Abstract
Background:
Articular cartilage repair has been a challenge in orthopedic practice due to the limited self-regenerative capability. Optimal treatment method for cartilage defects has not been defined. We investigated the effect of decellularized human placental (DHP) scaffold, mesenchymal stem cells (MSC) and platelet-rich plasma (PRP) on hyaline cartilage regeneration in a rat model.
Methods:
An osteochondral defect was created in trochlea region of the femur in all groups, bilaterally. No additional procedure was performed in control group (n = 14). Only the DHP scaffold was applied to the P group (n = 14). The DHP scaffold and 1 × 106 MSCs were applied to the PS group (n = 14). The DHP scaffold and PRP were applied to the PP group (n = 14). The DHP scaffold, 1 × 106 MSCs and PRP were applied to the PSP group (n = 14). Outcome measures at 12 weeks included Pineda histology score and qualitative histology.
Results:
The mean Pineda scores of P, PS, PP, and PSP groups were significantly better than the control group (p = 0.031, p = 0.002, p < 0.001, p < 0001, respectively). There was no statistically difference in mean Pineda scores of P, PS, PP, and PSP groups (p > 0.05).
Conclusion:
In conclusion, the DHP scaffold appears to be a promising scaffold on hyaline cartilage regeneration. The augmentation of DHP scaffold with MSCs and PRP combinations did not enhance its efficacy on articular cartilage regeneration.
Keywords: Cartilage, Placenta decellularization, Platelet-rich plasma, Mesenchymal stem cells
Introduction
Articular cartilage defects are a common problem in orthopedic practice. These defects mostly occur due to trauma or degenerative process [1]. They result in joint pain and degeneration which lead to decreased function at the associated extremity [2]. Treatment options of articular cartilage defects include debridement, osteochondral graft transfers, autologous chondrocyte implantation (ACI), and bone marrow stimulation techniques such as microfracture application [3]. Unfortunately, hyaline cartilage could not be regenerated with the utility of these methods [2].
Regeneration of native hyaline cartilage is the main goal of tissue engineering studies. Tissue engineering is based on the combination of suitable medium, stem cells, and growth factors [4]. Various materials including synthetic and biological scaffolds have been investigated for regeneration of articular cartilage, however, hyaline cartilage has not been regenerated successfully [5]. Decellularized extracellular matrix (ECM) containing scaffolds are of interest in recent literature [6]. The ECM creates a proliferation medium for cells and regulates cellular behavior [7]. Placenta is an easily accessible tissue as a waste material after delivery and it has a rich ECM content [8]. Promising results have been reported with the use of decellularized human placenta (DHP) xenografts in animal models for tissue regeneration [9, 10].
Stem cell-based tissue engineering is an alternative method that has been intensively studied in recent years. It has been demonstrated that mesenchymal stem cells (MSCs) can transform into chondrocytes in vitro [11]. In addition, encouraging results have been reported in tissue engineering studies using xenotransplanted human MSCs in animal models [12, 13]. Growth factors such as TGF-B, FGF, IGF-1 are involved in cartilage regeneration [14, 15]. These growth factors have been shown to be packaged in microsomes within platelets [16]. Platelet-rich plasma (PRP) contains concentrated form of platelets above baseline levels thus these growth factors can be applied at a higher concentration with use of PRP [17].
Following these rationales, it has been hypothesized that the DHP may be an effective scaffold in articular cartilage regeneration. In addition, it has been hypothesized that its effect can be enhanced with the application of MSCs and PRP combinations. The aim of this study is to investigate the effectiveness of DHP scaffold on articular cartilage regeneration and evaluate the impact of MSC and PRP applications on DHP scaffold.
Materials and methods
Decellularization of human placenta
The placenta fragments were obtained from residues that had been used in previous experiments with different purposes. Tissues that were stored in − 80 °C were thawed gradually; first to − 20 °C in the freezer and then to + 4 °C refrigerator. The thawed tissues were then taken into PBS solution, which was prepared in a 1-liter flask. Tissues in PBS solution were intermittently shaken in a shaker for 24 h. PBS solution was replaced three times during the 24 h shaking process. After this step, tissues were taken into 1% (w/v) sodium dodecyl sulfate (SDS) and shaken for 24 h at a constant shaking rate. Tissues were then extracted from the SDS solution and washed with PBS solution at least three times to remove excess SDS remaining in the tissues. Tissues were then treated with isopropanol in separate tubes for 48 h to remove lipids. Afterwards, tissues were washed with PBS solution at least three times. In the last stage, the enzymatic release of DNA and RNA was performed. For this purpose, tissues were taken into a single tube and incubated with DNAse and RNAse enzyme mixture at 37 °C for 1 h. Finally, the tissues were washed with PBS solution, poured into petri dishes and lyophilized for 48 h [8].
