Abstract
Background:
The breast cancer microenvironment contains a variety of stromal cells that are widely implicated in worse patient outcomes. While many in vitro models of the breast tumor microenvironment have been published, only a small fraction of these feature adipocytes. Adipocytes are a cell type increasingly recognized to have complex functions in breast cancer.
Methods:
In this review, we examine findings from recent examples of in vitro experiments modeling adipocytes within the local breast tumor microenvironment.
Results:
Both two-dimensional and three-dimensional models of adipocytes in the breast tumor microenvironment are covered in this review and both have uncovered interesting phenomena related to breast tumor progression.
Conclusion:
Certain aspects of breast cancer and associated adipocyte biology: extracellular matrix effects, cell-cell contact, and physiological mass transport can only be examined with a three-dimensional culture platform. Opportunities remain for innovative improvements to be made to in vitro models that further increase what is known about adipocytes during breast cancer progression.
Keywords: Adipocyte, Breast cancer, 3D culture, Microenvironment, Phenotypic assay
Introduction
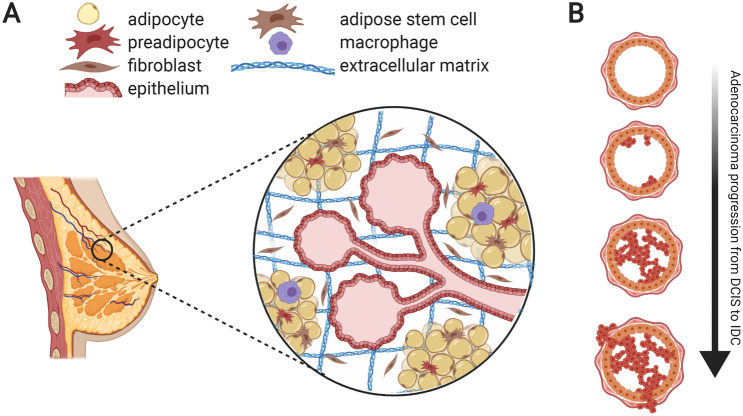
The human breast is an organ containing a wide range of cell types. Epithelial cells, the organ’s primary functional cell type, comprise a network of nutrient-secreting lobules and ducts. The architecture of the breast positions epithelial cells in close proximity to numerous other stromal cell types, which support and regulate breast epithelium (Fig. 1A). Fibroblasts [1], immune cells such as macrophages [2], adipocytes [3], and stromal stem cells [4] all function together within layers of surrounding connective tissue.
Fig. 1.
A The microenvironment around the epithelial breast lobule and duct contains a 3D arrangement of diverse cell types and ECM molecules, creating a unique challenge for tissue engineering in this geometry. B These components have been hypothesized to each possess a role in the progression of primary adenocarcinomas, as early as the transition of ductal carcinoma in situ to invasive ductal carcinoma, through paracrine signaling and matrix remodeling
Adipocytes, specifically, have been shown to play important roles in breast tissue development and maintenance [5, 6]. In circumstances where carcinogenesis occurs in the breast, most tumors are adenocarcinomas, meaning they arise from the epithelial cell structures. Adipocytes regulate both early and late stages of tumor progression, not only in breast cancers (BCs), but in a variety of other solid tumors [7–9].
In breast epithelial tissue, a tumor begins as uncontrolled proliferation of epithelial cells, forming a mass that begins to occlude the luminal space. This is called a ductal carcinoma in situ (DCIS) [10]. In a majority of cases the tumor remains non-invasive, however, extracellular matrix degradation and myoepithelial cell degeneration may contribute towards eventual breach of the basement membrane dividing epithelial and stromal tissue [11]. The tumor cells consequently invade into the surrounding stromal tissue and the tumor progresses from DCIS to invasive ductal carcinoma (IDC) (Fig. 1B).
The role of adipocytes in breast tumor progression has risen in interest, in part, due to the complex relationship between clinical obesity (body mass index > 30) and BC. Generally, obesity is considered a risk factor for BC, but even earlier studies with less patient stratification have been conflicted about obesity and BC outcomes: BC metastasis is higher, survival worse, and disease-free intervals shorter [12], but incidence correlations vary depending on race, age, and time of weight gain [13]. In a more recent study in the United States, the AMBER study, race was shown to be a factor for both obesity and triple-negative breast cancer (TNBC) risk, but it failed to generally link this most aggressive BC sub-type to obesity among African Americans [14]. The strongest conclusion from the AMBER study was that obesity may affect BC progression in different ways, suggesting a need for scientists to grapple with these mechanisms on a deeper level. A thorough review of studies on the link between obesity and TNBC is available [15].
Another reason that obesity does not always directly link to BC is the heterogeneity in associated medical co-morbidities in obese persons. Individuals with excessive adipose tissue, as many as thirty percent, can still retain normal metabolic health, as measured by insulin sensitivity and cytokine levels [16]. Excessive adipose tissue does not always indicate pro-inflammatory, metabolically-diseased adipose tissue, which is known to enhance breast tumor progression [7].
Numerous review articles and original research articles have addressed the subject of how adipose tissue influences solid tumor progression and worsens prognosis across cancers [7–9, 17–19], and BC specifically [3, 20–23]. Others touch on adipokines, such as leptin, pro-inflammatory [24] and pro-angiogenic [25] cytokines, free fatty acid metabolite transfer to provide alternative energy sources and adjust tumor metabolism [26], ECM degradation and remodeling such as with matrix metalloproteinases (MMPs), and adipocyte lipolysis and de-differentiation into a cancer-associated adipocyte or even cancer-associated fibroblast phenotype [27]. Adipocyte dysregulation and paracrine signaling with malignant cells may also contribute to drug resistance in BC [28]. These adipocyte-derived paracrine signals have been shown to be responses to early sensing of nearby tumor progression, but much less is understood about which initial signals adipocytes from tumor cells at first contact.
Stromal tissue that surrounds invasive BC has emerged as a much-needed topic of study for better understanding the tumor microenvironment. Previous reviews highlight contributions of in vitro systems to studies of breast cancer interactions with stroma, including ECM, fibroblasts, vasculature, and immune cells [29–31]. These studies have greatly illuminated understanding of the breast tumor microenvironment. By nature, systems that contain more than one cell type are difficult to design and refine, which limits the complexity that any particular in vitro system can re-create. However, increasing complexity within in vitro systems with thorough characterization opens the scope of questions that can be answered by design, and potentially increases the impact of data obtained from them.
Our review, in turn, focuses specifically on what has been learned about adipocytes in the breast tumor microenvironment using in vitro systems. It then brings up considerations in choosing how to study the breast tumor microenvironment in vitro, though with less examination on choosing the right adipocyte cell model, which other references provide [3, 32–35]. Finally, we focus on how these particular in vitro models can be improved for mechanistic studies and potentially diagnostic analyses of patient tumors.
Two-dimensional platforms
Much of what has been uncovered about adipocytes-BC cell interactions has been done with two-dimensional (2D) culture models, as they are relatively simple to utilize. More sophisticated, 2D-inspired models also exist, however, and may be of interest.
