Abstract
Background:
Development of valvular substitutes meeting the performance criteria for surgical correction of congenital heart malformations is a major research challenge. The sheep is probably the most widely used animal model in heart valves regenerative medicine. Although the standard cardiopulmonary bypass (CPB) technique and various anesthetic and surgical protocols are reported to be feasible and safe, they are associated with significant morbidity and mortality rates. The premise of this paper is that the surgical technique itself, especially the perioperative animal care and management protocol, is essential for successful outcomes and survival.
Methods:
Ten juvenile and adult female sheep aged 7.8–37.5 months and weighing 32.0–58.0 kg underwent orthotopic implantation of tissue-engineered pulmonary valve conduits on beating heart under normothermic CPB. The animals were followed-up for 6 months before scheduled euthanasia.
Results:
Based on our observations, we established a guide for perioperative care, follow-up, and treatment containing information regarding the appropriate clinical, biological, and ultrasound examinations and recommendations for feasible and safe anesthetic, surgical, and euthanasia protocols. Specific recommendations were also included for perioperative care of juvenile versus adult sheep.
Conclusion:
The described surgical technique was feasible, with a low mortality rate and minimal surgical complications. The proposed anesthetic protocol was safe and effective, ensuring both adequate sedation and analgesia as well as rapid recovery from anesthesia without significant complications. The established guide for postoperative care, follow-up and treatment in sheep after open-heart surgery may help other research teams working in the field of heart valves tissue regeneration.
Keywords: Pulmonary valve, Sheep, Cardiopulmonary bypass, Tissue engineering, Heart valves
Introduction
Current research in heart valves tissue engineering is oriented toward development of ideal valvular substitutes that can meet the performance criteria for surgical correction of congenital heart malformations: low risk of thromboembolic events, degeneration, or calcification and the ability to grow with the somatic growth of the child. Although various types of mechanical or biological prostheses have been proposed and evaluated on different animal models, they have yielded variable results in terms of performance, safety, and in vivo hemodynamics. In attempts to identify the ideal animal model, these candidates have been tested on sheep, cattle, dogs, swine, and primates [1]. The pros and cons for each species have been reported, starting with reduced availability of the species, difficulties in animal handling (weight, large size, rapid growth rate), up to anatomical and physiological differences in comparison with humans (short ascending aorta, such as in calves, sheep, and goats), and greater predisposition to infections or thrombosis (e.g. dogs), etc. [2].
Given their similarities with human anatomy and cardiac physiology, sheep are probably the most widely used animal model in regenerative medicine, especially in the cardiovascular field. In comparison with other species, sheep appear to have almost similar blood pressure, heart rate, cardiac output, and intra-cardiac pressure as humans [3]. Although a standard CPB technique as well as various anesthetic and surgical protocols are considered to be feasible and safe for this animal [1], the reported morbidity and mortality rates were significant [4].
This study is part of a project conducted at the Tissue Engineering and Regenerative Medicine Laboratory “TERMLab” within the University of Medicine, Pharmacy, Science and Technology (UMFST) “George Emil Palade” of Tirgu Mures. We started with the hypothesis that an ideal valvular substitute could be manufactured from acellular valvular roots seeded with differentiated autologous stem cells. In the previous stage of the project, decellularized porcine pulmonary valve roots or roots seeded with adipose-derived stem cells (ADSCs) were extra-anatomically implanted into the pulmonary position as shunt RV-PA (right ventricle—pulmonary artery) in sheep [5]. The project continued with the aim of developing new biomaterials and technologies that can be integrated and transposed into clinical practice in heart surgery. In Romania, the orthotopic implantation of acellular valve conduit in the pulmonary position on beating heart in sheep under cardiopulmonary bypass (CPB) performed by our research group, was the first such attempt in the country.
The aim of this study was to establish and standardize several protocols for open-heart surgery on beating heart under CPB by using the sheep as a large animal model: 1) a guide for preoperative care and the anesthetic protocol, describing the surgical technique for acellular pulmonary valve conduit implantation in the orthotopic position, 2) a guide for postoperative care, follow-up, and treatment in sheep, 3) protocols for transesophageal echocardiography (TEE) for intraoperative evaluation of the native pulmonary valve and cardiac function as well as epicardial echocardiography (ECE) for implanted valve evaluation, 4) transthoracic echocardiography (TTE) for postoperative evaluation of the implanted valve conduits and cardiac performance, at predefined time intervals, and 5) establishment of the euthanasia protocol.
Materials and methods
All procedures and perioperative care of the experimental animals were performed in accordance with the “Guide for the care and use of laboratory animals” and Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes. Euthanasia was performed in compliance with the Convention on the experiments on live vertebrate animals and all the legal norms referring to the protection and welfare of animals: Law No. 9 of January 11, 2008 on the animals’ protection and Decision no. 19 of 01. July 2011 adopted by the National Council of the Veterinary Doctors College regarding the “Guide for the euthanasia of animals.” This work is part of a research grant conducted in accordance with the protocol no. 131/21.10.2016 approved by the Ethics Committee of the UMFST “George Emil Palade” of Tirgu Mures.
Biological scaffold preparation
Eleven pulmonary valves were carefully dissected and isolated from fresh ovine hearts provided by a local abattoir. The valves were subjected to a perfusion decellularization protocol described in our previous work on porcine pulmonary valves. Randomly chosen valves were used for a microbiological sterility assay and the efficiency of the decellularization process was assessed by DAPI staining and DNA extraction [6].
Animal selection
Ten female sheep (“Tigaie metis” breed) aged 7.8–37.5 months and weighing 32.0–58.0 kg were used. Animals were followed-up for 6 months before the planned euthanasia date. Animals that showed cardiorespiratory decompensation or other life-threatening conditions during the follow-up period were sacrificed before the scheduled date.
The protocols described in this study were established after a detailed review of data available in the human cardiovascular field and veterinary medicine, and from our experience acquired in the previous stage of the project.
Results
Preoperative care
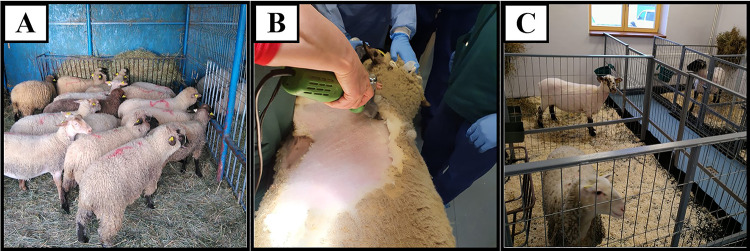
At the outdoor facilities (Fig. 1A), the sheep were trimmed and washed, and then transferred to an isolation box into the indoor facility (Fig. 1C), but in the same room with other sheep. Twenty-four hours before the surgery, the skin was cleaned with antiseptic and shaved in the area where the surgical procedure was to be conducted (Fig. 1B). Solid foods were restricted 24 h before the surgery, and liquid intake was stopped 8–12 h before the procedure. The animal was weighed and clinically examined by heart auscultation to exclude possible rhythm disturbances and/or pathological cardiac murmurs and bilateral auscultation of the lungs to exclude any pathological crackles. Animals with signs of contamination or pulmonary, digestive, and other dysfunctions were treated before undergoing the procedure. In order to establish the appropriate anesthetic algorithm, the laboratory parameters (blood gas, serum electrolytes, complete blood count [CBC], biochemical profile, coagulation tests) were evaluated, and any possible imbalances were corrected.
Fig. 1.
A Experimental animals in the outdoor facilities; B animal preparation before moving into the indoor facility; C indoor facility, before the surgery day
Anesthetic protocol
Pre-anesthetic sedation of the animal
In order to facilitate the animal’s handling and its transfer to the operating room, 30 min before the operation, the sheep was sedated with 0.015 mg/kg body weight (bw) detomidine or medetomidine hydrochloride intramuscularly (i.m.) and 0.01–0.02 mg/kg.bw atropine subcutaneously (s.c.) (Fig. 2A). Prior to anesthetic induction, it was positioned on the operating table in right lateral recumbency and secured with harnesses and straps. Vital parameters were monitored as follows: heartbeat rate and heart rhythm by three-lead electrocardiography (ECG); oxygen saturation (SpO2) using a lingual pulse oximeter (Fig. 2E-1); non-invasive blood pressure (BP) by placing a cuff on the forelimb at the brachial artery level.
Fig. 2.

A Preoperative i.m. detomidine administration; B ventilation on face mask; C peripheral venous catheter placement; D OTI cannula connected to the mechanical ventilation circuit; E-1 lingual pulse oximeter; E-2 esophageal thermometer; E-3. orogastric tube; F. the anesthesia and mechanical ventilation machine
Anesthetic induction: The animal was administered 1.0 mg/kg.bw propofol, 1.0 mg/kg.bw ketamine, 0.02 mg/kg.bw atropine, and 0.5 mg/kg.bw atracurium intravenously (i.v.) administered through a peripheral venous catheter placed on the left forelimb (Fig. 2C). During anesthetic induction, the animal was monitored and ventilated with a bag valve mask with 100% oxygen (Fig. 2B).
