Abstract
Introduction
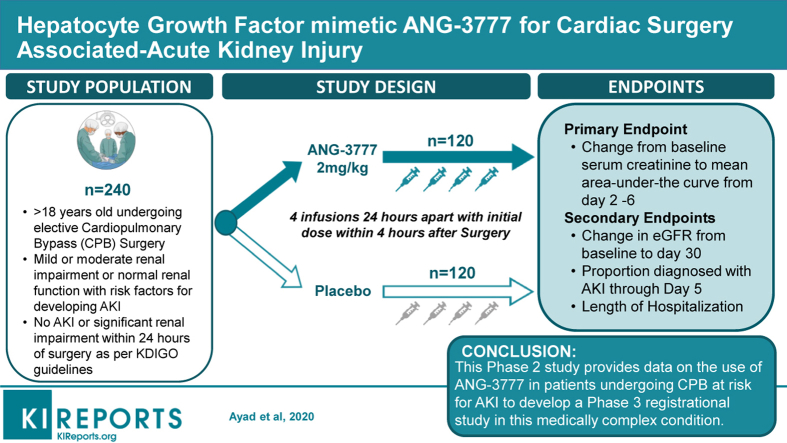
Nearly one-third of patients undergoing cardiac surgery involving cardiopulmonary bypass (CPB) experience cardiac surgery–associated (CSA) acute kidney injury (AKI); 5% require renal replacement therapy. ANG-3777 is a hepatocyte growth factor mimetic. In vitro, ANG-3777 reduces apoptosis and increases cell proliferation, migration, morphogenesis, and angiogenesis in injured kidneys. In animal models, ANG-3777 mitigates the effects of renal damage secondary to ischemia reperfusion injury and nephrotoxic chemicals. Phase 2 data in AKI of renal transplantation have shown improved renal function and comparable safety relative to placebo. The Guard Against Renal Damage (GUARD) study is a phase 2 proof of concept trial of ANG-3777 in CSA-AKI.
Methods
GUARD is a 240-patient, multicenter, double-blind, randomized placebo-controlled trial to assess the efficacy and safety of ANG-3777 in patients at elevated pre-surgery risk for AKI undergoing coronary artery bypass graft (CABG) or heart valve repair/replacement requiring CPB. Subjects are randomized 1:1 to receive ANG-3777 (2 mg/kg) or placebo. Study drug is dosed via 4 daily intravenous 30-minute infusions. The first dose is administered less than 4 hours after completing CPB, second at 24 ± 2 hours post-CPB, with two subsequent doses at 24 ± 2 hours after the previous dose.
Results
The primary efficacy endpoint is percent change from baseline serum creatinine to mean area under the curve from days 2 through 6. Secondary endpoints include change in estimated glomerular filtration rate from baseline to day 30, the proportion of patients diagnosed with AKI by stage through day 5, and the length of CSA-AKI hospitalization. Safety will include adverse events and laboratory measures.
Conclusion
This phase 2 study of ANG-3777 provides data to develop a phase 3 registrational study in this medically complex condition.
Keywords: acute kidney injury, cardiac surgery, cardiopulmonary bypass, ANG-3777, hepatocyte growth factor, clinical trial
Graphical abstract
AKI is an abrupt decrease in renal function resulting from a variety of types of injury. The 2012 Kidney Disease: Improving Global Outcomes guidelines established the Acute Kidney Injury Network classification as the standard for diagnosing AKI in the following manner: A ≥0.3 mg/dl (≥26.5 μmol/l) increase in serum creatinine (SCr) within 48 hours, or an increase in SCr ≥1.5 times over baseline levels occurring within 7 days, or urine volume <0.5 ml/kg per hour for 6 hours.1 AKI has numerous causes including urinary tract obstruction, exposure to nephrotoxic drugs and chemicals, acute infection, inflammation, or thromboembolic events.2, 3, 4, 5, 6, 7, 8 Another common cause of AKI is ischemia reperfusion injury associated with vascular surgeries, especially those involving CPB, known as CSA-AKI.
Approximately one-third of patients undergoing cardiac procedures involving CPB, such as CABG and heart valve replacement/repair, experience CSA-AKI.3,4 Approximately 5% of patients with CSA-AKI require renal replacement therapy, and mortality is high among those who require dialysis.9 Patients with CSA-AKI have longer hospital stays and are more likely to require intensive care.10 Even a small increase in SCr (<0.3 mg/dl) is associated with higher mortality, progression of chronic kidney disease, and requirement for renal replacement therapy.1,11, 12, 13, 14, 15 The longer the duration of elevated SCr, the greater the risk of significant morbidity and mortality. Compared to patients without AKI, those with a diagnosis lasting 1 to 2 days had increased risk for mortality hazard ratio (HR) of 1.66; at 3 to 6 days the HR risk increased to 1.94; and at ≥7 days the incremental HR risk was 3.40.16 This association has been independently replicated.17 The incremental cost of CSA-AKI is considerable, including $16,000 to $22,000 in initial hospital costs alone.10,14 Treatment for the underlying cause of AKI and supportive care remain the mainstay of clinical management.1
Common risk factors for AKI include older age, hypertension, diabetes mellitus, heart failure, chronic kidney disease, sepsis, chronic obstructive pulmonary disease, use of nephrotoxic drugs, and use of vasopressors/inotropes.18, 19, 20 More than 50% of all cardiac surgery patients may have one or more of these risk factors, and a combination of CPB with one or more of these risks probably increases the incidence of AKI more than 50%. Given the related adverse clinical and cost consequences of CSA-AKI, an effective treatment for patients at high risk would address a significant unmet clinical need.
