Abstract
Introduction
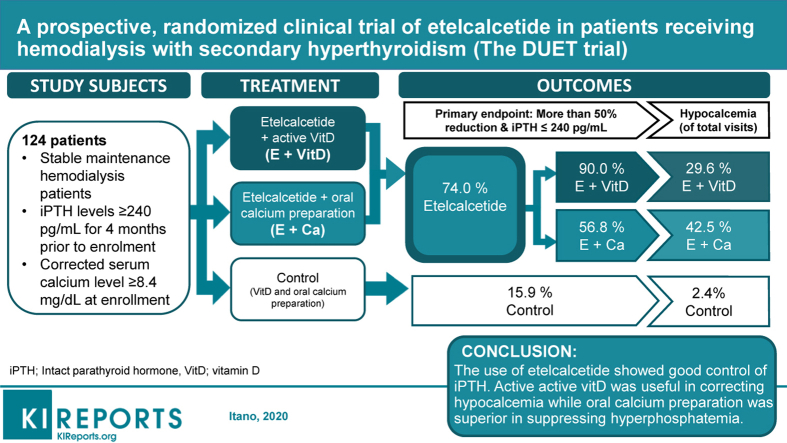
The clinical trial on the Development of a treatment strategy for chronic kidney disease‒mineral and bone disorder by a mUltilateral mechanism of ETelcalcetide hydrochloride, or the DUET trial, was designed to determine the efficacy of etelcalcetide, an intravenous calcimimetic, for control of secondary hyperparathyroidism (SHPT).
Methods
Eligible SHPT maintenance hemodialysis patients (n = 124) were randomized (1:1:1) for inclusion in the DUET trial, a 12-week, multicenter, open-label, parallel-group study (jRCTs041180108), and assigned to either an etelcalcetide + active vitamin D group (group E+D), an etelcalcetide + oral calcium preparation group (group E+Ca), or a control group (group C). The primary endpoint was number of patients with a 50% reduction from baseline of intact parathyroid hormone (iPTH) levels, and iPTH levels ≤ 240 pg/mL at 12 weeks after start of the trial.
Results
The proportion of patients reaching the primary endpoint (95% confidence interval [CI]) was 90.0% (76.3%–97.2%) in group E+D, 56.8% (39.5%–72.9%) in group E+Ca, and 19.5% (8.8%–34.9%) in group C. Etelcalcetide treatment led to a significant increase in the number of patients achieving the endpoint (odds ratio, 13.4; 95% CI, 5.10–35.3) on logistic regression analysis, with iPTH, corrected serum calcium, and phosphate at baseline as covariates. Significantly more patients achieved the endpoint in group E+D compared with group E+Ca (odds ratio, 6.35; 95% CI, 1.79–22.48). There were fewer hypocalcemic visits in group E+D compared with group E+Ca (P = 0.018), yet the former group was prone to hyperphosphatemia.
Conclusion
Etelcalcetide showed good control of iPTH for maintenance hemodialysis patients with SHPT. Active vitamin D was useful in correcting hypocalcemia, but the oral calcium preparation was superior for suppression of hyperphosphatemia.
Keywords: calcimimetic, etelcalcetide, hemodialysis, randomized study, secondary hyperthyroidism
Graphical abstract
Secondary hyperparathyroidism (SHPT), a major complication in advanced chronic kidney disease (CKD), may lead to imbalances in mineral metabolism (such as hypocalcemia and hyperphosphatemia) and, in turn, vascular calcification and high mortality.1 Intact parathyroid hormone (iPTH) levels are monitored as an index to determine the course of SHPT treatment in the clinical setting.2 Parathyroid hormone (PTH)-lowering therapy, calcimimetics, calcitoriol, vitamin D analogs, and the combination of a calcimimetic with calcitoriol and vitamin D analogs have been recommended for dialysis patients.2,3 Before calcimimetics use was widespread, poor control of SHPT had been far more common.4 Based on satisfactory results from the various global clinical studies using calcimimetics,5,6 we expect that use of these agents will lead to improved control of SHPT, and that the new lower therapeutic target of iPTH levels will improve the prognosis of maintenance dialysis patients in the near future.
Chronic kidney disease‒mineral and bone disorder (CKD-MBD) is a complex condition that occurs over the course of declining kidney function.7 Most patients with advanced kidney disease develop SHPT as a consequence of CKD-MBD and have markedly elevated iPTH levels, exceeding the upper normal limit.8 Nephrologists are in agreement that poor control of serum iPTH levels is associated with poor outcomes,9 but the target level of serum iPTH for dialysis patients has varied.10 The iPTH target level for maintenance dialysis patients from recent clinical practice guidelines published in 200911 and 201712 is broader than the recommendation from 2003.13 The new level is approximately 2 to 9 times the upper normal limit for the assay rather than 150 to 300 pg/mL. Until 2006, the same target range was also accepted in Japan. However, because Japanese dialysis patients have naturally lower iPTH levels than patients in the United States and Europe,10 we now have a stricter iPTH target level (60–240 pg/mL) for demonstrating improved survival.14
Because calcimimetics amplify the sensitivity of the calcium (Ca)-sensing receptor, the use of calcimimetics suppresses serum Ca, which results in hypocalcemia and a decrease in PTH secretion. Control of hypocalcemia induced by calcimimetics poses a significant problem. Therefore, we also compared vitamin D and Ca preparations to determine which is the safer approach for correction of hypocalcemia induced by etelcalcetide treatment.
The DUET (Development of treatment strategy for chronic kidney disease-mineral and bone disorder by mUltilateral mechanism of ETelcalcetide hydrochloride) study addressed the efficacy and safety of etelcalcetide for control of iPTH levels and also evaluated its optimal use for treatment of etelcalcetide-induced hypocalcemia in maintenance dialysis patients with SHPT.
