Abstract
Background:
Skeletal muscle injuries are frequent clinical challenges due to associated fibrosis and disability. Regenerative medicine is an emerging promising strategy for such cases. The aim of this study was to compare between the effects of bone marrow-mesenchymal stem cells (BM-MSCs) versus adipose tissue stromal cells (ADSCs) on regeneration and re-innervation of skeletal muscle laceration injury in Wistar rats at different time intervals.
Methods:
Six young male rats were used as a source of allogenic MSCs. Eighty-four adult female rats were divided into: Group I (control), Group II (Untreated Laceration): right gluteal muscle was lacerated and left for spontaneous healing, Group III (BM-MSCs): right gluteal muscle was lacerated with concomitant local intramuscular injection of 1 × 106 BM-MSCs in the lacerated muscle, Group IV (ADSCs): right gluteal muscle was lacerated with concomitant local intramuscular injection of 1 × 106 ADSCs in lacerated muscle. Rats were sacrificed after one, two and eight weeks. Muscles were processed to prepare sections stained with H&E, Mallory’s trichrome and immune-histochemical staining (neurofilament light chain).
Results:
A significant increase in collagen fibers and failure of re-innervation were noticed in untreated laceration group. BM-MSCs-treated groups showed regeneration of muscle fibers but with increased collagen fibers. Meanwhile, ADSCs showed better regenerative effects evidenced by significant increase in the number of myotubes and significant decrease in collagen deposition. Re-innervation was noticed in MSCs-injected muscles after 8 weeks of laceration.
Conclusion:
Both BM-MSCs and ADSCs improved regeneration of skeletal muscle laceration injury at short- and long-term durations. However, fibrosis was less in ADSCs-treated rats. Effective re-innervation of injured muscles occurred only at the long-term duration.
Keywords: Muscle laceration, Bone marrow-derived mesenchymal stem cells, Adipose tissue-derived stem cells, Re-innervation, Neurofilament light chain
Introduction
Skeletal muscle is a dynamic tissue that is responsible for voluntary movement and postural maintenance. It is the most abundant tissue in the human body, representing approximately 40% of body mass [1]. Skeletal muscle injury can be encountered clinically as contusion, strain, laceration or a combination of these injuries [2]. The capacity of skeletal muscle tissue to regenerate resides in a reserve population of mononucleated precursors, termed satellite cells [3]. Physiologically, satellite cells are quiescent. Upon muscle injury, satellite cells become activated, proliferated, differentiated into myocytes and fuse either with each other or with damaged myofibers to repair injured muscle. Satellite cells have three myogenic statuses: quiescent, activated/proliferated (myoblasts) or differentiated status (myocytes which subsequently form generated multinucleated myotubes) during muscle healing [4]. However, paucity of satellite cells in skeletal muscle fibers limits its regenerative capacity [5]. After severe injuries, muscle healing is incomplete, resulting in the formation of fibrous tissue and subsequently mechanical dysfunction.
The integrity of neuromuscular junctions has an important role in skeletal muscle maintenance. Neuromuscular disruptions elicit severe myofiber atrophy and are frequently associated with skeletal muscle dysfunction [6].
Although researchers have extensively investigated various approaches to improve muscle healing, there is still no gold standard treatment [7]. Over the last couple of decades, the field of regenerative medicine has emerged as a novel promising strategy to treat many diseases. Mesenchymal stem cells (MSCs) are present in many adult tissues and can be easily isolated from different sources, including the bone marrow and adipose tissue. This makes them an ideal target for cell-based therapy [8].
Several researchers have reported that bone marrow-derived mesenchymal stem cells (BM-MSCs) have the potential to fuse with myoblasts in vitro and to contribute to muscle healing and treatment of muscle disorders. Like bone marrow, adipose tissue contains a heterogeneous stromal cell population as endothelial cells, smooth muscle cells, pericytes and mesenchymal stem cells [3].
In this study, we compared between the possible effects of MSCs derived from bone marrow versus those derived from adipose tissue in regeneration and re-innervation of experimentally induced skeletal muscle laceration injury in female Wistar rats at different time intervals.
The clinical significance this study could provide preclinical data about safety and effectiveness of using stem cells in management of patients with traumatic muscle injuries.
Materials and methods
Animals
This study included 84 adult female Wister rats weighing 200–250 g. In addition to six young male rats weighing 56–80 g which were used as a source of allogenic bone marrow and adipose tissue. Animals were housed in clean plastic cages with mesh wire covers. They were given a free access to standard rat chow diet and tap water for the period of the experiment. The experiment was conducted in the Research Center institute (MASRI), Faculty of Medicine, Ain Shams University, Research Ethics Committee (FMASU REC) with Federal Wide Assurance No. FWA 00017585. All the experimental procedures were performed in accordance with international standards for animal care.
Experimental protocol
After 7-day acclimatization period, female rats were randomly divided into the following groups:
Group I (Sham control) included 21 rats. They were anesthetized, and skin incision was done over the right gluteal muscle. Rats received local intramuscular injection (IM) of 1 ml phosphate buffer saline (PBS). This group was subdivided equally into three subgroups: Ia, Ib and Ic, in which muscle specimens were taken 1 week, 2 weeks and 8 weeks, respectively, after the beginning of the experiment.
Group II (Laceration group) included 21 rats that were subjected to right gluteal muscle laceration injury [3]. Rats concomitantly received local IM injection of 1 ml PBS at site of injury. Suturing was done, and then, they were left for spontaneous healing. Rats were then subdivided equally into three subgroups: IIa, IIb and IIc, in which muscle specimens were taken 1 week, 2 weeks and 8 weeks, respectively, after the beginning of the experiment.
Group III (BM-MSCs-treated group) included 21 rats that were subjected to right gluteal muscle laceration injury. Rats concomitantly received local IM injection of 1 ml PBS containing 1 × 106 BM-MSCs at the site of injury before suturing. Rats were then subdivided equally into three subgroups: IIIa, IIIb and IIIc, in which muscle specimens were taken 1 week, 2 weeks and 8 weeks, respectively, after the beginning of the experiment.
Group IV (ADSCs-treated group) included 21 rats that were subjected to right gluteal muscle laceration injury. Rats concomitantly received local intramuscular injection of 1 ml PBS containing 1 × 106 ADSCs at the site of injury before suturing. Rats were then subdivided equally into three subgroups: IVa, IVb and IVc, in which muscle specimens were taken 1 week, 2 weeks and 8 weeks, respectively, after the beginning of the experiment.
Donor group included six young rats for preparation of BM-MSCs and ADSCs. BM-MSCs were taken from the femur and tibia, while ADSCs were taken from fat in the back of rats after adding collagenase.
Steps of skeletal muscle laceration
Rats were anaesthetized by intra-peritoneal injection of a mixture of ketamine (50 mg/kg) and xylazine (5 mg/kg) [3]. Skin over right gluteal muscle was shaved and sterilized. Laceration injury was done in the middle of the right gluteal muscle—about 0.5 cm depth—using scissors. A suture was taken between the ends of the lacerated gluteal muscle. Then, the skin was sutured using silk surgical suture (2-0) size.
Culture and isolation of stem cells
Preparation of stem cells was done from the six rats of the donor group. All steps of isolation and culture of BM-MSCs and ADSCs were carried out at Stem cell research unit, Histology and Cell Biology department, Faculty of Medicine, Ain Shams University, Cairo, Egypt, according to McFarlin et al. [9] and Cakici et al. [10] protocol. Cells of the 3rd passage were suspended in PBS at a concentration of 1 × 106/ml and were used for intramuscular injection.
