (− 0.444, 0.717) | 0.461 (0.645) |
| Nematoda | 37.544 (0.162) | 33 | 22 | 20 | 0.388 (0.004, 0.773) | 3.918 (0.048) |
| Trematoda | 20.086 (0.389) | 21 | 11 | 8 | − 0.252 (− 0.885, 0.380) | − 0.782 (0.434) |
| Protozoa | 12.537 (0.484) | 15 | 15 | 5 | − 0.596 (− 1.708, 0.516) | − 1.050 (0.294) |
Meta-analyses were performed using multilevel random-effect meta-analysis with restricted maximum likelihood (REML). Fixed-effect variables: period of sampling and method of parasite detection. Random-effect variables: phylogenetic relatedness and study. QREML = test for heterogeneity; k = number of effect sizes; n = number of host species; Studies = number of studies.
Parasite prevalence and annual adult mortality
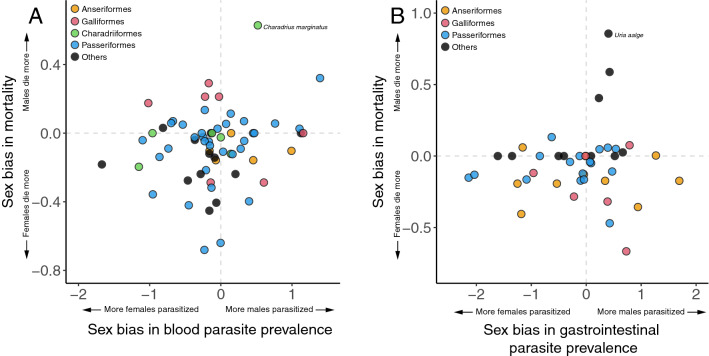
We found no association between annual mortality and prevalence in either blood parasites or gastrointestinal parasites (Table 2). The lack of association was consistent when each sex was tested separately (Table 2) and also when analyzing the sex bias (Fig. 2 and Table 2).
Table 2.
Phylogenetic generalized least squares (PGLS) showing single-predictor and multi-predictor relationships between annual mortality and prevalence of (a) blood parasites and (b) gastrointestinal parasites. Multi-predictor models include two additional life history variables: body mass and mating system. First each sex was analyzed separately, then we tested the relationship between sex bias in the response and predictor variables (see Material and methods).
| Response variable | Explanatory variable | Slope | P |
|---|---|---|---|
| (a) Blood parasites | |||
| Single-predictor models (n = 63) | |||
|
Male annual mortality Adjusted R2 = 0.00; λ = 0.805 |
Male overall blood parasite prevalence | 0.009 | 0.899 |
|
Female annual mortality Adjusted R2 = 0.00; λ = 0.735 |
Female overall blood parasite prevalence | 0.039 | 0.560 |
|
Sex bias in annual mortality Adjusted R2 = 0.02; λ = < 0.001 |
Sex bias in blood parasite prevalence | 0.064 | 0.167 |
| Multi-predictor models | |||
|
Male annual mortality (n = 56) Adjusted R2 = 0.18; λ = 0.994 |
Male overall blood parasite prevalence | 0.021 | 0.554 |
| Male body mass | − 0.208 | 0.002 | |
| Male mating system | 0.109 | 0.025 | |
|
Female annual mortality (n = 55) Adjusted R2 = 0.18; λ = 0.925 |
Female overall blood parasite prevalence | 0.018 | 0.718 |
| Female body mass | − 0.242 | 0.007 | |
| Female mating system | 0.252 | 0.045 | |
|
Sex bias in annual mortality (n = 55) Adjusted R2 = 0.03; λ = < 0.001 |
Sex bias in blood parasite prevalence | 0.046 | 0.223 |
| Sexual size dimorphism | 0.154 | 0.159 | |
| Sex bias in mating system | 0.029 | 0.056 | |
| (b) Gastrointestinal parasites | |||
| Single-predictor models (n = 43) | |||
|
Male annual mortality Adjusted R2 = 0.00; λ = 0.917 |
Male overall gastrointestinal parasite prevalence | − 0.008 | 0.889 |
|
Female annual mortality Adjusted R2 = 0.02; λ = 0.999 |
Female overall gastrointestinal parasite prevalence | 0.055 | 0.173 |
|
Sex bias in annual mortality Adjusted R2 = 0.00; λ = 0.384 |
Sex bias in gastrointestinal parasite prevalence | 0.034 | 0.414 |
| Multi-predictor models (n = 43) | |||
|
Male annual mortality Adjusted R2 = 0.31; λ = 0.900 |
Male overall gastrointestinal parasite prevalence | 0.013 | 0.791 |
| Male body mass | − 0.415 | < 0.001 | |
| Male mating system | 0.138 | 0.130 | |
|
Female annual mortality Adjusted R2 = 0.170; λ = 0.950 |
Female overall gastrointestinal parasite prevalence | 0.005 | 0.913 |
| Female body mass | − 0.353 | 0.005 | |
| Female mating system | 0.107 | 0.435 | |
|
Sex bias in annual mortality Adjusted R2 = 0.44; λ = 0.999 |
Sex bias in gastrointestinal parasite prevalence | 0.007 | 0.749 |
| Sexual size dimorphism | − 0.936 | < 0.001 | |
| Sex bias in social mating system | − 0.009 | 0.779 |
Figure 2.