Methods
Trial Design and Oversight
The trial design and methods of the DUET study have been described elsewhere.15 The trial was registered with the Japan Registry of Clinical Trials (jRCTs041180108). The protocol was approved by the clinical research review board of Nagoya University (CRB4180004) and is consistent with the 1964 Helsinki Declaration. All patients provided written informed consent for participation in this study.
The DUET study is a 12-week, multicenter, open-label, randomized (1:1:1), parallel-group study comparing the control of SHPT with 3 groups: group E+D---etelcalcetide and additional active vitamin D on top of the original medications if hypocalcemia is induced by etelcalcetide; group E+Ca---etelcalcetide and additional oral precipitated calcium carbonate on top of the original medications if hypocalcemia is induced by etelcalcetide; or group C---control (standard therapy using vitamin D and precipitated calcium carbonate). The main inclusion criteria were stable maintenance hemodialysis patients, iPTH level ≥ 240 pg/mL for 4 months before enrollment, and corrected serum calcium level of ≥ 8.4 mg/dL at enrollment. The stratifying factors for randomization included iPTH (> 400 pg/mL or ≤ 400 pg/mL), corrected serum Ca (> 9 mg/dL or ≤ 9 mg/dL), serum phosphate (> 5 mg/dL or ≤ 5 mg/dL within 2 weeks before start of the trial), and the institution to which the patient belonged. The dose algorithm of etelcalcetide was applied as follows: starting dose was 5 mg and then increased in 2.5- or 5-mg increments at 4 and 8 weeks based on the target iPTH level calculated to achieve the primary outcome.
Primary and Secondary Endpoints
The primary endpoint of the protocol was a 50% reduction of iPTH levels and reduction of iPTH levels to < 240 pg/mL after 12 weeks of treatment. As per the guidelines from the Japanese Society for Dialysis Therapy, we set PTH 60 ≤ iPTH ≤ 240 pg/mL as the target range for the control of SHPT in our study patients.14 The secondary endpoints after 12 weeks of treatment included > 50% reduction in iPTH level, > 30% reduction in iPTH level, 60 ≤ iPTH ≤ 240 pg/mL, and > 30% or > 50% reduction in iPTH levels and 60 ≤ iPTH ≤ 240 pg/mL. This endpoint was a comparison between the group treated with etelcalcetide and the control group. We evaluated changes in iPTH levels and corrected serum Ca levels (relative to the baseline) every 2 weeks during treatment of the 3 groups. Initially, we planned to study the normalized ratios of hypocalcemia induced by etelcalcetide between group E+D and group E+Ca. However, due to recurrence in some patients, it became difficult to count the number of the patients who became hypocalcemic. Therefore, we identified the proportion of total visits in which patients elicited hypocalcemia. Finally, we assessed safety and adverse events during the intervention period.
Statistical Analysis
The statistical analysis used in this trial has been described elsewhere.15 In summary, we calculated the endpoint achievement proportions and their 95% confidence intervals (CIs) based on binomial distribution for each treatment group, and compared these between the groups treated with etelcalcetide (group E+D and group E+Ca) and the control group (group C) by logistic regression analysis with iPTH, corrected Ca, and phosphate as covariates. In addition, statistically significant differences were then compared between group E+D and group E+Ca. We assumed that 35% of patients in the intervention group would achieve a lower than iPTH normalization rate (iPTH 240 pg/mL). After assuming a 10% normalization rate in the control group, we calculated that at least 76 individuals would be necessary in the intervention group to detect a significant difference with a power of 0.85 and significance level of 0.05. We speculated that the normalization rate would be higher than that of the previous report (cited in Kato et al.15) and set the number of intervention patients to 80, with 40 control patients, and thus, a total of 120 patients had been foreseen.
To test the changes in parameters, we calculated the adjusted mean and the 95% CI for changes at each time-point using a linear mixed model. The treatment group, time-point, and interaction of treatment group and time-point were used as fixed effects. Changes in each index among treatment groups at each time-point were then compared using the Tukey-Kramer method to correct for multiplicity.
To test the proportion of visits in which patients showed hypocalcemia, we performed a covariance analysis with iPTH, corrected Ca, and serum phosphate levels as covariates at baseline. The groups treated with etelcalcetide (group E+D and group E+Ca) were initially compared with the control group (group C), and then compared versus each other.
Results
Overview of Trial Conduct
One hundred twenty-four Japanese patients were randomized to the trial groups from May 2018 to February 2019; 118 patients were included in the statistical analysis of the primary outcome. The flow diagram of the study is shown in Supplementary Figure S1. The baseline data of all the enrolled patients are shown in Table 1. The clinical characteristics and baseline laboratory data were similar in these 3 groups.
Table 1.