Proliferation of mesenchymal stem cells
Culture and characterization of mesenchymal stem cells
Mononuclear cells obtained from healthy bone marrow transplant donors and isolated in our archive cryostocks were used with the decision of the local ethics committee. Mononuclear cells obtained from a healthy, eight-year-old female bone marrow donor that were cryopreserved at − 196 °C were thawn at 37 °C. Then, 20 × 106 cells were cultured in complete medium consisting of 10 ml of growth medium (DMEM-LG), 10% fetal bovine serum, 1% L-Gutamine, and 1% antibiotic (Penicillin–Streptomycin) in T75 flask and incubated at 37 °C in 5% CO2. Cell culture was continued until adherent cells revealed an adherent fibroblastic morphology at the 4th passage. Culture mediums were exchanged with fresh mediums after 48–72 h to remove non-adherent cells. The culture was continued by checking with an inverted microscope every day and changing the culture medium every three days. When cells reached 70–80% confluence, cells were harvested with 0.25% trypsin/1 ml EDTA at 7–12 days. In subsequent passages, cells were cultured in T75 flask with of a density 2 × 103 cells/cm2. In this way, proliferation was carried out until the 3rd passage.
Cells isolated from human bone marrow were identified by antibodies in flow cytometry. CD29 (BD Biosciences, Bedford, MA, USA), CD34 (BD Biosciences), CD44 (e-bioscience), CD45 (BD Biosciences), CD73 (BD Biosciences), CD90 (BD Biosciences), CD105 (e-bioscience), CD146 (BD Biosciences) were used as an antibody panel in Navios (Beckman Coulter, Inc., Brea, CA, USA) flow cytometry.
MSCs were evaluated in Passage 4 by flow cytometry according to cell size, granularity and antibody expression. The differentiation capacity of the cells was tested in osteogenic and adipogenic differentiation assays according to standard protocols [18].
MSC characterization
Adherent cells at passage 4 showed fibroblastic morphology and a homogenous cell population was obtained in all cultures. The cells demonstrated 95% positivity for stromal markers including CD105, CD90, CD73, CD29 and CD44. Hematopoietic markers, CD146, CD45 and CD34, were negative. The differentiation capacity of MSCs was confirmed by positive staining in osteogenic and adipogenic differentiation assays after 21 days of culture.
Labeling of MSCs by iron-particles
MSCs were allowed to grow until 80–90% confluence, before changing culture medium. Cells were magnetically labeled to trace their survival after the implantation in vivo with 50 μg/ml ferrum oxide (Endorem; Guerbet, Villepinte, France) and complexed to 0.375 g/ml poly-l-lysine (Sigma-Aldrich Chemical Co., St. Louis, MO, USA). Labeled MSCs were prepared for administration in PBS in a concentration of 1 × 106 cells for each cartilage defect.
Preparation of the PRP
10 ml of intracardiac blood was drawn from one rat. Then 1 ml sodium gluconate anticoagulant was added to the blood and the erythrocytes were separated by centrifugation at 1200 rpm for 5 min. After the erythrocytes were separated remaining plasma was centrifuged again at 1200 rpm for 10 min. Allogenic PRP was used during the surgical procedure.
Animal model
The study was carried out in an animal research laboratory with male, average weight of 350 grams, inbred Wistar albino rats after obtaining local ethics committee approval. The study was conducted in accordance with the Declaration of Helsinki. The rats were randomly divided into 5 groups containing 7 animals in each group. Prior to surgical procedure, all animals were allowed to acclimate to the laboratory environment for a period of 10 days. All rats were kept in 22 ± 2 °C environmental temperature in 12 h light and 12 h darkness cycle in plastic cages with access to food and water. None of the rats had been subjected to any experiments prior to this study.