Sex bias in annual mortality in relation to the sex bias in prevalence of (A) blood and (B) gastrointestinal parasites (see Table 2 for statistics). Sex bias in mortality was expressed as log(male mortality/female mortality), whereas the sex bias in parasite prevalence was expressed as the weighted average effect size of all comparisons (see Material and methods). Represented in colors are the avian orders with the greatest numbers of species in each of the analyses (full species list in supplementary material). Outliers are specified. Dashed lines indicate no sex difference, positive values represent male bias and negative values female bias.
These results remained qualitatively unchanged after conducting multi-predictor analyses incorporating body mass and mating competition into the models (Table 2). In these latter analyses only body mass and mating competition had a significant effect on mortality, although the relationship with mating competition was significant only in the blood parasite analyses (Table 2a).
In most cases the phylogenetic signal (λ) was moderate to high, indicating important variation associated to phylogenetic relatedness, however, further examination considering avian orders show no clear clustering for sex bias analyses (Fig. 2 and S2).
Discussion
To our knowledge, this work represents the largest comparative study of sex-specific parasite prevalence in birds, based on 96 species with sex-specific blood parasite prevalence data and 54 species with gastrointestinal parasite prevalence data. Taken together, our results showed little evidence supporting sex biases in parasite prevalence, with no overall sex bias in blood or gastrointestinal parasites prevalence in birds. Additionally, no relationship was found between sex bias in mortality and sex bias in parasite prevalence, even after controlling for possible confounding life history variables, i.e. mating system, body size and sexual size dimorphism.
Our findings do not support the prediction of male-biased parasitism generated by the sexual size dimorphism1,30 and sex-different hormonal immunosuppression31,33. One possible explanation is that in birds the magnitude of the difference in size between sexes tends to be smaller compared to mammals77, where an association between sexual size dimorphism and parasite prevalence has been shown1. Furthermore, some evidence shows little and no relationship between body size and blood parasites across avian species47,78,79. Scheuerlein and Ricklefs80 found an association in parasite prevalence and body size in passerines, however, after controlling for phylogeny, the association was marginal. On the other hand, although stress and sex hormones were not part of our analysis, our results give little support to the idea of sex-differences in corticosterone immunosuppression, and seem to be in line of with recent research finding inconclusive results in the immunocompetence handicap theory in birds26,37,81.
Specifically, we found no sex bias in the overall prevalence of blood parasites, consistent with the overall results of a previous meta-analysis of blood parasites in birds79. Sex differences in blood parasites are generally thought to occur due to unequal exposure of the sexes to vectors82,83 and differences between males and females in the immune-endocrine system84. Perhaps the lack of sex differences seen here could be attributed to these processes balancing each other out. For example, in males, the persistent pressure of male-male competition could generate stress-induced corticosterone which due to its immunodepressive effect could make them more prone to infection34, at the same time that the elevated exposure of females to vectors while incubating35. Poulin27 found a strong male-biased infection of Acanthocephalan and Nematodes parasites, consistent with our results in the overall parasite prevalence in Nematoda. Nematodes are a very diverse group of round worms. Male-biased parasite prevalence in this group could be due to many non-exclusive variables including those previously suggested for overall gastrointestinal parasites (mainly based on differences in body size; see Introduction), in addition to sex-specific foraging behavior as result of niche specialization or competitive exclusion by the dominant sex85,86. However, more studies are needed to test these hypotheses.
