Abstract
Direct reprogramming/direct conversion/transdifferentiation is a process that induces conversion between completely different matured (differentiated) cells in higher organisms. Unlike the process of reprogramming of differentiated cells into induced pluripotent stem cells (iPSCs) and re-differentiation into the desired cell types, differentiated cells undergo the conversion into another type of differentiated cells without going through the iPSCs state. Osteoarthritis (OA) is the most common type of arthritis that causes a significant deterioration in patients’ quality of life. The high prevalence of OA as well as the current lack of disease-modifying drugs has led to a rise in regenerative strategy for OA treatment. Regenerative therapy in OA started with the concept of engraftment of the administered cells within the cartilage lesion and differentiation to chondrocytes after the engraftment. However, recent studies show that cells, particularly when injected in suspension, rapidly undergo apoptosis after exerting a transient paracrine effect. In this perspective review, the general overview and current status of direct conversion are introduced along with the conceptual strategy and future directions for possible application of regenerative therapy using stem cells in OA. In vivo direct conversion may open a new stage of regenerative medicine for OA treatment. Recent advances in in vivo gene transfer and smart biomaterials can bring the concept into reality in near future. Direct conversion can be a new type of treatment technology that has the potential to overcome the limitations of current cell therapy.
Keywords: Direct conversion, Osteoarthritis, Regenerative medicine, Chondrogenesis
Overview of direct conversion
Direct reprogramming/direct conversion/trans-differentiation is a process that induces conversion between completely different matured (differentiated) cells in higher organisms. Unlike the process of reprogramming of differentiated cells into induced pluripotent stem cells (iPSCs) and re-differentiation into the desired cell types, in this process, the differentiated cells undergo the conversion into another type of differentiated cells without going through the iPSCs state. Reprogramming refers to deletion of the original cell’s traits and development of new characteristics [1]. This concept was first established by Gurdon’s somatic cell nuclear transfer (SCNT) [2].
One major difference between direct converted cells and iPSCs is the presence of epigenetic memory from cell origin. In iPSCs, almost all the epigenetic markers from derived cells are erased. On the other hand, there exists a possibility of “not resetting” in cells obtained through direct conversion. Target cells are more easily converted into the cells of closely related lineage in direct conversion while there is no lineage-dependent disparity in differentiation potential in iPSCs. For possible clinical applications, direct conversion can be considered safer than iPSCs because directly converted cells are not pluripotent and this property would make them more advantageous for regenerative medicine [1, 3].
There are two broad categories of direct conversion methods. The traditional method is somatic cell-specific factor-mediated direct reprogramming (SDR). In this process, direct conversion is performed by over-expressing factors that are frequently expressed in the target cells. On the contrary, pluripotent cell-specific factor-mediated direct reprogramming (PDR) de-differentiates cells into incomplete pluripotent stem cell type, rather than true iPSCS, employing the factors (Oct4, Sox2, Klf4, and C-Myc) used for reprogramming iPSCs for direct conversion. While PDR can induce differentiation of stem cells into various tissue cells based on differentiation inducer, a risk of teratoma formation still exists. Although SDR does not pose a high risk of teratoma, discovering new tissue-specific factors is time-consuming and costly. In addition, a wide variety of transcription factors have been proposed by different authors for SDR [1].
Development of direct conversion methods
The concept of direct conversion was first introduced by Davis [4]. MyoD was successfully overexpressed in mouse embryonic fibroblasts to convert them into another cell type, myoblast, of the same lineage [4]. In 2010, Wernig group converted mouse fibroblasts into neurons by overexpressing Brn2, Ascl1, and Myt1l genes in fibroblasts and were named as iN cells [5]. In the same year, Srivastava group differentiated fibroblasts into cardiomyocyte-like cells by overexpression of Gata4, Mef2c, and Tbx5 genes [6]. In 2011, the Suzuki group overexpressed Hnf4α, Foxa1, Foxa2, and Foxa3 genes in mouse fibroblasts to induce direct conversion of the cells into hepatocyte-like cells [7]. In 2012, Jaenisch group induced direct conversion of fibroblasts into Sertoli-like cells with the combination of Nr5a1, Wt1, Dmrt1, Gata4, and Sox9 genes [8]. Ding group, using the four reprogramming Yamanaka factors, succeeded in inducing direct conversion of fibroblasts to myocardial and neuronal cells without going through iPSC state by changing the culture medium to a neural stem cell culture medium during the process [9]. In the same year, Scholer group induced direct conversion of mouse fibroblasts into proliferative neural stem cells through the transfer of genes Brn4, Sox2, Klf4, c-Myc, and E47 [10]. Wernig group succeeded in directly converting fibroblasts in mice into proliferative neural stem cells by overexpressing Sox2, Brn2, and FoxG1, suggesting the possibility of cell therapy for degenerative neurological diseases in the brain [11].
