Abstract
Introduction
In humans, heterozygous mutations of hepatocyte nuclear factor 1beta (HNF1B) are responsible for a dominant inherited disease with both renal and extrarenal phenotypes. HNF1B nephropathy is the umbrella term that includes the various kidney phenotypes of the disease, ranging from congenital anomalies of the kidney and urinary tract (CAKUT), to tubular transport abnormalities, to chronic tubulointerstitial and cystic renal disease.
Methods
We describe 7 families containing 13 patients with ascertained HNF1B nephropathy. All patients underwent genetic testing and clinical, laboratory, and instrumental assessment, including renal imaging and evaluation of extrarenal HNF1B manifestations.
Results
Significant inter- and intrafamilial variability of HNF1B nephropathy has been observed. In our cohort, HNF1B pathogenic variants presented with renal cysts and diabetes syndrome (RCAD); renal cystic phenotype mimicking autosomal dominant polycystic kidney disease (ADPKD); autosomal dominant tubulointerstitial kidney disease (ADTKD) with or without hyperuricemia and gout; CAKUT; and nephrogenic diabetes insipidus (NDI). Of note, for the first time, we describe the occurrence of medullary sponge kidney (MSK) in a family harboring the HNF1B whole-gene deletion at chromosome 17q12. Genotype characterization led to the identification of an additional 6 novel HNF1B pathogenic variants, 3 frameshift, 2 missense, and 1 nonsense.
Conclusion
HNF1B nephropathy may present with a highly variable renal phenotype in adult patients. We expand the HNF1B renal clinical picture to include MSK as a potential new finding. Finally, we expand the allelic repertoire of the disease by adding novel HNF1B pathogenic variants.
Keywords: ADPKD, ADTKD, CAKUT, cystic kidney disease, HNF1B, medullary sponge kidney, nephrogenic diabetes, RCAD, tubulointerstitial nephritis
Graphical abstract
See Commentary on Page 2133
HNF1B gene, also known as transcription factor-2 (TCF2), is a developmental gene located on chromosome 17q12, coding for protein hepatocyte nuclear factor 1 homeobox B, with a crucial role in early embryonic development. The TFC2 protein is required for tissue-specific gene expression in the epithelial cells of many organs, including the kidney, pancreas, liver, and genitourinary tract.1,2 HNF1B is also transiently expressed in the neural tube, epididymis, seminal vesicles, prostate, and uterus.1,2 This expression pattern explains the frequently highly variable presentation of HNF1B-related disorder.
In 1997, Horikawa et al.3 first reported, in a Japanese family, the association of pathogenic variants of HNF1B gene with maturity onset diabetes of the young 5 (MODY-5), a monogenic form of diabetes mellitus characterized by onset before 25 years of age and autosomal dominant inheritance.3 Renal disease was also reported in some affected members of the family.3
Since then, renal involvement has emerged as the earliest and most prevalent finding described in almost all patients with HFN1B variants, which are now considered to be the most frequent monogenic cause of developmental renal disease.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17
In the last decade, various kidney phenotypes have been described among patients with HFN1B pathogenic variants, encompassing CAKUT and tubular transport disorders.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 To date, HFN1B-nephropathy phenotype includes the following: pre-natal hyperechogenic kidney; chronic tubulointerstitial kidney disease; renal cystic disease; renal hypo-dysplasia; glomerulocystic kidney disease; single, horseshoe, and duplex kidneys; bilateral hydronephrosis; nephro-calcinosis; nephrogenic diabetes insipidus; and hypomagnesemia due to renal magnesium wasting.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17
Although the association of MODY with a heterogenous clinical kidney disease is the core phenotype of HFN1B-associated disease, an ever-expanding spectrum of extrarenal manifestations have been reported, such as exocrine pancreatic failure, pancreatic hypoplasia, fluctuating liver tests abnormality, early-onset gout, and genital tract malformations.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 Therefore, HNF1B disease must be considered as an autosomal, dominant multi-system disorder (MIM 137920).
In approximately 40% to 50% of patients, the molecular defect is a whole HNF1B gene deletion, occurring in the context of the 17q12 recurrent deletion syndrome that includes several other genes (OMIM 614527); the 17q12 recurrent deletion syndrome can be associated with mild dysmorphic features and neurodevelopmental/neuropsychiatric disorders, attributable to deletion of the genes other than HNF1B located in the 17q12 region.16 In the remaining cases, heterozygous HNF1B variants are detected: about 52% are missense, 29% are frameshift, and 15% are nonsense (LOVD database; https://databases.lovd.nl/shared/genes/HNF1B/graphs).
De novo HNF1B variants are encountered in approximately 30% to 50% of cases.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 A close genotype−phenotype correlation is lacking. Some authors have suggested that patients with HNF1B sequence pathogenic variant may have a poorer renal prognosis than those with whole-gene deletion. This observation is based mainly on the evidence that in a large cohort of adult HNF1B-disease patients, those with sequence variants showed a higher frequency of chronic kidney disease stage 3 to 4 or end-stage renal disease and a lower median estimated glomerular filtration rate (eGFR) at follow-up compared with patients with whole-gene deletion.15,17 The mechanism underlying this possible genotype−phenotype correlation remains unexplained; it can be hypothesized that some HNF1B variants may have a dominant negative effect worsening the phenotype.17
Here we describe 13 patients from 7 families of Italian ancestry with HNF1B nephropathy, showing a great inter- and intrafamilial variability in the renal disease, including the following: RCAD; renal cystic phenotype mimicking ADPKD; ADTKD with hyperuricemia and gout; CAKUT; and prenatal hyperechogenic kidney in association with NDI. Interestingly, for the first time, we describe, in a family with 17q12 recurrent deletion syndrome, a renal phenotype consistent with MSK, a rare developmental disorder for which the underlying defect is not fully understood.
Despite the single-genetic etiology, HNF1B nephropathy has a protean clinical presentation. Diagnosis of HNF1B nephropathy is often challenging, and the differential diagnosis is complex, as the disease may mimic a variety of renal disorders. For this reason, HNF1B nephropathy can be considered as one of the “great masqueraders” in nephrology.
