Abstract
Background
Pharmacological augmentation is a recommended strategy for patients with treatment-resistant depression. A range of guidelines provide advice on treatment selection, prescription, monitoring and discontinuation, but variation in the content and quality of guidelines may limit the provision of objective, evidence-based care. This is of importance given the side effect burden and poorer long-term outcomes associated with polypharmacy and treatment-resistant depression. This review provides a definitive overview of pharmacological augmentation recommendations by assessing the quality of guidelines for depression and comparing the recommendations made.
Methods
A systematic literature search identified current treatment guidelines for depression published in English. Guidelines were quality assessed using the Appraisal of Guidelines for Research and Evaluation II tool. Data relating to the prescription of pharmacological augmenters were extracted from those developed with sufficient rigor, and the included recommendations compared.
Results
Total of 1696 records were identified, 19 guidelines were assessed for quality, and 10 were included. Guidelines differed in their quality, the stage at which augmentation was recommended, the agents included, and the evidence base cited. Lithium and atypical antipsychotics were recommended by all 10, though the specific advice was not consistent. Of the 15 augmenters identified, no others were universally recommended.
Conclusions
This review provides a comprehensive overview of current pharmacological augmentation recommendations for major depression and will support clinicians in selecting appropriate treatment guidance. Although some variation can be accounted for by date of guideline publication, and limited evidence from clinical trials, there is a clear need for greater consistency across guidelines to ensure patients receive consistent evidence-based care.
Keywords: Augmentation, depression, guideline, pharmacology, systematic review
Introduction
Patients with major depression who do not respond to initial antidepressant treatment(s) may be regarded as treatment resistant and are more likely to experience poorer long-term outcomes (Fekadu et al., 2009). However, for the 25% to 50% of patients reported to have a poor response to at least 2 different antidepressant treatments (Fava and Davidson, 1996; Rush et al., 2006), outcomes can be improved with successive and multimodal treatment (Rush et al., 2006; Wooderson et al., 2014). Pharmacological augmentation—the addition of a second agent to a continued antidepressant—is one such approach, and several recent meta-analyses support the efficacy of a number of augmentation agents (Zhou et al., 2015; Strawbridge et al., 2019).
When treating patients with treatment-resistant depression (TRD), clinicians may refer to a range of guidelines published independently by local, national, and international bodies for advice on treatment selection, monitoring, and discontinuation. Such guidelines are not standardized, and a universal or “first-line” option does not exist. Therefore, treatment recommendations may vary, as may the evidence on which they are based and the overall guideline quality.
Given this potential for variation between guidelines, the poor outcomes associated with TRD, and the number of pharmacological augmentation options available, an overview of relevant guideline recommendations could prove highly valuable to both clinicians and researchers. This would help to ensure that patients receive the most appropriate, evidence-based treatment(s) and guide future research by highlighting areas of need. A recent review of treatment guidelines for depression included pharmacotherapy and neuro-stimulation but very little information pertaining to pharmacological augmentation (Bayes and Parker, 2018). The present review will therefore examine and compare guidelines for the prescription of pharmacological augmentation treatments in patients with unipolar resistant depression to provide a comprehensive overview of the recommendations made, identify consistencies and inconsistencies between them, and assess their quality. Recommendations for the development of future treatment guidelines are also made.
Methods
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines (Moher et al., 2009), and the study protocol was registered with PROSPERO a priori (reference: CRD42018112343).
Search Strategy and Selection Criteria
Literature searches were conducted in Embase, MEDLINE, and PsycINFO to identify guidelines published between 2008 and August 23, 2019. Titles, abstracts, and key words were searched using terms relating to guidelines, treatment, and depression as per the study protocol. All articles were evaluated for suitability by 2 independent reviewers (L.M., R.W.T.), and any discrepancies were discussed until a consensus was reached. A third review author (A.J.C.) was consulted as necessary. In addition, reference lists of identified guidelines or relevant reviews were also manually searched, and guideline websites were checked where necessary to ensure the inclusion of current guideline versions. Records identified by the search were assessed for eligibility in 2 stages.
Stage 1: Selection
All current versions of guidelines meeting the following criteria were included at this stage:
Treatment guidelines for clinicians, meeting the following definition: statements that include recommendations intended to optimize patient care that are informed by a review of evidence and an assessment of the benefits and harms of alternative care options. This definition was adapted from Verdolini et al. (2018) and based on one created by the Institute of Medicine (Institute of Medicine [U.S.]; Graham, 2011);
Guidelines for the management of adults (18+ years) with unipolar major depressive disorder (MDD). Recommendations for specific subsets of patients, for example, pregnant or breastfeeding women, were not included;
Pharmacological augmentation treatment options discussed, defined as the addition of a second pharmacological agent to a continuation antidepressant. Consideration of combination therapies and augmentation with a second antidepressant medication was deemed beyond the scope of this review; and
Published since 2008 and fully available in English.
Stage 2: Guideline Selection
All guidelines meeting stage 1 eligibility were quality assessed using the Appraisal of Guidelines for Research and Evaluation (AGREE) II tool (Brouwers et al., 2010), conducted independently by 2 of 6 review authors (R.W.T., L.M., E.O., V.A., B.V., and S.M.). All raters first completed the AGREE II online tutorial video (http://www.agreetrust.org), and all appraisals were made in line with the user manual. All items were scored from 1 (strongly disagree) to 7 (strongly agree). Scaled scores were calculated for each of the 6 domains according to the instruction manual (Hoffmann-Eßer et al., 2018). Guidelines with 3 or more domains scoring μ ≥ 60% were included, as this cut-off has been used by previous appraisals to indicate that a guideline is of reasonable quality (Brosseau et al., 2014).
Data Analysis
For guidelines reaching stage 2 inclusion, data relating to pharmacological augmentation were extracted and narratively synthesized according to the study outcomes. This included recommendations for indication/contraindication, pre-prescribing and monitoring tests, dosage, and withdrawal. Data were extracted for all pharmacological augmentation treatments recommended as first or second line (or equivalent) by at least 1 of the included guidelines. Data extraction was conducted by L.M. and R.W.T. and any discrepancies resolved through consensus.
Outcomes
Primary Outcomes
Our primary outcome is the augmentation treatments recommended by the included guidelines, including indication/contraindication, pre-prescribing and monitoring tests, dosage, and tapering/withdrawal recommendations.
Secondary Outcomes
Our secondary outcomes are discrepancies between guideline recommendations and the quality of the guidelines as assessed by the AGREE II tool.