Orotracheal intubation (OTI): The ventilation mask was removed and salivary secretions were aspirated. A laryngoscope with a straight blade of at least 5 cm in size and a 125° handle blade opening was used. After vocal cord visualization, 1–2 puffs of 1% lidocaine were administered and a cuffed endotracheal tube (ETT) was inserted until the cuff passed 2–3 cm beyond the vocal cords. The ETT was then connected to the ventilation circuit (Fig. 2D). To exclude esophageal intubation, end-tidal CO2 (EtCO2) was checked and the animal was examined by auscultation. ETTs of different sizes were used depending on the animal’s body weight: an ETT with an internal diameter of 7–10 mm for animals weighing < 30 kg and one with an internal diameter of 8–12 mm for animals weighing > 30 kg.
Mechanical ventilation: The sheep was ventilated for a tidal volume (Vt) of 5–10 mL/kg.bw (maximum, 15 mL/kg.bw) and respiratory frequency of 12–20 breaths/minute to maintain pCO2 within the limit of 30–40 mmHg. In order to avoid barotrauma and hypoxemia, the animal was ventilated under pressure (pressure mode). A maximum ventilation pressure of 30 mmHg and PEEP of 5–10 cm H2O were maintained (Fig. 2F).
Orogastric tube placement
A large Faucher tube was inserted through the buccal cavity up to the rumen level and connected to a collection bag (Fig. 2E-2) to minimize ruminal tympany and free-gas bloat during the procedure. The esophageal thermometer was inserted through the nasal cavity (Fig. 2E-3).
Central venous line placement
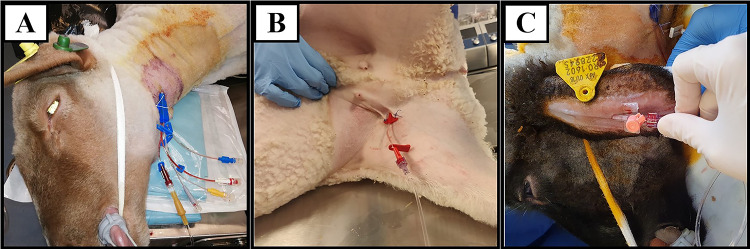
Under sterile conditions, a central venous catheter (CVC) of 7.5–8.5 Fr and 20 cm length with three or four lumens was inserted into the external jugular vein (Fig. 3A). A line was used to monitor the central venous pressure (CVP), while the other two free lumens were used for intravenous infusions.
Fig. 3.
A Central venous catheter placed in the left external jugular vein; B arterial catheter placed in the right femoral artery for intraoperative invasive BP measurement; C arterial catheter placed in the left auricular artery
Arterial line placement
A 20 G arterial catheter was placed at the level of the right femoral artery (Fig. 3B) or left auricular artery (Fig. 3C) (depending on the animal’s size) and connected to a BP transducer. The arterial line was used for invasive, continuous monitoring of BP and regular arterial blood sampling.
Maintenance of anesthesia
During the surgery, anesthesia was maintained by 1–2.5% sevoflurane in 50% oxygen (1 MAC = 2.3%), using a standard disposable circuit. In addition, 0.5 mg/kg.bw ketamine every 15–25 min and 10.0 mg atracurium every 30 min were used as adjuvants for maintaining anesthesia.
Intra-anesthetic monitoring
During anesthesia, vital functions were continuously monitored and controlled in order to maintain homeostasis: maintenance of sinus rhythm, heart rate within ± 20% of the baseline value (70–80 beats/minute in sheep [7]); mean BP of at least 60 mmHg; CVP of 8–15 cmH2O; SpO2 over 90%; CO2 below 55 mmHg; and constant body temperature (normal rectal temperature in sheep is 38.5°-40.0 °C [8]; average, 39.0 °C). During the surgery, the animal’s eyes were covered with ophthalmic ointment or wet pads.
Surgical protocol
Under general anesthesia, a 10 cm incision was performed, followed by left lateral thoracotomy (LLT) in the 3rd/4th intercostal space, depending on the animal’s weight. We observed that in larger animals weighing more than 45–50 kg, the pulmonary trunk is more accessible through the fourth intercostal space. After a rib spreader (Finochietto retractor) was placed, the left internal mammary artery and vein were highlighted and eventually ligated when necessary. For pericardium exposure, the left middle pulmonary lobe was laterally dislocated and a “reversed T”-shaped pericardiotomy was performed avoiding phrenic nerve injury, thus exposing the right side of the heart (Fig. 4A). Systemic heparinization was performed with 250 IU/kg.bw heparin, the dose being supplemented as needed, for an activated clotting time (ACT) of at least 400 s. The pulmonary trunk was dissected and mobilized, ensuring optimal access to the ascending aorta.
Fig. 4.
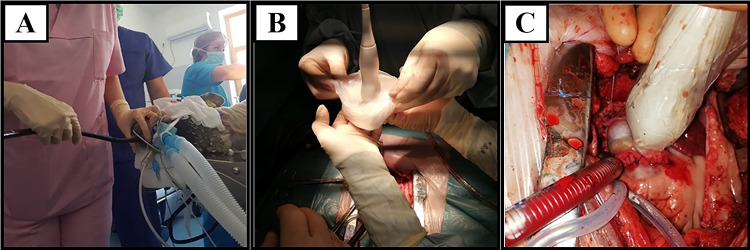
A Exposure of the right side of the heart after pericardiotomy; B placement of the aortic purse string at the level of the ascending aorta; C in situ pulmonary artery with arterial and venous cannulas introduced to initiate the CPB; D termino-terminal anastomosis of the scaffold’s ventricular portion to the sinotubular junction of the pulmonary trunk; E termino-terminal anastomosis of the scaffold’s distal portion to the pulmonary trunk; F final appearance of the pulmonary valve conduit implanted in orthotopic position
Cardiopulmonary bypass
Because of the increased tissue friability in older animals, including the aortic wall tissue, a different arterial cannulation technique was performed: a triple purse string suture using 4.0 Prolene reinforced with Teflon patches on the whole circumference of the purse string (Fig. 4B). Considering the extremely short length of the ascending aorta in sheep (approximately 2 cm), the triple purse string was placed immediately below the brachiocephalic trunk emergency, on the left antero-lateral side of the aorta. For venous cannulation, a 2.0 Ticron suture purse string was placed at the level of the right atrial appendage. Using a sharp no. 11 scalpel blade, a breach was created in the center of the purse string, through which an angled arterial cannula of 16–18 Fr was inserted, with the peak oriented toward the aortic arch. The arterial cannula was connected to an arterial line, with the pressure being checked in order to exclude iatrogenic dissection of the aorta. A “two-stage” venous cannula of 26–30 Fr. was inserted through the right atrial appendage (Fig. 4C).
Before CPB establishment, the decellularized pulmonary valve conduit was prepared by dissecting its proximal and distal portions to obtain a suitable length. Primer solution (750–1200 mL) was introduced and partial CPB was established, with anesthesia being maintained with 1%-2% sevoflurane administered directly into the circuit. During the surgery, pulmonary mechanical ventilation with reduced volumes was maintained in order to prevent postoperative pulmonary atelectasis. After establishing partial CPB, transverse pulmonary arteriotomy was performed at the level of the sinotubular junction, distal to the pulmonary sinus. The native pulmonary valve was identified and excised. The decellularized pulmonary valve conduit was implanted by termino-terminal anastomoses at the proximal level of the pulmonary trunk (Fig. 4D-proximal anastomosis, Fig. 4E-distal anastomosis), with 5.0 monofilament running suture reinforced with Teflon strips (Fig. 4F).