Characterization of cultured stem cells
This was done in the third passage to see whether cells exhibit properties of stem cells:
Giemsa stain was performed to adherent BM-MSCs and ADSCs on passage three.
Immunohistochemical study for cultured BM-MSCs and ADSCs was done using streptavidin–biotin immunoperoxidase technique for detection of CD133 expression [11]. The primary antibody (CD133 mouse monoclonal antihuman antibody) was purchased from Lap Vision Cor., Fremont, CA, USA. Secondary antibody was biotylinated goat anti-mouse antibody. Positive expression appeared as brown cytoplasmic reaction. CD133 is a characteristic marker for ADSCs.
Characterization of BM-MSCs and ADSCs was also done using flow cytometry for CD90 [12].
Sample collection
At the end of each time point, animals were sacrificed by decapitation after ether inhalation anesthesia. Right gluteal muscle was taken from all rats including the injured area in the middle.
Preparation of tissue
Muscle specimens from all rats were fixed in 10% buffered formalin followed by dehydration, clearing and embedding in paraffin [13]. Serial sections of 5 µm thickness were cut and stained with hematoxylin and eosin (H&E) and Mallory’s trichrome stain. At 2 weeks and 8 weeks duration, paraffin sections were cut on positively charged slides and were subjected to immune-histochemical study using neurofilament light chain (NFL) mouse monoclonal antibody [14] for detection of nerve fibers. Secondary antibody was biotylinated goat anti-mouse antibody. Antibodies were purchased from Dako, Santa Clara, CA, USA (Catalog number: M0762).
Morphometric and statistical Study
In all groups, the following parameters were measured: (1) Mean number of myotubes with central nuclei per field was counted in longitudinal H&E Sections. (2) Mean area percentage of collagen fibers in the interstitium was measured in Mallory’s trichrome-stained Section. (3) Mean area percentage of positive expression of NFL protein was measured in immune-histochemical-stained sections.
Measurements were taken in three different slides obtained from each animal in each group. Five haphazardly selected nonoverlapping fields were examined in each slide using objective lens X20. Measurements were taken using image analyzer Leica Q win V.3 program installed on a computer in Histology and Cell Biology Department, Faculty of Medicine, Ain Shams University, was used. The computer was connected to a Leica DM2500 microscope (Wetzlar, Germany).
Data were statistically analyzed using one-way analysis of variance (ANOVA) performed with SPSS.21 program (IBM Inc., Chicago, IL, USA). Post hoc LSD test was used for pair-wise comparison between the different subgroups. The significance of the data was determined by p value < 0.05; p < 0.05 is significant; and p > 0.05 is nonsignificant.
Results
General health conditions of rats during the experiment
All rats subjected to laceration were limping for 3 days postoperatively. Rats treated with stem cells were minimally affected. No deaths occurred in all groups.
Morphology of cultured BM-MSCs and ADSCs
Examination of the primary cultures of BM-MSCs on day 4 showed a large number of attached cells of different shapes (Fig. 1A). In ADSCs, an apparent increased number of attached cells were noticed together with appearance of whorly shaped colonies. Mitotic figures were occasionally seen (Fig. 1B).
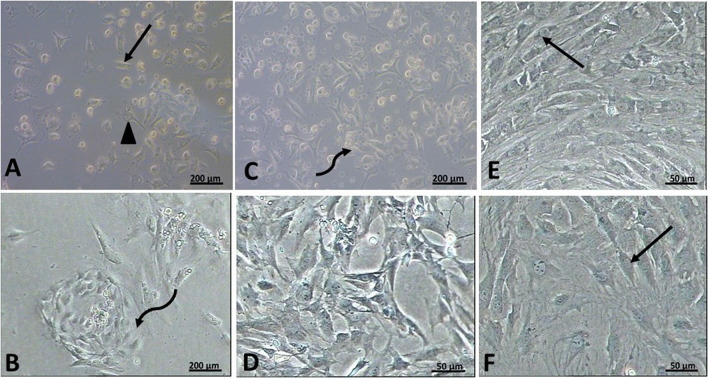
Fig. 1.
Phase contrast micrographs rat BM-MSCs rat ADSCs. A, B day four of primary culture rat BM-MSCs showing a large number of attached cells of different shapes. Some cells appeared spindle-shaped (↑), and others appeared polygonal (▲). Notice the presence of many unattached cells. Rat ADSCs showing whorl shaped appearance of a starting colony (curved arrow). C, D day six of primary culture rat BM-MSCs showing further increased in the number of attached cells. The attached cells appeared of variable shapes. There is the evidence of start of colony formation (curved arrow). ADSCs culture showing further increased in the number of attached cells. Notice that the cells show multiple interdigitating cell processes. E Rat BM-MSCs on the 5th day of passage one showing the whorl appearance of a colony formed of spindle-shaped cells (↑). F Rat ADSCs on the 3rd day of passage one showing colonies of spindle-shaped attached cells (↑) with polygonal cells in between
On day six, cultured BM-MSCs showed an increased number of attached cells. There was evidence of start of colony formation (Fig. 1C). In ADSCs, there was increase in number of attached cells to reach more than 75% confluence (Fig. 1D). ADSCs proliferated and reached confluency earlier than BM-MSCs in the primary culture and during the first passage.
During the first passage of MSCs, the cells proliferated at a higher rate, forming colonies formed of whorls of spindle-shaped attached cells with polygonal cells in between. The cells have central vesicular nuclei, and most of them showed 1 or 2 nucleoli. BM-MSCs reached > 95% confluency within 5 days of subculture (Fig. 1E). While in ADSCs, cells reached about 95% confluency within 3 days from the start of the subculture (Fig. 1F).
Characterization of the cultured stem cells
It was done for stem cells obtained from the 3rd subculture. Giemsa stain of BM-MSCs was examined by phase contrast microscope. Colonies were seen arranged in whorls and formed of spindle-shaped cells that reached > 95% confluency within 4 days of the third subculture (Fig. 2A). On the contrary, the third subculture of ADSCs showed whorly appearance of colonies of spindle-shaped cells that reached > 95% confluency within 2 days (Fig. 2B).
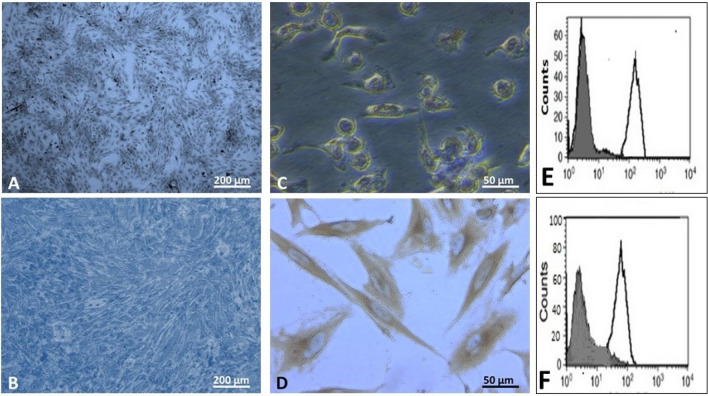
Fig. 2.
A, B phase contrast micrographs of Giemsa stain X 100, in the third passage, showing colonies of spindle-shaped cells that are seen in rat BM-MSCs on the 4th day rat ADSCs on 2nd day. C, D phase contrast micrographs of CD133 X400 rat BM-MSCs showing weak brownish cytoplasmic reaction in some cultured cell rat ADSCs showing strong positive brown cytoplasmic reaction in all cells. E, F Flow cytometric analysis of cell-surface antigens, showing most cells positive for CD90 rat BM-MSCs rat ADSCs
Immunostaining of cultured BM-MSCs taken in the 3rd passage showed weak positive cytoplasmic reaction for CD133 in some cultured cells (Fig. 2C), while high positive reaction was noticed in most cultured ADSCs (Fig. 2D).
