Among children with nephrotic syndrome, 10% to 15% are steroid resistant (steroid-resistant nephrotic syndrome [SRNS]), and of these 30% may develop refractory SRNS that is nonresponsive to the first-choice immunosuppressant for SRNS (i.e., calcineurin inhibitors [CNIs]).1 These children carry a guarded prognosis with up to 50% progressing to end-stage renal disease, and almost half developing disease recurrence posttransplant.2 The treatment protocol for refractory SRNS is not well established. Rituximab, a chimeric anti–CD20 antibody, did generate initial enthusiasm,3 which was somewhat dampened by the negative results from the only published randomized controlled trial.4 Primarily based on this, the International Pediatric Nephrology Association guidelines gave rituximab a weak recommendation for use in SRNS.1 On the other hand, various reviews, which have included retrospective cohort studies, have been supportive of rituximab.5,6 Some smaller studies have even proposed its earlier use.7,8 We hereby present our experience with the use of rituximab among a multicentric cohort of refractory SRNS.
Results
Thirty-one children (median age 78 months, interquartile range [IQR] 48–110 months, 52% male) with refractory SRNS received a total of 36 cycles of rituximab. Just over half were secondary SRNS (n = 18, 58%), and the most common renal pathology was focal segmental glomerulosclerosis (n = 15, 48%) and minimal change (n = 10, 32%). Other pathologies found were IgM nephropathy (n = 4, 13%) and mesangioproliferative glomerulonephritis (n = 2, 7%). At the initiation of rituximab, all were on a dual immunosuppressant of alternate-day steroids (median 1.5 mg/kg [IQR 1.2–1.7 mg/kg) with either tacrolimus (n = 20) or mycophenolate mofetil (n = 11). Postinfusion steroids were tapered as per the discretion of the treating physician, but CNIs or mycophenolate mofetil was continued. Among those on mycophenolate mofetil, all previously received CNIs without benefit (tacrolimus = 4, cyclosporine = 7). CD19 depleted (<1%) in all and replenished by a median of 8 months (IQR 5–8.5 months). Adverse events related to rituximab were rare (n = 4) and were limited to infusion reactions.
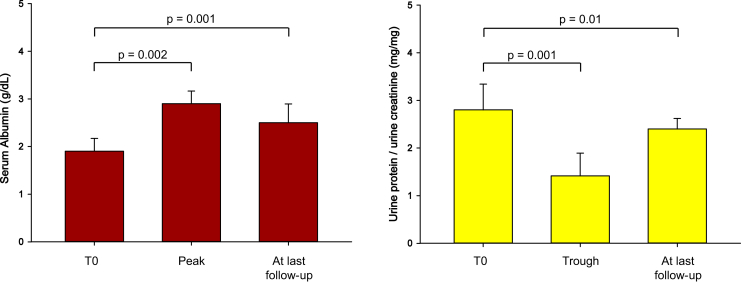
The median follow-up after a cycle of rituximab was 17.5 months (IQR 15–21.5 months). Postrituximab serum albumin showed a significant peak (median 1.9 g/dl, IQR 1.7–2.1 g/dl increased to 2.9 g/dl, IQR 2.3–3.4 g/dl; P = 0.002) and urine protein/creatinine a significant decrease (median 2.8 mg/mg, IQR 2.2–3.4 mg/mg dropped to 1.4 mg/mg, IQR 0.6–2.2 mg/mg; P = 0.001; Figure 1). Peak improvement was noted at a median of 6 months (albumin IQR 4.5–7 months and urine protein/creatinine IQR 5–8 months). Even though subsequent deterioration was noted, levels of both serum albumin (median 2.5 g/dl, IQR 2.1–3.2 g/dl) and urine protein/creatinine (median 2.4 mg/mg, IQR 1.5–2.9 mg/mg) at the last follow-up were significantly better compared with prerituximab values (P = 0.001 and P = 0.01, respectively; Figure 1).
Figure 1.
The postrituximab trend of serum albumin and spot urine protein/urine creatinine ratio. T0, at start.