Mortality was not related to parasite prevalence across all analyses conducted, even in multi-predictor analyses where mortality was tested against parasite prevalence, body mass and mating system. Only body mass was consistently associated with mortality as found in previous studies13,52,87. Although parasite burden has often been linked to mortality in species-specific studies in birds23,44,88 (but see89), here we found that such association seems to be less clear at interspecific level. Nevertheless, our results should be treated cautiously because in most cases parasitism and mortality data did not come from the same population, and because parasite data for males and females is more likely to be reported in studies investigating sexually dimorphic birds, therefore, we cannot discard a possible bias toward sexually dimorphic species over monomorphic ones. In addition, prevalence, as an index of parasitism, could be problematic because it informs about the proportion of infected individuals in relation to the number examined90, generating uncertainty whether the individuals found positive only correspond to infection-resistant animals that survived the infection82. For example, a previous study found that males had lower survival than females to influenza A virus infection91, therefore, in the hypothetical situation of sampling this population in the wild without knowing this sex-different viral susceptibility beforehand, and assuming a similar infection rate between sexes, females would have a higher prevalence than males because a larger proportion of infected males died.
In contrast to the findings of Moore and Wilson1 in mammals, sex-biased parasitism in birds did not seem to be a consistent driver of sex-specific mortality. The pressure that parasites impose on birds not only appeared to be low between sexes but also within sexes as no increase nor diminution of mortality were seen when tested males and females separately. Perhaps, juveniles should be the target by further studies to obtain a thorough understanding of mortality patterns. Accordingly, a recent study suggests that juvenile mortality rather than chick and adult mortality corresponded to the main contributor of sex biases in ASR in six plover populations (Charadrius)92. Unfortunately, juvenile sex-specific parasitism data in birds is scant.
In conclusion, our analyses showed that birds do not exhibit overall sexual difference in parasite prevalence, and parasite prevalence do not predict sex-specific mortality, thus suggesting that other processes may drive the sex-differences in adult mortalities reported from numerous bird species. Though, perhaps the limitations in our analysis (mentioned above) contributed to this lack of association. Although life history traits (e.g. mating system, parental care, and body mass) have been shown as important predictors of mortality in birds13,52,87, the actual etiology that originates female-biased mortality in birds is still poorly explored. Perhaps mortality events during migration93, predation94, susceptibility to stress95, or simply resilience to starvation are more important determining sex-specific mortality than parasites. In addition to this, understanding male versus female immune systems undoubtedly is highly relevant. We call for further comparative and single-species studies to understand the causes of sex different mortality patterns.
Supplementary information
Acknowledgements
We would like to thank Matthieu Guillemain and Andy Green for kindly providing additional parasite data when requested, and Judit Mokos, Kathryn Maher, Zsolt Végvári, Laura Gangoso and Michael Jennions for their comments on various stages of this work, and to the many authors that kindly gave us additional data when it was requested. J.O.V. was funded by the Agencia Nacional de Investigación y Desarrollo de Chile (ANID; former CONICYT), BECAS CHILE 72170569; T.S. by Royal Society Wolfson Merit Award (WM170050), and by the Hungarian scientific funding agency—HKFIH (ÉLVONAL KKP-126949, K 116310); A.L. was funded by an NKFIH grant (KH 130430) and by the NKFIH’s TKP2020-IKA-07 project financed under the 2020-4.1.1-TKP2020 Thematic Excellence Programme by the National Research, Development and Innovation Fund of Hungary.
Author contributions
J.O.V. and T.S. conceived and designed the research; J.O.V. conducted the data analysis and wrote the paper; A.L. and T.S. provided part of the mortality and life history data; all authors contributed substantially to revisions of the paper and gave final approval for publication.
Data availability
The full list of references consulted to extract the parasite data is given in the supplementary material. The dataset and R code can be accessed on 10.6084/m9.figshare.13232435.v1.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-77410-6.