Kidney Injury Associated with Cardiac Surgery
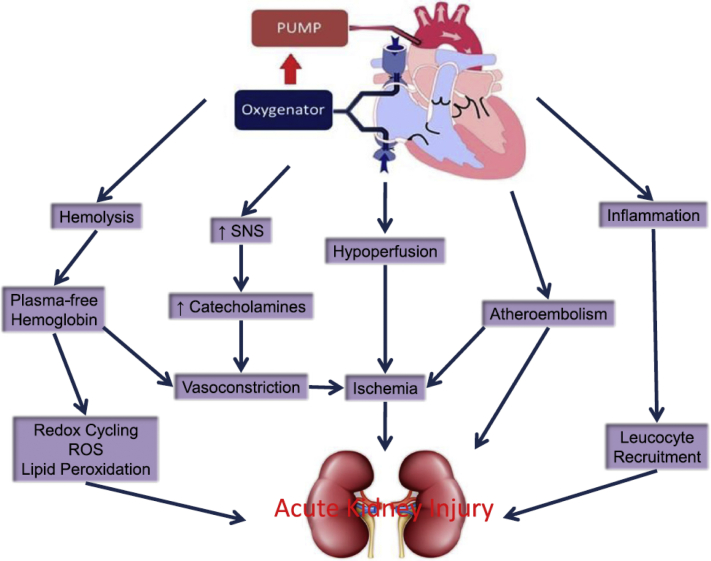
There are several mechanisms by which AKI occurs secondary to cardiac surgery. The contrast medium used in cardiac imaging is known to be directly cytotoxic to renal tubular cells, and can also induce medullary vasoconstriction and hypoxia, increase blood and renal tubular viscosity, impair tubuloglomerular feedback, and increase oxidative stress.21 The switch from high osmolal to low-osmolar and iso-osmolar contrast medium has reduced but not eliminated the AKI risk of contrast media,21 and has been shown by the Preserve Trial.22 O’Neal et al.9 has outlined additional mechanisms underlying CSA-AKI (Figure 1).9 Although oxidative stress, vasoconstriction, and thromboembolic events all play a role, ischemia reperfusion injury associated with CSA-AKI appears to be a significant cause, with time on CPB, return to CPB, hypovolemia, and hypoperfusion among the contributing factors.
Figure 1.
Pathophysiology of acute kidney injury after cardiac surgery. Reprinted from O’Neal JB, Shaw AD, Billings FT. Acute kidney injury following cardiac surgery: current understanding and future directions. Crit Care. 2016;20:187.9 © 2016 The Author(s), http://creativecommons.org/licenses/by/4.0/. ROS, reactive oxygen species; SNS, sympathetic nervous system.
Although no systematic studies of the pathologic changes in the kidney have been undertaken in patients with AKI associated with CPB, it is largely assumed that the pathologic lesion is acute tubular necrosis.23 In a physiologic study of 10 patients with protracted severe AKI after CPB, the transmembrane gradient for glomerular ultrafiltration was significantly diminished, likely from intratubular obstruction and hypertension due to sloughing from injured tubular epithelial cells.24 In addition, significant transtubular back-leak of glomerular ultrafiltrate across the injured epithelium was observed. The pathology is similar to other, better studied causes of AKI, such as AKI of transplantation.25
The Role of Hepatocyte Growth Factor and ANG-3777 in Renal Repair
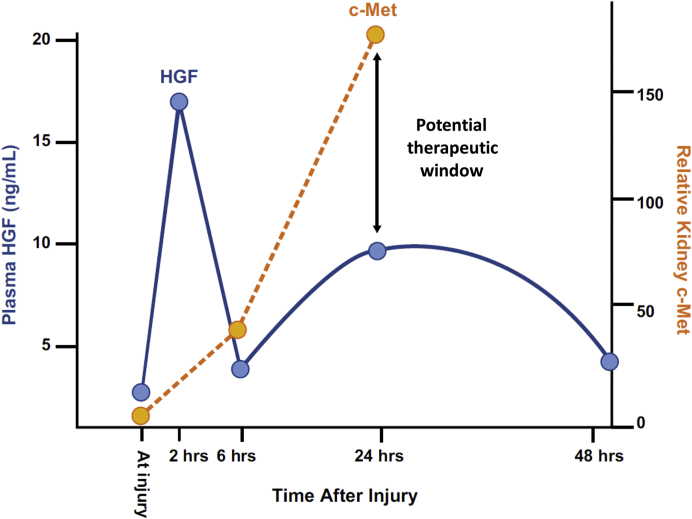
One of the primary mechanisms of solid organ tissue repair is the hepatocyte growth factor (HGF)/c-Met pathway. HGF binds exclusively with its receptor, c-Met, initiating a cascade that decreases apoptosis and increases proliferation, migration, morphogenesis, and angiogenesis.26, 27, 28, 29 When tissue is damaged, chemokines stimulate the proximal and distal release of HGF.30,31 In the kidney, HGF reduces tubular necrosis, decreases renal epithelial apoptosis, and augments renal regeneration, ultimately attenuating renal dysfunction.32, 33, 34, 35, 36 There is, however, a mismatch in the upregulation of HGF and c-Met. HGF levels increase within minutes of tissue damage and peak at approximately 2 hours, with a second, smaller peak at 24 hours.37 c-Met receptor expression is also upregulated shortly after injury, however, the surface density of c-Met peaks at 24 hours, leaving a mismatch between peak surface receptor expression and ligand levels.37 This presents a potential therapeutic window for exogenous stimulation (Figure 2).
