Abstract
Introduction
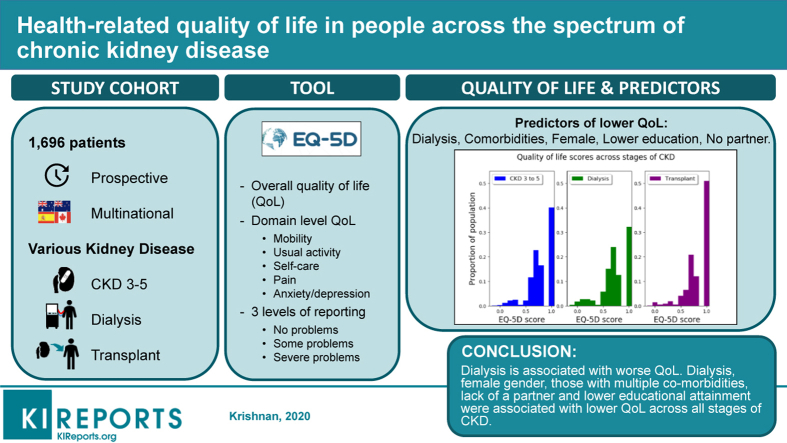
People with chronic kidney disease (CKD) experience reduced quality of life (QoL) because of the high symptom and treatment burden. Limited data exist on the factors associated with overall and domain-specific QoL across all CKD stages.
Methods
Using data from a prospective, multinational study (Australia, New Zealand, Canada, and Spain) in 1696 participants with CKD, we measured overall and domain-specific QoL (pain, self-care, activity, mobility, anxiety/depression) using the EuroQoL, 5 dimension, 3 level. Multivariable linear regression and logistic modeling were used to determine factors associated with overall and domain-specific QoL.
Results
QoL for patients with CKD stages 3 to 5 (n = 787; mean, 0.81; SD, 0.20) was higher than in patients on dialysis (n = 415; mean, 0.76; SD, 0.24) but lower than in kidney transplant recipients (n = 494; mean, 0.84; SD, 0.21). Factors associated with reduced overall QoL (β [95% confidence intervals]) included being on dialysis (compared with CKD stages 3–5: –0.06 [–0.08 to –0.03]), female sex (–0.03 [–0.05 to –0.006]), lower educational attainment (– 0.04 [–0.06 to –0.02), lacking a partner (–0.04 [–0.06 to –0.02]), having diabetes (–0.05 [–0.07 to –0.02]), history of stroke (–0.09 [–0.13 to –0.05]), cardiovascular disease (–0.06 [–0.08 to –0.03]), and cancer (–0.03 [–0.06 to –0.009]). Pain (43%) and anxiety/depression (30%) were the most commonly affected domains, with dialysis patients reporting decrements in all 5 domains. Predictors for domain-specific QoL included being on dialysis, presence of comorbidities, lower education, female sex, and lack of a partner.
Conclusions
Being on dialysis, women with CKD, those with multiple comorbidities, lack of a partner, and lower educational attainment were associated with lower QoL across all stages of CKD.
Keywords: chronic kidney disease, domain-specific QoL, EuroQoL-5D3L, quality of life
Graphical abstract
People with CKD have an increased risk of mortality. The excess risk extends from 1.2 times for patients with CKD not requiring kidney replacement therapy to over 100-fold among those with end-stage kidney disease compared with the general population.1 The impact of impaired kidney function on overall QoL is also profound, particularly among those with advanced kidney disease.1 Compared with other chronic conditions, utility-based QoL is lowest for patients with end-stage kidney disease, with estimates even lower than in patients with cancer or heart failure.2, 3, 4 Although kidney transplant recipients may experience improved QoL compared with those on dialysis,2 their overall QoL remains lower than the general population.2,5
Previous studies of QoL assessment in CKD have generally been small, and most have not provided details of domain-specific QoL data.2 Few have examined the factors associated with domain-specific QoL in patients with CKD6,7 and none across the entire spectrum of CKD. Knowledge of the contributing factors that negatively influence health-related QoL (HR-QoL) is important because those factors will inform the development and delivery of interventions targeting modifiable factors in patients with CKD. The purpose of this study was to compare the overall and domain-specific QoL in a large cohort of patients in different stages of CKD and to determine the factors associated with overall and domain-specific QoL in patients with CKD (stages 3–5), on dialysis and after kidney transplantation.
Methods
Study Population
This is a substudy of the DETECT (Detecting Bowel Cancer in CKD) study. The DETECT study is a prospective, multicenter, cohort study of 1706 patients that aimed to determine the consequences of a 1-time screening for advanced colorectal neoplasia using fecal immunohistochemistry test across a broad spectrum of CKD stages.8 We screened patients with CKD (stages 3–5 [not on kidney replacement therapy], dialysis and transplant recipients) aged between 35 and 74 years, for study eligibility and enrolled participants at 11 sites across Australia, New Zealand, Spain, and Canada including hospital, academic, and private practice clinics. The initial enrollment period for the cohort was from June 2010 to November 2015 with biennial follow-up for up to 4 years after the initial recruitment. QoL data were collected from all enrolled participants at baseline using the EuroQol 5 domain, 3 level (EQ-5D-3L) multiattribute utility measure before performing the fecal immunohistochemistry test.
The study protocol9 was approved by the Human and Research Ethics Committee of all participating centers (Westmead, Gosford, Royal North Shore, Blacktown, Nepean, Sir Charles Gairdner, Concord, Royal Prince Alfred, Toronto General, Christchurch Hospitals, and the Hospital Clinic of Barcelona). All participants provided written informed consent.