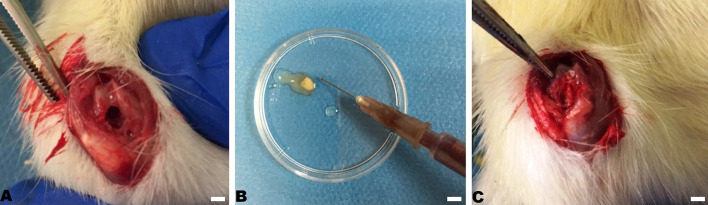
Anesthesia was provided with 50 mg/kg of ketamine hydrochloride and 5 mg/kg xylazine injected intraperitoneally. After the completion of anesthesia, arthrotomy was performed with medial parapatellar approach to the knee joint to reveal the trochlea region of the femur. An osteochondral defect, 2 mm diameter and 3 mm depth, was created with a 2 mm drill bit in subjects’ trochlea region of the femur in all groups, bilaterally. 1 × 106 MSCs and 1 ml PRP were combined with 3 × 3 × 3 mm sized DHP scaffold before the application to the defected area (Fig. 1). No additional procedure was performed in the control group (n = 14). Only the DHP scaffold was applied to the P group (n = 14). The DHP scaffold and 1 × 106 MSCs were applied to the PS group (n = 14). The DHP scaffold and PRP were applied to the PP group (n = 14). The DHP, 1 × 106 MSCs and PRP were applied to the PSP group (n = 14). Rats were then put into separate cages with no restriction of activities. Rats were euthanized after 12 weeks of follow-up for histological evaluation.
Fig. 1.
2mm osteochondral defect on A rat trochlea, B combination of MSCs with DHP scaffold, C application of DHP scaffold to the defect
Histological evaluation
Tissue samples were fixed in buffered 10% neutral formaldehyde solution for 48 h and then were decalcified via immersion in De Castro solution, then rinsed in buffer and dehydrated in a graded ethanol series before embedding in paraffin. 5 micron thick serial sections were cut by a rotary microtome (Thermo Fisher Scientific, Dreieich, Germany). Sections were stained with Hematoxylin–Eosin, Masson’s trichrome and Safranin-O stains for evaluation of cartilage regeneration. Prussian blue stain was used for the cell tracing of labeled MSCs. Preparations were examined with Leica DMi8 research light microscope. Digital images were captured with Leica DFC 7000 digital color camera. Histological scoring was performed with Pineda score [19].
Statistical analysis
Distribution of variables was measured with the Kolmogorov–Smirnov test. The mean values of Pineda scores among groups were analyzed by Kruskal–Wallis test. Dunn’s test was performed to make pairwise comparisons. Confidence interval was accepted as 0.05. The data were made by SPSS v23 (IBM Corporation, Armonk, NY, USA).
Results
Since one rat from the control group was dead during the surgical procedure, 12 femurs were available to be taken into histological evaluation in the control group. 14 femurs were analyzed in P, PS, PP and PSP groups.
Qualitative cartilage histology
In some sections stained with Prussian blue stain, we observed iron labeled particles within chondrocytes which may be an indicator of survival of iron-labeled MSCs and transformation into chondrocytes (Fig. 2).
Fig. 2.
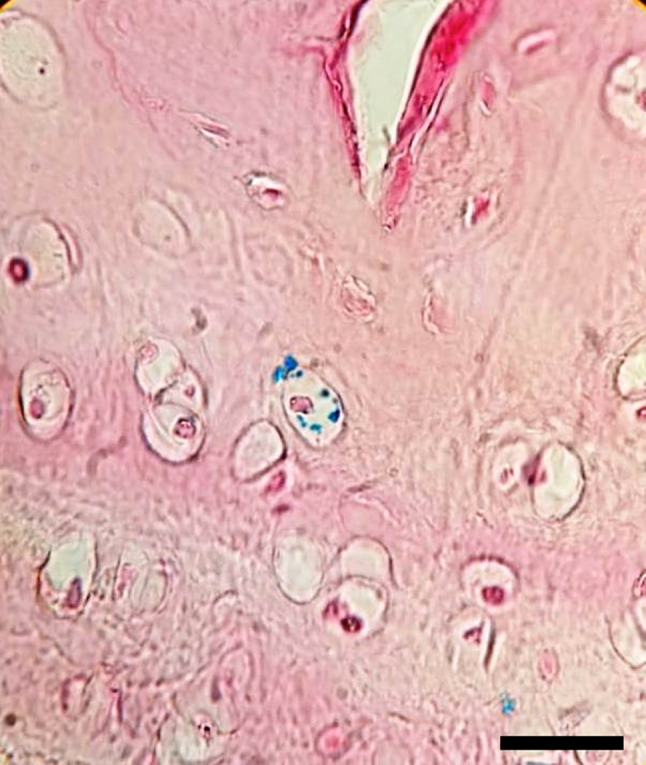
Histology Sect. (40×) stained with Prussian blue stain representing the iron labeled particles within a chondrocyte which may be a signal for the survival of iron-labeled mesenchymal stem cell
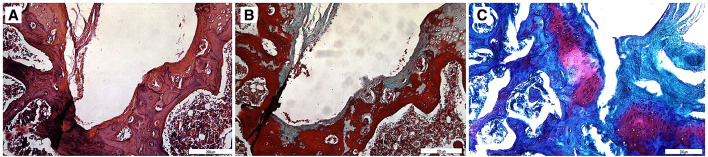
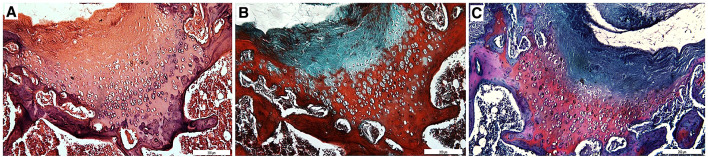
The defects created during the surgical procedure was left to natural healing in the control group. Minimal cartilage formation was observed after 12 weeks. (Fig. 3).