Figure 2.
Hepatocyte growth factor (HGF) plasma levels and c-Met receptor expression levels.
ANG-3777 is a small molecule HGF mimetic. In vivo studies have shown that it binds to renal c-Met receptors, producing the same decreases in apoptosis and increases in proliferation induced by endogenous HGF. In a series of pre-clinical studies, Narayan et al.38 induced renal ischemia reperfusion injury in rats and randomized animals to receive 2 mg/kg of ANG-3777 or placebo at 24 hours after injury. Compared to vehicle, ANG-3777 reduced tubular epithelial apoptosis preserving nephron mass, and also reduced hemorrhage, tubular dilation, and acute tubular necrosis. Additionally, animals treated with ANG-3777 showed increased urine output, decreased SCr and blood urea nitrogen, and reduced mortality.38 In a phase 2 randomized placebo-controlled double-blind proof-of-concept study in renal transplantation patients showing signs of delayed graft function (a form of AKI in kidney transplantation), patients treated with ANG-3777 showed improvements in multiple endpoints compared to placebo. ANG-3777–treated patients had higher urine output over 28 days after transplantation, lower SCr, higher estimated glomerular filtration rate (eGFR) up to 1 year, greater reduction in markers of renal damage (C-reactive protein and neutrophil gelatinase-associated lipocalin), and shorter duration of dialysis and transplant hospitalization.39
With approximately 50% of patients undergoing cardiac surgery at high risk of AKI, and with the significant increase in morbidity and mortality among those who experience AKI, there is a significant unmet need for preventive treatment.
Rationale and Design for a Randomized Controlled Trial in CSA-AKI: The Prevention and Amelioration of CSA-AKI
The goal of this phase 2 proof-of-concept study is to assess the safety and efficacy of ANG-3777 in patients at high risk for AKI undergoing cardiac surgery involving cardiopulmonary bypass.
Trial Name
The trial name is Guard Against Renal Damage (GUARD); Angion study 002-15; Clinicaltrials.gov identifier NCT02771509.
Study design
This is a randomized, double-blind, placebo-controlled, multicenter phase 2 trial.
Patient population
Participants are male and female patients 18 years of age or older undergoing a non-emergent cardiac surgical procedure involving CPB. Inclusion and exclusion criteria were selected to first enrich the sample for risk of CSA-AKI, then to minimize confounders that could obscure efficacy signal detection and safety assessment (Table 1). The first set of inclusion criteria are specific to the AKI risks of cardiac surgery itself. The requirement for CPB was based on its association with significant increased risk for CSA-AKI: 24% to 26%.3,4 The cardiac procedures were selected based upon the requirement for CPB, as well as their own associated risk of AKI: CABG; aortic, mitral, tricuspid, or pulmonic valve replacement or repair; valve repair/replacement with concurrent CABG. Emergency cardiac surgery was excluded as it introduces significant confounding clinical complications, including acute cardiac ischemia.
Table 1.