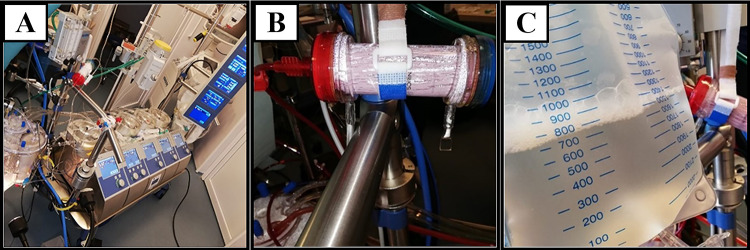
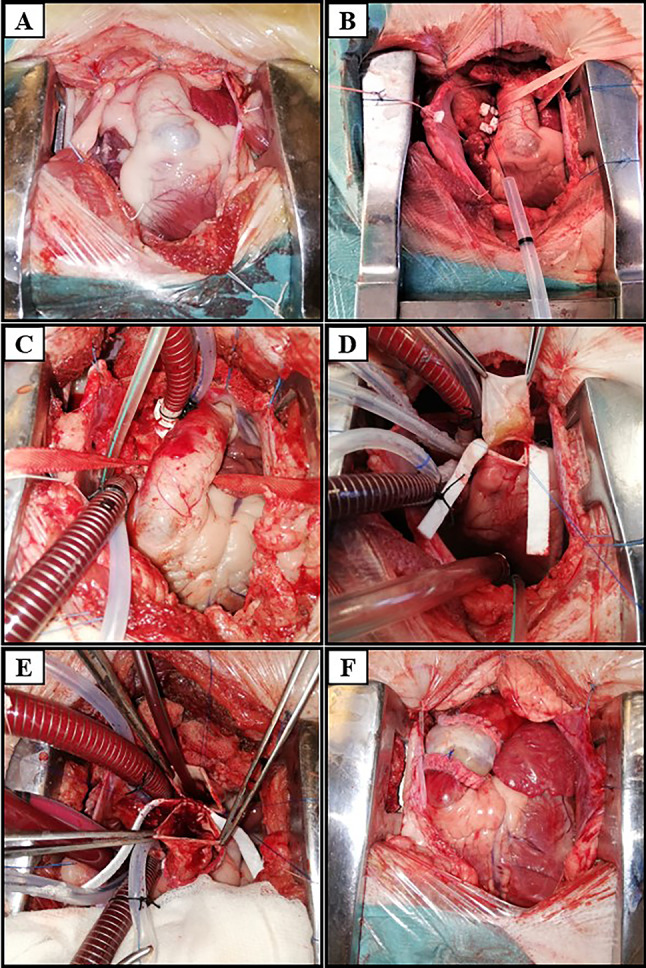
Serum electrolytes, arterial blood gas, and acid–base status were monitored intraoperatively. After ensuring hemodynamic stability and anastomosis tightness, the sheep was gradually weaned off CPB; in some cases, inotropic drug support was required. Prior to cannula removal, “modified ultrafiltration” (MUF) was performed. A specific amount of blood (approx. 10–20% of cardiac output) was actively extracted from the arterial line and passed through a special hemofilter (Hemofiltration DHFO.2 System, Sorin Group, Italy) mounted on a separate module in the extracorporeal circulation machine (Fig. 5A, B). Filtered and concentrated blood was returned into the animal’s body through the venous line, and excess fluid was drained into a collection bag (Fig. 5C).
Fig. 5.
A Extracorporeal circulation machine; B MUF module; C collection bag with excess fluid collected along with inflammatory factors and metabolites, filtered through MUF
Given the aortic wall’s friability, which is mainly attributable to a lack of elasticity in older animals, when sealing the aortic purse string, the suture can cut the arterial wall. In order to avoid major blood loss, the arterial cannula was suppressed before the venous one. Thus, the blood lost during aortic cannula suppression can be sucked into the cardiotomy reservoir, allowing its gradual reintroduction into the animal’s body through the arterial line connected to the venous cannula (a technique developed by our team). After weaning off CPB, the effects of heparin were reversed by protamine administered at a dose of 1:1.3 of the initial dose of heparin. After controlling all major sources of bleeding, a thoracic drainage tube was placed, followed by costal adduction with isolated sutures, wound closure, skin antiseptic treatment, and bandage.
Awakening/reversal from anesthesia
The last dose of intravenous anesthetic and neuromuscular blocking agent were given at least 30 min before the end of surgery. Sevoflurane concentration was reduced and maintained to 1% until skin suture, and then stopped. To ensure postoperative pain control, i.v. analgesic and anti-inflammatory drugs were given: 1000.0 mg metamizole, 30.0 mg ketorolac, 100.0 mg tramadol, and 0.3 mg/kg.bw dexamethasone. For awakening, the animal was ventilated with 100% O2, with the ventilation parameters being reduced gradually until CO2 increased, after which it was kept on manual ventilation until spontaneous breathing, with a Vt of at least 5 mL/kg.bw and respiratory frequency of approximately 40 breaths/min. Oral and pharyngeal secretions were aspirated. Awakening from anesthesia was evaluated by the presence of ciliary reflex, eye opening, swallowing, rumination, and breathing movements. The sheep was extubated when it was able to lift and keep its head raised for at least 5 s. The Faucher tube, pulse oximeter, and thermometer were removed. Oxygen was maintained on the ventilation mask with a flow rate of 8–10 L/min. Monitoring was gradually dropped when the Aldrete score was greater than eight points. The arterial catheter and then peripheral venous line were suppressed, followed by compression hemostasis for 10 min. The thoracic drainage tube was connected to a mobile aspirator and CVC lumens were closed with plugs. The CVC was kept for the next seven postoperative days, being bandaged with an elastic, non-compressive gauze around the neck. ECG electrodes were removed, and the sheep was moved to the postoperative room in the containment cage. It was placed in ventral recumbency in order to avoid traction of the drainage tube, being continuously monitored until it rose in orthostatism without unbalancing. Over the following hours, it was periodically monitored for the state of consciousness, presence of rumination and voice, state of agitation, urine and fecal production, drainage tube’s permeability, respiratory pattern, surgical wound condition, and presence of possible bleeding sources. Bilateral lung auscultation was performed to exclude postoperative pneumothorax or hemothorax. SpO2 and rectal temperature were measured.
Postoperative care protocol
We established a postoperative care, follow-up, and treatment guide in sheep after open-heart surgery under CPB. For the first seven postoperative days, all animals received treatment with anticoagulant therapy with low molecular weight heparin, 0.6 mL enoxaparin sodium s.c. every 12 h, diuretic (20.0 mg furosemide i.v. once daily), analgesic (1.0 g/2 mL metamizole i.v. and 30.0 mg/mL ketorolac i.v. once daily), and steroidal anti-inflammatory medication (4.0 mg/mL dexamethasone i.v. once daily) (Fig. 6A). Prophylactic antibiotic therapy was started intraoperatively, with the first dose being of 15.0 mg/kg.bw., and then continued for 7 days with 500.0 mg cefuroxime i.v. twice daily. Oral antiaggregant therapy with 75.0 mg/day acetylsalicylic acid was maintained for one month. The thoracic drainage tube was suppressed 24 h postoperatively and the animal was moved from the containment cage into the indoor facility, along with other sheep. Animals were clinically examined, and their temperature, heart rate, respiratory rate, and SpO2 were noted. The clinical examination also included thoracic auscultation along with evaluation of attitude, activity, appetite, urine and fecal production, and the surgical wound. Blood gas analyses and serum electrolyte analyses were performed daily, and hydro-electrolytic and/or acid–base imbalances were corrected with intravenous fluids and electrolytes (5% glucose, Ringer lactate, gelofusine, potassium, magnesium, calcium, sodium bicarbonate) (Fig. 6B). When it was considered necessary, BP measurements, ECG and blood analyses were performed. Under conditions of favorable evolution, 7–10 days postoperatively, CVC was removed and biological samples were collected from the tip of the catheter for bacteriological examination.
Fig. 6.
A Sheep on the first postoperative day; B acid–base and hydro-electrolytic rebalancing; C sheep at approx. one month postoperatively, in the indoor facility
Postoperatively, water was given after 6 h and food after 12 h. The sheep was kept in the indoor facility for one month, especially in case of cold weather with outdoor temperatures below 0°C (Fig. 6C), after which it was moved into the outdoor facilities along with other operated sheep.
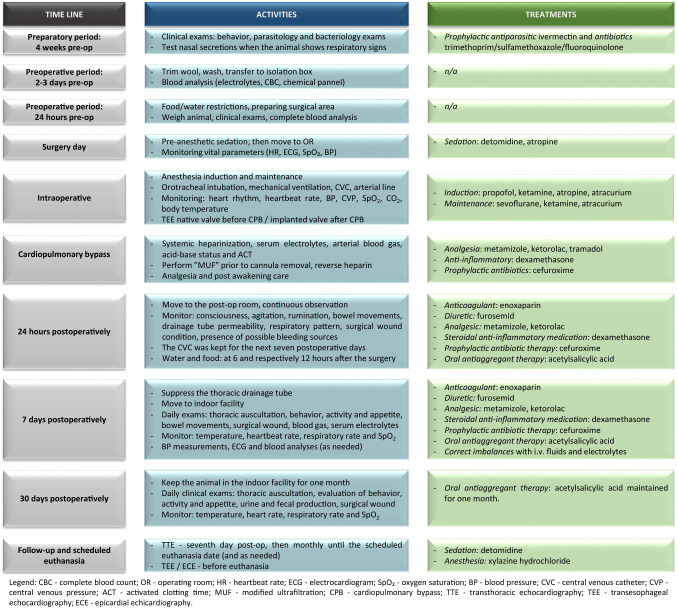
A flowchart describing the animal care protocol was presented in Fig. 7.
Fig. 7.