Methods
Since 2014, a total of 120 patients with clinical diagnosis of tubulo-interstitial kidney disease have been evaluated at the Division of Nephrology, University of Brescia (Brescia, Italy); 92 patients have undergone genetic testing. Diagnosis of tubulo-interstitial kidney disease was established on the basis of renal phenotype characterized by chronic tubulo-interstitial nephritis with nonsignificant urinalysis and slowly progressive renal failure, with or without hyperuricemia and gout. Genetic testing for ADTKD genes was offered to patients with a positive family history for tubulo-interstitial kidney disease or sporadic cases with renal disease onset at less than 50 years of age.
The genetic testing protocol included targeted gene panel NGS analysis to detect sequence variants in the following genes related to ADTKD or cystic kidney diseases: PKD2, UMOD, HNF1B, REN, GANAB, PARN, ALG8, and DNAJB11. To detect HNF1B gene deletion, multiple ligation probe amplification (MPLA) analysis was performed in all patients (Supplementary Materials and Methods).
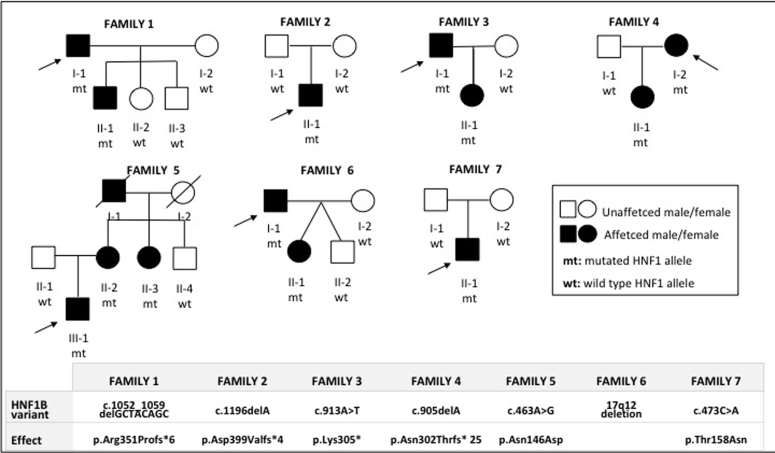
In our series, 7 of 92 probands had pathogenic variants in HNF1B (7.6%). All family members underwent genetic testing and clinical, laboratory, and instrumental assessments, including renal function tests and renal imaging. At-risk family members were offered presymptomatic genetic testing. Extension of the clinical and molecular study to relatives allowed us to find an additional 6 affected patients, for a total of 7 families with 13 affected members (Figure 1).
Figure 1.
Pedigrees of families showing affected and unaffected individuals and HNF1B testing results. In the lower part of the figure, the HNF1B gene variants for each family are listed.
Medical histories were obtained as part of all patients’ clinical workup. Clinical and genetic data were collected according to national laws (Supplementary Materials and Methods).
Results
Clinical and molecular data of the patients are summarized in Figure 1 and Table 1, Table 2, Table 3.8
Table 1.
Renal manifestations in reported HNF1B patients
| Pedigree ID (sex) | Family 1 |
Family 2 |
Family 3 |
Family 4 |
Family 5a |
Family 6 |
Family 7 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I-1 (M) | II-1 (M) | II-1 (M) | I-1 (M) | II-1 (F) | I-2 (F) | II-1 (F) | III-1a (M) | II-2a (F) | II-3a (F) | I-1 (M) | II-1 (F) | II-1 (M) | |
| First evaluation (age) | 56 yr | 2 yr | 28 yr | 26 yr | 28 yr | 16 yr | 47 yr | 47 yr | 23 yr | 18 yr | |||
| Serum creatinine (mg/dl) | 0.80 | 0.8 | 2.27 | 1.78 | N/A | 1.32 | N/A | 1.4 | 1.2 | 1.04 | 1.2 | N/A | 1.5 |
| eGFRb (ml/min per 1.73 m2) | 99 | 46c | 38 | 51.5 | 54 | 73.9 | 53.8 | 63.9 | 84.7 | 67 | |||
| Suspected diagnosis at presentation | ADPKD | NPH | NPH | ADTKD | N/A | ADTKD | N/A | ARPKD | MCKD | MCKD | CAKUT/MSK | NC | NPH |
| Last follow-up (age) | 77 yr | 35 yr | 54 yr | 48 yr | 19 yr | 38 yr | 9 yr | 25 yr | 58 yr | 57 yr | 51 yr | 16 yr | 38 yr |
| Serum creatinine (mg/dl) | 1.5 | 2.17 | 4.3 | 5.5 | 0.96 | 1.49 | 0.72 | 1.56 | 1.41 | 1.20 | 1.2 | 1.0 | 2.36 |
| eGFRb (ml/min per 1.73 m2) | 44.3 | 38 | 14.4 | 12 | 114.4 | 44 | >75c | 60.8 | 41 | 50.3 | 69.6 | 110 | 33.7 |
| Hyperuricemia/gout | Yes/no | No/no | Yes/yes | Yes/no | Yes/no | Yes/no | No/no | No/no | No/no | No/no | No/no | No/no | No/no |
| Magnesemia (n.v. 0.66−1.07 mEq/l) | 0.70 | 0.60 | 0.31 | 0.56 | 0.61 | 0.60 | 0.66 | 0.78 | 0.75 | 0.90 | 0.66 | 0.90 | |
| Kalemia/serum calcium (K+ n.v. 3.5−5.3 mEq/l) (Ca++ n.v. 9.3−10.6 mg/dl) |
3.4/9.3 | 3.6/9.5 | 3.1/6.79 | 3.3/9.6 | 3.3/9.9 | 3.6/9.36 | 3.6/10.6 | 5.1/10 | 4.9/8.4 | 4.9/9 | 3.8 /8.4 | 4,8/9.7 | 4.9/10.1 |
| Renal biopsy (age) | No | Yesd (2 yr) | Yesd (28 yr) | No | No | No | No | No | No | No | No | No | Yesd (31 yr) |
| Other renal features | No | POL | No | No | No | No | No | 11POL 12NDI | No | No | No | No | No |
| Type of HNF1B nephropathy | ADTKD | Early-onset ADTKD |
ADTKD | ADTKD | ADTKD | ADTKD | ADTKD | Early-onset ADTKD |
RCAD (ADTKD) | RCAD (ADTKD) | CAKUT/MSK | NC/MSK | ADTKD |
ADPKD, autosomal dominant polycystic kidney disease; ADTKD, autosomal dominant tubulo-interstitial kidney disease; ARPKD, autosomal recessive polycystic kidney disease; CAKUT, congenital anomalies of kidney and urinary tract; DI, nephrogenic diabetes insipidus; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; F, female; M, male; MCKD, medullary cystic kidney disease; MSK, medullary sponge kidney; N/A, not available; NC, nephrocalcinosis; No, absent; NPH, nephronophthisis; n.v., normal value; POL, polyuria in infancy; RCAD, renal cysts and diabetes; Yes, present.