Fig. 3.
Sections (10×) stained with A Hematoxylin–Eosin, B Masson’s trichrome and C Safranin-O. Minimal cartilage formation was observed in control group
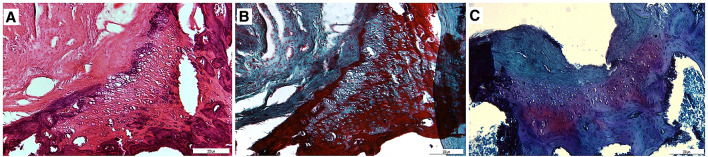
In P group, the chondrocytes were observed as quadruple isogenous groups, not separate from each other in the lacuna, indicating that the regenerated cartilage was in intramembranous growth stage. Morphologically, chondrocytes started to make their own lacuna. Matrix staining was weak thus cartilage matrix production was relatively slow. Regenerated cartilage was not fully connected to bone. P group was more developed than the control group (Fig. 4).
Fig. 4.
Sections (10×) stained with A Hematoxylin–Eosin, B Masson’s trichrome and C Safranin-O. Chondrocytes were found to be quadruple isogenous groups and matrix staining was weak in P group
In PS group, addition of MSCs to the DHP scaffold increased chondrocyte proliferation and matrix production compared to the C group. However, cartilage regeneration of PS group was inferior to PP and PSP groups while it was slightly better than P group. (Figure 5).
Fig. 5.
Sections (10x) stained with A Masson’s trichrome, B Hematoxylin–Eosin and C Safranin-O. Better chondrocyte proliferation and matrix production in PS group compared to the C group
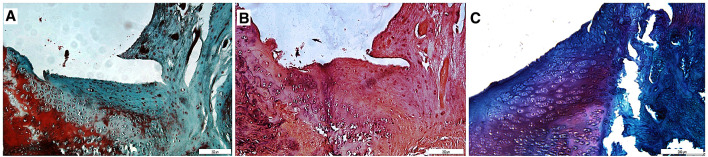
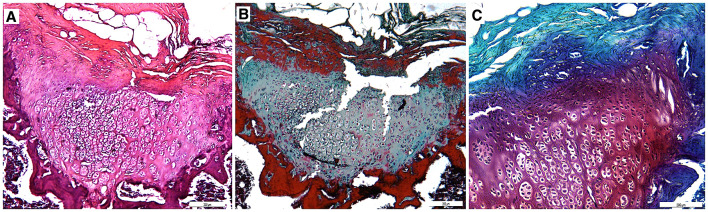
In PP group, chondrocyte clustering was reduced compared to the P group demonstrating that PRP stimulated chondrocyte proliferation. Furthermore, the proportion of chondrocytes observed in developed lacunae was increased which expresses cell development. Matrix production was increased indicating an advanced level of cartilage production. Matrix staining and reconstruction of osteochondral junction were better than C and P groups (Fig. 6).
Fig. 6.
Sections (10×) stained with A Hematoxylin–Eosin, B Masson’s trichrome and C Safranin-O. Chondrocyte clustering was reduced, matrix production and reconstruction of osteochondral junction was significantly better than C group in PP group
In PSP group, cartilage regeneration was better for matrix production, chondrocyte differentiation and restoration of osteochondral junction compared to other groups due to stimulation of matrix production and mutagenicity by PRP and increased number of potential chondrocytes by addition of MSCs. Chondrocytes were individually observed in developed lacunae as mature hyaline cartilage. Furthermore, metachromatic territorial matrix that is rich in sulphated glycosaminoglycans and interterritorial matrix that is rich in collagen and poor in sulphated glycosaminoglycan were observed indicating regeneration of mature articular cartilage (Fig. 7).
Fig. 7.