Transwell migration assays
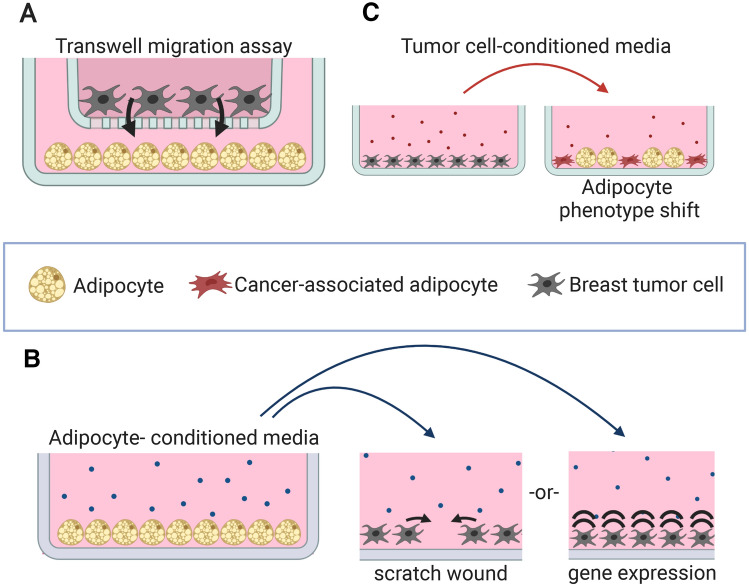
Transwells afford the ability for multiple cell populations to sense each other through soluble biochemical signals and respond via cell migration through a porous membrane (Fig. 2A). Previously-published assays utilize BC cells seeded in the upper chamber with either adipocytes or adipocyte-conditioned media in the lower chamber [25, 36, 37]. These studies have implicated adipocyte-secreted molecules with inducing increased BC cell migration through the porous membrane in a contact-free manner. Multiple studies have identified important proteins that increase BC cell migration and are attributed to adipocyte proximity. Adipocyte-secreted IL-6 and leptin modulate lysyl hydroxylase-2 in MDA-MB-231 cells, increasing migratory behavior in co-culture [38]; preadipocyte-secreted IL-6 increases migration of the ductal carcinoma in situ (DCIS) cell line MCF10DCIS.com, suggesting that IL-6-secreting local adipose tissue cells may promote progression in the earliest stages of BC [39]; and S100A7 modulation in MCF-7 cells has a role in increased migration upon signaling from adipocytes [37].
Fig. 2.
Examples of previously-published 2D adipose/breast tumor cell culture systems. A Transwell migration assay to measure tumor cell migration towards adipocyte-secreted chemoattractants. B Using adipocyteconditioned media for modulation of invasiveness in a scratch-wound assay or modulation of other phenotypic markers of breast tumor cells in 2D culture. C Testing the effects of tumor cell-secreted factors on adipocytes using tumor cell-conditioned media in a 2D adipocyte differentiation assay
Transwell co-culture assays
Assays using transwells have revealed other pro-invasive and metastatic phenotype shifts in BC cells induced by adipocytes. In one paper this included proliferation increase, increased epithelial-mesenchymal transition caused by increased expression of Twist1, and greater MMP9 expression [36]. Another used radio-labeled lipids to show direct free fatty acid transfer from adipocyte to BC cells, which were revealed to reprogram the tumor cell metabolism from a normal aerobic respiratory state to a glycolysis/fatty acid oxidation-heavy state [26].
Conditioned media
Using conditioned media (CM) in a basic culture platform enables one cell type to respond to secretory signals of another cell type without the confounding nature of two-way communication (Fig. 2B). Adipocyte CM increases MDA-MB-231 cell closure motility in a scratch-wound assay, caused in part by the chemokine CCL5 [40], which is known to promote invasion and metastasis of BC in vivo [41]. CM from adipocyte/MCF-7 co-cultures increases MCF-7 scratch wound closure compared to both adipocyte CM and control media, with IGFBP2 having a role [42].
Adipocyte phenotypic assays
While the preceding paragraphs focused on how adipocytes alter BC cell behavior, other assays have studied how BC cells impact adipocyte behavior. Using either co-culture with BC cells or CM, adipocyte phenotype has been examined as a measure of the deleterious effect of a breast tumor on adipocyte maturation (Fig. 2C), as observed in vivo [43]. CM from BC/adipocyte co-cultures reduces lipid droplet formation in new preadipocyte differentiation with highly-invasive MDA-MB-231 cells showing a stronger effect than less-aggressive MCF-7 cells [42]. In differentiated adipocytes, co-culture with ZR 75.1 BC cells results in delipidation and reduced expression of healthy adipokines and transcription factors [27]. Instead, adipocytes co-cultured with ZR 75.1 BC cells increase pro-inflammatory gene transcription [27]. In a monoculture test of adipocyte phenotypes, adipose stromal vascular fraction cells isolated from invasive cancer stroma showed less lipid accumulation, less adipogenic gene expression, and increased pro-inflammatory gene expression than controls from benign stromal tissue [44]. Another monoculture experiment showed that MMP11-knockout adipocytes demonstrated better adipogenesis than wild type cells, suggesting that BC-induced MMP11 expression may directly impede adipocyte phenotype maintenance [45].
Microfluidic platforms
Microfluidic devices have been used extensively to study the breast tumor microenvironment and make use of compartmentalized architectures with 2D interfaces to mimic separations between the epithelial lumen, stromal compartment, vascular lumen, or even lymphatic vessels [46]. Examples have modeled chemotherapeutic drug transport from microvasculature into the stromal tumor compartment [47], and DCIS embedding in epithelial cell layers in the presence of stromal cells [48]. While included in this section, these examples also feature 3D architectures, such as where cells are encapsulated in hydrogels within the device channel. Published microfluidic platforms that examine the role of adipocytes in the breast tumor microenvironment are not well-represented, however, perhaps because adipocytes do not experience fluid shear forces in native tissue, a common feature in microfluidic devices. Nevertheless, cell encapsulation in a 3D gel layer as done with human mammary fibroblasts in reference 48 could serve as a feasible means to examine the role of adipocytes in DCIS progression on a similar platform.
Three-dimensional platforms to study adipocytes and breast cancer in vitro
Three-dimensional (3D) experimental platforms recapitulate particular features of microenvironment that breast tumor cells experience in their progression into surrounding tissue. While 3D tumor models have become more common and accessible [49], platforms that recreate adipose tumor stroma in 3D remain somewhat rare, perhaps owing to of the time-intensive nature of culturing adipose tissue and early stage of developing therapeutics which target adipose stroma. We examine published platforms from the simplest to most complex. These platforms are additionally summarized in Table 1.
Table 1.
Three-dimensional culture platforms for studying the role of adipose tissue in the breast tumor microenvironment
| Platform description | Key finding | References |
|---|---|---|
| Spheroid-forming assay | Adipose stem cells, in vitro adipocytes, primary adipocytes increase spheroid-forming capacity of MDA-MB-231, MCF7, T47D and other cell lines via co-culture pre-treatment or co-culture | [50] |
| Transwell Matrigel invasion assay | Cancer-associated adipocyte conditioned media increases serum gradient-induced MCF7 invasion | [42] |
| Adipocytes increase invasion of MDA-MB-231, MCF7 and other cell lines during co-culture | [26, 36] | |
| Adipocytes in serum-free media induce similar invasiveness to serum in MDA-MB-231 cells | [40] | |
| Adipocyte-induced fibroblasts induce more invasion than mature adipocytes | [51] | |
| Blocking IL-6 signaling during adipocyte pre-conditioning step reduces invasion of ZR75.1 cells | [27] | |
| Radial matrix invasion assay | Leader cells in the invasive protrusions of primary tumor samples, across human BC sub-types, embedded in collganen hydrogels express the basal epithelial marker cyokeratin-14, shown to be an important attribute of leading invasive cells | [52] |
| Adipocyte co-culture prior to spheroid encapsulation in Matrigel increases outward invasion in MDA-MB-231 and MDA-MB-468 cells | [38] | |
| Spheroid co-culture of SUM159-PT cells plus adipocyte-derived fibroblasts, but not mature adipocytes, increases outward invasion | [51] | |
| Angiogenesis assay | Adipocyte-conditioned media induces greater angiogenesis than fibroblast-conditioned and control media for bovine endothelial cells encapsulated in Matrigel | [25] |
