Abstract
Microfluidic technologies have emerged as a powerful tool that can closely replicate the in-vivo physiological conditions of organ systems. Assisted reproductive technology (ART), while being able to achieve successful outcomes, still faces challenges related to technical error, efficiency, cost, and monitoring/assessment. In this review, we provide a brief overview of the uses of microfluidic devices in the culture, maintenance and study of ovarian follicle development for experimental and therapeutic applications. We discuss existing microfluidic platforms for oocyte and sperm selection and maintenance, facilitation of fertilization by in-vitro fertilization/intracytoplastimc sperm injection, and monitoring, selection and maintenance of resulting embryos. Furthermore, we discuss the possibility of future integration of these technologies onto a single platform and the limitations facing the development of these systems. In spite of these challenges, we envision that microfluidic systems will likely evolve and inevitably revolutionize both fundamental, reproductive physiology/toxicology research as well as clinically applicable ART.
Keywords: Microfluidics, In-vitro oocyte culture, In-vitro sperm culture, Assisted reproductive technology, Ovarian follicle development
Introduction
Microfluidic systems, or micro-physiological systems (MPS), precisely position collections of cells in a three-dimensional (3D) manner that mimics the structure and function of organs in the body [1]. The overall objective of these systems is to model the physiological aspects of naturally occurring functional organ units when exposed to pharmaco-therapeutics, hormones, cell signaling molecules and various biomechanical stressors [2]. These devices have wide variability in their design, organization, and size depending on the experimental objective and the organ or tissue that is being modeled. While some devices allow for self-assembly of organoids, others provide a scaffolding biomaterial matrix for cells to self-incorporate and proliferate in a structurally defined way [3]. The complexity of such designs increases significantly when specific cell types need to be positioned relative to each other in spatially defined compartments to accurately recapitulate the functional unit of the organ, such as in the kidney nephron or liver sinusoid [3]. Furthermore, the range of the overall platform size can vary from the use of small cell compartments of a few hundred micrometers in size to scaled-up designs consisting of multi-organ systems measuring several centimeters.
MPS platforms possess major design features that provide advantages to studying functional organ units and tissues. First, by utilizing microfluidic technologies, precise volumes of fluid can be supplied to the system to deliver nutrients while simultaneously removing cellular waste, while replicating physiologically relevant pressures and gradients. Second, by precisely positioning multiple cell types spatially with the maintenance of their three-dimensional arrangement, intricate cell-cell interfaces and interactions can more accurately recapitulated in vitro. Furthermore, these two design features, microfluidic flow and 3D cellular arrangement in combination, provide shear/stretch forces that more accurately mimic in vivo conditions.
MPS systems confer various advantages when designing, managing, and collecting data for a proposed study. MPS devices are often designed using transparent/translucent materials that enable cells to be visualized by real-time microscopy. This feature allows the investigator latitude in assessing and monitoring cellular function, cell-cell interactions, structural changes and an assortment of other visually defined parameters over the course of longer experiments [4]. Longer longitudinal monitoring, when applied to an ovarian follicle, would allow for more intricate cell function analysis, the determination of time course response and recovery to specific pharmaco-therapeutics, and the ability to elucidate the effects of cyclical hormone patterns on drug response patterns over time [2]. Additionally, the active, continuous flow of fluid media through the system allows for collection of platform effluent for further enzymatic or biochemical assays.
Microfluidic platforms for drug discovery, efficacy assessment and toxicology studies
Currently, animal models, especially rodent, are the gold standard for pre-clinical assessment of pharmaco-therapeutics [5]. However, the distinct interspecies physiological differences between animals and humans, overall poor characterization of these models, and lack of sufficient experimental quality and reproducibility have proven to be problematic [6, 7]. Additionally, 80% of potential therapeutics that progress past this form of preclinical testing ultimately fail during clinical trials furthermore proving to be costly [8]. In response to these limitations, movement towards modeling physiological systems using 3D engineered organoids with perfusion by a microfluidic platform could provide a valuable alternative.
One particular study showed the power of utilizing a microfluidic approach for supporting and characterizing individual human pre-antral follicles encapsulated in an alginate-based biomaterial and cultured over a period of 8 days [9]. Estradiol and testosterone were measured at regular intervals assessing the paracrine response to stimulatory media supplied by the microfluidic device [9]. Furthermore, the relative changes in follicle diameter, which reflected follicle maturation, specific cell-cell interactions, and molecular changes in gap-junction enrichment could be assessed using captured images [9]. This study displayed the power of a microfluidic approach to study normal folliculogenesis at the individual follicle level, assess molecular and structural changes, as well as hormonally mediated changes. Furthermore, this experimental design could be used to assess follicle development and function due to changes in the embedding biomaterial, varying media stimulatory factors, or by the addition of specific environmental drugs or toxins for testing.
As described, utilizing a microfluidics approach affords the investigator more precise control over the extended culture and observation of the response of an individual or collection of ovarian follicles. Thus far, most studies have relied on the extraction of ovarian follicles from murine, bovine, and human tissue for culturing on microfluidic platforms. In one study, a microfluidic device was developed to culture ovarian follicles in order to demonstrate the potential of such systems to answer more complex questions related to a drug’s toxicology profile, mechanism of action, and changes in gene expression [10]. The microfluidic chip used in this study consisted of three inlets funneling culture medium and drugs with one outlet for fluid collection. With the cultured ovarian follicles perfused from below the PDMS base layer into the follicle containing compartment, the volume and flow rate of culture medium could be precisely regulated. This control of flow is fundamental to delivering nutrients and removing waste present in the medium, thus better mimicking the in vivo ovarian microenvironment. For this particular study, doxorubicin was utilized due to its well-known toxicity to secondary oocytes, antral follicles, and granulosa cells (GCs) [11, 12]. Doxorubicin treatment showed dose-dependent toxic effects including the inhibition of ovarian follicle growth, oocyte development, overall follicle maturation, GC degradation as well as hormone secretion [10, 11]. The mechanisms by which doxorubicin elicited these various deleterious effects were investigated using a single, intact ovarian follicle placed onto the chip, prior to the addition of culture medium containing doxorubicin and selected inhibitors (Src kinase, ER-calcium release, and PIM kinase) of signaling pathways thought to be involved in mediating doxorubicin toxicity [10, 11].
Using the microfluidic system allowed for continuous culture of both control and treated follicles in parallel and allowed for various parameters to be assessed over time to give a more complete picture of the structural and functional aspects of the ovarian follicle [13]. Overall results showed that doxorubicin reduced ovarian follicle diameter and estradiol (E2) secretion compared to control groups [10]. Furthermore, TUNEL staining and up-regulation of key genes demonstrated that ovarian follicles underwent apoptosis after doxorubicin exposure. The combination of assessing follicle size by imaging with assessment of relative gene expression when selected inhibitors were introduced, showed contrasting but fundamentally important results [10].
Overall, this was an ideal model experiment that effectively displayed the possibilities and power of using a microfluidic approach to study the physiological effect of existing or novel experimental medications and therapeutics. This experiment also showed that various other physiologically relevant stimuli could be assessed simultaneously using this approach such as active biological agents and toxins, viruses and bacteria, disease models, cancer models, radiation, and a host of other possibilities by tailoring the end point assays to the experimental objective.