Sections (10×) stained with A Hematoxylin–Eosin, B Masson’s trichrome and C Safranin-O. Chondrocytes were individually observed in developed lacunae in PSP group
Quantitative histology
The mean Pineda scores of P, PS, PP, and PSP groups were significantly better than control group (p = 0.031, p = 0.002, p < 0.001, p < 0001, respectively) (Fig. 8). There was no statistical difference at mean Pineda scores between P and PS, PP, PSP groups (p = 1.000, p = 0.710, p = 0.192, respectively).
Fig. 8.
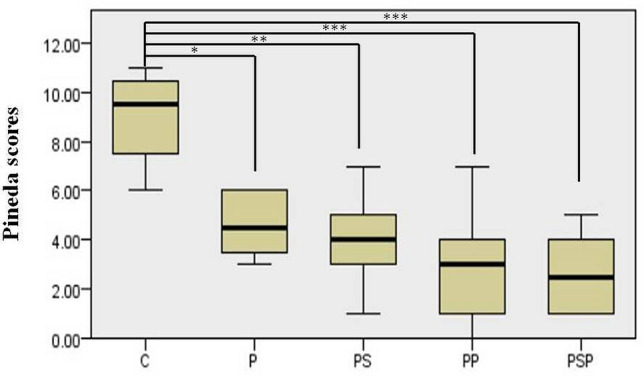
Distribution of mean Pineda scores among groups. *p < 0.05, **p < 0.01, ***p < 0.001
C: control, P: DHP scaffold, PS: DHP scaffold and MSCs, PP: DHP scaffold and PRP, PSP: DHP scaffold, MSCs and PRP
Discussion
The original structure of articular cartilage could not be regenerated with any of the methods described in the literature [2]. Our study was designed based on the triad of bioengineering including a suitable scaffold, stem cells and growth factors [11]. This is the first study examining the effect of MSCs and PRP together with DHP scaffold on a defected articular cartilage model and has shown qualitative and quantitative improvement of the defected cartilage.
Since articular cartilage is a highly specialized tissue and its regeneration capacity is limited, creation of a suitable medium (niche) for regeneration is crucial [20]. The human placenta was chosen as an universal scaffold due to its rich ECM content [8]. In the previous literature, promising results have been reported with use of DHP scaffolds in non-cartilage tissues. Francis et al. [9] demonstrated that DHP hydrogel reduced cardiac scarring in a rat model with ischemic myocardium. Kakabadze et al. [10] reported DHP vessels were re-endothelialized by adjacent native cells and bridged vessel defects in rats. In the current study, DHP scaffold was found to be a promising scaffold on hyaline cartilage regeneration. Human placenta could be transformed from being a waste material to a scaffold for cartilage regeneration by decellularization process.
Stem-cell based treatments have been intensively investigated in cartilage regeneration. However, the xenotransplantation of MSCs in cartilage tissue engineering is an issue to debate. The xenotransplatation of MSCs to immune-competent subjects could potentially start an immune reaction. On the other hand, MSCs are considered to be non-immunogenic because they do not express histocompatibility complex II, they can release non-inflammatory paracrine factors such as TGF-B and prostoglandin E2 [21]. Besides, the immunogenicity of the host tissue might be an important factor in xenotransplantation of MSCs. Articular cartilage has reduced vascularity which can decrease the immunogenicity of the tissue. Wang et al. used human adipose-derived MSCs to treat knee osteoarthritis in a rat model. They reported that all the rats were alive and they did not observe any side effects [22]. In addition, many previous study have shown that human MSCs can survive and be used in xenogenic immun-competent subjects [12, 13, 23]. The xenotransplantation of MSCs have some potential advantages over autotransplantation such as ease of use, timing of harvesting and reduced donor site morbidity [23]. In the current study, human bone marrow derived MSCs were used in a rat model and none of the rats were dead during 12 weeks follow-ups. We acknowledge that further studies utilizing MSCs from the same the recipients would be beneficial to obtain more reliable results.
The survival of xenotransplanted human MSCs remains unclear. A number of studies evaluated the survival of human MSCs in animal models. Li et al. [24] reported that the signals of 2.5 × 106 DiD-labeled MSCs could be observed up to 70 days following injection to knee joint. On the contrary, McKinney et al. [25] could not detect bioluminescent signals of human bone marrow derived MSCs in the rat knee joint at day 7 after injection. Besides, iron labeled MSCs have been reported to be traced in vivo up to 12 weeks [26]. Various survival days of MSCs may be emerged due to the different labeling methods, number of injected cells and different follow-up durations. In the current study, we labeled MSCs with iron particles prior to application to cartilage defect. At the 12 week follow-up, we observed that some particles within chondrocytes were stained with Prussian blue which could be a signal for the survival of the MSCs.