| Endotrophin, an adipocyte-secreted molecule enhances MS-1 endothelial cell migration across a Transwell membrane, among other effects | [53] | |
| Adipose tissue derived from a murine tumor model develops greater vessel structures than normal adipose tissue when embedded in Matrigel | [54] | |
| Scaffold and hydrogel-free models | A biobank of organoids derived from > 100 BC tumor samples highlights disease heterogeneity and usefulness for pre-clinical drug development studies | [55] |
| Mammary organoids cultured from MCF10A epithelial cells show collagen deposition and EMT responses to EMT agents such as TGF-β and CoCl2 | [56] | |
| MCF10A-comprised organoids acquire a geometrically inverted morphology when formed in dilute Matrigel, enabling use for observing MDA-MB-231 cell invasion as a DCIS progression model | [57] | |
| Co-culture with scaffold | Tri-culture of MCF10A, mammary fibroblasts and adipose stem cells reduces epithelial proliferation, yet enhances duct formation and casein milk protein production | [58] |
| 3D bioprinted tissue | Encapsulation of hASCs in a decellularized adipose tissue-based bio-ink induces adipogenesis in bioprinted structures suitable for implantation | [59] |
| Human preadipocytes in a geletin-based bio-ink undergo adipogenesis after 3D-printing | [60] | |
| Direct co-culture of adipocytes and MCF7 cells is feasible in alginate bio-ink, with viability maintained | [61] | |
| Biofabricated microenvironment | MCF7 duct with hASC stroma architecture predicts anastrazole sensitivity w.r.t. hASC Body Mass Index better than 2D co-culture | [62] |
| Adipocytes encapsulated and differentiated in a collagen plug gel enhance inward invasion of MDA-MB-231 cells | [63] |
Spheroid forming assay
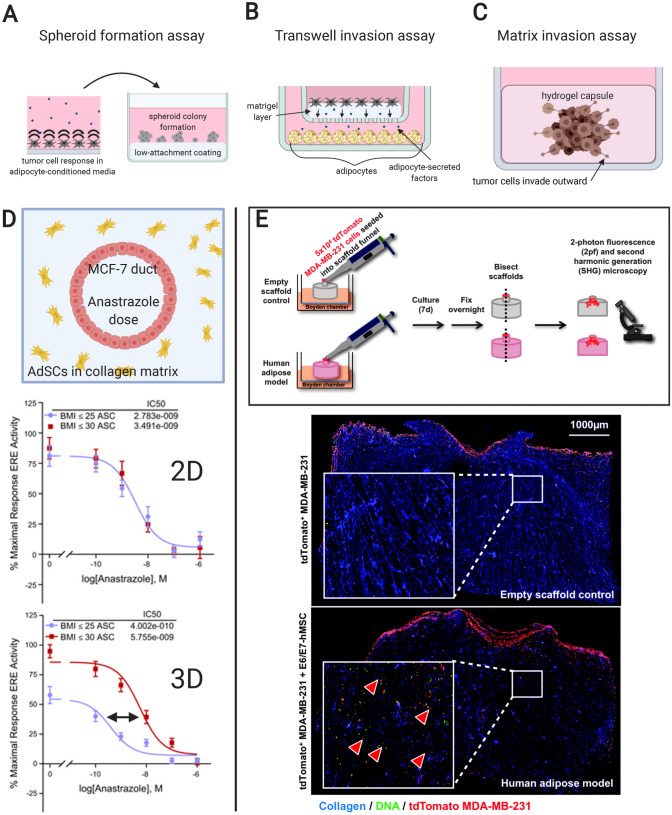
Tumor cell aggregates, or spheroids, have a wide number of uses in cancer research and drug development [64]. One way that spheroids have been used to study paracrine signaling between adipocytes and BC cells is the spheroid forming assay, a kind of 3D proliferation assay. In this system, treated tumor cells are sparsely seeded on a low-attachment surface, such as agarose, and spheroid formation is tallied (Fig. 3A) [50]. This quantifies stemness, a property of tumor cells based on their capability to singly propagate a tumor or re-populate it after treatment [64]. Several breast tumor cell lines, including MDA-MB-231, MCF7, T47D, DT28 and HMLER3 show increased spheroid formation after co-culture with adipocytes, suggesting a paracrine signaling mechanism exists in the tumor microenvironment resulting in increased tumor cell stemness [50].
Fig. 3.
Previously-published adipose/breast tumor experiments that incorporate elements unique to 3D cultures. A Tumor cells exposed to adipocyte-conditioned media passaged into an agarose-coated surface to test spheroid-forming capability. B Linear invasion assay for breast tumor cells using a transwell platform with layered Matrigel. C Radial invasion assay for a breast tumor spheroid embedded in Matrigel. D Schematic and data from Morgan et al. displaying the cross-section of a tissue-engineered cancerous duct surrounded by adipose stromal cells (AdSCs) from donor patients to demonstrate AdSC modulation of anastrazole sensitivity in an intra-ductal MCF-7 cell population (reproduced with permission from reference 64). The 3D model shows clear distinction between obese and normal weight adipose cell donors compared to data collected from a simple 2D model. Note: The data in red should be correctly recognized as “BMI ≥ 30”. E Schematic and data from Hume et al. displaying how an adipocyte-laden collagen gel stimulates increased interior invasion than a blank control by MDA-MB-231 cells seeded on the upper surface of the gel (reproduced with permission from reference 72). Invaded cells are indicated by red arrowheads in the inset higher magnification view
Linear Matrigel invasion assay
These experiments bear similarity to transwell migration assays, except that a layer of Matrigel coats the porous membrane of the upper chamber for both soluble factors and cells to traverse (Fig. 3B). In basic experiments, researchers have shown that adipocyte co-culture increases invasion of multiple cell lines through Matrigel in a transwell system whether in the absence [40] or presence of serum [26, 36, 51]. The latter reference further presents an adipocyte-derived fibroblast model with even greater BC cell attraction than normal adipocytes. The Matrigel invasion assay also distinguishes reduced invasiveness of murine BC cells co-cultured with adipocytes in the presence of IL-6 antibody, compared to no antibody, suggesting a key role for IL-6 in BC progression in the tumor microenvironment [27].
Radial matrix invasion assay
As an alternative to the transwell-based linear Matrigel invasion assay, this assay begins with a tumor cell spheroid encapsulated in a hydrogel such as collagen or Matrigel (Fig. 3C). Instead of linear invasion as with a transwell, radial cell invasion outward from the spheroid is measured. In BC cell monocultures encapsulated in collagen, this assay pinpoints cytokeratin-14 as a key protein expressed in cells leading outward invasion [52]. Using adipocyte co-culture as a pre-treatment, He et al. [38] demonstrates that breast tumor spheroids are more invasive when encapsulated in Matrigel than monoculture controls. Bochet et al. [51] shows that while normal adipocyte co-culture increased SUM159PT BC cell invasion in a Matrigel invasion assay, only adipocytes having undergone delipidation to become adipose-derived fibroblasts increase collagen matrix invasion from a co-culture spheroid.
Three-dimensional angiogenesis assays
Stromal cells, particularly adipocytes, have been implicated in angiogenesis within the tumor microenvironment, providing tumors with the means of sustenance and growth [18, 53, 54, 65]. Published experiments measuring endothelial cell function use Matrigel encapsulation and endothelial migration. Adipocyte-conditioned media has been shown to enhance angiogenesis of endothelial cells encapsulated in Matrigel over fibroblast-conditioned media [25]. Additionally, as a cleavage product of adipocyte-derived Collagen Type VI in the breast tumor-microenvironment, endotrophin is shown to enhance endothelial cell migration in vitro [53]. In an experiment with resected adipose tissues embedded in Matrigel, Wagner et al. [54] shows that adipose tissue proximal to a murine tumor model demonstrates extensive vascular sprouting, compared to normal adipose tissue controls which demonstrate none.
Scaffold-free tissue models
The term ‘organoid’ is often used loosely to indicate any miniaturized 3D in vitro-cultured tissue with spontaneous self-organization in absence of a scaffold or encapsulation material. Collecting patient tumor samples, Sachs et al. [55] presents a breast cancer organoid biobank to catalog patient-to-patient heterogeneity with respect to histological features, DNA mutations, gene expression and drug sensitivity. Spherical enveloping structures of normal mammary epithelial cells have also demonstrated usefulness for studying epithelial-mesenchymal transition within the mammary epithelial duct [56], as well as DCIS progression into IDC through the epithelial layer and adjacent basement membrane [57]. Currently, scaffold-free 3D models of BC have only been investigated using normal epithelial or tumor cells, and have not seen inclusion of a stromal cell type. Expanding organoid cultures to include BC cells and relevant stromal cells could make scaffold-free models that are conducive to high-throughput analyses of tumor microenvironments and potential therapeutic interventions.