Current approaches in assisted reproductive technologies (ART)
In vitro fertilization (IVF)
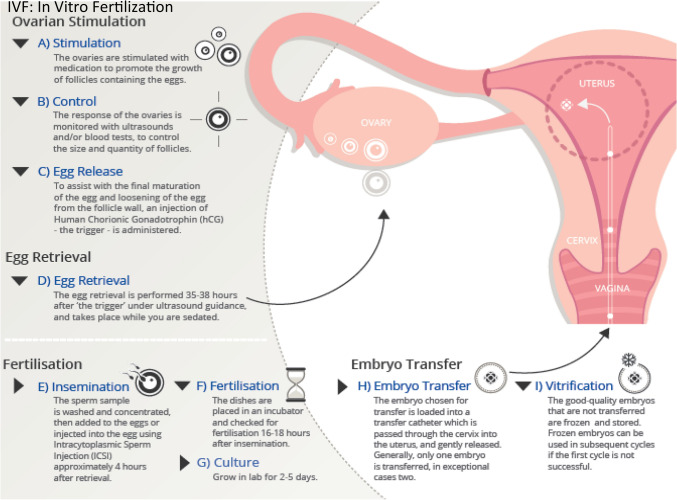
For IVF the requisite gametes needed for fertilization must be acquired separately but in a parallel and time-sensitive fashion. As shown in Fig. 1, the IVF requires hormonal stimulation of the patient to produce eggs which are subsequently fertilized with acquired sperm to form embryos which are then transferred to the uterus for gestation. Typically, the ovaries are stimulated by a hormonal protocol that promotes the maturation of follicles forming oocytes. During this period, the ovaries are monitored by noninvasive ultrasound and/or blood tests to determine the size quantity of the follicles. For the final maturation of the oocytes, human chorionic gonadotropin (hCG) is administered to trigger the release of the mature oocytes. Oocyte pick-up (OPU) is usually scheduled for 34–36 h following hCG administration. During the early days of IVF, OPU was performed laparoscopically under general anesthesia but is now performed by transvaginal ultrasound guided follicle aspiration [14]. Once ovarian follicular aspirates are acquired, they are transferred to a HEPES based buffered media to ensure the maintenance of a physiologic pH [15]. The aspirates are the visualized by microscopy to identify and clear the cumulus oophorous complex (COC) of any surrounding blood clots and debris [16]. Oocyte maturity is determined based on the morphological appearance of the COC, with mature oocytes identified by highly dispersed cumulous cells and the radiating coronal layer [17].
Fig. 1.
Diagram depicting in vitro fertilization (IVF) procedure. IVF requires hormonal stimulation of the patient to produce eggs which are subsequently fertilized with acquired sperm to form embryos which are then transferred to the uterus for gestation
Sperm selection
For the sperm selection, a semen specimen is obtained, and sperm preparation is performed by either a “swim-up” or “density gradient” method to isolate a high concentration of motile sperm [14]. Subsequently, the isolated motile sperm are incubated in media containing a high concentration of protein for 30 min to 4 h to achieve capacitation [18]. Conventionally, IVF is usually performed 4–6 h after OPU with each mature oocyte incubated with 50–100,000 sperm/mL for 12–18 h. A fertilization event is confirmed by the presence of 2 pronuclei (PN) and the extrusion of a second polar body ~18 h post-IVF [19]. The fertilization rate achieved by IVF is between 50 and 70%, but this rate drops if the oocytes are less mature [14].
Intracytoplasmic sperm injection (ICSI)
ICSI circumvents the need for sperm to penetrate the Zona Pellucida (ZP), thus removing a critical step that could impair proper fertilization. This procedure was developed for couples with infertility traced to impairment in sperm characteristics and failed prior attempts with IVF. The most common indications of severely impaired sperm characteristics include oligospermia, asthenospermia, and teratospermia. ICSI is also indicated for patients with a history of failed IVF, those with Klinefelter syndrome, or those with high sperm antibody titers [14].
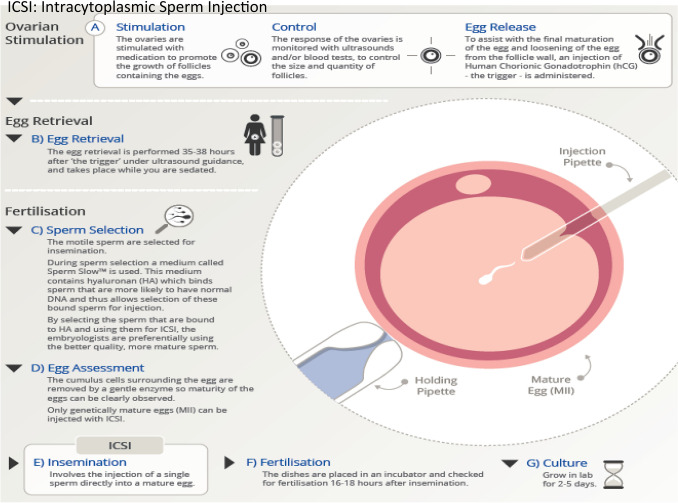
The procedure described in Fig. 2 is performed on mature oocytes obtained by OPU as described in Fig. 1. A healthy motile, morphologically normal-appearing spermatozoan is selected with an injection pipette while a holding pipette secures a selected oocyte in position with its polar body-oriented at the 12 o’clock position [14, 16]. The injection pipette pierces the ZP, passing through the oolema at the 3 o’clock position [14] and the sperm is released at a 90° angle from the first polar body, in order to avoid the meiotic spindle apparatus [20].
Fig. 2.
Diagram depicting intracytoplasmic sperm injection (ICSI). ICSI is performed on mature oocytes obtained by oocyte pick-up as depicted in Fig. 1. A healthy motile, morphologically normal-appearing spermatozoan is selected with an injection pipette while a holding pipette secures a selected oocyte in position with its polar body-oriented at the 12 o’clock position. The injection pipette pierces the zona pelucida, passing through the oolema at the 3 o’clock position and the sperm is released at a 90° angle from the first polar body, in order to avoid the meiotic spindle apparatus
Embryo culture and maturation
Once the fertilization event has occurred, the resultant zygotes are transferred to an embryo culture media. The culture media strategies are categorized as either monoculture or sequential culture systems. The monoculture media is formulated to mimic naturally occurring human tubal fluid (HTF) which supports zygote maturation to the blastocyst stage without the need to change the media. However, most current and commercially available embryo culture systems use sequential culture media. The sequential media is conceptually formulated to support the dynamically changing nutrient and energy needs of cleavage and blastocyst stage embryos [14] as it has been noted that pre-compacting embryos require pyruvate and nonessential amino acids as nutrient sources while post-compacting embryos require glucose and essential amino acids for nourishment [14]. As a result, the sequential culture system involves two different culture media given in succession. The first media supports the development of cleavage stage embryos, which are then transferred to a second media to support the development of the blastocyst stage embryo. Besides nutrient composition, the pH, gas concentration, temperature, type of protein supplement, and presence of reactive oxygen species all play a role in the maturation of the embryo [14]. Currently, most embryos are matured to between the day 3 cleavage and day 5 blastocyst stage embryos to increase the success of implantation as well as reduce the risks of multiple births [15]. Extended culture to the blastocyst stage allows for the selection of embryos with the highest implantation and developmental potential, therefore reducing the number of embryos transferred and decreasing the risk for multiple births [14].
Microfluidic-based strategies for assisted reproductive technologies (ART)
The various steps of assisted reproductive technologies (ART) could be integrated into a single platform using microfluidic technologies. The individual steps of the ART protocol have been demonstrated separately on microfluidic platforms, including the analysis of semen for the procurement of sperm [21], oocyte characterization [22], the removal of cumulus cells [23, 24], fertilization [25–27], and embryo culture and characterization. The advantage of using a fully integrated microfluidic platform would allow for minimal human intervention in the form of manipulation of gametes and resulting embryos, with the overall reduction in the risk of error and contamination. Additionally, the use of an integrated, standardized, and fully automated platform to perform ART techniques would improve overall success rates of embryo implantation [28].
The application of microfluidic-based platforms could specifically improve a subset of techniques that comprise the complete ART protocol, thereby enhancing the success of rate of the entire procedure. Identification of viable immotile sperm, incapable of proper penetration of oocytes, could selectively be removed, leaving the best sperm for fertilization [29]. The microfluidic technology could further be applied to ICSI, by enhancing control of the individual spermatid and improving oocyte manipulation and guidance to an integrated needle, which has previously been demonstrated for use with somatic cells [30].