Results
Search Results and Quality Assessment
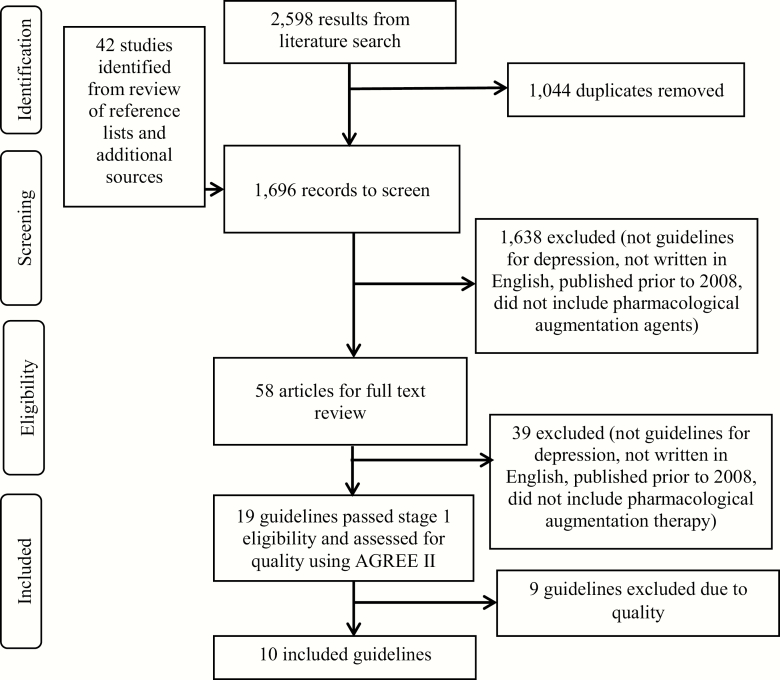
Embase returned 1176, MEDLINE 646, and PsycINFO 513 results. The process of guideline selection can be seen in Figure 1.
Figure 1.
Guideline selection
Ten guidelines met the quality cut-off for inclusion (at least 3 domains with a scaled score of ≥60%). Details of included guidelines can be found in Table 1 and the scaled domain scores in Table 2. Domain scores for the 9 guidelines excluded at this stage can be found in supplementary Table 1.
Table 1.
Included Guidelines for Augmentation Treatments in Unipolar Depression
| Guideline | Region | Year |
|---|---|---|
| APA (American Psychiatric Association, 2010) | North America | 2010 |
| BAP (Cleare et al., 2015) | Europe | 2015 |
| CANMAT (Kennedy et al., 2016) | North America | 2016 |
| CPG-S (Ministry of Health, Social Services and Equality, Galician Agency for Health et al., 2014) | Europe | 2014 |
| ICSI (Trangle et al., 2016) | North America | 2016 |
| MPG (Taylor, David M. et al., 2018) | Europe | 2018 |
| NICE (NICE, 2009) | Europe | 2009 |
| RANZCP (Malhi et al., 2015) | Australasia | 2015 |
| TMAP (Suehs et al., 2008) | North America | 2008 |
| WFSBP (Bauer et al., 2013b, 2015)a | Worldwide | 2013/2015 |
Abbreviations: APA, American Psychiatric Association; BAP, British Association of Psychopharmacology; CANMAT, Canadian Network for Mood and Anxiety Disorders; CPG-S, Clinical Practice Guidelines in the Spanish National Health Service; ICSI, Institute for Clinical Systems Improvement; MPG, Maudsley Prescribing Guidelines in Psychiatry; NICE, National Institute for Health and Clinical Excellence; RANZCP, Royal Australian and New Zealand College of Psychiatrists; TMAP, Texas Medication Algorithm Project; WFSBP, World Federation of Societies of Biological Psychiatry.
aGuideline parts I and II are considered together in this review
Table 2.
AGREE II Scaled Domain Scores For Included Guidelines
| Domain score (%) | |||||||
|---|---|---|---|---|---|---|---|
| Guideline | Scope and purpose | Stakeholder involvement | Rigor of development | Clarity of presentation | Applicability | Editorial independence | Mean (SD) |
| APA | 89.9 | 62.5 | 70.0 | 77.8 | 43.8 | 75.0 | 69.7 (14.1) |
| BAP | 80.6 | 61.1 | 47.0 | 75.0 | 50.0 | 79.2 | 65.4 (13.7) |
| CANMAT | 80.6 | 62.5 | 53.0 | 94.4 | 27.1 | 66.7 | 64.1 (21.2) |
| CPG-S | 100.0 | 93.1 | 83.0 | 80.6 | 85.4 | 83.3 | 87.6 (6.8) |
| ICSI | 94.4 | 80.6 | 72.0 | 83.3 | 83.3 | 83.3 | 82.8 (6.7) |
| MPG | 80.6 | 40.3 | 45.0 | 88.9 | 68.8 | 25.0 | 58.1 (22.9) |
| NICE | 100.0 | 93.1 | 78.0 | 91.7 | 91.7 | 75.0 | 88.3 (8.7) |
| RANZCP | 94.4 | 81.9 | 63.0 | 86.1 | 54.2 | 62.5 | 73.8 (14.5) |
| TMAP | 72.2 | 31.9 | 27.0 | 88.9 | 60.4 | 41.7 | 53.6 (22.3) |
| WFSBP | 86.1 | 63.9 | 70.0 | 80.6 | 39.6 | 70.8 | 68.5 (14.8) |
| Mean (SD) | 87.8 (8.9) | 67.1 (19.5) | 60.8 (16.7) | 84.7 (6.0) | 60.4 (20.4) | 66.3 (18.0) | |
Abbreviations: APA, American Psychiatric Association; BAP, British Association of Psychopharmacology; CANMAT, Canadian Network for Mood and Anxiety Disorders; CPG-S, Clinical Practice Guidelines in the Spanish NHS; ICSI, Institute for Clinical Systems Improvement; MPG, Maudsley Prescribing Guidelines; NICE, National Institute for Health and Care Excellence; RANZCP, Royal Australian and New Zealand College of Psychiatrists; TMAP, Texas Medication Algorithm Project; WFSBP, World Federation of Societies of Biological Psychiatry.
General Treatment Guidance
When to Augment? Indications and Contraindications
Table 3 shows the treatment stage at which augmentation was recommended. Six guidelines recommended augmentation following 1 failed antidepressant treatment, and 4 recommended it after 2 antidepressant treatments. Varying criteria and terminology were also used to define response to prior treatments (Table 3).
Table 3.