The efficacy of MSCs on cartilage regeneration alone or combination with scaffolds has been investigated in preclinical studies. Kim et al. [27] reported that MSCs with hydrogels inhibit osteoarthritis progression in a rat model. Soulner et al. [28] demonstrated that human neonatal MSCs could prevent osteoarthritis in a rabbit model. However, Dhinsa et al. [29] reported that the use of MSCs alone to treat cartilage injury has very limited effect. In the present study, 1 × 106 MSCs were combined with DHP scaffold. Chondrocyte proliferation and matrix production were significantly better in PS group compared to the control group. PS group had better qualitative histology than P group, however, there was no statistical difference at mean Pineda scores between P and PS groups. The xenogenic origin and the non-optimized number of MSCs could be the reason that MSCs did not enhance the effect of the DHP scaffold.
Growth factors help stem cell transformation into chondrocytes as well as maintaining ECM synthesis [15]. PRP is widely used as a source of growth factors [17]. The mechanism of PRP on cartilage regeneration is still debating. There are controversial studies regarding the effect of PRP on cartilage regeneration. Sun et al. showed that PRP with poly-lactic-glycolic acid improves osteochondral healing in a rabbit model while Kon et al. reported addition of PRP to a triphasic hydroxyapatite-collagen scaffold had a negative effect on osteochondral lesion repair [30, 31]. In the current study, cartilage regeneration was at an advanced stage in the PP group compared to the control group. However, there was no difference at mean Pineda score of P and PP groups. Administration of 1 ml PRP with DHP scaffold did not had a positive effect on articular cartilage regeneration. The discrepancies in the efficacy of PRP regarding cartilage regeneration in the current study and the existing literature may be due to the non-standardized dosage of PRP.
The PSP group was designed based on triad of tissue engineering. It was hypothesized that MSCs would be distributed within the three-dimensional structure of DHP scaffold and cartilage regeneration would be enhanced with the growth factors provided by PRP. In PSP group, chondrocytes were observed individually within the lacuna. Cartilage regeneration was superior to the control group but administration of 1 × 106 MSCs and PRP did not significantly improve the effect of DHP scaffold. The difference in the origins of MSCs and PRP may have influenced the results. A further study using the same species would be valuable to reveal the combined effect of MSCs and PRP.
In the current orthopaedic practice, mosaicplasty and ACI are the most preferred methods aiming cartilage regeneration. Hyaline-like cartilage is obtained from these methods [32, 33]. These methods have major drawbacks. Mosaicplasty could be used in restricted surface area due to its donor site morbidities and ACI requires two-stage surgery [32, 34]. Considering the limitations of existing treatment options, cartilage tissue engineering studies are promising in the treatment of articular cartilage defects. In the present study, P group showed superior cartilage regeneration compared to the control group, and the administration of MSCs and PRP combinations did not significantly improve the effect of DHP. Thus DHP scaffold may have potential in producing hyaline cartilage.
Our study has some limitations. The amount of administered MSCs and PRP were not optimized. Further studies are needed to determine the optimal amount of MSCs and PRP. Present study was conducted on an acute cartilage lesion. A further study would be beneficial to determine the healing capacity of chronic lesions. Since our study was an animal model further studies are required to translate our findings to human applications.
In conclusion, the DHP scaffold appears to be a promising scaffold on hyaline cartilage regeneration. The utility of MSC and PRP combinations with the DHP scaffold did not improve the efficacy of the DHP scaffold.
Acknowledgements
This study was funded by Hacettepe University Research Fund (Grant No. THD-2018-16887). Erdi Özdemir, Abdülsamet Emet and Egemen Turhan decellularized the human placenta and performed the animal experiment. Emine Kılıç and Duygu Uçkan Çetinkaya proliferated the mesenchymal stem cells used in the study. Histological analysis was performed by Ramin Hashemihesar and Ali Celalettin Sinan Yürüker. All the authors had an outstanding contribution to the design of the study, analysis of the data and writing of the report. All authors reviewed final version of the manuscript.
Compliance with ethical standards
Conflict of interest
Authors have declared that there is no conflict of interest.