References
- 1.Moore SL, Wilson K. Parasites as a viability cost of sexual selection in natural populations of mammals. Science. 2002;297:2015–2018. doi: 10.1126/science.1074196. [DOI] [PubMed] [Google Scholar]
- 2.Le Galliard JF, Fitze PS, Ferriere R, Clobert J. From the cover: sex ratio bias, male aggression, and population collapse in lizards. Proc. Natl. Acad. Sci. USA. 2005;102:18231–18236. doi: 10.1073/pnas.0505172102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Székely T, Weissing FJ, Komdeur J. Adult sex ratio variation: implications for breeding system evolution. J. Evol. Biol. 2014;27:1500–1512. doi: 10.1111/jeb.12415. [DOI] [PubMed] [Google Scholar]
- 4.Ancona S, Dénes FV, Krüger O, Székely T, Beissinger SR. Estimating adult sex ratios in nature. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017 doi: 10.1098/rstb.2016.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams PD, Day T, Fletcher Q, Rowe L. The shaping of senescence in the wild. Trends Ecol. Evol. 2006;21:458–463. doi: 10.1016/j.tree.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Calder WA. Size, Function and Life History. Harvard: Harvard Univiversity Press; 1984. [Google Scholar]
- 7.Boukal DS, Berec L, Křivan V. Does sex-selective predation stabilize or destabilize predator-prey dynamics? PLoS ONE. 2008;3:e2687. doi: 10.1371/journal.pone.0002687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pauly D. On the interrelationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. ICES J. Mar. Sci. 1980;39:175–192. doi: 10.1093/icesjms/39.2.175. [DOI] [Google Scholar]
- 9.Healy K, et al. Ecology and mode-of-life explain lifespan variation in birds and mammals. Proc. R. Soc. B. 2014;281:20140298. doi: 10.1098/rspb.2014.0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valcu M, Dale J, Griesser M, Nakagawa S, Kempenaers B. Global gradients of avian longevity support the classic evolutionary theory of ageing. Ecography. 2014;37:930–938. doi: 10.1111/ecog.00929. [DOI] [Google Scholar]
- 11.Scharf I, et al. Late bloomers and baby boomers: ecological drivers of longevity in squamates and the tuatara. Glob. Ecol. Biogeogr. 2015;24:396–405. doi: 10.1111/geb.12244. [DOI] [Google Scholar]
- 12.Darwin C. The Descent of Man, and Selection in Relation to Sex. London: J. Murray; 1871. [Google Scholar]
- 13.Liker A, Székely T. Mortality costs of sexual selection and parental care in natural populations of birds. Evolution. 2005 doi: 10.1554/04-560. [DOI] [PubMed] [Google Scholar]
- 14.Jennions MD, Kokko H. In: Evolutionary Behavioral Ecology. Westneat D, Fox C, editors. Oxford: Oxford Univiversity Press; 2010. pp. 343–364. [Google Scholar]
- 15.Hays JN. Epidemics and Pandemics: Their Impacts on Human History. Santa Barbara: ABC-CLIO; 2005. [Google Scholar]
- 16.Peckham, H. et al. Sex-bias in COVID-19: a meta-analysis and review of sex differences in disease and immunity. 17 April 2020, PREPRINT (Version 1) available at Research Square. 10.21203/rs.3.rs-23651/v1 (2020).
- 17.Kleindorfer S, Peters KJ, Custance G, Dudaniec RY, O’Connor JA. Changes in Philornis infestation behavior threaten Darwin’s finch survival. Curr. Zool. 2014;60:542–550. doi: 10.1093/czoolo/60.4.542. [DOI] [Google Scholar]
- 18.Roelke-Parker ME, et al. A canine distemper virus epidemic in Serengeti lions (Panthera leo) Nature. 1996;379:441–445. doi: 10.1038/379441a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wyatt KB, et al. Historical mammal extinction on Christmas Island (Indian Ocean) correlates with introduced infectious disease. PLoS ONE. 2008;3:e3602. doi: 10.1371/journal.pone.0003602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Møller A, Erritzøe J. Predation against birds with low immunocompetence. Oecologia. 2000;122:500–504. doi: 10.1007/s004420050972. [DOI] [PubMed] [Google Scholar]