Further downstream, a finely tuned biopsy needle integrated into the microfluidic device could be used to isolate one or more cells from different cleavage or blastocyst stage embryos, which could be funneled by fluid flow to a separate compartment within the device, leaving the embryo to continue to grow and develop within the first compartment. The isolated cells could be examined by molecular, genetic, and chromosomal analyses to assess for inherited disease, mutations, genetic errors, and other possible factors influencing normal embryo development [31]. Furthermore, as the embryo continues to develop and advance into later stages, additional sampling of fluid by needle biopsy would provide an even more accurate analysis of chromosomal arrangement owing to the lower impact of mosaicism in blastocysts compared to cleavage-stage embryos [32]. While the combination of all ART protocols onto a single platform would revolutionize the efficiency and hopefully success of the process, each individual technique within the whole process has its own challenges and barriers that must be overcome.
Semen analysis and separation of quality sperm for fertilization
For human IVF purposes, isolation of sperm of sufficient quality and quantity is required to facilitate better success rates of fertilization events by IVF or ICSI. Various parameters must be assessed when examining semen composition including total volume as well as sperm concentration, motility, viability, and overall morphology [33]. Typically sperm processing has been performed by simple media washing, a semen overlay with medium and sperm swimming out of the seminal plasma, density gradient centrifugation or some combination of these methods (WHO, 2010). It was only in the early to mid-1990s that investigators assessed sperm through branching micro channels and suggested microfluidic devices could be used to assess sperm motility during semen analysis [18, 34]. In 2003, investigators used a microfluidic approach to process human semen and isolate motile sperm with the idea that parallel, laminar flow of streams of semen and media would allow for better separation of motile from non-motile sperm and cellular debris [35]. This sorting system was comprised of two inlets, two outlets, a sorting channel, and arrays of horizontal passageways for maintaining parallel, laminar streams perfused by a passively driven, constant-flow-rate pump. The sperm with higher motility were selectively sorted and separated out from the rest of the semen sample based on their enhanced ability to swim through the interface between adjacent, laminar streams into a common “collecting stream” while the less motile sperm and debris remained restricted in the initial stream [35]. The simple, self-contained device has the added advantage of selecting the healthiest sperm which are often the most-motile [33].
Oocytes
Currently, oocyte collection for the purposes of clinical ART applications involves the use of a combination of intricate procedures and specialized materials including macro- and micro-processing needles, tubing, vacuums, test tubes, and Petri dishes. In addition, microscopic observation over sustained periods of time is required to isolate individual oocyte cumulus masses. During IVF/ICSI, the removal of the majority of cumulus cells is important for determining oocyte maturity, polar body positioning in relation to the site of intended sperm injection, and the overall process of sperm injection. Typically these cumulus cells are removed using a combination of enzymatic and mechanical manipulation [36], which works rather well. However a microfluidic approach could be utilized to mechanically remove cumulus cells from the oocyte using fluid flow shearing and in turn reduce exposure of the oocytes to enzymes which can cause cellular degradation as has been shown in bovine oocyte cumulus complexes [23]. The shearing process relies on precisely directing minute volumes of fluid at a tangent to the oocyte-cumulus cell complex, peeling away the surrounding cells. Compared to the more stress inducing methods previously described, this microfluidic shearing method showed accelerated zygote development by day 2 and blastocyst development by day 8 [24]. Furthermore, the study employed in situ transcription assays used to measure biochemical stress/activity of zygotes which were markedly reduced with the shearing method, strongly supporting the microfluidic method as a way to enhance production efficiencies [24].
Ovarian follicles for production of oocytes
A microfluidic approach could have a distinct advantage in supporting the development of early-stage follicles into mature late-stage follicles capable of producing significantly higher numbers of oocytes. The culture conditions must be suitable to promote timely interaction between the oogonia and supportive cells. This has proven to be especially challenging with isolated primary follicles cultured on flat surfaces since physical manipulation is often needed to prevent cell attachment and migration of somatic cells in an attempt to maintain follicle structure which could be more efficiently provided using a microfluidic approach [37]. The development of specifically defined microenvironments that maintain an expanding microarchitecture surrounding cultured follicles and continuously deliver factors would promote more efficient follicle maturation. These 3D culture systems encapsulate follicles and provide the necessary mechanical stress which is required to preserve oocyte–somatic cell connections and promote survival of early-stage follicles, without the need for manipulation during culture [3, 38]. Specific design criteria and principles for these three-dimensional hydrogel-based follicle culture systems have been identified. First, the encapsulation and culture conditions must be mild, as years of experience with IVF have established that oocyte quality is significantly impacted by the culture medium and conditions. Second, the growth of the follicle within the hydrogel must maintain the cell-cell connectivity and overall architecture of the follicle while enabling the follicle to expand. Human secondary follicles, with an initial diameter of 120 μm, undergo a 4.7 × 106–fold increase in volume to their final diameter of 20 mm [39]. Third, the mechanical properties of the hydrogel and/or matrix degradation must accommodate this exponential increase in size during culture. The follicle and the oocyte must be easily retrieved from the hydrogel upon the termination of culture to study biological endpoints or for use of the recovered oocyte for IVF. Finally, the culture system environment must be easily modified to contain biological signals such as growth factors, hormones, or ECM peptides or proteins [40].
The guiding principle in the design of these 3D culture environments is the accurate presentation of the various signals (growth factors, hormones, ECM mechanics, etc.) that will allow for coordinated growth of the multiple cellular compartments of the follicle—the oocyte, granulosa cells, and theca cells—in vitro [40]. The ovarian environment is dynamic, governed by cyclic changes in endocrine factors in the systemic circulation and local signals produced by the ovary [41]. To mimic the ovarian environment in vitro, these factors must be supplemented at the appropriate times and concentrations in a means that can be transported to the follicle by diffusion through the hydrogel to recapitulate the changes that occur during folliculogenesis. The signals presented to the follicle must promote the growth of the somatic cells in conjunction with, and not at the expense of, the oocyte [41]. As a result, two specific culture systems have emerged as possible methods to efficaciously mature follicles to a stage where viable oocytes could be retrieved: the alginate hydrogel culture system and the cell responsive culture system.
The alginate hydrogel culture system
Alginate is a linear polysaccharide derived from algae and composed of repeating units of β-D-mannuronic acid and α-L-guluronic acid [42] that solidifies by ionic cross-linking of the guluronic residues [43, 44]. The mild gelation process has led to the use of alginate in a number of applications [42] Conversely, the hydrogel can be dissolved through the addition of calcium chelators [44, 45] or degraded with alginate lyase, a bacterially derived enzyme that has had no observable effects on the follicle. Alginate lyase has been employed to retrieve intact follicles from the matrix for subsequent in vitro maturation of the oocyte. Granulosa and theca cells do not have adhesion receptors for alginate and thus do not interact with the hydrogel; however, ECM peptides or proteins can be immobilized or entrapped within the hydrogel matrix to interact with the follicle [46, 47]. Molecules with molecular weights less than 20 kDa are able to diffuse through alginate gels with the same diffusivity as in water [48, 49]. Larger molecules diffuse through the alginate matrix pores, which have diameters ranging from 5 to 200 nm, in a molecular weight–dependent manner [48, 49]. The alginate matrix also helps to maintain paracrine signaling by factors produced by the follicle and can be modified to mimic ovarian stroma [46].
Follicles cultured within alginate maintain both an in vivo–like morphology, with a centrally located oocyte and surrounding layers of granulosa and theca cells, and connections between the somatic cells and the granulosa cells and oocyte are maintained [47] Follicle growth and hormone secretion patterns of secondary follicles cultured in vitro in the alginate system mimic those of follicles in vivo [39] and oocytes retrieved following the culture of secondary follicles in alginate hydrogels develop the capacity for fertilization similar to that of in vivo–matured oocytes.
