Abstract
Introduction
There is increasing interest in the relationship between cannabinoids and psychosis. While individual human laboratory studies have been critical in demonstrating that cannabinoids (e.g., delta-9-tetrahydrocannabinol [THC]) can induce acute transient psychosis-like effects in healthy human volunteers, combining data from multiple studies offers a fine-grained view of these effects.
Methods
THC-induced psychosis-relevant effects were examined using a data repository of 10 double-blind, randomized, placebo-controlled, crossover studies with 400 i.v. THC infusions in healthy human volunteers. The Positive and Negative Syndrome scale was used to measure psychotomimetic effects. The profile of symptoms, frequency of a response, its relationship to THC dose and substance use, latent structure in Positive and Negative Syndrome scale response, and the relationships between psychotomimetic and perceptual alteration symptoms were evaluated.
Results
Clinically meaningful increases in positive symptoms were noted in 44.75% infusions; conceptual disorganization, hallucinations, blunted affect, somatic concern, motor retardation, and poor attention were the items most frequently altered by THC. The increase in Positive and Negative Syndrome scale positive symptoms was positively associated with THC dose (beta = 11.13, SE = 4.94, Wald χ 2 = 19.88, P < .001) and negatively associated with frequent cannabis use (beta = −0.575, SE = 0.14, Wald χ 2 = 18.13, P < .001). Furthermore, positive symptoms were strongly correlated with Clinician Administered Dissociative States Scale perceptual alterations score (rs = 0.514, P < .001).
Conclusion
Intravenous administration of THC consistently induces psychotomimetic effects that include symptoms across Positive and Negative Syndrome scale domains. Moreover, healthy individuals who frequently use cannabis have a blunted psychotomimetic response.
Keywords: cannabinoid, delta-9-THC, human laboratory model, PANSS, psychosis, schizophrenia
Significance Statement
Human laboratory studies of drug-induced psychosis such as the delta-9-tetrahydrocannabinol (THC) model have made significant contributions to our understanding of psychosis. Taking advantage of the largest repository of individual participant data from 10 double-blind, randomized, placebo-controlled studies of THC-induced acute psychosis, we examine the symptom profile, factor structure, and determinants of psychosis severity. Acute administration of THC induces a transient psychosis characterized by specific symptoms from positive, negative, excitement, and emotional dimensions of psychopathology in a dose-dependent fashion. This psychotomimetic response is blunted by prior frequent cannabis use and tobacco smoking. The results define the aspects of schizophrenia phenomenology that can be successfully modelled with THC and are relevant to the design of future human laboratory studies with THC and other pharmacological agents.
Introduction
Drug-induced psychosis models in humans have substantially contributed to our current understanding of the underlying neurobiology (Murray et al., 2013). These models permit an objective evaluation of schizophrenia-like phenotype in healthy human volunteers in safe and well-controlled laboratory environments. The ability to study human behavioral and subjective effects in real time, while engaging specific neuroreceptors with known physio-chemical properties and tissue distribution, renders these models suitable to investigate network-level changes that may mimic the psychopathology in psychosis. Drugs acting on diverse neurotransmitter systems, including dopamine (amphetamine), glutamate (ketamine), serotonin (lysergic acid diethylamide, psilocybin, dimethyltryptamine), and cannabinoid (delta-9-tetrahydrocannabinol [THC]), have been used to induce transient psychosis states in healthy human volunteers to “model” the symptoms of psychosis (Paparelli et al., 2011; Murray et al., 2013; Steeds et al., 2015). Given the heterogeneity in the expression of psychoses, manipulation of a specific neurotransmitter system is likely to recapitulate only some aspects of the rich phenomenology that characterizes psychoses (Murray et al., 2013). Furthermore, the different drug-induced psychotomimetic effects may reflect potential neurotransmitter and circuit-level biology that are most relevant to a specific symptom dimension. This calls for an in-depth examination of the phenotype induced by each drug.
A potential role of endocannabinoid system dysfunction in the pathophysiology of psychosis, leading to an “endocannabinoid hypothesis,” was proposed more than 2 decades ago (Emrich et al., 1997). Accumulating evidence (listed below) regarding the relationship between cannabis, cannabinoids, and psychosis have further given rise to the “exo-cannabinoid hypothesis” (Tikka and D’ Souza, 2019), which complements the endocannabinoid hypothesis. First, the administration of cannabinoid receptor agonists to healthy humans induces transient behavioral and cognitive effects, and electrophysiological information-processing abnormalities akin to those seen in schizophrenia (D’Souza et al., 2009; Cortes-Briones et al., 2015; Broyd et al., 2016; Skosnik et al., 2016). Second, a small yet significant proportion of individuals who abuse phyto- and synthetic cannabinoids manifest psychosis characterized by paranoid ideations, delusions, and hallucinations resembling the positive symptoms of schizophrenia (Núñez and Gurpegui, 2002). Third, epidemiological studies demonstrate a strong positive relationship between cannabis use (frequency and potency) and the incidence of first-episode psychosis (Di Forti et al., 2019). Fourth, among the cases of drug-induced psychosis, the highest rate of conversion to schizophrenia is noted for cannabis (Starzer et al., 2018). Fifth, in laboratory studies, THC exacerbates symptoms in those with established schizophrenia, and, related to this, cannabis use is known to have a negative impact on the course and expression of schizophrenia (Foti et al., 2010). Sixth, genome-wide association studies adopting Mendelian randomization methods demonstrate possible bidirectional causal relationships between schizophrenia and lifetime cannabis use (Pasman et al., 2018; Vaucher et al., 2018). Lastly, studies in animal models demonstrate the emergence of schizophrenia-relevant behavioral abnormalities on chronic and subchronic cannabinoid administration (Chesworth and Karl, 2017). Collectively, the evidence supports a relationship between cannabinoids and psychosis and suggests that a “human laboratory model-cannabinoids (HLM-cannabinoids)” has utility in understanding the contributions of the cannabinoid system to the pathophysiology of psychosis.
Several research groups, including ours, have examined acute, transient psychosis-like effects induced by administration of THC, the principal psychoactive constituent of cannabis, in healthy human volunteers [for comprehensive reviews, see Englund et al. (Englund et al., 2012) and Sherif et al. (Sherif et al., 2016)]. Briefly, laboratory studies of cannabis and cannabinoids (THC, cannabidiol, nabilone, dronabinol) in human volunteers have been undertaken over the past several decades (Domino, 1971; Hollister and Gillespie, 1973; Emrich et al., 1991; Leweke et al., 2000). These studies have adopted different routes of administration (smoking, inhalation, oral, and i.v.) and have examined diverse cognitive and behavioral outcomes. Notwithstanding the heterogeneity in study design, many of these early studies noted psychosis-relevant behavioral and cognitive responses such as frank hallucinations (Domino, 1971), temporal disorganization and delusion-like ideas (Melges et al., 1974; Mathew et al., 1998), attention and memory impairment (Hooker and Jones, 1987), alterations in binocular depth perception (Leweke et al., 1999), and emotional word recognition (Leweke et al., 1998). More recent double-blind, randomized, placebo-controlled studies have adopted tools used to assess psychosis of schizophrenia to measure psychotomimetic effects of THC (vide infra): Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987), Brief Psychiatric Rating Scale (Overall and Gorham, 1962), Community Assessment of Psychic Experience (Konings et al., 2006), Psychotomimetic States Inventory (Mason et al., 2008), and Clinician Administered Dissociative States Scale (CADSS) (Bremner et al., 1998). Among these scales, the PANSS has been most frequently utilized both by our group and several others.
While the results of individual THC studies from our group have been reported elsewhere (D’Souza et al., 2004, 2008a, 2008b, 2009, 2012), pooling individual participant data provides the necessary sample size to examine, for the first time to our knowledge, the PANSS symptom profile, correlates of symptoms severity, latent dimensions in the symptoms, and the relationship between psychotic and perceptual alteration following i.v. THC administration in healthy human volunteers.