Characterizing the cultured BM-MSCs and ADSCs cells after the third passage using flow cytometry for CD90 revealed that most of the cultured cells were positive for CD90 (Fig. 2E, F respectively).
Histological and statistical results
Examination of longitudinal sections of right gluteal muscle from all subgroups in group I (control) showed similar results with nonsignificant change.
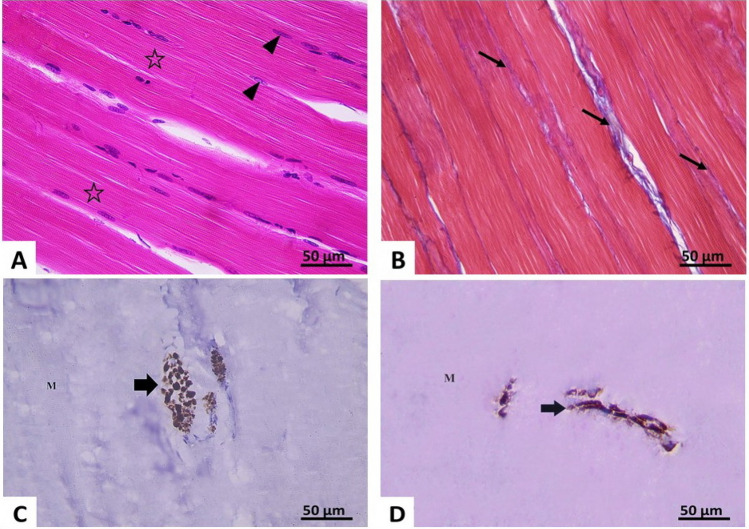
Examination of longitudinal H&E-stained sections of gluteal muscle in control rats showed parallel muscle fibers having nearly the same diameter. The sarcoplasm of each muscle fiber appeared acidophilic with well-defined transverse striations. Multiple elongated peripheral nuclei were seen (Fig. 3A). Examination of Mallory’s trichrome-stained sections showed few collagen fibers in the endomysium and perimesium (Fig. 3B). The mean collagen area percentage was 1.97% ± 0.6 (Table 1). Immune-histochemical study showed strong positive expression of NFL protein in nerve fibers between muscle fibers (Fig. 3C, D). Its mean area percentage was 1.24% ± 0.60 (Table 1).
Fig. 3.
Photomicrographs of section of right gluteal muscle of the control group X400 A H&E-stained section showing well-defined cross striations (*) and peripheral elongated nuclei (▲).B Mallory’s trichrome: thin bundles of collagen fibers (↑) are seen in the interstitium between the muscle fibers. C, D Immune-histochemical reaction for neurofilament light chain: showing positive expression (↑) in transverse (C) and longitudinal nerves (D) between muscle fibers (M)
Table 1.
Showing the mean ± SD of: number of myotubes with central nuclei per field, mean area percentage of collagen fibers in the interstitium and mean area percentage of positive expression of neurofilament light chain protein (NFL) in different subgroups
| Groups | Mean number of myotubes/field (no. 15) |
Mean area % of collagen fibers (no. 15) |
Mean area % of positive NFL immune reaction (no. 15) |
|---|---|---|---|
| Group I (Control) | |||
| Ia | 0 | 1.97 ± 0.6 | 1.24 ± 0.6 |
| Ib | 0 | 1.81 ± 0.4 | 1.31 ± 0.4 |
| Ic | 0 | 2.04 ± 0.5 | 1.62 ± 0.6 |
| Group II (Laceration) | |||
| IIa (1 week) | 2.64 ± 0.57* | 10.65 ± 2.6* | – |
| IIb (2 weeks) | 3.66 ± 0.58* | 12.02 ± 1.68* | 0 |
| IIc (8 weeks) | 0 | 20.22 ± 6.72* | 0 |
| Group III (Laceration + BM-MSCs) | |||
| IIIa (1 week) | 8.50 ± 2.03 *▲ | 21.7 ± 1.52 *▲ | – |
| IIIb (2 weeks) | 6.39 ± 2.34 *▲ | 18.3 ± 2.17 *▲ | 0.8 ± 0.14* |
| IIIc (8 weeks) | 0 | 5.54 ± 1.46 *▲ | 1.1 ± 0.26 |
| Group IV (Laceration + ADSCs) | |||
| IVa (1 week) | 22.00 ± 4.3 *▲ Δ | 5.3 ± 1.11 *▲ Δ | – |
| IVb (2 weeks) | 15.50 ± 2.5 *▲ Δ | 8.96 ± 1.61 *▲ Δ | 0.79 ± 0.13* |
| IVc (8 weeks) | 0 | 2.36 ± 1.44 ▲ Δ | 1.18 ± 0.29 |
*Significant change compared to control subgroups
▲significant change compared to corresponding untreated laceration subgroup
Δ significant change compared to corresponding BM-MSCs-treated subgroup
Examination of gluteal muscle in untreated laceration group:
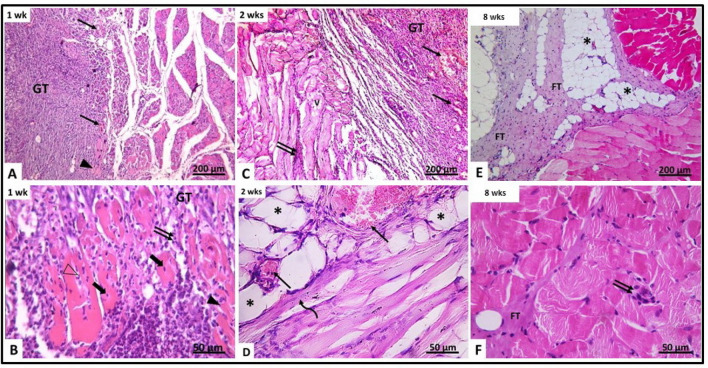
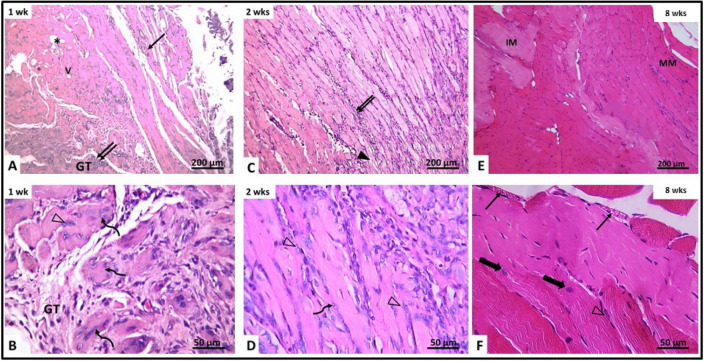
After 1 week (subgroup IIa), granulation tissue was seen filling the site of laceration injury. The granulation tissue showed congested blood vessels and cells with deeply stained nuclei, macrophages and fibroblasts and few neutrophils. The muscle fibers around the granulation tissue appeared discontinuous with marked disruption. Their cytoplasm showed vacuolations and lack of transverse striations. Most of them contained deeply stained pyknotic nuclei. The interstitium between muscle fibers showed mononuclear cellular infiltration formed of macrophages, lymphocyte, few neutrophils and some fibroblast (Fig. 4A, B). Few myotubes containing a chain of centrally located nuclei were observed. The mean number of myotubes/field was 2.64 ± 0.57 (Table 1). After 2 weeks (subgroup IIb), dissection of the muscle was difficult as a result of adhesions. Histological examination showed the site of laceration still filled with granulation tissue containing congested blood vessels. Most muscle fibers appeared vacuolated, disrupted and disorganized. Their cytoplasm showed vacuolations and loss of striations. Some myotubes of small caliber with basophilic cytoplasm and centrally located vesicular nuclei were observed (Fig. 4C, D). Their mean number was 3.66 ± 0.58 (Table 1, Fig. 5). After 8 weeks (subgroup IIc), the site of injury was filled with fibrous tissue containing many fat cells and remnants of granulation tissue. The fibrous tissue extended between disorganized muscle fibers. Some muscle fibers appeared pale (Fig. 4E, F). No myotubes could be detected.