Physical properties of the alginate hydrogel determine whether the environment is permissive for follicle growth and development [42, 47]. These properties include factors such as pore size, which determines the transport of hormones and growth factors from the culture medium through the hydrogel, and mechanical properties, which cells translate to a biochemical signal in a process known as mechano-transduction. They varied the concentration of alginate, which influences both transport and mechanical properties, and then determined the impact on follicle growth in vitro [47]. A 0.25% alginate hydrogel, which creates relatively soft beads, was more permissive for follicle growth than the other concentrations tested (0.5, 1, and 1.5%). The 0.25% alginate hydrogel improved growth, increased steroidogenesis (estradiol and androstenedione), and produced a greater yield of MII oocytes.
Cell-responsive culture systems
The use of degradable matrices applied to follicle culture that allows for matrix remodeling in response to follicle growth have recently been reported [50]. The diameter of follicles increase as they grow in a 3D environment in vitro, and an encapsulating hydrogel such as alginate, which is not readily degradable, will exert a compressive force on the follicle in response to the expansion [51, 52]. The compressive force is dependent upon the elastic strength of the hydrogel, as well as the change in the size of the follicle. The volume of the hydrogel that is displaced by the developing follicle increases as r3, where r is the radius of the follicle, but the surface area that is acted on by the compressive force increases only as r2 [53, 54]. Although nondegradable alginate hydrogel systems support the culture of mouse secondary follicles, translation of these systems to either earlier-stage follicles or follicles of larger animal species may be challenging owing to the significantly greater volumetric increase that must occur during culture. The stress profile in a human follicle may significantly differ from that in a murine follicle and may partially contribute to the challenge of culturing human follicles in vitro [54]. Naturally derived polymers such as collagen and fibrin have been used extensively in regenerative medicine applications and can degrade in response to growing follicles to allow for expansion of the follicle, yet the success of these materials for in vitro follicle culture has been modest [39, 55]. Collagen was one of the first biomaterials used for three-dimensional in vitro follicle culture [56] Follicles cultured in collagen gels survived in vitro for 2 weeks with the formation of multilayered follicles, but they were unable to proceed to the antral stage [54]. More recently, however, buffalo pre-antral follicles encapsulated in collagen were shown to develop an antrum [50]. Fibrin has also been employed for follicle culture; this protein is responsible for blood clotting and is formed via enzymatic cross-linking [54]. Follicles encapsulated and cultured in fibrin hydrogels secreted enzymes that rapidly degraded the matrix, and the integrity of the follicle architecture was lost once the follicle fell from the degraded gel onto the culture surface. Thus, fibrin alone cannot support the 3D in vitro culture of follicles [54].
A hydrogel consisting of degradable and non-degradable components was developed based on a combination of fibrin and alginate to create a fibrin–alginate interpenetrating network (FA-IPN) [54]. An interpenetrating network (IPN) is a combination of polymers in network form, in which at least one polymer is synthesized and/or cross-linked in the presence of the other, either simultaneously or sequentially. Chains of the individual polymers are completely entangled, and there may or may not be chemical bonds between the combined networks. The overall IPN structure behavior reflects the characteristics of each polymer [46, 54]. In the FA-IPN hydrogel, the fibrin component is bioactive and can be degraded by the follicle to create space for outward expansion, whereas the alginate component provides long-term stability to maintain the follicle architecture [39, 54]. Also, the alginate concentration is significantly reduced relative to the alginate-only hydrogels described earlier. Importantly, the FA-IPN material has dynamic mechanical properties consistent with the needs of the growing follicle. Initially, the combination of fibrin and alginate is relatively rigid, which supports the follicular structure [39, 54]. As the follicle grows, the fibrin component is gradually degraded, thereby decreasing the elastic modulus and providing less resistance to follicle expansion. Fibrin and alginate can be gelled simultaneously under mild conditions to facilitate cell encapsulation, and the initial mechanics of the hydrogel can be modified depending on the properties of each component [54]. The FA-IPNs promoted follicle growth and increased the number of meiotically competent oocytes relative to follicles cultured in either fibrin or alginate alone [54].
Encapsulating matrices were subsequently developed that provided even greater control over the degradation rate of the hydrogel, allowing it to be matched more closely to the growth rate of the follicle. Naturally occurring biomaterials, such as collagen and fibrin, have the advantage of intrinsic biological activity; however, these materials are difficult to modify for desired physical properties, such as degradation [12, 57]. Hydrogels were developed that were based on peptide crosslinking of a non-degradable polymer with peptide cross-linkers, and the peptide sequences could be tuned to modulate the degradation rate of the hydrogel. Poly(ethylene glycol) (PEG) was modified with vinyl sulfone groups to allow cross-linking through peptides that contain multiple cysteine residues. Initial studies of follicle culture with four-arm PEG and bifunctional peptides, which have been successful for the culture of many cell types, were unsuccessful for the culture of ovarian follicles [52]. The bifunctional peptides required extended gelation times that led to dehydration of the oocyte, and the buffer conditions were harmful to the follicles [52]. A trifunctional peptide, which avoids elastically inactive loops and minimizes defects in the network, reduced gelation time to prevent oocyte dehydration and enabled the use of more cell-compatible buffer conditions. Tunable degradation of the plasmin-sensitive PEG gels was achieved using peptide sequences with varying plasmin sensitivity. Sequences that were highly sensitive to plasmin (YKNR) resulted in a loss of follicle from the gel [52, 54]. Conversely, sequences that were relatively insensitive to plasmin (YKND) did not allow follicle growth. However, peptide sequences with moderate sensitivity (YKNS) limited hydrogel degradation to the area immediately around the follicles, forming a soft pocket inside an otherwise rigid matrix, suggesting that the cell-mediated proteolysis was localized to the follicle–hydrogel interface [58, 59]. Tuning the peptide sequences to achieve the appropriate degradation rate of the hydrogel allowed for the coordinated growth of the multiple cellular compartments of the encapsulated follicle [54].
While extensive challenges exist with attempting to isolate and mature ovarian follicles in vitro, the potential and possibilities of this approach are significant. Once follicles are immobilized and encapsulated within a supporting matrix, the microfluidic approach can be utilized to deliver stimulatory factors and hormones in a precise and timely manner. Furthermore, monitoring follicles for oocyte maturation, staging, production, and ultimate selection for reproductive purposes can be performed with greater accuracy and efficiency.
IVF/ICSI
Currently sperm is placed within a tightly constricted volume of media (ranging from 10 μL to 1 mL) with either a single or several oocyte-cumulus masses. This technique for insemination has essentially remained unchanged over the past 30–40 years with fertilization rates ranging from 50 to 70% [60, 61]. However, the true fertilization rate is difficult to know with uncertainty in the presence of cumulus cells and the inability to determine the actual maturity stage of the oocyte with the cumulus mass attached. Immature oocytes can also be penetrated by sperm, using this conventional technique at the time of insemination, but lack the ability to form pronuclei or to develop competent embryos. However, they still progress to metaphase II of meiosis and interfere with the calculation of a “true fertilization rate” making the efficiency of human IVF/ICSI difficult to quantitate. Microfluidics could be used to assess and isolate the most mature oocytes by using flow shearing to remove the cumulus mass cells while also maintaining a platform that can trap the oocyte in a separate compartment for precise micro-insemination with selected sperm. As detailed previously, the most motile and healthy sperm could be introduced via a microchannel or by microinjection into the chamber containing the “trapped” oocyte for insemination. Indeed, one single but rather complex device, was utilized to trap mouse oocytes, simultaneously isolate motile sperm, and micro-inseminate for embryo development over a period of 96 h [26]. A separate study evaluated microfluidic devices for ICSI using porcine derived oocytes with successful results and a reduction in overall procedure time and human error [62]. In this particular study, polystyrene microspheres were utilized within the microchannel structure, and by gravity descend and become evenly spaced at the bottom of the sperm collection chamber [62]. Once a sperm sample is added, these microspheres due to being evenly spaced, maintain a relatively constant, concentrated motile sperm reservoir completely independent of whether the introduced sample is diluted or concentrated initially [62]. This proves highly useful since male caused infertility can often be due to a dilute semen sample. Study results showed that by using the microfluidic channel design with microspheres, the microsphere could decrease the overall search time for motile sperm and therefore decrease the overall ICSI treatment time resulting in viable zygotes [62].