Patients reported in Izzi et al.8
eGFR calculated with Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula.
eGFR calculated with Schwartz formula.
Renal biopsy findings: interstitial fibrosis and tubular atrophy.
Table 2.
Extrarenal manifestations in reported HNF1B patients
| Pedigree ID (sex) | Family 1 |
Family 3 |
Family 5a |
Family 6 |
||
|---|---|---|---|---|---|---|
| I-1 (M) | II-1 (M) | I-1 (M) | II-2a (F) | II-3a (F) | I-1 (M) | |
| Pancreas abnormalities | Hypoplasia | Hypoplasia | Hypoplasia | — | — | Hypoplasia |
| Genital tract abnormalities | — | — | — | Septate uterus | — | EDO |
| Glycemic status: diabetes | — | Yes | Yes | Yes | GD | Yes |
| Neurologic impairment | — | — | — | — | — | Epilepsy, mild cognitive impairment |
| Cardiovascular disease | Severe aortic insufficiency | Mild aortic bulb ectasia, MVP |
— | — | — | MVP |
—, not present; EDO, ejaculatory duct obstruction; F, female; GD,gestational diabetes; M, male; MVP, mitral valve prolapse; No, absent; Yes, present.
Patients reported in Izzi et al.8
Table 3.
Renal imaging (US, CT, or MRI scan) in reported HNF1B patients
| Family | Pedigree ID | Presentation | Last follow-up |
|---|---|---|---|
| Family 1 | I-1 | Multiple bilateral cysts, increased TKV, renal stone (56 yr) | Multiple bilateral cysts, increased TKV (73 yr) |
| II-1 | Normal kidneys (2 yr) | Multiple bilateral cysts, normal kidneys (36 yr) | |
| Family 2 | II-1 | Few bilateral cysts, normal kidneys (28 yr) | Bilateral small cysts, small kidneys, renal stones (54 yr) |
| Family 3 | I-1 | Few cysts, normal kidneys (35 yr) | Few cysts, small kidneys (49 yr) |
| II-1 | Normal kidneys (19 yr) | NA | |
| Family 4 | I-2 | Normal kidneys (28 yr) | Few cortical cysts, small kidneys (38 yr) |
| II-1 | Few bilateral cysts (9 yr) | NA | |
| Family 5 | III-1 | Antenatal: HE, enlarged kidneys (22 wk); bilateral cysts, normal kidneys (3 yr) | Multiple bilateral cysts (25 yr) |
| II-2 | Few bilateral cysts | Few small bilateral cysts (45 yr) | |
| II-3 | NA | Bilateral cysts (40 yr) | |
| Family 6 | I-1 | MSK,, few left renal cysts, right hypoplastic kidney (23 yr) | MSK, bilateral cysts, right hypoplastic kidney (45 yr) |
| II-1 | Echogenic foci in renal medulla suggestive for calcifications (8 yr) | MSK, few small cysts (16 yr) | |
| Family 7 | II-1 | NA | Bilateral cysts, small kidneys (33 yr) |
Age in parentheses is age at evaluation. CT, computed tomography; HE, hyperechogenic kidneys; MRI, magnetic resonance imaging; MSK, medullary sponge kidney; NA, not available; TKV, total kidney volume; US, ultrasound; wk, week of gestation.
Family 1
The index case (I-1, Figure 1), a 73-year-old man, was referred to the nephrologist because of large bilateral renal cysts. His medical history revealed severe aortic insufficiency and aortic bulb dilatation requiring cardiac surgery at age 55 years; serum creatinine (sCr) was 0.8 mg/dl (eGFR 99 ml/min per 1.73 m2, calculated with the Chronic Kidney Disease Epidemiology Collaboration [CKD-EPI] formula). At referral, sCr was 1.14 mg/dl (eGFR 63 ml/min per 1.73 m2) and uric acid 8.4 mg/dl (Table 1). Urinalysis findings was nonsignificant, and hemoglobin A1c was in the normal range. A computed tomography (CT) scan revealed 2 enlarged kidneys with numerous bilateral cysts, no liver cysts, and mild pancreatic hypoplasia without alteration of glycemic status (Tables 2 and 3, Figure 2a). For the cystic renal involvement and the vascular phenotype, sequence, and MLPA analysis of PKD1 and PKD2 genes, results were negative.
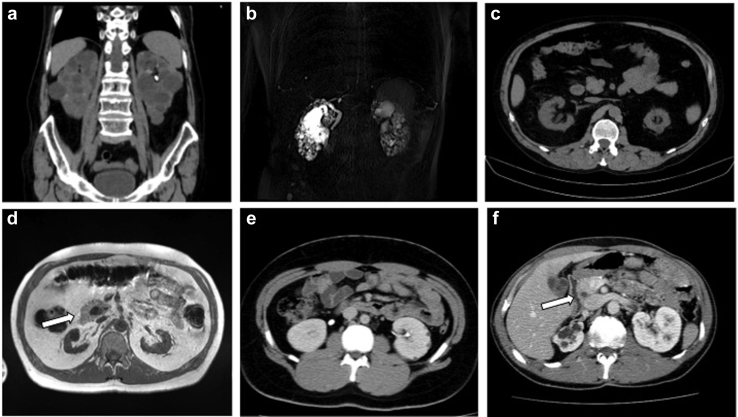
Figure 2.
(a) I-1, family 1: computed tomography (CT) scan showing enlarged bilateral cysitc kidneys, left renal stone. (b) II-1, family 1: magnetic resonance imaging (MRI) scan showing multiple small bilateral kidney cysts in normal sized kidneys. (c) II-1, family 2: CT scan showing bilateral small kidneys and few cysts. (d) I-1, family 3: MRI scan showing bilateral small kidneys and annular pancreas (white arrow). (e) III-1, family 5: CT scan showing bilateral normal sized kidneys and several cysts. (f) I-1, family 6: CT scan showing right kidney hypoplasia with cysts, including multiple cysts in the left kidney and annular pancreas (white arrow).