Patient population
| Inclusion criteria | Exclusion criteria |
|---|---|
| ≥18 years of age undergoing CPB surgerya | Emergency cardiac surgery |
| eGFR ≥20 and <30 ml/min per 1.73 m2 | AKI or significant renal impairment within 24 hours of surgery: a diagnosis of AKI as defined by KDIGO criteria or |
| eGFR ≥30 and <60 ml/min per 1.73 m2 and one additional risk factor from Table 2 (other than age ≥75 years) or | eGFR <20 ml/min per 1.73 m2 or |
| eGFR ≥60 ml/min per 1.73 m2 and two additional risk factors from Table 2 | An acute rise in SCr >0.3 mg/dl or |
| 50% increase in SCr between the time of screening visit and pre-surgery | |
| Patients receiving iodinated contrast material within 24 hours of surgery | |
| Active sepsis or current active infection requiring antibiotic treatment | |
| Treatment with cytochrome P450 1A2 (CYP1A2) inhibitors; | |
| HIV seropositivity | |
| Active malignancy or history within the previous 5 years before screening visit | |
| Cardiogenic shock or hemodynamic instability within 24 hours before randomization | |
| Use of a pacemaker, mechanical ventilation, any form of mechanical circulatory support, or cardiopulmonary resuscitation 7 days before surgery | |
| Clinical or laboratory diagnosis of shock liver | |
| BMI >40 kg/m2 at screening |
AKI, acute kidney injury; BMI, body mass index; CPB, cardiopulmonary bypass; eGFR, estimated glomerular filtration rate; KDIGO, Kidney Disease: Improving Global Outcomes; SCr, serum creatinine.
Age ≥75 years can be considered an additional risk factor only for patients with eGFR ≥60 ml/min per 1.73 m2.
The second set of enrichment criteria involved pre-surgical patient characteristics associated with AKI. The first risk factor was eGFR ≥20 and <30 ml/min per 1.73 m2. In a study of CSA-AKI risk factors, Hsu et al.40 reported that, after controlling for other patient and surgical risk factors, a pre-surgery eGFR 15 to <30 ml/min per 1.73 m2 was associated with an HR of 20.4 to 28.5 for AKI (the reference group was eGFR ≥60 ml/min per 1.73 m2). Thus, an eGFR indicative of chronic kidney disease stage 4 is highly predictive of CSA-AKI. The decision to reduce the lower bound to 20 ml/min per 1.73 m2 was made to avoid the enrollment of patients on the cusp of requiring renal replacement therapy, where advanced chronic kidney disease would significantly confound CSA-AKI–specific treatment effects.
Patients with an eGFR ≥30 and <60 ml/min per 1.73 m2 had an HR of 1.7 to 6.5 for CSA-AKI compared to eGFR ≥60 ml/min per 1.73 m2.40 Although this represented significant incremental risk, the decision was made to further enrich this population by requiring at least one additional AKI risk factor. Those risk factors and associated HRs are shown in Table 2.
Table 2.
AKI risk factors
| Risk (enrichment) factors | HR |
|---|---|
| Combined valve and CABG surgery5,7,8 | >3 |
| Previous open heart surgery5, 6, 7, 8 | 1.8 |
| Left ventricular ejection fraction <35%5,6,8 | 1.5 |
| Diabetes mellitus requiring insulin or with at least moderate (+2) proteinuria7,8,13 | 1.8; 2.0 |
| NYHA functional class IV5 | 1.55 |
AKI, acute kidney injury; CABG, coronary artery bypass graft; HR, hazard ratio; NYHA, New York Heart Association.
Patients with eGFR ≥60 ml/min per 1.73 m2 were required to have at least two additional risk factors shown in Table 2. For these patients, age ≥75 years was also considered an additional risk factor, as it is generally accepted that advanced age is a risk factor for AKI,1,41, 42, 43 and there is an increased risk of AKI for those who are ≥75 years compared to those who are younger than 75 years.41,44,45
Exclusion criteria were established to ensure that AKI was associated with cardiac surgery and no other causes, in other words, to reduce confounding factors that would unnecessarily interfere with efficacy signal detection and safety assessment. To ensure AKI was associated with cardiac surgery and not existing renal insufficiency, patients were excluded who had existing AKI or significant renal impairment within 24 hours of surgery: a diagnosis of AKI as defined by Kidney Disease: Improving Global Outcomes criteria or eGFR <20 ml/min per 1.73 m2, or; an acute rise in SCr >0.3 mg/dl or 50% increase in SCr between the time of screening visit and pre-surgery. Patients receiving iodinated contrast material within 24 hours of surgery are also excluded for the reasons previously discussed.
To reduce confounding, patients with the following comorbidities were excluded: active sepsis or current active infection requiring antibiotic treatment; treatment with cytochrome P450 1A2 (CYP1A2) inhibitors; HIV seropositivity; active malignancy or history within the previous 5 years before screening visit; cardiogenic shock or hemodynamic instability within 24 hours prior to randomization; use of a pacemaker, mechanical ventilation, any form of mechanical circulatory support or cardiopulmonary resuscitation 7 days before surgery; clinical or laboratory diagnosis of shock liver; and body mass index >40 kg/m2 at screening.
Treatment
Patients are randomized 1:1 to placebo or ANG-3777. This study uses the same 2-mg/kg ANG-3777 dose as the phase 2 trial in delayed graft function,39 which was determined based upon pre-clinical and phase 1 studies showing efficacy and safety at this dose. The study drug is administered via peripheral venous infusion over a period of 30 minutes (±5 minutes), with initial dose administered within 4 hours after CPB (i.e., at the time the patient is taken off pump). The second dose is administered 24 ± 2 hours after CPB, and the third and fourth doses 24 ± 2 hours after the previous dose.