Covariates of Interest
Demographic details were obtained from each patient at the time of enrollment, and prespecified clinical data were extracted from the healthcare records. Demographic data included age, gender, ethnicity, education level, and marital status. Higher education status was defined as those who attended college/university, whereas lower education status was defined as attending high school or below. Clinical information included CKD stage, comorbidities (diabetes mellitus, cardiovascular disease, and cerebrovascular disease), type of kidney replacement therapy, a personal or family history of cancer, and medications (including immunosuppressants, antiplatelets, anticoagulants, antihypertensives, hypoglycemics, antidepressants, iron replacement therapy, and erythropoiesis-stimulating agents). Glomerular filtration rate for stages 3 to 5 CKD patients and kidney transplant recipients was estimated using the Chronic Kidney Disease Epidemiology Collaboration formula.10
Outcomes of Interest
The EQ-5D-3L multiattribute utility instrument was used to assess the baseline QoL of all participants before bowel cancer screening with the fecal immunochemical test and was administered in English and Spanish. The validity and reliability of the EQ-5D instrument has been previously established in both general and disease-specific populations,11 including adults with CKD.2,6,12, 13, 14
The EQ-5D-3L questionnaire assesses the 5 individual domains of mobility, self-care, usual activities, pain, and anxiety/depression at 3 response categories: no problems, some problems, and extreme problems.15 For example, mobility (walking) may be described as having no problems, some problems, or a lot of problems walking. The combination of all possible levels and dimensions results in 243 unique health states. The EQ-5D levels are then converted into a single summary utility value by applying a formula that attaches the weights to each reported level in each domain. Scoring is based on an Australian value set, which results in utility values over the range of –0.217 (worse than dead) to 1.0 (perfect health).16 Because it is a multiattribute utility measure, the EQ-5D-3L provides utility weights; however, we use the term “QoL score” throughout for simplicity. Domain levels were also dichotomized into those with “any problems” and those with “no problems” based on the response categories of the instrument.
Statistical Analysis
Baseline demographic data with continuous distributions were presented as means with SDs or medians with interquartile ranges (IQRs) for normally and non-normally distributed variables, respectively. Characteristics with binary distributions were reported as counts and percentages. QoL scores were expressed both as medians (IQRs) and means (SDs) to enable comparison with other studies and for use in future health economic evaluations.
Given the skewed distribution of the QoL scores, comparisons by CKD stage were made using the Mann-Whitney U test. Comparisons between the EQ-5D-3L domain levels and CKD stages were performed using χ2 tests.
Multiple linear regression models assessed the risk factors for changes in overall QoL scores, whereas logistic regression models examined the risk factors for reported problems in the individual domain levels. Covariates with P < 0.25 in the unadjusted association for QoL scores were included in the multivariable analyses. Several 2-way interactions between baseline characteristics and CKD stages were tested. The final model retained the covariates that remained significant after adjustment using a backward stepwise strategy. The same approach was undertaken for the logistic regression models. A random effects model was used to assess variation by country. Data were processed and scored using SPSS v25 (IBM North America, New York, NY). Data analyses were performed using SPSS v25, R, and SAS 9.4 (SAS Institute, Cary, NC), whereas Python was used to create graphs. P < 0.05 was considered statistically significant.
Results
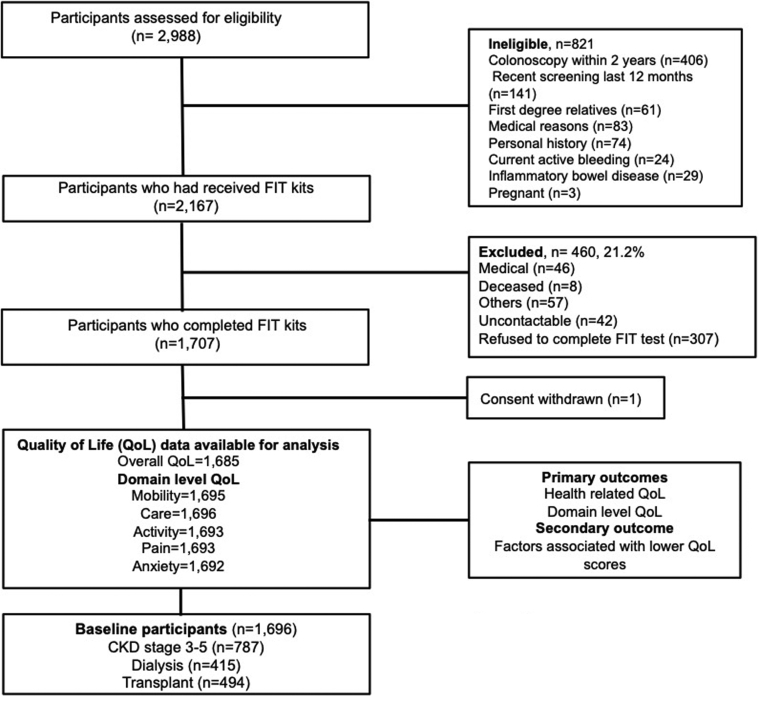
The inclusion criteria and screening protocol for this study have been described previously.8 Of the 2988 patients with CKD who were screened, 2167 (72.5%) were eligible. The 460 (21.2%) who were ineligible did not undergo screening because of death, refusal, or medical reasons including hospitalization after screening kits were given. Overall, 1707 patients (78.8%) completed the fecal immunohistochemistry test. One withdrew consent after screening, and 21 participants had missing QoL data, leaving 1685 participants with overall QoL scores, whereas domain-level responses were available in up to 1696 participants (Figure 1).
Figure 1.
Participant flowchart. ∗Sites, 11 centers across Australia, New Zealand, Canada, and Spain. CKD, chronic kidney disease; FIT, fecal immunohistochemistry test.
Baseline Characteristics of the Study Cohort
Of the 1696 participants, 787 (46.4%) had CKD stages 3 to 5, 415 (24.5%) were on dialysis, and 494 (29.1%) were kidney transplant recipients. Overall, 1027 (60.5%) were men, with a median age of 59.9 years. Most were white (n = 1208, 71.2%), and most (n = 1,523, 89.8%) had at least 1 comorbidity (diabetes mellitus, cardiovascular, or cerebrovascular disease). Three hundred fifty patients (20.6%) had a history of prior cancer (skin and nonskin cancers), and 892 (52.6%) were either ex- or current smokers. Of the 415 patients receiving dialysis, 69.9% were maintained on hemodialysis and 30.1% were maintained on peritoneal dialysis; 62.2% of all kidney transplant recipients received deceased donor kidneys. Seven hundred fourteen participants (42.1%) had a higher education, and 1217 (71.8%) were married or partnered (Table 1).
