Introduction
Transplant renal artery stenosis (TRAS) is a well-recognized vascular complication following kidney transplantation, affecting 1% to 23% of recipients.1 It is associated with poor outcomes, including transplant loss and death, but is potentially amenable to intervention with endovascular revascularization.
We present the case of a patient who developed an unusual distal transplant artery stenosis after receiving a cadaveric kidney from a young female individual that may have occurred as a result of undiagnosed fibromuscular dysplasia (FMD) in the donor. We then consider the risk factors, diagnosis, and management of TRAS.
Case Presentation
A 47-year-old woman with IgA nephropathy and controlled hypertension received a pre-emptive, cadaveric renal transplant from an 18-year-old female donor with a 0-0-1 mismatch and a cold ischemic time of 20 hours. The donor died of anaphylactic shock, with no other medical history. The donor was cytomegalovirus (CMV) IgG negative and the recipient CMV IgG positive. Basiliximab was used as induction immunosuppression, and regular tacrolimus, mycophenolate mofetil, and prednisolone were started at the time of transplantation as maintenance immunosuppression alongside valganciclovir as CMV prophylaxis. In the context of initial delayed transplant function, a Doppler ultrasound showed good transplant perfusion, and a transplant biopsy on day 7 post-transplantation showed acute tubular necrosis only. Transplant function subsequently improved, and the patient’s creatinine was 1.23 mg/dl by day 32.
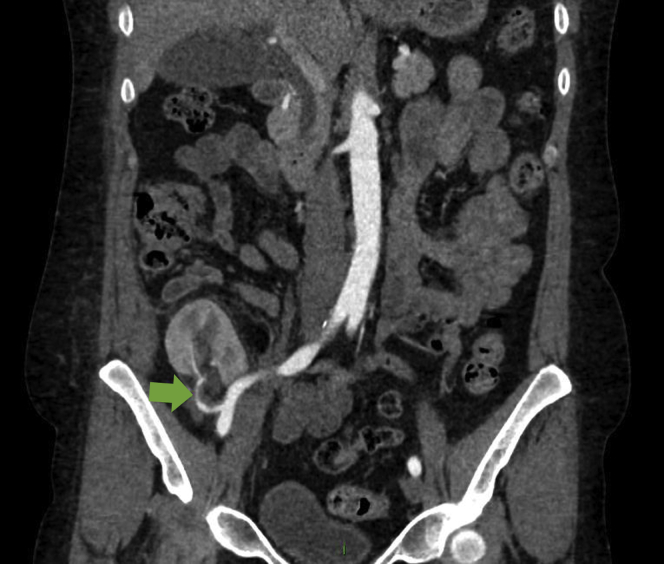
Following discharge, she was well but noted to have hypertension on routine clinic reviews over the first few months, and antihypertensives were titrated. Four months post-transplantation, she developed significant peripheral edema with uncontrolled hypertension, severe headache, vomiting, and transplant dysfunction. There was no audible renal transplant bruit. Serum creatinine rose to 2.67 mg/dl, urinary protein:creatinine ratio was 716 mg/mmol, and serum albumin fell to 29 g/l. Initial Doppler ultrasound imaging showed good transplant perfusion and a resistive index of 0.54 (0.5−0.8). On full-dose amlodipine, doxazosin, bisoprolol, furosemide 80 mg once daily and methyldopa 250 mg 3 times daily, she remained hypertensive with a blood pressure of 170/109 mm Hg. In the context of new nephrotic-range proteinuria and transplant dysfunction, a transplant biopsy was performed. This showed acute tubular injury and mild microvascular disease, but no other pathology. A repeat ultrasound showed a low resistive index of 0.36. A magnetic resonance renal angiogram showed a small filling defect at the renal hilum but good distal perfusion and a normal surgical anastomosis. Subsequently, a computed tomographic angiogram demonstrated a caliber change at the renal hilum strongly suggestive of a distal transplant artery stenosis (Figure 1).
Figure 1.
Image from computed tomographic angiogram showing a caliber change at the renal hilum (indicated by the green arrow) suggestive of distal transplant artery stenosis.
Subsequent invasive arteriography confirmed a smooth stenosis from the mid to distal portion of the transplant artery, and angioplasty to a 3.2-mm segment was performed (Figure 2). This led to an immediate improvement in blood pressure, and a drop in creatinine from 2.44 mg/dl pre-procedure to 1.78 mg/dl after 12 hours and 1.48 mg/dl at discharge 4 days later.
