Abstract
Introduction
The incidence of kidney replacement therapy (KRT) for kidney failure varies internationally much more than chronic kidney disease (CKD) prevalence. This ecologic study investigated the relation of CKD prevalence to KRT and mortality risks by world region.
Methods
We used data from Global Burden of Disease and KRT registries worldwide with linear models to estimate the percentages of variance in KRT incidence and all-cause mortality explained by age-adjusted prevalence of CKD stages 3 to 5, overall and by gender, in 61 countries classified in 3 regions: high income (n = 28), Eastern and Central Europe (n = 15), and other (n = 18).
Results
The incidence of KRT ranged from 89 to 378 per million population in high-income regions, 32 to 222 per million population in Central and Eastern Europe, and 22 to 493 per million population in the other region; age-adjusted CKD prevalence ranged from 5.5% to 10.4%, 7.6% to 13.7%, and 7.4% to 13.1%, respectively. The relation between these indicators was positive in high-income countries, negative in Central and Eastern Europe, and null in the other region. Age-adjusted CKD prevalence explained 40% of the variance in KRT incidence (P < 0.001) in high-income countries. The explained variance of age-adjusted mortality was close to 0 in high-income countries and positive at 19% (P = 0.10) in Central and Eastern Europe and at 11% (P = 0.17) in the other region. Results were consistent by gender.
Conclusion
This study raises awareness on the significant part of the gaps in KRT incidence across countries not explained by the number of individuals with CKD, even in high-income countries where access to KRT is not limited.
Keywords: chronic kidney disease, disparity, ecologic study, international comparison, kidney replacement therapy
Over the past 2 decades, knowledge about the epidemiology of chronic kidney disease (CKD) and its complications has advanced substantially worldwide.1, 2, 3, 4, 5, 6 The increasing availability of national registries of kidney replacement therapy (KRT) for kidney failure and the work by the Global Burden of Disease (GBD) consortium in recent years to search for and standardize CKD prevalence data from the many available population-based studies have made it possible to obtain a global picture of CKD burden from moderate to advanced stages.7, 8, 9 The most striking finding from these estimates is that the disparities across countries are much greater for KRT incidence than for CKD prevalence.
The Global Kidney Health Atlas, led by the International Society of Nephrology, recently provided a comprehensive overview of the capacity of health systems to deliver care for kidney failure worldwide.10 This capacity remains a major determinant of the variations observed in KRT incidence in low- and middle-income countries. Large variations in KRT incidence may also exist, however, across countries where access to KRT is not restricted. Hallan et al.11 first pointed out this discrepancy in 2006, showing that KRT incidence in the United States was 3 times higher than in Norway, despite a similar prevalence of CKD. They suggested that faster progression to kidney failure rather than a higher number of individuals at risk likely explains this difference.11 Since then, 2 studies have investigated potential explanatory factors for differences in CKD progression across countries.12,13 Nevertheless, no overview is available of the gaps in KRT incidence between countries with their links to CKD prevalence within and across world regions, although it would help in understanding the possible sources of variation. We therefore studied the relations of CKD prevalence with the incidence of KRT for kidney failure and with all-cause and CKD-related mortality rates across countries, by region, both overall and by gender, and estimated the proportion of variance in these three latter health indicators that is explained by CKD prevalence.