Current status of in vivo direct conversion
In vivo direct conversion may provide a simpler form of treatment by eliminating cell implantation, provided that the effectiveness and safety are guaranteed. The process was first started in the pancreas, an organ that has related progenitors among different constituent cell types. Later, this concept was introduced into the myocardium and nervous system [12–14]. Zhou et al. [15] in vivo differentiated exocrine pancreatic cells into insulin-secreting cells, using adenoviral transduction of three factors Ngn3, Pdx1, and Mafa among 20 or more transcription factors expressed in β cells of the pancreas.
Unlike the pancreas, myocardial and fibroblast cells of the heart come from different progenitor cells. In Qian et al. [13] reported the direct conversion of mouse fibroblasts into cardiomyocytes by gene transfer of GMT transcription factors (Gata4, Mef2c, and Tbx5) in vivo. Pulsation was observed in most of the cells, and the transcripts were more similar to those from intrinsic cardiomyocytes than seen in in vitro reprogramming. According to Song et al. [16] GHMT gene transfer with the addition of the Hand2 transcription factor enhances the efficiency of gene transfer in vivo and heart function. Direct differentiation of cells with special functions other than cardiomyocytes is also being studied. According to Kapoor, TBX18 gene transfer converts myocardial cells into cells with electrical signal transduction function [17].
In the nervous system, in vivo direct differentiation has been studied to convert glial cells into functional neurons using a single transcription factor rather than a combination of several transcription factors. Niu et al. [12] reported that SOX2 was sufficient to reprogram resident astrocytes into proliferative neuroblasts in the adult mouse brain. According to De la Rossa et al. [18] the transfer of Fezf2 transcription factor was used to convert glial cells to neurons with different functions.
Viral transduction remains the main method of in vivo direct conversion. Retrovirus [13, 16], lentivirus [12], adenovirus [15, 17], adeno-associated (AAV) virus [14] were used to transfer key transcription factors that induce direct conversion in vivo. On the other hand, De la Rossa et al. [18] used iontoporation to achieve in vivo rapid gene delivery into postmitotic neocortical neurons. When we apply direct conversion for nonlethal disease in musculoskeletal system in vivo, safety would matter most. In this sense, the use of AAV would be favored over other viral transduction methods. Also, the development of effective nonviral gene delivery method for in vivo direct conversion should be considered in the future research.
Needs for regenerative medicine for articular cartilage and osteoarthritis
Articular cartilage (AC), composed of chondrocytes and extracellular matrix (ECM), has the function of lubrication and shock absorption in the joints. Hyaline cartilage which constitutes AC lacks the capacity of self-renewal. Therefore, AC loss due to trauma or degeneration mostly leads to osteoarthritis. AC damage is sometimes healed with fibrocartilage, a type of scar tissue, which expresses type I and type II collagen, unlike hyaline cartilage that only expresses type II collagen. As type I collagen prevents the development of ECM and biomechanical properties specific to AC, the fibrocartilage cannot properly restore the function of the joint. Therefore, the goal of treating cartilage damage is to regenerate hyaline cartilage [19].
Transplantation of autologous chondrocytes and mesenchymal stem cells has been attempted to regenerate hyaline cartilage. With the former, it is difficult to obtain an adequate number of autologous chondrocytes. Moreover, when culture-expanded, the cells lose chondrocytic phenotypes and dedifferentiate into fibroblasts. With the latter, mesenchymal stem cells show a tendency to progress towards hypertrophy and differentiate into bone. It has recently been recognized that implanted MSCs rapidly undergo cell death inside the joint cavity [20].
Osteoarthritis (OA), also called degenerative arthritis or degenerative joint disease, is a quite common disease characterized by the loss of articular cartilage, resulting in joint pain and functional loss. OA deteriorates the quality of life in the involved patients. Joint injury in younger people leads to increased incidence of OA because once damaged, AC has little regeneration ability. Currently, the number of patients with OA is increasing due to the ageing of the world population. A great deal of medical resources is being spent on symptom-relieving medication and joint replacement surgery for OA. The cost is expected to increase further with an increase in average life expectancy. Therefore, to prevent the increase in medical expenses due to the progression of osteoarthritis, it is considered essential to change the paradigm of the existing treatment strategies to stopping the progression of OA before joint destruction occurs [21].