Bioprinted adipose tissue and three-dimensional microphysiological systems
Several bioengineered culture platforms have been developed to study breast epithelial and stromal interactions [29], though far fewer have incorporated an adipocyte component [58]. 3D bioprinting is one technology becoming increasingly favored in tissue engineering for its ability to produce unique tissue architectures. In recent years decellularized matrix and gelatin hydrogel have both shown promise as suitable substrates for adipose bioprinting [59, 60]. Basic characterization of adipocyte-breast tumor cell co-culture in a gelatin/alginate-based bio-ink has also been attempted [61].
A biofabricated device published by Morgan et al. [62] describes an MCF-7 cell mammary duct surrounded by undifferentiated human adipose stromal cells, and shows how the body mass index (BMI) of the donor source affects resistance to aromatase therapy by the BC cells (Fig. 3D). Interestingly, a simplified 2D co-culture model fails to distinguish between BMI of the donors to the same degree.
Adipocyte differentiation and maintenance in a 3D bioengineered platform is a relatively new concept [66–72]. One recent example applies 3D-differentiated adipocytes to the study of BC invasion [63]. Hume et al. describes seeding human breast-derived mesenchymal stem cells in a collagen plug gel and, after allowing for proliferation and adipocyte differentiation, testing the adipocyte-laden gel against a blank gel in an invasion assay where MDA-MB-231 cells are seeded on the top surface (Fig. 3E). The results show that adipocytes enhanced interior invasion of the MDA-MB-231 cells compared to a blank control gel.
Considerations for selecting two-dimensional and three-dimensional culture models
The experimental question of interest ultimately determines whether a 2D or 3D culture works best. In some situations, 2D culture may require less optimization and still produce meaningful results. In other cases, only 3D culture provides accurate and realistic results. There are several considerations for choosing between 2D and 3D systems.
Proliferative constraints
Most tumor cells have more proliferative constraints when cultured in 3D compared to a 2D monolayer [73, 74]. In conventional monolayer 2D cultures, all cells have the same access to nutrients enabling them to proliferate until reaching confluence. 3D culture is more complex than 2D due the gradients formed for nutrients, waste, and dissolved gases like oxygen and carbon dioxide, as found with spheroid structures [75–78]. The proliferative constraints on 3D culture are thus based on tissue geometry. The surface area-to-volume ratio decreases as size increases, causing slower diffusion of nutrients and gases in larger 3D tissues. In 3D tumor cell cultures especially, the inverse gradients of nutrient access and waste buildup make cell proliferation feasible on the tissue periphery, approximately a 100 micron-thick layer, while the interior is mostly filled with necrotic and quiescent cells [79, 80]. Because breast tumors in vivo begin developing vasculature upon reaching the millimeter scale in diameter [81], 3D tumor cultures without vasculature remain physiologically relevant at the micro scale.
3D-cultured adipose tissue may be generated with encapsulated cells in hydrogel as conducted in Ref. [63]. Such a hydrogel allows ample exchange of nutrients and waste between the construct and culture medium to maintain cell viability. Culturing adipocytes in 3D without encapsulation, but instead through aggregation followed by differentiation, as conducted in references [66, 68, 70], will be more subject to similar gradients as with tumor spheroids, where adipocytes on the interior of larger aggregates will mature less than peripheral cells and possibly lose viability.
Drug efficacy in 3D cultures
In general, 3D culture architectures not only affect cell access to nutrients and waste metabolism, but also delivery of therapeutic agents. Spheroid culture, with layers of cells enveloping each other, significantly impacts the rate of molecular mass transport to constituent cells [82]. 3D-cultured BC cell lines within a dense spheroid can be more resistant toward certain chemotherapeutic drugs such as 5-fluorouracil and doxorubicin. These drugs, which inhibit proliferation, only act most effectively on tumor cells proliferating on the cultured tumor surface, leaving the quiescent cells in the interior relatively unharmed [77, 83].
Reduced therapeutic effect is also observed in 3D models of BC cell lines, compared to 2D when exposed to paclitaxel, an apoptotic drug, because it enables the surviving interior cells to re-populate the tumor construct after surviving treatment [77]. This heterogeneity in cell behavior and drug exposure observed in 3D cultures contrasts with the more uniform hyper-proliferation and drug exposure observed in 2D culture. The geometrical constraints and resulting heterogeneity in 3D models can make them a better predictor for in vivo outcomes [79, 84]. These factors should be considered during studies such as drug screening, in which the rate of drug uptake may be important.
Intra-cellular signaling and gene expression
Some tumor cell signaling cascades are more evident in 3D models [85]. Classic examples can be found in signaling cascades between β1-integrin and epidermal growth factor receptors that are activated in 3D models but not in 2D models of breast cell cultures [86]. Another study of cancer cells reports that the mTOR1-AKT pathway response to drug inhibition is present only in 3D culture [87]. Lee et al. [88] shows self-organization of mammary epithelial cells (MECs) when 2D surface culture is disrupted, and modulation of casein and the milk protein butyrophilin [89]. In these experiments, MECs assemble into a cuboidal shape and begin expressing milk proteins due to interaction with ECM. These 3D culture effects are due to a combination of ECM composition, biomechanical environments that are less stiff than 2D cultures, and cellular interaction changes that result.
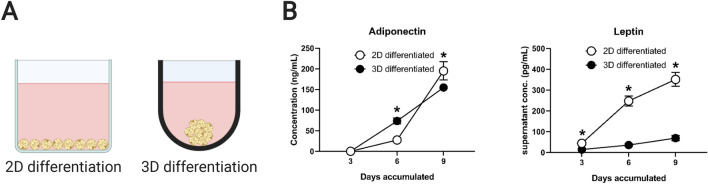
Adipocytes can also show different behavior in 3D compared to 2D. Figure 4 presents original adipokine secretion data from human adipocytes differentiated either in a 2D monolayer or as a 3D spheroid in an ultra-low attachment well. In both platforms human preadipocytes (Lonza, PT-5001) were cultured according to the manufacturer’s protocol, and 10,000 cells per well were seeded overnight in the respective culture plates (2D: Corning 3701; 3D: SBIO 9384UZ). Adipogenic differentiation with insulin, dexamethasone, indomethacin and 3-isobutyl-1-methylxanthine (IBMX) was initiated the following day, using the media formulation (Lonza, PT-8002) recommended by the manufacturer. Due to contact inhibition via confluent seeding in the 2D well (surface area = 0.056 mm2) and in spheroid formation, cell number was assumed constant for the duration of the experiment. The data show that relative to the accumulated concentration of adiponectin, leptin in the supernatant is significantly reduced in spheroid culture by Day 9 as assayed by ELISA kits for leptin and adiponectin (R&D Systems, DLP00 and DRP300). For leptin-relevant studies of BC, choosing between monolayer-cultured and spheroid-cultured adipocytes may be of importance.
Fig. 4.
A simple example of how selecting a culture platform may influence adipocyte phenotype. A Example 2D (flat bottom well) and 3D (ultra-low attachment spheroid well) culture platforms for adipose tissue culture. B During differentiation of primary human preadipocytes, the culture platform dictates accumulation rate of the key adipokines adiponectin and leptin in the supernatant. Interestingly, 3D culture severely downregulates production of leptin. All conditions were assayed in triplicate. (*) indicates p < 0.05 when same-day data are compared using a Student’s t test
Biophysical properties and mechano-transduction
While cell mechanics can be analyzed in both 2D and 3D, foundational studies have shown that the forces which cells experience and enact differ significantly. An average measurement of total contractility of 63 different MDA-MB-231 cells was found to be 47.6 nanonewtons (nN) when suspended in a 1.2 mg/mL collagen hydrogel, compared to 270 nN for cells on a 2D collagen-coated surface [90]. Others have also shown higher traction forces in 2D cultures compared to 3D [91, 92]. While 2D-cultured cells may show greater total contractile force, this force is only imparted on the surface on which they rest, whereas 3D-cultured cells experience forces in a greater number of directions, potentially influencing intracellular properties and signaling.