Embryo culture
The pre-implantation embryo is known to develop within a dynamic, constantly moving environment within the fallopian tube and uterus. In contrast, with the use of conventional methods, pre-implantation embryos are often grown in a steady state environment within the lab. Microfluidics, when applied to embryo culture, could provide unique capabilities and advantages that more accurately model the in vivo embryonic environment with a dynamically changing chemical and fluid microenvironment [3]. A microfluidic approach was first applied to assess the micro-environmental effect of no flow vs. minimal fluid flow on mouse embryos within micro-channels [63]. It was demonstrated that the mouse embryos cultured within the micro-channels showed faster cleavage rates, yielded more blastocysts, and had reduced overall embryo degeneration compared with the controlled micro-drop culture. However, when media flow was applied to the micro-channel, embryo development was not improved and resulted in a high incidence of abnormal embryos [64]. Further studies showed that fluid flow required precise regulation, with a more complex pulsatile flow rather than a traditional steady state flow. Utilizing a completely redesigned microfluidic device with a “braille pin” actuator and a sequential computer programmed pin controller, periodic pulses of media at physiological frequencies of roughly ~0.1 Hz and an average flow rate of ~18 nl/min resulted in greater success [65]. The cultured mouse embryos displayed faster rates of pre-implementation embryo development, development of blastocysts with more cells, and significantly improved implantation rates [65].
Embryo analysis and selection
Over the past decades, little has changed in the evaluation and selection for transfer and overall assessment of embryos. Typically, embryo analysis and selection has been done manually through microscopic observation of cleavage and division rates, blastocyst formation and staging, and monitoring of the relative cellularity of the inner cell mass vs. the trophoectoderm [3]. Most manually performed microscopic observations are subjective, inconsistent and lack predictive value for the success of embryo implantation [3]. A microfluidic-based platform would provide a unique ability to revolutionize this field by allowing for real-time sampling and analysis of specific, biochemical and genetic factors as well as imaging for morphology and morphokinetic staging that contribute to overall embryo implantation competence [2]. Currently, few microfluidic approaches have been utilized to perform noninvasive embryo marker measurements [2].
Biomarkers of embryo health require noninvasive analysis of molecules produced from the embryo. Major biomarkers would include but are not limited to oxygen consumption, amino acid turnover, and overall energy metabolism. In regard to energy metabolism, glucose consumption, lactate production, and overall rate of glycolytic activity have been shown to be the most useful parameters for selection of embryos.
Oxygen consumption was one of the first parameters used to characterize the overall metabolic activity of the embryo. Oxygen consumption can be determined indirectly by monitoring dissolved oxygen concentrations in medium either using electrochemical or optical means with both strategies implemented in microfluidic devices. In one particular study, researchers employed a series of four independent microelectrodes placed at varying distances from the central bovine embryo to characterize the oxygen concentration gradient resulting from respiratory activity [1]. This design later evolved into a ring shaped electrochemical sensor, integrated into the underlying PDMS microwell surrounding an individual embryo. Yet another platform consisted of incorporating both 2-cell and more advanced blastocyst stage mouse embryos onto a chip with capillary, passive fluid flow while and using a respirometric microfluidic cartridge to actively monitor oxygen consumption [66]. Alternatively, real-time changes in dissolved oxygen concentration can be measured using oxygen sensitive phosphorescent reactions based on transition metal complexes. In one instance a water soluble oxygen sensitive probe was added to culture medium to follow the oxygen consumption of 5–20 embryos at different developmental stages during 1 h on a microfluidic device. Similarly a platinum based porphyrin based oxygen sensitive dye was encapsulated in a polymer material patterned into a thin film at the bottom of a micro array culture device for monitoring embryos over a period of 4 days. All these strategies successfully measured the different developmental stages of the embryo using oxygen as the metabolic marker.
For metabolic assessment, three basic substrates (glucose, pyruvate, and lactate) are vitally important parameters to assess in pre-implantation embryos. Pyruvate and glucose are main energy sources for the pre-implantation embryo with their consumption directly associated with lactate production. Consumption and production of these substrates can be analyzed by quantifying secondary products of enzymatic reactions. One study utilized dehydrogenases to produce fluorescent probes such as NAD(P)H so that substrates present in the micro scale volumes of media circulating in the microfluidic device could be directly measured by examining fluorescent intensity [67]. In this study, 10 morphologically identical murine embryos were examined and shown to have significantly different metabolic activity [67]. Another group utilized oxidases to examine substrates producing H2O2 which could then be directly measured by amperometry. In a separate study, a more highly integrated microfluidic chip was designed to measure glucose, pyruvate, and lactate, from micro-scale volumes of circulating media, all on one single device [67]. In addition, the chip integrated an automated computer controlled microfluidic platform that incorporated embryo culture and metabolic analysis with sampling, media mixing, data collection and analysis [67]. This device was novel in that it abandoned previously used UV light detection methods that could be detrimental to embryo DNA. Instead, this device was developed to allow for real-time glucose measurement using fluorescent wavelengths that would be harmless to embryos. The device quantified glucose that was automatically sampled from close proximity to the embryo, through an enzymatically mediated glucose oxidase—peroxidase reaction producing red—fluorescent resorufin from a colorless Amplex Red compound [67]. Furthermore, by utilizing the previously mentioned “braille pin” actuator to create pulsatile flow, this device could sample, pump, mix, wash, and detect glucose from individual pre-implantation embryos every hour [68].
Microfluidic devices can also be used for time lapse imaging throughout embryo pre-implantation culture. This has been explored using a microwell containing bovine, mouse, and human embryos. In one study utilizing mouse embryos, two parameters, the 4-cell cleavage stage and 8-cell cleavage stage were identified which importantly indicated the embryo’s developmental competence and ultimately, those embryos gave rise to viable offspring after transfer [69]. In another study, using human embryos to determine kinetic parameters, the time required to complete the second and third cleavage divisions during pre-implantation development, were examined in individual embryos [70]. This important study allowed embryos conceived via different fertilization and cryopreservation conditions to be compared. For example, ICSI vs. IVF and slow freezing vs. vitrification could be assessed in parallel fashion.
Current limitations of microfluidic technologies
While microfluidic technology has advanced tremendously in the past few decades, much work still needs to be done to utilize it in active IVF clinics worldwide. The technology is still relatively new and must be validated first in animal models prior to human models and randomized clinical trials [71]. The technology also requires acceptance amongst the community of basic science embryologists and infertility clinic physicians and technical staff. They must be convinced of the superior performance and efficiency of an integrated microfluidic platform compared to the current techniques and protocols. With this in mind, embracing this new technology will require changes in daily practices, treatment procedures and considerable effort in training new clinic employees.
Currently, most microfluidic based research takes place in academic settings with limited numbers of industrial or commercial partners investing in these platforms [72]. One of the most basic barriers involves selection of materials for fabricating the microfluidic platform [73]. In research settings, the platforms intended for embryo culture and characterization have been made using PDMS. While this elastomer plastic material has been proven not to be toxic to embryos in some studies [63], other studies have raised concerns over its biocompatibility [74]. Furthermore, PDMS displays hydrophobicity and the soft lithography fabrication technique commonly used to produce PDMS devices is not conducive to large-scale production needed for commercialization [73]. Currently, most commercial ART techniques are performed using single use, sterilized polymer material dishes (polystyrene) which have a proven track record of safety and compatibility with embryos.