Fig. 4.
H&E-stained section of gluteal muscle from laceration group (group II) at different time intervals. A, B After 1 week (IIa) (A) showing granulation tissue (GT) containing mononuclear inflammatory cells, congested blood vessels (↑) and disrupted muscle fibers (▲). The granulation tissue (GT) contains fragmented muscles with pyknotic nuclei (thick arrow). Other muscles contain neutrophils (Δ). C, B After 2 weeks (IIb) showing disrupted vacuolated muscles (V), congested blood vessels (↑) and inflammatory cellular infiltration (↑↑). D Showing congested blood vessels (↑), fat cells (*) and regenerating myotubes containing centrally located nuclei (curved arrow). E, F After 8 weeks (IIc) showing the area of injury containing fibrous tissue and fat cells (*). Minimal inflammatory cellular infiltration (↑↑) and fibrous tissue (FT) are seen between muscle fibers
Fig. 5.
Showing the mean number of myotubes/field in different groups
Examination of lacerated gluteal muscle treated with BM-MSCs:
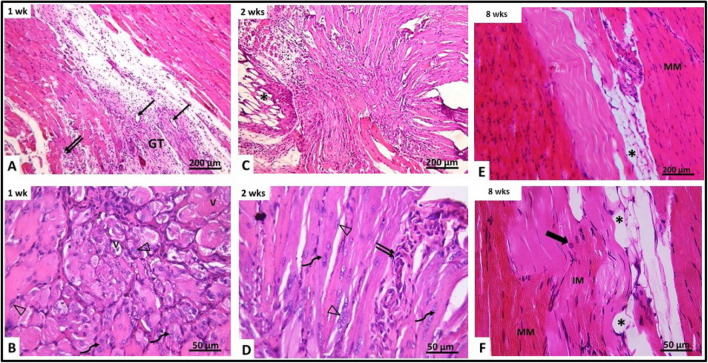
After 1 week (subgroup IIIa), examination of gluteal muscle showed granulation tissue in the area of injury that was less in amount and more vascular than untreated lacerated group. It was seen penetrated by irregularly arranged myotubes containing slightly basophilic cytoplasm and a chain of centrally located vesicular nuclei. The mean number of myotubes was 8.50 ± 2.03 with significant increase compared to untreated laceration group (Table 1, Fig. 5). Some myofibers appeared with vacuolated cytoplasm. Many congested blood vessels were observed. They were seen surrounded by curved cells with flat nuclei (most probably pericytes). Groups of active cells with central vesicular nuclei were seen in the interstitium near the surface of the muscle fibers, in addition to the presence of many fat cells between the regenerating myofibers. Mononuclear cellular infiltration was occasionally seen in the connective tissue between the distorted muscle fibers (Fig. 6A, B). After 2 weeks (subgroup IIIb), gluteal muscle showed appearance of parallelly arranged regenerating muscle fibers in the granulation tissue that appeared cellular with increase cellularity of the connective tissue between muscle fibers. They included many macrophages and cells with flat nuclei. Myotubes with basophilic cytoplasm and centrally located vesicular nuclei were observed. Their mean number was 6.39 ± 2.34 with significant increase compared to untreated laceration group (Table 1, Fig. 5). However, some muscle fibers were still seen disrupted and disorganized with apparent splitting and loss of transverse striation (Fig. 6C, D). After 8 weeks (subgroup IIIc), the site of injury was filled with new muscle fibers. The muscle fibers were of two types. Mature muscle fibers with dark acidophilic sarcoplasm with well-defined cross striations. Also, immature muscle fibers with pale acidophilic sarcoplasm and absent cross striations were present. Some muscle fibers were seen with central nuclei and the cross striations were not completely restored. Some cells were seen at the periphery of the muscle fibers most probably active satellite cells. Congested blood vessels were also seen in the interstitium (Fig. 6E, F).
Fig. 6.
Photomicrographs of right gluteal muscle from laceration group treated with BM-MSCs (group III). A, B After 1 week (IIIa) showing vacuolated muscles (V), cellular granulation tissue (GT), congested blood vessels (↑) and inflammatory cellular infiltration (↑↑). The granulation tissue (GT) is seen containing myotubes with centrally located nuclei (curved arrow) and activated cells with vesicular nuclei (Δ). C, D After 2 weeks (IIIb), disrupted muscle fibers are seen with apparent splitting and loss of striations (▲). Inflammatory cellular infiltration (↑↑) is also noticed. Showing regenerating myotubes containing centrally located vesicular nuclei and basophilic cytoplasm (curved arrow). Activated cells with vesicular nuclei (Δ) are also seen. E, F After 8 weeks (IIIc) showing mature muscle fibers (MM) and immature muscle fibers (IM) at area of injury showing congested blood vessels (↑) and muscle fibers with central nuclei and incomplete striations (thick arrow). Active cells with vesicular nuclei are seen at the periphery of some muscle fibers (Δ)
Examination of lacerated gluteal muscle treated with ADSCs:
After 1 week (subgroup IVa), there were little vascular granulation tissue. Parallelly arranged, slightly distorted and widely separated muscle fibers of variable length and diameter were seen at the area of injury (Fig. 7A). Many myotubes of apparently small caliber were observed with basophilic cytoplasm and centrally located vesicular nuclei (Fig. 7B). The mean number of myotubes was 22.00 ± 4.30 with significant increase compared to corresponding untreated and BM-MSCs groups (Table 1, Fig. 5). Some of the surrounding myofibers showed small vacuoles with no apparent transverse striations. The interstitium between the muscle fibers showed spindle-shaped cells with flat nuclei. Small groups of active cells with rounded vesicular nuclei were seen. Small areas of mononuclear cell infiltration containing macrophages were noticed (Fig. 7B). After 2 weeks (subgroup IVb), the site of laceration injury showed parallelly arranged muscle fibers. However, disoriented muscle fibers of different caliber and running in different directions were also seen. Some of the muscle fibers showed transverse striations. Only few myofibers showed vacuolations. The mean number of myotubes was 15.50 ± 2.5 with significant increase compared to laceration and BM-MSCs-treated groups (Table 1, Fig. 5). The interstitium between the muscle fibers contained spindle-shaped cells with flat nuclei. Small groups of active cells with rounded vesicular nuclei and prominent nucleoli were seen on the surface of some muscle fibers. Groups of fat cells were also observed between bundles of muscle fibers. Mononuclear cell infiltration was rarely seen (Fig. 7C, D). After 8 weeks from administration of ADSCs, (subgroup IVc), the site of the injury was filled with new muscle fibers. The muscle fibers were of two types. Mature muscle fibers with acidophilic sarcoplasm and well-defined cross striations. Also, immature muscle fibers with pale acidophilic sarcoplasm and absent cross striations and muscle fibers were seen with central nuclei and incomplete cross striations (Fig. 7E, F).