Methods
Data Sources
CKD Prevalence
Crude (all ages) and age-standardized CKD prevalence rates in 2016, expressed as percentages, were extracted overall and by gender from the Global Health Data exchange, GBD Results Tool website (http://ghdx.healthdata.org/gbd-results-tool). In the GBD Study, CKD is defined by an estimated glomerular filtration rate (eGFR) <60 ml/min per 1.73 m2 or a need for KRT. Data that inform GBD estimates include microdata or tabulated data obtained from publications (systematic literature reviews), as well as other relevant data sources such as vital registrations, hospital data, and disease registries (citations available on http://ghdx.healthdata.org/gbd-2017/data-input-sources). Prevalence is estimated with a compartmental model that allows data to be incorporated for various epidemiologic indicators, such as incidence and mortality rates, through an integrative Bayesian metaregression.14 It is based on (one or multiple) individual (national representative or local) studies or other data sources within a country. When there are no available data for that country, modeling may consider information from other countries with similar epidemiologic backgrounds. Prevalence estimates were age-standardized by the GBD 2017 world standard population.14
Incidence of Kidney Replacement Therapy for Kidney Failure
Kidney replacement therapy included dialysis or pre-emptive kidney transplantation and its incidence was expressed per million population (pmp). We used data gathered by the US Renal Data System from national KRT registries worldwide that were willing to contribute data to the international comparison chapter of this report. We extracted data for the crude incidence of KRT for the year 2016, overall and by gender, from the US Renal Data System website (https://www.usrds.org/2018/view/v2_11.aspx). Age-adjusted estimates were not available.7
All-Cause Mortality
Age-standardized all-cause and CKD-related mortality rates per 100 000 population, overall and by gender, from 2016 were extracted from the World Health Organization (WHO), Global Health Estimates website (http://origin.who.int/healthinfo/global_burden_disease/estimates/en/).15 WHO 2016 mortality rates are based on the latest available national-level information about mortality and causes of death at the beginning of 2018. WHO also uses estimates from the 2016 GBD study for countries without usable vital registration data or other nationally representative sources of information for causes of death.16 CKD-related mortality included deaths from diabetes-related CKD and from CKD due to other causes. Mortality rates were age-standardized according to the WHO 2001 standard population.17
Global Burden of Disease Regional Classification System
The 61 countries or regions included in this study, because of the availability of KRT incidence, were classified into 3 regions or categories, using GBD super regions, defined by a combination of geographic location and country gross domestic product (GDP), and based on cause of death patterns (http://www.healthdata.org/terms-defined), as follows14:
-
•
High-income regions. High-income regions included those from Asia Pacific (Japan, South Korea, and Singapore), Australasia (Australia and New Zealand), Western Europe (Andorra, Austria, Belgium, Cyprus, Denmark, Finland, France, Germany, Greece, Iceland, Ireland, Israel, Italy, Luxembourg, Malta, Netherlands, Norway, Portugal, Spain, Sweden, Switzerland, and the United Kingdom), Southern Latin America (Argentina, Chile, and Uruguay), and North America (Canada and the United States).
-
•
Central and Eastern Europe. This region included Lithuania, Slovakia, Latvia, Hungary, Russia, Bulgaria, Romania, Belarus, Serbia, Albania, Bosnia and Herzegovina, Macedonia, and Ukraine.
-
•
Other regions. Other regions included those from Latin America (Jalisco [Mexico], Brazil, Peru, Colombia, and Guatemala), North Africa and Middle East (Qatar, Saudi Arabia, Kuwait, Iran, Turkey, and Jordan), South, East, and Southeast Asia (Hong Kong, Malaysia, Taiwan, Thailand, Philippines, Indonesia, and Bangladesh), and Southern sub-Saharan Africa (South Africa).
Statistical Analysis
Estimates of CKD prevalence (%), KRT incidence (pmp), and all-cause and CKD-related mortality (per 100,000 population) were tabulated and are presented as median and ranges according to region, as described above (high-income, Central and Eastern Europe, or other), both overall and by gender. To study the relations with KRT incidence, we plotted crude KRT incidence rates against crude and age-adjusted CKD prevalence, by region, overall and by gender, and for the relations with mortality risk, age-adjusted all-cause or CKD-related mortality rates against age-standardized CKD prevalence, again by region, overall and by gender. In addition, we used linear regression models to estimate the proportion of variance (R2) in KRT incidence and mortality rates explained by CKD prevalence. We tested the linearity assumption for all regression models, which was not satisfied for the analysis of the association with mortality rates and required log-transformation. Other model assumptions (normality of residuals, link function, and homoscedasticity) were checked with the Global Validation of Linear Models Assumptions “glvma” package. Analyses were performed in R software (version 3.4.2; available at http://www.R-project.org/).
Results
Geographic Variations in Age-Standardized CKD Prevalence, by Gender and Region
The age-standardized prevalence of CKD stages 3 to 5 ranged from 5.5% to 10.4% (median 6.3%) in the high-income region, 7.6% to 13.7% (median 8.7%) in Central and Eastern Europe, and 7.4% to 13.1% (median 10.7%) in the other region (Supplementary Table S1). The prevalence of CKD was higher in women than in men in all countries except Sweden (Supplementary Table S2).