In 2D, mechano-transduction is dependent on sensing ECM stiffness gradients and ligand density with the response often being migration towards the stiffer substrate [93]. However, in a 3D environment, ECM stiffness can be highly variable owing to other ECM-dependent factors such as cross-linking, fibril alignment, and ECM pore size [94].
A combination of stiffness and composition of ECM can alter mechano-transduction pathways as well [31]. In a co-culture of TNBC cells with preadipocytes, hydrogel stiffness directly impacts adipogenesis [95]. Inhibition of adipogenesis by a breast tumor spheroid was greater at higher versus lower hydrogel stiffness, where the hydrogels were tuned to mimic fibrotic mammary tissue associated with a more aggressive malignancy and normal mammary tissue, respectively. These data suggest that surrounding ECM stiffness may be a cofactor in breast tumor impedance on adipocyte differentiation and function.
Extracellular matrix composition and microenvironment dynamics
Two-dimensional culture models have provided evidence that adipocytes are a producer of collagen in the breast tumor microenvironment [51, 96] because collagen production increases with BC cell co-culture [51]. Collagen has been shown to form scaffolding structures that facilitate BC cell migration into adipose tissue, increasing invasiveness [63, 96]. In addition to collagen, adipocytes have also shown increased fibronectin expression in co-culture with breast tumor cells [51, 97]. Chandler et al. [97] found that tumor cell-conditioned media also increased the stiffness of adipocyte-deposited fibronectin.
3D culture enables researchers to create relevant cell-ECM interactions that promote biochemical and biomechanical activities of interest [79], applicable to BC, where important changes in ECM composition have been observed. Growing mammary epithelial cells in laminin-1-rich ECM, a key basement membrane component, induces growth arrest [98]. Accordingly, in the breast microenvironment, laminin and basement membrane degradation is a feature of tumor progression [99, 100].
Improving in vitro adipocyte breast tumor models
With development of the models described above in its early stages, there is much room for refinement to improve ease of use, throughput, complexity and translatability. This section offers some perspective as to how this might be achieved.
Cell sources
BC cell lines are relatively easy to obtain and manipulate. In using them, as well as with primary tumor cells, knowing the disease sub-type and characteristics such as doubling time and gene mutations are critical for drawing informative conclusions from experiments. As mentioned in Sect. 1, much literature has also been published about adipocyte culture and choosing the right cell model. One limiting factor for using adipocytes in vitro is the time and resources required to obtaining mature adipocytes. Most protocols require at least1 to 2 weeks differentiation time in culture. For researchers looking for a quick means to produce adipocytes where using human cells is not a high priority, the mouse cell line OP9 achieves mature adipocytes in 3 days of differentiation [101].
Culture platform throughput
Throughput is another potential area for innovation in adipocyte-BC culture models. Increasing production of uniformly-manufactured adipose tissues [70] and using automated liquid handling workflows will reduce barriers to using them for larger screening-type experiments. Real-time, automated, high content imaging could enable acquisition of useful data sets on adipose-tumor dynamics such as tumor cell migration, proliferation and invasion and adipocyte phenotypic shift within compatible platforms such as multi-well plates.
Increasing sophistication
To improve mechanistic understanding of the early-stage breast tumor microenvironment, adding complexity to current standards for in vitro platforms may answer more nuanced questions in a controlled experiment. With recent interest in the role of adipose tissue in breast tumor progression, other cell types in the microenvironment that signal to and receive signals from adipocytes may also be added, such as immune cells. Because local inflammation among adipocytes and immune cells is a theorized causation for breast tumorigenesis in individuals with obesity, such in vitro models could potentially find better evidence for these mechanisms [7, 24].
As was discussed in Sect. 3.5, at the time of this review no published in vitro model has shown live interactions between breast tumor cells, adipocytes and endothelial cells. Designing an experimental platform that features these cells in simultaneous culture would provide stronger evidence for the role of, and means to study adipocytes in breast tumor angiogenesis.
Additionally, building models of unique dimension and shape to better replicate geometries in the tumor microenvironment is more feasible with the contributions of microfabrication and bioprinting. As it is a stated goal of microphysiological system designers for the most-sophisticated platforms to parallel and even replace animal studies, taking the steps listed above can improve the translatability from in vitro experiments to in vivo.
Clinical relevance
Inflammatory and fibrotic adipose stroma has emerged as a potential indicator for the rate of breast tumor progression [21, 44]. The greatest contribution that in vitro systems can offer to advance clinical treatment might be the development and refinement of high-throughput phenotypic assays. For example, testing patient breast tumor cells for adipocyte de-differentiation ex vivo in a phenotypic assay may reveal tumors most likely to re-model nearby stroma.
BC progression is also potentially subject to proximal adipocyte signaling from as early a stage as DCIS [39]. DCIS has divided the medical community as to how to prevent overtreatment while still identifying patients at risk of developing invasive BC [11]. Therefore, testing DCIS patient samples of adipocyte-containing stroma with standard tumor cell lines as well as patient tumor samples on laboratory-synthesized adipose tissues in current or new phenotypic invasion assays may emerge as a useful platform to better understand which tumor or microenvironment factor predisposes a patient to invasive BC. Determining the best platforms to test patient samples for possible adipocyte-induced BC malignancy will be critical to advance understanding and to potentially even use such systems as diagnostics for assessing patient risk at the earliest stages of BC.
Concluding remarks
Much remains to be learned about initial bi-directional signaling between BC tumor cell signaling and adipocytes. It remains unclear which tumor-derived signals alter adipocyte phenotype at first contact, but findings from in vitro models clearly suggest a detrimental cycle of tumor cell-adipocyte interactions that lead to tumor progression.
With increased interest in understanding the role of stromal cells, particularly adipocytes, within the breast tumor microenvironment the outlook for obtaining novel findings that could enhance the therapeutic arsenal against BC is bright. The in vitro models described here support the existence of numerous mechanisms by which mammary adipose tissue can encourage tumor progression and diminish therapeutic response. Such solutions for resolving adipocyte-BC interactions will surely prove a critical component to acquiring this important information.