Figure 2.
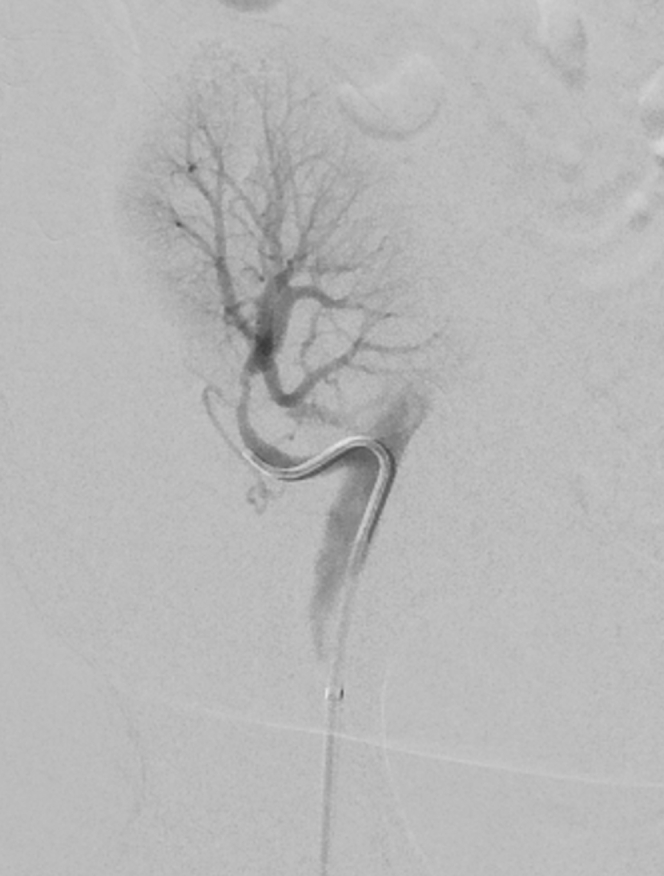
Image from catheter angiogram showing renal artery stenosis.
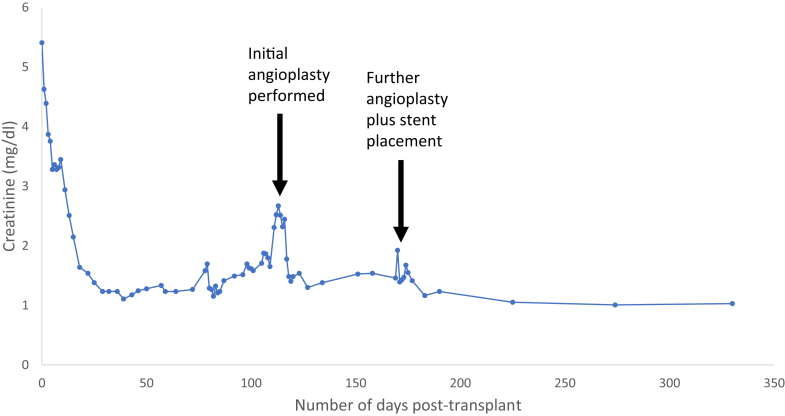
Two months later, however, the patient again developed hypertension and worsening peripheral edema, with a rise in creatinine to 1.92 mg/dl. A repeat catheter angiogram confirmed recurrence of the stenosis, and further angioplasty followed by stent placement was carried out. Following this intervention, there was an improvement in blood pressure control and serum creatinine (Figure 3), which has now been sustained for greater than 12 months.
Figure 3.
Trend in creatinine following kidney transplantation. The initial angioplasty was performed on day 116. The repeat angioplasty with stent placement was performed on day 172.
Discussion
Hypertension is very common following kidney transplantation, with a prevalence of 80% to 85%, whereas resistant hypertension (defined as a blood pressure that remains above target despite the use of 3 or more antihypertensives) affects 7% of transplant recipients.2 Transplant renal artery stenosis is a potentially reversible cause of refractory post-transplantation hypertension, whereby narrowing of the transplant renal artery impedes blood flow and results in renal hypoperfusion. This leads to activation of the renin−angiotensin−aldosterone system (RAAS), resulting in sodium retention, volume expansion, and sustained hypertension in a mechanism analogous to the “1-kidney, 1-clip” animal model used in Goldblatt’s seminal work on hypertension.S1
TRAS usually becomes apparent between 3 months and 2 years following transplantation but occurs most frequently in the first 6 months. It presents, as in this case, with hypertension, salt and water retention, and transplant dysfunction. At its most severe, this can result in hypertensive crises and flash pulmonary edema.3 Transplant dysfunction in the absence of any other cause is indicative of a critical stenosis.