Ethical statement
The animal studies were performed after receiving approval of the Institutional Animal Care and Use Committee (IACUC) in Hacettepe University (IACUC approval No. 2017/48-07).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lories RJ, Luyten FP. The bone-cartilage unit in osteoarthritis. Nat Rev Rheumatol. 2011;7:43–49. doi: 10.1038/nrrheum.2010.197. [DOI] [PubMed] [Google Scholar]
- 2.Makris EA, Gomoll AH, Malizos KN, Hu JC, Athanasiou KA. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol. 2015;11:21–34. doi: 10.1038/nrrheum.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamplot JD, Schafer KA, Matava MJ. Treatment of failed articular cartilage reconstructive procedures of the knee: a systematic review. Orthop J Sports Med. 2018;6:2325967118761871. doi: 10.1177/2325967118761871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Brien FJ. Biomaterials & scaffolds for tissue engineering. Mater Today (Kidlington) 2011;14:88–95. doi: 10.1016/S1369-7021(11)70058-X. [DOI] [Google Scholar]
- 5.Ge Z, Li C, Heng BC, Cao G, Yang Z. Functional biomaterials for cartilage regeneration. J Biomed Mater Res A. 2012;100:2526–2536. doi: 10.1002/jbm.a.34147. [DOI] [PubMed] [Google Scholar]
- 6.Spang MT, Christman KL. Extracellular matrix hydrogel therapies: In vivo applications and development. Acta Biomater. 2018;68:1–14. doi: 10.1016/j.actbio.2017.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoshiba T, Lu H, Kawazoe N, Chen G. Decellularized matrices for tissue engineering. Expert Opin Biol Ther. 2010;10:1717–1728. doi: 10.1517/14712598.2010.534079. [DOI] [PubMed] [Google Scholar]
- 8.Leonel LCPC, Miranda CMFC, Coelho TM, Ferreira GAS, Caaada RR, Miglino MA, et al. Decellularization of placentas: establishing a protocol. Braz J Med Biol Res. 2017;51:e6382. doi: 10.1590/1414-431x20176382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francis MP, Breathwaite E, Bulysheva AA, Varghese F, Rodriguez RU, Dutta S, et al. Human placenta hydrogel reduces scarring in a rat model of cardiac ischemia and enhances cardiomyocyte and stem cell cultures. Acta Biomater. 2017;52:92–104. doi: 10.1016/j.actbio.2016.12.027. [DOI] [PubMed] [Google Scholar]
- 10.Kakabadze Z, Kakabadze A, Chakhunashvili D, Karalashvili L, Berishvili E, Sharma Y, et al. Decellularized human placenta supports hepatic tissue and allows rescue in acute liver failure. Hepatology. 2018;67:1956–1969. doi: 10.1002/hep.29713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher JN, Tessaro I, Bertocco T, Peretti GM, Mangiavini L. The application of stem cells from different tissues to cartilage repair. Stem Cells Int. 2017;2017:2761678. doi: 10.1155/2017/2761678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niemeyer P, Szalay K, Luginbühl R, Südkamp NP, Kasten P. Transplantation of human mesenchymal stem cells in a non-autogenous setting for bone regeneration in a rabbit critical-size defect model. Acta Biomater. 2010;6:900–908. doi: 10.1016/j.actbio.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Lin CC, Lin SC, Chiang CC, Chang MC, Lee OK. Reconstruction of bone defect combined with massive loss of periosteum using injectable human mesenchymal stem cells in biocompatible ceramic scaffolds in a porcine animal model. Stem Cells Int. 2019;2019:6832952. doi: 10.1155/2019/6832952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tatari H. The structure, physiology, and biomechanics of articular cartilage: injury and repair. Acta Orthop Traumatol Turc. 2007;41(Suppl 2):1–5. [PubMed] [Google Scholar]
- 15.Goldring MB. Chondrogenesis, chondrocyte differentiation, and articular cartilage metabolism in health and osteoarthritis. Ther Adv Musculoskelet Dis. 2012;4:269–285. doi: 10.1177/1759720X12448454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94:3791–3799. doi: 10.1182/blood.V94.11.3791. [DOI] [PubMed] [Google Scholar]
- 17.Smyth NA, Murawski CD, Fortier LA, Cole BJ, Kennedy JG. Platelet-rich plasma in the pathologic processes of cartilage: review of basic science evidence. Arthroscopy. 2013;29:1399–1409. doi: 10.1016/j.arthro.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Uckan D, Kilic E, Sharafi P, Kazik M, Kaya F, Erdemli E, et al. Adipocyte differentiation defect in mesenchymal stromal cells of patients with malignant infantile osteopetrosis. Cytotherapy. 2009;11:392–402. doi: 10.1080/14653240802582083. [DOI] [PubMed] [Google Scholar]