One year later, we evaluated the 35-year-old son (II-1, Figure 1) with chronic kidney disease stage IIIB (sCr 2.17 mg/dl; eGFR 38 ml/min per 1.73 m2). His past history revealed onset of polyuria and polydipsia at age 2. Nephronophthisis was hypothesized by renal biopsy, which revealed a picture of chronic tubulointerstitial nephritis (Table 1). At referral, diabetes, hypomagnesemia, and hypokalemia were documented (Tables 1 and 2). A CT scan showed bilateral cortical cysts with normal kidney volume and pancreatic hypoplasia (Table 3, Figure 2b). A mild aortic bulb dilation was also recognized. Multigene NGS panel analysis for ADKTD revealed a novel heterozygous disease−causing frameshift variant in HNF1B: c.1052_1059delGCTACAGC; p. Arg351Profs∗6. Segregation analysis revealed that the father also harbored the familial pathogenic HNF1B variant.
Family 2
The only affected family member of this family was a 54-year-old man (II-1, Figure 1) who was diagnosed with chronic renal disease stage III at age 27 (sCr 2.27 mg/dl, eGFR 38 ml/min per 1.73 m2). Urinalysis was not significant. Renal biopsy was performed at age 28 and showed diffuse tubulo-interstitial fibrosis (Table 1). A diagnosis of nephronopthisis was made. At referral, renal function deterioration was documented (sCr 4.3 mg/dl, eGFR 14.4 ml/min per 1.73 m2) (Table 1). A CT scan revealed 2 small kidneys with few small cortical cysts (Figure 2c, Table 3). His medical history revealed hyperuricemia and recurrent gouty attacks, unexplained elevated liver enzymes, and normal serum magnesium levels (Table 1). Multigene NGS panel analysis for ADKTD disclosed a novel pathogenic heterozygous frameshift mutation in HNF1B: c.1196delA; p. Asp399Valfs∗4 (Table 2).
Family 3
The index case, a 52-year-old man (I-1, Figure 1), was diagnosed with chronic renal disease stage III at age 26 (sCr 1.78 mg/dl, eGFR 51.5 ml/min per 1.73 m2). Abdominal ultrasound (US) revealed normal kidney volume and left renal cysts, and urinalysis was nonsignificant (Table 3). Hypomagnesemia, uncomplicated hyperuricemia, and diabetes were also found (Table 1). At age 48, sCr 5.5 mg/dl was documented (eGFR 12 ml/min per 1.73 m2), and at age 49, a living-donor kidney transplantation was performed (Table 1). A CT scan revealed 2 small kidneys with few cysts and pancreas hypoplasia (Figure 2d, Table 3). A multigene NGS panel analysis for ADKTD revealed a heterozygous nonsense mutation in HNF1B gene c.913A>T; p. Lys305∗ (Table 2). The 19-year-old daughter (II-1, Figure1) inherited the familial pathogenic variant of HN1FB. A tailored clinical evaluation was performed, which showed normal renal function, hypomagnesemia, and hyperuricemia (Table 1). Abdominal US findings were normal (Table 3).
Family 4
The 38-year-old proband (I-2, Figure 1) was incidentally found to have sCr 1.32 mg/dl (eGFR 54 ml/min per 1.73 m2) at age 28; urinalysis was negative except for the low specific gravity (Table 1). At last follow-up, at age 38, sCr was 1.49 mg/dl and eGFR 44 ml/min per 1.73 m2 (Table 1). Renal US showed moderately reduced kidneys and few cortical cysts (Table 3). Multigene panel for ADTKD was scheduled and revealed a novel heterozygous frameshift mutation in HNF1B: c.905delA; p.Asn302Thrfs∗ 25 (Table 2). In the 10-year old daughter (II-1, Figure 1), US showed few bilateral cortical cysts (Table 3). Renal function was in the normal range for age, and magnesium level was in the lower range (Table 1). The maternal HNF1B variant was identified (Figure 1).
Family 5
This family has already been described in a previous report.8 Briefly, in the index case (III-1, Figure 1), renal disease was diagnosed prenatally, when fetal US disclosed enlarged hyperechogenic kidneys and polyhydramnios (Table 3). After birth, the clinical course was characterized by normal-sized kidneys showing numerous bilateral renal cysts; severe polyuria and polydipsia, consistent with a diagnosis of partial NDI, was present; slowly progressive chronic kidney disease was also evident (Tables 1 and 3). At last follow-up, at age 25, sCr was 1.56 mg/dl and eGFR 60 ml/min per 1.73 m2) (Table 1). Diagnosis of early-onset HNF1B nephropathy was made following the detection of a novel heterozygous missense variant in HNF1B c.463A>G; p.Asn146Asp (Table 2). Family history and segregation analysis revealed an affected 58-year-old mother (II-2, Figure 1) (eGFR 41 ml/min per 1.73 m2, bilateral renal cysts by US, diabetes mellitus, and septate uterus) and an affected 57-year-old maternal aunt (II-3, Figure 1) (eGFR 50.3 ml/min per 1.73 m2, bilateral renal cysts, and gestational diabetes) (Table 1, Table 2, Table 3).
Family 6
In the index case (I-1, Figure 1), hypoplastic right kidney and MSK were diagnosed at age 23 by urography; sCr was 1.2 mg/dl and eGFR 84.7 ml/min per 1.73 m2 (Table 1). At last follow-up, at age 51, sCr was 1.2 mg/dl and eGFR 69.6 ml/min per 1.73 m2 (Table 1). Clinical history was also remarkable for multifaceted extrarenal manifestations, including mild cognitive impairment, juvenile diabetes mellitus, generalized epilepsy, ejaculatory duct obstruction (recognized during ascertainment for infertility) (Table 2). A CT scan showed nephrocalcinosis, cystic dilatation of the renal medullary collecting ducts, and pancreas hypoplasia (Figure 3a, Tables 2 and 3). Because of the diagnosis of MSK, a nephropathy of unknown cause, the patient was enrolled 5 years ago for whole-exome sequencing in a research project dedicated to families with genetic nephropathy of unknown origin. The analysis did not identify pathogenic sequence variants. Recently, on suspicion of genomic syndrome because of neurologic extrarenal manifestations in the proband, we searched for HNF1B whole-gene deletion with MLPA analysis. Because MPLA analysis revealed a whole-gene HNF1B deletion, CGH-array analysis was performed to define the extension of the chromosomal deletion, revealing a 17q12 deletion of 1.77 to 2.08 Mb, including the HNF1B gene (arr[GRCh37]17q12(34438350_36207539)x1), enabling the diagnosis of the recurrent 17q12 microdeletion syndrome.