Methods
Studies conducted at the Schizophrenia Neuropharmacology Research Group at Yale University to characterize acute psychotomimetic response to intravenous THC in healthy human volunteers were included in this analysis. The data were anonymized and curated in a secure online database with a common protocol approved by the local institutional review boards of VA Connecticut Healthcare System (VACHS), West Haven, CT, and Yale University School of Medicine (YUSM). Demographic information, cannabis use measures, experimental conditions (THC/placebo dose, duration of infusion), and behavioral measures were extracted from the database. The studies were conducted under an IND (#51671: DSouza) at the VACHS Neurobiological Studies Unit. The individual studies were approved by the institutional review boards of VACHS and Yale University School of Medicine. Capitalizing on this largest known data repository of HLM-cannabinoid studies, we aimed to address the following 5 questions:
1) Does i.v. THC reliably induce a psychotomimetic response in healthy individuals?
2) What is the dose-response relationship?
3) Do demographic variables and history of recent cannabis use influence the severity of the psychotomimetic response?
4) Are there similarities in the PANSS symptom profile and factor structure between the THC psychotomimetic response and schizophrenia?
5) Are the THC response PANSS scores related to the subjective experience of high and perceptual alterations?
Studies and Setting
The methods employed in participant screening, inclusion and exclusion criteria, recruitment, randomization, blinding, THC/placebo infusion and pre- and post-infusion assessments, and safety monitoring have been published elsewhere (D’Souza et al., 2004, 2008a, 2008b; Carbuto et al., 2012; Ranganathan et al., 2012b, 2019; Radhakrishnan et al., 2015). Study participants had a wide range of cannabis exposure (D’Souza et al., 2008a); cannabis-naïve individuals were not permitted to participate in the studies. Participants were required to abstain from drugs and medications 1 week prior to the test day to avoid any potential interactions. Studies of frequent users of cannabis were allowed cannabis use no closer than 8 hours from the start of a test session (8 am). Six of the 10 studies examined the interaction between THC and other compounds such as cannabidiol (D. C. D’Souza, unpublished observations), haloperidol (D’Souza et al., 2008b), iomazenil (Radhakrishnan et al., 2015), naltrexone (Ranganathan et al., 2012b), tolcapone (Ranganathan et al., 2019), and physostigmine (D. C. D’Souza, unpublished observations). In these studies, only the data from THC alone and placebo-alone test days were used for the analysis. Of the 239 participants, 114 (47.7%) received more than 1 dose of THC (2–6 infusions) on different test days.
THC and placebo (<2 mL of sterile vehicle: 190-proof USP ethanol) were administered i.v. into a rapidly flowing saline infusion by manual injection or via an infusion pump over 2 to 5 minutes (“rapid”), 10 minutes (“brief”), or 20 minutes (“extended”) (Table 1). The dose of THC ranged from 0.015 mg/kg to 0.070 mg/kg, with some studies testing 2 doses of THC in the same participants on 2 separate test days (D’Souza et al., 2004, 2008a). This dose range is roughly equivalent to the amount of THC delivered by one-quarter to a full joint (Lee et al., 2015; Boggs et al., 2018).
Table 1.
Summary of Studies Included in Analysis
| Study# | Study title | Sample sizea | THC doses in mg/kg | Duration of infusion in minutes | Other drugs if tested | THC infusions with PANSS - sample size (n) | Placebo infusions with PANSS - sample size (n) |
|---|---|---|---|---|---|---|---|
| 1 | THC response in healthy frequent and infrequent cannabis users | 44 | 0.035 and 0.07 | 2–5 | n/a | 87 | 44 |
| 2 | Interactions between haloperidol and THC | 31 | 0.0286 | 20 | Haloperidol | 31 | 31 |
| 3 | Genetics of THC response and interaction with tolcapone | 78 | 0.05 | 20 | Tolcapone | 78 | 78 |
| 4 | THC effects in people with family history of alcoholism | 30 | 0.018 and 0.036 | 20 | n/a | 60 | 30 |
| 5 | Electrophysiological effects of THC | 33 | 0.015 and 0.030 | 10 | n/a | 66 | 30 |
| 6 | Interactions between iomazenil and THC | 23 | 0.015 | 10 | Iomazenil | 23 | 23 |
| 7 | Interactions between naltrexone and THC | 6 | 0.025 | 20 | Naltrexone | 6 | 6 |
| 8 | Interactions between physostigmine and THC | 6 | 0.015 | 10 | Physostigmine | 6 | 6 |
| 9 | Interactions between cannabidiol and THC | 29 | 0.035 | 20 | Cannabidiol | 29 | 29 |
| 10 | THC effects on information processing | 7 | 0.015 and 0.030 | 10 | n/a | 14 | 7 |
| Total | 287b | 400 | 284 |
Abbreviations: n/a, not applicable; PANSS, Positive and Negative Syndrome scale.
a Sample sizes represent participants with complete PANSS information on the 28 items for THC condition in each study.
b39 individuals participated in more than 1 study, resulting in a total sample size of 287.
Assessment Scales
Demographic variables including age, gender, race, and ethnicity were collected. Lifetime and past 30-day cannabis exposure were assessed using the Scale for the Assessment of Lifetime Cannabis Use developed in our laboratory and/or the Time Line Follow Back Approach (Sobell and Sobell, 1992; Hjorthøj et al., 2012a, 2012b). Current and past history of psychiatric disorders and use of alcohol and tobacco products were assessed using the Structured Clinician Interview for DSM IIIR or IV (First and Gibbon, 2004). The National Adult Reading Test was used to rule out participants with diminished capacity to provide consent.
Positive, negative, and general symptoms were assessed using relevant subscales of a modified version of the PANSS that excluded irrelevant items (N4: passive/apathetic social withdrawal, and G16: active social avoidance) to repeated measurements within a short time (D’Souza et al., 2004). We adopted an empirical cut-off of a 2-point change in each PANSS item to define a positive psychotomimetic response on that item. PANSS items are scored on a 7-point Likert scale ranging from 1 to 7 (1 = absent, 2 = minimal, 3 = mild, 4 = moderate, 5 = moderate severe, 6 = severe, and 7 = extreme) (Kay et al., 1987). The 2-point cutoff in item change scores was chosen to ensure a definitive and clinically meaningful change. For the 3 PANSS subscales, we set an empirical cut-off of 2-point change in ≥2 PANSS items amounting to a minimum change of 4 in each subscale. This threshold is more stringent than some of the previous HLMs drug studies that have used a cutoff score of 3 in PANSS positive subscale to define a positive psychotomimetic response (D’Souza et al., 2004).
THC-induced quasi-psychotic perceptual alterations were measured using the 27-item CADSS (Bremner et al., 1998). Subjective effects of “high” induced by THC were measured with a 100-point visual analog scale (VAS) (0 = not at all to 100 = most of all). Participants were assessed at baseline prior to THC/placebo infusion and at regular intervals up to 5 hours after the infusion. The maximum score noted on each item following THC infusion was used to define the post infusion score. Pre-infusion baseline scores were subtracted from the post infusion scores on each item to derive the change scores as measures of THC/placebo response.
Statistical Analysis
A clinically significant psychotomimetic response was defined as a ≥2-point increase in at least 2 items on the PANSS positive subscale (primary outcome). A ≥2-point change in at least 2 items of negative and general psychopathology subscales and at least 5 items of the full PANSS scale were measured as secondary outcomes. The proportion of infusions that resulted in response above these threshold scores were computed.