Summary Of Defined Treatment Failures Prior To Recommendation Of Augmentation
| Guideline | No. failed AD trials | Length per AD trial (weeks) | AD dose | AD response | Additional indicators for augmentation |
|---|---|---|---|---|---|
| APA | 2 | 4–8 | Sufficient | Minimal/no improvement | Consider in patients with significant side effects, i.e., if AD dose increase would not be tolerable |
| BAP | ≥1 | 4 | Adequate | Insufficient/partial | Good AD tolerability, AD switching unsuccessful |
| CANMAT | ≥2 | 2–4 | – | Partial (25–49%) or no response (<25%) reduction in severity score, or nonremission at >6–8 weeks | Current AD well tolerated, patient preference for augmentation, specific residual symptoms, or side effects to be targeted |
| CPG-S | ≥1 | 3–4 | – | Partial/nonresponse | When deciding best treatment option, consider prior treatment resistance, risk factors, symptom profile, and severity |
| ICSI | ≥2 | 6–12 | – | Partial/treatment resistant | Consider augmentation after other stepped care options |
| MPG | 2 + consideration of 3rd choice AD optionsa | 3–4 | – | Failed/no effect | – |
| NICE | Initial treatment | – | – | Nonresponse | Patient willing to tolerate increased side effect burden |
| RANZCP | ≥1 | 3 | Adequate | Failed | Severe/debilitating symptoms of depression |
| TMAP | ≥1 | ≥4 (augmentation recommended at 6) | – | Partial (QIDS-CR 6–8) to ≥1 AD/nonresponse (QIDS-CR ≥9), or intolerance to 2 ADsb | – |
| WFSBP | ≥1 | 2–4 | Adequate | Failure/partial | – |
Abbreviations: –, not stated; AD, antidepressant; QIDS-CR, Quick Inventory of Depressive Symptomatology – Clinician Rated;(Rush et al., 1996) APA, American Psychiatric Association; BAP, British Association of Psychopharmacology; CANMAT, Canadian Network for Mood and Anxiety Disorders; CPG-S, Clinical Practice Guidelines in the Spanish NHS; ICSI, Institute for Clinical Systems Improvement; MPG, Maudsley Prescribing Guidelines; NICE, National Institute for Health and Care Excellence; RANZCP, Royal Australian and New Zealand College of Psychiatrists; TMAP; Texas Medication Algorithm Project; WFSBP, World Federation of Societies of Biological Psychiatry.
aThese included mirtazapine, vortioxetine, and agomelatine.
bAntidepressants must be different classes/mechanism of action.
Who Should Prescribe Augmentation Treatment?
The National Institute for Health and Care Excellence (NICE) guidelines stated that combinations of medications initiated in primary care should be done in consultation with a psychiatrist, and referral to specialist services/an individual with specialist interest may be appropriate. The Royal Australian and New Zealand College of Psychiatrists (RANZCP), Clinical Practice Guidelines in the Spanish NHS (CPG-S), and the World Federation of Societies of Biological Psychiatry (WFSBP) offered similar advice.
The British Association for Psychopharmacology (BAP) and the Maudsley Prescribing Guidelines (MPG) recommended the initiation of certain augmenters in specialist centers with careful monitoring. The BAP included stimulants, estrogen in perimenopausal women, and testosterone in men with low levels, while the MPG specified lithium, ketamine, and triiodothyronine (T3). The Canadian Network for Mood and Anxiety Disorders (CANMAT) stated that their guidance was intended for psychiatrists and other mental health professionals. The Institute for Clinical Systems Improvement (ICSI), American Psychiatric Association (APA), and Texas Medication Algorithm Project (TMAP) did not advise.
Augmentation Selection/Pre-Prescription
All guidelines (except the MPG) offered general guidance about treatment selection in depression, and some included general pre-prescription assessments. General pharmacological augmentation guidance is included here, and details of pre-prescription assessments for individual augmenters are included in the relevant sections below.
TMAP took an algorithmic approach to treatment selection, while APA and RANZCP advised consideration of the side effect profile, as well as personality, lifestyle, and social factors (RANZCP). BAP recommended consideration of options with the largest evidence base and, in cases of severe TRD, consideration of multiple pharmacological combinations. NICE advocated following General Medical Council advice when prescribing augmentation off-label and recommended that clinicians document the rationale. NICE and RANZCP advised that most decisions are based on both clinical judgement and patient preference. The ICSI recommended consideration of brain imaging and pharmacogenetic testing prior to prescription in refractory disorders.
Monitoring
None of the guidelines offered general monitoring advice for augmentation beyond that relevant to all psychotropic medications (see guidelines). RANZCP was the only guideline to mention assessing for early response despite an absence of early response being the best supported predictor of augmentation treatment outcome in TRD (Taylor et al., 2019). All augmentation-specific monitoring is included in the relevant sections below.
Discontinuation
In cases of response, NICE advocated continuing both medications and, if necessary, the augmenter should be stopped before the antidepressant. For those with a higher risk of relapse, NICE recommended continuation for up to 2 years. No other general discontinuation advice was provided.
Pharmacological Augmentation Recommendations
Table 4 summarizes the augmentation agents recommended as first or second line (or equivalent) by at least 1 of the 10 included guidelines. However, guidelines varied in their categorization criteria: CANMAT’s first-, second-, or third-line options were based on the level of evidence available and graded according to specific criteria; BAP, TMAP, the MPG, RANZCP, and WFSPB used similar systems, though their grading criteria were less explicit or not explained (TMAP); RANZCP distinguished between evidence-based recommendations (where there was sufficiently consistent evidence) and consensus-based recommendations (based on the collective knowledge and experience of the committee). The ICSI used the Grading of Recommendations Assessment, Development and Evaluation methodology (http://www.gradeworkinggroup.org/) and CPG-S used the Scottish Intercollegiate Guidelines Network system to assess evidence (Scottish Intercollegiate Guidelines Network, 2012). NICE used both Scottish Intercollegiate Guidelines Network and Grading of Recommendations Assessment, Development and Evaluation as well as considering factors such as evidence from depression in the general population, economic factors, and the values of the Guideline Development Group.
Table 4.