Fig. 7.
Photomicrographs of right gluteal muscle from laceration group treated with ADSCs (group IV). A, B After 1 week (IVa) granulation tissue (GT) contains blood vessels (↑) and inflammatory cellular infiltration (↑↑). Showing vacuolated muscles (V) and regenerating myotubes with central vesicular nuclei (curved arrow). Activated cells with vesicular nuclei (Δ) are also seen. C, D After 2 weeks (IVb) showing fat cells (*) at area of injury showing myotubes with basophilic cytoplasm and centrally located chain of vesicular nuclei (curved arrow). E, F Active cells with vesicular nuclei (Δ) and inflammatory cellular infiltration (↑↑) are seen after 8 weeks (IVc) showing pale-stained muscle fibers near mature muscle fibers (MM) and fat cells (*) muscle fibers with well-defined striations (MM), immature muscle fibers with ill-defined striations (IM) and muscle fibers with central nuclei and incomplete striations (thick arrow) are seen
Examination of collagen fiber deposition
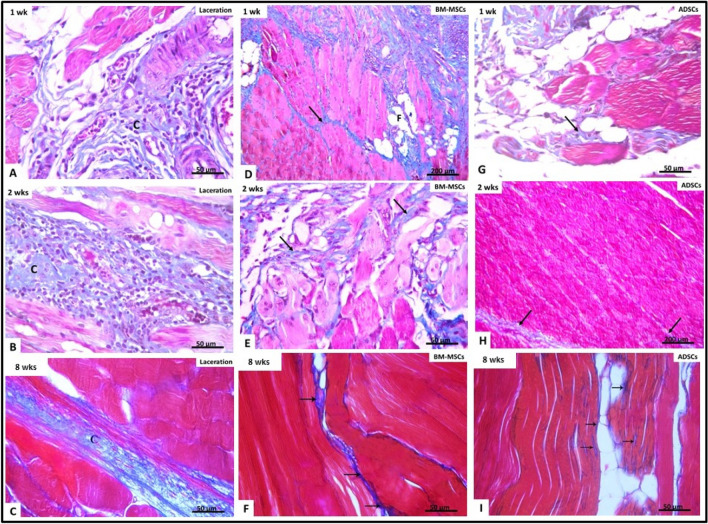
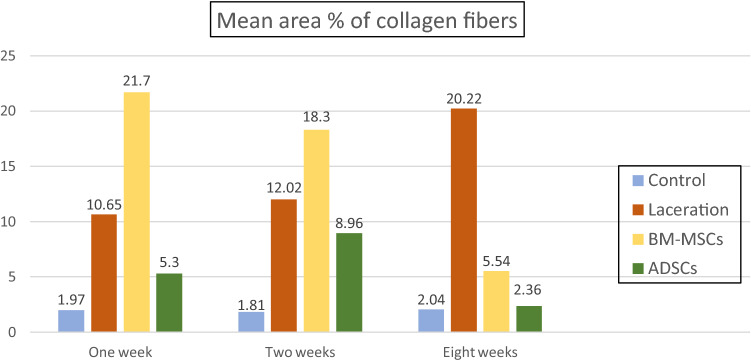
Examination of Mallory’s trichrome-stained sections after 1 week in untreated lacerated muscle subgroup (IIa) showed increase in collagen fiber deposition in between the myofibers and around the blood vessels (Fig. 8A). The mean area percentage of collagen fibers was 10.65% ± 2.6 (Table 1, Fig. 9). In BM-MSCs-treated subgroup (IIIa) (Fig. 8D), significant increase in the mean area percentage of collagen fibers 21.7% ± 1.52 was noticed in the granulation tissue and in the connective tissue between the disrupted muscle fibers (p < 0.05) as compared to untreated laceration group (Table 1, Fig. 9). While in ADSCs-treated subgroup (IVa), fine collagen bundles were noticed in between the distorted myofibers (Fig. 7G). The mean area parentage of collagen fibers was 5.3% ± 1.11 which was significantly decreased compared to untreated laceration and BM-MSCs-treated groups at same duration (Table 1, Fig. 9).
Fig. 8.
Mallory’s trichrome-stained sections in different groups. (A, D, G) after 1 week (B, E, H) after 2 weeks (C, F, I) after 8 weeks. Laceration group (group II) D–F BM-MSCs-treated group (group III) ADSCs-treated group (group IV). Collagen fibers (↑) are seen between muscle fibers. At every time point, the amount of collagen fibers decreased in stem cell-treated groups (III, IV) compared to untreated laceration group (II). Also notice minimal amounts of collagen fibers in ADSCs-treated group (IV) compared to BM-MSCs-treated groups (III) at each time point. Fat cells (*) are seen between muscle fibers
Fig. 9.
Showing the mean area percentage of collagen fibers in different groups
After 2 weeks in untreated laceration subgroup (IIb), increased deposition of collagen fibers was noticed in granulation tissue in between the disrupted myofibers and surrounding the congested blood vessels (Fig. 8B). The mean area percentage of collagen fibers was 12.02% ± 1.68 (Table 1, Fig. 9). In BM-MSCs-treated subgroup (IIIb) (Fig. 8E), the mean area percentage of collagen fibers was 18.3% ± 2.17 which was significantly more than in untreated laceration group (p < 0.05) (Table 1, Fig. 9), while in ADSCs subgroup (IVb) very thin collagen fibers deposition in between the muscle fibers and in the surrounding connective tissue (Fig. 8H) was noticed. The mean area percentage of collagen fibers was 8.96% ± 1.61 with significant decrease as compared to untreated laceration and BM-MSCs-treated groups (Table 1, Fig. 9).
After 8 weeks in untreated laceration subgroup (IIc), marked increase in deposition of collagen fibers at the site of injury and in the interstitium between the muscle fibers was seen (Fig. 8C). The mean area percentage of collagen fibers was 20.22% ± 6.72 (Table 1, Fig. 9). In BM-MSCs-treated subgroup (IIIc) (Fig. 8F), the mean area percentage of collagen fibers was 5.54% ± 1.46 which was significantly less than that of untreated laceration group (Table 1, Fig. 9). While in ADSCs-treated subgroup (IVc), minimal deposition of collagen fibers was noticed between the new muscle fibers (Fig. 8I). The mean area percentage of collagen fibers was 2.36% ± 1.44 which was significantly less than that of untreated laceration group and laceration treated with BM-MSCs (Table 1, Fig. 9).
Examination of re-innervation in the different groups
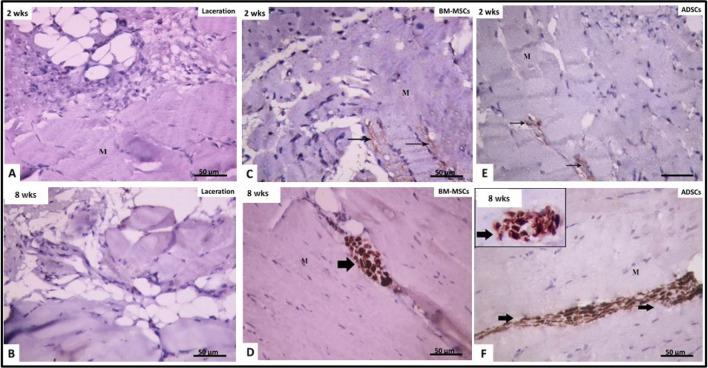
Immunohistochemical examination of laceration group after 2 weeks (IIb) and 8 weeks (IIc) showed negative expression of NFL protein in the nerve fibers of injured muscle (Fig. 10A, B), which indicated no re-innervation. However, 2 weeks after administration of either BM-MSCs (IIIb) or ADSCs (IVb), positive expression of NFL protein was seen in individual very thin nerve fibers (Fig. 10C, E). This could be an early indication of re-innervation. The mean area percentage of NFL protein expression was 0.80% ± 0.14 in BM-MSCs group and 0.79% ± 0.13 in ADSCs group, with no significant difference between them (Table 1).