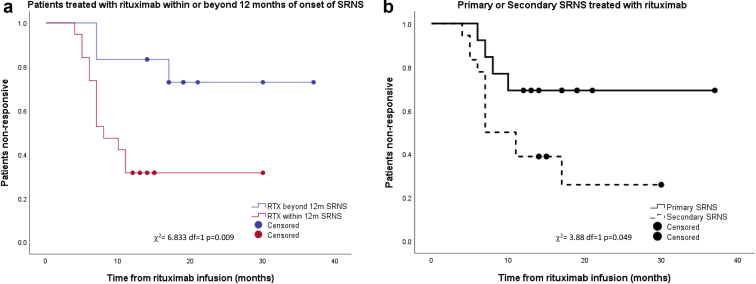
Complete remission (CR) was observed in 8 children (26%), partial remission (PR) in 10 (32%), and nonremission (NR) in 13 (42%). Four children received additional cycles of rituximab (3 children received 2 cycles and 1 child received 3 cycles); only 1 showed an improved response (NR became CR). Table 1 shows the correlation of relevant variables with the postrituximab response. The median interval from the onset of SRNS to the first rituximab cycle was 9 months (IQR 7–13 months), and a better response (CR + PR) was noted among those with early rituximab therapy, particularly when given within 12 months of SRNS diagnosis (Table 1 and Figure 2a and b). This correlation remained significant even on binary logistic regression analysis (P = .014). Overall, no significant difference were noted in pre- (median 0.66 mg/dl; IQR 0.54–0.78 mg/dl) or postrituximab (median 0.61 mg/dl; IQR 0.41–1.1 mg/dl) serum creatinine. Serum creatinine among NR showed a nonsignificant rising trend (P = 0.2), but it was significantly higher at the last follow-up vis-à-vis responders (Table 1). Children with secondary SRNS were more likely to respond (CR + PR) to rituximab (72%, n = 13), in contrast to primary SRNS (38%, n = 5; Figure 2). Overall, half of the response was achieved by 6.8 months and most by 11 months.
Table 1.
Postrituximab response variables
| Response variables | Responder | Nonresponder (NR, n = 13) | P value |
|---|---|---|---|
| Age at first dose of RTX, mo | 97 (48–112) | 71 (45.5–102.5) | 0.23 |
| Duration of follow-up postrituximab, mo | 19 (15.5–25) | 17 (14–20) | 0.2 |
| Male | 6 (33) | 10 (77) | 0.01 |
| Histopathology: FSGS/MCNS/others | 8 (44)/5 (28)/5 (28) | 7 (54)/5 (38)/1 (8) | 0.1 |
| Creatinine at initiation of rituximab, mg/dl | 0.67 (0.5–0.76) | 0.66 (0.59–1.1) | 0.08 |
| Creatinine at last follow-up, mg/dl | 0.46 (0.37–0.69) | 0.9 (0.48–1.35) | 0.01 |
| Primary SRNS/secondary SRNS | 5 (28)/13 (72) | 8 (62)/5 (38) | 0.06 |
| Duration from diagnosis of SRNS to first RTX infusion, mo | 8 (6–10) | 13 (7.5–14) | 0.04 |
| First RTX infusion within 12 mo/beyond 12 mo of diagnosis of SRNS | 16 (89)/2 (11) | 4 (31)/9 (60) | 0.002 |
CR, complete remission; FSGS, focal segmental glomerulosclerosis; MCNS, minimal change nephrotic syndrome; NR, nonremission; NS, nonsignificant; PR, partial remission; RTX, rituximab; SRNS, steroid-resistant nephrotic syndrome.
Data are n (%) or interquartile range, unless othewise noted.
Figure 2.
The Kaplan-Meier curve comparing the response to rituximab of (a) children treated within (red) or beyond (blue) 12 months of the onset of steroid-resistant nephrotic syndrome (SRNS) and (b) children with primary (solid line) or secondary SRNS (dashed line). RTX, rituximab.
Discussion
Similar to systematic reviews,5,6 more than half of our children with refractory SRNS (58%) achieved either CR or PR. The importance of this result should be interpreted via the PodoNet registry analysis9 wherein renal survival was better for CR (93%) and PR (73%) in comparison with NR (43%). In agreement with this, our cohort of NR at last the follow-up showed significantly worse creatinine (Table 1). Hence, even the achievement of PR should be highlighted because it delays/prevents the onset of end-stage renal disease, which is often a death sentence for many in resource-constrained countries.