Summary of Pharmacological Augmentation Recommendations by Guideline
| APA | BAP | CANMAT | CPGS | ICSI | NICE | MPG | RANZCP | TMAP | WFSBP | |
|---|---|---|---|---|---|---|---|---|---|---|
| AAPs1 | 2nd | 1st | 1st | 1st | ✔ | 1st | 1st | 1st | 2nd | 1st |
| Lithium | 2nd | 1st | 2nd | 1st | ✔ | ✔ | 1st | 1st | ✔ | 1st |
| Other mood stabilisers | ✔ | 2nd 1 | ✘ | ✘ | – | ✘ | 2nd 1 | – | ✔a | – |
| Thyroid hormones | 2nd | 2nd | 2nd | ✘ | ✔ | ✘ | 2nd | 1st | 1st | 2nd |
| Stimulants | ✔ | ✔ | 2ndb | – | ✔ | ✘ | ✔ | ✘ | – | – |
| Bupropion | ✔ | ✔ | 2nd | ✘ | ✔ | ✘ | 1st | ✘ | 1st | ✘ |
| Buspirone | ✔ | ✔ | ✘ | ✘ | ✔ | ✘ | 2nd | ✘ | 1st | ✘ |
| Ketamine | – | ✘ | ✔ | – | ✔ | – | 2nd | ✘ | – | ✘ |
Abbreviations: 1st, first-line recommendation or equivalent; 2nd, second-line recommendation or equivalent; ✔, other recommendation/level not specified, ✘, not recommended, —, treatment not discussed by guideline; AAPs, atypical antipsychotics; APA, American Psychiatric Association; BAP, British Association of Psychopharmacology; CANMAT, Canadian Network for Mood and Anxiety Disorders; CPG-S, Clinical Practice Guidelines in the Spanish NHS; ICSI, Institute for Clinical Systems Improvement; NICE, National Institute for Health and Care Excellence; RANZCP, Royal Australian and New Zealand College of Psychiatrists; MPG, Maudsley Prescribing Guidelines; TMAP; Texas Medication Algorithm Project; WFSBP, World Federation of Societies of Biological Psychiatry.
aSpecific combination/medication subtype recommendation.
bAt least 1 of this class of drug recommended at indicated level.
APA employed 3 levels of confidence (substantial, moderate, or may be recommended on the basis of individual circumstances), which were not clearly linked to an assessment of the evidence. Recommendations were not ranked as first or second line (or equivalents). As none of the pharmacological augmenters were recommended with “substantial confidence,” they were considered either second line or “other” as appropriate in this review. As TMAP is a medication algorithm, step-by-step recommendations were divided into stages, employed according to patient response. It is not clear how closely these recommendations aligned to the evidence used, as TMAP stated “the treatment algorithms are evidence-based to the extent that evidence is available to guide treatment decisions” (Suehs et al., 2008).
Atypical Antipsychotics
AAPs: General Recommendations
First line
BAP advised that the most appropriate atypical antipsychotic (AAP) may be decided on an individual basis. An alternative may prove effective if one has failed (based on clinical experience). BAP highlighted the paucity of long-term studies and lack of research in severely treatment-resistant populations. They cited a meta-analysis in which AAPs showed benefit over placebo, though adverse effects were fourfold higher (Nelson and Papakostas, 2009). BAP also discussed a more recent meta-analysis in which response and remission rates were higher for quetiapine, aripiprazole, and risperidone, while response to olanzapine-fluoxetine combination (OFC) did not significantly differ from placebo (Spielmans et al., 2013).
AAPs were first-line equivalents in NICE, specifically aripiprazole, olanzapine, quetiapine, and risperidone, though it was not clear that this list is exhaustive. NICE concluded that the evidence for clinical efficacy was “moderate” and cautioned that dropout and side-effect reports were more likely than for antidepressant monotherapy, particularly for quetiapine (Shelton et al., 2001; Corya et al., 2006; Berman et al., 2007; Mahmoud et al., 2007; McIntyre et al., 2007; Song et al., 2007; Thase et al., 2007a; Marcus et al., 2008; Keitner et al., 2009). The absence of head-to-head trials was highlighted, and NICE suggested that similar results between treatments may be due to the low number of studies. At the time of publication, AAPs did not have UK marketing authorization for use in depression. AAP augmentation was also first line in the CPG-S, who highlighted the randomized controlled trials (RCTs) included by NICE plus an additional 8. RANZCP recommended “some” AAPs as level I augmenters and referenced placebo-controlled evidence for aripiprazole, olanzapine, quetiapine, and risperidone efficacy (Papakostas et al., 2007; Philip et al., 2008). CANMAT stated that AAPs have the most consistent evidence base of all augmentation options, though there is not enough evidence comparing with alternative adjuncts.
Second line
APA considered AAPs to be second-line augmenters and did not make agent-specific recommendations. They stated that most trials report rapid improvements in mood, but the difference is modest compared with placebo (Shelton et al., 2001; Corya et al., 2006; Berman et al., 2007; Dorée et al., 2007; Mahmoud et al., 2007; McIntyre et al., 2007; Thase et al., 2007a; Garakani et al., 2008; Keitner et al., 2009). They also referred to trials in which OFC therapy was not significantly more effective than continued monotherapy with nortriptyline (Shelton et al., 2005) or venlafaxine (Corya et al., 2006). APA referenced the same meta-analysis as BAP (Nelson and Papakostas, 2009) in which AAP augmentation was significantly more effective than placebo. APA stressed that long-term studies were lacking at the time of publication.
TMAP recommended AAP augmentation at stage 3 of their 8-stage algorithm, making it a second-line option. TMAP included a flow chart, text descriptions, and summary medication charts in the appendix. AAPs were not included in the algorithm flow chart at stage 3 but were in the full text description. TMAP stated that there is strong evidence for the efficacy of olanzapine, aripiprazole (both Level A), and risperidone (Level B), though they are associated with adverse effects. Other AAPs, including quetiapine, were included in the appendix.
Other general recommendations
As mentioned, the ICSI did not explicitly rank their recommendations. They highlighted that AAPs were second line in the APA guidelines, and aripiprazole, quetiapine, and OFC were approved in the United States at the time of publication. The ICSI referred to a review of 3 placebo-controlled RCTs of quetiapine extended release (XR), reporting it to be effective for response and remission (Maneeton et al., 2012), and stressed that efficacy and safety should be assessed frequently (Wright et al., 2013). They also referred to 2 meta-analyses in which response and remission rates were significantly better for AAP augmentation than placebo (Papakostas et al., 2007; Nelson and Papakostas, 2009).
TMAP also included AAP augmentation of an selective serotonin reuptake inhibitor (SSRI) as an option at stage 4 if a tricyclic antidepressant (TCA) (with or without lithium or a monoamine oxidase inhibitor [MAOI]) was used at stage 3. OFC treatment was mentioned at this stage. AAPs were also included at stage 6, though the authors stated that little evidence supported recommendations at this stage, which were based on expert opinion and the consensus of the TMAP panel. Here, AAPs were recommended as part of a triple therapy approach, alongside an SSRI and bupropion.