Conclusions and future directions
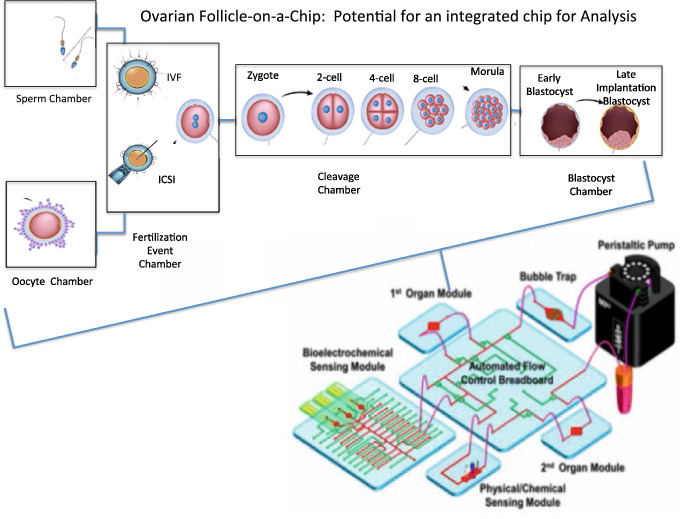
Conventional human IVF/ICSI can be an expensive, labor-intensive undertaking, which often limits its widespread use for infertility treatment. ART is an intricate and delicate, procedure. Furthermore, several steps are interdependent or dependent on the success of prior steps in order for the entire procedure to be successful. As a result the major challenge ahead, is perfecting these various steps in separate modules whilst also integrating them onto a single microfluidic platform capable of efficiently performing IVF/ICSI [75, 76]. An example of this modular integrated design is shown in Fig. 3, as a closed circuit design with a media reservoir and pump capable of continuous flow. While, as mentioned, this is an exceptional challenge at face value, the overall potential benefits in efficiency and reproducibility long-term would undoubtedly revolutionize the field [75, 76]. These benefits include reducing the overall procedural time, reducing technical human error or variability, and reducing cost, while at the same time maintaining similar or improved results [13]. The microfluidic platform for reproductive medicine is not only a worthwhile endeavor, but could revolutionize the field of reproductive medicine. In order to move forward with this technology for reproductive purposes, the designs for the various subset procedures such as sperm and oocyte separation analysis and processing must show improved consistency, greater successful outcomes, enhanced efficiency, and reduced cost.
Fig. 3.
Modular microfluidic platform for reproductive medicine. A depiction of a modular integrated design, consisting of a closed circuit with a media reservoir and pump capable of continuous flow
Furthermore, the use of a microfluidic system to culture and sustain follicles, will allow for the elucidation of the in vivo maturation process of ovarian follicles and the effect of various positive and/or negative stimuli on pathophysiological disease processes with greater efficiency and accuracy [13]. Scientists and physicians studying fertility, molecular and genetic pathologies, pharmaco-therapeutics, and other relevant applicable fields must be willing to embrace and contribute to the success of this future approach which may have a steep initial learning curve but tremendous long-term potential.
Acknowledgement
Wake Forest Institute for Regenerative Medicine Departmental Funding.
Compliance with ethical standards
Conflict of interest
The authors have no financial conflicts of interest.
Ethical statement
No animal studies or experiments were carried out for this article.
Footnotes
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wu Q, Liu J, Wang X, Feng L, Wu J, Zhu X, et al. Organ-on-a-chip: recent breakthroughs and future prospects. Biomed Eng Online. 2020;19:9. doi: 10.1186/s12938-020-0752-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith GD, Takayama S. Application of microfluidic technologies to human assisted reproduction. Mol Hum Reprod. 2017;23:257–268. doi: 10.1093/molehr/gaw076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yanez LZ, Camarillo DB. Microfluidic analysis of oocyte and embryo biomechanical properties to improve outcomes in assisted reproductive technologies. Mol Hum Reprod. 2017;23:235–247. doi: 10.1093/molehr/gaw071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mark D, Haeberle S, Roth G, von Stetten F, Zengerle R. Microfluidic lab-on-a-chip platforms: requirements, characteristics and applications. Chem Soc Rev. 2010;39:1153–1182. doi: 10.1039/b820557b. [DOI] [PubMed] [Google Scholar]
- 5.Osuchowski MF, Remick DG, Lederer JA, Lang CH, Aasen AO, Aibiki M, et al. Abandon the mouse research ship? Not just yet! Shock. 2014;41:463–475. doi: 10.1097/SHK.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perrin S. Preclinical research: make mouse studies work. Nature. 2014;507:423–425. doi: 10.1038/507423a. [DOI] [PubMed] [Google Scholar]
- 7.Couzin-Frankel J. When mice mislead. Science. 2013;342:922–3–925. doi: 10.1126/science.342.6161.922. [DOI] [PubMed] [Google Scholar]
- 8.Pound P, Bracken MB. Is animal research sufficiently evidence based to be a cornerstone of biomedical research? BMJ. 2014;348:g3387. doi: 10.1136/bmj.g3387. [DOI] [PubMed] [Google Scholar]
- 9.Aziz AUR, Fu M, Deng J, Geng C, Luo Y, Lin B, et al. A microfluidic device for culturing an encapsulated ovarian follicle. Micromachines (Basel). 2017;8:335. [DOI] [PMC free article] [PubMed]
- 10.Xiao S, Zhang J, Liu M, Iwahata H, Rogers HB, Woodruff TK. Doxorubicin has dose-dependent toxicity on mouse ovarian follicle development, hormone secretion, and oocyte maturation. Toxicol Sci. 2017;157:320–329. doi: 10.1093/toxsci/kfx047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aziz AUR, Yu X, Jiang Q, Zhao Y, Deng S, Qin K, et al. Doxorubicin-induced toxicity to 3D-cultured rat ovarian follicles on a microfluidic chip. Toxicol In Vitro. 2020;62:104677. doi: 10.1016/j.tiv.2019.104677. [DOI] [PubMed] [Google Scholar]
- 12.Carvalho MR, Truckenmuller R, Reis RL, Oliveira JM. Biomaterials and microfluidics for drug discovery and development. Adv Exp Med Biol. 2020;1230:121–135. doi: 10.1007/978-3-030-36588-2_8. [DOI] [PubMed] [Google Scholar]
- 13.Weng L. IVF-on-a-chip: recent advances in microfluidics technology for in vitro fertilization. SLAS Technol. 2019;24:373–385. doi: 10.1177/2472630319851765. [DOI] [PubMed] [Google Scholar]
- 14.Huang JY, Rosenwaks Z. Assisted reproductive techniques. Methods Mol Biol. 2014;1154:171–231. doi: 10.1007/978-1-4939-0659-8_8. [DOI] [PubMed] [Google Scholar]
- 15.Healy MW, Hill MJ, Levens ED. Optimal oocyte retrieval and embryo transfer techniques: where we are and how we got here. Semin Reprod Med. 2015;33:83–91. doi: 10.1055/s-0035-1545365. [DOI] [PubMed] [Google Scholar]
- 16.Wang C, Feng G, Shu J, Zhou H, Zhang B, Chen H, et al. Cumulus oophorus complexes favor physiologic selection of spermatozoa for intracytoplasmic sperm injection. Fertil Steril. 2018;109:823–831. doi: 10.1016/j.fertnstert.2017.12.026. [DOI] [PubMed] [Google Scholar]
- 17.Naknam W, Salang L, Sothornwit J, Amnatbuddee S, Seejorn K, Pongsritasana T, et al. Effect of sperm selection method by cumulus oophorus complexes and conventional sperm preparation method on sperm quality and DNA fragmentation for assisted reproduction techonology. Eur J Obstet Gynecol Reprod Biol. 2019;243:46–50. doi: 10.1016/j.ejogrb.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Oseguera-López I, Ruiz-Díaz S, Ramos-Ibeas P, Pérez-Cerezales S. Novel techniques of sperm selection for improving IVF and ICSI outcomes. Front Cell Dev Biol. 2019;7:298. doi: 10.3389/fcell.2019.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Labs EGGGPI, De los Santos MJ, Apter S, Coticchio G, Debrock S, Lundin K, et al. Revised guidelines for good practice in IVF laboratories (2015) Hum Reprod. 2016;31:685–686. doi: 10.1093/humrep/dew016. [DOI] [PubMed] [Google Scholar]