Current status of regenerative cell therapy for OA and need for improvement
Clinical studies of stem cells for OA treatment mostly used mesenchymal stem cells (MSCs) isolated from bone marrow. Clinical improvements have been reported after single MSC injection in small patient series [22–25]. Also, the results of two repeated MSCs injection were reported. No severe adverse events encountered during 24 months of follow-up, and functional scores improved significantly. Mean knee cartilage thickness measured by MRI also improved significantly after 12 months [26]. Clinical application of allogeneic MSCs were also reported. In a randomized, double-blind, controlled study, 5 to 15 × 107 allogeneic MSCs were injected 7 to 10 days after meniscectomy. Patients experienced significant reductions in pain as compared with controls [27]. In a controlled multicenter study, 30 patients with chronic knee OA unresponsive to conservative treatments were randomized into 2 groups of 15 patients. The test group was treated with IA allogeneic MSCs and hyaluronic acid (HA). Injection group displayed significant improvements in algofunctional indices as compared with active controls treated with HA alone. Cartilage quality from MRI using T2 relaxation showed improvements in MSC-treated patients after 12 months [28]. On the other hand, in another larger study, allogeneic IA MSCs (Stempeucel®) were tested in 60 OA patients randomized to receive different doses of cells (25, 50, 75, or 150 million cells) or placebo. Whole-organ MRI scores of knees did not reveal any differences in score between the placebo group and the experimental groups or between the experimental groups and baseline after 12 months. Overall, IA MSCs did not overall show clinically significant improvement in pain, function or structure [29].
Stem cell-based and chondrocyte-based cell therapeutics to treat OA have been introduced into the Korean market. Cartistem®, developed using cord blood-derived stem cells mixed with hyaluronic acid, has been indicated for advanced late-stage OA in Korea. Open surgery is necessary for application of the cell therapeutics. While engraftment of implanted cells was first expected in the healing of damaged AC, the therapeutic effect in structural improvement was found to be of paracrine action [30]. Invossa®, an allogeneic TGF-β gene-transferred chondrocytes that has been developed in Korea was recently suspended in the market due to the issue of cell line contamination. While this cell therapeutics was originally designed for direct cartilage tissue regeneration by engraftment, it has been reported that the cells disappeared within 2 weeks after injection. The improvement in clinical symptoms including pain relief and suppression of inflammation was also attributed to the secretory effect [31]. However, the expected effects on cartilage regeneration and structural improvement were not observed. These results bring home a new technology that can answer the unmet clinical needs in OA that the therapeutics developed so far failed to achieve.
Direct conversion for cartilage regeneration and OA treatment
So far, from the result of through extensive research, factors that induce chondrogenic differentiation include transcription factors Sox5, Sox6, Sox9, Nkx3.2 and growth factors BMPs and TGFβs. Overexpression of Sox5, Sox6 and Sox9 or treatment of TGF-β differentiates fibroblasts into chondrocyte-like cells [32]. Our group has tried to transform chondrocytes by transferring the key transcription factor of cartilage differentiation to various types of somatic cells [33, 34]. This strategy can function as a source technology to induce tissue regeneration at low cost while significantly replacing the need for existing cell therapy. While these gene transfer of chondrogenic genes transform non-chondrogenic cells into chondrocyte-like cells, it is not expected that their transformed characteristics are transmitted to their progeny cell because their epigenetic signatures are not modified with transfection of chondrogenic genes.
Tsukmaki group reported the generation of induced chondrocytes (iChon) from mouse fibroblasts using two reprogramming factors (c-Myc and Klf4) and one cartilage forming factor (Sox9) using retrovital transduction. Induced cells expressed marker genes of chondrocytes, not those of fibroblasts. The promoter regions of collagen type I genes were extensively methylated. Although some induced cell lines formed tumors when subcutaneously injected into nude mice, other induced cell lines generated stable homogenous hyaline cartilage–like tissue. Also, induced cells responded to chondrogenic medium by expressing endogenous Sox9 and maintain chondrogenic potential after substantial reduction of transgene expression [35].