The data distribution of PANSS scores as continuous measures were examined with histograms and box plots. The PANSS data exhibited positive skewness and floor effects. PANSS positive total scores were fitted with generalized estimating equations (GEE) with main effects of dose, infusion-rate, age, gender, and cannabis use frequency. The GEE used tweedie distribution with a log-link function (to model zero-inflated data with positive skew) with an unstructured working correlation matrix. GEE is a robust statistical method to handle correlated measures, missing data, and non-normal distributions (D’ Souza et al., 2019). The effect of dose*infusion-rate interaction was further examined in the GEE model. The resulting marginal means for PANSS positive scores were plotted using bar graphs in the 3 infusion-duration categories. Holm-Bonferroni correction was used to adjust for multiple comparisons when appropriate. Further, dose response was also examined in a subgroup of participants who received 2 different doses of THC. The difference in PANSS positive scores between past-month users and nonusers and between tobacco smokers and nonsmokers were examined with the Mann-Whitney-U test.
An exploratory factor analysis was conducted to examine the latent structure in the THC-induced psychotomimetic response as measured by the 28-item PANSS scale. Data characteristics were assessed prior to factor analysis with the Kaiser-Mayer-Olkin test for sampling adequacy and Bartlett’s test for sphericity. Orthogonal varimax rotation was used to derive the rotated component matrix; this has been most frequently used to derive latent PANSS dimensions in schizophrenia (Wallwork et al., 2012). The resulting factor loadings were compared with the PANSS factor solutions in schizophrenia literature. Lastly, the relationships between PANSS and CADSS scores, and PANSS and VAS (high) scores were assessed using Spearman’s rank correlation. Statistical analyses were carried out using IBM SPSS statistics version 26.
Results
Sample Characteristics
The sample consisted of 239 healthy individuals (71 females, 29.71%) who received a total of 400 i.v. THC infusions and 284 placebo infusions (supplementary Table 1). Four of the 10 studies tested 2 doses of THC against a single placebo infusion (Table 1). Seventy-six (31.8%) participants were characterized as “frequent” cannabis users with a history of use ≥2 times/wk. The remaining 163 (68.2%) participants reported occasional (≤1 use/mo) to very occasional (≤1 use/4 mo) cannabis use. Thirty-nine (15.5%) participants had participated in more than 1 study, and 114 (47.7%) of the participants had THC response data available on at least 2 different doses. The mean (SD) age of the sample was 25.29 (7.49) years, and only 32 (13.4%) participants were older than 30 years.
Psychotomimetic Response to THC
A clinically significant psychotomimetic response was noted in 179 (44.75%) of the 400 THC infusions. A clinically significant increase in THC-induced PANSS negative, general psychopathology, and global symptoms was noted in 177 (44.25%), 253 (63.25%), and 223 (55.8%) infusions, respectively. In contrast, no clinically significant psychotomimetic responses were noted with the placebo infusions. In the subsample of 114 participants who received 2 or more doses of THC, 24 (21.05%) participants had a clinically significant psychotomimetic response on every THC dose they received, while 41 (35.96%) did not have a psychotomimetic response on any of the infusions. An additional 27 (23.68%) participants had a clinically significant psychotomimetic response only with higher THC doses (>0.03 mg/kg).
Among the individual PANSS items, a significant response was most frequently noted for conceptual disorganization (P2) (46.25%), hallucinations (P3) (45%), blunted affect (N1) (46.75%), somatic concern (G1) (54.5%), motor retardation (G7) (43.75%), and poor attention (G11) (46.5%). In contrast, the items of grandiosity (P5), hostility (P7), stereotyped thinking (N7), guilt feelings (G3), depression (G6), uncooperativeness (4.5), disorientation (G10), lack of judgment and insight (G12), and poor impulse control (G14) yielded a positive response in <5% of the infusions. The proportion of infusions resulting in a positive psychotomimetic response for each PANSS item, the 3 subscales, and PANSS total using different cut-off thresholds are presented in supplementary Tables 2.1 and 2.2.
Relationship of Dose and Rate of Infusion to Psychotomimetic Response
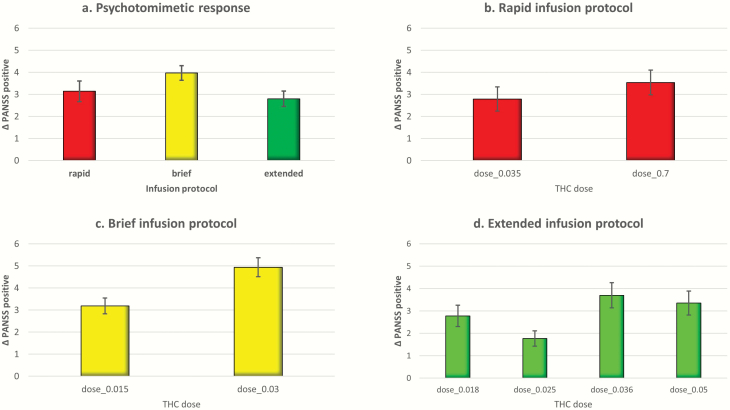
There was a significant effect of dose on the PANSS positive symptom response to THC (beta = 11.13, SE = 4.94, Wald χ 2 = 19.88, P < .001). A greater psychotomimetic response was noted for “brief” followed by “rapid” and “extended” infusion protocols, respectively (Figure 1a). Plotting the estimated marginal means of dose categories across the 3 infusion-rate protocols demonstrated a doserelated increase in the psychotomimetic response (PANSS positive) in the “rapid” and “brief” infusion protocols, while the pattern of response was complex in the “extended” infusion protocol (Figure 1b–d). A similar pattern of dose-response relationships was noted across the PANSS negative and general psychopathology symptoms and PANSS total scores (supplementary Figure 1). Lastly, we compared the PANSS positive symptoms in participants who had THC response data available on 2 different doses of THC from 2 different time-points. The magnitude of response was significantly higher for the higher dose of THC (mean [SD] = 4.73 [3.71]) compared with the lower dose (3.59 [3.73], P = .002).
Figure 1.
Bar graph presenting estimated marginal means of Positive and Negative Syndrome scale (PANSS) positive symptom change score, error bars (±SE). (a) Three delta-9-tetrahydrocannabinol (THC) infusion protocols. (b) Two THC doses in rapid infusion protocol. (c) Two doses in the brief infusion protocols. (d) Four THC doses in the extended infusion protocol.
Factors Influencing the THC Response
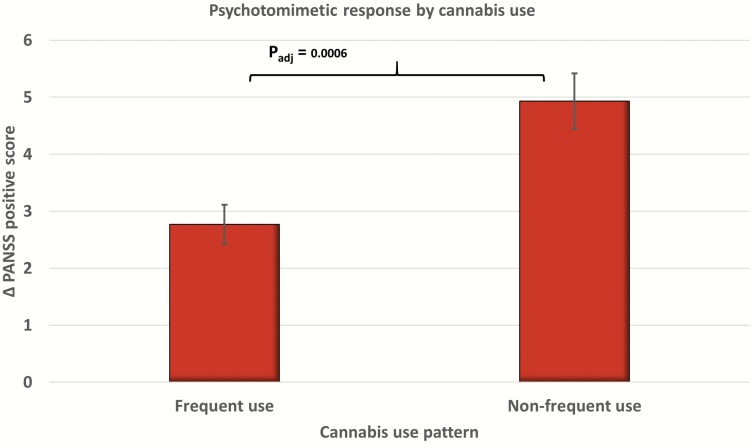
Frequent cannabis use (beta = −0.575, SE = 0.14, Wald χ 2 = 18.13, P < .001) (Figure 2) and age (beta = −0.14, SE = 0.006, Wald χ 2 = 5.05, P = .025) were associated with a blunted psychotomimetic response. There was no significant effect of gender. In the subsample with available past-month cannabis use data (n = 159 infusions, 39.75 %), past month users had a blunted PANSS positive response compared with nonusers, and the mean difference was marginally significant. Similarly, in a subsample with data about current tobacco smoking (n = 269 infusions, 67.25%), smokers had a significantly lower psychotomimetic response (supplementary Table 3). The main effects of THC dose and frequent cannabis use on PANSS negative, general symptom scores, and PANSS total scores were also significant (supplementary Table 4).