Characteristics of study patients at baseline
| Characteristic | Etelcalcetide |
Control | |
|---|---|---|---|
| Etelcalcetide + Vit D | Etelcalcetide + Ca | ||
| Number of patients | 41 | 41 | 42 |
| Age, years, mean (SD) | 66.5 (11.6) | 66.8 (14.1) | 66.4 (10.5) |
| Men, n (%) | 32 (78.0) | 27 (65.9) | 25 (59.5) |
| Dialysis vintage, years, median (IQR) | 7.0 (2.3–13.6) | 5.8 (2.4–9.5) | 5.8 (2.4–12.0) |
| Underlying kidney disease, n (%) | |||
| Diabetes mellitus | 16 (39.0%) | 12 (29.3%) | 21 (50.0%) |
| Nephrosclerosis | 8 (19.5%) | 9 (22.0%) | 4 (9.5%) |
| Glomerulonephritis | 10 (24.4%) | 9 (22.0%) | 9 (21.4%) |
| Polycystic kidney disease | 1 (2.4%) | 0 (0.0%) | 2 (4.8%) |
| Unknown/other | 6 (14.6%) | 11 (26.8%) | 6 (14.3%) |
| Dialysis modality, n (%) | |||
| Hemodiafiltration | 17 (41.4%) | 16 (39.0%) | 18 (42.9%) |
| Hemodialysis | 24 (58.5%) | 25 (61.0%) | 24 (57.1%) |
| History of cinacalcet use, n (%) | 6 (14.6%) | 6 (14.6%) | 10 (23.8%) |
| History of parathyroidectomy, n (%) | 0 (0.0%) | 1 (2.4%) | 1 (2.4%) |
| Use of phosphate binders, n (%) | |||
| Oral calcium carbonate | 21 (51.2%) | 14 (34.1%) | 14 (34.1%) |
| Non–calcium-containing phosphate binders | 32 (78.0%) | 29 (70.7%) | 32 (78.0%) |
| Intact parathyroid hormone, pg/mL | |||
| Median (IQR) | 258 (223.5–336) | 266 (195.5–368.5) | 286 (212–357) |
| Mean (SD) | 284.3 (109.6) | 293.7 (138.5) | 311.3 (136.1) |
| Ca (albumin-corrected), mg/dL, mean (SD) | 9.2 (0.6) | 9.3 (0.7) | 9.2 (0.4) |
| Phosphate, mg/dL, mean (SD) | 5.1 (1.2) | 5.7 (1.7) | 5.4 (0.9) |
| BAP, μg/L, mean (SD) | 16.8 (9.2) | 22.0 (18.2) | 18.7 (9.1) |
| TRACP-5b, mU/dL, mean (SD) | 697.6 (404.4) | 800.3 (658.2) | 690.9 (326.5) |
BAP, bone-specific alkaline phosphatase; IQR, interquartile range; SD, standard deviation; TRACP-5b, tartrate-resistant acid phosphatase 5b; Vit D, vitamin D.
Dialysate calcium = 3 mEq/L.
Primary Endpoint: > 50% Reduction and Reduction of iPTH Levels to < 240 pg/mL After 12 Weeks of Treatment
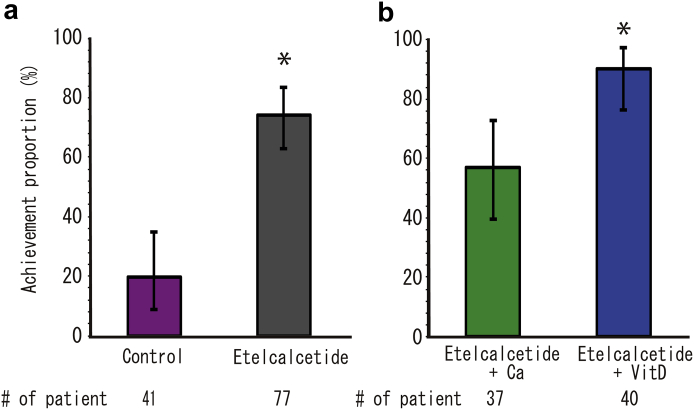
The proportion of patients reaching the primary endpoint was 74.0% (95% CI, 62.8%–83.4%) for those treated with etelcalcetide, compared with 19.5% (8.8%–34.9%) of control patients (Figure 1a). The proportions of patients achieving the primary endpoint in group E+D and group E+Ca were 90.0% (95% CI, 76.3%–97.2%) and 56.8% (95% CI, 39.5%–72.9%), respectively (Figure 1b). The upper row of Table 2 shows the odds ratios for the primary outcome. Treatment with etelcalcetide demonstrated a significant increase in the number of patients achieving the primary endpoint. Significantly fewer patients reached the primary endpoint in group E+Ca when compared with group E+D. Supplementary Figure S2 shows the distributions of iPTH at the end of the intervention.
Figure 1.
Achievement of iPTH 50% reduction and iPTH ≤ 240 pg/mL at 12 weeks after administration and 95% confidence intervals on a binomial distribution for each treatment group: (a) Comparison of etelcalcetide patients versus controls. (b) Etelcalcetide users corrected hypocalcemia by active vitamin D versus oral calcium preparation (Ca). ∗P < 0.05.
Table 2.
Odds ratios of primary and secondary endpoints for control of SHPT
| Etelcalcetide vs. control |
E+D vs. E+Ca |
|||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P value | Odds ratio | 95% CI | P value | |
| Primary outcome | ||||||
| > 50% reduction and iPTH ≤ 240 pg/dL | 13.42 | 5.10–35.34 | 0.000a | 6.35 | 1.79–22.48 | 0.004a |
| Secondary outcome | ||||||
| > 50% reduction of iPTH | 16.14 | 5.99–43.49 | 0.000a | 5.31 | 1.49–18.91 | 0.010a |
| > 30% reduction of iPTH | 30.10 | 9.53–95.06 | 0.000a | NP | ||
| 60 ≤ iPTH ≤ 240 pg/dL | 0.35 | 0.16–0.78 | 0.010a | 0.41 | 0.15–1.14 | 0.089 |
| > 50% reduction and 60 ≤ iPTH ≤ 240 pg/dL | 1.33 | 0.48–3.70 | 0.585 | |||
| > 30% reduction and 60 ≤ iPTH ≤ 240 pg/dL | 1.28 | 0.55–2.98 | 0.569 | |||
CI, confidence interval; E+Ca, etelcalcetide + oral calcium preparation; E+D, etelcalcetide + active vitamin D; iPTH, intact parathyroid hormone; NP, analysis not possible.
P < 0.05.