Flowchart presenting the animal care protocol
Transesophageal (TEE) and epicardial echocardiography (ECE)
Intraoperative TEE was performed using a Mindray DC-7 ultrasound machine (Shenzhen Mindray Bio-medical Electronics Co. Ltd., Nansha, Shenzhen, P.R. China) with a 3–7 MHz echo probe (Mindray P7-3T) (Fig. 8A). Before establishment of CPB, cardiac function and cavities and the functionality of native valves were evaluated. A standardized protocol for TEE was established: the probe was inserted up to the 60 cm mark in relation to the animal’s mouth at a 125° rotation relative to the investigation plan in the great vessels section and aortic and pulmonary valves were examined. Physiological velocities were assessed by continuous-wave Doppler (CW) examination. After weaning off CPB, the implanted valve conduit was morphologically and functionally evaluated by TEE and ECE, and the adaptation to the hemodynamic conditions was also assessed. Valve regurgitation was evaluated as follows: no regurgitation (grade 0), mild (grade I), moderate (grade II), and severe (grade III and IV) (Fig. 9A, B). ECE was performed after sterile isolation of the probe (Fig. 8B) and positioning it directly on the implanted conduit (Fig. 8C), and valve motion was examined in the cross and longitudinal sections (Fig. 9C, D).
Fig. 8.
A Placement of the TEE echo probe; B sterile isolation of the ECE echo probe; C ECE of the implanted valve conduit
Fig. 9.
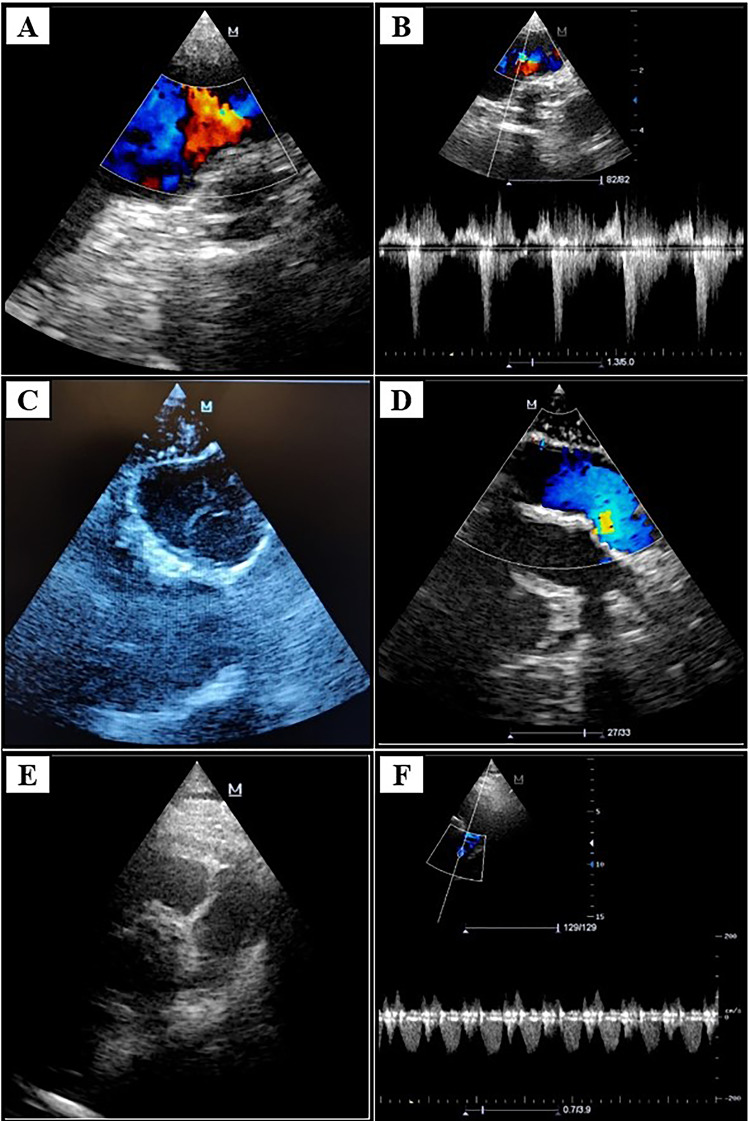
A Intraoperative color Doppler TEE, after conduit implantation—no turbulences detected; B pulsed-wave color Doppler TEE; C ECE—cross section; D color Doppler ECE—longitudinal section; E TTE 1 week postoperatively—short axis parasternal view at the great vessels level; F pulsed-wave Doppler TTE
Transthoracic echocardiography (TTE)
The examination was performed preoperatively and postoperatively using the same Mindray ultrasound unit with a 3–7 MHz TEE echo probe (Mindray P 7-3) at predefined time intervals: first day, seventh day, and then monthly until the scheduled euthanasia date. Preoperatively, the investigations aimed to detect possible pre-existing cardiac pathologies that could influence the subsequent postoperative evolution. The following aspects were evaluated: cardiac cavities and walls (right and left ventricles, interventricular septum, left ventricle posterior wall, right ventricle free wall, left and right atria), aortic and pulmonary annulus. Postoperative TTE: Under light sedation, the animal was positioned in right lateral recumbency. The left forelimb was pulled forward and the probe was placed on the exposed axilla. The implanted valve was identified in pulmonary position between the right ventricle ejection tract and pulmonary artery trunk (Fig. 9E). Morphological changes of the valve leaflets were evaluated, specifically the conduit walls and anastomoses sites. The hemodynamic performance of the implanted valve (Fig. 9F) and other cardiac valves were evaluated using color Doppler echocardiography: maximum and mean systolic pressure gradients, regurgitation grade and degree of stenosis (if any), valve opening (in mm), and the respective valvular areas in cm2. As indirect signs, impairment degree of the four cardiac chambers was quantified by evaluating ventricular pressures and volumes, specifically the left and right ventricular regional kinetics and global systolic function. Subsequently, the pericardial and bilateral pleural space was examined to detect possible postoperative pathological effusions.
Euthanasia/necropsy protocol
After about 6 months, the animals were euthanized and necropsied. Under sterile conditions, the sheep was placed in right lateral recumbency and anesthesia was performed with 0.2 mL/10 kg.bw i.m. xylazine hydrochloride (Narcoxyl 2—Intervet International BV, Netherlands). Left re-thoracotomy was performed in the third intercostal space (Fig. 10A), followed by pericardiotomy and meticulous adhesiolysis, exposing the right side of the heart (Fig. 10B) and the previously implanted pulmonary valve conduit (Fig. 10C). In parallel, TEE was performed (Fig. 10D) and after exposure of the pulmonary valve conduit, ECE was performed as well (Fig. 10E,F). Cardiorespiratory arrest was induced by i.v. administration of 1.2 mL/10 kg.bw T 61 solution (MSD Animal Health GmbH, Germany). The explanted valve conduit was macroscopically analyzed, after which it was placed in a sterile container for further bacteriological and histological analysis. After euthanasia, tissue samples were taken for morphological, histopathological, and immunohistochemical evaluations. All euthanized animals underwent complete necropsy (to assess associated pathologies).
Fig. 10.
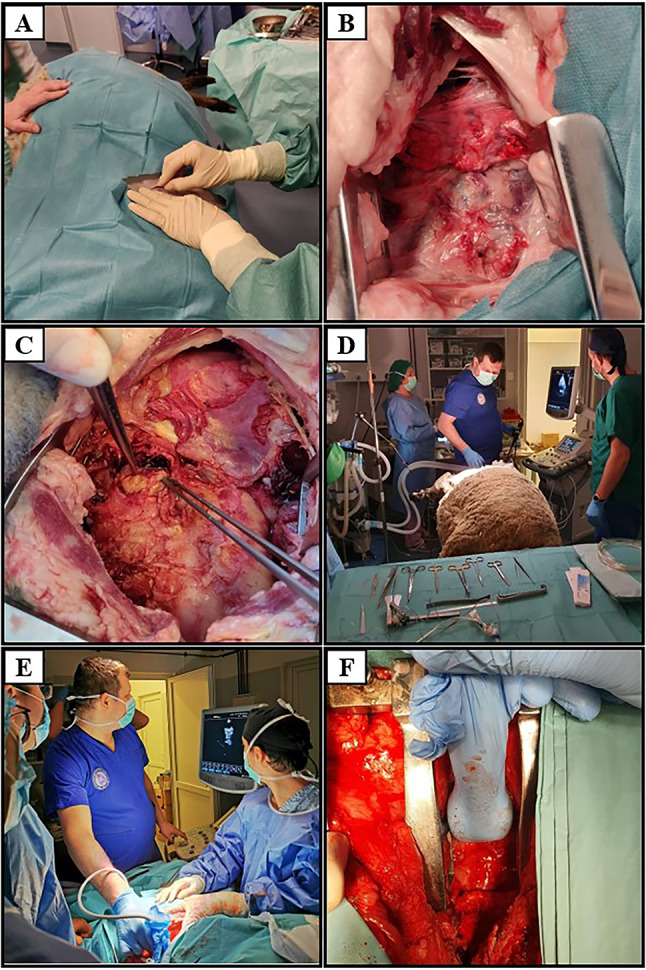
A Left re-thoracotomy; B adhesiolysis followed by exposure of the heart; C in situ appearance of the pulmonary valve conduit; D TEE before euthanasia; E, F ECE after exposure of the valve conduit
Surgical outcomes
Using the described protocol, decellularized pulmonary valve conduits were successfully implanted in 10 sheep. The average operation time was 214 ± 14 min. whereas the average CPB time was 70 ± 11 min. With respect to intraoperative complications, there were two cases of aortic wall rupture, which occurred at the site of the aortic purse string, after arterial cannula suppression. Both animals survived, with their complications being solved by aortorrhaphy. Except these two cases, all the other procedures were conducted without any unusual blood loss. Usual hemorrhage sites were observed, as in any human cardiac surgical procedure, and were easily controlled by hemostasis. One death occurred on the 16th day postoperatively (Table 1).