Figure 2.
Bar graph presenting estimated marginal means of Positive and Negative Syndrome scale (PANSS) positive symptom change score between frequent and nonfrequent cannabis users with error bars (±SE). padj, adjusted P value in the generalized estimating equations (GEE) model.
Factor Structure of PANSS Items in THC Psychotomimetic Response
Exploratory factor analysis was conducted on THC response measured with 28-item PANSS. A Kaiser-Mayer-Olkin value of 0.848 suggested adequate sample size to carry out a factor analysis. Further, a highly significant Bartlett’s test (approx. χ 2 = 5033.75, P < .001) suggested redundancy between variables that can be summarized with factor analysis. An initial analysis yielded 8 components with eigen values above the Kaiser’s criteria of 1. Analysis of the scree plot (supplementary Figure 2) suggested an optimal fit for a 5-factor solution that cumulatively explained 53.94% of variance, and these 5 factors were retained in the final analysis. Table 2 presents the rotated component matrix with factor loadings of individual PANSS items. These results suggest that THC-induced psychotomimetic response is characterized by positive/THC-response (factor 1), negative (factor 2), emotional (factor 3), excitement (factor 4), and disorganization like (factor 5) symptoms that broadly resemble the PANSS dimensions noted in schizophrenia literature (van der Gaag et al., 2006; Wallwork et al., 2012).
Table 2.
Rotated component matrix presenting factor loadings of PANSS items
| Rotated component matrix (Varimaxa) | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| PANSS items | Positive | Negative | Emotional | Excitement | Disorganization |
| P3 Hallucinations | 0.778 | ||||
| G1 Somatic concern | 0.682 | ||||
| G9 Unusual thought content | 0.677 | 0.422 | |||
| P1 Delusions | 0.666 | ||||
| G11 Poor attention | 0.614 | 0.454 | |||
| G7 Motor retardation | 0.596 | 0.433 | |||
| G4 Tension | 0.56 | 0.475 | |||
| N1 Blunted affect | 0.559 | 0.454 | |||
| P2 Conceptual disorganization | 0.532 | ||||
| G13 Disturbance of volition | 0.445 | ||||
| N3 Poor rapport | 0.697 | ||||
| N2 Emotional withdrawal | 0.643 | ||||
| N6 Lack of spontaneity and flow of conversation conversation | 0.631 | ||||
| G15 Preoccupation | 0.569 | ||||
| G8 Uncooperativeness | 0.552 | ||||
| G10 Disorientation | 0.441 | ||||
| P7 Hostility | 0.413 | 0.669 | |||
| N7 Stereotyped thinking | 0.636 | ||||
| P6 Suspiciousness/persecution | 0.582 | ||||
| G2 Anxiety | 0.441 | 0.568 | |||
| G6 Depression | 0.482 | ||||
| G3 Guilt feelings | |||||
| G14 Poor impulse control | 0.746 | ||||
| G12 Lack of judgement and insight | 0.743 | ||||
| P4 Excitement | 0.597 | ||||
| P5 Grandiosity | 0.558 | ||||
| N5 Difficulty in abstract thinking | 0.735 | ||||
| G5 Mannerisms and posturing | 0.401 | 0.452 |
Abbreviations: PANSS, Positive and Negative Syndrome scale.
aItems are ordered on the magnitude of factor loadings and values <0.4 are suppressed.
Relationship of PANSS Response With Perceptual Alteration and Subjective Experience Measures
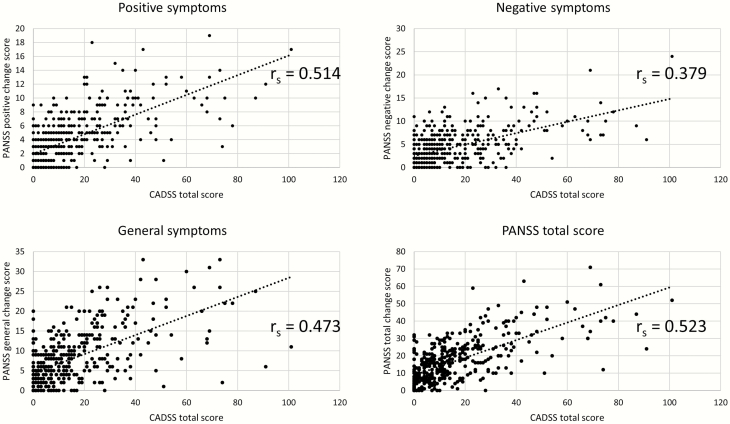
A dose response relationship was also noted for perceptual alterations measured by the CADSS (supplementary Figure 3). PANSS positive symptoms had the strongest correlation (rs = 0.514, P < .001) with CADSS scores followed by PANSS general psychopathology (rs = 0.473, P < .001) and PANSS negative symptoms (rs = 0.379, P < .001). Further, there was a strong positive correlation between CADSS scores and PANSS total scores (rs = 0.523, P < .001) (Figure 3). In contrast, the strength of the relationship between subjective effects of high (VAS-high) and the PANSS positive [rs = 0.293 (z = 3.75)], negative [rs = 0.207 (z = 2.66)], and general psychopathology [rs = 0.291 (z = 3.02)] subscales and the PANSS total scores [rs = 0.31 (z = 3.66)] was significantly smaller than for the perceptual alterations (CADSS).
Figure 3.
Scatter plots depicting relationship between Clinician Administered Dissociative States Scale (CADSS) total scores with (a) Positive and Negative Syndrome scale (PANSS) positive, (b) PANSS negative, (c) PANSS general psychopathology, and (d) PANSS total scores. rs, Spearman’s rank order correlation coefficient.
Discussion
Capitalizing on the largest known repository of individual participant data on the psychotomimetic response to i.v. THC, we have characterized this phenotype at individual symptom-level resolution, leading to some novel insights. In addition, these results provide some useful directions for future studies using HLM cannabinoids to examine the behavioral, cognitive, and biological aspects of psychosis. THC induces a clinically meaningful psychotomimetic response in a substantial proportion of the participants; this response is often driven by specific PANSS items (conceptual disorganization, hallucinations, affective blunting, somatic concern, motor retardation, and poor attention). Furthermore, exploratory factor analysis suggests an overlap in the symptom domains between the psychotomimetic response to THC and schizophrenia captured by the PANSS (van der Gaag et al., 2006). A strong relationship is noted between the measures of positive symptoms (PANSS) and perceptual alterations (CADSS), suggesting a continuum between these non-normative quasi-psychotic perceptual alterations that may precede the onset of outright positive symptoms. In contrast, we note a weak correlation between THC-induced feelings of “high” and positive symptoms suggesting a dissociation in these symptom domains.
With respect to the experimental design, while some individuals experienced a psychotomimetic effect at a dose as low as 0.015 mg/kg, our results suggest that a 10-minute dosing protocol with THC doses in the range of 0.03 to 0.036 mg/kg will be optimal for HLM-cannabinoid studies of psychosis. Based on our previous studies, this dose range results in a plasma THC concentration more than 60ng/dL, approximating the concentrations reached by smoking three-quarters to a full standard joint (Lee et al., 2015; Boggs et al., 2018). While we demonstrate a linear dose-response relationship in the severity of the psychosis-relevant phenomena, it is moderated by rate of THC infusion, frequency and recency of cannabis use, and ongoing tobacco use. It will be vital to account for these factors in future studies of HLM cannabinoids. The findings of blunted psychotomimetic response in persons with a history of frequent and recent cannabis use (D’Souza et al., 2008a) confirm some of the previous findings reported by our group related to the symptom profile, severity, and predictors of THC response (D’Souza et al., 2004).