Selection of primary endpoint
The primary endpoint is the percent increase in SCr above baseline (24 hours after end of CPB) area under the curve from days 2 through 6. This is calculated as: area under the curve = (½ day 2 + day 3 + day 4 + day 5 + ½ day 6)/number of non-missing days. The selection of this endpoint reflects SCr as a fundamental criteria in the Acute Kidney Injury Network diagnostic criteria, and allows for the assessment of change in SCr both as a continuous measure, and as categorical stage as defined by Kidney Disease: Improving Global Outcomes (Table 3).1
Table 3.
KDIGO AKI stages1
| Stage | Serum creatinine | Urine output |
|---|---|---|
| 1 | 1.5–1.9 times baseline or ≥0.3 mg/dl increase | <0.5 ml/kg/h for 6–12 hours |
| 2 | 2.0–2.9 times baseline | <0.5 ml/kg/h for ≥12 hours |
| 3 | 3.0 times baseline or Increase in serum creatinine to ≥4.0 mg/dl (≥353.6 μmol/l) or Initiation of renal replacement therapy or In patients <18 years, decrease in eGFR to <35 ml/min per 1.73 m2 | < 0.3 ml/kg/h for ≥24 hours, or; Anuria for ≥12 hours |
AKI, acute kidney injury; eGFR, estimated glomerular filtration rate; KDIGO, Kidney Disease: Improving Global Outcomes.
The primary endpoint will be an analyzed as a between group difference using an analysis of variance.
Selection of secondary endpoints
There are three secondary efficacy endpoints: (i) change in eGFR from baseline to day 30; (ii) proportion of patients diagnosed at each AKI stage per Kidney Disease: Improving Global Outcomes criteria through day 5 (see Table 2); and (iii) length of hospitalization starting from 24 hours after end of CPB.
Twenty-eight–day eGFR was an endpoint in the phase 2 delayed graft function trial, and 12-month eGFR is the primary endpoint in the phase 3 delayed graft function trial. eGFR was selected both for clinical meaningfulness and to allow for comparison of data across AKI indications. The choice of AKI stage is self-evident. The choice of hospital length of stay was selected to begin to understand the potential cost offsets associated with treatment efficacy in this population.
Safety
Safety will be assessed through physician- and patient-reported adverse events, physical examination and vital signs, clinical laboratory tests (hematology, serum chemistry, coagulation profile, and hepatic profile), and electrocardiogram. A Data and Safety Monitoring Board is undertaking ongoing monitoring of the safety data for this trial.
Procedures
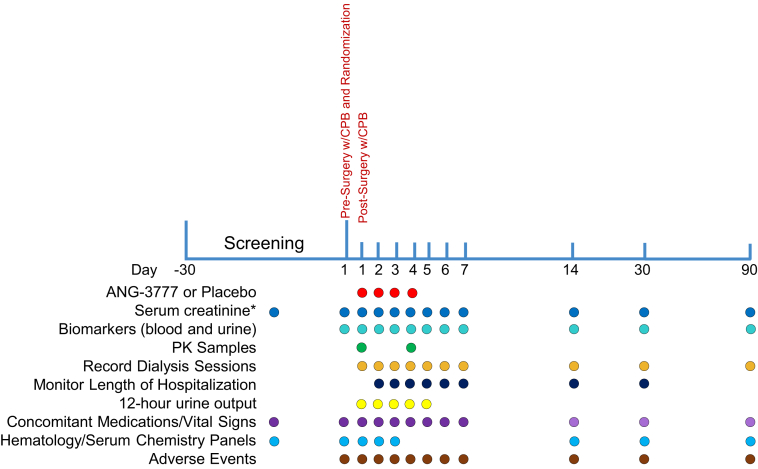
The GUARD study will enroll approximately 240 eligible subjects at 30 sites in the United States, Canada, and Brazil over approximately 4 years. With 90 days of follow-up, total study time will be ∼4.5 years. As shown in Figure 3, subjects are screened for eligibility, consented, and enrolled up to 30 days before surgery. Baseline demographics, height and weight, medical history, concomitant medications, vital signs, physical examination, and all safety measures are assessed at this time. On the day of surgery, eligibility is confirmed and all safety measures are again collected, as well as baseline SCr for calculation of the primary endpoint. Patients then undergo cardiac surgery. Within 4 hours of the completion of CPB, patients receive the first dose of study drug, and receive their second dose 24 (±2) hours after CPB. Two additional doses are administered at subsequent 24 (±2)–hour intervals, for a total of 4 doses. SCr is measured daily though day 7, and again at days 14, 30, and 90, as are adverse events and vital signs. Full hematology and serum chemistry panels are conducted on days 1, 2, 3, 14, 30, and 90.
Figure 3.