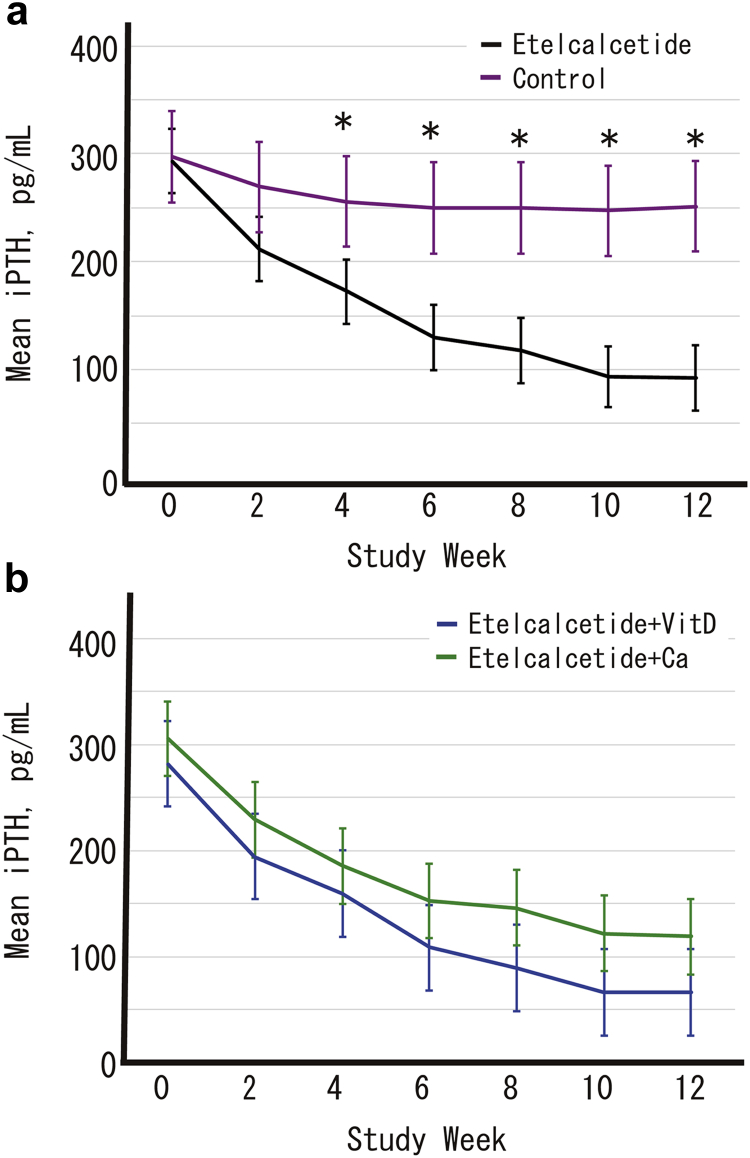
Secondary Endpoint: Change in iPTH and Corrected Ca in a Linear Mixed Model
Estimated iPTH levels using a linear mixed model are shown in Figure 2. The iPTH levels in patients treated with etelcalcetide (group E+D and group E+Ca) were significantly reduced compared with those of the control patients (group C) at 4, 6, 8, 10, and 12 weeks after the start of the trial. However, there were no statistical differences in iPTH levels between group E+D and group E+Ca.
Figure 2.
Adjusted mean intact parathyroid hormone levels and 95% confidence intervals calculated using a linear mixed model with each treatment group, time-point, and interaction of treatment group and time-point as fixed effects. Changes in each index are compared among treatment groups at each time-point using the Tukey-Kramer method to correct for multiplicity. (a) Comparison of etelcalcetide patients versus controls. (b) Etelcalcetide users corrected hypocalcemia by active vitamin D versus oral calcium preparation (Ca). ∗P < 0.05. iPTH, intact parathyroid hormone.
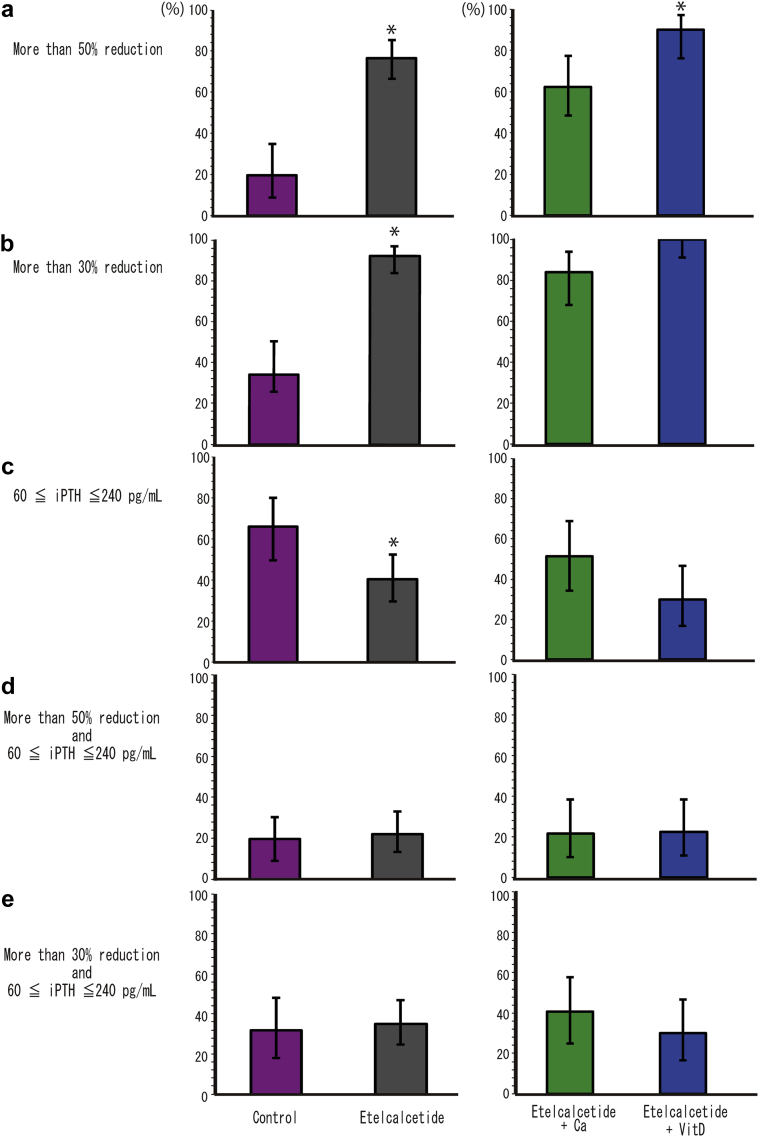
Secondary Endpoint: Achievement Proportions for Different Targets for Control of SHPT
The achieved proportions (95% CIs) for secondary outcomes are shown in Figure 3. In this trial, there were more patients with PTH oversuppression than expected, and approximately 52% of patients of the etelcalcetide treatment groups had iPTH < 60 pg/mL at 12 weeks after the intervention (the lower limit of target value for the Japanese Society for Dialysis Therapy).