Fig. 10.
Immune-histochemical reaction for neurofilament light chain × 400: at 2 weeks (A, C, E) and 8 weeks (B, D, F). A, B Laceration group (II): showing negative reaction for neurofilament tight chain between muscle fibers (M). C, D BM-MSCs-treated group (III): showing faint positive immune reaction (↑) at 2 weeks and strong positive reaction after 8 weeks. E, F ADSCs-treated group (IV): showing faint positive reaction for neurofilament light chain after 2 weeks and strong reaction after 8 weeks
After 8 weeks from administration of either BM-MSCs (subgroup IIIb) or ADSCs (subgroup IIIc), positive expression of NFL protein was noticed in nerve fibers between muscle fibers (Fig. 10D, F). The mean area percentage of NFL expression was 1.10% ± 0.26 in BM-MSCs-treated group and 1.18% ± 0.29 in ADSCs group, no significant difference between them and the control (p > 0.05 (Table 1).
Summary of results:
In H&E-stained sections, after one and 2 weeks from laceration, infiltration of site of injury with cellular granulation tissue was more obvious in untreated laceration group. In BM-MSCs- and ADSCs-treated groups, the number of regenerating myotubes was significantly increased compared to untreated laceration group. The number of myotubes was significantly higher in ADSCs group compared to BM-MSCs group. After 8 weeks, fibrous tissue and inflammatory cells were still seen in untreated laceration group. Meanwhile, formation of new muscle fibers was noticed in BM-MSCs- and ADSCs-treated groups. However, some of these fibers were seen with ill-defined cross striations.
Examination of Mallory’s trichrome-stained sections showed that administration of ADSCs resulted in significant decrease in collagen fibers at all time intervals compared to corresponding untreated laceration group and BM-MSCs-treated group.
Immunohistochemical examination for NFL showed negative reaction in untreated laceration group after 2 weeks and 8 weeks. Faint positive reaction was noticed in BM-MSCs and ADSCs after 2 weeks, while strong positive reaction was noticed in stem cells-treated groups after 8 weeks compared to laceration group indicating re-innervation. No significant difference was noticed between BM-MSCS- and ADSCs-treated groups.
Discussion
Skeletal muscle injury occurs in many clinical scenarios. Surgical reconstruction is always associated with high costs, and even if successful, there is frequently a residual muscle dysfunction [15].
In the present study, we used two different types of MSCs; BM-MSCs and ADSCs therapies for regeneration and re-innervation of experimental skeletal muscle laceration injury were evaluated after injection of. Evaluation of the regenerative changes was done after 1 week, 2weeks and 8 weeks of injection.
On culturing isolated BM-MSCs and ADSCs, we observed higher proliferative capacity of ADSCs which reached confluency faster than that of BM-MSCs. Similarly, Li et al. [16] noticed that ADSCs had greater proliferative potential compared to BM-MSCs. Moreover, we observed that cultured ADSCs revealed strong positive cytoplasmic expression of the protein CD133 as compared to BM-MSCs, which showed only weak expression of CD133. This is in accordance with Baer et al. [17] who reported that cells adherent fraction of cultured cells separated after digestion of adipose tissue were CD133 positive.
On examining untreated lacerated muscle 1- and 2-weeks post-laceration, we noticed marked mononuclear cellular infiltration in the granulation tissue. The chemokines, cytokines and growth factors produced by macrophages and fibroblasts in the injured area attracted more circulating inflammatory cells, including T and B lymphocytes [18].
However, evidence of early regeneration was noticed, with appearance of basophilic muscle fibers containing chains of centrally located nuclei in the sarcoplasm. These myotubes resulted from activation, proliferation and fusion of satellite cells [2]. Satellite cells can be activated by inflammatory cytokines, chemokines and growth factors which are secreted by the inflammatory cells as macrophage and lymphocytes [19]. Moreover, the vascular endothelial cells of damaged vessels release growth factors as vascular endothelial growth factor and platelet-derived growth factor [20]. These factors promote satellite cells activation and proliferation.
Activated satellite cells fuse to form myotubes then withdraw from the cell cycle to become terminally differentiated myocytes. In the current study, the statistical results revealed a significant increase in the number of these myotubes after 1 and 2 weeks compared to control group. This was concordant with Pecanha et al. [3].
Meanwhile, we observed deposition of fat cells and collagen fibers in the interstitium were noticed in the untreated laceration group. This could be attributed to the presence of fibro adipogenic progenitors in the interstitium of muscle cells that could differentiate into fat cells [21]. On the other hand, Yin et al. [22] attributed that to the trans-differentiation of satellite cells to adipocytes and fibroblasts.
In the current study, when BM-MSCs were used in treatment of lacerative muscle injury, there was a significant increase in the number of myotubes as compared to untreated group indicating a prominent regenerative activity. This was in accordance with Andrade et al. [25]. It was reported that BM-MSCs promoted the regeneration of muscle fibers either by differentiating to satellite cells then regenerating skeletal muscle fibers, or that a proportion of these cells fuse directly with muscle fibers and that both mechanisms coexist in the same tissue [23]. Kholodenko et al. [24] added that therapeutic efficacy of BM-MSCs was mainly dependent on their ability to produce paracrine factors through secretion of a variety of growth factors and cytokines, which could play a role for repairing tissue damage.
The present work also showed the presence of cells with vesicular nuclei near the muscle surface in the BM-MSC-treated group. This might be BM-MSCs or satellite cells. Andrade et al. [25] also proved the presence of injected BM-MSCs into the injured muscle. Meanwhile, Shabbir et al. [26] reported that injection of BM-MSCs promoted the activation and proliferation of satellite cells in skeletal muscle and stimulated proliferation of the resident muscle progenitor cells. Activated satellite cells appeared rounded with basophilic cytoplasm and central rounded vesicular nuclei [27].
In ADSCs-treated group, we found that the skeletal muscle laceration injury showed less inflammatory cellular infiltration in the surrounding connective tissue especially after 1 and 2 weeks. It seems that ADSCs inhibit the generation of pro-inflammatory cytokines and stimulate the production of anti-inflammatory IL-10 cytokine [28]. Immunomodulatory properties of ADSCs have been confirmed both in vitro and in vivo. ADSCs could mediate immunosuppression through inhibiting proliferation and activation of T helper lymphocyte subtypes which lead to suppression of T cell responses [29]. Meanwhile, we found significant increase in the number of myotubes in ADSCs-treated lacerations. This was consistent with Pecanha et al. [3].
After 8 weeks in untreated lacerated muscle, high amount of fibrous tissue was noticed at the site of the laceration injury. On the other hand, after 8 weeks from administration of BM-MSCS and ADSCs to the lacerated muscles, the site of the injury was filled with newly regenerated muscle fibers. Some of these regenerating muscle fibers contained central vesicular nuclei. They were seen with indistinct cross striations. This is in accordance with Karalaki et al. [30] who added that new muscle fibers were formed as a result of the myogenic proliferation phase, and then, the myoblasts differentiated and fused to existing damaged fibers for repair or to one another for new myofiber formation. Some characteristics of muscle regeneration are that newly formed fibers are basophilic, and this reflects a high protein synthesis. However, Kowalski et al. [31] mentioned that even after the myogenic commitment was induced in BM-MSCs by their contact with the satellite cell niche, their ability to form new myofibers in regenerating muscle was still low.