Geographic Variations in KRT Incidence, by Gender and Region
Overall, KRT incidence rates were highest in Taiwan (493 pmp), the United States (378 pmp), Mexico (355 pmp), and Thailand (345 pmp) and lowest in South Africa (22 pmp), Ukraine (32 pmp), Bangladesh, and Belarus (51 pmp). The median KRT incidence was 155 pmp, ranging from 89 to 378 in the high-income region, 107 pmp ranging from 32 to 222 in Central and Eastern Europe, and 140 pmp, ranging from 22 to 493 in the other region. The incidence was higher in men than women in all countries but one, with sex ratios ranging from 1.04 in Bulgaria to 2.5 in Estonia. In Jordan, more women (70 pmp) than men (51 pmp) received treatment for KRT.
Geographic Variations in Age-Adjusted All-Cause and CKD-Related Mortality Rate, by Gender
The age-standardized all-cause mortality rate per 100,000 ranged from 299 in Japan to 555 in Argentina in the high-income region (median 360), from 525 in Estonia and Bosnia Herzegovina to 798 in Russia in Central and Eastern Europe (median 647), and from 523 in Qatar to 1213 in South Africa in the other region (median 640; Supplementary Table S3). All-cause mortality rates were higher in men than in women in all countries (Supplementary Table S4), with sex ratios ranging from 1.15 in Kuwait to 2.2 in Lithuania. The age-standardized CKD-related mortality rate ranged from 2.5 to 42.4 per 100,000 in the high-income region, from 3.5 to 17 in Central and Eastern Europe, and from 2.6 to 45.2 in the other region.
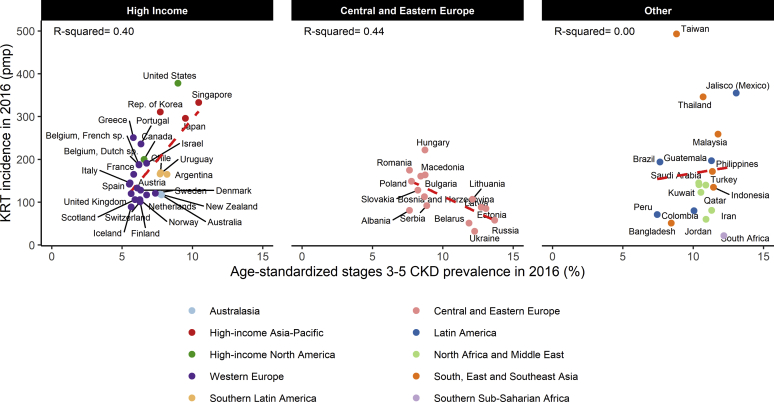
Ecologic Associations Between Age-Adjusted CKD Prevalence and KRT Incidence
In the high-income region, there was a positive and statistically significant relation between the age-adjusted prevalence of CKD and KRT incidence (Figure 1). CKD prevalence explained 40% of the variation in KRT incidence, while 60% remained unexplained. Relations were similar when crude CKD prevalence was used (Supplementary Figure S1). In a subset of the 23 (of the 28 studied) high-income countries with available KRT incidence data stratified by gender, the percentage of explained variance was slightly lower in men than women (49% vs. 56%; Supplementary Table S5). In Central and Eastern Europe, KRT incidence was negatively and statistically significantly related to both the crude and age-adjusted prevalence of CKD and did not differ by gender. In contrast, in countries from the other region, crude CKD prevalence was positively and significantly related to KRT incidence (explained variance = 37%; P = 0.007), but the relation was null with age-standardized prevalence.
Figure 1.
Incidence of kidney replacement therapy (KRT) according to age-standardized chronic kidney disease (CKD) prevalence, by region. pmp, per million population.
Although differences in KRT incidence between countries with similar CKD prevalence were usually larger across than within regions, substantial intraregional variations were also observed. Western Europe is the region with the smallest differences across countries, but KRT incidence was nonetheless 3 times higher in Greece than in Iceland despite their similar CKD prevalence. In South, East, and Southeast Asia, KRT incidence varied from 1 to 10, for small differences in CKD prevalence.