- 19.Pineda S, Pollack A, Stevenson S, Goldberg V, Caplan A. A semiquantitative scale for histologic grading of articular cartilage repair. Acta Anat. 1992;143:335–340. doi: 10.1159/000147272. [DOI] [PubMed] [Google Scholar]
- 20.Burdick JA, Mauck RL, Gorman JH, 3rd, Gorman RC. Acellular biomaterials: an evolving alternative to cell-based therapies. Sci Transl Med. 2013;5:176ps4. doi: 10.1126/scitranslmed.3003997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin CS, Lin G, Lue TF. Allogeneic and xenogeneic transplantation of adipose-derived stem cells in immunocompetent recipients without immunosuppressants. Stem Cells Dev. 2012;21:2770–2778. doi: 10.1089/scd.2012.0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z, Zhu H, Dai S, Liu K, Ge C. Alleviation of medial meniscal transection-induced osteoarthritis pain in rats by human adipose derived mesenchymal stem cells. Stem Cell Investig. 2020;7:10. doi: 10.21037/sci-2020-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jang KM, Lee JH, Park CM, Song HR, Wang JH. Xenotransplantation of human mesenchymal stem cells for repair of osteochondral defects in rabbits using osteochondral biphasic composite constructs. Knee Surg Sports Traumatol Arthrosc. 2014;22:1434–1444. doi: 10.1007/s00167-013-2426-y. [DOI] [PubMed] [Google Scholar]
- 24.Li M, Luo X, Lv X, Liu V, Zhao G, Zhang X, et al. In vivo human adipose-derived mesenchymal stem cell tracking after intra-articular delivery in a rat osteoarthritis model. Stem Cell Res Ther. 2016;7:160. doi: 10.1186/s13287-016-0420-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKinney JM, Doan TN, Wang L, Deppen J, Reece DS, Pucha KA, et al. Therapeutic efficacy of intra-articular delivery of encapsulated human mesenchymal stem cells on early stage osteoarthritis. Eur Cell Mater. 2019;37:42–59. doi: 10.22203/eCM.v037a04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bull E, Madani SY, Sheth R, Seifalian A, Green M, Seifalian AM. Stem cell tracking using iron oxide nanoparticles. Int J Nanomedicine. 2014;9:1641–1653. doi: 10.2147/IJN.S48979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim JE, Lee SM, Kim SH, Tatman P, Gee AO, Kim DH, et al. Effect of self-assembled peptide-mesenchymal stem cell complex on the progression of osteoarthritis in a rat model. Int J Nanomedicine. 2014;9(Suppl 1):141–157. doi: 10.2147/IJN.S54114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saulnier N, Viguier E, Perrier-Groult E, Chenu C, Pillet E, Roger T, et al. Intra-articular administration of xenogeneic neonatal Mesenchymal Stromal Cells early after meniscal injury down-regulates metalloproteinase gene expression in synovium and prevents cartilage degradation in a rabbit model of osteoarthritis. Osteoarthritis Cartilage. 2015;23:122–133. doi: 10.1016/j.joca.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Dhinsa BS, Adesida AB. Current clinical therapies for cartilage repair, their limitation and the role of stem cells. Curr Stem Cell Res Ther. 2012;7:143–148. doi: 10.2174/157488812799219009. [DOI] [PubMed] [Google Scholar]
- 30.Sun Y, Feng Y, Zhang CQ, Chen SB, Cheng XG. The regenerative effect of platelet-rich plasma on healing in large osteochondral defects. Int Orthop. 2010;34:589–597. doi: 10.1007/s00264-009-0793-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kon E, Filardo G, Delcogliano M, Fini M, Salamanna F, Giavaresi G, et al. Platelet autologous growth factors decrease the osteochondral regeneration capability of a collagen-hydroxyapatite scaffold in a sheep model. BMC Musculoskelet Disord. 2010;11:220. doi: 10.1186/1471-2474-11-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hangody L, Dobos J, Baló E, Pánics G, Hangody LR, Berkes I. Clinical experiences with autologous osteochondral mosaicplasty in an athletic population: a 17-year prospective multicenter study. Am J Sports Med. 2010;38:1125–1133. doi: 10.1177/0363546509360405. [DOI] [PubMed] [Google Scholar]
- 33.Henderson I, Francisco R, Oakes B, Cameron J. Autologous chondrocyte implantation for treatment of focal chondral defects of the knee–a clinical, arthroscopic, MRI and histologic evaluation at 2 years. Knee. 2005;12:209–216. doi: 10.1016/j.knee.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 34.Gooding CR, Bartlett W, Bentley G, Skinner JA, Carrington R, Flanagan A. A prospective, randomised study comparing two techniques of autologous chondrocyte implantation for osteochondral defects in the knee: Periosteum covered versus type I/III collagen covered. Knee. 2006;13:203–210. doi: 10.1016/j.knee.2006.02.011. [DOI] [PubMed] [Google Scholar]