Table 1.
Baseline characteristics
| Characteristics | CKD 3–5 (n = 787, 46.4%) | Dialysis (n = 415, 24.4%) | Transplant (n = 494, 29.1%) | All (n = 1696) |
|---|---|---|---|---|
| Age, yr | ||||
| 35–49 | 108 (13.7) | 76 (18.3) | 166 (33.6) | 350 (20.6) |
| 50–64 | 283 (36.0) | 234 (56.4) | 255 (51.6) | 772 (45.5) |
| ≥65 | 396 (50.3) | 105 (25.3) | 73 (14.8) | 574 (33.8) |
| Sex | ||||
| Female | 335 (42.6) | 152 (36.6) | 182 (37.0) | 669 (39.4) |
| Male | 452 (57.4) | 263 (63.4) | 312 (63.1) | 1027 (60.5) |
| Race or ethnic groups | ||||
| White | 543 (69.0) | 275 (66.3) | 389 (78.7) | 1207 (71.2) |
| Asian | 81 (10.3) | 60 (14.5) | 44 (8.9) | 185 (10.9) |
| Middle eastern | 30 (3.8) | 16 (3.9) | 18 (3.6) | 64 (3.8) |
| Aboriginal/Torres Strait Islander/Maori/Pacific Islanders | 18 (2.3) | 21 (5.1) | 13 (2.6) | 52 (3.1) |
| Others | 115 (14.6) | 43 (10.1) | 30 (6.1) | 188 (11.1) |
| Education level | ||||
| High school graduate or less | 316 (40.2) | 159 (38.3) | 249 (48.4) | 724 (42.6) |
| College/university | 457 (58.1) | 244 (58.8) | 239 (50.4) | 940 (55.4) |
| Unknown | 14 (1.8) | 12 (2.9) | 6 (1.2) | 32 (1.9) |
| Marital status | ||||
| Married/partnered | 560 (71.2) | 276 (66.5) | 370 (74.8) | 1217 (71.8) |
| Single/divorced/widowed | 225 (28.5) | 136 (32.8) | 121 (24.4) | 471 (27.8) |
| Unknown | 2 (0.3) | 3 (0.7) | 3 (0.8) | 8 (0.4) |
| Smoking status | ||||
| Current | 81 (10.3) | 26 (6.2) | 17 (3.4) | 124 (7.3) |
| Ex-smoker | 357 (45.4) | 197 (47.5) | 214 (43.3) | 768 (45.2) |
| Never | 339 (43.1) | 185 (44.6) | 255 (51.6) | 779 (45.9) |
| Unknown | 10 (1.3) | 7 (1.7) | 8 (1.6) | 25 (1.5) |
| Diabetes mellitus | ||||
| Yes | 317 (40.3) | 149 (35.9) | 109 (22.1) | 575 (33.9) |
| No | 470 (59.7) | 266 (64.1) | 385 (77.9) | 1121 (66.0) |
| Cardiovascular disease | ||||
| Yes | 207 (26.3) | 112 (27.0) | 72 (14.6) | 391 (23.1) |
| No | 580 (73.7) | 303 (73.0) | 422 (85.4) | 1305 (76.9) |
| Cerebrovascular disease | ||||
| Yes | 58 (7.3) | 29 (7.0) | 23 (4.7) | 110 (6.4) |
| No | 729 (92.6) | 386 (93.0) | 471 (95.3) | 1586 (93.5) |
| Body mass index, kg/m2 | ||||
| <20 | 20 (2.5) | 32 (7.7) | 18 (3.6) | 70 (4.1) |
| 20.1–25 | 156 (19.8) | 127 (30.6) | 158 (32.0) | 441 (26.0) |
| 25.1–30 | 277 (35.2) | 127 (30.6) | 179 (36.2) | 583 (34.4) |
| >30 | 301 (38.2) | 108 (26.0) | 123 (24.9) | 532 (31.4) |
| Unknown | 33 (4.2) | 21 (5.1) | 16 (3.2) | 70 (4.1) |
| Prior cancer | ||||
| Yes | 149 (18.9) | 82 (20.0) | 118 (23.9) | 349 (20.5) |
| No | 638 (81.1) | 332 (80.0) | 376 (76.1) | 1346 (79.4) |
| Prior lower gastrointestinal endoscopy | ||||
| Yes | 281 (35.7) | 111 (26.7) | 134 (27.1) | 526 (31.0) |
| No | 502 (63.8) | 304 (73.3) | 360 (72.9) | 1166 (68.8) |
| Unknown | 4 (0.5) | 0 | 0 | 4 (0.2) |
| Daily use of antiplatelet agents | ||||
| Yes | 239 (30.4) | 147 (35.4) | 138 (27.9) | 524 (30.9) |
| No | 548 (69.6) | 268 (64.6) | 356 (72.1) | 1172 (69.1) |
| Daily use of anticoagulation | ||||
| Yes | 67 (8.5) | 65 (15.7) | 26 (5.3) | 158 (9.3) |
| No | 720 (91.5) | 350 (84.3) | 468 (94.7) | 1538 (90.7) |
| Types of dialysis | ||||
| Hemodialysis | NA | 290 (69.9) | NA | 290 (69.9) |
| Peritoneal dialysis | NA | 125 (30.1) | NA | 125 (30.1) |
| Donor types | ||||
| Deceased | NA | NA | 308 (62.3) | 308 (62.3) |
| Living | NA | NA | 186 (37.7) | 186 (37.7) |
| Daily immunosuppression use | ||||
| Prednisone | 40 (5.1) | 29 (7.0) | 448 (90.7) | 517 (30.5) |
| Azathioprine | 9 (1.1) | 4 (1.0) | 52 (10.5) | 65 (3.8) |
| Mycophenolate mofetil | 9 (1.1) | 3 (0.7) | 381 (77.1) | 393 (23.2) |
| Tacrolimus | 1 (0.1) | 3 (0.7) | 303 (61.3) | 3097(18.1) |
| Cyclosporine | 5 (0.6) | 1 (0.2) | 104 (21.1) | 110 (6.5) |
| Regular use of erythropoiesis-stimulating agents | ||||
| Yes | 38 (4.8) | 201 (48.4) | 31 (6.3) | 270 (15.9) |
| No | 749 (95.2) | 214 (51.6) | 463 (93.7) | 1426 (84.1) |
CKD, chronic kidney disease; NA, not applicable.