The group also generated induced chondrogenic (iChon) cells from human dermal fibroblast culture with the same factors as those used in mice using retroviral transduction. They also developed chondrocyte-specific COL11A2 promoter/enhancer lentiviral reporter vector to select iChon cells. The human iChon cells expressed marker genes for chondrocytes. As iChon cells did not express Nanog, a marker of pluripotent cells, these cells would not theoretically cause teratoma [36].
Ishii et al. identified a combination of only five genes BCL6, T (also called brachyury), c-myc, MITF, and BAF60C (also called SMARCD3) that rapidly and efficiently convert postnatal human chorion and decidual cells into chondrocytes. The cells thus generated expressed cartilage-specific genes collagen type II α1, link protein-1, and aggrecan, and exhibited characteristics of cartilage both in vivo and in vitro. Expression of the endogenous genes for T and MITF was initiated, implying that the cell conversion is due to not only the forced expression of the transgenes, but also to cellular reprogramming by the transgenes [37].
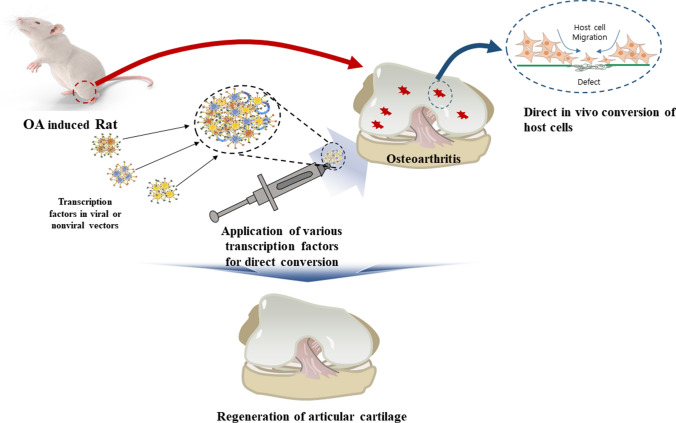
While in vivo direct conversion for the treatment of OA has not been reported until date, the technique is likely to be materialized in near future with recent advancements in in vivo gene transfer and smart biomaterials (Fig. 1). In vivo direct conversion can be applied to rejuvenate diseased chondrocytes that have lost chondrocytes phenotypes or to convert synovial or bone marrow-derived stem cells into chondrocytes when used in combination with microfracture.
Fig. 1.
Concept of direct conversion to regenerate articular cartilage in surgically-induced osteoarthritis model in rats
In vivo direct conversion will be a relatively non-invasive method for cartilage regeneration because converting vectors can be applied into the OA lesion by injection or by arthroscopic placement. On the other hand, as multiple types of cells exist in knee joint, the possibility and efficiency of direct conversion to chondrocyte would vary depending on the cell type that is induced to differentiate into chondrocyte. In addition, given that the efficiency of direct conversion is known to be generally lower than differentiation of iPSCs, the success of application will depend on raising the conversion efficiency enough to lead to clinical detectable differences.
Conclusion and perspective
When cell therapy was first reported to treat cartilage defects and osteoarthritis, an optimistic view prevailed which held that implanted cells could be incorporated into defects and regenerate AC. The focus was on how to ensure that the implanted stem cells possess all the properties of articular chondrocytes. However, it became evident that almost all intra-articularly administered stem cells rapidly undergo apoptosis, and that their principal mode of action is paracrine. With early cell death, durable structural modification of diseased joint may not be achieved as originally intended with cell therapy.
Given these dilemmas with cell therapy for OA treatment, it is likely that in vivo direct conversion become a new type of treatment technology which overcomes the limitations of the currently developed regenerative therapies for OA. Direct conversion can be achieved in a shorter time than reprogramming, which is particularly useful for tissue engineering regenerative medicine as the cost required for development as a therapeutic agent is reduced. When in vivo application is realized, it can ultimately replace cell therapy. Direct conversion transforms cells in vivo without the need for cell transplantation, thereby avoiding problems with the immune response. Unlike cell therapy, off-the-shelf storage of vector is possible with a significant decrease in the treatment costs. Therefore, in vivo direct conversion may open a new stage of regenerative medicine for OA treatment. Recent advances in in vivo gene transfer and smart biomaterials can bring the concept into reality in near future.
Acknowledgements
Funding was provided by Korea Health Industry Development Institute (Grant No. HI20C0090) and National research Foundation of Korea (Grant No. NRF-2020R1A2C2008266).