- 21.Genovart M, et al. The young, the weak and the sick: evidence of natural selection by predation. PLoS ONE. 2010;5:e9774. doi: 10.1371/journal.pone.0009774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ingram WM, Goodrich LM, Robey EA, Eisen MB. Mice infected with low-virulence strains of Toxoplasma gondii lose their innate aversion to cat urine, even after extensive parasite clearance. PLoS ONE. 2013;8:e75246. doi: 10.1371/journal.pone.0075246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adelman JS, Mayer C, Hawley DM. Infection reduces anti-predator behaviors in house finches. J. Avian Biol. 2017;48:519–528. doi: 10.1111/jav.01058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Promislow DEL, Montgomerie R, Martin TE. Mortality cost of sexual dimorphism in birds. Proc. R. Soc. Lond. B. 1992;250:143–150. doi: 10.1098/rspb.1992.0142. [DOI] [Google Scholar]
- 25.Klein SL, Roberts CW. Sex Hormones and Immunity to Infection. Berlin: Springer; 2010. [Google Scholar]
- 26.Kelly CD, Stoehr AM, Nunn C, Smyth KN, Prokop ZM. Sexual dimorphism in immunity across animals: a meta-analysis. Ecol. Lett. 2018;21:1885–1894. doi: 10.1111/ele.13164. [DOI] [PubMed] [Google Scholar]
- 27.Poulin R. Sexual inequalities in helminth infections: a cost of being a male? Am. Nat. 1996;147:287–295. doi: 10.1086/285851. [DOI] [Google Scholar]
- 28.Kamiya T, O'Dwyer K, Nakagawa S, Poulin R. What determines species richness of parasitic organisms? A meta-analysis across animal, plant and fungal hosts. Biol. Rev. 2014;89:123–134. doi: 10.1111/brv.12046. [DOI] [PubMed] [Google Scholar]
- 29.Gutiérrez JS, Rakhimberdiev E, Piersma T, Thieltges DW. Migration and parasitism: habitat use, not migration distance, influences helminth species richness in Charadriiform birds. J. Biogeogr. 2017;44:1137–1147. doi: 10.1111/jbi.12956. [DOI] [Google Scholar]
- 30.Székely T, Lislevand T, Figuerola J. In: Sex, Size and Gender Roles. Fairbairn D, Blanckenhorn W, Szekely T, editors. Oxford: Oxford University Press; 2007. pp. 27–37. [Google Scholar]
- 31.Folstad I, Karter AJ. Parasites, bright males, and the immunocompetence handicap. Am. Nat. 1992;139:603–622. doi: 10.1086/285346. [DOI] [Google Scholar]
- 32.Adkins-Regan E. Hormones and Animal Social Behavior. Princeton: Princeton University Press; 2005. [Google Scholar]
- 33.Duffy D, Bentley GE, Drazen DL, Ball GF. Effects of testosterone on cell-mediated and humoral immunity in non-breeding adult Eruopean starlings. Behav. Ecol. 2000;11:654–662. doi: 10.1093/beheco/11.6.654. [DOI] [Google Scholar]
- 34.Gao S, Sanchez C, Deviche PJ. Corticosterone rapidly suppresses innate immune activity in the house sparrow (Passer domesticus) J. Exp. Biol. 2017;220:322–327. doi: 10.1242/jeb.144378. [DOI] [PubMed] [Google Scholar]
- 35.Korpimäki E, Hakkarainen H, Bennett GF. Blood parasites and reproductive success of Tengmalm’n Owls: detrimental effects on females but Not on males? Funct. Ecol. 1993;7:420–426. doi: 10.2307/2390029. [DOI] [Google Scholar]
- 36.Végvári Z, et al. Sex-biased breeding dispersal is predicted by social environment in birds. Ecol. Evol. 2018;8:6483–6491. doi: 10.1002/ece3.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts ML, Buchanan KL, Evans MR. Testing the immunocompetence handicap hypothesis: a review of the evidence. Anim. Behav. 2004;68:227–239. doi: 10.1016/j.anbehav.2004.05.001. [DOI] [Google Scholar]
- 38.Foo YZ, Nakagawa S, Rhodes G, Simmons LW. The effects of sex hormones on immune function: a meta-analysis. Biol. Rev. 2017;92:551–571. doi: 10.1111/brv.12243. [DOI] [PubMed] [Google Scholar]
- 39.Peters A, Delhey K, Nakagawa S, Aulsebrook A, Verhulst S. Immunosenescence in wild animals: meta-analysis and outlook. Ecol. Lett. 2019;22:1709–1722. doi: 10.1111/ele.13343. [DOI] [PubMed] [Google Scholar]