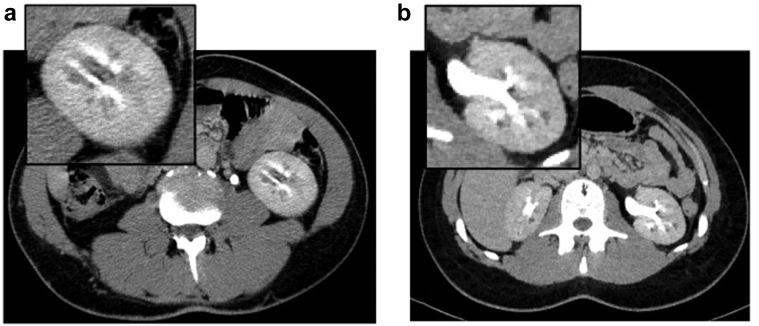
Figure 3.
Computed tomography scan of (a) I-1 and (b) I-2 of family 6: insets show magnification of left kidney with nephrocalcinosis and cystic dilatation of the renal medullary collecting ducts.
Molecular study documented that the daughter (I-2, Figure 1) inherited the 17q12 deletion from the father (Table 2). Her clinical history was unremarkable except for echogenic foci in the renal medulla suggestive for calcifications, revealed on abdominal US at 8 years of age (Table 3). At age 16, renal function was normal and urinalysis was negative (Figure 3b, Tables 1 and 3). A CT scan of the abdomen showed cystic dilatation of the renal medullary collecting ducts, consistent with a diagnosis of MSK; few cortical cysts were also recognized (Table 3). Neurologic involvement was not present (Table 2).
Family 7
The 33-year-old index case (II-1, Figure 1) was noted to have renal impairment at age of 18, when sCr was 1.4 mg/dl (eGFR 67 ml/min per 1.73 m2) (Table 1). Renal biopsy indicated a picture of tubulo-interstitial nephritis, and a diagnosis of nephronophthisis was made (Table 1). At referral, at age 33, sCr was 2.2 mg/dl and eGFR 38 ml/min per 1.73 m2 (Table 1). Renal imaging reveled reduced kidney volume and bilateral cysts (Table 3); urinalysis was normal except for a decreased urinary concentration ability. Multigene NGS analysis for ADTKD disclosed a de novo heterozygous missense mutation in HNF1B c.473C>A; p.Thr158Asn (Figure 1). Extrarenal manifestations were not recognized (Table 2).
Classification of Sequence Variants in HNF1B Gene
All HNF1B variants detected in families 1, 2, 3, 4, 5, and 7 are novel, not listed in any public repository of population-based exome sequencing project (ExAC, gnomAD) and not found in 169 ethnically matched controls. Phenotype is consistent with the disease associated with HNF1B, and the variants co-segregated with the disease in family affected members. These data provide strong evidence of pathogenicity for the nonsense variants p.Arg351Profs∗6, p.Asp399Valfs∗4, p.Lys305∗, and p.Asn302Thrfs∗25, according to the proposed classification of pathogenicity by the American College of Medical Genetics (ACMG).18
The p. Asn146Asp variant co-segregated with the disease in family affected members and has been excluded in nonaffected members. Segregation analysis in the unaffected parents in family 7 revealed that the p. Thr158Asn variant is de novo, supporting evidence of probable pathogenicity, according to the ACMG classification.19 The most common in silico prediction tools (Align-GVGD, SIFT, PolyPhen-2, MutationTaster2) based on sequence homology, protein structure, and evolutionary conservation classified the 2 missense variants as deleterious.
Discussion
HNF1B nephropathy is a condition characterized by an autosomal dominant inheritance and a highly variable renal phenotype, including cystic abnormalities, CAKUT, tubular transport disorders, and slowly progressive renal decline.1, 2, 3, 4, 5, 6 Renal presentation varies widely according to age at recognition. In the fetus, hyperechogenic kidneys with normal or slightly enhanced size and normal amniotic fluid or polyhydramnios is the more frequent renal phenotype.20,21 In childhood, HNF1B nephropathy is typically characterized by renal cystic hypo-dysplasia and CAKUT.19,22 In adults, renal phenotype is less frequently described, and is usually characterized by slowly progressive chronic renal failure with a tubulointerstitial pattern.12, 13, 14, 15,17,23
In our study, we focused on HNF1B nephropathy in 11 affected adults and 2 young patients belonging to 7 families, providing some new clinical and imaging findings useful for the recognition of HNF1B nephropathy. With respect to the renal phenotype, the key findings were 3-fold.