Figure 3.
Achievement (95% confidence interval) of secondary outcomes. Achievement proportions of (a) > 50% reduction, (b) > 30% reduction, (c) iPTH 60 ≤ iPTH ≤ 240 pg/mL, (d) > 50% reduction and iPTH 60 ≤ iPTH ≤ 240 pg/mL, and (e) > 30% reduction and iPTH 60 ≤ iPTH ≤ 240 pg/mL. ∗P < 0.05. iPTH, intact parathyroid hormone.
The lower row of Table 2 shows the odds ratios of the secondary endpoints for control of SHPT. Administration of etelcalcetide demonstrated a significant increase in patients achieving a > 50% reduction and a < 30% reduction in iPTH levels. Achievement of iPTH 60 ≤ iPTH ≤ 240 pg/mL was significantly lower when compared with control patients.
Group E+D and group E+Ca were compared with regard to achievement of secondary endpoints. In group E+D, achievement of a > 50% reduction, > 30% reduction, and iPTH 60 ≤ iPTH ≤ 240 pg/mL were 90.0% (95% CI, 76.3%–97.2%), 100% (95% CI, 91.1%–100%), and 30.0% (95% CI, 16.6%–46.5%), respectively. In group E+Ca, achievements of a > 50% reduction, a > 30% reduction, and iPTH 60 ≤ iPTH ≤ 240 pg/mL were 62.2% (95% CI, 44.8%–77.5%), 83.8% (95% CI, 68.0%–93.8%), and 51.4% (95% CI, 34.4%–68.1%), respectively. Treatment with etelcalcetide plus vitamin D resulted in a significant increase in patients achieving with a > 50% reduction in iPTH compared with treatment using etelcalcetide with oral calcium preparations (lower row of Table 2).
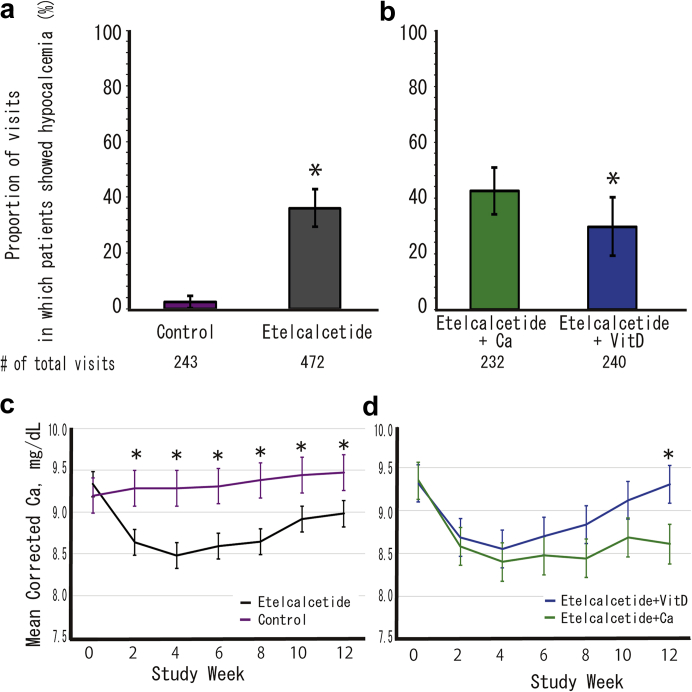
Exploratory Endpoints: Management of Hypocalcemia
Figure 4a and b shows the changes in serum-corrected Ca and the proportion of visits in which patients showed hypocalcemia. Covariance analysis showed there were significantly more hypocalcemic visits in patients treated with etelcalcetide (group E+D and group E+Ca) compared with controls (group C) (P < 0.0001). Moreover, number of hypocalcemic visits in group E+D was lower than in group E+Ca (P = 0.018). Estimated correct serum Ca levels according to a linear mixed model in patients treated with etelcalcetide (group E+D and group E+Ca) were significantly lower when compared with controls (group C) at all intervals after the start of the trial (Figure 4c). Although estimated correct serum Ca levels in group E+Ca were consistently low, those in group E+D reached a nadir at 4 weeks after the start of the trial and then recovered, and were significantly higher than those in group E+Ca at 12 weeks (Figure 4d).
Figure 4.
Visits in which patients showed hypocalcemia (corrected Ca level < 8.3 mg/dL) and 95% confidence intervals, based on a binomial distribution for each treatment group. (a) Comparison of etelcalcetide patients versus controls. (b) Etelcalcetide users corrected hypocalcemia by active vitamin D (Vit D) versus oral calcium preparation (Ca). The adjusted mean serum corrected calcium levels and 95% confidence interval calculated by a linear mixed model with each treatment group, time-point, and interaction of treatment group and time-point as fixed effects. Changes of each index compared among treatment groups at each time-point using the Tukey-Kramer method to correct for multiplicity. (c) Comparison of etelcalcetide patients versus controls, (d) Etelcalcetide users corrected hypocalcemia by active vitamin D (Vit D) versus oral calcium preparation (Ca). ∗P < 0.05. VitD, vitamin D.