- 40.Donald PF. Adult sex ratios in wild bird populations. Ibis. 2007;149:671–692. doi: 10.1111/j.1474-919X.2007.00724.x. [DOI] [Google Scholar]
- 41.Spalding M, Bancroft GT, Forrester DJ. The epizootiology of eustrongylidosis in wading birds (Ciconiiformes) in Florida. J. Wildl. Dis. 1993;29:237–249. doi: 10.7589/0090-3558-29.2.237. [DOI] [PubMed] [Google Scholar]
- 42.Robinson RA, et al. Emerging infectious disease leads to rapid population declines of common British birds. PLoS ONE. 2010;5:e12215. doi: 10.1371/journal.pone.0012215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martínez-de la Puente JM, et al. The blood parasite Haemoproteus reduces survival in a wild bird: a medication experiment. Biol. Lett. 2010;6:663–665. doi: 10.1098/rsbl.2010.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lachish S, Knowles SC, Alves R, Wood MJ, Sheldon BC. Infection dynamics of endemic malaria in a wild bird population: parasite species-dependent drivers of spatial and temporal variation in transmission rates. J. Anim. Ecol. 2011;80:1207–1216. doi: 10.1111/j.1365-2656.2011.01893.x. [DOI] [PubMed] [Google Scholar]
- 45.Dunn JC, et al. High rates of infection by blood parasites during the nestling phase in UK Columbids with notes on ecological associations. Parasitology. 2017;144:622–628. doi: 10.1017/S0031182016002274. [DOI] [PubMed] [Google Scholar]
- 46.Jovani R, Tella JL. Parasite prevalence and sample size: misconceptions and solutions. Trends Parasitol. 2006;22:214–218. doi: 10.1016/j.pt.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 47.Ellis VA, et al. Explaining prevalence, diversity and host specificity in a community of avian haemosporidian parasites. Oikos. 2020;129:1314–1329. doi: 10.1111/oik.07280. [DOI] [Google Scholar]
- 48.Maklakov AA, Lummaa V. Evolution of sex differences in lifespan and aging: causes and constraints. BioEssays. 2013;35:717–724. doi: 10.1002/bies.201300021. [DOI] [PubMed] [Google Scholar]
- 49.Immonen E, Hamalainen A, Schuett W, Tarka M. Evolution of sex-specific pace-of-life syndromes: genetic architecture and physiological mechanisms. Behav. Ecol. Sociobiol. 2018;72:60. doi: 10.1007/s00265-018-2462-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tarka M, Guenther A, Niemela PT, Nakagawa S, Noble DWA. Sex differences in life history, behavior, and physiology along a slow-fast continuum: a meta-analysis. Behav. Ecol. Sociobiol. 2018;72:132. doi: 10.1007/s00265-018-2534-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sæther B-E. Survival rates in relation to body weight in European birds. Ornis Scand. 1989;20:13–21. doi: 10.2307/3676702. [DOI] [Google Scholar]
- 52.Székely T, Liker A, Freckleton RP, Fichtel C, Kappeler PM. Sex-biased survival predicts adult sex ratio variation in wild birds. Proc. R. Soc. B. 2014;281:20140342. doi: 10.1098/rspb.2014.0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gusenbauer M, Haddaway NR. Which academic search systems are suitable for systematic reviews or meta-analyses? Evaluating retrieval qualities of Google Scholar, PubMed, and 26 other resources. Res. Synth. Method. 2020;11:181–217. doi: 10.1002/jrsm.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valkiūnas G, et al. A comparative analysis of microscopy and PCR-based detection methods for blood parasites. J. Parasitol. 2008;94:1395–1401. doi: 10.1645/GE-1570.1. [DOI] [PubMed] [Google Scholar]
- 55.Ishtiaq F, Rao M, Huang X, Bensch S. Estimating prevalence of avian haemosporidians in natural populations: a comparative study on screening protocols. Parasit. Vectors. 2017;10:127. doi: 10.1186/s13071-017-2066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.López G, Figuerola J, Soriguer R. Time of day, age and feeding habits influence coccidian oocyst shedding in wild passerines. Int. J. Parasitol. 2007;37:559–564. doi: 10.1016/j.ijpara.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 57.Liker A, Freckleton RP, Székely T. Divorce and infidelity are associated with skewed adult sex ratios in birds. Curr. Biol. 2014;24:880–884. doi: 10.1016/j.cub.2014.02.059. [DOI] [PubMed] [Google Scholar]