- 20.Malter HE. Micromanipulation in assisted reproductive technology. Reprod Biomed Online. 2016;32:339–347. doi: 10.1016/j.rbmo.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 21.Knowlton SM, Sadasivam M, Tasoglu S. Microfluidics for sperm research. Trends Biotechnol. 2015;33:221–229. doi: 10.1016/j.tibtech.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Wacogne B, Pieralli C, Roux C, Gharbi T. Measuring the mechanical behaviour of human oocytes with a very simple SU-8 micro-tool. Biomed Microdevices. 2008;10:411–419. doi: 10.1007/s10544-007-9150-7. [DOI] [PubMed] [Google Scholar]
- 23.Zeringue HC, Beebe DJ. Microfluidic removal of cumulus cells from mammalian zygotes. Methods Mol Biol. 2004;254:365–374. doi: 10.1385/1-59259-741-6:365. [DOI] [PubMed] [Google Scholar]
- 24.Zeringue HC, Rutledge JJ, Beebe DJ. Early mammalian embryo development depends on cumulus removal technique. Lab Chip. 2005;5:86–90. doi: 10.1039/b316494m. [DOI] [PubMed] [Google Scholar]
- 25.Suh RS, Zhu X, Phadke N, Ohl DA, Takayama S, Smith GD. IVF within microfluidic channels requires lower total numbers and lower concentrations of sperm. Hum Reprod. 2006;21:477–483. doi: 10.1093/humrep/dei323. [DOI] [PubMed] [Google Scholar]
- 26.Han C, Zhang Q, Ma R, Xie L, Qiu T, Wang L, et al. Integration of single oocyte trapping, in vitro fertilization and embryo culture in a microwell-structured microfluidic device. Lab Chip. 2010;10:2848–2854. doi: 10.1039/c005296e. [DOI] [PubMed] [Google Scholar]
- 27.Ma R, Xie L, Han C, Su K, Qiu T, Wang L, et al. In vitro fertilization on a single-oocyte positioning system integrated with motile sperm selection and early embryo development. Anal Chem. 2011;83:2964–2970. doi: 10.1021/ac103063g. [DOI] [PubMed] [Google Scholar]
- 28.Meseguer M, Kruhne U, Laursen S. Full in vitro fertilization laboratory mechanization: toward robotic assisted reproduction? Fertil Steril. 2012;97:1277–1286. doi: 10.1016/j.fertnstert.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 29.Nordhoff V. How to select immotile but viable spermatozoa on the day of intracytoplasmic sperm injection? An embryologist’s view. Andrology. 2015;3:156–162. doi: 10.1111/andr.286. [DOI] [PubMed] [Google Scholar]
- 30.Adamo A, Jensen KF. Microfluidic based single cell microinjection. Lab Chip. 2008;8:1258–1261. doi: 10.1039/b803212b. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y, Li P, Huang PH, Xie Y, Mai JD, Wang L, et al. Rare cell isolation and analysis in microfluidics. Lab Chip. 2014;14:626–645. doi: 10.1039/c3lc90136j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tšuiko O, Zhigalina DI, Jatsenko T, Skryabin NA, Kanbekova OR, Artyukhova VG, et al. Karyotype of the blastocoel fluid demonstrates low concordance with both trophectoderm and inner cell mass. Fertil Steril. 2018;109:1127–34.e1. doi: 10.1016/j.fertnstert.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Jeyendran RS, Caroppo E, Rouen A, Anderson A, Puscheck E. Selecting the most competent sperm for assisted reproductive technologies. Fertil Steril. 2019;111:851–863. doi: 10.1016/j.fertnstert.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 34.Kricka LJ, Faro I, Heyner S, Garside WT, Fitzpatrick G, McKinnon G, et al. Micromachined analytical devices: microchips for semen testing. J Pharm Biomed Anal. 1997;15:1443–1447. doi: 10.1016/s0731-7085(96)02046-8. [DOI] [PubMed] [Google Scholar]
- 35.Cho BS, Schuster TG, Zhu X, Chang D, Smith GD, Takayama S. Passively driven integrated microfluidic system for separation of motile sperm. Anal Chem. 2003;75:1671–1675. doi: 10.1021/ac020579e. [DOI] [PubMed] [Google Scholar]
- 36.Van de Velde H, Nagy ZP, Joris H, De Vos A, Van Steirteghem AC. Effects of different hyaluronidase concentrations and mechanical procedures for cumulus cell removal on the outcome of intracytoplasmic sperm injection. Hum Reprod. 1997;12:2246–2250. doi: 10.1093/humrep/12.10.2246. [DOI] [PubMed] [Google Scholar]
- 37.Lenie S, Cortvrindt R, Adriaenssens T, Smitz J. A reproducible two-step culture system for isolated primary mouse ovarian follicles as single functional units. Biol Reprod. 2004;71:1730–1738. doi: 10.1095/biolreprod.104.028415. [DOI] [PubMed] [Google Scholar]
- 38.Xiao S, Coppeta JR, Rogers HB, Isenberg BC, Zhu J, Olalekan SA, et al. A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat Commun. 2017;8:14584. doi: 10.1038/ncomms14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shea LD, Woodruff TK, Shikanov A. Bioengineering the ovarian follicle microenvironment. Annu Rev Biomed Eng. 2014;16:29–52. doi: 10.1146/annurev-bioeng-071813-105131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jensen C, Teng Y. Is it time to start transitioning from 2D to 3D cell culture? Front Mol Biosci. 2020;7:33. doi: 10.3389/fmolb.2020.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skory RM, Xu Y, Shea LD, Woodruff TK. Engineering the ovarian cycle using in vitro follicle culture. Hum Reprod. 2015;30:1386–1395. doi: 10.1093/humrep/dev052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amsden B, Turner N. Diffusion characteristics of calcium alginate gels. Biotechnol Bioeng. 1999;65:605–610. doi: 10.1002/(sici)1097-0290(19991205)65:5<605::aid-bit14>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 43.Nunamaker EA, Otto KJ, Kipke DR. Investigation of the material properties of alginate for the development of hydrogel repair of dura mater. J Mech Behav Biomed Mater. 2011;4:16–33. doi: 10.1016/j.jmbbm.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 44.Enck K, Rajan SP, Aleman J, Castagno S, Long E, Khalil F, et al. Design of an adhesive film-based microfluidic device for alginate hydrogel-based cell encapsulation. Ann Biomed Eng. 2020;48:1103–11. [DOI] [PMC free article] [PubMed]
- 45.Tamayol A, Najafabadi AH, Aliakbarian B, Arab-Tehrany E, Akbari M, Annabi N, et al. Bioactive fibers: hydrogel templates for rapid manufacturing of bioactive fibers and 3D constructs (Adv. Healthcare Mater. 14/2015) Adv Healthc Mater. 2015;4:2050. doi: 10.1002/adhm.201570082. [DOI] [PubMed] [Google Scholar]
- 46.Kreeger PK, Woodruff TK, Shea LD. Murine granulosa cell morphology and function are regulated by a synthetic Arg-Gly-Asp matrix. Mol Cell Endocrinol. 2003;205:1–10. doi: 10.1016/s0303-7207(03)00209-0. [DOI] [PubMed] [Google Scholar]
- 47.Pangas SA, Saudye H, Shea LD, Woodruff TK. Novel approach for the three-dimensional culture of granulosa cell-oocyte complexes. Tissue Eng. 2003;9:1013–1021. doi: 10.1089/107632703322495655. [DOI] [PubMed] [Google Scholar]