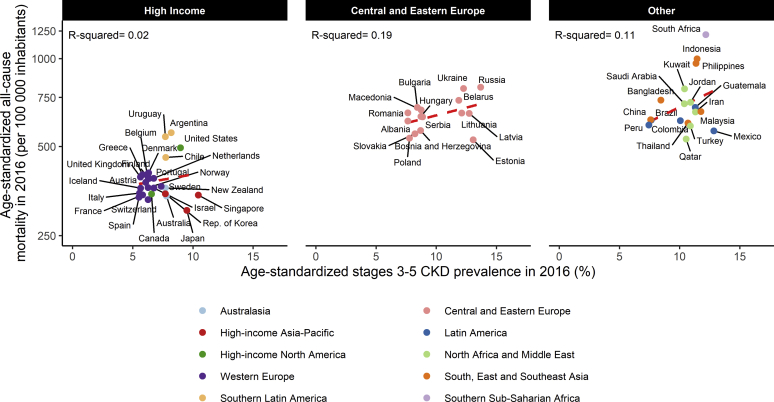
Ecologic Associations Between Age-Adjusted CKD Prevalence and Mortality Risk
In the high-income region, CKD prevalence explained a low percentage of the variation in mortality (2%), while it tended to explain a higher but still not significant percentage in Central and Eastern Europe and in the other region (Figure 2). This percentage was similar for both genders in the high-income and other regions, but differed strongly by gender, 47% in men versus 0% in women, in Central and Eastern Europe (Supplementary Table S5). Regarding CKD-related mortality, the association was positive and statistically significant (P = 0.016) in high-income countries, negative (P = 0.042) in Central and Eastern Europe, and positive but nonsignificant (P > 0.10) in the other region (Supplementary Figure S2).
Figure 2.
Age-standardized all-cause mortality according to age-standardized chronic kidney disease (CKD) prevalence, by region.
Discussion
We confirm the well-known greater gaps in KRT incidence than in CKD prevalence between countries in all the GBD regions. The main novelty of this study is to highlight the high percentage of variation in KRT incidence not explained by the prevalence of CKD, even in high-income countries (60%) where access to dialysis is not restricted. For similar CKD prevalence, KRT incidence rate can be up to 3 times higher from one country to another, as between Iceland and Greece. These results also show an unexpected negative association between these 2 kidney health indicators in Central and Eastern Europe, together with a weak but positive association of CKD prevalence with all-cause mortality. An overview of these ecologic associations we observed can help raise global awareness about the issues and challenges regarding kidney failure prevention and care.
Our results extend worldwide the observation of significant discrepancies in the KRT to CKD ratio between countries, as described previously between Norway and the US white population.11 Only a small fraction of individuals with CKD eventually progress to kidney failure, but if the variation in the number of individuals with CKD were the only explanation for that of KRT incidence, the ratio of KRT incidence to CKD prevalence would be similar across countries, and the percentage of variance explained would be 100%. In high-income countries, this percentage was 40%, which suggests that differences in the distribution of CKD risk factors play a substantial role in the variation of kidney failure incidence between countries. Nonetheless, although a meta-analysis of 19 general population-based studies from 13 European countries did find variations in CKD prevalence across these countries, differences in major CKD risk factors, including diabetes, hypertension, and obesity, did not appear to explain them well.6,8,18, 19, 20 The negative relation or lack of association observed in Central and Eastern Europe and in the other region, in contrast, cannot be interpreted as the absence of an impact of CKD risk factors, especially given that CKD prevalence is higher in these regions than in high-income countries.6,8,18, 19, 20 In Central and Eastern Europe, the association of higher CKD prevalence with lower KRT incidence together with higher all-cause mortality may instead reflect the combination, at the population level, of poor population health with a health care system with inadequate capacity. Several CKD risk factors are well established, including genetic, socioeconomic, and environmental risk factors, as well as poor maternal and fetal health. A landmark article reporting the conclusions of an International Society of Nephrology CKD summit on Global Kidney Health in 2016 updated these factors, which are summarized in Table 1.1,21 These risk factors might be attenuated by promoting healthy lifestyles, improved education of the public and of health practitioners, and strategies to improve maternal and fetal health.
Table 1.