Aripiprazole
First-Line Recommendations
BAP considered aripiprazole to be a first-line augmenter, referring to Berman et al. (2007), which demonstrated good tolerability and a higher response rate than placebo when added to a continuation antidepressant. CANMAT recommended aripiprazole supported by Level I evidence and discussed the network meta-analysis by Zhou and colleagues, which included 48 studies (n = 6645) examining the comparative efficacy of adjunctive strategies (Zhou et al., 2015). Aripiprazole and quetiapine were the only AAPs significantly more effective than placebo and were more efficacious than other options (lithium and T3). CANMAT referred to 4 additional meta-analyses (again including Nelson and Papakostas, 2009), each including 12–17 trials and reporting better efficacy for aripiprazole, quetiapine, olanzapine, and risperidone compared with placebo, with small to medium effect sizes (Komossa et al., 2010; Spielmans et al., 2013; Wen et al., 2014).
The CPG-S also specified aripiprazole as first line. They referenced 2 RCTs included by NICE (Berman et al., 2007; Marcus et al., 2008) in which the augmentation group had nonsignificantly better response and remission rates and no difference in discontinuation due to adverse effects compared with placebo. The CPG-S also referenced an RCT demonstrating the efficacy of low-dose aripiprazole in patients with an inadequate response to up to 3 antidepressants. Again, the difference in response and remission was not significant (Fava et al., 2012). An RCT of aripiprazole augmentation of clomipramine was also discussed, in which Hamilton Depression Rating Scale (HDRS) scores significantly decreased each week (Fabrazzo et al., 2012).
The WFSBP guidelines included aripiprazole as first line, citing a Cochrane study that reported aripiprazole to be significantly more effective than antidepressant monotherapy but associated with more side effects (Komossa et al., 2010). They also referenced the same double-blind RCT of low-dose aripiprazole augmentation as the CPG-S, which reported a nonsignificant effect but good tolerability (Fava et al., 2012). The MPG included aripiprazole as first line, advocating a good evidence base, good tolerability and safety, and the potential efficacy of low doses, though side effects may be disadvantageous (Papakostas et al., 2005; Simon and Nemeroff, 2005; Berman et al., 2007; Hellerstein et al., 2008; Marcus et al., 2008; Fava et al., 2012; Yoshimura et al., 2012; Jon et al., 2013).
No guidelines specifically listed aripiprazole as second line/other/not recommended.
Quetiapine
First-Line Recommendations
BAP recommended quetiapine as first line, citing evidence indicating equal efficacy to lithium (Bauer et al., 2013a) and noting that it was the only AAP licensed for use as an augmenter in the United Kingdom at the time of publication. BAP also referred to El-Khalili et al. (2010) in which quetiapine XR was significantly more effective than augmentation with placebo. CANMAT similarly recommended quetiapine as a first-line option, supported by Level I evidence.
The CPG-S referred to a comparison of quetiapine augmentation of an SSRI and venlafaxine also included by NICE in which response, remission, and depression severity did not significantly differ between groups (quetiapine did slightly better, albeit with a higher discontinuation rate; McIntyre et al., 2007). The CPG-S also mentioned Bauer et al. (2010), in which patients received quetiapine XR augmentation (150 or 300 mg) or placebo. Both groups had significantly higher remission rates compared with placebo, but only the 150-mg/d group did better in terms of response at 6 weeks. Another RCT in patients with comorbid anxiety and residual depressive symptoms demonstrated higher completion rates for quetiapine, and nonsignificantly higher response and remission rates compared with placebo were referenced as was an open-label comparison with lithium in patients unresponsive to at least 4 weeks of antidepressant treatment. HDRS scores were significantly reduced in both groups, but more so for quetiapine (Dorée et al., 2007; McIntyre et al., 2007).
The WFSBP included quetiapine as a first-line option, referencing a study in which it was significantly more effective than antidepressant monotherapy, though it has been associated with more weight gain and sedation (Komossa et al., 2010; Bauer et al. 2010), in line with CPG-S. The MPG included quetiapine as a first-line, well-tolerated augmenter with a good evidence base, advising that it should be used in addition to an SSRI or serotonin-norepinephrine reuptake inhibitors. They advised that quetiapine is possibly more effective than lithium (Montgomery et al., 2010).
No guidelines specifically included quetiapine as second line/other/not recommended.
Risperidone
First-line recommendations
The BAP recommended risperidone as first line, as did CANMAT (Level I evidence), and the CPG-S, who referred to 3 studies included in the current NICE guidelines in which there was no significant difference in depression severity or discontinuation due to adverse effects between risperidone and control (Mahmoud et al., 2007; Song et al., 2007; Keitner et al., 2009). Another study was discussed in which patients with 2 or more antidepressant failures were randomized to receive 1 of 5 augmenters, including risperidone, added to paroxetine (Fang et al., 2011). The risperidone response rate was 46.7% and remission 26.7%, with no significant difference between treatments (valproic acid buspirone, trazodone, or T3). However, the CPG-S recognized the lack of placebo arm, modest sample sizes, fixed doses, and exclusively Chinese sample as limitations.
Second-line recommendations
The MPG recommended risperidone as a second-line augmenter and cautioned that it has a small evidence base and less RCT support than other AAPs, though it is usually well tolerated. Several side effects were highlighted as disadvantages (Ostroff and Nelson, 1999; Stoll and Haura, 2000; Rapaport et al., 2006; Mahmoud et al., 2007; Yoshimura et al., 2008; Keitner et al., 2009).
Other recommendations
ICSI did not state whether they recommended the use of risperidone, but it was the only AAP highlighted as being efficacious despite not having US approval.
Not recommended
Though the WFSBP stated that risperidone was significantly more effective than antidepressant monotherapy, it was not recommended as benefits have not been sustained and it is associated with greater weight gain and prolactin change (Rapaport et al., 2006; Mahmoud et al., 2007; Reeves et al., 2008; Keitner et al., 2009).
Olanzapine
First-line recommendations
The CPG-S included olanzapine as a first-line option, referring to 2 RCTs examining OFC, also included by NICE, in which the olanzapine group had nonsignificantly better response and remission rates vs placebo (Shelton et al., 2005; Thase et al., 2007a). CPG-S also mentioned a review of 5 OFC trials that reported a significantly greater improvement in MADRS scores compared with either monotherapy with the same adverse effect levels (Trivedi et al., 2009).
The MPG recommended OFC as a first-line option, stating that it is well researched and generally well tolerated, and suggested that olanzapine augmentation of a TCA may also be effective, as may olanzapine monotherapy (Takahashi et al., 2008). The MPG highlighted the risk of weight gain and limited clinical experience outside the United States and the fact that most available data are related to bipolar disorder (Luan et al., 2017).