- 58.Piersma T. Do global patterns of habitat use and migration strategies co-evolve with relative investments in immunocompetence due to spatial variation in parasite pressure? Oikos. 1997;80:623–631. doi: 10.2307/3546640. [DOI] [Google Scholar]
- 59.Martínez-Abraín A, Esparza B, Oro D. Lack of blood parasites in bird species: Does absence of blood parasite vectors explain it all? Ardeola. 2004;51:225–232. [Google Scholar]
- 60.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010;36:1–48. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 61.Koricheva J, Gurevitch J, Mengersen K. Handbook of Meta-analysis in Ecology and Evolution. Princeton: Princeton University Press; 2013. [Google Scholar]
- 62.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sterne JA, Egger M. In: Publication Bias in Meta-analysis: Prevention, Assessment, and Adjustments. Rothstein HR, Sutton AJ, Borenstein M, editors. Hoboken: Wiley; 2005. pp. 99–110. [Google Scholar]
- 64.Viechtbauer W, Cheung MWL. Outlier and influence diagnostics for meta-analysis. Res. Synth. Method. 2010;1:112–125. doi: 10.1002/jrsm.11. [DOI] [PubMed] [Google Scholar]
- 65.Habeck CW, Schultz AK. Community-level impacts of white-tailed deer on understorey plants in North American forests: a meta-analysis. AoB Plants. 2015;7:119. doi: 10.1093/aobpla/plv119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jackson D, Turner R. Power analysis for random-effects meta-analysis. Res. Synth. Method. 2017;8:290–302. doi: 10.1002/jrsm.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Symonds MRE, Blomberg SP. In: Modern Phylogenetic Comparative Methods and Their Application in Evolutionary Biology: Concepts and Practice. Garamszegi LZ, editor. Berlin: Springer; 2014. pp. 105–130. [Google Scholar]
- 68.Molina-Venegas R, Rodriguez MA. Revisiting phylogenetic signal; strong or negligible impacts of polytomies and branch length information? BMC Evol. Biol. 2017;17:53. doi: 10.1186/s12862-017-0898-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Freckleton R, Harvey P, Pagel M. Phylogenetic analysis and comparative data: a test and review of evidence. Am. Nat. 2002;160:712–726. doi: 10.1086/343873. [DOI] [PubMed] [Google Scholar]
- 70.Warton DI, Hui FKC. The arcsine is asinine: the analysis of proportions in ecology. Ecology. 2011;92:3–10. doi: 10.1890/10-0340.1. [DOI] [PubMed] [Google Scholar]
- 71.Jetz W, Thomas GH, Joy JB, Hartmann K, Mooers AO. The global diversity of birds in space and time. Nature. 2012;491:444–448. doi: 10.1038/nature11631. [DOI] [PubMed] [Google Scholar]
- 72.Holder MT, Sukumaran J, Lewis PO. A justification for reporting the majority-rule consensus tree in Bayesian phylogenetics. Syst. Biol. 2008;57:814–821. doi: 10.1080/10635150802422308. [DOI] [PubMed] [Google Scholar]
- 73.Sukumaran J, Holder MT. DendroPy: a Python library for phylogenetic computing. Bioinformatics. 2010;26:1569–1571. doi: 10.1093/bioinformatics/btq228. [DOI] [PubMed] [Google Scholar]
- 74.Rubolini D, Liker A, Garamszegi LZ, Møller AP, Saino N. Using the BirdTree.org website to obtain robust phylogenies for avian comparative studies: a primer. Curr. Zool. 2015;61:959–965. doi: 10.1093/czoolo/61.6.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Paradis E, Claude J, Strimmer K. APE: analyses of phylogenetics and evolution in R language. Bioinformatics. 2004;20:289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- 76.Orme, D. et al. Caper: Comparative analyses of phylogenetics and evolution in R. R package version 0.5.2 (2013).
- 77.Lindenfors, P., Gittleman, J. L. & Jones, K. E. in Sex, Size and Gender Roles 16–26 (2007).