Potential sources of international variations in the relation between chronic kidney disease prevalence and the risk to progress to kidney failure requiring kidney replacement therapy
| Variation in methods to estimate CKD prevalence |
|
| Variations in life expectancy and distribution of CKD risk factors |
Life expectancy
|
| Variation in risk factors for CKD progression (GFR decline) to kidney failure |
|
| Variations in access to KRT |
|
ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; CKD, chronic kidney disease; GDP, gross domestic product; GFR, glomerular filtration rate; KRT, kidney replacement therapy.
Although heterogeneity in the prevalence of CKD risk factors explains part of the variance in KRT incidence, this study shows that a significant part of this variance remains unexplained. Several hypotheses can be generated, including variations across countries in risk factors for CKD progression or in access to KRT (Table 1). Hallan et al.11 first suggested that slower progression to kidney failure rather than a smaller number of individuals could account for the lower incidence of KRT in Norway than in the United States. Two recent meta-analyses of CKD cohort studies have investigated this hypothesis with individual-level data.12,13 The European CKD Burden Consortium, which used a joint model to compare estimated eGFR decline and mortality risk across 9 cohorts from 5 European countries, showed that baseline patient profiles and CKD progression rates differed substantially between studies.12,13 These variations in CKD progression, however, persisted despite adjustment for baseline eGFR, age, sex, albuminuria, comorbidities, and use of renin-angiotensin system inhibitors. The international network of CKD cohort has also observed different baseline profiles and highly heterogeneous risks of CKD progression, KRT incidence, and mortality among individuals with CKD participating in 8 cohort studies around the globe.12,13 This study also observed that adjustment for several patient characteristics and laboratory markers did not substantially attenuate the differences in outcomes. Its finding that the magnitude of the associations of individual-level predictors with clinical outcomes varied substantially across studies suggests that the setting or differences in populations may influence these differences. Another international network of CKD cohorts study finding of considerable heterogeneity in blood pressure control and antihypertensive prescription patterns in CKD worldwide also indicates that clinical practices may have an effect.22,23
Finally, because kidney failure incidence is assessed from dialysis and transplantation registries, factors linked to the management of transition to kidney failure or access to KRT may play a major role in the gaps observed between countries (Table 1). It is well-known that access to KRT is influenced by local policies and that several countries, such as the United States, Japan, South Korea, and Taiwan, have liberal criteria for acceptance on dialysis, while many European countries, Australia, and New Zealand have a less liberal acceptance policy and most African, Asian, and Central or Eastern European countries have limited access. An ecologic study of 46 countries reported that GDP per capita and percentage of GDP spent on health care were independently associated with KRT incidence.3,4,10,13,24 It also showed that in more developed countries, the private for-profit share of hemodialysis facilities was also associated with higher KRT incidence. This study and ours raise the issue of overtreatment related to early-start dialysis in countries with extremely high KRT incidence. In France, eGFR level at dialysis start explained a substantial part of the variations in KRT incidence between regions.25 Variations in the availability of conservative therapy may also have an impact in the elderly population, as suggested by the Global Kidney Health Atlas study.10,26 Overall, these meta-analyses highlight the complexity of the relations between CKD and KRT risk and the need for further investigation.
This study has several strengths including the large number of countries spread over the different GBD super regions with standardized estimates of kidney health indicators. Another strength is that we were able to assess the relations between CKD prevalence and KRT incidence by gender in many of these countries. We also looked at the relation between CKD prevalence and all-cause and CKD-related mortality risk, a major competing risk for KRT in CKD.
Our study also has a number of limitations. First, it is an ecologic study, and the associations observed at the country level may be different at the individual level. Further studies comparing the relationship of CKD to kidney failure risk at the individual level are needed to identify potentially modifiable risk factors and racial disparities. Second, the use of KRT registry data did not enable us to describe the association of CKD prevalence with kidney failure risk because KRT registries cannot capture patients who progress to kidney failure but do not have access to KRT, refuse dialysis, or receive conservative care. Moreover, age-standardized KRT incidence was not available. Third, CKD prevalence may have been affected by the use of different creatinine assays or GFR estimation equations across studies,6,8,18, 19, 20 although the GBD correction methods should have reduced these disparities by standardizing these estimates. Fourth, the available reference population for age standardization was different for the WHO and the GBD statistics. However, while this may have had an impact on mortality rate and CKD prevalence estimates, it should not have affected the associations we observed. Finally, the aggregation of countries with diverse CKD epidemiology in one single “other” group may be controversial, but the small number of these countries precluded a more detailed stratification.