We found that treatment of muscle laceration with ADSCs promoted the healing more effectively than BM-MSCs as evidenced by the presence of significantly more regenerating myotubes than that observed in the group treated with BM-MSCs. Acceleration of muscle repair occurred in ADSCs-treated group could result from the paracrine mechanism of ADSCs. They were found to secrete different growth factors, as vascular endothelial growth factor, insulin-like growth factor and hepatocyte growth factor. These growth factors could play important role in activation, proliferation and differentiation of satellite cells [3]. In addition, Cui et al. [32] reported that muscle repair and regain of function were enhanced by ADSCs therapy, suggesting that ADSCs administration might accelerate muscle repair. Furthermore, ADSCs have several advantages over bone marrow, as easy accessibility, abundance, higher stem cell proliferation, less immunogenic and immune-suppressive effects [33].
Regarding the degree of fibrosis, we found extensive fibrosis at the site of laceration in untreated rats. Significant increase in collagen fibers deposition in the granulation tissue as well as around the congested blood vessels in rats of untreated muscle laceration injury in the early and late phases. This was in accordance with Desmoulière et al. [34] who reported that the matrix deposition in the skeletal muscle injury site could be seen within a week post-injury and could continue for several weeks. Excessive deposition of extracellular matrix components leads to the loss of proper muscle architecture and function [35].
We observed also significant increase in collagen fiber deposition in BM-MSCs-treated group than in ADSCs-treated group. This was in accordance with Liu et al. [36] and Andrade et al. [25]. Kang et al. [37] reported that BM-MSCs can produce extracellular matrix proteins as: collagen I, collagen IV, fibronectin, and laminin. However, BM-MSCs could restore muscle function through acceleration of regeneration processes, despite scar tissue development in cases of severe re-injury [25].
Our statistical results showed a significant decrease collagen fiber deposition in between the disrupted myofibers in all subgroups treated with ADSCs in comparison with untreated group and BM-MSCs-treated group. This was in accordance with Xiong et al. [38] who reported that ADSCs secrete many paracrine factors, including IGF-1 which inhibit excessive collagen deposition.
We studied the re-innervation process by examining the expression of neurofilament Light chain (NFL) protein. Untreated laceration group showed negative expression of NFL protein, after 2 weeks and 8 weeks. This indicated failure of re-innervation of injured muscle.
In lacerated muscle treated with BM-MSCs or ADSCs, positive expression of NFL was noticed. After 2 weeks, it was seen in the form of very thin individual nerve fibers but after 8 weeks, thick bundles were seen. This indicated early muscle re-innervation and then abundant restoration of muscle innervation.
It was reported that BM-MSCs possess the ability to differentiate into non mesodermal lineages, such as astrocytes, neurons and Schwann cells [39]. BM-MSCs also stimulate glial cells to produce neurotrophic factors as nerve growth factor and Brian-derived nerve factor [40]. These cytokines are known to be essential factors for the survival and differentiation of neuronal progenitor cells. Therefore, there was good evidence to support the hypothesis that transplantation of BM-MSCs may repair peripheral nerve injuries [37]. It was reported that ADSCs can differentiate into a Schwann cell-like phenotype, thus representing a valid Schwann cell alternative [41]. ADSCs transplantation also facilitates myelination of axons and reduces inflammation [42].
In the current study, both BM-MSCs and ADSCs improved the healing process of skeletal muscle laceration injury at short- and long-term durations. But effective re-innervation of injured muscles was achieved only on the long-term follow-up.
ADSCs could have better effects in treating muscle laceration injury than BM-MSCs as evidenced by significant increase in the number of regenerating myotubes and significant decrease in collagen deposition.
Further studies are needed to assess the functional recovery of the skeletal muscle and effective vascularization of the muscle. It is also recommended to extend the experiment for longer time to test safety of stem cells injection.
Compliance with ethical standards
Conflicts of interest
The authors have no conflicting financial or competing interests.
Ethical statement
The animal experiment was conducted in the Research Center institute (MASRI), Faculty of Medicine, Ain Shams University. The FMASU REC is organized and operated according to guidelines of the International Council on Harmonization (ICH) and the Islamic Organization for Medical Science (IOMS), the United States Office for Human Research Protections and the United States Code of Federal regulations and operates under Federal Wide Assurance N. FWA 00017585.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Beldjilali-Labro M, Garcia A, Farhat F, Bedoui F, Grosset J, Dufresne M, Legallais C. Biomaterials in tendon and skeletal muscle tissue engineering: current trends and challeng. Materials (Basel) 2018;11:1116. doi: 10.3390/ma11071116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pereira T, Gärtner A, Amorim I, Armada-da-Silva P, Gomes R, Pereira C, et al. Biomaterials and stem cell therapies for injuries associated to skeletal muscle tissues. Adv Biomater Sci Biomed Appl. 2013;23:329–363. [Google Scholar]
- 3.Peçanha R, Bagno LL, Ribeiro MB, Robottom Ferreira AB, Moraes MO, Zapata-Sudo G, et al. Adipose-derived stem-cell treatment of skeletal muscle injury. J Bone Joint Surg Am. 2012;94:609–17. doi: 10.2106/JBJS.K.00351. [DOI] [PubMed] [Google Scholar]
- 4.Tian ZL, Jiang SK, Zhang M, Wang M, Li JY, Zhao R, et al. a7nAChR is expressed in satellite cells at different myogenic status during skeletal muscle wound healing in rats. J Mol Histol. 2015;46:499–509. doi: 10.1007/s10735-015-9641-4. [DOI] [PubMed] [Google Scholar]
- 5.Filareto A, Rinaldi F, Arpke RW, Darabi R, Belanto JJ, Toso EA, et al. Pax3-induced expansion enables the genetic correction of dystrophic satellite cells. Skelet Muscle. 2015;5:36. doi: 10.1186/s13395-015-0061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moloney EB, de Winter F, Verhaagen J. ALS as a distal axonopathy: molecular mechanisms affecting neuromuscular junction stability in the presymptomatic stages of the disease. Front Neurosci. 2014;8:252. doi: 10.3389/fnins.2014.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laumonier T, Menetrey J. Muscle injuries and strategies for improving their repair. J Experimen Orthopaed. 2016;3:15. doi: 10.1186/s40634-016-0051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feisst V, Meidinger S, Locke MB. From bench to bedside: use of human adipose-derived stem cells. Stem Cells Cloning. 2015;8:149–162. doi: 10.2147/SCCAA.S64373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McFarlin K, Gao X, Liu YB, Dulchavsky DS, Kwon D, Arbab AS, et al. Bone marrow-derived mesenchymal stromal cells accelerate wound healing in the rat. Wound Repair Regen. 2006;14:471–478. doi: 10.1111/j.1743-6109.2006.00153.x. [DOI] [PubMed] [Google Scholar]