Compared with earlier studies, we used stringent cut-off criteria to define psychotomimetic response to THC in the PANSS positive subscale (D’Souza et al., 2004). We note that a substantial proportion of healthy participants (~45%) experience clinically meaningful psychotomimetic responses. This finding confirms the previously reported rates of response in healthy volunteers to i.v. THC from independent research groups (Bhattacharyya et al., 2010). A substantial proportion of the participants had consistent responses to THC (i.e., those who did experience a clinically significant psychotomimetic response on a THC study had the same response on other THC studies). Similarly, there was consistency to those who did not have a clinically significant response across studies. This suggests that a participant’s unique vulnerabilities attributable to genetic or developmental factors may determine the psychotomimetic response to cannabinoids. Studies that examine biological endophenotypes and polygenic risk burden for psychosis in the context of HLM cannabinoids may further our understanding of these vulnerabilities.
Dose-related psychotomimetic effects were evident in 2 of the 3 dosing protocols (“rapid” and “brief” infusions). Likewise, higher psychotomimetic responses were observed with higher doses of THC. Lastly, THC dose was one of the significant predictors of severity of psychotomimetic response in PANSS total and all 3 subscale scores in the regression model after controlling for the effects of infusion rate and other covariates such as age, gender, and frequent cannabis use. These results support a dose-dependent effect of THC in inducing transient psychotic states. Thus, the well-known biphasic effects of cannabinoids (e.g., anxiety with low dose are anxiolytic while high doses are anxiogenic) do not extend to their psychotomimetic effects. The complex dose-response relationship in the psychotomimetic effects observed in the “extended” infusion protocol can be attributed to the variation in the sample profiles (recent cannabis use, family history of substance use) for the first 2 doses of this protocol.
Greater cannabis exposure was associated with lower THC-induced PANSS positive symptoms. This was further corroborated in a subsample of participants for whom detailed past 30-day cannabis use data were available; there was a blunted THC-induced psychotomimetic response in cannabis users compared with nonusers. These results suggest the development of tolerance to THC-induced psychotomimetic response, confirming our previous findings (D’Souza et al., 2008a) or that these reflect inherent resilience to cannabinoids that are unrelated to tolerance. The mechanism of tolerance in the context of HLM is intriguing as this brings to the fore potential differences between dopamine and cannabinoid models of psychosis. In the case of the former, the mechanism of “sensitization” has been proposed in the development of psychosis (Peleg-Raibstein et al., 2009). Thus, studying the neural substrate of cannabinoid-induced psychosis might provide valuable clues in contrast to other drug models of schizophrenia.
Differences in the profile of psychotomimetic effects induced by different drugs may offer clues to the contributions of different neurotransmitter systems underlying the neurobiology of these phenomena. Acute administration of stimulants that act on dopaminergic pathways model some positive symptoms of psychosis. However, stimulants do not induce negative symptoms acutely and might even improve some negative symptoms (e.g., emotional withdrawal) and cognitive deficits (e.g., attention) (Lindenmayer et al., 2013). Serotonergic drugs such as lysergic acid diethylamide and psilocybin induce a range of positive symptoms of psychosis marked by visual hallucinations (Geyer and Vollenweider, 2008). The NMDA receptor antagonist ketamine models disorganization and negative symptoms in addition to positive symptoms (Abi-Saab et al., 1998). THC appears to induce a range of positive and negative symptoms, specifically conceptual disorganization, hallucinations, affective blunting, somatic concern, motor retardation, and poor attention. In addition, while not analyzed in this paper, THC reliably induces cognitive deficits when administered acutely (D’Souza et al., 2008b, 2012). The profile of THC-induced symptoms encompassed the symptoms frequently disrupted by dopaminergic, serotonergic, and glutamatergic agents, suggesting that the THC model of psychosis is distinct from other drug models (see supplementary Table 5).
While this study did not investigate the precise mechanism/s by which THC induces acute psychotomimetic effects, we provide some potential explanations based on preclinical and human studies. One obvious candidate neurotransmitter system implicated in acute psychotomimetic states is dopamine (DA). As stated by Bloomfield et al. (Bloomfield et al., 2016), the effects of THC on the DA system are complex and diverse. Fitoussi et al. demonstrated that administration of THC into the shell of the nucleus accumbens in rats resulted in dose-dependent potentiation of emotional salience for fear conditioning cues. This was paralleled by increased activity of dopaminergic neurons in the ventral tegmental area, suggesting that acute administration of THC could modulate dopaminergic transmission in the ventral tegmental area (Fitoussi et al., 2018). In contrast, relative to DA stimulants such as amphetamine, which induce significant DA release and also induce positive symptoms of psychosis in healthy individuals and exacerbate psychosis in schizophrenia patients (Laruelle et al., 1995; Abi-Dargham et al., 2009), the effects of THC on dopamine release in humans are very small (Bossong et al., 2009; Stokes et al., 2009; Barkus et al., 2011) and not commensurate with its capacity to induce psychosis.
The interplay between GABA and CB1-R systems (Eggan et al., 2010) provides another mechanism underlying the capacity of cannabinoids to induce psychosis in healthy individuals and exacerbate psychosis in schizophrenia. Pharmacological induction of a GABA deficit can enhance the psychosis-relevant behavioral and psychophysiological effects of THC (Radhakrishnan et al., 2015). This could further be explained by the disruption of endocannabinoid signaling by exogenous THC (Wilson et al., 2001; Wilson and Nicoll, 2001) and the interplay between GABA and CB1-R systems (Eggan et al., 2010). For instance, if CB1-R activation could lead to disinhibition and desynchronization of pyramidal cell activity, leading to perturbations in gating, associative functions, and neurocognition, this could culminate in psychotic symptoms as suggested by Radhakrishnan et al. (Radhakrishnan et al., 2015). If this were to occur in the presence of a GABAergic deficit, as may be the case in schizophrenia, it would explain why cannabinoids exacerbate psychosis in schizophrenia.
The mechanisms underlying the blunted psychotomimetic response noted in chronic users merits discussion. We and others have reported that chronic cannabis users show in vivo evidence of downregulation of cannabinoid receptor (Hirvonen et al., 2012; Ceccarini et al., 2015; D’Souza et al., 2016) that would explain a blunted response to THC. Additionally, Bloomfield et al. have demonstrated in vivo evidence of reduced dopamine synthesis in the striatal and limbic region in cannabis users (Bloomfield et al., 2014) that may explain the blunted response to THC. Future investigations that examine neurobiological correlates of psychotomimetic states in acute, chronic, and acute-on-chronic cannabis exposure would better elucidate the underlying mechanisms in the relationship between THC, cannabinoids, and schizophrenia.
The factor structure of THC-related psychosis was comparable with schizophrenia with an optimal 5-factor solution and factors corresponding to positive, negative, emotional, excitement, and disorganization symptom domains. However, there were specific differences in the item loading, with items that measure positive, negative, and disorganization symptoms in the 5-factor solutions of schizophrenia, loading on a common factor (factor 1, Table 2) in THC-induced transient psychosis. These findings suggest that while PANSS captures levels of psychopathology along multiple schizophrenia relevant items in THC-induced psychosis, the 2 phenotypes differ in the covariation of these symptoms.
Lastly, we observed large correlations between PANSS positive symptoms and the measures of perceptual alterations captured with CADSS. Hence, the acute experience induced by THC involves several perceptual and cognitive alterations that are not typically classified as psychotic experiences but co-vary with these symptoms. Collectively, the CADSS and PANSS together capture a wide range of THC-induced effects. In contrast, only modest correlations were observed between THC-induced positive symptoms and subjective effects of high. These data suggest some dissociation between the euphoric and psychotomimetic effects of THC.