In conclusion, this ecologic study highlights the large proportion of the global variation in KRT incidence that is apparently not explained by the number of individuals with CKD. While differences in health care resources are an obvious explanation of disparities in access to KRT, at least in part, in low- and middle-income countries, other hypotheses should be assessed for high-income countries, such as variations in clinical practice patterns intended to slow CKD progression or to manage the transition to KRT.27,28 The issue of dialysis overtreatment is scarcely discussed and may receive greater attention from both the patients and public health perspective. International comparisons at both patient and provider levels are necessary to investigate the relative impact of individual characteristics, physician practices, and local policies on the incidence of KRT.
Disclosure
All the authors declared no competing interests.
Acknowledgments
MHCvR was supported by grants from the Radboud Institute for Health Sciences and the Dutch Kidney Foundation (150KK109). We thank Joe Coresh for his helpful comment in this study and Jo-Ann Cahn for her editorial assistance.
Footnotes
Table S1. Age-adjusted chronic kidney disease prevalence and kidney replacement therapy incidence, by region (61 countries).
Table S2. Age-adjusted chronic kidney disease prevalence and kidney replacement therapy incidence, by gender and by region (41 countries).
Table S3. Age-adjusted chronic kidney disease prevalence and all-cause and kidney disease–related mortality rates (59 countries).
Table S4. Age-adjusted CKD prevalence and adjusted all-cause mortality, by gender and region (59 countries).
Table S5. Linear regression estimates for the association between age-adjusted chronic kidney disease prevalence and (A) kidney replacement therapy incidence or (B) age-adjusted mortality rates, by gender and by region.
Figure S1. Incidence of kidney replacement therapy according to crude chronic kidney disease prevalence, by region
Figure S2. Age-standardized chronic kidney disease–related mortality according to age-standardized chronic kidney disease prevalence, by region.
Supplementary Material
References
- 1.Jha V., Garcia-Garcia G., Iseki K. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 2.Nitsch D., Grams M., Sang Y. Associations of estimated glomerular filtration rate and albuminuria with mortality and renal failure by sex: a meta-analysis. BMJ. 2013;346:f324. doi: 10.1136/bmj.f324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas B., Matsushita K., Abate K.H. Global cardiovascular and renal outcomes of reduced GFR. J Am Soc Nephrol. 2017;28:2167–2179. doi: 10.1681/ASN.2016050562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caskey F.J., Jager K.J. A population approach to renal replacement therapy epidemiology: lessons from the EVEREST study. Nephrol Dial Transplant. 2014;29:1494–1499. doi: 10.1093/ndt/gft390. [DOI] [PubMed] [Google Scholar]
- 5.Liyanage T., Ninomiya T., Jha V. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385:1975–1982. doi: 10.1016/S0140-6736(14)61601-9. [DOI] [PubMed] [Google Scholar]
- 6.Hill N.R., Fatoba S.T., Oke J.L. Global prevalence of chronic kidney disease - a systematic review and meta-analysis. PloS One. 2016;11 doi: 10.1371/journal.pone.0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saran R., Robinson B., Abbott K.C. US Renal Data System 2018 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2019;73(suppl 1):S1–S772. doi: 10.1053/j.ajkd.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mills K.T., Xu Y., Zhang W. A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int. 2015;88:950–957. doi: 10.1038/ki.2015.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bikbov B., Perico N., Remuzzi G. on behalf of the GBDGDEG. Disparities in chronic kidney disease prevalence among males and females in 195 countries: analysis of the Global Burden of Disease 2016 Study. Nephron. 2018;139:313–318. doi: 10.1159/000489897. [DOI] [PubMed] [Google Scholar]
- 10.Bello A.K., Levin A., Lunney M. Status of care for end stage kidney disease in countries and regions worldwide: international cross sectional survey. BMJ. 2019;367:l5873. doi: 10.1136/bmj.l5873. [DOI] [PubMed] [Google Scholar]