Values are n (%).
Overall HR-QoL Score by CKD Stage
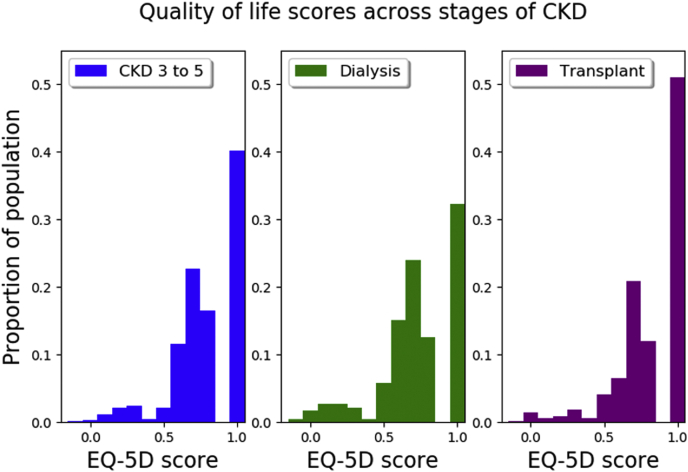
Most of the cohort (n = 987, 58.2%) reported decrements in QoL (EQ-5D-3L overall score < 1). The overall mean QoL score was 0.80 (SD, 0.22), and the median QoL score was 0.83 (IQR, 0.70–1.00). QoL scores for participants on dialysis (median, 0.79; IQR, 0.68–1.00; mean [SD], 0.76 [0.20]) were lower compared with kidney transplant recipients (median, 1.00; IQR, 0.73–1.00; P < 0.001; mean [SD], 0.84 [0.21]) and participants with CKD stages 3 to 5 (median, 0.83; IQR, 0.71–1.00; P < 0.001; mean [SD], 0.81 [0.20]) (Figure 2, Table 2). Compared with those with CKD stages 3 to 5, a greater proportion of participants on dialysis experienced any decrement from perfect health (CKD stages 3–5 vs. dialysis: 59.5% vs. 67.7%, P = 0.005), whereas a lower proportion of transplant recipients experienced a decrement from perfect heath (CKD stages 3–5 vs. transplant: 59.5% vs. 49.4%, P < 0.001). A sensitivity analysis comparing the QoL of the Spanish participants using normative Spanish EQ-5D data was conducted and found similar results to the Australian set (Supplementary Sensitivity Analysis).
Figure 2.
Quality of life in participants with chronic kidney disease (CKD), stratified by stage. EQ-5D, EuroQoL, 5 dimension.
Table 2.
Summary quality of life scores
| Summary statistics | CKD stages 3–5 (n = 787) | Dialysis (n = 415) | Transplant (n = 494) |
|---|---|---|---|
| Median (interquartile range) | 0.83 (0.71–1.00) | 0.79 (0.68–1.00) | 1.00 (0.73–1.00) |
| Mean (SD) | 0.81 (0.20) | 0.76 (0.24) | 0.84 (0.21) |
| P valuea | <0.001 | 0.001 |
CKD, chronic kidney disease.
Data are missing for 21 participants.
Compared with CKD stages 3–5 by the Mann-Whitney U test.
Domain-Specific QoL by CKD Stage
A large proportion of participants experienced pain (43%), 29% experienced anxiety/depression, 29% reported decreased mobility, and 28% reported problems with performing daily activity (Table 3). Only 9% of the participants reported any deficit in the domain of self-care. Compared with participants with CKD stages 3 to 5, a greater proportion of participants receiving dialysis reported any problems with self-care (7% vs. 17%, P < 0.001), usual activities (26% vs. 40%, P < 0.001), and anxiety/depression (27% vs. 39%, P < 0.001). Transplant recipients were less likely to experience pain (36% vs. 45%, P = 0.002) or impairments in mobility (22% vs. 31%, P = 0.001) and usual activities (21% vs. 26%, P = 0.05) compared with those with CKD stages 3 to 5, but there were no differences in the domains of self-care (P = 0.99) and anxiety/depression (P = 0.65) (Supplementary Figure S1). Compared with kidney transplant recipients, a greater proportion of participants receiving dialysis reported problems in all 5 domains: mobility (35% vs. 22%, P < 0.001), self-care (17% vs. 7%, P < 0.001), activity (40% vs. 21%, P < 0.001), pain (48% vs. 36%, P < 0.001), and anxiety/depression (39% vs. 26%, P < 0.001).
Table 3.
Proportion of participants reporting decrements in each domain-specific area, stratified by chronic kidney disease stage
| Domain | CKD stages 3–5 (n = 787) | Dialysis (n = 415) | Pa | Transplant (n = 494) | Pa | All (n = 1696) |
|---|---|---|---|---|---|---|
| Mobility | 243 (31) | 146 (35) | 0.13 | 109 (22) | 0.001 | 498 (29) |
| Self-care | 54 (7) | 69 (17) | <0.001 | 34 (7) | 0.99 | 157 (9) |
| Activity | 207 (26) | 165 (40) | <0.001 | 106 (21) | 0.05 | 478 (28) |
| Pain | 354 (45) | 198 (48) | 0.33 | 179 (36) | 0.002 | 731 (43) |
| Anxiety/depression | 214 (27) | 160 (39) | <0.001 | 129 (26) | 0.65 | 503 (29) |
CKD, chronic kidney disease.