Study schematic. ∗Serum creatinine (Scr) must be captured twice prior to surgery and after paitent goes off cardiopulmonary bypass (CPB). A sample for SCr must be obtained after the patient goes off CPB and before the first administration of the study drug, then at 12 ± 2 hours, at 24 hours ± 2 hours, and at 48 ± 2 hours after the patient has gone off CPB. PK, pharmacokinetics.
This study is being conducted in accordance with ethical principles that have their origin in the Declaration of Helsinki and are consistent with International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use/good clinical practice, applicable regulatory requirements and the sponsor policy on bioethics. All sites received Institutional Review Board/Institutional Ethics Committee approval before screening and enrolling into the study.
Discussion
Cardiac surgery is a life-saving procedure, with 470,000 coronary bypass and valve replacement surgeries performed each year.9,46 These procedures include the use of CPB, which is required for these surgeries, but also runs the risk of inducing ischemia reperfusion injury in the kidney. CSA-AKI is associated with renal tubular damage, and treatment largely consists of supportive care. More than half of patients undergoing cardiovascular surgery have one or more risk factors for CSA-AKI, and those diagnosed have significantly increased morbidity and mortality and associated health care resource use and cost.
An endogenous mechanism by which renal tissues repair and regenerate is through the HGF/c-Met pathway. Released soon after renal injury, HGF binds to c-Met on renal cells, stimulating a cascade that decreases apoptosis and increases proliferation, migration, morphogenesis, and angiogenesis. However, HGF peaks 2 hours after injury whereas c-Met surface receptor density peaks at 24 hours. This timing mismatch represents a potential therapeutic window for exogenous c-Met stimulation.
ANG-3777 is an HGF mimetic that in vitro induces the same c-Met cascades and cellular effects as endogenous HGF. In animals, ANG-3777 stimulates tubular repair and regeneration, resulting in improved renal function following ischemia reperfusion injury. In humans, ANG-3777 has been shown to improve renal function up to 1 year after kidney transplantation in patients with signs of delayed graft function.
This study will test the hypothesis that ANG-3777 improves renal function and reduces AKI in patients undergoing cardiac surgery. Inclusion criteria were selected to enrich the sample for risk for AKI. It will include patients undergoing CABG and or valve replacement/repair requiring CPB, as this increases the risk for renal ischemia reperfusion injury. Inclusion criteria also include patient-levels factors known to increase risk for CSA-AKI. An eGFR >20 to 30 ml/min per 1.73 m2 represents sufficient incremental risk; an eGFR >30 to 60 ml/min per 1.73 m2 requires at least one additional risk factor; and eGFR >60 ml/min per 1.73 m2 requires two or more additional risk factors. Conditions known to introduce significant clinical risk beyond CSA-AKI, such as active viral or bacterial infection or active malignancy, were excluded. The primary endpoint is percent change from baseline SCr, using mean area under the curve from days 2 through 6 post-surgery. SCr is a primary sign of AKI, and change in SCr defines AKI stage which is a secondary endpoint in this study. Additional endpoints include renal function at day 30 measured via eGFR, as well as length of hospital stay, which will provide data to begin to understand the cost-offsets associated with this treatment. It is hoped that GUARD produces incremental efficacy with no associated difference in safety, which will progress to a phase 3 registrational study in this costly and medically complex condition.
Disclosure
SA and MS are both principal investigators for the GUARD study. DG and TJM were employees of Angion Biomedica at the time of writing and original submission. JN is an employee of Angion Biomedica and owns stock/stock options.
Acknowledgments
This work was supported by Angion Biomedica Corporation, Uniondale, New York. All authors were involved in the review and approval of this manuscript and are involved in the conduct of The GUARD study (NCT02771509). The authors thank Springer Nature for the use of Figure 1 from O’Neal et al.,9 which was published under the terms of Creative Commons CC BY license. Also, the authors thank Eric Krauter, PhD, for his editorial assistance with this manuscript.