Compliance with ethical standards
Conflict of interest
All the authors confirmed that, there was no conflict of interest regarding publication of this manuscript.
Ethical statement
There are no animal experiments carried out for this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Xu J, Du Y, Deng H. Direct lineage reprogramming: strategies, mechanisms, and applications. Cell Stem Cell. 2015;16:119–134. doi: 10.1016/j.stem.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Gurdon JB, Elsdale TR, Fischberg M. Sexually mature individuals of Xenopus laevis from the transplantation of single somatic nuclei. Nature. 1958;182:64–65. doi: 10.1038/182064a0. [DOI] [PubMed] [Google Scholar]
- 3.Papp B, Plath K. Epigenetics of reprogramming to induced pluripotency. Cell. 2013;152:1324–1343. doi: 10.1016/j.cell.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-X. [DOI] [PubMed] [Google Scholar]
- 5.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–41. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sekiya S, Suzuki A. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature. 2011;475:390–393. doi: 10.1038/nature10263. [DOI] [PubMed] [Google Scholar]
- 8.Buganim Y, Itskovich E, Hu YC, Cheng AW, Ganz K, Sarkar S, et al. Direct reprogramming of fibroblasts into embryonic Sertoli-like cells by defined factors. Cell Stem Cell. 2012;11:373–386. doi: 10.1016/j.stem.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Efe JA, Hilcove S, Kim J, Zhou H, Ouyang K, Wang G, et al. Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nat Cell Biol. 2011;13:215–22. [DOI] [PubMed]
- 10.Han DW, Tapia N, Hermann A, Hemmer K, Höing S, Araúzo-Bravo MJ, et al. Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell. 2012;10:465–472. doi: 10.1016/j.stem.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 11.Yang N, Zuchero JB, Ahlenius H, Marro S, Ng YH, Vierbuchen T, et al. Generation of oligodendroglial cells by direct lineage conversion. Nat Biotechnol. 2013;31:434–439. doi: 10.1038/nbt.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niu W, Zang T, Zou Y, Fang S, Smith DK, Bachoo R, et al. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nat Cell Biol. 2013;15:1164–1175. doi: 10.1038/ncb2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593–598. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torper O, Ottosson DR, Pereira M, Lau S, Cardoso T, Grealish S, et al. In vivo reprogramming of striatal NG2 glia into functional neurons that integrate into local host circuitry. Cell Rep. 2015;12:474–481. doi: 10.1016/j.celrep.2015.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song K, Nam YJ, Luo X, Qi X, Tan W, Huang GN, et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485:599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapoor N, Liang W, Marbán E, Cho HC. Direct conversion of quiescent cardiomyocytes to pacemaker cells by expression of Tbx18. Nat Biotechnol. 2013;31:54–62. doi: 10.1038/nbt.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De la Rossa A, Bellone C, Golding B, Vitali I, Moss J, Toni N, et al. In vivo reprogramming of circuit connectivity in postmitotic neocortical neurons. Nat Neurosci. 2013;16:193–200. doi: 10.1038/nn.3299. [DOI] [PubMed] [Google Scholar]
- 19.Ulrich-Vinther M, Maloney MD, Schwarz EM, Rosier R, O’Keefe RJ. Articular cartilage biology. J Am Acad Orthop Surg. 2003;11:421–430. doi: 10.5435/00124635-200311000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Im GI. Perspective on intra-articular injection cell therapy for osteoarthritis treatment. Tissue Eng Regen Med. 2019;16:357–363. doi: 10.1007/s13770-018-00176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park YB, Ha CW, Lee CH, Yoon YC, Park YG. Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: results from a clinical trial for safety and proof-of-concept with 7 years of extended follow-up. Stem Cells Transl Med. 2017;6:613–621. doi: 10.5966/sctm.2016-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emadedin M, Aghdami N, Taghiyar L, Fazeli R, Moghadasali R, Jahangir S, et al. Intra-articular injection of autologous mesenchymal stem cells in six patients with knee osteoarthritis. Arch Iran Med. 2012;15:422–428. [PubMed] [Google Scholar]