First, the most common clinical renal presentation was that of chronic tubulo-interstitial nephritis, characterized by nonsignificant urinalysis and slowly progressive renal failure; when performed, renal biopsy confirmed a picture of interstitial fibrosis and tubular atrophy. In some patients, this picture was associated with hyperuricemia and gout. Varying degrees of renal impairment were found, from mild to moderate renal involvement to end-stage renal disease. A similar phenotype is usually found in patients with heterozygous variants of UMOD, MUC1, and REN genes, causing an autosomal dominant form of tubulo-interstitial nephritis.24, 25, 26, 27 For this, HNF1B nephropathy is now categorized as part of the group of disorders named ADTKD, together with ADTKD-UMOD, ADTKD-MUC1, ADTKD-REN, and ADTKD-SEC61A1.24, 25, 26, 27 In our series, 9 of 13 patients had a renal presentation of chronic tubulo-interstitial nephritis; only 2 of these patients had diabetes, making the phenotype indistinguishable from that of other ADTKD subtypes. The renal phenotypes associated with HNF1B variants, however, only partly overlap with mutations in REN, UMOD, and MUC1. Indeed, although the clinical manifestations of diseases caused by variants in UMOD, MUC1, and REN are usually confined to the kidney, HNF1B variants result in variable renal and extrarenal manifestations.12, 13, 14, 15,24, 25, 26, 27 For this, it appears reasonable to confine the term “ADTKD” to those HNF1B-related cases in which tubulo-interstitial fibrosis is the leading renal manifestation. The mechanism whereby mutated HNF1b leads to renal fibrosis is not fully understood. In ADTKD-UMOD, impaired intracellular trafficking and retention of mutant uromodulin may initiate an inflammatory process, resulting in progressive interstitial fibrosis, suggesting a gain-of-function effect of UMOD variants.27 Although HNF1b regulates the transcription of UMOD, it is unlikely that a mechanism similar to the gain-of-function effect of UMOD mutations in ADTKD-UMOD is responsible for the phenotype of ADTKD-HNF1B, as patients with HNF1B-related ADTKD have normal UMOD expression.26,27 On the other hand, HNF1b has been found to be implicated in epithelial–mesenchymal transition, a process by which epithelial cells acquire mesenchymal characteristics. Epithelial–mesenchymal transition is essential for tissue regeneration and, when sustained, is associated with fibrogenesis.28 Recently, Chan et al. found that loss of HNF1b may induce epithelial–mesenchymal transition both in vitro and in vivo, leading to up-regulation of expression of transforming growth factor−β ligands in renal epithelial cells, which can ultimately cause renal fibrosis.29 These findings reveal a potential novel mechanism of renal fibrosis in HNF1B nephropathy.2,29
The second important finding of our study was that cystic disease was the predominant renal imaging phenotype, being found in 92% of patients (Table 3). This is consistent with previous reports describing cystic phenotype in 62% to 81% of adult patients and in 73% of a large case series including both pediatric and adult patients.5,6,15,17,22 The finding of cystic kidneys is not unexpected in patients with HNF1B mutation. In an animal model, HNF1b regulates the transcription of numerous cystic disease genes, including PKHD1, PKD2, UMOD, GLIS2, and KIF12, a polycystic kidney disease (PKD) modifier gene.2,30 Moreover, it is of note that humans carrying heterozygous mutations of both PKD1 and HNF1B have a more severe cystic phenotype, suggesting that HNF1B may virtually function as a modifier gene in ADPKD.31,32 Taken together, these data indicate that HNF1b produces kidney cysts by downregulating the expression of multiple cystic disease genes.2,30 In keeping with previous reports, renal cysts were usually small.7,12 However, in the index case of family 1, massively enlarged cystic kidneys mimicking those of ADPKD were found (Tables 1 and 3). This uncommon renal presentation, already described by Faguer et al. in two 20-year-old monozygous twins,33 suggests that phenocopies of the ADPKD phenotype can be due to HNF1B variants. In our case, the identification of the affected son with a picture of chronic tubulointerstitial disease since infancy and diabetes paved the way for accurate diagnosis and confirmed that, in HNF1B nephropathy, phenotypic variability is often extensive, even within the same family (Tables 1 and 3). A more severe cystic phenotype was also found in the index case of family 5; in this patient, the kidneys, although normal in size, showed numerous bilateral cysts, which were probably responsible for a secondary form of polycythemia with inappropriately high serum erythropoietin produced by renal cysts (Tables 1 and 3).
The third major finding was the occurrence of 2 unusual renal phenotypes in the context of HNF1B nephropathy, such as severe polyuria attributable to NDI and MSK. In the index case of family 5, already reported by our group as a case report,8 the course of the renal disease since infancy was characterized by severe polyuria, increase in size and number of renal cysts, and slow progression of chronic kidney disease (Table 1). In the absence of mutations in AVPR2 and AQP2 genes, for the first time a diagnosis of partial NDI in the context of HNF1B nephropathy was made.8 Polyuria has already been observed in humans with mutations of HNF1B2,4; however, it is not a universal finding, particularly in adult patients.12, 13, 14, 15,24,26 Usually, the causes of polyuria include glycosuria due to early-onset diabetes, and renal sodium and potassium wasting. Recently, however, a murine model with HNF1B deletion in the renal collecting ducts exhibited persistent polyuria due to partial nephrogenic diabetes insipidus through multiple mechanisms, including impaired intracellular trafficking of AQP2.34 These experimental findings disclose a role of HNF1b in osmoregulation, expanding the spectrum of renal disorders caused by HNF1B mutations.34
Possibly the most intriguing finding of our study was the presence, in the index case of family 6, of a clinical and imaging picture of MSK (Table 1, Table 2, Table 3). MSK is a rare developmental disorder, characterized by ectasia and cystic formation in the medullary collecting ducts, which usually manifest with nephro-calcinosis and recurrent kidney stones.35 Both the proband and the daughter of family 6 carrying the recurrent 17q12 deletion showed the MSK phenotype (Tables 1 and 3). This deletion comprises several genes, and none of these are known to be involved in renal development, apart from HNF1B1.6 To our knowledge, MSK has never been reported in association with HNF1B variants or deletion; only 2 cases of nephro-calcinosis, apparently in the absence of MSK, have been reported by Faguer et al.6 The underlying defect of MSK is not fully understood. Interestingly, MSK may occur in association with a number of renal and urinary tract abnormalities and with hemihypertrophy and in Beckwith−Wiedemann syndrome,35 reinforcing the hypothesis that MSK is a developmental abnormality resulting from alterations in critical developmental genes. Familial clustering of MSK, with an apparent autosomal dominant inheritance, further suggests a genetic component in the pathogenesis of the disease.36 In 2010, Torregrossa et al.37 demonstrated that variants in glial cell–derived neurotrophic factor (GDNF), a ligand for the tyrosine kinase receptor RET (HGCN-approved name: ret proto-oncogene), the most important inducer of the ureteric bud (UB) emergence and the branching process,38 cosegregated with MSK in a few families.37 However, although suggestive,39 the role of the GDNF variants in MSK remains questionable, as the proportion of cases attributable to GDNF or RET mutations is small.40,41 In our family the proband underwent whole-exome sequencing, and no pathogenic variants were identified in the GDNF or RET gene. Whether HNF1b plays a direct role in the occurrence of MSK is currently unknown. Recent experimental studies revealed that HNF1B deletion can lead to defective expression of key regulators of UB branching, including GDNF−RET pathway components.42 Hence we might envisage HNF1B as an additional candidate for MSK. Although further studies are required to explore the role of the HNF1B gene in MSK, our data further suggest the genetic background of this developmental disorder expanding the spectrum of renal malformation observed in the context of HNF1B nephropathy.