Values are n (%). Number of missing data: overall health-related quality of life = 21, mobility = 11, self-care = 10, activity = 13, pain = 13, anxiety/depression = 14.
Compared with CKD stages 3–5 using the χ2 test.
Factors Associated With Overall HR-QoL Across All CKD Stages
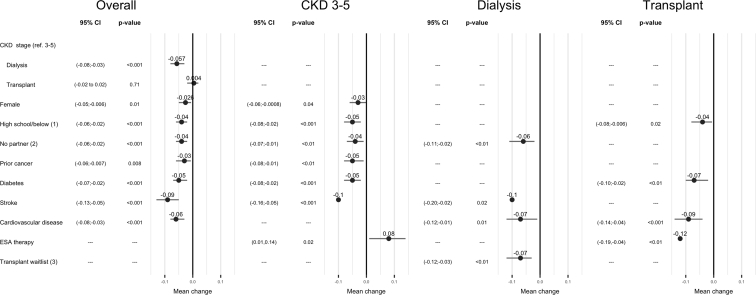
The adjusted risk factors associated with reduced overall HR-QoL (β [95% confidence interval]) included being on dialysis (compared with CKD stages 3–5: –0.06 [–0.08 to –0.03]), female sex (–0.03 [–0.05, –0.006]), lower educational attainment (–0.04 [–0.06, –0.02]), and lack of a partner (compared with having a spouse/partner: –0.04 [–0.06, –0.02]). Comorbidities were also associated with lower QoL: diabetes (–0.05 [–0.07, –0.02]), stroke (– 0.09 [–0.13, –0.05]), cardiovascular disease (– 0.06 [–0.08, –0.03]), and history of prior cancer (– 0.03 [–0.06, –0.009]) (Figure 3). Univariate analysis of the predictors of QoL are shown in Supplementary Table 1. There was no interaction between CKD stages and other factors including sex and marital and education status.
Figure 3.
Forest plot of overall quality of life (QoL) and QoL by chronic kidney disease (CKD) stage. ∗Multivariate-adjusted model; 1, compared with college/university; 2, compared with married/partnered; 3, compared with waitlisted; CI, confidence interval; ESA, erythropoiesis-stimulating agent.
Factors Associated With Reduced HR-QoL Stratified by CKD Stage
The presence of coexisting comorbidities was the key predictor for reduced QoL across all CKD stages. Although lack of a partner was associated with worse QoL in those with CKD stages 3 to 5 and on dialysis, lower education attainment was associated with worse QoL in transplant recipients. Dialysis participants on the transplant waitlist experienced better QoL compared with unlisted participants. Additionally, for patients with CKD stages 3 to 5, the use of erythropoiesis-stimulating agents was associated with better QoL but was associated with worse QoL in kidney transplant recipients (Figure 3).
Factors Associated With Decrements in Domain-Specific QoL
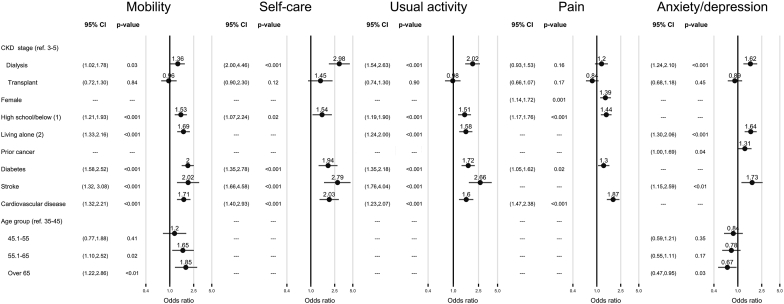
Being on dialysis was associated with reduced mobility (adjusted odds ratio [95% confidence interval]): 1.36 [1.02–1.78]), problems with self-care (2.98 [2.00–4.46]), problems with usual activities (2.02 [1.54–2.63]), and worse anxiety/depression (1.62 [1.24–2.10]) when compared with CKD stages 3 to 5 (Figure 4). Similar to overall QoL, the key determinants associated with the QoL domains of reduced mobility, problems with self-care, and usual activities were a lower education attainment, lack of a partner, or comorbid conditions such as diabetes, stroke, or cardiovascular disease. Women were also more likely to experience pain than men. Although advancing age was associated with poor mobility, it was inversely associated with anxiety/depression.
Figure 4.
Forest plot of domain-level quality of life. ∗Multivariate-adjusted model; 1, compared with college/university; 2, compared with married/partnered; CI, confidence interval; CKD, chronic kidney disease.
Discussion
In this large study of 1696 participants from 4 countries and 11 centers and across the spectrum of CKD from stages 3 to 5 and dialysis and transplant recipients, overall, we found a considerable decrement in QoL. Consistent with previous studies,2 being on dialysis has the greatest impact on overall QoL. The 3 key domains that were commonly affected were pain, anxiety/depression, and mobility, with dialysis patients reporting more problems in almost all domains compared with other CKD stages. The most striking finding was that the relative decrements in the utility-based QoL were similar across patients of female sex, lower education status, lacking a partner, and with comorbidities such as diabetes, stroke, and cardiovascular disease.