Acknowledgements
The authors gratefully acknowledge funding from the National Institutes of Health (R01 CA196018) and National Science Foundation’s Center for Emergent Behaviors of Integrated Cellular Systems (CBET 0939511). All figures were created using BioRender.com.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
There were no animal or human subject experiments carried out for this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Parmar H, Cunha GR. Epithelial-stromal interactions in the mouse and human mammary gland in vivo. Endocr Relat Cancer. 2004;11:437–458. doi: 10.1677/erc.1.00659. [DOI] [PubMed] [Google Scholar]
- 2.Ward R, Sims AH, Lee A, Lo C, Wynne L, Yusuf H, et al. Monocytes and macrophages, implications for breast cancer migration and stem cell-like activity and treatment. Oncotarget. 2015;6:14687–14699. doi: 10.18632/oncotarget.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang YY, Lehuédé C, Laurent V, Dirat B, Dauvillier S, Bochet L, et al. Adipose tissue and breast epithelial cells: a dangerous dynamic duo in breast cancer. Cancer Lett. 2012;324:142–151. doi: 10.1016/j.canlet.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Pinilla S, Alt E, Abdul Khalek FJ, Jotzu C, Muehlberg F, Beckmann C, et al. Tissue resident stem cells produce CCL5 under the influence of cancer cells and thereby promote breast cancer cell invasion. Cancer Lett. 2009;284:80–85. doi: 10.1016/j.canlet.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Zwick RK, Rudolph MC, Shook BA, Holtrup B, Roth E, Lei V, et al. Adipocyte hypertrophy and lipid dynamics underlie mammary gland remodeling after lactation. Nat Commun. 2018;9:3592. doi: 10.1038/s41467-018-05911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang QA, Song A, Chen W, Schwalie PC, Zhang F, Vishvanath L, et al. Reversible de-differentiation of mature white adipocytes into preadipocyte-like precursors during lactation. Cell Metab. 2018;28:282–288.e3. doi: 10.1016/j.cmet.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilbert CA, Slingerland JM. Cytokines, obesity, and cancer: new insights on mechanisms linking obesity to cancer risk and progression. Annu Rev Med. 2013;64:45–57. doi: 10.1146/annurev-med-121211-091527. [DOI] [PubMed] [Google Scholar]
- 8.Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and cancer mechanisms: tumor microenvironment and inflammation. J Clin Oncol. 2016;34:4270–4276. doi: 10.1200/JCO.2016.67.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park J, Morley TS, Kim M, Clegg DJ, Scherer PE. Obesity and cancer—mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol. 2014;10:455–465. doi: 10.1038/nrendo.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong YK, McMasters KM, Egger ME, Ajkay N. Ductal carcinoma in situ current trends, controversies, and review of literature. Am J Surg. 2018;216:998–1003. doi: 10.1016/j.amjsurg.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 11.van Seijen M, Lips EH, Thompson AM, Nik-Zainal S, Futreal A, Hwang ES, et al. Ductal carcinoma in situ: to treat or not to treat, that is the question. Br J Cancer. 2019;121:285–292. doi: 10.1038/s41416-019-0478-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Majed B, Moreau T, Senouci K, Salmon RJ, Fourquet A, Asselain B. Is obesity an independent prognosis factor in woman breast cancer? Breast Cancer Res Treat. 2008;111:329–342. doi: 10.1007/s10549-007-9785-3. [DOI] [PubMed] [Google Scholar]
- 13.Berstad P, Coates RJ, Bernstein L, Folger SG, Malone KE, Marchbanks PA, et al. A case-control study of body mass index and breast cancer risk in white and African–American women. Cancer Epidemiol Biomarkers Prev. 2010;19:1532–1544. doi: 10.1158/1055-9965.EPI-10-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bandera EV, Chandran U, Hong CC, Troester MA, Bethea TN, Adams-Campbell LL, et al. Obesity, body fat distribution, and risk of breast cancer subtypes in African American women participating in the AMBER consortium. Breast Cancer Res Treat. 2015;150:655–666. doi: 10.1007/s10549-015-3353-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dietze EC, Chavez TA, Seewaldt VL. Obesity and triple-negative breast cancer: disparities, controversies, and biology. Am J Pathol. 2018;188:280–290. doi: 10.1016/j.ajpath.2017.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blüher M. Are there still healthy obese patients? Curr Opin Endocrinol Diabetes Obes. 2012;19:341–346. doi: 10.1097/MED.0b013e328357f0a3. [DOI] [PubMed] [Google Scholar]
- 17.Lengyel E, Makowski L, DiGiovanni J, Kolonin MG. Cancer as a matter of fat: the crosstalk between adipose tissue and tumors. Trends Cancer. 2018;4:374–384. doi: 10.1016/j.trecan.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67:378–397. doi: 10.3322/caac.21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gyamfi J, Eom M, Koo JS, Choi J. Multifaceted roles of interleukin-6 in adipocyte-breast cancer cell interaction. Transl Oncol. 2018;11:275–285. doi: 10.1016/j.tranon.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan J, Buache E, Chenard MP, Dali-Youcef N, Rio MC. Adipocyte is a non-trivial, dynamic partner of breast cancer cells. Int J Dev Biol. 2011;55:851–859. doi: 10.1387/ijdb.113365jt. [DOI] [PubMed] [Google Scholar]
- 21.Choi J, Cha YJ, Koo JS. Adipocyte biology in breast cancer: from silent bystander to active facilitator. Prog Lipid Res. 2018;69:11–20. doi: 10.1016/j.plipres.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Hoy AJ, Balaban S, Saunders DN. Adipocyte-tumor cell metabolic crosstalk in breast cancer. Trends Mol Med. 2017;23:381–392. doi: 10.1016/j.molmed.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Mentoor I, Engelbrecht AM, van Jaarsveld PJ, Nell T. Chemoresistance: intricate interplay between breast tumor cells and adipocytes in the tumor microenvironment. Front Endocrinol (Lausanne) 2018;9:758. doi: 10.3389/fendo.2018.00758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arendt LM, McCready J, Keller PJ, Baker DD, Naber SP, Seewaldt V, et al. Obesity promotes breast cancer by CCL2-mediated macrophage recruitment and angiogenesis. Cancer Res. 2013;73:6080–6093. doi: 10.1158/0008-5472.CAN-13-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iyengar P, Combs TP, Shah SJ, Gouon-Evans V, Pollard JW, Albanese C, et al. Adipocyte-secreted factors synergistically promote mammary tumorigenesis through induction of anti-apoptotic transcriptional programs and proto-oncogene stabilization. Oncogene. 2003;22:6408–6423. doi: 10.1038/sj.onc.1206737. [DOI] [PubMed] [Google Scholar]
- 26.Wang YY, Attané C, Milhas D, Dirat B, Dauvillier S, Guerard A, et al. Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells. JCI Insight. 2017;2:e87489. doi: 10.1172/jci.insight.87489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71:2455–2465. doi: 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- 28.Mentoor I, Engelbrecht AM, Nell T. Fatty acids: adiposity and breast cancer chemotherapy, a bad synergy? Prostaglandins Leukot Essent Fat Acids. 2019;140:18–33. doi: 10.1016/j.plefa.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Morgan MM, Schuler LA, Ciciliano JC, Johnson BP, Alarid ET, Beebe DJ. Modeling chemical effects on breast cancer: the importance of the microenvironment in vitro. Integr Biol (Camb) 2020;12:21–33. doi: 10.1093/intbio/zyaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sung KE, Su X, Berthier E, Pehlke C, Friedl A, Beebe DJ. Understanding the impact of 2D and 3D fibroblast cultures on in vitro breast cancer models. PLoS One. 2013;8:e76373. doi: 10.1371/journal.pone.0076373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belgodere JA, King CT, Bursavich JB, Burow ME, Martin EC, Jung JP. Engineering breast cancer microenvironments and 3D bioprinting. Front Bioeng Biotechnol. 2018;6:66. doi: 10.3389/fbioe.2018.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruiz-Ojeda FJ, Rupérez AI, Gomez-Llorente C, Gil A, Aguilera CM. Cell models and their application for studying adipogenic differentiation in relation to obesity: a review. Int J Mol Sci. 2016;17:1040. doi: 10.3390/ijms17071040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the international federation for adipose therapeutics and science (IFATS) and the international society for cytotherapy (ISOC) Cytotherapy. 2013;15:641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Novakofski J. Adipogenesis: usefulness of in vitro and in vivo experimental models. J Anim Sci. 2004;82:905–915. doi: 10.2527/2004.823905x. [DOI] [PubMed] [Google Scholar]