Secondary Endpoint: Change in Phosphate and Ca-phosphate Product in a Linear Mixed Model
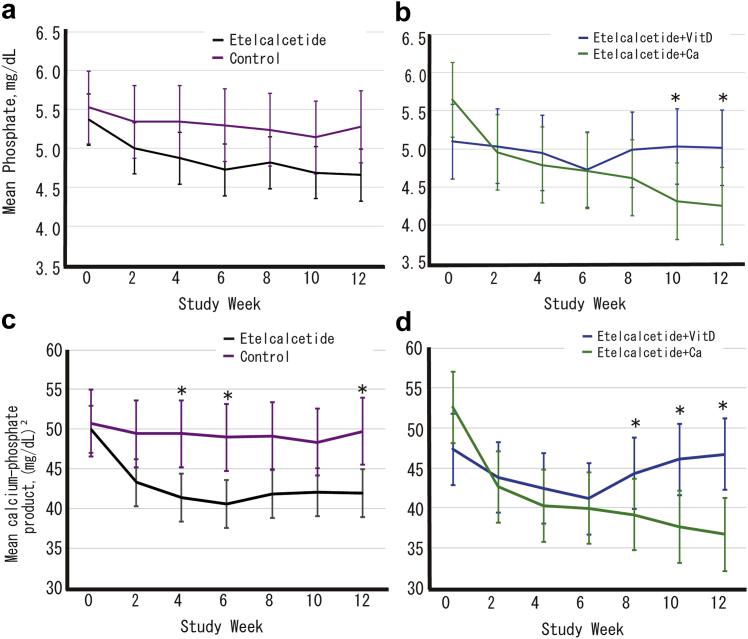
Estimated phosphate and Ca-phosphate product levels using a linear mixed model are shown in Figure 5. Although there were no significant differences for E+Ca in phosphate levels between patients treated with etelcalcetide (group E+D and group E+Ca) and controls (group C), phosphate levels in group E+D increased significantly compared with group E+Ca at 10 and 12 weeks after start of the trial. Ca-phosphate product levels in patients treated with etelcalcetide (group E+D and group E+Ca) were reduced compared with those in controls (group C), and there were significant differences at 4, 6, and 12 weeks after the start of the trial between these 2 groups. When comparing group E+D and group E+Ca, Ca-phosphate product levels in the former group increased significantly at 8, 10, and 12 weeks after start of the trial. Supplementary Figures S3 and S4 show the achievement of targeted corrected calcium and phosphate levels and change in magnesium in a linear mixed model, respectively. Supplementary Figures S5–S9 show the summary of the dose level of medication during the study.
Figure 5.
Adjusted mean phosphate levels and 95% confidence intervals (a, b) and calcium-phosphate products and the 95% confidence intervals (c, d) calculated using a linear mixed model with each treatment group, time-point, and interaction of treatment group and time-point as fixed effects. Changes in each index are compared among treatment groups at each time-point using the Tukey-Kramer method to correct for multiplicity. (a, c) Comparison of etelcalcetide patients versus controls. (b, d) Etelcalcetide users corrected hypocalcemia by active vitamin D (Vit D) versus oral calcium preparation (Ca). ∗P < 0.05. VitD, vitamin D.
Adverse Events
A full list of treatment-emergent adverse events with frequency in each treatment group is shown in Table 3. Information on all severe adverse events was collected by the individual attending doctors, and the correlation with etelcalcetide administration was evaluated by the safety monitoring committee. One patient died from septic shock during the study (group E+Ca). One patient had been hospitalized due to unstable angina and another for fever before entry into the study, but both safely entered the trial after recovery.
Table 3.
Treatment-emergent adverse events
| Adverse events | Etelcalcetide (n = 82) |
Control (n = 42) | |
|---|---|---|---|
| Etelcalcetide + Vit D (n = 41) | Etelcalcetide + Ca (n = 41) | ||
| Blood calcium decreasea | 29 (70.7%) | 32 (82.1%) | 5 (12.2%) |
| Severe blood Ca decreaseb | 0 (0.0%) | 5 (12.2%) | 0 (0.0%) |
| AVF-site complication | 2 (4.9%) | 4 (9.8%) | 3 (7.1%) |
| Infection | 1 (2.4%) | 4 (9.8%) | 0 (0.0%) |
| CVD/heart failure | 1 (2.4%) | 4 (9.8%) | 2 (4.8%) |
| Cancer | 0 (0.0%) | 2 (4.9%) | 0 (0.0%) |
| Fracture | 0 (0.0%) | 0 (0.0%) | 1 (2.4%) |
| Joint pain | 1 (2.4%) | 1 (2.4%) | 0 (0.0%) |
| Rash | 0 (0.0%) | 1 (2.4%) | 0 (0.0%) |
| Nausea | 1 (2.4%) | 0 (0.0%) | 0 (0.0%) |
AVF, arteriovenous fistula; CVD, cardiovascular disease; E+Ca, etelcalcetide + oral calcium preparation; E+D, etelcalcetide + active vitamin D; Vit D, vitamin D.
Data expressed as number (%).
Blood calcium decrease defined as a corrected Ca level of < 8.3 mg/dL (need medical intervention: add/increase active Vit D or oral calcium preparations according to allocation).
Severe blood Ca decrease defined as a corrected Ca level of < 7.4 mg/dL (stop etelcalcetide).
Although neoplasms were discovered in 2 patients (allocated to group E+Ca after starting the study), their cancer was already advanced at diagnosis. During the intervention, 3 patients received planned percutaneous coronary intervention, 1 received emergency hemodialysis due to overflow, and 1 was hospitalized due to fracture after a fall. None of the severe adverse events were found to be related to the study.
Discussion
In this multicenter, prospective, open-label, randomized, controlled trial of etelcalcetide administration in patients on maintenance hemodialysis therapy with moderate SHPT, we found that etelcalcetide had a powerful PTH-lowering action. Use of etelcalcetide also induced lower serum Ca levels. The addition of active vitamin D to the current treatment was more effective in the correction of hypocalcemia induced by etelcalcetide than the addition of an oral Ca preparation. However, in many cases, the PTH-lowering effect of etelcalcetide caused oversuppression.