Variability in the reported incidence of TRAS can be attributed to the different definitions and diagnostic techniques used in studies. Hurst et al., using the United States Renal Data System registry from 2000 to 2005, found that the cumulative incidence of TRAS was 2% at 3 years and was strongly associated with an increased risk of delayed transplant function, transplant loss, and death.4 Notably, the donor characteristics associated with an increased risk of TRAS in the Hurst et al. study (i.e., older age, extended-criteria donors, and ischemic heart disease) were absent in our case. Other factors found to be associated with TRAS included older age of recipients (particularly those >65 years of age); diabetes mellitus, ischemic heart disease and hypertension in the recipient; use of induction immunosuppression; and use of mycophenolate at the time of discharge.4 Other studies have also found CMV infectionS2 and acute cellular rejectionS3 to be associated with a higher rate of TRAS.
Most commonly, TRAS occurs close to the site of surgical anastomosis and relates to the suture line or postanastomotic turbulence of blood flow. Atherosclerotic disease may be evident in older donors, or may develop many years post-transplantation, most commonly in the proximal transplant artery. Traction injury of the transplant artery at the time of retrieval can cause distal transplant artery narrowing but would be expected to present earlier than in this case. The unusual distal location and relative length of stenosis seen here raises the possibility of fibromuscular dysplasia, a nonatherosclerotic, noninflammatory disease that may have been pre-existent but not yet clinically apparent in the young female donor (Table 1). Assessment of living donors has shown that up to 2.6% had computed tomographic angiographic evidence of fibromuscular dysplasia, of whom 87% were female.5 This suggests that fibromuscular dysplasia may be a more common cause of TRAS following cadaveric transplantation than previously appreciated.
Table 1.
Key learning points
| Fibromuscular dysplasia may be an underappreciated cause of TRAS following cadaveric renal transplantation. |
| Ultrasound is frequently used as the first-line imaging modality in transplant renal artery stenosis, but it is highly operator dependent and may miss cases. |
| Percutaneous transluminal angioplasty is an effective treatment for patients with TRAS. The additional use of stenting may reduce the need for re-intervention. |
TRAS, transplant renal artery stenosis.
The diagnosis of TRAS is not always straightforward and depends upon having a high index of suspicion and choosing an appropriate imaging modality. Doppler ultrasound imaging is a readily accessible and noninvasive tool that can identify TRAS, but it is highly operator dependent and requires assessment for low resistance indices (<0.5) and a high peak systolic velocity (>200 cm/s) in the renal artery. In a similar case report by Venturini et al.,6 Doppler ultrasound imaging proved challenging in a case of TRAS that was thought to be caused by fibromuscular dysplasia of the mid-distal portion of the renal artery. The authors describe 2 negative Doppler ultrasound examinations before a third ultrasound showed unexpectedly lower resistance indices, prolonged acceleration time, and increased peak systolic velocity in the distal third of the renal artery, suggestive of significant stenosis. Their case report highlights the importance of being vigilant for indirect signs suggestive of a renal artery stenosis. In our case report, the patient was also investigated with 3 Doppler ultrasound scans, the first 2 intended as diagnostic investigations and the third scan being completed for the purpose of an ultrasound-guided graft biopsy. Indirect signs suggestive of TRAS were present on the second Doppler scan, which found a dampened waveform within the kidney. The third scan reported a very flat Doppler trace with a resistive index of 0.36.