Some limitations should be considered in the interpretation of these results. As these data were collected over 2 decades, we have included those variables that were common across the studies in our analysis. While the analysis-related variables were standardized, there was heterogeneity in the questionnaires used to capture cannabis use, family history of psychiatric disorders, and other substance use. Based on these scales, participants were dichotomized as frequent and nonfrequent cannabis users and as past month users and nonusers. But across both categorical variables, there was a consistent pattern of blunted psychotomimetic response. In the absence of definite cut-offs defining a positive psychotomimetic response, we used empirical criteria to define a positive response on global PANSS measures and the 3 subscales. To overcome this limitation, we have also presented the data with multiple cut-off thresholds. The PANSS was developed for typological and dimensional assessment in schizophrenia. We used a modified PANSS (without items N4: passive/apathetic social withdrawal, and G16: active social avoidance) removing 2 items that are not relevant to capturing acute psychotomimetic effects in healthy individuals. This modified version has been used extensively to capture the effects of a range of psychotomimetic drugs, including ketamine (Krystal et al., 2005), amphetamine (Krystal et al., 2005), and salvinorin A (Ranganathan et al., 2012a). We analyzed the PANSS in this report as it was the commonly used measure in the 10 primary studies included in the analysis. While formal studies have not established sensitivity of PANSS in the context of acute psychotomimetic response, this has been the most commonly used scale in HLM studies of drug-induced psychosis. Furthermore, the factor structure presented in our analysis provides vital clues to the PANSS domains that are most representative of acute psychotomimetic response to THC.
Conclusions
Intravenous administration of THC reliably induces a psychotomimetic response in a substantial proportion of the participants in a dose-dependent fashion. Frequent cannabis users demonstrate a blunted psychotomimetic response. While this experience broadly mimics the psychosis in schizophrenia, specific symptoms spanning the positive, negative, excitement, and emotional domains are most frequently elicited by THC. In sum, human laboratory model with cannabinoids can be useful to examine specific aspects of the phenomenology and neurobiology of psychosis.
Supplementary Materials
Supplementary data are available at International Journal of Neuropsychopharmacology (IJNPPY) online.
Acknowledgments
We acknowledge the critical contributions of the research nurses (Angelina Genovese, Elizabeth O’Donnell, Margaret Dion-Marovitz) and pharmacists (Rachel Galvan, Sonah Perry, and Robert Sturwold) supporting the Neurobiological Studies Unit at VA Connecticut Healthcare System where these studies were conducted. We also acknowledge support from the Department of Veterans Affairs, National Institute of Drug Abuse, National Institute of Alcoholism and Alcohol Abuse, National Institute of Mental Health (NIMH), and the National Alliance for Research in Schizophrenia and Depression (NARSAD).
Funding and support from National Institute on Drug Abuse RO1 DA 12382, R21 DA020750 to D.C.D., R21 DA030696-01A1 to M.R., R21 DA031853-01 to P.D.S. National Institute of Mental Health MH086769 to D.C.D. National Institute of Alcohol Abuse and Alcoholism R21 16311 to D.C.D. S.G. is supported by NARSAD young investigator award (#27340) from the Brain and Behavior Research Foundation.
Statement of Interest
Rajiv Radhakrishnan is supported by the Dana Foundation David Mahoney program and CTSA grant number UL1 TR001863 from the National Center for Advancing Translational Science (NCATS), components of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. He has also received funding from Neurocrine Biosciences, Inc. Mohini Ranganathan has or currently receives material or funding research support administered through Yale University School of Medicine currently from the National Institute of Drug Abuse, The National Center for Advancement of Translational Science, INSYS, Roche, and Bioxcel Therapeutics. Mohini Ranganathan has or currently receives material or funding research support from the Department of Veterans Affairs. Mohini Ranganathan has or currently serves as a consultant to Bioxcel Therapeutics. DCD’SOUZA has or currently receives material or funding research support administered through Yale University School of Medicine currently from the National Institute of Drug Abuse, the National Institute of Alcohol Abuse and Alcoholism, the National Institute of Mental Health, The National Center for Advancement of Translational Science, the Brain and Behavior Foundation (formerly NARSAD), the Heffter Institute, the Wallace Foundation, CH TAC, Pfizer, Soring Works, Takeda, and Roche. The other authors have no disclosures.
References
- First MB, Gibbon M (2004) The structured clinical interview for DSM-IV axis I disorders (SCID-I) and the structured clinical interview for DSM-IV axis II disorders (SCID-II). In: Comprehensive handbook of psychological assessment, Vol. 2 Personality assessment (Hilsenroth MJ, Segal DL, eds), pp134–143. Hoboken, NJ: John Wiley & Sons Inc. [Google Scholar]
- Abi-Dargham A, van de Giessen E, Slifstein M, Kegeles LS, Laruelle M (2009) Baseline and amphetamine-stimulated dopamine activity are related in drug-naïve schizophrenic subjects. Biol Psychiatry 65:1091–1093. [DOI] [PubMed] [Google Scholar]
- Abi-Saab WM, D’Souza DC, Moghaddam B, Krystal JH (1998) The NMDA antagonist model for schizophrenia: promise and pitfalls. Pharmacopsychiatry 31 Suppl 2:104–109. [DOI] [PubMed] [Google Scholar]
- Barkus E, Morrison PD, Vuletic D, Dickson JC, Ell PJ, Pilowsky LS, Brenneisen R, Holt DW, Powell J, Kapur S, Murray RM(2011) Does intravenous Delta9-tetrahydrocannabinol increase dopamine release? A SPET study. J Psychopharmacol 25:1462–1468. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Morrison PD, Fusar-Poli P, Martin-Santos R, Borgwardt S, Winton-Brown T, Nosarti C, O’ Carroll CM, Seal M, Allen P, Mehta MA, Stone JM, Tunstall N, Giampietro V, Kapur S, Murray RM, Zuardi AW, Crippa JA, Atakan Z, McGuire PK (2010) Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology 35:764–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield MA, Morgan CJ, Egerton A, Kapur S, Curran HV, Howes OD (2014) Dopaminergic function in cannabis users and its relationship to cannabis-induced psychotic symptoms. Biol Psychiatry 75:470–478. [DOI] [PubMed] [Google Scholar]
- Bloomfield MA, Ashok AH, Volkow ND, Howes OD (2016) The effects of Δ9-tetrahydrocannabinol on the dopamine system. Nature 539:369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs DL, Cortes-Briones JA, Surti T, Luddy C, Ranganathan M, Cahill JD, Sewell AR, D’Souza DC, Skosnik PD(2018) The dose-dependent psychomotor effects of intravenous delta-9-tetrahydrocannabinol (Delta(9)-THC) in humans. J Psychopharmacol 32:1308–1318. [DOI] [PubMed] [Google Scholar]
- Bossong MG, van Berckel BN, Boellaard R, Zuurman L, Schuit RC, Windhorst AD, van Gerven JM, Ramsey NF, Lammertsma AA, Kahn RS (2009) Delta 9-tetrahydrocannabinol induces dopamine release in the human striatum. Neuropsychopharmacology 34:759–766. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, Mazure CM (1998) Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS). J Trauma Stress 11:125–136. [DOI] [PubMed] [Google Scholar]