- 11.Hallan S.I., Coresh J., Astor B.C. International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. J Am Soc Nephrol. 2006;17:2275–2284. doi: 10.1681/ASN.2005121273. [DOI] [PubMed] [Google Scholar]
- 12.Bruck K., Jager K.J., Zoccali C. Different rates of progression and mortality in patients with chronic kidney disease at outpatient nephrology clinics across Europe. Kidney Int. 2018;93:1432–1441. doi: 10.1016/j.kint.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Orlandi P.F., Huang J., Fukagawa M. A collaborative, individual-level analysis compared longitudinal outcomes across the International Network of Chronic Kidney Disease (iNETCKD) cohorts. Kidney Int. 2019;96:1217–1233. doi: 10.1016/j.kint.2019.07.024. [DOI] [PubMed] [Google Scholar]
- 14.Global Burden of Disease Injury, Incidence Prevalence, Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Global Health Estimates 2016 . World Health Organization; Geneva, Switzerland: 2018. Disease burden by cause A, sex, by country and by region, 2000-2016. [Google Scholar]
- 16.World Health Organization website WHO methods and data sources for global causes of death 2000-2016. https://www.who.int/healthinfo/global_burden_disease/GlobalCOD_method_2000-2016.pdf Available at:
- 17.Ahmad O.B., Boschi-Pinto C., Lopez A.D. Age standardization of rates: a new WHO standard. https://www.who.int/healthinfo/paper31.pdf Available at:
- 18.Brück K., Stel V.S., Gambaro G. CKD prevalence varies across the European general population. J Am Soc Nephrol. 2016;27:2135–2147. doi: 10.1681/ASN.2015050542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stel V.S., Brück K., Fraser S. International differences in chronic kidney disease prevalence: a key public health and epidemiologic research issue. Nephrol Dial Transplant. 2017;32(suppl 2) doi: 10.1093/ndt/gfw420. ii129–ii35. [DOI] [PubMed] [Google Scholar]
- 20.De Nicola L., Zoccali C. Chronic kidney disease prevalence in the general population: heterogeneity and concerns. Nephrol Dial Transplant. 2016;31:331–335. doi: 10.1093/ndt/gfv427. [DOI] [PubMed] [Google Scholar]
- 21.Levin A., Tonelli M., Bonventre J. Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. Lancet. 2017;390:1888–1917. doi: 10.1016/S0140-6736(17)30788-2. [DOI] [PubMed] [Google Scholar]
- 22.Pecoits-Filho R., Fliser D., Tu C. Prescription of renin-angiotensin-aldosterone system inhibitors (RAASi) and its determinants in patients with advanced CKD under nephrologist care. J Clin Hypertens (Greenwich) 2019;21:991–1001. doi: 10.1111/jch.13563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alencar de Pinho N., Levin A., Fukagawa M. Considerable international variation exists in blood pressure control and antihypertensive prescription patterns in chronic kidney disease. Kidney Int. 2019;96:983–994. doi: 10.1016/j.kint.2019.04.032. [DOI] [PubMed] [Google Scholar]
- 24.Caskey F.J., Kramer A., Elliott R.F. Global variation in renal replacement therapy for end-stage renal disease. Nephrol Dial Transplant. 2011;26:2604–2610. doi: 10.1093/ndt/gfq781. [DOI] [PubMed] [Google Scholar]
- 25.Couchoud C., Guihenneuc C., Bayer F. The timing of dialysis initiation affects the incidence of renal replacement therapy. Nephrol Dial Transplant. 2010;25:1576–1578. doi: 10.1093/ndt/gfp675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davison S.N., Levin A., Moss A.H. Executive summary of the KDIGO Controversies Conference on Supportive Care in Chronic Kidney Disease: developing a roadmap to improving quality care. Kidney Int. 2015;88:447–459. doi: 10.1038/ki.2015.110. [DOI] [PubMed] [Google Scholar]
- 27.Dienemann T., Fujii N., Orlandi P. International Network of Chronic Kidney Disease cohort studies (iNET-CKD): a global network of chronic kidney disease cohorts. BMC Nephrol. 2016;17:121. doi: 10.1186/s12882-016-0335-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mariani L., Stengel B., Combe C. The CKD Outcomes and Practice Patterns Study (CKDopps): rationale and methods. Am J Kidney Dis. 2016;68:402–413. doi: 10.1053/j.ajkd.2016.03.414. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.