- 78.Ricklefs RE. Embryonic development period and the prevalence of avian blood parasites. Proc. Natl. Acad. Sci. USA. 1992;89:4722–4725. doi: 10.1073/pnas.89.10.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McCurdy DG, Shutler D, Mullie A, Forbes MR. Sex-biased parasitism of avian hosts: relations to blood parasite taxon and mating system. Oikos. 1998;82:303–321. doi: 10.2307/3546970. [DOI] [Google Scholar]
- 80.Scheuerlein A, Ricklefs RE. Prevalence of blood parasites in European passeriform birds. Proc. R. Soc. Lond. B. 2004;271:1363–1370. doi: 10.1098/rspb.2004.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boyd RJ, Kelly TR, MacDougall-Shackleton SA, MacDougall-Shackleton EA. Alternative reproductive strategies in white-throated sparrows are associated with differences in parasite load following experimental infection. Biol. Lett. 2018 doi: 10.1098/rsbl.2018.0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van Oers K, Richardson DS, Sæther SA, Komdeur J. Reduced blood parasite prevalence with age in the Seychelles Warbler: selective mortality or suppression of infection? J. Ornithol. 2010;151:69–77. doi: 10.1007/s10336-009-0427-x. [DOI] [Google Scholar]
- 83.Egizi AM, Farajollahi A, Fonseca DM. Diverse host feeding on nesting birds may limit early-season West Nile virus amplification. Vector Borne Zoonotic Dis. 2014;14:447–453. doi: 10.1089/vbz.2013.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schuurs AH, Verheul HA. Effects of gender and sex steroids on the immune response. J. Steroid Biochem. 1990;35:157–172. doi: 10.1016/0022-4731(90)90270-3. [DOI] [PubMed] [Google Scholar]
- 85.Mancini PL, Bond AL, Hobson KA, Duarte LS, Bugoni L. Foraging segregation in tropical and polar seabirds: testing the intersexual competition hypothesis. J. Exp. Mar. Biol. Ecol. 2013;449:186–193. doi: 10.1016/j.jembe.2013.09.011. [DOI] [Google Scholar]
- 86.Pereira JM, Paiva VH, Phillips RA, Xavier JC. The devil is in the detail: small-scale sexual segregation despite large-scale spatial overlap in the wandering albatross. Mar. Biol. 2018;165:55. doi: 10.1007/s00227-018-3316-0. [DOI] [Google Scholar]
- 87.Benito MM, González-Solis J. Sex ratio, sex-specific chick mortality and sexual size dimorphism in birds. J. Evol. Biol. 2007;20:1522–1530. doi: 10.1111/j.1420-9101.2007.01327.x. [DOI] [PubMed] [Google Scholar]
- 88.Møller A, Nielsen JT. Malaria and risk of predation: a comparative study of birds. Ecology. 2007;88:871–881. doi: 10.1890/06-0747. [DOI] [PubMed] [Google Scholar]
- 89.Podmokła E, et al. Effect of haemosporidian infections on host survival and recapture rate in the blue tit. J. Avian Biol. 2017;48:796–803. doi: 10.1111/jav.01108. [DOI] [Google Scholar]
- 90.Margolis L, Esch GW, Holmes JC, Kuris AM, Schad GA. The use of ecological terms in parasitology (report of an Ad Hoc Committee of the American Society of Parasitologists) J. Parasitol. 1982;68:131–133. doi: 10.2307/3281335. [DOI] [Google Scholar]
- 91.Krementsov DN, et al. Genetic variation in chromosome Y regulates susceptibility to influenza A virus infection. Proc. Natl. Acad. Sci. USA. 2017;114:3491. doi: 10.1073/pnas.1620889114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Eberhart-Phillips LJ, et al. Demographic causes of adult sex ratio variation and their consequences for parental cooperation. Nat. Commun. 2018;9:1651. doi: 10.1038/s41467-018-03833-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hewson CM, Thorup K, Pearce-Higgins JW, Atkinson PW. Population decline is linked to migration route in the Common Cuckoo. Nat. Commun. 2016;7:12296. doi: 10.1038/ncomms12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xenophontos M, Cresswell W. Survival and dispersal of the Cyprus wheatear Oenanthe cypriaca, an endemic migrant. J. Ornithol. 2016;157:707–719. doi: 10.1007/s10336-015-1315-1. [DOI] [Google Scholar]
- 95.Losdat S, et al. Additive genetic variance and effects of inbreeding, sex and age on heterophil to lymphocyte ratio in song sparrows. Funct. Ecol. 2015;30:1185–1195. doi: 10.1111/1365-2435.12586. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The full list of references consulted to extract the parasite data is given in the supplementary material. The dataset and R code can be accessed on 10.6084/m9.figshare.13232435.v1.