Approximately two thirds of our patients on dialysis (68%) reported reduced QoL, compared with 60% of those with CKD stages 3 to 5 and 49% with kidney transplants. On average, patients on dialysis experienced larger mean decrements of 0.24 in the overall QoL score (from a score of 1.0 representing full health) compared with decrements of 0.16 and 0.19 among those with kidney transplants and with CKD stages 3 to 5, respectively. Changes in QoL are important when they reflect a meaningful alteration in a patient’s experience of an illness, and this important difference was found to be 0.06 to 0.08 in EQ-5D measurements, particularly in patients with malignancy.17,18 These differences could be extrapolated in patients with CKD because those with CKD, particularly advanced-stage kidney disease, may experience comparable decrement in QoL as in patients with cancer.4 Although kidney transplant recipients had higher mean QoL scores (by 0.08) compared with those on dialysis, this was still inferior when compared with the general Australian or American population.19, 20, 21 Although offering improved survival and freedom from dialysis, transplant recipients still live with the affliction of chronic disease, with the need to comply with a strict medication regimen that is typically associated with multiple adverse effects and the requirement of ongoing medical care and support.
Deficits in mobility, usual activities, and anxiety/depression were reported in one third of our cohort regardless of CKD stage. Pain was pervasive across the cohort, with nearly 50% of those with CKD stages 3 to 5 and on dialysis reporting moderate to severe pain compared with 36% of kidney transplant recipients. Although prior studies using the EQ-5D-3L have reported substantial problems in these domains,6,7 we also demonstrated that a large proportion of this cohort had concerns with anxiety/depression, with nearly 40% of dialysis patients reporting moderate to severe problems.
Identifying and understanding the key factors that may impact on the overall and domain-specific QoL are crucial because targeted treatments and interventions could be implemented to reduce risk and increase protective factors for this vulnerable group of patients with CKD. Additionally, worse QoL may be associated with increased mortality.22
Living alone without a spouse or partner is becoming more prevalent in today’s societies, although this is not necessarily associated with loneliness.23,24 People living alone may also be at different stages of their lives, and conflicting findings between the relationship between living alone and overall QoL have been observed in the general population. Although prior work has suggested that married persons experience better QoL,25 more recent work in a Korean cohort26 has noted that this differed by age and gender. Although older married men and women reported higher QoL as compared with younger single men who reported the lowest QoL, interestingly younger single women reported the highest QoL in the general population.25,26 Additionally, being alone could be considered a surrogate for social isolation, especially in older individuals. Patients with CKD, particularly dialysis, are at a greater risk of social isolation, occurring possibly from a loss of independence, financial stress from early retirement, and a lack of control over daily activities.27 Social isolation may be associated with higher mortality, higher rates of depression, poor self-rated health, and unfavorable health behaviors such as poor diet and noncompliance with treatment,23,28,29 likely because of the lack of social support that facilitates health-promoting behaviors.
In this cohort of patients with CKD, we have shown that the lack of a spouse or partner (single, widowed, or divorced) is associated with worse overall QoL as well as problems in mobility, daily activity, and anxiety/depression, suggesting that social support (including virtual or online support, focus groups, patient/community navigators) may have beneficial effects on QoL and the management of serious illnesses.30,31 Although social contact is difficult to prescribe, recognizing isolation itself and putting in place measures to support these individuals may benefit them. Further work quantifying social isolation and evaluation of measures against perceived social isolation may be a useful health promotion strategy in these patients. Interestingly, there was no evidence of interaction between living alone without a partner or spouse and age or gender, suggesting that living alone has a similar effect on QoL in both genders.
Similarly, lower education status was associated with worse overall QoL and domain-level QoL. The chronic nature of kidney disease requires a sufficient amount of understanding by patients to enable them to actively participate in the management of their health. Limited health literacy may result in patients becoming overwhelmed, resulting in decreased compliance and poorer health outcomes.32 Although certain social structures cannot be changed without enormous political action, health literacy is susceptible to social environments and could be improved by targeted health education.33,34 These socioeconomic measures such as education and marital status may influence life opportunities such as access to health care and the individual’s perception of his or her QoL.35,36 Additionally, we have shown that female sex was associated with worse QOL,18,37,38 reflecting the vulnerability of women with chronic disease as well as their different psychosocial perspective on life (such as experiencing more psychological distress, stigmatization, having family roles that compete for time and resources, and a feeling of disempowerment in their interactions with health professionals) when compared with men.39, 40, 41 Although the use of erythropoiesis-stimulating agents was associated with better QoL in CKD stages 3 to 5, it was associated with worse QoL in transplant recipients, suggesting this may be a surrogate for declining allograft function along with the additional burden of needing subcutaneous injections.
Pain is common in CKD, with nearly 50% of this population reporting moderate to severe pain, regardless of being on dialysis or managed conservatively.42 Women seemed to experience more pain compared with men, and this is in keeping with the population prevalence of chronic pain conditions that seem to more frequently affect women. This is likely because of a complex interplay of biologic (effect of sex hormones and endogenous opioids) and psychosocial (pain coping techniques, gender roles) mechanisms.43 Chronic pain is associated with poor QoL, depression, and missed medical treatment. This symptom is often under-recognized and poorly managed by physicians because chronic pain is a multidimensional phenomenon with both physical and psychosocial components. Identifying pain and focusing therapy in a multimodal manner (physical, behavioral, and pharmacotherapy) is vital in managing this issue.44 Similarly, the burden of a chronic illness and the rigors associated with dialysis treatment and its intrusiveness into social life domains can result in higher rates of anxiety/depression in patients with kidney disease. Assessments need to extend beyond simply questioning a patient regarding his or her mood. If an anxiety/depressive disorder is suspected, formal assessment should be considered along with psychotherapy and pharmacotherapy.45
Our study has several strengths. Because it is a prospective, multicenter study conducted across 11 centers in 4 countries in a routine screening setting, the study population is likely to be representative of the general CKD population. Although most other studies focused on moderate to severe CKD and/or dialysis populations, our study investigated QoL across moderate CKD, end-stage kidney disease, and transplant recipients. Our response rate was high with almost all participants (99.4%) completing the QoL questionnaire. Additionally, we assessed both QoL data and domain-specific data.