References
- 1.KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012;2 [Google Scholar]
- 2.Conlon P.J., Stafford-Smith M., White W.D. Acute renal failure following cardiac surgery. Nephrol Dial Transplant. 1999;14:1158–1162. doi: 10.1093/ndt/14.5.1158. [DOI] [PubMed] [Google Scholar]
- 3.D’Onofrio A., Cruz D., Bolgan I. RIFLE criteria for cardiac surgery-associated acute kidney injury: risk factors and outcomes. Congest Heart Fail. 2010;16(suppl 1):S32–S36. doi: 10.1111/j.1751-7133.2010.00170.x. [DOI] [PubMed] [Google Scholar]
- 4.Karkouti K., Wijeysundera D.N., Yau T.M. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation. 2009;119:495–502. doi: 10.1161/CIRCULATIONAHA.108.786913. [DOI] [PubMed] [Google Scholar]
- 5.Chertow G.M., Lazarus J.M., Christiansen C.L., Cook E.F. Preoperative renal risk stratification. Circulation. 1997;95:878–884. doi: 10.1161/01.cir.95.4.878. [DOI] [PubMed] [Google Scholar]
- 6.Thakar C.V., Arrigain S., Worley S., Yared J.-P. A clinical score to predict acute renal failure after cardiac surgery. JASN. 2005;16:162–168. doi: 10.1681/ASN.2004040331. [DOI] [PubMed] [Google Scholar]
- 7.Mehta R.H., Grab J.D., O’Brien S.M. Bedside tool for predicting the risk of postoperative dialysis in patients undergoing cardiac surgery. Circulation. 2006;114:2208–2216. doi: 10.1161/CIRCULATIONAHA.106.635573. quiz 2208. [DOI] [PubMed] [Google Scholar]
- 8.Wijeysundera D.N., Karkouti K., Dupuis J.-Y. Derivation and validation of a simplified predictive index for renal replacement therapy after cardiac surgery. JAMA. 2007;297:1801–1809. doi: 10.1001/jama.297.16.1801. [DOI] [PubMed] [Google Scholar]
- 9.O’Neal J.B., Shaw A.D., Billings F.T. Acute kidney injury following cardiac surgery: current understanding and future directions. Crit Care. 2016;20:187. doi: 10.1186/s13054-016-1352-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hobson C., Ozrazgat-Baslanti T., Kuxhausen A. Cost and mortality associated with postoperative acute kidney injury. Ann Surg. 2015;261:1207–1214. doi: 10.1097/SLA.0000000000000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishani A., Nelson D., Clothier B. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch Intern Med. 2011;171:226–233. doi: 10.1001/archinternmed.2010.514. [DOI] [PubMed] [Google Scholar]
- 12.Lassnigg A., Schmidlin D., Mouhieddine M. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol. 2004;15:1597–1605. doi: 10.1097/01.asn.0000130340.93930.dd. [DOI] [PubMed] [Google Scholar]
- 13.Chertow G.M., Burdick E., Honour M., Bonventre J.V. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. JASN. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 14.Dasta J.F., Kane-Gill S.L., Durtschi A.J., Pathak D.S. Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol Dial Transplant. 2008;23:1970–1974. doi: 10.1093/ndt/gfm908. [DOI] [PubMed] [Google Scholar]
- 15.Hobson C.E., Yavas S., Segal M.S. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119:2444–2453. doi: 10.1161/CIRCULATIONAHA.108.800011. [DOI] [PubMed] [Google Scholar]
- 16.Brown J.R., Kramer R.S., Coca S.G., Parikh C.R. Duration of acute kidney injury impacts long-term survival after cardiac surgery. Ann Thorac Surg. 2010;9:1142–1148. doi: 10.1016/j.athoracsur.2010.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coca S.G., King J.T., Rosenthal R.A., Perkal M.F. The duration of postoperative acute kidney injury is an additional parameter predicting long-term survival in diabetic veterans. Kidney Int. 2010;78:926–933. doi: 10.1038/ki.2010.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siew E.D., Davenport A. The growth of acute kidney injury: a rising tide or just closer attention to detail? Kidney Int. 2015;87:46–61. doi: 10.1038/ki.2014.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calvert S., Shaw A. Perioperative acute kidney injury. Perioper Med (Lond) 2012;1:6. doi: 10.1186/2047-0525-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cartin-Ceba R., Kashiouris M., Plataki M., Kor D.J. Risk factors for development of acute kidney injury in critically ill patients: a systematic review and meta-analysis of observational studies. Crit Care Res Pract. 2012;2012:691013. doi: 10.1155/2012/691013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozkok S., Ozkok A. Contrast-induced acute kidney injury: a review of practical points. World J Nephrol. 2017;6:86–99. doi: 10.5527/wjn.v6.i3.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weisbord S.D., Gallagher M., Jneid H. Outcomes after angiography with sodium bicarbonate and acetylcysteine. N Engl J Med. 2018;378:603–614. doi: 10.1056/NEJMoa1710933. [DOI] [PubMed] [Google Scholar]
- 23.Rosner M.H., Okusa M.D. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol. 2006;1:19–32. doi: 10.2215/CJN.00240605. [DOI] [PubMed] [Google Scholar]