- Broyd SJ, van Hell HH, Beale C, Yücel M, Solowij N (2016) Acute and chronic effects of cannabinoids on human cognition-a systematic review. Biol Psychiatry 79:557–567. [DOI] [PubMed] [Google Scholar]
- Carbuto M, Sewell RA, Williams A, Forselius-Bielen K, Braley G, Elander J, Pittman B, Schnakenberg A, Bhakta S, Perry E, Ranganathan M, D’Souza DC; Yale THC Study Group (2012) The safety of studies with intravenous Δ 9-tetrahydrocannabinol in humans, with case histories. Psychopharmacology (Berl) 219:885–896. [DOI] [PubMed] [Google Scholar]
- Ceccarini J, Kuepper R, Kemels D, van Os J, Henquet C, Van Laere K (2015) [18F]MK-9470 PET measurement of cannabinoid CB1 receptor availability in chronic cannabis users. Addict Biol 20:357–367. [DOI] [PubMed] [Google Scholar]
- Chesworth R, Karl T (2017) Molecular basis of cannabis-induced schizophrenia-relevant behaviours: insights from animal models. Current Behavioral Neuroscience Reports 4:254–279. [Google Scholar]
- Cortes-Briones JA, Cahill JD, Skosnik PD, Mathalon DH, Williams A, Sewell RA, Roach BJ, Ford JM, Ranganathan M, D’Souza DC (2015) The psychosis-like effects of Δ(9)-tetrahydrocannabinol are associated with increased cortical noise in healthy humans. Biol Psychiatry 78:805–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu YT, Braley G, Gueorguieva R, Krystal JH (2004) The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology 29:1558–1572. [DOI] [PubMed] [Google Scholar]
- D’Souza DC, Ranganathan M, Braley G, Gueorguieva R, Zimolo Z, Cooper T, Perry E, Krystal J (2008a) Blunted psychotomimetic and amnestic effects of delta-9-tetrahydrocannabinol in frequent users of cannabis. Neuropsychopharmacology 33:2505–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza DC, Braley G, Blaise R, Vendetti M, Oliver S, Pittman B, Ranganathan M, Bhakta S, Zimolo Z, Cooper T, Perry E (2008b) Effects of haloperidol on the behavioral, subjective, cognitive, motor, and neuroendocrine effects of Delta-9-tetrahydrocannabinol in humans. Psychopharmacology (Berl) 198:587–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza DC, Sewell RA, Ranganathan M (2009) Cannabis and psychosis/schizophrenia: human studies. Eur Arch Psychiatry Clin Neurosci 259:413–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza DC, Fridberg DJ, Skosnik PD, Williams A, Roach B, Singh N, Carbuto M, Elander J, Schnakenberg A, Pittman B, Sewell RA, Ranganathan M, Mathalon D (2012) Dose-related modulation of event-related potentials to novel and target stimuli by intravenous Δ 9-THC in humans. Neuropsychopharmacology 37:1632–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza DC, Cortes-Briones JA, Ranganathan M, Thurnauer H, Creatura G, Surti T, Planeta B, Neumeister A, Pittman B, Normandin MD, Kapinos M, Ropchan J, Huang Y, Carson RE, Skosnik PD (2016) Rapid changes in cannabinoid 1 receptor availability in cannabis-dependent male subjects after abstinence from cannabis. Biol Psychiatry Cogn Neurosci Neuroimaging 1:60–67. [DOI] [PubMed] [Google Scholar]
- D’Souza DC, Cortes-Briones J, Creatura G, Bluez G, Thurnauer H, Deaso E, Bielen K, Surti T, Radhakrishnan R, Gupta A, Gupta S, Cahill J, Sherif MA, Makriyannis A, Morgan PT, Ranganathan M, Skosnik PD (2019) Efficacy and safety of a fatty acid amide hydrolase inhibitor (PF-04457845) in the treatment of cannabis withdrawal and dependence in men: a double-blind, placebo-controlled, parallel group, phase 2a single-site randomised controlled trial. Lancet Psychiatry 6:35–45. [DOI] [PubMed] [Google Scholar]
- Di Forti M, et al. ; EU-GEI WP2 Group (2019) The contribution of cannabis use to variation in the incidence of psychotic disorder across Europe (EU-GEI): a multicentre case-control study. Lancet Psychiatry 6:427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domino EF. (1971) Neuropsychopharmacologic studies of marijuana: some synthetic and natural thc derivatives in animals and man. Annals of the New York Academy of Sciences 191:166–191. [Google Scholar]
- Eggan SM, Melchitzky DS, Sesack SR, Fish KN, Lewis DA (2010) Relationship of cannabinoid CB1 receptor and cholecystokinin immunoreactivity in monkey dorsolateral prefrontal cortex. Neuroscience 169:1651–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emrich HM, Weber MM, Wendl A, Zihl J, von Meyer L, Hanisch W (1991) Reduced binocular depth inversion as an indicator of cannabis-induced censorship impairment. Pharmacol Biochem Behav 40:689–690. [DOI] [PubMed] [Google Scholar]
- Emrich HM, Leweke FM, Schneider U (1997) Towards a cannabinoid hypothesis of schizophrenia: cognitive impairments due to dysregulation of the endogenous cannabinoid system. Pharmacol Biochem Behav 56:803–807. [DOI] [PubMed] [Google Scholar]
- Englund A, Stone JM, Morrison PD (2012) Cannabis in the arm: what can we learn from intravenous cannabinoid studies? Curr Pharm Des 18:4906–4914. [DOI] [PubMed] [Google Scholar]
- Fitoussi A, Zunder J, Tan H, Laviolette SR (2018) Delta-9-tetrahydrocannabinol potentiates fear memory salience through functional modulation of mesolimbic dopaminergic activity states. Eur J Neurosci 47:1385–1400. [DOI] [PubMed] [Google Scholar]
- Foti DJ, Kotov R, Guey LT, Bromet EJ (2010) Cannabis use and the course of schizophrenia: 10-year follow-up after first hospitalization. Am J Psychiatry 167:987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Vollenweider FX (2008) Serotonin research: contributions to understanding psychoses. Trends Pharmacol Sci 29:445–453. [DOI] [PubMed] [Google Scholar]
- Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, Morse C, Pike VW, Volkow ND, Huestis MA, Innis RB (2012) Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry 17:642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjorthøj CR, Hjorthøj AR, Nordentoft M (2012a) Validity of timeline follow-back for self-reported use of cannabis and other illicit substances—systematic review and meta-analysis. Addic Behav 37:225–233. [DOI] [PubMed] [Google Scholar]
- Hjorthøj CR, Fohlmann A, Larsen AM, Arendt M, Nordentoft M (2012b) Correlations and agreement between delta-9-tetrahydrocannabinol (THC) in blood plasma and timeline follow-back (TLFB)-assisted self-reported use of cannabis of patients with cannabis use disorder and psychotic illness attending the CapOpus randomized clinical trial. Addiction 107:1123–1131. [DOI] [PubMed] [Google Scholar]
- Hollister LE, Gillespie HK (1973) Delta-8- and delta-9-tetrahydrocannabinol; comparison in man by oral and intravenous administration. Clin Pharmacol Ther 14:353–357. [DOI] [PubMed] [Google Scholar]
- Hooker WD, Jones RT (1987) Increased susceptibility to memory intrusions and the Stroop interference effect during acute marijuana intoxication. Psychopharmacology (Berl) 91:20–24. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13:261–276. [DOI] [PubMed] [Google Scholar]
- Konings M, Bak M, Hanssen M, van Os J, Krabbendam L (2006) Validity and reliability of the CAPE: a self-report instrument for the measurement of psychotic experiences in the general population. Acta Psychiatr Scand 114:55–61. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Perry EB Jr, Gueorguieva R, Belger A, Madonick SH, Abi-Dargham A, Cooper TB, Macdougall L, Abi-Saab W, D’Souza DC (2005) Comparative and interactive human psychopharmacologic effects of ketamine and amphetamine: implications for glutamatergic and dopaminergic model psychoses and cognitive function. Arch Gen Psychiatry 62:985–994. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, van Dyck CH, Rosenblatt W, Zea-Ponce Y, Zoghbi SS, Baldwin RM, Charney DS, Hoffer PB, Kung HF (1995) SPECT imaging of striatal dopamine release after amphetamine challenge. J Nucl Med 36:1182–1190. [PubMed] [Google Scholar]