Our study also has a number of potential limitations. First, this was a cross-sectional study without serial measurements. As such, we were unable to compare the changes in QoL scores over time, particularly in relation to events related to life-changing events such as change in dialysis modalities or transition between dialysis and transplantation across the lifespan of a person with CKD. Second, there is a possibility of selection or volunteer bias, leading to recruitment of a relatively healthier patient population. Third, for the purposes of this study, the Australian scoring system of the Euro-QoL was used for all participants, although the sensitivity analyses conducted using the Spanish version of the Euro-QoL were aligned with the findings observed using the Australian version of the Euro-QoL.
In conclusion, in this multicenter cohort of adults with CKD stages 3 to 5, on dialysis and with kidney transplants, there were widespread deficits of QoL. Being on dialysis was associated with poor overall QoL as well as pervasive deficits in domain-specific scores. In addition, a lower educational status, concurrent comorbidities, and lack of a partner were associated with reduced QoL in almost all dimensions. Knowledge of this triad of factors will provide valuable means of identifying those who are most at risk of poor QoL and defining novel social and medical interventions and the resources to support them, which are of benefit to this group of individuals.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This study was funded by the Australian National Health and Medical Research Council, Australia and New Zealand Trial Registry Number ACTRN12611000538943.
We thank all participants in the DETECT study for generously participating in this research. We also thank the clinicians, nurses, and clinical research staff who have kindly assisted us by collecting information from participants for this study. We extend our deepest gratitude to the late Dr Richard Hope for his unfailing support throughout the study. Without his dedication and perseverance over the many years, the DETECT study would have not been possible. He is sadly missed by all members of the DETECT team, his patients, and fellow researchers. The preliminary results of this study were presented at the World Congress of Nephrology held in Melbourne in April 2019.
Footnotes
Figure S1. Domain-specific quality of life by chronic kidney disease stage.
Table S1. Univariate analysis of the predictors of quality of life.
Supplementary Sensitivity Analysis.
Strobe Statement.
Supplementary Material
References
- 1.Go A.S., Chertow G.M., Fan D. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Wyld M., Morton R.L., Hayen A. A systematic review and meta-analysis of utility-based quality of life in chronic kidney disease treatments. PLoS Med. 2012;9 doi: 10.1371/journal.pmed.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mittal S.K., Ahern L., Flaster E. Self-assessed physical and mental function of haemodialysis patients. Nephrol Dial Transplant. 2001;16:1387–1394. doi: 10.1093/ndt/16.7.1387. [DOI] [PubMed] [Google Scholar]
- 4.Wong G., Howard K., Chapman J. How do people with chronic kidney disease value cancer-related quality of life? Nephrology. 2012;17:32–41. doi: 10.1111/j.1440-1797.2011.01531.x. [DOI] [PubMed] [Google Scholar]
- 5.Ogutmen B., Yildirim A., Sever M.S. Health-related quality of life after kidney transplantation in comparison intermittent hemodialysis, peritoneal dialysis, and normal controls. Transplant Proc. 2006;38:419–421. doi: 10.1016/j.transproceed.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen N.T.Q., Cockwell P., Maxwell A.P. Chronic kidney disease, health-related quality of life and their associated economic burden among a nationally representative sample of community dwelling adults in England. PLoS One. 2018;13 doi: 10.1371/journal.pone.0207960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee H., Oh Y.J., Kim M. The association of moderate renal dysfunction with impaired preference-based health-related quality of life: third Korean national health and nutritional examination survey. BMC Nephrol. 2012;13:19. doi: 10.1186/1471-2369-13-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong G., Hope R.L., Howard K. One-time fecal immunochemical screening for advanced colorectal neoplasia in patients with CKD (DETECT study) J Am Soc Nephrol. 2019;30:1061–1072. doi: 10.1681/ASN.2018121232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong G., Howard K., Chapman J.R. Test performance of faecal occult blood testing for the detection of bowel cancer in people with chronic kidney disease (DETECT) protocol. BMC Public Health. 2011;11:516. doi: 10.1186/1471-2458-11-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDowell I. Oxford University Press; 2006. Measuring Health: A Guide to Rating Scales and Questionnaires. [Google Scholar]
- 12.Tajima R., Kondo M., Kai H. Measurement of health-related quality of life in patients with chronic kidney disease in Japan with EuroQol (EQ-5D) Clin Exp Nephrol. 2010;14:340–348. doi: 10.1007/s10157-010-0304-1. [DOI] [PubMed] [Google Scholar]
- 13.van Loon I.N., Goto N.A., Boereboom F.T.J. Quality of life after the initiation of dialysis or maximal conservative management in elderly patients: a longitudinal analysis of the Geriatric assessment in OLder patients starting Dialysis (GOLD) study. BMC Nephrol. 2019;20:108. doi: 10.1186/s12882-019-1268-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park J.I., Baek H., Jung H.H. CKD and health-related quality of life: the Korea National Health and Nutrition Examination Survey. Am J Kidney Dis. 2016;67:851–860. doi: 10.1053/j.ajkd.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 15.EuroQol Group. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 16.Viney R., Norman R., King M.T. Time trade-off derived EQ-5D weights for Australia. Value Health. 2011;14:928–936. doi: 10.1016/j.jval.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Pickard A.S., Neary M.P., Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70. doi: 10.1186/1477-7525-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chow F.Y., Briganti E.M., Kerr P.G. Health-related quality of life in Australian adults with renal insufficiency: a population-based study. Am J Kidney Dis. 2003;41:596–604. doi: 10.1053/ajkd.2003.50121. [DOI] [PubMed] [Google Scholar]
- 19.Luo N., Johnson J.A., Shaw J.W. Self-reported health status of the general adult U.S. population as assessed by the EQ-5D and Health Utilities Index. Med Care. 2005;43:1078–1086. doi: 10.1097/01.mlr.0000182493.57090.c1. [DOI] [PubMed] [Google Scholar]
- 20.Clemens S., Begum N., Harper C. A comparison of EQ-5D-3L population norms in Queensland, Australia, estimated using utility value sets from Australia, the UK and USA. Qual Life Res. 2014;23:2375–2381. doi: 10.1007/s11136-014-0676-x. [DOI] [PubMed] [Google Scholar]
- 21.McCaffrey N., Kaambwa B., Currow D.C., Ratcliffe J. Health-related quality of life measured using the EQ-5D-5L: South Australian population norms. Health Qual Life Outcomes. 2016;14:133. doi: 10.1186/s12955-016-0537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wyld M.L.R., Morton R.L., Clayton P. The impact of progressive chronic kidney disease on health-related quality-of-life: a 12-year community cohort study. Qual Life Res. 2019;28:2081–2090. doi: 10.1007/s11136-019-02173-1. [DOI] [PubMed] [Google Scholar]
- 23.Hammig O. Health risks associated with social isolation in general and in young, middle and old age. PLoS One. 2019;14 doi: 10.1371/journal.pone.0219663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Vaus D and Qu L. Demographics of living alone, Australian Institute of Family Studies Melbourne, Australia, In: Australian Family Trends; no. 6, 2015. Available at: https://nla.gov.au/nla.obj-400853003/view. Accessed November 12, 2020.