In summary, our data confirm the heterogenic presentation of the renal phenotype of HNF1B-associated disease. In addition, we describe 2 unusual renal phenotypes, namely, nephrogenic diabetes insipidus and MSK, enlarging the spectrum of HNF1B nephropathy. A considerable diagnostic delay is frequent, as demonstrated by our cases; in this context, extrarenal manifestations may be considered an important tool to make an early diagnosis.
We could also speculate that, in our era, clinical criteria could be surpassed by genetic testing availability. Nowadays, cost-effective NGS-based strategies allow simultaneous and rapid sequencing of a number of genes; thus, we suggest that all patients with familial tubulo-interstitial kidney disease or early-onset disease should be investigated for ADTKD genes including HNF1B. Diagnosing the syndrome is important, because this enable us to prevent unnecessary examinations, including renal biopsy and screening for diabetes and other extrarenal manifestations, and to offer genetic counseling to patients and family members.
Disclosure
All authors declared no competing interests.
Footnotes
Supplementary Materials and Methods.
Supplementary Material
References
- 1.Kolatsi-Joannou M., Bingham C., Ellard S. Hepatocyte nuclear factor-1beta: a new kindred with renal cysts and diabetes and gene expression in normal human development. J Am Soc Nephrol. 2001;12:2175–2180. doi: 10.1681/ASN.V12102175. [DOI] [PubMed] [Google Scholar]
- 2.Ferrè S., Igarashi P. New insights into the role of HNF-1β in kidney (patho)physiology. Pediatr Nephrol. 2019;34:1325–1335. doi: 10.1007/s00467-018-3990-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horikawa Y., Iwasaki N., Hara M. Mutation in hepatocyte nuclear factor-1 beta gene (TCF2) associated with MODY. Nat Genet. 1997;17:384–385. doi: 10.1038/ng1297-384. [DOI] [PubMed] [Google Scholar]
- 4.Adalat S., Woolf A.S., Johnstone K.A. HNF1B mutations associate with hypomagnesemia and renal magnesium wasting. Am Soc Nephrol. 2009;20:1123–1131. doi: 10.1681/ASN.2008060633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heidet L., Decramer S., Pawtowski A. Spectrum of HNF1B mutations in a large cohort of patients who harbor renal diseases. Clin J Am Soc Nephrol. 2010;5:1079–1790. doi: 10.2215/CJN.06810909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faguer S., Decramer S., Chassaing N. Diagnosis, management, and prognosis of HNF1B nephropathy in adulthood. Kidney Int. 2011;80:768–776. doi: 10.1038/ki.2011.225. [DOI] [PubMed] [Google Scholar]
- 7.Faguer S., Chassaing N., Bandin F. The HNF1B score is a simple tool to select patients for HNF1B gene analysis. Kidney Int. 2014;86:1007–1015. doi: 10.1038/ki.2014.202. [DOI] [PubMed] [Google Scholar]
- 8.Izzi C., Dallera N., Manenti C. The Case / Cystic renal disease, nephrogenic diabetes insipidus, and polycytemia. Kidney Int. 2014;86:863–864. doi: 10.1038/ki.2013.445. [DOI] [PubMed] [Google Scholar]
- 9.Clissold R., Hamilton A.J., Hattersley A.T. HNF1B-associated renal and extra-renal disease—an expanding clinical spectrum. Nat Rev Nephrol. 2015;11:102–112. doi: 10.1038/nrneph.2014.232. [DOI] [PubMed] [Google Scholar]
- 10.Raaijmakers A., Corveleyn A., Devriendt K. Criteria for HNF1B analysis in patients with congenital abnormalities of kidney and urinary tract. Nephrol Dial Transplant. 2015;30:835–842. doi: 10.1093/ndt/gfu370. [DOI] [PubMed] [Google Scholar]
- 11.van der Made C.I., Hoorn E.J., de la Faille R. Hypomagnesemia as first clinical manifestation of ADTKD-HNF1B: a case series and literature review. Am J Nephrol. 2015;42:85–90. doi: 10.1159/000439286. [DOI] [PubMed] [Google Scholar]
- 12.Bockenhauer D., Jaureguiberry G. HNF1B-associated clinical phenotypes: the kidney and beyond. Pediatr Nephrol. 2016;31:707–714. doi: 10.1007/s00467-015-3142-2. [DOI] [PubMed] [Google Scholar]
- 13.Verhave J.C., Bech A.P., Wetzels J.F. Hepatocyte nuclear factor 1β-associated kidney disease: more than renal cysts and diabetes. J Am Soc Nephrol. 2016;27:345–353. doi: 10.1681/ASN.2015050544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madariaga L., García-Castaño A., Ariceta G. Variable phenotype in HNF1B mutations: extrarenal manifestations distinguish affected individuals from the population with congenital anomalies of the kidney and urinary tract. Clin Kidney J. 2018;12:373–379. doi: 10.1093/ckj/sfy102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagano C., Morisada N., Nozu K. Clinical characteristics of HNF1B-related disorders in a Japanese population. Clin Exp Nephrol. 2019;23:1119–1129. doi: 10.1007/s10157-019-01747-0. [DOI] [PubMed] [Google Scholar]
- 16.Mitchel M.W., Moreno-De-Luca D., Myers S.M. 17q12 Recurrent deletion syndrome. In: Adam M.P., Ardinger H.H., Pagon R.A., editors. GeneReviews. University of Washington; Seattle: 2016. [PubMed] [Google Scholar]
- 17.Dubois-Laforgue D., Cornu E., Saint-Martin C. Monogenic Diabetes Study Group of the Société Francophone du Diabète. Diabetes, associated clinical spectrum, long-term prognosis, and genotype/phenotype correlations in 201 adult patients with hepatocyte nuclear factor 1B (HNF1B) molecular defect. Diabetes Care. 2017;40:1436–1443. doi: 10.2337/dc16-2462. [DOI] [PubMed] [Google Scholar]
- 18.Richards S., Aziz N., Bale S. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishiwa S., Sato M., Morisada N. Association between the clinical presentation of congenital anomalies of the kidney and urinary tract (CAKUT) and gene mutations: an analysis of 66 patients at a single institution. N Pediatr Nephrol. 2019;34:1457–1464. doi: 10.1007/s00467-019-04230-w. [DOI] [PubMed] [Google Scholar]