Second-line recommendations
The BAP recommended olanzapine as second line and concluded that olanzapine augmentation of an SSRI may be most effective when patients have been nonresponsive to SSRIs, rather than serotonin-norepinephrine reuptake inhibitors or TCAs. They referred to 2 large OFC studies in which it was more effective than fluoxetine monotherapy, but not nortriptyline continuation (Shelton et al., 2005) or venlafaxine (Corya et al., 2006), and an additional study that combined data from 1 positive and 1 negative OFC trial, which demonstrated that OFC was more effective than monotherapy (Thase et al., 2007a).
CANMAT also recommended olanzapine as a second-line augmenter, supported by Level I evidence, as did the WFSBP, concluding that the evidence was more ambiguous than for aripiprazole or quetiapine, and olanzapine was associated with more side effects, including weight gain and increase in prolactin (Komossa et al., 2010).
Brexpiprazole
Second-Line Recommendations
CANMAT recommended brexpiprazole as a second-line augmenter, supported by Level I evidence from placebo controlled trials (Thase et al., 2015a, 2015b), but highlighted that all AAPs were worse tolerated than placebo.
General AAP Dosage Recommendations
The APA, BAP, RANZCP, and the WFSBP guidelines advised that lower doses of AAPs are generally used for augmentation in TRD compared with psychosis. RANZCP cited 3 studies (Berman et al., 2007; Marcus et al., 2008; Philip et al., 2008) and highlighted that use for augmentation in unipolar TRD was off-label in Australasia at the time of publication. The ICSI recommended that dosing should be individualized, and the MPG advised that there is considerable individual variation in plasma levels, meaning monitoring is required to reach the median optimal range, if known.
CANMAT, CPG-S, NICE, and TMAP did not include general dosage guidance, but specific recommendations can be found in Tables 7–11. NICE did not make any dosage recommendations for AAPs.
Table 7.
Aripiprazole Dosage and Monitoring Guidance
| Guideline | Dose | Patient monitoring parameters | Specific side effects | Specific drug interactions |
|---|---|---|---|---|
| APA | 2.5–5 mg/d, titrated to a max of 15 mg/d (Berman et al., 2007) | General AAP guidance only | General AAP guidance only | General AAP guidance only |
| BAP | 2.5–10 mg/d (Taylor et al., 2015) | General AAP guidance only | General AAP guidance only | – |
| CANMAT | 2–15 mg/d | – | – | Moderate potential for drug-drug interactions (2D6, 3A4 substrate) |
| CPG-S | – | – | Weight gain, dry mouth, constipation (Fava et al., 2012) | – |
| ICSI | General AAP guidance only | – | Higher rates of akathisia and fatigue compared with placebo (Berman et al., 2007; Marcus et al., 2008) | – |
| MPG | 2–10 mg/d | General AAP guidance, plus caution required in cases of severe hepatic impairment. General monitoring advice applies, but lipids and blood pressure may not be required, and less monitoring of weight compared with other AAPs. | General AAP guidance, plus akathisia and restlessness common, and insomnia may be problematic. Caution required in cases of severe hepatic impairment due to findings of increased LFTs, hepatitis, and jaundice (Mallikaarjun et al., 2008; Datapharm Communications Ltd, 2017; Truven Health Analytics, 2018). Very low risk (in relation to other AAPs) for sedation, weight gain, parkinsonism, anticholinergic effects, hypotension, and prolactin level changes. Low risk of akathisia. Low relative effect on QTc. No reported AEs on sexual function. Cases of hypersexuality have been reported (Chen et al., 2011; Vrignaud et al., 2014). | General AAP guidance/refer to guidelines |
| NICE | – | – | – | – |
| RANZCP | General AAP guidance only | General AAP guidance only | General AAP guidance only | – |
| TMAP | 10 mg/d, titrated by 5 mg/d to 10–20 mg/d | General AAP guidance only | General AAP guidance, plus agitation, constipation, EPS, Insomnia, nausea, somnolence | Carbamazepine, fluoxetine, ketoconazole, paroxetine, quinidine, St John’s wort |
| WFSBP | 2–5 mg/d initially, adjustments of up to 5 mg/d no less than once p/w, maximum 15 mg/d | – | Weight gain, akathisia | – |
Abbreviations: –, not reported by guideline; AAPs, atypical antipsychotics; AEs, adverse effects; APA, American Psychiatric Association; BAP, British Association of Psychopharmacology; CANMAT, Canadian Network for Mood and Anxiety Disorders; CPG-S, Clinical Practice Guidelines in the Spanish NHS; ICSI, Institute for Clinical Systems Improvement; EPS, extrapyramidal side-effects; LFTs, liver function tests; NICE, National Institute for Health and Care Excellence; RANZCP, Royal Australian and New Zealand College of Psychiatrists; MPG, Maudsley Prescribing Guidelines; p/w, per week; QTc, corrected Q-T interval; TMAP, Texas Medication Algorithm Project; WFSBP, World Federation of Societies of Biological Psychiatry.
Table 8.
Quetiapine Dosage and Monitoring
| Guideline | Dose | Patient monitoring parameters | Specific side effects | Specific drug interactions |
|---|---|---|---|---|
| APA | 25-400 mg/d | General AAP guidance only | General AAP guidance only | General AAP guidance only |
| BAP | No specific recommendations but cited Vieta et al., 2013 and not El-Khalili et al., 2010 in relation to dosing (El-Khalili et al., 2010; Vieta et al., 2013) | General AAP guidance only | General AAP guidance only | – |
| CANMAT | 150–300 mg/d | – | Cautioned in patients with prolonged QT interval | High potential for drug-drug interactions (3A4 substrate). Increases serum levels of CYP34A CYP substrates. |
| CPG-S | – | – | – | – |
| ICSI | General AAP guidance only | – | – | – |
| MPG | 150 or 300 mg/d. See guidelines for recommendations in cases of hepatic impairment | General AAP guidance, plus annual thyroid function tests | General AAP guidance, plus dry mouth, sedation, constipation, weight gain risk in longer term. Transient rises in AST, ALT and GGT, rarely jaundice and hepatitis. Severe cases of fatal hepatic failure and hepatocellular damage reported (Preskorn, 2012; Das et al., 2017; Datapharm Communications Ltd, 2017; Truven Health Analytics, 2018). Very low relative risk of akathisia, parkinsonism, and prolactin elevation. Low relative risk of anticholinergic effects, moderate relative risk of sedation, weight gain and hypotension. Moderate relative risk of effect on QTc. Small risk of TFT abnormality (Remington et al., 2007; Leucht et al., 2009). Moderate propensity for increasing plasma lipids (Smith et al., 2010). Low risk of sexual dysfunction (Bobes et al., 2003; Byerly et al., 2004; Knegtering et al., 2004; Montejo González et al., 2005), but studies conflicting(Atmaca et al., 2005; Kelly and Conley, 2006). | General AAP guidance/see guidelines |
| NICE | – | – | – | – |
| RANZCP | General AAP guidance only | General AAP guidance only | General AAP guidance, plus high levels of sedation | – |
| TMAP | 100 mg/d x 3 days, then 200 mg/d, max 400 mg/d (target range 150–400 mg/d) | General AAP guidance only | General AAP guidance, plus cataract formation, dry mouth, glucose dysregulation, headache, hyperlipidaemia, increased appetite, orthostatic hypotension, sedation, weight gain | Erythromycin, fluconazole, ketoconazole, phenytoin, St John’s wort, thioridazine, valproate |
| WFSBP | 50 mg/d, increased to 150 mg at day 3, increased to 300 mg/d dependent on response. Higher doses not studied as add on for TRD | – | Sedation, weight gain | – |
Abbreviations: –, not reported by guideline; AAPs, atypical antipsychotics; ALT, alanine aminotransferase; APA, American Psychiatric Association; AST, aspartate aminotransferase; BAP, British Association of Psychopharmacology; CANMAT, Canadian Network for Mood and Anxiety Disorders; CPG-S, Clinical Practice Guidelines in the Spanish NHS; GGT, gamma-glutamyl transpeptidase; ICSI, Institute for Clinical Systems Improvement; MPG, Maudsley Prescribing Guidelines; NICE, National Institute for Health and Care Excellence; QTc, corrected Q-T interval; RANZCP, Royal Australian and New Zealand College of Psychiatrists; TMAP, Texas Medication Algorithm Project; TFT, thyroid function test; TRD, treatment resistant depression; WFSBP, World Federation of Societies of Biological Psychiatry.