- 10.Cakici C, Buyrukcu B, Duruksu G, Haliloglu A, Aksoy A, Isik A, et al. Recovery of fertility in azoospermia rats after injection of adipose-tissue derived mesenchymal stem cells: the sperm generation. Biomed Res Int. 2013;2013:529589. doi: 10.1155/2013/529589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li H, Fu X, Ouyang Y, Cai C, Wang J, Sun T. Adult bone marrow-derived mesenchymal stem cells contribute to wound healing of skin appendages. Cell Tissue Res. 2006;326:725–736. doi: 10.1007/s00441-006-0270-9. [DOI] [PubMed] [Google Scholar]
- 12.Karaoz E, Aksoy A, Ayhan S, Sariboyaci AE, Kaymaz F, Kasap M. Characterization of mesenchymal stem cells from rat bone marrow: ultrastructural properties, differentiation potential and immunophenotypic markers. Histochem Cell Biol. 2009;132:533–546. doi: 10.1007/s00418-009-0629-6. [DOI] [PubMed] [Google Scholar]
- 13.Suvarna K, Layton C, Bancroft J. Theory and practice of histological Techniques. 7. London: Churchill Livingston; 2013. [Google Scholar]
- 14.Petrosyan K, Tamayo R, Joseph D. Sensitivity of a novel Biotin- free Detection Reagent (powervision+™) for immune histochemistry. J Histotechnol. 2002;25:247–250. [Google Scholar]
- 15.Liu J, Saul D, Böker KO, Ernst J, Lehman W, Schilling AF. Current methods for skeletal muscle tissue repair and regeneration. Biomed Res Int. 2018;2018:1984879. doi: 10.1155/2018/1984879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li CY, Wu XY, Tong JB, Yang XX, Zhao JL, Zheng GF, et al. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res Ther. 2015;6:55. doi: 10.1186/s13287-015-0066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baer PC, Geiger H. Adipose-derived mesenchymal stromal/stem cells: tissue localization, characterization, and heterogeneity. Stem Cells Int. 2012;2012:812693. doi: 10.1155/2012/812693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kharraz Y, Guerra J, Mann CJ, Serrano AL, Muñoz-Cánoves P. Macrophage plasticity and the role of inflammation in skeletal muscle repair. Mediators Inflamm. 2013;2013:491497. doi: 10.1155/2013/491497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quintero AJ, Wright VJ, Fu FH, Huard J. Stem cells for the treatment of skeletal muscle injury. Clin Sports Med. 2009;28:1–11. doi: 10.1016/j.csm.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montarras D, L'honoré A, Buckingham M. Lying low but ready for action: the quiescent muscle satellite cell. FEBS J. 2013;280:4036–4050. doi: 10.1111/febs.12372. [DOI] [PubMed] [Google Scholar]
- 21.Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev. 2013;93:23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi X, Garry DJ. Muscle stem cells in development, regeneration, and disease. Genes Dev. 2006;20:1692–1708. doi: 10.1101/gad.1419406. [DOI] [PubMed] [Google Scholar]
- 24.Kholodenko IV, Konieva KAA, Kholodenko RV, Yarygin KN. Molecular mechanism of migration and homing of intravenously transplanted mesenchymal stem cells. J Regen Med Tissue Eng. 2013;1:1–4.
- 25.Andrade BM, Baldanza MR, Ribeiro KC, Porto A, Peçanha R, Fortes FS, et al. Bone marrow mesenchymal cells improve muscle function in a skeletal muscle re-injury model. PLoS One. 2015;10:e0127561. doi: 10.1371/journal.pone.0127561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shabbir A, Zisa D, Leiker M, Johnston C, Lin H, Lee T. Muscular dystrophy therapy by nonautologous mesenchymal stem cells: muscle regeneration without immunosuppression and inflammation. Transplantation. 2009;87:1275–1282. doi: 10.1097/TP.0b013e3181a1719b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dumont NA, Wang YX, von Maltzahn J, Pasut A, Bentzinger CF, Brun CE, et al. Dystrophin expression in muscle stem cells regulates their polarity and asymmetric division. Nat Med. 2015;21:1455–1463. doi: 10.1038/nm.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagaya R, Mizuno-Kamiya M, Takayama E, Kawaki H, Onoe I, Tanabe T, et al. Mechanisms of the immunosuppressive effects of mouse adipose tissue derived mesenchymal stromal cells on mouse alloreactively stimulated spleen cells. Exp Ther Med. 2014;7:17–22. doi: 10.3892/etm.2013.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez-Rey E, Gonzalez MA, Varela N, O’Valle F, Hernandez-Cortes P, Rico L, et al. Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann Rheum Dis. 2010;69:241–248. doi: 10.1136/ard.2008.101881. [DOI] [PubMed] [Google Scholar]
- 30.Karalaki M, Fill S, Philppou A, Koutsilieris M. Muscle regeneration: cellular and molecular events. In Vivo. 2009;23:779–796. [PubMed] [Google Scholar]
- 31.Kowalski K, Dos Santos M, Maire P, Ciemerych MA, Brzoska E. Induction of bone marrow-derived cells myogenic identity by their interactions with the satellite cell niche. Stem Cell Res Therapy. 2018;9:258. doi: 10.1186/s13287-018-0993-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cui L, Yin S, Liu W, Li N, Zhang W, Cao Y. Expanded adipose-derived stem cells suppress mixed lymphocyte reaction by secretion of prostaglandin E2. Tissue Eng. 2007;13:1185–1195. doi: 10.1089/ten.2006.0315. [DOI] [PubMed] [Google Scholar]
- 33.Forcales SV. Potential of adipose derived stem cells inmuscular regenerative therapies REVIEW; volum 7/article 123 published: 13 July. Front Aging Neurosci. 2015;7:123. doi: 10.3389/fnagi.2015.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Desmoulière A, Darby IA, Gabbiani G. Normal and pathologic soft tissue remodeling: role of the myofibroblast, with special emphasis on liver and kidney fibrosis. Lab Invest. 2003;83:1689–1707. doi: 10.1097/01.lab.0000101911.53973.90. [DOI] [PubMed] [Google Scholar]
- 35.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu X, Zheng L, Zhou Y, Chen Y, Chen P, Xiao W. BMSC transplantation aggravates inflammation oxidative stress and fibrosis and impairs skeletal muscle regeneration. Front Physiol. 2019;10:87. doi: 10.3389/fphys.2019.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang SB, Olson JL, Atala A, Yoo JJ. Functional recovery of completely denervated muscle: implications for innervation of tissue-engineered muscle. Tissue Eng Part A. 2012;18:1912–1920. doi: 10.1089/ten.tea.2011.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiong CJ, Li PF, Song YL, Xue LX, Jia ZQ, Yao CX, et al. Insulin induces C2C12 cell proliferation and apoptosis through regulation of cyclin D1 and BAD expression. J Cell Biochem. 2013;114:2708–2717. doi: 10.1002/jcb.24619. [DOI] [PubMed] [Google Scholar]
- 39.Fairbairn NG, Meppelink AM, Ng-Glazier J, Randolph MA, Winograd JM. Augmenting peripheral nerve regeneration using stem cells: a review of current opinion. World J Stem Cells. 2015;7:11–26. doi: 10.4252/wjsc.v7.i1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delk NA, Farach-Carson MC. Interleukin-6: a bone marrow stromal cell paracrine signal that induces neuroendocrine differentiation and modulates autophagy in bone metastatic PCa cells. Autophagy. 2012;8:650–663. doi: 10.4161/auto.19226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tse KH, Novikov LN, Wiberg M, Kingham PJ. Intrinsic mechanisms underlying the neurotrophic activity of adipose derived stem cells. Exp Cell Res. 2015;331:142–151. doi: 10.1016/j.yexcr.2014.08.034. [DOI] [PubMed] [Google Scholar]
- 42.Marconi S, Castiglione G, Turano E, Bissolotti G, Angiari S, Farinazzo A, et al. Human adipose-derived mesenchymal stem cells systemically injected promote peripheral nerve regeneration in the mouse model of sciatic crush. Tissue Eng Part A. 2012;18:1264–1272. doi: 10.1089/ten.TEA.2011.0491. [DOI] [PubMed] [Google Scholar]