- 25.Hu Y.R., Goldman N. Mortality differentials by marital status: an international comparison. Demography. 1990;27:233–250. [PubMed] [Google Scholar]
- 26.Han K.T., Park E.C., Kim J.H. Is marital status associated with quality of life? Health Qual Life Outcomes. 2014;12:109. doi: 10.1186/s12955-014-0109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tezel A., Karabulutlu E., Sahin O. Depression and perceived social support from family in Turkish patients with chronic renal failure treated by hemodialysis. J Res Med Sci. 2011;16:666–673. [PMC free article] [PubMed] [Google Scholar]
- 28.Karamanidou C., Clatworthy J., Weinman J., Horne R. A systematic review of the prevalence and determinants of nonadherence to phosphate binding medication in patients with end-stage renal disease. BMC Nephrol. 2008;9:2. doi: 10.1186/1471-2369-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clarke A.L., Young H.M., Hull K.L. Motivations and barriers to exercise in chronic kidney disease: a qualitative study. Nephrol Dial Transplant. 2015;30:1885–1892. doi: 10.1093/ndt/gfv208. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y.R., Schulz P.J. The effect of information communication technology interventions on reducing social isolation in the elderly: a systematic review. J Med Internet Res. 2016;18:e18. doi: 10.2196/jmir.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mann F., Bone J.K., Lloyd-Evans B. A life less lonely: the state of the art in interventions to reduce loneliness in people with mental health problems. Soc Psychiatry Psychiatr Epidemiol. 2017;52:627–638. doi: 10.1007/s00127-017-1392-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor D.M., Fraser S., Dudley C. Health literacy and patient outcomes in chronic kidney disease: a systematic review. Nephrol Dial Transplant. 2018;33:1545–1558. doi: 10.1093/ndt/gfx293. [DOI] [PubMed] [Google Scholar]
- 33.Nutbeam D. The evolving concept of health literacy. Soc Sci Med. 2008;67:2072–2078. doi: 10.1016/j.socscimed.2008.09.050. [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Vargas P.A., Tong A., Howell M., Craig J.C. Educational interventions for patients with CKD: a systematic review. Am J Kidney Dis. 2016;68:353–370. doi: 10.1053/j.ajkd.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 35.Augustus J.S., Kwan L., Fink A. Education as a predictor of quality of life outcomes among disadvantaged men. Prostate Cancer Prostatic Dis. 2009;12:253–258. doi: 10.1038/pcan.2008.58. [DOI] [PubMed] [Google Scholar]
- 36.Ashing-Giwa K.T., Padilla G., Tejero J. Understanding the breast cancer experience of women: a qualitative study of African American, Asian American, Latina and Caucasian cancer survivors. Psychooncology. 2004;13:408–428. doi: 10.1002/pon.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mujais S.K., Story K., Brouillette J. Health-related quality of life in CKD patients: correlates and evolution over time. Clin J Am Soc Nephrol. 2009;4:1293–1301. doi: 10.2215/CJN.05541008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paniagua R., Amato D., Vonesh E. Health-related quality of life predicts outcomes but is not affected by peritoneal clearance: the ADEMEX trial. Kidney Int. 2005;67:1093–1104. doi: 10.1111/j.1523-1755.2005.00175.x. [DOI] [PubMed] [Google Scholar]
- 39.Bonomi A.E., Anderson M.L., Reid R.J. Medical and psychosocial diagnoses in women with a history of intimate partner violence. Arch Intern Med. 2009;169:1692–1697. doi: 10.1001/archinternmed.2009.292. [DOI] [PubMed] [Google Scholar]
- 40.Seear K. The etiquette of endometriosis: stigmatisation, menstrual concealment and the diagnostic delay. Soc Sci Med. 2009;69:1220–1227. doi: 10.1016/j.socscimed.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 41.DiGiacomo M., Green A., Rodrigues E. Developing a gender-based approach to chronic conditions and women's health: a qualitative investigation of community-dwelling women and service provider perspectives. BMC Womens Health. 2015;15:105. doi: 10.1186/s12905-015-0264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davison S.N., Koncicki H., Brennan F. Pain in chronic kidney disease: a scoping review. Semin Dial. 2014;27:188–204. doi: 10.1111/sdi.12196. [DOI] [PubMed] [Google Scholar]
- 43.Bartley E.J., Fillingim R.B. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth. 2013;111:52–58. doi: 10.1093/bja/aet127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davison S.N. Clinical pharmacology considerations in pain management in patients with advanced kidney failure. Clin J Am Soc Nephrol. 2019;14:917–931. doi: 10.2215/CJN.05180418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cohen S.D., Cukor D., Kimmel P.L. Anxiety in patients treated with hemodialysis. Clin J Am Soc Nephrol. 2016;11:2250–2255. doi: 10.2215/CJN.02590316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.