- 23.Davatchi F, Abdollahi BS, Mohyeddin M, Shahram F, Nikbin B. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int J Rheum Dis. 2011;14:211–215. doi: 10.1111/j.1756-185X.2011.01599.x. [DOI] [PubMed] [Google Scholar]
- 24.Davatchi F, Sadeghi Abdollahi B, Mohyeddin M, Nikbin B. Mesenchymal stem cell therapy for knee osteoarthritis: 5 years follow-up of three patients. Int J Rheum Dis. 2016;19:219–225. doi: 10.1111/1756-185X.12670. [DOI] [PubMed] [Google Scholar]
- 25.Centeno CJ, Busse D, Kisiday J, Keohan C, Freeman M, Karli D. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician. 2008;11:343–353. [PubMed] [Google Scholar]
- 26.Al-Najar M, Khalil H, Al-Ajlouni J, Al-Antary E, Hamdan M, Rahmeh R, et al. Intra-articular injection of expanded autologous bone marrow mesenchymal cells in moderate and severe knee osteoarthritis is safe: a phase I/II study. J Orthop Surg Res. 2017;12:190. doi: 10.1186/s13018-017-0689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vangsness CT, Jr, Farr J, 2nd, Boyd J, Dellaero DT, Mills CR, LeRoux-Williams M. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy: a randomized, double-blind, controlled study. J Bone Joint Surg Am. 2014;96:90–98. doi: 10.2106/JBJS.M.00058. [DOI] [PubMed] [Google Scholar]
- 28.Vega A, Martín-Ferrero MA, Del Canto F, Alberca M, García V, Munar A, et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation. 2015;99:1681–1690. doi: 10.1097/TP.0000000000000678. [DOI] [PubMed] [Google Scholar]
- 29.Gupta PK, Chullikana A, Rengasamy M, Shetty N, Pandey V, Agarwal V, et al. Efficacy and safety of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells (Stempeucel®): preclinical and clinical trial in osteoarthritis of the knee joint. Arthritis Res Ther. 2016;18:301. doi: 10.1186/s13075-016-1195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ha CW, Cho JJ, Elmallah RK, Cherian JJ, Kim TW, Lee MC, et al. A multicenter, single-blind, Phase IIa clinical trial to evaluate the efficacy and safety of a cell-mediated gene therapy in degenerative knee arthritis patients. Hum Gene Ther Clin Dev. 2015;26:125–130. doi: 10.1089/humc.2014.145. [DOI] [PubMed] [Google Scholar]
- 31.Johnstone B, Stoddart MJ, Im GI. Multi-disciplinary approaches for cell-based cartilage regeneration. J Orthop Res. 2020;38:463–472. doi: 10.1002/jor.24458. [DOI] [PubMed] [Google Scholar]
- 32.Ikeda T, Kamekura S, Mabuchi A, Kou I, Seki S, Takato T, et al. The combination of SOX5, SOX6 and SOX9 (the SOX trio) provides signals sufficient for induction of permanent cartilage. Arthritis Rheum. 2004;50:3561–3573. doi: 10.1002/art.20611. [DOI] [PubMed] [Google Scholar]
- 33.Lee JM, Im GI. SOX trio-co-transduced adipose stem cells in fibrin gel to enhance cartilage repair and delay the progression of osteoarthritis in the rat. Biomaterials. 2012;33:2016–2024. doi: 10.1016/j.biomaterials.2011.11.050. [DOI] [PubMed] [Google Scholar]
- 34.Kim HJ, Im GI. Electroporation-mediated transfer of SOX trio genes (SOX-5, SOX-6, and SOX-9) to enhance the chondrogenesis of mesenchymal stem cells. Stem Cells Dev. 2011;20:2103–2114. doi: 10.1089/scd.2010.0516. [DOI] [PubMed] [Google Scholar]
- 35.Hiramatsu K, Yoshikawa H, Tsumaki N. Generation of hyaline cartilaginous tissue from mouse adult dermal fibroblast culture by defined factors. J Clin Invest. 2011;121:640–657. doi: 10.1172/JCI44605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Outani H, Okada M, Yamashita A, Nakagawa K, Yoshikawa H, Tsmaki N. Direct induction of chondrogenic cells from human dermal fibroblast culture by defined factors. PLoS One. 2013;8:e77365. doi: 10.1371/journal.pone.0077365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishii R, Kami D, Toyoda M, Makino H, Gojo S, Ishii T, et al. Placenta to cartilage: direct conversion of human placenta to chondrocytes with transformation by defined factors. Mol Biol Cell. 2012;23:3511–3521. doi: 10.1091/mbc.e11-10-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]