- 35.Ojima K, Oe M, Nakajima I, Muroya S, Nishimura T. Dynamics of protein secretion during adipocyte differentiation. FEBS Open Bio. 2016;6:816–826. doi: 10.1002/2211-5463.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee Y, Jung WH, Koo JS. Adipocytes can induce epithelial-mesenchymal transition in breast cancer cells. Breast Cancer Res Treat. 2015;153:323–335. doi: 10.1007/s10549-015-3550-9. [DOI] [PubMed] [Google Scholar]
- 37.Sakurai M, Miki Y, Takagi K, Suzuki T, Ishida T, Ohuchi N, et al. Interaction with adipocyte stromal cells induces breast cancer malignancy via S100A7 upregulation in breast cancer microenvironment. Breast Cancer Res. 2017;19:70. doi: 10.1186/s13058-017-0863-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He JY, Wei XH, Li SJ, Liu Y, Hu HL, Li ZZ, et al. Adipocyte-derived IL-6 and leptin promote breast Cancer metastasis via upregulation of Lysyl Hydroxylase-2 expression. Cell Commun Signal. 2018;16:100. doi: 10.1186/s12964-018-0309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim HS, Jung M, Choi SK, Woo J, Piao YJ, Hwang EH, et al. IL-6-mediated cross-talk between human preadipocytes and ductal carcinoma in situ in breast cancer progression. J Exp Clin Cancer Res. 2018;37:200. doi: 10.1186/s13046-018-0867-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D’Esposito VD, Liguoro D, Ambrosio MR, Collina F, Cantile M, Spinelli R, et al. Adipose microenvironment promotes triple negative breast cancer cell invasiveness and dissemination by producing CCL5. Oncotarget. 2016;7:24495–24509. doi: 10.18632/oncotarget.8336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robinson SC, Scott KA, Wilson JL, Thompson RG, Proudfoot AE, Balkwill FR. A chemokine receptor antagonist inhibits experimental breast tumor growth. Cancer Res. 2003;63:8360–8365. [PubMed] [Google Scholar]
- 42.Wang C, Gao C, Meng K, Qiao H, Wang Y. Human adipocytes stimulate invasion of breast cancer MCF-7 cells by secreting IGFBP-2. PLoS One. 2015;10:e0119348. doi: 10.1371/journal.pone.0119348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dobbs JL, Shin D, Krishnamurthy S, Kuerer H, Yang W, Richards-Kortum R. Confocal fluorescence microscopy to evaluate changes in adipocytes in the tumor microenvironment associated with invasive ductal carcinoma and ductal carcinoma in situ. Int J Cancer. 2016;139:1140–1149. doi: 10.1002/ijc.30160. [DOI] [PubMed] [Google Scholar]
- 44.Wang F, Gao S, Chen F, Fu Z, Yin H, Lu X, et al. Mammary fat of breast cancer: gene expression profiling and functional characterization. PLoS One. 2014;9:e109742. doi: 10.1371/journal.pone.0109742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andarawewa KL, Motrescu ER, Chenard MP, Gansmuller A, Stoll I, Tomasetto C, et al. Stromelysin-3 is a potent negative regulator of adipogenesis participating to cancer cell-adipocyte interaction/crosstalk at the tumor invasive front. Cancer Res. 2005;65:10862–10871. doi: 10.1158/0008-5472.CAN-05-1231. [DOI] [PubMed] [Google Scholar]
- 46.Portillo-Lara R, Annabi N. Microengineered cancer-on-a-chip platforms to study the metastatic microenvironment. Lab Chip. 2016;16:4063–4081. doi: 10.1039/c6lc00718j. [DOI] [PubMed] [Google Scholar]
- 47.Shin K, Klosterhoff BS, Han B. Characterization of cell-type-specific drug transport and resistance of breast cancers using tumor-microenvironment-on-chip. Mol Pharm. 2016;13:2214–2223. doi: 10.1021/acs.molpharmaceut.6b00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi Y, Hyun E, Seo J, Blundell C, Kim HC, Lee E, et al. A microengineered pathophysiological model of early-stage breast cancer. Lab Chip. 2015;15:3350–3357. doi: 10.1039/c5lc00514k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pampaloni F, Reynaud EG, Stelzer EH. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol. 2007;8:839–845. doi: 10.1038/nrm2236. [DOI] [PubMed] [Google Scholar]
- 50.Picon-Ruiz M, Pan C, Drews-Elger K, Jang K, Besser AH, Zhao D, et al. Interactions between adipocytes and breast cancer cells stimulate cytokine production and drive Src/Sox2/miR-302b-mediated malignant progression. Cancer Res. 2016;76:491–504. doi: 10.1158/0008-5472.CAN-15-0927. [DOI] [PubMed] [Google Scholar]
- 51.Bochet L, Lehuédé C, Dauvillier S, Wang YY, Dirat B, Laurent V, et al. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res. 2013;73:5657–5668. doi: 10.1158/0008-5472.CAN-13-0530. [DOI] [PubMed] [Google Scholar]
- 52.Cheung KJ, Gabrielson E, Werb Z, Ewald AJ. Collective invasion in breast cancer requires a conserved basal epithelial program. Cell. 2013;155:1639–1651. doi: 10.1016/j.cell.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park J, Scherer PE. Adipocyte-derived endotrophin promotes malignant tumor progression. J Clin Invest. 2012;122:4243–4256. doi: 10.1172/JCI63930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wagner M, Bjerkvig R, Wiig H, Melero-Martin JM, Lin RZ, Klagsbrun M, et al. Inflamed tumor-associated adipose tissue is a depot for macrophages that stimulate tumor growth and angiogenesis. Angiogenesis. 2012;15:481–495. doi: 10.1007/s10456-012-9276-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sachs N, de Ligt J, Kopper O, Gogola E, Bounova G, Weeber F, et al. A living biobank of breast cancer organoids captures disease heterogeneity. Cell. 2018;172:373–86.e10. doi: 10.1016/j.cell.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 56.Djomehri SI, Burman B, Gonzalez ME, Takayama S, Kleer CG. A reproducible scaffold-free 3D organoid model to study neoplastic progression in breast cancer. J Cell Commun Signal. 2019;13:129–143. doi: 10.1007/s12079-018-0498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parigoris E, Lee S, Mertz D, Turner M, Liu AY, Sentosa J, et al. Cancer cell invasion of mammary organoids with basal-in phenotype. Adv Healthc Mater. 2020 doi: 10.1002/adhm.202000810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang X, Sun L, Maffini MV, Soto A, Sonnenschein C, Kaplan DL. A complex 3D human tissue culture system based on mammary stromal cells and silk scaffolds for modeling breast morphogenesis and function. Biomaterials. 2010;31:3920–3929. doi: 10.1016/j.biomaterials.2010.01.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pati F, Ha DH, Jang J, Han HH, Rhie JW, Cho DW. Biomimetic 3D tissue printing for soft tissue regeneration. Biomaterials. 2015;62:164–175. doi: 10.1016/j.biomaterials.2015.05.043. [DOI] [PubMed] [Google Scholar]
- 60.Contessi Negrini N, Celikkin N, Tarsini P, Farè S, Święszkowski W. Three-dimensional printing of chemically crosslinked gelatin hydrogels for adipose tissue engineering. Biofabrication. 2020;12:025001. doi: 10.1088/1758-5090/ab56f9. [DOI] [PubMed] [Google Scholar]
- 61.Chaji S, Al-Saleh J, Gomillion CT. Bioprinted three-dimensional cell-laden hydrogels to evaluate adipocyte-breast cancer cell interactions. Gels. 2020;6:10. doi: 10.3390/gels6010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morgan MM, Arendt LM, Alarid ET, Beebe DJ, Johnson BP. Mammary adipose stromal cells derived from obese women reduce sensitivity to the aromatase inhibitor anastrazole in an organotypic breast model. FASEB J. 2019;33:8623–8633. doi: 10.1096/fj.201802347RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hume RD, Berry L, Reichelt S, D’Angelo M, Gomm J, Cameron RE, et al. An engineered human adipose/collagen model for in vitro breast cancer cell migration studies. Tissue Eng Part A. 2018;24:1309–1319. doi: 10.1089/ten.TEA.2017.0509. [DOI] [PubMed] [Google Scholar]
- 64.Weiswald LB, Bellet D, Dangles-Marie V. Spherical cancer models in tumor biology. Neoplasia. 2015;17:1–15. doi: 10.1016/j.neo.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arner E, Forrest AR, Ehrlund A, Mejhert N, Itoh M, Kawaji H, et al. Ceruloplasmin is a novel adipokine which is overexpressed in adipose tissue of obese subjects and in obesity-associated cancer cells. PLoS One. 2014;9:e80274. doi: 10.1371/journal.pone.0080274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moraes C, Labuz JM, Leung BM, Inoue M, Chun TH, Takayama S. On being the right size: scaling effects in designing a human-on-a-chip. Integr Biol (Camb) 2013;5:1149–1161. doi: 10.1039/c3ib40040a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Turner PA, Tang Y, Weiss SJ, Janorkar AV. Three-dimensional spheroid cell model of in vitro adipocyte inflammation. Tissue Eng Part A. 2015;21:1837–1847. doi: 10.1089/ten.TEA.2014.0531. [DOI] [PubMed] [Google Scholar]