Table 1.
Short-term surgical outcomes
| Animal number | Age at surgery (months) | Weight at acquisition (kg) | Weight at surgery (kg) | 30-day survival | Cause of death | Complications during the first 7 post-op days | Complications during the first 30 post-op days |
|---|---|---|---|---|---|---|---|
| 1 | 28.7 | 41.0 | 50.0 | Survived | – | – | – |
| 2 | 27.4 | 29.5 | 45.0 | Survived | – | – | – |
| 3 | 37.5 | 40.0 | 58.0 | Survived | – | – | – |
| 4 | 27.7 | 29.0 | 50.0 | Died—16th post-op day | Cardiac tamponade |
Dyspnea Reduced appetite and rumination |
Fever Pleural and pericardial effusion |
| 5 | 29.0 | 28.0 | 52.0 | Survived | – | Tachypnea | – |
| 6 | 7.8 | 35.5 | 32.0 | Survived | – |
Reduced Appetite and rumination Pleural effusion |
Fever Reduced appetite |
| 7 | 8.0 | 32.7 | 35.6 | Survived | – | Chills (1st day) | – |
| 8 | 9.6 | 29.7 | 38.0 | Survived | – | Tachycardia | – |
| 9 | 12.0 | 30.6 | 36.0 | Survived | – |
Hypokalemia Hypocalcemia Abnormal fecal production Tachycardia |
– |
| 10 | 11.8 | 29.7 | 38.0 | Survived | – |
Hypokalemia Hypocalcemia Anemia Tachycardia |
– |
A major complication of this type of surgery could be endocarditis, caused by inefficient sterilization of the autograft. In our study, autografts sterilization was performed efficiently, without any postoperative complication related to this issue.
Discussion
Given the similarities in the anatomical and hemodynamic parameters between ovine and human races, sheep are one of the most widely used large animal models in numerous investigations assessing the degeneration and calcification of bioprosthetic valves [3, 10, 11]. Sheep have a relatively rapid growth rate, allowing the evaluation of valvular implants in terms of growth and remodeling in relation to the recipient’s heart growth [12]. In our group of animals, we observed a tendency to overweight, especially in older animals (Table 1), between the acquisition time and the surgery date. This finding emphasizes the importance of a streamlined diet and avoiding hyper-care by the staff involved.
Perioperative protocol
Orotracheal intubation, mechanical ventilation
A special situation that may arise from OTI, which was not experienced by our group, is called “cannot intubate, cannot ventilate.” In such cases, emergency percutaneous tracheostomy was reported through cricothyroidotomy [13]. However, the probability of failure is low, especially after reaching the learning curve plateau.
Arterial line placement
There are multiple options for arterial line placement in sheep. Our first choice for this maneuver was the right femoral artery. However, we stopped considering this location because we encountered various difficulties, such as small-sized femoral arteries, difficult access to the cannulation site, hematoma formation after arterial line suppression, and increased predisposition to infections during the postoperative period. Therefore, we decided to place the arterial line at the level of the left auricular artery, because it is much more accessible, has the appropriate size, and is associated with a low risk of hematoma formation. Schweiger et al. performed aortic valve replacement in adult sheep (59–65 kg) either by sternotomy or by right lateral thoracotomy through the fourth intercostal space [14]. For invasive BP measurement, they chose to mount the arterial line on the femoral or auricular artery, for the same reasons.
Maintaining hydro-electrolyte and coagulation homeostasis
In our cases, fluid losses were replaced by colloidal or crystalloid solutions, as needed. Intraoperatively, blood loss was recovered in the extracorporeal circulation machine through cardiotomy suction. Another way for recovery of blood loss after weaning off CPB is by auto-transfusion with filtered blood through a cell-salvage device, a method that has been used in human patients and reported in canine subjects [15, 16].
Maintaining constant body temperature
Most anesthetic procedures cause depression of the thermoregulatory mechanism at the hypothalamic level, predisposing the animals to hypothermia [17]. Despite performing normothermic CPB, an electrical heating surgery table as well as pre-warmed intravenous fluids were used to prevent hypothermia caused by general anesthesia.
Anesthetic protocol
Various anesthetic protocols have been successfully used in surgical procedures performed on large animals, such as sheep. In all our cases, we used detomidine or medetomidine hydrochloride as pre-anesthetic medication, ensuring sufficient sedation and analgesia. Detomidine is an α2 adrenoceptor agonist with dose-dependent sedative and analgesic effects [18], presenting the advantage of allowing intramuscular administration and preventing the need for prior placement of a venous catheter. This maneuver could be a challenge for the team, because some animals are difficult to handle. The same drug was reported by our team in a previous study describing minimally invasive procedures performed for fat harvesting in sheep [19]. Abrahamsen suggested that an opioid may be used along with the α2 receptor agonist, such as 0.05–0.1 mg/kg butorphanol, for systemic analgesia enhancement, especially in situations when animal cooperation needs improvement [20]. A combination of ketamine/midazolam/glycopyrrolate mixed in the same syringe and given through a catheter placed on the day before surgery in the external jugular vein was reported by Di Vicenti et al. [3]. For induction and maintenance of anesthesia, we used propofol and ketamine. Propofol is a short-acting drug with amnestic and anti-emetic properties. It is known to have dose-dependent adverse effects, such as respiratory depression and hemodynamic impairment [21]. It is usually used in combination with opioids or other analgesic agents, since it does not have analgesic properties [22]. It has been suggested that a combination of ketamine and propofol provides both deep sedation and analgesia. Moreover, ketamine reduces the risk of respiratory depression, which occurs when propofol is combined with opioids [23–25]. Lin and co-workers reported that 3 mg/kg.bw propofol bolus followed by continuous infusion had comparable anesthetic effects to the xylazine-ketamine-halotan combination in sheep, while also ensuring rapid recovery of the animal [26]. Intraoperatively, systemic analgesia was performed with metamizole, ketorolac, and tramadol in the doses mentioned in the results section. Metamizole and ketorolac were continued for 5–7 days after the surgery to provide postoperative analgesia. Metamizole (20 mg/kg) in combination with carprofen (4.0 mg/kg) was reported by Knirsch et al. [27] for pain control. To avoid tracheal and/or laryngeal damage and facilitate endotracheal intubation, a non-depolarizing neuromuscular blocking agent (atracurium) was used in all cases. According to Harper et al., the incidence of anaphylactic reactions in human patients following atracurium administration has been reported to be 4.2/100,000 cases, but this incidence was lower than that seen with rocuronium [28]. However, no anaphylactic reactions or other adverse events were noted in our experience. Published data regarding the use of anticholinergic drugs in cardiovascular procedures in ruminants are controversial, due to potential for arrhythmia, gastric stasis, and paralytic ileus. On the other hand, the heavy salivation caused by ketamine may lead to difficult intubation; therefore, an anticholinergic drug may be useful [29]. Atropine was used in all cases (0.02 mg/kg.bw) for decreasing salivary and bronchial secretions during ketamine anesthesia. Ruminants have high levels of atropinase and therefore require higher and repeated doses of atropine [30]. Nevertheless, no significant cardiac arrhythmias were detected, and all animals resumed their appetite, rumination, and fecal production after surgery. Other types of anticholinergic drugs, such as glycopyrrolate, were reported to be safely used without changes in heart rate [3]. Generally, any inhalation anesthetic may be used to maintain anesthesia, isoflurane being the most commonly used in veterinary anesthesia. Hikasa et al. compared sevoflurane with isoflurane during short- and long-term surgery in sheep. They observed a faster recovery time when using sevoflurane compared to that obtained for isoflurane, but no differences were noted for respiratory and cardiac frequencies, tidal volume, and respiratory acidosis degree between the two inhalational anesthetics [31]. Rapid recovery without significant complications was observed in all cases using sevoflurane, especially in older animals. Another protocol for maintaining anesthesia in ruminants is TIVA (total intravenous anesthesia), but it is limited to procedures under 45 min and consists of a “triple-drip” technique—a combination of xylazine + ketamine + guaifenesin and propofol 50 mcg/kg.bw/min [32].