In this study, we set 60 to 240 pg/mL iPTH as the therapeutic target level. As we anticipated the powerful PTH-lowering action of etelcalcetide, 69 of 77 patients (89.6%) showed iPTH levels < 240 pg/mL. All 6 patients who failed to achieve this target had to stop or not increase the dose of etelcalcetide due to hypocalcemia. Because etelcalcetide is administered intravenously, it is not involved in self-withdrawal due to gastrointestinal symptoms. Therefore, etelcalcetide shows promise as a therapeutic agent, but there remains a risk of hypocalcemia.
The iPTH-lowering effect of etelcalcetide was significant enough to induce oversuppression, that is, iPTH < 60 pg/mL, in many cases in this study. Several studies reported a J-curve relationship between iPTH levels and mortality.9,10 One reason for the high mortality rate is the association with a low iPTH level, below the lower limit, which may indicate low bone turnover and lead to accelerated vascular calcification.16 It was reported that excessively low iPTH was an independent risk factor for aortic arch calcification progression and also for adverse cardiac and cerebrovascular events.17 Another reason for the high mortality is malnutrition and inflammation inhibiting PTH secretion.18 Thus, the detrimental impact of hypoparathyroidism induced by calcimimetics remains unclear. The administration of etelcalcetide, as well as other calcimimetics, must be constantly monitored for oversuppression while following the clinical course of such hypoparathyroidism.
Hypocalcemia induced by calcimimetics is a major risk. Among calcimimetics, etelcalcetide is associated with hypocalcemia19 due to its longer elimination half-life.20 In this study, 61 of 82 patients (74.39%) treated with etelcalcetide developed hypocalcemia. Although previous studies demonstrated that hypocalcemia induced by etelcalcetide occurred in 60% to 70% of patients,19,21 the incidence in this study was higher. We speculate that the lower corrected serum Ca at baseline in our patients was due to a higher incidence of hypocalcemia compared with patients in other RCTs.19,21 The lower iPTH levels in our study were also suspected to be due to milder SHPT. Although there have been reports that mild and asymptomatic hypocalcemia due to calcimimetics is harmless,2,12 severe hypocalcemia, although rare, remains fatal. Hypocalcemia should be corrected in a cautious manner, as low serum Ca is associated with mortality in the dialysis population.9 The concurrent therapies for correcting hypocalcemia were active vitamin D, oral Ca preparation, and dialysate Ca. For avoiding hypercalcemia that may induce vascular calcification and result in mortality,9 restriction of a Ca-based phosphate binder has been recommended.12,14 In this study, we compared the Ca-correcting ability of active vitamin D and an oral calcium preparation. Active vitamin D was more effective in correcting Ca levels than the oral Ca preparation. We assessed risk factors of etelcalcetide-associated hypocalcemia at the end of the intervention. We found that higher baseline iPTH levels as well as use of an oral Ca preparation, as compared with use of active vitamin D, contributed to hypocalcemia, whereas age, sex, dialysis vintage, previous cinacalcet use, and baseline-corrected Ca did not (Supplementary Table S1). We speculated that the high risk of hypocalcemia induced by etelcalcetide in patients with high iPTH levels at baseline was due to the study protocol that dose of etelcalcetide was to be increased until iPTH level of each patient was to achieve his/her target of iPTH.
However, we demonstrated that an oral Ca preparation is superior for suppression of phosphate and Ca-phosphate product levels. In the practical setting, the combination of active vitamin D, Ca-free phosphate binder, and an oral Ca preparation may be necessary for management of hypocalcemia induced by calcimimetics.
The DUET trial has some limitations. As SHPT is a chronic complication often requiring long-term control, a 12-week observation may be too short to evaluate the efficacy of etelcalcetide. Due to the relatively long period from screening to the start of the intervention, 41 patients (33%) reported baseline iPTH levels < 240 pg/mL. Patients who did not require step up for treatment of SHPT were included. A strength of this trial is that few patients were lost to follow-up.
In conclusion, the DUET study is the first randomized, controlled study to assess the ability of etelcalcetide to control iPTH and investigate the correction of hypocalcemia. Among maintenance hemodialysis patients with moderate SHPT, etelcalcetide had high potential to lower iPTH levels. Because active active vitamin D was useful in correcting hypocalcemia while oral calcium preparation was superior in suppressing hyperphosphatemia, the combination of active vitamin D, Ca-free phosphate binder, and an oral calcium preparation may be necessary for management of hypocalcemia induced by calcimimetics. Further study is needed to evaluate vascular calcification over a long period in dialysis patients using etelcalcetide.
Disclosure
YI, SK, MH, TI, TK, and SM declare that the Department of Nephrology, Nagoya University Graduate School of Medicine, receives research promotion grants from Astellas, Alexion, Otsuka, Kyowa Hakko Kirin, Takeda, Torii, Pfizer, and MSD. However, the research topics of these donation grants are not restricted. All the other authors declared no competing interests.
Acknowledgments
The following researchers from the Kaikoukai Healthcare Group contributed to this study: Hirohisa Kawahara, Takashi Sato, Hajime Inoue, Takeshi Onogi, Shinji Watabe, Satoshi Yamaguchi, Katsura Hamaguchi, Ryo Takahashi, Masayuki Hirata. Safety Monitoring Committee, Takayuki Katsuno, Hiroki Hayashi, and Hideki Ishii. Clinical research coordinators and data management group included Kayo Torii, Miki Abe, and Yumi Fujihara. The authors thank Editage (www.editage.jp) for English language editing. This study was funded by Ono Pharmaceutical Co, Ltd., Japan.
Author Contributions
YI was responsible for the research idea, study concept, and design, and was primarily responsible for researching and writing the article. SK took part in composing the study protocol, analysis, and interpretation of the data; constructing the study management team; and writing the article. MT, MH, TI, and TK took part in composing the study protocol and acquisition, analysis, and interpretation of the data. HK, YT, and FS enrolled the patients and took part in analysis of the data. YK constructed the statistical analysis. MA compiled the data management system. SM was also responsible for the research idea, the study concept, designing and organizing the study as a principal investigator, as well as supervising the writing of the manuscript.