- 48.Laronda MM, Duncan FE, Hornick JE, Xu M, Pahnke JE, Whelan KA, et al. Alginate encapsulation supports the growth and differentiation of human primordial follicles within ovarian cortical tissue. J Assist Reprod Genet. 2014;31:1013–1028. doi: 10.1007/s10815-014-0252-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brito IR, Lima IM, Xu M, Shea LD, Woodruff TK, Figueiredo JR. Three-dimensional systems for in vitro follicular culture: overview of alginate-based matrices. Reprod Fertil Dev. 2014;26:915–930. doi: 10.1071/RD12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berkholtz CB, Shea LD, Woodruff TK. Extracellular matrix functions in follicle maturation. Semin Reprod Med. 2006;24:262–269. doi: 10.1055/s-2006-948575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Albertini DF, Akkoyunlu G. Ovarian follicle culture systems for mammals. Methods Enzymol. 2010;476:107–121. doi: 10.1016/S0076-6879(10)76007-9. [DOI] [PubMed] [Google Scholar]
- 52.Desai N, Alex A, AbdelHafez F, Calabro A, Goldfarb J, Fleischman A, et al. Three-dimensional in vitro follicle growth: overview of culture models, biomaterials, design parameters and future directions. Reprod Biol Endocrinol. 2010;8:119. doi: 10.1186/1477-7827-8-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Telfer EE, McLaughlin M. In vitro development of ovarian follicles. Semin Reprod Med. 2011;29:15–23. doi: 10.1055/s-0030-1268700. [DOI] [PubMed] [Google Scholar]
- 54.Telfer EE, Zelinski MB. Ovarian follicle culture: advances and challenges for human and nonhuman primates. Fertil Steril. 2013;99:1523–1533. doi: 10.1016/j.fertnstert.2013.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Herta AC, Lolicato F, Smitz JEJ. In vitro follicle culture in the context of IVF. Reproduction. 2018;156:F59–73. doi: 10.1530/REP-18-0173. [DOI] [PubMed] [Google Scholar]
- 56.Woodruff TK, Shea LD. The role of the extracellular matrix in ovarian follicle development. Reprod Sci. 2007;14:6–10. doi: 10.1177/1933719107309818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.D’Costa K, Kosic M, Lam A, Moradipour A, Zhao Y, Radisic M. Biomaterials and culture systems for development of organoid and organ-on-a-chip models. Ann Biomed Eng. 2020;48:2002–27. [DOI] [PMC free article] [PubMed]
- 58.Amorim CA, Shikanov A. The artificial ovary: current status and future perspectives. Future Oncol. 2016;12:2323–2332. doi: 10.2217/fon-2016-0202. [DOI] [PubMed] [Google Scholar]
- 59.Green LJ, Shikanov A. In vitro culture methods of preantral follicles. Theriogenology. 2016;86:229–238. doi: 10.1016/j.theriogenology.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 60.Bhattacharya S, Hamilton MP, Shaaban M, Khalaf Y, Seddler M, Ghobara T, et al. Conventional in-vitro fertilisation versus intracytoplasmic sperm injection for the treatment of non-male-factor infertility: a randomised controlled trial. Lancet. 2001;357:2075–2079. doi: 10.1016/s0140-6736(00)05179-5. [DOI] [PubMed] [Google Scholar]
- 61.Foong SC, Fleetham JA, O'Keane JA, Scott SG, Tough SC, Greene CA. A prospective randomized trial of conventional in vitro fertilization versus intracytoplasmic sperm injection in unexplained infertility. J Assist Reprod Genet. 2006;23:137–140. doi: 10.1007/s10815-005-9008-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matsuura K, Uozumi T, Furuichi T, Sugimoto I, Kodama M, Funahashi H. A microfluidic device to reduce treatment time of intracytoplasmic sperm injection. Fertil Steril. 2013;99:400–407. doi: 10.1016/j.fertnstert.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 63.Raty S, Walters EM, Davis J, Zeringue H, Beebe DJ, Rodriguez-Zas SL, et al. Embryonic development in the mouse is enhanced via microchannel culture. Lab Chip. 2004;4:186–190. doi: 10.1039/b316437c. [DOI] [PubMed] [Google Scholar]
- 64.Hickman DL, Beebe DJ, Rodriguez-Zas SL, Wheeler MB. Comparison of static and dynamic medium environments for culturing of pre-implantation mouse embryos. Comp Med. 2002;52:122–126. [PubMed] [Google Scholar]
- 65.Heo YS, Cabrera LM, Bormann CL, Shah CT, Takayama S, Smith GD. Dynamic microfunnel culture enhances mouse embryo development and pregnancy rates. Hum Reprod. 2010;25:613–622. doi: 10.1093/humrep/dep449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O’Donovan C, Twomey E, Alderman J, Moore T, Papkovsky D. Development of a respirometric biochip for embryo assessment. Lab Chip. 2006;6:1438–1444. doi: 10.1039/b607622j. [DOI] [PubMed] [Google Scholar]
- 67.Urbanski JP, Johnson MT, Craig DD, Potter DL, Gardner DK, Thorsen T. Noninvasive metabolic profiling using microfluidics for analysis of single preimplantation embryos. Anal Chem. 2008;80:6500–6507. doi: 10.1021/ac8010473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heo YS, Cabrera LM, Bormann CL, Smith GD, Takayama S. Real time culture and analysis of embryo metabolism using a microfluidic device with deformation based actuation. Lab Chip. 2012;12:2240–2246. doi: 10.1039/c2lc21050a. [DOI] [PubMed] [Google Scholar]
- 69.Chung YH, Hsiao YH, Kao WL, Hsu CH, Yao DJ, Chen C. Microwells support high-resolution time-lapse imaging and development of preimplanted mouse embryos. Biomicrofluidics. 2015;9:022407. doi: 10.1063/1.4918642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hashimoto S, Kato N, Saeki K, Morimoto Y. Selection of high-potential embryos by culture in poly(dimethylsiloxane) microwells and time-lapse imaging. Fertil Steril. 2012;97:332–337. doi: 10.1016/j.fertnstert.2011.11.042. [DOI] [PubMed] [Google Scholar]
- 71.Harper J, Magli MC, Lundin K, Barratt CL, Brison D. When and how should new technology be introduced into the IVF laboratory? Hum Reprod. 2012;27:303–313. doi: 10.1093/humrep/der414. [DOI] [PubMed] [Google Scholar]
- 72.Domachuk P, Tsioris K, Omenetto FG, Kaplan DL. Bio-microfluidics: biomaterials and biomimetic designs. Adv Mater. 2010;22:249–260. doi: 10.1002/adma.200900821. [DOI] [PubMed] [Google Scholar]
- 73.Ahadian S, Civitarese R, Bannerman D, Mohammadi MH, Lu R, Wang E, et al. Organ-on-a-chip platforms: a convergence of advanced materials, cells, and microscale technologies. Adv Healthc Mater. 2018. 10.1002/adhm.201700506
- 74.Berthier E, Young EW, Beebe D. Engineers are from PDMS-land, biologists are from Polystyrenia. Lab Chip. 2012;12:1224–1237. doi: 10.1039/c2lc20982a. [DOI] [PubMed] [Google Scholar]
- 75.Sackmann EK, Fulton AL, Beebe DJ. The present and future role of microfluidics in biomedical research. Nature. 2014;507:181–189. doi: 10.1038/nature13118. [DOI] [PubMed] [Google Scholar]
- 76.Zhao Y, Kankala RK, Wang SB, Chen AZ. Multi-organs-on-chips: towards long-term biomedical investigations. Molecules. 2019;24:675. [DOI] [PMC free article] [PubMed]