- 20.Decramer S., Parant O., Beaufils S. Anomalies of the TCF2 gene are the main cause of fetal bilateral hyperechogenic kidneys. J Am Soc Nephrol. 2007;18:923–933. doi: 10.1681/ASN.2006091057. [DOI] [PubMed] [Google Scholar]
- 21.Jones G.E., Mousa H.A., Rowley H. Should we offer prenatal testing for 17q12 microdeletion syndrome to all cases with prenatally diagnosed echogenic kidneys? Prenatal findings in two families with 17q12 microdeletion syndrome and review of the literature. Prenat Diagn. 2015;35:1336–1341. doi: 10.1002/pd.4701. [DOI] [PubMed] [Google Scholar]
- 22.Ulinski T., Lescure S., Beaufils S. Renal phenotypes related to hepatocyte nuclear factor-1 (TCF2) mutations in a pediatric cohort. J Am Soc Nephrol. 2006;17:497–503. doi: 10.1681/ASN.2005101040. [DOI] [PubMed] [Google Scholar]
- 23.Kompatscher A., de Baaij J.H.F., Aboudehen K. Loss of transcriptional activation of the potassium channel Kir5.1 by HNF1β drives autosomal dominant tubulointerstitial kidney disease. Kidney Int. 2017;92:1145–1156. doi: 10.1016/j.kint.2017.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bleyer A.J., Hart P.S., Kmoch S. Autosomal dominant tubulointerstitial kidney disease, UMOD-related. In: Adam M.P., Ardinger H.H., Pagon R.A., editors. GeneReviews. University of Washington; Seattle: 2016. pp. 1993–2019. [PubMed] [Google Scholar]
- 25.Kmoch S., Zivna M., Bleyer A.J. Autosomal dominant tubulointerstitial kidney disease, REN-related. In: Adam M.P., Ardinger H.H., Pagon R.A., editors. GeneReviews. University of Washington; Seattle: 2016. [Google Scholar]
- 26.Devuyst O., Olinger E., Weber S. Autosomal dominant tubulointerstitial kidney disease. Nat Rev Dis Primers. 2019;5:60. doi: 10.1038/s41572-019-0109-9. [DOI] [PubMed] [Google Scholar]
- 27.Eckardt K.U., Alper S.L., Antignac C. Autosomal dominant tubulointerstitial kidney disease: diagnosis, classification, and management—a KDIGO consensus report. Kidney Int. 2015;88:676–683. doi: 10.1038/ki.2015.28. [DOI] [PubMed] [Google Scholar]
- 28.Carew R.M., Wang B., Kantharidis P. The role of EMT in renal fibrosis. cell tissue res. 2012;347:103–116. doi: 10.1007/s00441-011-1227-1. [DOI] [PubMed] [Google Scholar]
- 29.Chan S.C., Zhang Y., Shao A.J. Mechanism of fibrosis in HNF1B-related autosomal dominant tubulointerstitial kidney disease. Am Soc Nephrol. 2018;29:2493–2509. doi: 10.1681/ASN.2018040437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hiesberger T., Bai Y., Shao X.J. Mutation of hepatocyte nuclear factor-1beta inhibits Pkhd1 gene expression and produces renal cysts in mice. Clin Invest. 2004;113:814–825. doi: 10.1172/JCI20083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergmann C. Early and severe polycystic kidney disease and related ciliopathies: an emerging field of interest. Nephron. 2019;141:50–60. doi: 10.1159/000493532. [DOI] [PubMed] [Google Scholar]
- 32.Bergmann C. ARPKD and early manifestations of ADPKD: the original polycystic kidney disease and phenocopies. Pediatr Nephrol. 2015;30:15–30. doi: 10.1007/s00467-013-2706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faguer S.1, Bouissou F., Dumazer P. Massively enlarged polycystic kidneys in monozygotic twins with TCF2/HNF-1beta (hepatocyte nuclear factor-1beta) heterozygous whole-gene deletion. Am J Kidney Dis. 2007;50:1023–1027. doi: 10.1053/j.ajkd.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 34.Aboudehen K., Noureddine L., Cobo-Stark P. Hepatocyte nuclear factor-1β regulates urinary concentration and response to hypertonicity. J Am Soc Nephrol. 2017:2887–2900. doi: 10.1681/ASN.2016101095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fabris A., Anglani F., Lupo A. Medullary sponge kidney: state of the art. Nephrol Dial Transplant. 2013;28:1111–1119. doi: 10.1093/ndt/gfs505. [DOI] [PubMed] [Google Scholar]
- 36.Goldfarb D.S. Evidence for inheritance of medullary sponge kidney. Kidney Int. 2013 Feb;83(2):193–196. doi: 10.1038/ki.2012.417. [DOI] [PubMed] [Google Scholar]
- 37.Torregrossa R., Anglani F., Fabris A. Identification of GDNF gene sequence variations in patients with medullary sponge kidney disease. Clin J Am Soc Nephrol. 2010;5:1205–1210. doi: 10.2215/CJN.07551009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Costantini F., Shakya R. GDNF/Ret signaling and the development of the kidney. Bioessays. 2006;28:117–127. doi: 10.1002/bies.20357. [DOI] [PubMed] [Google Scholar]
- 39.Ria P., Fabris A., Dalla Gassa A., Zaza G. New non-renal congenital disorders associated with medullary sponge kidney (MSK) support the pathogenic role of GDNF and point to the diagnosis of MSK in recurrent stone formers. Urolithiasis. 2017;45:359–362. doi: 10.1007/s00240-016-0913-6. [DOI] [PubMed] [Google Scholar]
- 40.Mezzabotta F., Cristofaro R., Ceol M. Spontaneous calcification process in primary renal cells from a medullary sponge kidney patient harbouring a GDNF mutation. J Cell Mol Med. 2015;19:889–902. doi: 10.1111/jcmm.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janjua M.U., Long X.D., Mo Z.H. Association of medullary sponge kidney and hyperparathyroidism with RET G691S/S904S polymorphism: a case report. J Med Case Rep. 2018 doi: 10.1186/s13256-018-1736-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Desgrange A., Heliot C., Skovorodkin I. HNF1B controls epithelial organization and cell polarity during ureteric bud branching and collecting duct morphogenesis. Development. 2017;144:4704–4719. doi: 10.1242/dev.154336. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.