Table 9.
Risperidone Dosage and Monitoring
| Guideline | Dose | Patient monitoring parameters | Specific side effects | Specific drug interactions |
|---|---|---|---|---|
| APA | Up to 3mg/d | General AAP guidance only | General AAP guidance, plus prolactin related side effects | General AAP guidance only |
| BAP | 0.5-2mg/d.(Taylor et al., 2015) | General AAP guidance only | General AAP guidance only | – |
| CANMAT | 1-3mg/d | – | – | Moderate potential for drug-drug interactions (2D6, 3A4 substrate). Increases serum levels of CYP1A2 CYP substrates and CYP2D6. |
| CPG-S | – | – | – | – |
| ICSI | General AAP guidance only | – | – | – |
| MPG | General AAP guidance, plus 0.5-3mg/d, see guidelines for recommendations in cases of hepatic impairment | General AAP guidance only | Transient asymptomatic elevations in LFTs, cholestatic hepatitis, jaundice and rare cases of hepatic failure reported (Atasoy et al., 2007; Preskorn, 2012; Datapharm Communications Ltd, 2017; Truven Health Analytics, 2018). Low relative risk of sedation, akathisia, parkinsonism, and anticholinergic effects. Moderate relative risk of weight gain and hypotension, and high incidence of prolactin elevation (avoid in patients under 25/osteoporosis/history of hormone dependent breast cancer). Low relative risk of effect on QTc. Moderate propensity for increasing plasma lipids.(Perez-Iglesias et al., 2009) Moderately high reported incidence of ejaculatory problems (Dubé et al., 2007; Tohen et al., 2012). Priapism reported rarely (Baldwin and Mayers, 2003). Prevalence of sexual dysfunction reported to be 60–70% (Serretti and Chiesa, 2011). | General AAP guidance/see guidelines |
| NICE | – | – | – | – |
| RANZCP | General AAP guidance only | General AAP guidance only | General AAP guidance only | May be less effective in poor CYP2D6 metabolisers (de Leon et al., 2010). |
| TMAP | 0.25-0.5mg/d, target range 1-2mg/d | General AAP guidance only | General AAP guidance, plus EPS, glucose dysregulation, galactorrhoea, hyperlipidaemia, menstrual irregularity, orthostatic hypotension, prolactin elevation, sedation, sexual dysfunction, tardive dyskinesia, weight gain | Carbamazepine, cimetidine, fluoxetine, paroxetine, phenytoin, rifampin, tricyclic antidepressants. |
| WFSBP | Not recommended |
Abbreviations: –, not reported by guideline; AAPs, atypical antipsychotics; APA, American Psychiatric Association; BAP, British Association of Psychopharmacology; CANMAT, Canadian Network for Mood and Anxiety Disorders; CPG-S, Clinical Practice Guidelines in the Spanish NHS; ICSI, Institute for Clinical Systems Improvement; LFTs, liver function tests; NICE, National Institute for Health and Care Excellence; MPG, Maudsley Prescribing Guidelines; QTc, corrected Q-T interval; RANZCP, Royal Australian and New Zealand College of Psychiatrists; TMAP, Texas Medication Algorithm Project; WFSBP, World Federation of Societies of Biological Psychiatry.
Table 10.
Olanzapine Dosage and Monitoring
| Guideline | Dose | Patient monitoring parameters | Specific side effects | Specific drug interactions |
|---|---|---|---|---|
| APA | 25 mg/d fluoxetine plus 6 mg/d olanzapine, 18 mg/d max | General AAP guidance only | General AAP guidance plus long-term weight gain | General AAP guidance only |
| BAP | 2.5–10 mg/d olanzapine | General AAP guidance only | General AAP guidance only | – |
| CANMAT | 2.5–10 mg/d olanzapine | – | – | Moderate potential for drug-drug interactions (1Ae substrate) and is also metabolized through the uridine diphosphate glucuronosyltransferase pathway. Increases serum levels of CYP1A2 CYP substrates and CYP2D6. |
| CPG-S | – | – | Weight gain, dry mouth, appetite increase, fatigue, drowsiness, headache, and edema occurring in ≥10% of patients (Trivedi et al., 2009) | – |
| ICSI | General AAP guidance only | – | – | – |
| MPG | 6.25–12.5 mg/d olanzapine plus 25–50 mg/d fluoxetine | Special precaution to be taken with plasma glucose, which should be checked at baseline, 1 month, and then every 4–6 months | Sedation, anticholinergic, caution advised in hepatic impairment. Reports of transient asymptomatic elevations in ALT and AST in physically healthy adults (Atasoy et al., 2007; Preskorn, 2012; Datapharm Communications Ltd, 2017; Truven Health Analytics, 2018). Very low risk (in relation to other AAPs) of akathisia and parkinsonism. Low relative risk of anticholinergic effects, hypotension, and prolactin elevation. Moderate relative risk of sedation, high relative risk of weight gain. Low relative risk of effects on QTc. Highest propensity for increasing plasma lipids (Chaggar et al., 2011). Prevalence of sexual dysfunction reported to be >50% (Serretti and Chiesa, 2011), priapism reported rarely (Dossenbach et al., 2006). | General AAP guidance/refer to guidelines |
| NICE | – | – | – | – |
| RANZCP | General AAP guidance and lower dose in elderly | General AAP guidance only | General AAP guidance, plus very sedating, increased appetite, metabolic syndrome | – |
| TMAP | 5–10 mg/d, titrated by 5 mg/d to 10–20 mg/d | General AAP guidance only | General AAP guidance, plus constipation, dizziness, dry mouth, glucose dysregulation, hyperlipidaemia, increased appetite, sedation, weight gain | Carbamazepine, fluvoxamine, rifampicin, smoking, St John’s wort |
| WFSBP | General AAP guidance only | – | – | – |
Abbreviations: . ., not reported by guideline; AAPs, atypical antipsychotics; APA, American Psychiatric Association; BAP, British Association of Psychopharmacology; CANMAT, Canadian Network for Mood and Anxiety Disorders; CPG-S, Clinical Practice Guidelines in the Spanish NHS; ICSI, Institute for Clinical Systems Improvement; NICE, National Institute for Health and Care Excellence; MPG, Maudsley Prescribing Guidelines; RANZCP, Royal Australian and New Zealand College of Psychiatrists; TMAP, Texas Medication Algorithm Project; WFSBP, World Federation of Societies of Biological Psychiatry.