- 68.Turner PA, Gurumurthy B, Bailey JL, Elks CM, Janorkar AV. Adipogenic differentiation of human adipose-derived stem cells grown as spheroids. Process Biochem. 2017;59:312–320. doi: 10.1016/j.procbio.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Godwin LA, Brooks JC, Hoepfner LD, Wanders D, Judd RL, Easley CJ. A microfluidic interface for the culture and sampling of adiponectin from primary adipocytes. Analyst. 2015;140:1019–1025. doi: 10.1039/c4an01725k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Akama T, Leung BM, Labuz J, Takayama S, Chun TH. Designing 3-D adipospheres for quantitative metabolic study. Methods Mol Biol. 2017;1566:177–183. doi: 10.1007/978-1-4939-6820-6_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rinker TE, Hammoudi TM, Kemp ML, Lu H, Temenoff JS. Interactions between mesenchymal stem cells, adipocytes, and osteoblasts in a 3D tri-culture model of hyperglycemic conditions in the bone marrow microenvironment. Integr Biol (Camb) 2014;6:324–337. doi: 10.1039/c3ib40194d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Daquinag AC, Souza GR, Kolonin MG. Adipose tissue engineering in three-dimensional levitation tissue culture system based on magnetic nanoparticles. Tissue Eng Part C Methods. 2013;19:336–344. doi: 10.1089/ten.tec.2012.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spencer VA, Xu R, Bissell MJ. Gene expression in the third dimension: the ECM-nucleus connection. J Mammary Gland Biol Neoplasia. 2010;15:65–71. doi: 10.1007/s10911-010-9163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Härmä V, Virtanen J, Mäkelä R, Happonen A, Mpindi JP, Knuuttila M, et al. A comprehensive panel of three-dimensional models for studies of prostate cancer growth, invasion and drug responses. PLoS One. 2010;5:e10431. doi: 10.1371/journal.pone.0010431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cavnar SP, Salomonsson E, Luker KE, Luker GD, Takayama S. Transfer, imaging, and analysis plate for facile handling of 384 hanging drop 3D tissue spheroids. J Lab Autom. 2014;19:208–214. doi: 10.1177/2211068213504296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leung BM, Lesher-Perez SC, Matsuoka T, Moraes C, Takayama S. Media additives to promote spheroid circularity and compactness in hanging drop platform. Biomater Sci. 2015;3:336–344. doi: 10.1039/c4bm00319e. [DOI] [PubMed] [Google Scholar]
- 77.Imamura Y, Mukohara T, Shimono Y, Funakoshi Y, Chayahara N, Toyoda M, et al. Comparison of 2D- and 3D-culture models as drug-testing platforms in breast cancer. Canc Res. 2015;33:1837–1843. doi: 10.3892/or.2015.3767. [DOI] [PubMed] [Google Scholar]
- 78.Edmondson R, Broglie JJ, Adcock AF, Yang L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev Technol. 2014;12:207–218. doi: 10.1089/adt.2014.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Duval K, Grover H, Han LH, Mou Y, Pegoraro AF, Fredberg J, et al. Modeling physiological events in 2D vs. 3D cell culture. Physiology (Bethesda) 2017;32:266–277. doi: 10.1152/physiol.00036.2016. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 80.Kim Y, Stolarska MA, Othmer HG. A hybrid model for tumor spheroid growth in vitro I: theoretical development and early results. Math Models Methods Appl Sci. 2007;17:1773–1798. [Google Scholar]
- 81.Hillen F, Griffioen AW. Tumour vascularization: sprouting angiogenesis and beyond. Cancer Metastasis Rev. 2007;26:489–502. doi: 10.1007/s10555-007-9094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Antoni D, Burckel H, Josset E, Noel G. Three-dimensional cell culture: a breakthrough in vivo. Int J Mol Sci. 2015;16:5517–5527. doi: 10.3390/ijms16035517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tung YC, Hsiao AY, Allen SG, Torisawa Y, Ho M, Takayama S. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst. 2011;136:473–478. doi: 10.1039/c0an00609b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mertz DR, Ahmed T, Takayama S. Engineering cell heterogeneity into organs-on-a-chip. Lab Chip. 2018;18:2378–2395. doi: 10.1039/c8lc00413g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gu L, Mooney DJ. Biomaterials and emerging anticancer therapeutics: engineering the microenvironment. Nat Rev Cancer. 2016;16:56–66. doi: 10.1038/nrc.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang F, Weaver VM, Petersen OW, Larabell CA, Dedhar S, Briand P, et al. Reciprocal interactions between β1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc Natl Acad Sci U S A. 1998;95:14821–14826. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Riedl A, Schlederer M, Pudelko K, Stadler M, Walter S, Unterleuthner D, et al. Comparison of cancer cells in 2D vs 3D culture reveals differences in AKT-mTOR-S6K signaling and drug responses. J Cell Sci. 2017;130:203–218. doi: 10.1242/jcs.188102. [DOI] [PubMed] [Google Scholar]
- 88.Lee EY, Lee WH, Kaetzel CS, Parry G, Bissell MJ. Interaction of mouse mammary epithelial cells with collagen substrata: regulation of casein gene expression and secretion. Proc Natl Acad Sci U S A. . 1985;82:1419–1423. doi: 10.1073/pnas.82.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee EY, Parry G, Bissell MJ. Modulation of secreted proteins of mouse mammary epithelial cells by the collagenous substrata. Proc Natl Acad Sci U S A. 1984;98:146–155. doi: 10.1083/jcb.98.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Steinwachs J, Metzner C, Skodzek K, Lang N, Thievessen I, Mark C, et al. Three-dimensional force microscopy of cells in biopolymer networks. Nat Methods. 2016;13:171–176. doi: 10.1038/nmeth.3685. [DOI] [PubMed] [Google Scholar]
- 91.Legant WR, Miller JS, Blakely BL, Cohen DM, Genin GM, Chen CS. Measurement of mechanical tractions exerted by cells in three-dimensional matrices. Nat Methods. 2010;7:969–971. doi: 10.1038/nmeth.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dembo M, Wang YL. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophys J. 1999;76:2307–2316. doi: 10.1016/S0006-3495(99)77386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Palecek SP, Loftust JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- 94.Doyle AD, Yamada KM. Mechanosensing via cell-matrix adhesions in 3D microenvironments. Exp Cell Res. 2016;343:60–66. doi: 10.1016/j.yexcr.2015.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yue X, Nguyen TD, Zellmer V, Zhang S, Zorlutuna P. Stromal cell-laden 3D hydrogel microwell arrays as tumor microenvironment model for studying stiffness dependent stromal cell-cancer interactions. Biomaterials. 2018;170:37–48. doi: 10.1016/j.biomaterials.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 96.Iyengar P, Espina V, Williams TW, Lin Y, Berry D, Jelicks LA, et al. Adipocyte-derived collagen VI affects early mammary tumor progression in vivo, demonstrating a critical interaction in the tumor/stroma microenvironment. J Clin Invest. 2005;115:1163–1176. doi: 10.1172/JCI23424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chandler EM, Saunders MP, Yoon CJ, Gourdon D, Fischbach C. Adipose progenitor cells increase fibronectin matrix strain and unfolding in breast tumors. Phys Biol. 2011;8:015008. doi: 10.1088/1478-3975/8/1/015008. [DOI] [PubMed] [Google Scholar]
- 98.Fournier MV, Martin KJ, Kenny PA, Xhaja K, Bosch I, Yaswen P, et al. Gene expression signature in organized and growth-arrested mammary acini predicts good outcome in breast cancer. Cancer Res. 2006;66:7095–7102. doi: 10.1158/0008-5472.CAN-06-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chang J, Chaudhuri O. Beyond proteases: basement membrane mechanics and cancer invasion. J Cell Biol. 2019;218:2456–2469. doi: 10.1083/jcb.201903066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mehner C, Hockla A, Miller E, Ran S, Radisky DC, Radisky ES. Tumor cell-produced matrix metalloproteinase 9 (MMP-9) drives malignant progression and metastasis of basal-like triple negative breast cancer. Oncotarget. 2014;5:2736–2749. doi: 10.18632/oncotarget.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wolins NE, Quaynor BK, Skinner JR, Tzekov A, Park C, Choi K, et al. OP9 mouse stromal cells rapidly differentiate into adipocytes: characterization of a useful new model of adipogenesis. J Lipid Res. 2006;47:450–460. doi: 10.1194/jlr.D500037-JLR200. [DOI] [PubMed] [Google Scholar]