- 24.Moran S.M., Myers B.D. Pathophysiology of protracted acute renal failure in man. J Clin Invest. 1985;76:1440–1448. doi: 10.1172/JCI112122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwon O., Nelson W.J., Sibley R. Backleak, tight junctions, and cell-cell adhesion in postischemic injury to the renal allograft. J Clin Invest. 1998;101:2054–2064. doi: 10.1172/JCI772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cecchi F., C, Rabe D., P, Bottaro D. The hepatocyte growth factor receptor: structure, function and pharmacological targeting in cancer. Curr Signal Transduct Ther. 2011;6:146–151. doi: 10.2174/157436211795659955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamura T., Sakai K., Nakamura T., Matsumoto K. Hepatocyte growth factor twenty years on: much more than a growth factor: hepatocyte growth factor. J Gastroenterol Hepatol. 2011;26:188–202. doi: 10.1111/j.1440-1746.2010.06549.x. [DOI] [PubMed] [Google Scholar]
- 28.Kato T. Biological roles of hepatocyte growth factor-Met signaling from genetically modified animals (Review). Biom Rep. 20177:495–503. [DOI] [PMC free article] [PubMed]
- 29.Goyal L., Muzumdar M.D., Zhu A.X. Targeting the HGF/c-MET pathway in hepatocellular carcinoma. Clin Cancer Res. 2013;19:2310–2318. doi: 10.1158/1078-0432.CCR-12-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakamura T., Mizuno S. The discovery of hepatocyte growth factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proc Jpn Acad, Ser B, Phys Biol Sci. 2010;86:588–610. doi: 10.2183/pjab.86.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsumoto K., Funakoshi H., Takahashi H., Sakai K. HGF–Met pathway in regeneration and drug discovery. Biomedicines. 2014;2:275–300. doi: 10.3390/biomedicines2040275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Souza Durão M., Razvickas C.V., Gonçalves E.A.P. The role of growth factors on renal tubular cells submitted to hypoxia and deprived of glucose. Ren Fail. 2003;25:341–353. doi: 10.1081/jdi-120021149. [DOI] [PubMed] [Google Scholar]
- 33.Miller S.B., Martin D.R., Kissane J., Hammerman M.R. Hepatocyte growth factor accelerates recovery from acute ischemic renal injury in rats. Am J Physiol Renal Physiol. 1994;266:F129–F134. doi: 10.1152/ajprenal.1994.266.1.F129. [DOI] [PubMed] [Google Scholar]
- 34.Igawa T., Matsumoto K., Kanda S., Saito Y. Hepatocyte growth factor may function as a renotropic factor for regeneration in rats with acute renal injury. Am J Physiol Renal Physiol. 1993;265:F61–F69. doi: 10.1152/ajprenal.1993.265.1.F61. [DOI] [PubMed] [Google Scholar]
- 35.Yamasaki N., Nagano T., Mori-Kudo I. Hepatocyte growth factor protects functional and histological disorders of HgCl2-induced acute renal failure mice. Nephron. 2002;90:195–205. doi: 10.1159/000049042. [DOI] [PubMed] [Google Scholar]
- 36.Kawaida K., Matsumoto K., Shimazu H., Nakamura T. Hepatocyte growth factor prevents acute renal failure and accelerates renal regeneration in mice. Proc Natl Acad Sci U S A. 1994;91:4357–4361. doi: 10.1073/pnas.91.10.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y., Tolbert E.M., Lin L. Up-regulation of hepatocyte growth factor receptor: an amplification and targeting mechanism for hepatocyte growth factor action in acute renal failure. Kidney Int. 1999;55:442–453. doi: 10.1046/j.1523-1755.1999.00267.x. [DOI] [PubMed] [Google Scholar]
- 38.Narayan P., Duan B., Jiang K. Late intervention with the small molecule BB3 mitigates postischemic kidney injury. Am J Physioil Renal Physiol. 2016;311:F352–F361. doi: 10.1152/ajprenal.00455.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bromberg J.S., Weir M.R., Gaber A.O. Renal function improvement following ANG-3777 treatment in patients at high risk for delayed graft function after kidney transplantation. Transplantation. 2020 doi: 10.1097/TP.0000000000003255. [epub ahead of print] Accessed May 20, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsu C.Y., Ordoñez J.D., Chertow G.M., Fan D. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int. 2008;74:101–107. doi: 10.1038/ki.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson S., Eldadah B., Halter J.B. Acute kidney injury in older adults. J Am Soc Nephrol. 2011;22:28–38. doi: 10.1681/ASN.2010090934. [DOI] [PubMed] [Google Scholar]
- 42.Kane-Gill S.L., Sileanu F.E., Murugan R., Trietley G.S. Risk factors for acute kidney injury in older adults with critical illness: a retrospective cohort study. Am J Kidney Dis. 2015;65:860–869. doi: 10.1053/j.ajkd.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moreira R., Jacinto T., Neves P., Vouga L. Predictors of acute kidney injury in the postoperative period of cardiac surgery associated with cardiopulmonary bypass. Rev Port Cir Cardiotorac Vasc. 2017;24:154. [PubMed] [Google Scholar]
- 44.Bagshaw S.M., Laupland K.B., Doig C.J. Prognosis for long-term survival and renal recovery in critically ill patients with severe acute renal failure: a population-based study. Crit Care. 2005;9:R700–R709. doi: 10.1186/cc3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chao C.-T., Wang J., Wu H.-Y., Huang J.-W., Chien K.-L. Age modifies the risk factor profiles for acute kidney injury among recently diagnosed type 2 diabetic patients: a population-based study. GeroScience. 2018;40:201–217. doi: 10.1007/s11357-018-0013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benjamin E.J., Virani S.S., Callaway C.W. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]