Surgical protocol
From the anatomical point of view, the sheep heart is very similar in structure to the human heart. Due to the narrow sternum in sheep, median sternotomy is technically very difficult to perform [33]. After pericardiotomy, the ventral side of the heart is accessed, the aorta being on the right and pulmonary trunk on the left. Despite this favorable exposure, median sternotomy causes higher surgical stress, increased postoperative pain, and longer recovery time, and is therefore not an appropriate approach. Although right or left thoracotomy does not provide concomitant access to great vessels, this remains the easiest way for valvular replacement. LLT performed in the third intercostal space first exposes the right ventricle ejection tract and pulmonary trunk to its bifurcation, an approach through which interventions such as pulmonary valve replacement could be performed. In contrast, approaching the aortic root located posteriorly to the pulmonary artery is difficult with classical LLT. In aortic valve replacement, Schweiger et al. suggested that right thoracotomy in the fourth intercostal space ensures good exposure of the aorta and brachiocephalic trunk, as well as easy access to the aortic valve [14]. In our experience with pulmonary valve replacement, the disadvantage of right thoracotomy was the presence of pulmonary veins that drain into the left atrium, blocking the access to the great vessels. Therefore, the only viable option for interventions involving the pulmonary trunk was LLT, mentioning that the access to ascending aorta required for arterial cannulation can only be achieved by temporary traction of pulmonary trunk.
Cardiopulmonary bypass
To prevent thrombosis in the CPB circuit, systemic anticoagulation with 250 IU/kg.bw heparin was performed, with dose supplementation in case of low ACT, which was evaluated intraoperatively every 30 min. In human heart surgery, the initial dose of heparin is 300–500 IU/kg.bw. However, the target value of ACT for thrombosis prevention is not yet clear for humans, nor for animals [34]. After weaning off CPB, the effects of heparin were antagonized by protamine in a dose ratio of 1:1.3 of the initial heparin dose. The controversies regarding heparin-protamine interactions are well known. Protamine can cause pulmonary hypertension, decreased BP, decreased myocardial oxygen consumption, cardiac output, and heart rate [35], as well as anaphylactic reactions (< 1%) with an increased risk of cardiovascular collapse and death. On the other hand, improper dosing of protamine may increase the risk of postoperative bleeding and the need for blood transfusions [36]. Boer et al. suggested that an adequate dose of protamine ranging from 0.6 to 1.0 compared to the initial dose of heparin could reduce the risk of postoperative bleeding [37]. However, Di Vicenti, Carney, and Shofti and did not use protamine to reverse heparin effects, and no significant bleeding at the time of wound closure was reported [3, 38, 39]. Similar to human cardiac surgery practice, in our cases, 1/3rd of the required dose of protamine was initially administered before administering the total required dose for hemostatic effect, with no significant bleeding observed. The optimum blood flow during CPB is determined by body surface and temperature. Usually, in conditions involving moderate hypothermia to normothermia, the targeted cardiac output calculated and used by most perfusionists is between 2.2 and 2.8 L/min/m2 [40]. Recent studies have been suggested that not only body surface area and temperature, but also oxygen supply (“oxygen delivery – DO2”) should be considered while calculating the adequate pump flow [41–43].
In large animals, small-sized aortic cannula may be used. When choosing the aortic cannula, the aortic diameter should be considered at the cannulation site to allow maximum flow through the cannula. The aortic cannula should be small enough to not impede blood flow around it when it is inserted, but also large enough to provide adequate flow [3]. The arterial cannula of 16–18 Fr. used by our team provided a maximum flow of 4.2–5 L/min, which was more than adequate for an optimal flow rate during CPB in adult sheep. Venous cannula selection is extremely important, with the intervention becoming very difficult if the cannula does not drain properly due to the right ventricle blood ejection. In some cases, we had to use an angled cannula that drained more efficiently due to the small size of the right atrium.
The major benefits of CPB are accompanied by adverse effects that cannot be ignored. The primary adverse effect involves systemic inflammatory response syndrome (SIRS), which occurs through synthesis and release of inflammatory agents, production of oxygen free radicals [44], release of catecholamines, alteration of body fluids, acid–base and hydro-electrolytic imbalances, or myocardial, lung, or other organ dysfunction through various malperfusion mechanisms. This inflammatory response can cause intravascular fluid migration into the interstitial space due to changes in vascular permeability and decreased oncotic pressure. The most known and harmful complications include edema, respiratory dysfunction, leukocyte agglutination with microcirculation obstructions, neurological alterations, renal dysfunction, arrhythmias, low cardiac output syndrome, postoperative bleeding, infections, and difficulties in glycemic balance. To minimize the inflammatory process caused by blood exposure to the extracorporeal circuit, MUF was performed at the end of CPB. This method offers three major benefits that mitigate the effects of SIRS, namely: (a) reduction of pulmonary hypertension induced by CPB; (b) reduction of post-perfusion edema in the immediate postoperative period, by eliminating excess fluids accumulated in the body’s interstitial space during CPB; and (c) elimination of inflammatory mediators and toxic metabolites accumulated during CPB. The MUF technique was used with great success in pediatric cardiac surgery [45]. It was evaluated by Luciani et al. in 573 human patients, being associated with a significant reduction of postoperative respiratory failure [46]. In another study on 37 subjects, Torina et al., reported decreased postoperative intrapulmonary resistance when MUF was used at the end of CPB [47]. The correlation between CPB time and incidence of pulmonary complications has been proven in human patients [48]. A reduction in total operative and CPB time and implicit surgical trauma seemed to accelerate postoperative physical recovery in our experience.
Postoperative care
In the immediate postoperative period, a tendency for hypokalemia was observed in some animals, especially in juvenile sheep (Table 1), which was corrected with intravenous potassium chloride (KCl) in Ringer’s solution. In the first 7 days postoperatively, mild or moderate anemia occurred in some juvenile animals, with hemoglobin (Hgb) values below the normal limit, a manifestation that can be considered common and frequent after open-heart surgery in CPB, even in human patients [49]. In our cases, anemia was corrected by 100 mg/5 mL intravenous iron hydroxide complex III, with gradual improvement until normalization of Hgb values within approximately 10 days. Another method for anemia correction, reported by Sousa et al., was transfusion of autologous blood stored in special transfusion bags for 15 or 35 days prior to surgery [9]. They observed an improvement in blood volume, but transfusion reactions (hyperthermia, tachycardia) occurred in the majority of animals.
Dry cough with episodes of productive cough was another specific problem that occurred in some of the operated sheep in the first postoperative days, which affected the rumination process. The animals were treated with mucolytics (N-acetylcysteine), antispasmodics and bronchodilators (miofilin). Moreover, some of the sheep showed tachycardia and tachypnea, with a heartbeat rate of 140–160 beats/min. and a respiratory rate of 40–60 breaths/min. Rarely, we have noticed that some sheep, after awakening from anesthesia and a few hours later, showed continuous agitation and lack of conservation instinct, probably caused by the temporary brain damage suffered during the CPB. Due to the fact that they could easily injure themselves, the containment cages were adjusted to the size of the sheep and padded with polystyrene boards to attenuate the shocks and minimize the possibility of injury. As medication, medetomidine and sodium chloride 0.9% i.v infusion was administered, as well as dexamethasone, mannitol and MgSO4 over the next 24–48 h postoperatively. In almost a third of the operated sheep, difficulties in resuming the rumination process and intestinal transit were noted. The sheep being ruminant animals, due to the prolonged period of lateral recumbency during the surgery (5–6 h), stomach atony may occur, with temporary paralytic ileus, sometimes with large accumulations of gas in the rumen. As treatment, Rumdigestin (ruminant), Fermactiv (probiotic), vitamin B1, paraffin oil and glycerin suppositories were used. Furthermore, it is recommended that the operated animals should receive high quality hay, easy to digest, and pelleted feed in small quantities. For an increased intake of mineral salts and trace elements (Na, Ca, Mg, Zn, Cu, Fe, I, Se), special salt briquettes for ruminants were used as well.
Study limitations
The presented study has several limitations. The number of operated animals was relatively small; however sufficient to prove the feasibility of the surgical technique and perioperative care. The immediate postoperative period after the animal’s early extubation was quite challenging in some cases, realizing that a dedicated intensive care unit (ICU) with remote continuous monitoring would have been ideal, feasible, but expensive. The protamine to heparin ratio used in our study was adapted according to protocols used in human cardiac surgery. Various administration regimens assessment would have been helpful to understand the specific coagulation status in sheep; however, a greater number of experimental animals was avoided due to animal protection and welfare reasons. MUF technique is commonly used in human cardiac surgery, especially the pediatric cardiac surgery. A case control study within our group, assessing its specific advantages and/or disadvantages was not performed due to the same concerns regarding animal welfare. A long-term follow-up (6–12 months) should be addressed in the future, as we performed a descriptive analysis of the short-term surgical outcomes. The functionality, hemodynamic characteristics and remodeling potential of the tissue-engineered valves, as well as comparison with pre-seeded valve conduits with ADSCs were beyond the aim of this study, these data are subject to a further study within our project.