Footnotes
Figure S1. Flow diagram of this study.
Figure S2. Distribution of iPTH at the end of the intervention.
Figure S3. Achieved targeted corrected calcium and phosphate levels in line with the Japanese Society for Dialysis Therapy at the end of the intervention.
Figure S4. Change from baseline over time for magnesium.
Figure S5. Weekly dose of etelcalcetide over time.
Figure S6. Use of calcitoriol, alfacalcidol, or active vitamin D analogs over time.
Figure S7. Weekly dose of calcitoriol, alfacalcidol, or active vitamin D analogs over time. The patients in the Etercalcetide+VitD group and the control group included 5 patients and 1 patient who changed alfacalcidol to maxacalcitol, respectively.
Figure S8. Weekly dose of calcium preparation (oral calcium carbonate) over time.
Figure S9. Use of calcium-noncontaining phosphate binders over time.
Table S1. Risks of etelcalcetide-associated hypocalcemia at end of intervention.
CONSORT Checklist.
Supplementary Material
References
- 1.Cunningham J., Locatelli F., Rodriguez M. Secondary hyperparathyroidism: pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol. 2011;6:913–921. doi: 10.2215/CJN.06040710. [DOI] [PubMed] [Google Scholar]
- 2.Beto J., Bhatt N., Gerbeling T. Overview of the 2017 KDIGO CKD-MBD update: practice implications for adult hemodialysis patients. J Ren Nutr. 2019;29:2–15. doi: 10.1053/j.jrn.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Ketteler M., Block G.A., Evenepoel P. Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guideline Update: what's changed and why it matters. Kidney Int. 2017;92:26–36. doi: 10.1016/j.kint.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Bover J., Urena P., Ruiz-Garcia C. Clinical and practical use of calcimimetics in dialysis patients with secondary hyperparathyroidism. Clin J Am Soc Nephrol. 2016;11:161–174. doi: 10.2215/CJN.01760215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Messa P., Macario F., Yaqoob M. The OPTIMA study: assessing a new cinacalcet (Sensipar/Mimpara) treatment algorithm for secondary hyperparathyroidism. Clin J Am Soc Nephrol. 2008;3:36–45. doi: 10.2215/CJN.03591006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fishbane S., Shapiro W.B., Corry D.B. Cinacalcet HCl and concurrent low-dose vitamin D improves treatment of secondary hyperparathyroidism in dialysis patients compared with vitamin D alone: the ACHIEVE study results. Clin J Am Soc Nephrol. 2008;3:1718–1725. doi: 10.2215/CJN.01040308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cozzolino M., Urena-Torres P., Vervloet M.G. Is chronic kidney disease–mineral bone disorder (CKD-MBD) really a syndrome? Nephrol Dial Transplant. 2014;29:1815–1820. doi: 10.1093/ndt/gft514. [DOI] [PubMed] [Google Scholar]
- 8.Levin A., Bakris G.L., Molitch M. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71:31–38. doi: 10.1038/sj.ki.5002009. [DOI] [PubMed] [Google Scholar]
- 9.Floege J., Kim J., Ireland E. Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol Dial Transplant. 2011;26:1948–1955. doi: 10.1093/ndt/gfq219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tentori F., Wang M., Bieber B.A. Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: the DOPPS study. Clin J Am Soc Nephrol. 2015;10:98–109. doi: 10.2215/CJN.12941213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kidney Disease: Improving Global Outcomes CKD-MBD Update Work Group KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease‒mineral and bone disorder (CKD-MBD) Kidney Int Suppl. 2009:S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 12.Kidney Disease: Improving Global Outcomes CKD-MBD Update Work Group KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD) Kidney Int Suppl. 2017;7:1–59. doi: 10.1016/j.kisu.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Kidney Foundation K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(Suppl):S1–S201. [PubMed] [Google Scholar]
- 14.Fukagawa M., Yokoyama K., Koiwa F. Clinical practice guideline for the management of chronic kidney disease–mineral and bone disorder. Ther Apher Dial. 2013;17:247–288. doi: 10.1111/1744-9987.12058. [DOI] [PubMed] [Google Scholar]
- 15.Kato S., Tsuboi M., Ando M. Rationale and study design of a randomized controlled trial for development of a treatment strategy for chronic kidney disease–mineral and bone disorder by multilateral mechanism of etelcalcetide hydrochloride (the DUET study) Ren Replace Ther. 2019;5:39. [Google Scholar]
- 16.Cannata Andia J.B. Adynamic bone and chronic renal failure: an overview. Am J Med Sci. 2000;320:81–84. doi: 10.1097/00000441-200008000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Lee S.A., Lee M.J., Ryu G.W. Low serum intact parathyroid hormone level is an independent risk factor for overall mortality and major adverse cardiac and cerebrovascular events in incident dialysis patients. Osteoporos Int. 2016;27:2717–2726. doi: 10.1007/s00198-016-3636-1. [DOI] [PubMed] [Google Scholar]
- 18.Feroze U., Molnar M.Z., Dukkipati R. Insights into nutritional and inflammatory aspects of low parathyroid hormone in dialysis patients. J Ren Nutr. 2011;21:100–104. doi: 10.1053/j.jrn.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Block G.A., Bushinsky D.A., Cheng S. Effect of etelcalcetide vs cinacalcet on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: a randomized clinical trial. JAMA. 2017;317:156–164. doi: 10.1001/jama.2016.19468. [DOI] [PubMed] [Google Scholar]
- 20.Pereira L., Meng C., Marques D. Old and new calcimimetics for treatment of secondary hyperparathyroidism: impact on biochemical and relevant clinical outcomes. Clin Kidney J. 2018;11:80–88. doi: 10.1093/ckj/sfx125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Block G.A., Bushinsky D.A., Cunningham J. Effect of etelcalcetide vs placebo on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: two randomized clinical trials. JAMA. 2017;317:146–155. doi: 10.1001/jama.2016.19456. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.