Computed tomography provides 3-dimensional images of the vascular tree but does require the administration of iodinated contrast media. Magnetic resonance angiography has also been used, and although in our case computed tomographic angiography provided a more definitive result, it preceded magnetic resonance angiography by 3 days, during which time the stenosis may have progressed. There is no convincing evidence of superiority of computed tomographic angiography over magnetic resonance angiography in the setting of TRAS.7 Ultimately, invasive arteriography is the gold standard, often providing the definitive diagnosis while simultaneously allowing treatment by percutaneous transluminal angioplasty (PTA) with or without stent placement as the critical therapeutic intervention.S4
Most would advocate medical management with antihypertensive agents and diuretics, assuming that renal function is stable, blood pressure can be controlled, and imaging parameters do not indicate a hemodynamically significant stenosis. In more severe cases, however, as in our case, revascularization is indicated. A systematic review by Ngo et al. of outcomes following PTA included 26 case series and 6 cohort studies and reported a technical success rate of over 90%. Clinical success rates (defined by improvements in blood pressure and/or creatinine) ranged from 66% to 94%. The re-intervention rate was higher in studies in which patients had PTA only compared to studies in which patients underwent stenting (18.9% vs. 9.1%). The complication rate was 9.9%, with vessel dissection, thrombosis, and puncture site hematoma being the most common complications reported.8 A recent matched cohort study found that 10-year transplant survival and patient survival following revascularization for TRAS (including patients treated with PTA or stenting) was the same as patients who had never had TRAS.9
Disclosure
All the authors declared no competing interests.
Acknowledgments
We would like to thank Dr. Shona Methven (Renal Physician, Aberdeen Royal Infirmary), Dr. Laura Clark (Renal Physician, Aberdeen Royal Infirmary), Prof. Lorna Marson (Transplant Surgeon, Royal Infirmary of Edinburgh Kidney Transplant Endowment Fund, United Kingdom), Dr. Andrew Walker (Interventional Radiologist, Royal Infirmary of Edinburgh Kidney Transplant Endowment Fund, United Kingdom), and Dr. Graeme Reid (Pathologist, Royal Infirmary of Edinburgh Kidney Transplant Endowment Fund, United Kingdom) for their contribution to this article.
Footnotes
Supplementary References.
Supplementary Material
References
- 1.Fervenza F.C., Lafayette R.A., Alfrey E.J., Petersen J. Renal artery stenosis in kidney transplants. Am J Kidney Dis. 1998;31:142–148. doi: 10.1053/ajkd.1998.v31.pm9428466. [DOI] [PubMed] [Google Scholar]
- 2.Gago Fraile M., Fernandez Fresnedo G., Gómez-Alamillo C. Clinical and epidemiological characteristics of refractory hypertension in renal transplant patients. Transplant Proc. 2009;41:2132–2133. doi: 10.1016/j.transproceed.2009.06.078. [DOI] [PubMed] [Google Scholar]
- 3.Chen W., Kayler L.K., Zand M.S. Transplant renal artery stenosis: clinical manifestations, diagnosis and therapy. Clin Kidney J. 2015;8:71–78. doi: 10.1093/ckj/sfu132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hurst F.P., Abbott K.C., Neff R.T. Incidence, predictors and outcomes of transplant renal artery stenosis after kidney transplantation: analysis of USRDS. Am J Nephrol. 2009;30:459–467. doi: 10.1159/000242431. [DOI] [PubMed] [Google Scholar]
- 5.McKenzie G.A., Oderich G.S., Kawashima A., Misra S. Renal artery fibromuscular dysplasia in 2,640 renal donor subjects: a CT angiography analysis. J Vasc Interv Radiol. 2013;24:1477–1480. doi: 10.1016/j.jvir.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Venturini M., Querques G., Margari S. Renal artery stenosis due to fibromuscular dysplasia in a transplanted kidney from a deceased donor: a difficult diagnosis at color Doppler ultrasonography. J Clin Ultrasound. 2014;42:116–120. doi: 10.1002/jcu.22056. [DOI] [PubMed] [Google Scholar]
- 7.Gaddikeri S., Mitsumori L., Vaidya S. Comparing the diagnostic accuracy of contrast-enhanced computed tomographic angiography and gadolinium-enhanced magnetic resonance angiography for the assessment of hemodynamically significant transplant renal artery stenosis. Curr Probl Diagn Radiol. 2014;43:162–168. doi: 10.1067/j.cpradiol.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Ngo A.T., Markar S.R., De Lijster M.S. A systematic review of outcomes following percutaneous transluminal angioplasty and stenting in the treatment of transplant renal artery stenosis. Cardiovasc Intervent Radiol. 2015;38:1573–1588. doi: 10.1007/s00270-015-1134-z. [DOI] [PubMed] [Google Scholar]
- 9.Patel U., Kumar S., Johnson O.W. Long-term graft and patient survival after percutaneous angioplasty or arterial stent placement for transplant renal artery stenosis: a 21-year matched cohort study. Radiology. 2019;290:555–563. doi: 10.1148/radiol.2018181320. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.