From our experience we concluded some important findings:
Since the animals were quite diverse in age, both surgical technique and anesthetic procedure had to be adjusted accordingly. Juvenile sheep showed good tissue quality, but higher incidence of postoperative complications. Unlike human clinical practice, a post-CPB psychotic reaction was observed in these animals, requiring longer postoperative sedation and more careful supervision. In contrast, older animals presented tissues with increased friability as well as preoperative comorbidities (pneumopathy, anemia, intestinal dysbiosis), which influenced the postoperative evolution. Postoperative complications should be identified and treated promptly since dysfunction of any organ may compromise the entire experiment. Therefore, the animal should be monitored daily by the surgical team, anesthesiologist, cardiologist, veterinarian, and technicians, especially during the initial convalescence period. The difficulties involved in carrying out all perioperative paraclinical investigations that are currently performed in human clinical practice makes this experiment extremely challenging, and the relevant team should cover the entire pathology of the ovine organism.
In the first 24 h, animals should be monitored almost continuously or until hemodynamic and respiratory stability is achieved, as well as evaluate the state of consciousness and confirm the absence of active bleeding. Moreover, we noticed that lifting the animal from lateral to ventral recumbency, as well as avoiding dorsal recumbency, is important, especially when sheep are transferred to the containment cage. Abdominal distension with tympanism causing breathing difficulties was observed in cases where this rule was not followed. Preoperative diet should be carefully monitored; since sheep have a tetracameral stomach and assuming possible perioperative gastric stasis even in the context of diet, the digestive tract could be severely affected by surgery. At the same time, abdominal distension can be prevented by proper mobilization of the animal.
Sheep predisposition to respiratory infections is an aspect that can be determined by the originating environment of these animals (sometimes with improper hygiene conditions or even the natural environment that predisposes them to contamination). Therefore, preoperative paraclinical analysis (chest radiography, nasal swab assay, and stool culture), as well as anti-infective and anti-parasitic treatment may be beneficial. Monitoring blood gas levels, hemoglobin, hematocrit, serum electrolytes, as well as acid–base balance is essential during the perioperative period.
Short surgery duration, reduced CPB time, minimal blood loss, as well as early extubation are variables that can be controlled by the surgical team. Using MUF after CPB reduces the effects of SIRS. Arterial cannula suppression with proper hemostasis at this level, protamine administration, and blood recovery from the extracorporeal machine circuit through the venous cannula could minimize the total blood loss.
Early mobilization of the animal and reintegration into the initial group leads to a shorter convalescence period, allowing the animal to regain the behavior of an unoperated animal. A tendency to overweight in the context of hyper-care was observed. Since sheep typically live in herds, their isolation can lead to increased perioperative stress and anxiety; therefore, isolation should be avoided.
In conclusion, we suggest that the proposed anesthetic protocol is safe and effective, ensuring both adequate sedation and analgesia as well as rapid recovery from anesthesia without significant complications. Our surgical technique is a feasible technique and can be performed with a low mortality rate and minimal surgical complications after reaching the learning curve plateau. The established guide for postoperative care, follow-up, and treatment in sheep after open-heart surgery under CPB as well as the anesthetic and surgical protocols described in this study may help other research teams working in the field of heart valves tissue regeneration.
Acknowledgments
This work was supported by a grant from the Competitiveness Operational Programme 2014-2020, Tissue engineering technologies for cardiac valve regeneration, valve-regen, ID:P_37_673, Mysmis code:103431, contract 50/05.09.2016, from grant 1P30GM131959 from NIGMS and from the Dempsey Endowment. We would like to thank Editage (www.editage.com) for English language editing.
Compliance with ethical standards
Conflicts of interest
The authors have no financial conflicts of interest.
Ethical statement
All procedures and perioperative care of the experimental animals were performed in accordance with the “Guide for the care and use of laboratory animals” and Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes. Euthanasia was performed in compliance with the Convention on the experiments on live vertebrate animals and all the legal norms referring to the protection and welfare of animals: Law No. 9 of January 11, 2008 on the animals’ protection and Decision no. 19 of 01. July 2011 adopted by the National Council of the Veterinary Doctors College regarding the “Guide for the euthanasia of animals.” This work is part of a research grant conducted in accordance with the protocol no. 131/21.10.2016 approved by the Ethics Committee of the UMFST “George Emil Palade” of Tirgu Mures.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hussam Al Hussein and Marius M. Harpa have contributed equally to this work.
References
- 1.Salerno CT, Droel J, Bianco RW. Current state of in vivo preclinical heart valve evaluation. J Heart Valve Dis. 1998;7:158–162. [PubMed] [Google Scholar]
- 2.Choo SJ, Kim KI, Park NH, Song JM, Choi IC, Shim JY, et al. Development of an animal experimental model for a bileaflet mechanical heart valve prosthesis. J Korean Med Sci. 2004;19:37–41. doi: 10.3346/jkms.2004.19.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DiVincenti L, Jr, Westcott R, Lee C. Sheep (Ovis aries) as a model for cardiovascular surgery and management before, during, and after cardiopulmonary bypass. J Am Assoc Lab Anim Sci. 2014;53:439–448. [PMC free article] [PubMed] [Google Scholar]
- 4.Leroux AA, Moonen ML, Pierard LA, Kolh P, Amory H. Animal models of mitral regurgitation induced by mitral valve chordae tendineae rupture. J Heart Valve Dis. 2012;21:416–423. [PubMed] [Google Scholar]
- 5.Harpa MM, Movileanu I, Sierad LN, Cotoi SO, Suciu H, Sircuta C, et al. Pulmonary heart valve replacement using stabilized acellular xenogeneic scaffolds; effects of seeding with autologous stem cells. Rev Rom Med Lab. 2015;23:415–430. [Google Scholar]
- 6.Ionela M, Klara B, Marius H, Dan N, Ovidiu C, Preda T, et al. Pressurized perfusion system for obtaining completely acellular pulmonary valve scaffolds for tissue engineering. ARS Medica Tomitana. 2019;25:149–156. [Google Scholar]
- 7.Valverde A, Doherty JT. Anesthesia and analgesia of ruminants. In: Fish ER, Brown JM, Danneman JP, Karas ZA, editors. Anesthesia and analgesia in laboratory animals. 2. London: Elsevier; 2008. pp. 406–407. [Google Scholar]
- 8.Jackson GGP, Cockroft DP. Clinical examination of farm animals. Oxford: Blackwell Science; 2002. [Google Scholar]
- 9.Sousa RS, Minervino AH, Araújo CA, Rodrigues FA, Oliveira FL, Mori CS, et al. Clinical response and transfusion reactions of sheep subjected to single homologous blood transfusion. ScientificWorldJournal. 2014;2014:734397. doi: 10.1155/2014/734397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Souza HJ, Palma JH, Casagrande IS, Christo SC, Alves-Silva LS, Almeida MA, et al. Replacement of pulmonary artery trunk in sheep using tubular valved heterograft in non-aldehydic preservation. Rev Bras Cir Cardiovasc. 2012;27:419–428. doi: 10.5935/1678-9741.20120071. [DOI] [PubMed] [Google Scholar]
- 11.Flameng W, Jashari R, De Visscher G, Mesure L, Meuris B. Calcification of allograft and stentless xenograft valves for right ventricular outflow tract reconstruction: an experimental study in adolescent sheep. J Thorac Cardiovasc Surg. 2011;141:1513–1521. doi: 10.1016/j.jtcvs.2010.08.082. [DOI] [PubMed] [Google Scholar]
- 12.Mendelson K, Schoen FJ. Heart valve tissue engineering: concepts, approaches, progress, and challenges. Ann Biomed Eng. 2006;34:1799–1819. doi: 10.1007/s10439-006-9163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heard AM, Green RJ, Eakins P. The formulation and introduction of a ’can’t intubate, can’t ventilate’ algorithm into clinical practice. Anaesthesia. 2009;64:601–608. doi: 10.1111/j.1365-2044.2009.05888.x. [DOI] [PubMed] [Google Scholar]