- Lee D, Bergamaschi MM, Milman G, Barnes AJ, Queiroz RH, Vandrey R, Huestis MA (2015) Plasma cannabinoid pharmacokinetics after controlled smoking and ad libitum cannabis smoking in chronic frequent users. J Anal Toxicol 39:580–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leweke M, Kampmann C, Radwan M, Dietrich DE, Johannes S, Emrich HM, Münte TF (1998) The effects of tetrahydrocannabinol on the recognition of emotionally charged words: an analysis using event-related brain potentials. Neuropsychobiology 37:104–111. [DOI] [PubMed] [Google Scholar]
- Leweke FM, Schneider U, Thies M, Münte TF, Emrich HM (1999) Effects of synthetic delta9-tetrahydrocannabinol on binocular depth inversion of natural and artificial objects in man. Psychopharmacology (Berl) 142:230–235. [DOI] [PubMed] [Google Scholar]
- Leweke FM, Schneider U, Radwan M, Schmidt E, Emrich HM (2000) Different effects of nabilone and cannabidiol on binocular depth inversion in man. Pharmacol Biochem Behav 66:175–181. [DOI] [PubMed] [Google Scholar]
- Lindenmayer JP, Nasrallah H, Pucci M, James S, Citrome L (2013) A systematic review of psychostimulant treatment of negative symptoms of schizophrenia: challenges and therapeutic opportunities. Schizophr Res 147:241–252. [DOI] [PubMed] [Google Scholar]
- Mason OJ, Morgan CJ, Stefanovic A, Curran HV (2008) The psychotomimetic states inventory (PSI): measuring psychotic-type experiences from ketamine and cannabis. Schizophr Res 103:138–142. [DOI] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH, Turkington TG, Coleman RE (1998) Cerebellar activity and disturbed time sense after THC. Brain Res 797:183–189. [DOI] [PubMed] [Google Scholar]
- Melges FT, Tinklenberg JR, Deardorff CM, Davies NH, Anderson RE, Owen CA (1974) Temporal disorganization and delusional-like ideation. Processes induced by hashish and alcohol. Arch Gen Psychiatry 30:855–861. [DOI] [PubMed] [Google Scholar]
- Murray RM, Paparelli A, Morrison PD, Marconi A, Di Forti M (2013) What can we learn about schizophrenia from studying the human model, drug-induced psychosis? Am J Med Genet B Neuropsychiatr Genet 162B:661–670. [DOI] [PubMed] [Google Scholar]
- Núñez LA, Gurpegui M (2002) Cannabis-induced psychosis: a cross-sectional comparison with acute schizophrenia. Acta Psychiatr Scand 105:173–178. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR(1962) The brief psychiatric rating scale. Psychological Reports 10:799–812. [Google Scholar]
- Paparelli A, Di Forti M, Morrison PD, Murray RM (2011) Drug-induced psychosis: how to avoid star gazing in schizophrenia research by looking at more obvious sources of light. Front Behav Neurosci 5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasman JA, et al. ; 23andMe Research Team; Substance Use Disorders Working Group of the Psychiatric Genomics Consortium; International Cannabis Consortium (2018) GWAS of lifetime cannabis use reveals new risk loci, genetic overlap with psychiatric traits, and a causal influence of schizophrenia. Nat Neurosci 21:1161–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg-Raibstein D, Yee BK, Feldon J, Hauser J (2009) The amphetamine sensitization model of schizophrenia: relevance beyond psychotic symptoms? Psychopharmacology (Berl) 206:603–621. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan R, Skosnik PD, Cortes-Briones J, Sewell RA, Carbuto M, Schnakenberg A, Cahill J, Bois F, Gunduz-Bruce H, Pittman B, Ranganathan M, D’Souza DC (2015) GABA deficits enhance the psychotomimetic effects of Δ9-THC. Neuropsychopharmacology 40:2047–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan M, Schnakenberg A, Skosnik PD, Cohen BM, Pittman B, Sewell RA, D’Souza DC (2012a) Dose-related behavioral, subjective, endocrine, and psychophysiological effects of the κ opioid agonist Salvinorin A in humans. Biol Psychiatry 72:871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan M, Carbuto M, Braley G, Elander J, Perry E, Pittman B, Radhakrishnan R, Sewell RA, D’Souza DC (2012b) Naltrexone does not attenuate the effects of intravenous Δ9-tetrahydrocannabinol in healthy humans. Int J Neuropsychopharmacol 15:1251–1264. [DOI] [PubMed] [Google Scholar]
- Ranganathan M, De Aquino JP, Cortes-Briones JA, Radhakrishnan R, Pittman B, Bhakta S, D’Souza DC (2019) Highs and lows of cannabinoid-dopamine interactions: effects of genetic variability and pharmacological modulation of catechol-O-methyl transferase on the acute response to delta-9-tetrahydrocannabinol in humans. Psychopharmacology (Berl) 236:3209–3219. [DOI] [PubMed] [Google Scholar]
- Sherif M, Radhakrishnan R, D’Souza DC, Ranganathan M (2016) Human laboratory studies on cannabinoids and psychosis. Biol Psychiatry 79:526–538. [DOI] [PubMed] [Google Scholar]
- Skosnik PD, Cortes-Briones JA, Hajós M (2016) It’s all in the rhythm: the role of cannabinoids in neural oscillations and psychosis. Biol Psychiatry 79:568–577. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB(1992) Timeline follow-back: a technique for assessing selfreported alcohol consumption. In: Measuring Alcohol Consumption: Psychosocial and Biological Methods (Allen RZLJ, ed), pp 41–72. New Jersey, NJ: Humana Press. [Google Scholar]
- Starzer MSK, Nordentoft M, Hjorthøj C (2018) Rates and predictors of conversion to schizophrenia or bipolar disorder following substance-induced psychosis. Am J Psychiatry 175:343–350. [DOI] [PubMed] [Google Scholar]
- Steeds H, Carhart-Harris RL, Stone JM (2015) Drug models of schizophrenia. Ther Adv Psychopharmacol 5:43–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes PR, Mehta MA, Curran HV, Breen G, Grasby PM (2009) Can recreational doses of THC produce significant dopamine release in the human striatum? Neuroimage 48:186–190. [DOI] [PubMed] [Google Scholar]
- Tikka SK, D’Souza DC(2019) The association between cannabinoids and psychosis. In: Cannabis Use Disorders (Montoya ID, Weiss SRB, eds), pp 127–155: Gewerbestrasse, Switzerland: Springer. [Google Scholar]
- van der Gaag M, Hoffman T, Remijsen M, Hijman R, de Haan L, van Meijel B, van Harten PN, Valmaggia L, de Hert M, Cuijpers A, Wiersma D (2006) The five-factor model of the positive and negative syndrome scale II: a ten-fold cross-validation of a revised model. Schizophr Res 85:280–287. [DOI] [PubMed] [Google Scholar]
- Vaucher J, Keating BJ, Lasserre AM, Gan W, Lyall DM, Ward J, Smith DJ, Pell JP, Sattar N, Paré G, Holmes MV (2018) Cannabis use and risk of schizophrenia: a Mendelian randomization study. Mol Psychiatry 23:1287–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallwork RS, Fortgang R, Hashimoto R, Weinberger DR, Dickinson D (2012) Searching for a consensus five-factor model of the positive and negative syndrome scale for schizophrenia. Schizophr Res 137:246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Kunos G, Nicoll RA (2001) Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron 31:453–462. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA (2001) Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 410:588–592. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.