Our encouraging results contrast the negative outcome of Magnasco et al.4 Even though a randomized controlled trial is considered as higher-grade evidence, it needs to be highlighted that Magnasco et al. analyzed the postrituximab response at 3 months, whereas we demonstrated the peak response beyond 3 months (Figure 2). An important observation was the better response seen if rituximab was given earlier, particularly within 12 months of steroid resistance (Table 1 and Figure 2). Similar data have been reported by Fujinaga et al.8 (n = 6) and Kamei et al.7 (n = 10), albeit in small cohorts. The improved response may be because of the initiation of rituximab before major irreversible damage has occurred to the kidneys from the disease process or from the prolonged use of CNIs.8 Along with early initiation, Fujinaga et al.8 also postulated that repeated doses of rituximab with concomitant use of high-dose steroids including pulse methylprednisolone are likely to aid in a better outcome. We used repeated doses in only 4 children and did not routinely use pulse methylprednisolone, although our cohort commenced on a higher steroid dose (1.5 mg/kg alternate day) at the start of rituximab, which was subsequently slowly tapered. It has to be noted that repeated rituximab doses come with the added baggage of increased side effects. Fujinaga et al.8 reported severe neutropenia in 2 children and hypogammaglobulinemia in 4 children. Although we did not check Ig levels routinely, none of our children developed neutropenia. Another observation was the better response noted among secondary SRNS (Figure 2), which was similar to the trend reported by Magnasco et al.4 Because secondary SRNS is unlikely to harbor genetic etiology, the better response is not surprising. We would also like to highlight the nearly 30% response among primary SRNS. A similar response rate has been reported in previous reviews5,6 and underscores the utility of rituximab even in primary SRNS. The response of rituximab in primary SRNS should be interpreted while acknowledging the difficulty in differentiating primary versus secondary SRNS, particularly in retrospective studies, but it can also be a manifestation of the direct stabilizing action of rituximab on the podocytes.
While acknowledging limitations inherent to any retrospective study, we did demonstrate a significant utility of rituximab among a multicenter cohort of refractory SRNS with earlier use likely to augment a better response. A major limitation is our lack of genetic data because we were able to look for this in only 4 children (2 tested negative for Wilms' tumor 1 and podocin mutations and 2 negative for SRNS genes by next-generation sequencing). Local resource constraint including the lack of facilities for genetic testing and their high cost was the main limiting factor at the time. However, we can postulate that if we had undertaken genetic study among our full cohort and excluded children with known SRNS mutation as per our current practice, this could have further improved our rituximab response results. Finally, our results should be interpreted keeping in mind our relatively small cohort size and hence needs revalidation by a larger trial. In conclusion, any epitaph on the use of rituximab in SRNS should be on hold until we have better designed randomized controlled trials looking at various minutiae associated with the rituximab response among childhood SRNS.
Disclosure
All the authors declared no competing interests.
Footnotes
Supplementary Methods.
Supplementary Material
References
- 1.Trautmann A., Vivarelli M., Samuel S. IPNA clinical practice recommendations for the diagnosis and management of children with steroid-resistant nephrotic syndrome. Pediatr Nephrol. 2020;35:1529–1561. doi: 10.1007/s00467-020-04519-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mekahli D., Liutkus A., Ranchin B. Long-term outcome of idiopathic steroid-resistant nephrotic syndrome: a multicenter study. Pediatr Nephrol. 2009;24:1525–1532. doi: 10.1007/s00467-009-1138-5. [DOI] [PubMed] [Google Scholar]
- 3.Bagga A., Sinha A., Moudgil A. Rituximab in patients with the steroid-resistant nephrotic syndrome. N Engl J Med. 2007;356:2751–2752. doi: 10.1056/NEJMc063706. [DOI] [PubMed] [Google Scholar]
- 4.Magnasco A., Ravani P., Edefonti A. Rituximab in children with resistant idiopathic nephrotic syndrome. J Am Soc Nephrol. 2012;23:1117–1124. doi: 10.1681/ASN.2011080775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jellouli M., Charfi R., Maalej B., Mahfoud A., Trabelsi S., Gargah T. Rituximab in the management of pediatric steroid-resistant nephrotic syndrome: a systematic review. J Pediatr. 2018;197:191–197.e1. doi: 10.1016/j.jpeds.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Kamei K., Ishikura K., Sako M., Ito S., Nozu K., Iijima K. Rituximab therapy for refractory steroid-resistant nephrotic syndrome in children. Pediatr Nephrol. 2020;35:17–24. doi: 10.1007/s00467-018-4166-1. [DOI] [PubMed] [Google Scholar]
- 7.Kamei K., Okada M., Sato M. Rituximab treatment combined with methylprednisolone pulse therapy and immunosuppressants for childhood steroid-resistant nephrotic syndrome. Pediatr Nephrol. 2014;29:1181–1187. doi: 10.1007/s00467-014-2765-z. [DOI] [PubMed] [Google Scholar]
- 8.Fujinaga S., Nishino T., Umeda C., Tomii Y., Watanabe Y., Sakuraya K. Long-term outcomes after early treatment with rituximab for Japanese children with cyclosporine- and steroid-resistant nephrotic syndrome. Pediatr Nephrol. 2019;34:353–357. doi: 10.1007/s00467-018-4145-6. [DOI] [PubMed] [Google Scholar]
- 9.Trautmann A., Schnaidt S., Lipska-Ziętkiewicz B.S. Long-term outcome of steroid-resistant nephrotic syndrome in children. J Am Soc Nephrol. 2017;28:3055–3065. doi: 10.1681/ASN.2016101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.