- 14.Schweiger M, Knirsch W, Cesarovic N, Krüger B, Schmiady M, Frauenfelder T, et al. Surgical technique: establishing a pre-clinical large animal model to test aortic valve leaflet substitute. J Thorac Dis. 2016;8:3733–3738. doi: 10.21037/jtd.2016.12.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashworth A, Klein AA. Cell salvage as part of a blood conservation strategy in anaesthesia. Br J Anaesth. 2010;105:401–416. doi: 10.1093/bja/aeq244. [DOI] [PubMed] [Google Scholar]
- 16.Kellett-Gregory LM, Seth M, Adamantos S, Chan DL. Autologous canine red blood cell transfusion using cell salvage devices. J Vet Emerg Crit Care (San Antonio) 2013;23:82–86. doi: 10.1111/vec.12017. [DOI] [PubMed] [Google Scholar]
- 17.Smith CJ, Danneman JP. Monitoring of anesthesia. In: Fish ER, Brown JM, Danneman JP, Karas ZA, editors. Anesthesia and analgesia in laboratory animals. 2. London: Elsevier; 2008. pp. 176–177. [Google Scholar]
- 18.England GC, Clarke KW. Alpha 2 adrenoceptor agonists in the horse—a review. Br Vet J. 1996;152:641–657. doi: 10.1016/s0007-1935(96)80118-7. [DOI] [PubMed] [Google Scholar]
- 19.Al Hussein H, Harpa M, Movileanu I, Al Hussein H, Suciu H, Branzaniuc K, et al. Minimally invasive surgical protocol for adipose derived stem cells collection and isolation—ovine model. Rev Chim. 2019;70:1826–1828. [Google Scholar]
- 20.Abrahamsen EJ. Chemical restraint and injectable anesthesia of ruminants. Vet Clin North Am Food Anim Pract. 2013;29:209–227. doi: 10.1016/j.cvfa.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Phillips W, Anderson A, Rosengreen M, Johnson J, Halpin J. Propofol versus propofol/ketamine for brief painful procedures in the emergency department: clinical and bispectral index scale comparison. J Pain Palliat Care Pharmacother. 2010;24:349–355. doi: 10.3109/15360288.2010.506503. [DOI] [PubMed] [Google Scholar]
- 22.Miner JR, Burton JH. Clinical practice advisory: emergency department procedural sedation with propofol. Ann Emerg Med. 2007;50:182–187. doi: 10.1016/j.annemergmed.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 23.Shah A, Mosdossy G, McLeod S, Lehnhardt K, Peddle M, Rieder M. A blinded, randomized controlled trial to evaluate ketamine/propofol versus ketamine alone for procedural sedation in children. Ann Emerg Med. 2011;57:425–33.e2. doi: 10.1016/j.annemergmed.2010.08.032. [DOI] [PubMed] [Google Scholar]
- 24.Andolfatto G, Willman E. A prospective case series of pediatric procedural sedation and analgesia in the emergency department using single-syringe ketamine-propofol combination (ketofol) Acad Emerg Med. 2010;17:194–201. doi: 10.1111/j.1553-2712.2009.00646.x. [DOI] [PubMed] [Google Scholar]
- 25.Willman EV, Andolfatto G. A prospective evaluation of “ketofol” (ketamine/propofol combination) for procedural sedation and analgesia in the emergency department. Ann Emerg Med. 2007;49:23–30. doi: 10.1016/j.annemergmed.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Lin HC, Purohit RC, Powe TA. Anesthesia in sheep with propofol or with xylazine-ketamine followed by halothane. Vet Surg. 1997;26:247–252. doi: 10.1111/j.1532-950x.1997.tb01494.x. [DOI] [PubMed] [Google Scholar]
- 27.Knirsch W, Krüger B, Fleischmann T, Malbon A, Lipiski M, Lemme F, et al. Establishing a pre-clinical growing animal model to test a tissue engineered valved pulmonary conduit. J Thorac Dis. 2020;12:1070–1078. doi: 10.21037/jtd.2019.12.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harper NJN, Cook TM, Garcez T, Lucas DN, Thomas M, Kemp H, et al. Anaesthesia, surgery, and life-threatening allergic reactions: management and outcomes in the 6th National Audit Project (NAP6) Br J Anaesth. 2018;121:172–188. doi: 10.1016/j.bja.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 29.Prassinos NN, Galatos AD, Raptopoulos D. A comparison of propofol, thiopental or ketamine as induction agents in goats. Vet Anaesth Analg. 2005;32:289–296. doi: 10.1111/j.1467-2995.2005.00204.x. [DOI] [PubMed] [Google Scholar]
- 30.Green AS. Veterinary anesthesia and pain management secrets. Philadelphia: Hanley & Belfus; 2002. [Google Scholar]
- 31.Hikasaa Y, Saitob K, Takaseb K, Ogasawarab S. Clinical, cardiopulmonary, hematological and serum biochemical effects of sevoflurane and isoflurane anesthesia in oxygen under spontaneous breathing in sheep. Small Rumin Res. 2000;36:241–249. doi: 10.1016/s0921-4488(99)00121-2. [DOI] [PubMed] [Google Scholar]
- 32.Davidson GS. Equine anesthesia: triple drip. Int J Pharm Compd. 2008;12:402–404. [PubMed] [Google Scholar]
- 33.Ali ML, Kumar SP, Bjornstad K, Duran CM. The sheep as an animal model for heart valve research. Cardiovasc Surg. 1996;4:543–549. doi: 10.1016/0967-2109(95)00142-5. [DOI] [PubMed] [Google Scholar]
- 34.Finley A, Greenberg C. Review article: heparin sensitivity and resistance: management during cardiopulmonary bypass. Anesth Analg. 2013;116:1210–1222. doi: 10.1213/ANE.0b013e31827e4e62. [DOI] [PubMed] [Google Scholar]
- 35.Carr JA, Silverman N. The heparin-protamine interaction. A review. J Cardiovasc Surg (Torino) 1999;40:659–666. [PubMed] [Google Scholar]
- 36.Hoenicka M, Rupp P, Müller-Eising K, Deininger S, Kunert A, Liebold A, et al. Anticoagulation management during multivessel coronary artery bypass grafting: a randomized trial comparing individualized heparin management and conventional hemostasis management. J Thromb Haemost. 2015;13:1196–1206. doi: 10.1111/jth.12999. [DOI] [PubMed] [Google Scholar]
- 37.Boer C, Meesters MI, Veerhoek D, Vonk ABA. Anticoagulant and side-effects of protamine in cardiac surgery: a narrative review. Br J Anaesth. 2018;120:914–927. doi: 10.1016/j.bja.2018.01.023. [DOI] [PubMed] [Google Scholar]
- 38.Carney EL, Clark JB, Myers JL, Peterson R, Wilson RP, Weiss WJ. Animal model development for the Penn State pediatric ventricular assist device. Artif Organs. 2009;33:953–957. doi: 10.1111/j.1525-1594.2009.00896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shofti R, Zaretzki A, Cohen E, Engel A, Bar-El Y. The sheep as a model for coronary artery bypass surgery. Lab Anim. 2004;38:149–157. doi: 10.1258/002367704322968821. [DOI] [PubMed] [Google Scholar]
- 40.Murphy GS, Hessel EA, 2nd, Groom RC. Optimal perfusion during cardiopulmonary bypass: an evidence-based approach. Anesth Analg. 2009;108:1394–1417. doi: 10.1213/ane.0b013e3181875e2e. [DOI] [PubMed] [Google Scholar]
- 41.Magruder JT, Crawford TC, Harness HL, Grimm JC, Suarez-Pierre A, Wierschke C, et al. A pilot goal-directed perfusion initiative is associated with less acute kidney injury after cardiac surgery. J Thorac Cardiovasc Surg. 2017;153:118–25.e1. doi: 10.1016/j.jtcvs.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ranucci M, Romitti F, Isgrò G, Cotza M, Brozzi S, Boncilli A, et al. Oxygen delivery during cardiopulmonary bypass and acute renal failure after coronary operations. Ann Thorac Surg. 2005;80:2213–2220. doi: 10.1016/j.athoracsur.2005.05.069. [DOI] [PubMed] [Google Scholar]
- 43.Magruder JT, Dungan SP, Grimm JC, Harness HL, Wierschke C, Castillejo S, et al. Nadir oxygen delivery on bypass and hypotension increase acute kidney injury risk after cardiac operations. Ann Thorac Surg. 2015;100:1697–1703. doi: 10.1016/j.athoracsur.2015.05.059. [DOI] [PubMed] [Google Scholar]
- 44.Levy JH, Tanaka KA. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 2003;75:S715–S720. doi: 10.1016/s0003-4975(02)04701-x. [DOI] [PubMed] [Google Scholar]
- 45.Manrique AM, Vargas PD, Palmer D, Kelly K, Litchenstein SE. The effects of cardiopulmonary bypass following pediatric cardiac surgery. In: Munoz R, Morell V, da Cruz E, Vetterly C, da Silva J, editors. Critical care of children with heart disease. Cham: Springer; 2020. pp. 113–129. [Google Scholar]
- 46.Luciani GB, Menon T, Vecchi B, Auriemma S, Mazzucco A. Modified ultrafiltration reduces morbidity after adult cardiac operations: a prospective, randomized clinical trial. Circulation. 2001;104:I253–I259. doi: 10.1161/hc37t1.094931. [DOI] [PubMed] [Google Scholar]
- 47.Torina AG, Petrucci O, Oliveira PP, Severino ES, Vilarinho KA, Lavagnoli C, et al. The effects of modified ultrafiltration on pulmonary function and transfusion requirements in patients underwent coronary artery bypass graft surgery. Rev Bras Cir Cardiovasc. 2010;25:59–65. doi: 10.1590/s0102-76382010000100014. [DOI] [PubMed] [Google Scholar]
- 48.Naveed A, Azam H, Murtaza HG, Ahmad RA, Baig MAR. Incidence and risk factors of pulmonary complications after cardiopulmonary bypass. Pak J Med Sci. 2017;33:993–996. doi: 10.12669/pjms.334.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lako S, Dedej T, Nurka T, Ostreni V, Demiraj A, Xhaxho R, et al. Hematological changes in patients undergoing coronary artery bypass surgery: a prospective study. Med Arch. 2015;69:181–186. doi: 10.5455/medarh.2015.69.181-186. [DOI] [PMC free article] [PubMed] [Google Scholar]