Table 11.
Brexpiprazole Dosage and Monitoring
| Guideline | Dose | Patient monitoring parameters | Specific side effects | Specific drug interactions |
|---|---|---|---|---|
| APA | Not recommended | |||
| BAP | Not recommended | |||
| CANMAT | 1–3 mg/d | – | – | – |
| CPG-S | Not recommended | |||
| ICSI | Not recommended | |||
| MPG | Not recommended | |||
| NICE | Not recommended | |||
| RANZCP | Not recommended | |||
| TMAP | Not recommended | |||
| WFSBP | Not recommended |
Abbreviations: –, not reported by guideline; AAPs, atypical antipsychotics; APA, American Psychiatric Association; BAP, British Association of Psychopharmacology; CANMAT, Canadian Network for Mood and Anxiety Disorders; CPG-S, Clinical Practice Guidelines in the Spanish NHS; ICSI, Institute for Clinical Systems Improvement; NICE, National Institute for Health and Care Excellence; RANZCP, Royal Australian and New Zealand College of Psychiatrists; MPG, Maudsley Prescribing Guidelines; TMAP, Texas Medication Algorithm Project; WFSBP, World Federation of Societies of Biological Psychiatry.
General AAP Monitoring and Adverse Effect Recommendations
General side effect and/or monitoring/drug interaction guidance for AAPs was provided by APA, TMAP, the MPG, RANZCP, BAP, and CANMAT (Tables 5 and 6). The MPG stated that at least 1 abnormal liver function test is common and rarely results in significant liver damage (Marwick et al., 2012), and the majority of side effects are dose dependent. The MPG offered extensive guidance on the interaction between AAPs and street drugs, and risks of toxicity in overdose. They advised that carbamazepine may reduce the levels of most antipsychotics (Crawford, 2002; Patsalos et al., 2002; Spina and Perucca, 2002; Citrome et al., 2007).
Table 5.
General Side Effects for AAPs
| Side effect | Guideline(s) |
|---|---|
| Weight gain | APA (Andersen et al., 2005), TMAP, RANZCP |
| Other metabolic complications/metabolic syndrome | RANZCP |
| Glucose dysregulation/diabetes mellitus | APA |
| Dyslipidaemia | Hypertriglyceridemia (APA), hypercholesterolemia and hyperglycemia (particularly for olanzapine; TMAP) |
| Hyperprolactinemia | APA, MPG |
| QTc prolongation | APA, BAP (Haddad and Anderson, 2002; Leucht et al., 2013), MPG |
| Coronary heart disease/risk of sudden cardiac death | MPG (Ray et al., 2001; Hennessy et al., 2002; Reilly et al., 2002; Straus et al., 2004; Liperoti et al., 2005; Osborn et al., 2007; Stroup et al., 2009; Murray-Thomas et al., 2013) |
| Extrapyramidal side effects (including tardive dyskinesia and neuroleptic malignant syndrome)a | NICE, MPG (Baldessarini et al., 1988; Peluso et al., 2012), RANZCP, APA, TMAP |
| Acute kidney injury | MPG |
| Sedation | MPG |
| Postural hypotension | MPG |
| Anticholinergic effects | MPG |
| Hyponatraemia | MPG (Littrell et al., 1997; Kawai et al., 2002; Montgomery and Tekell, 2003; Meulendijks et al., 2010) |
| Sexual dysfunction | MPG |
| Increased risk of pneumonia | MPG |
| Increased risk of thromboembolism | MPG (Zhang et al., 2011; Barbui et al., 2014) |
Abbreviations: APA, American Psychiatric Association; BAP, British Association of Psychopharmacology; CANMAT, Canadian Network for Mood and Anxiety Disorders; CPG-S, Clinical Practice Guidelines in the Spanish NHS; ICSI, Institute for Clinical Systems Improvement; NICE, National Institute for Health and Care Excellence; QTc, corrected QT interval; RANZCP, Royal Australian and New Zealand College of Psychiatrists; MPG, Maudsley Prescribing Guidelines; TMAP; Texas Medication Algorithm Project; WFSBP, World Federation of Societies of Biological Psychiatry.
aSee individual guidelines for specific symptoms.
Table 6.
General Pre-Prescription and Monitoring Tests for AAPs
| Test/examination | Guidelines(s) |
|---|---|
| ECG | BAP, TMAP, MPG |
| Pregnancy test (as indicated) | TMAP |
| Extrapyramidal side effects | TMAP |
| Weight/BMI | NICE, TMAP, MPG |
| Glucose levels | NICE, TMAP, MPG |
| Lipid levels | NICE, TMAP, MPG |
| Sexual function enquiry | TMAP |
| Prolactin level | TMAPa, MPG |
| Ocular evaluations | TMAP |
| Urea and electrolytes | MPG (Leucht et al., 2005; Agid et al., 2006; Zhang et al., 2013; Subotnik et al., 2015; Zhu et al., 2017; Robinson et al., 2018) |
| Full blood count | MPG (Leucht et al., 2005; Agid et al., 2006; Zhang et al., 2013; Subotnik et al., 2015; Zhu et al., 2017; Robinson et al., 2018) |
| Blood pressure | MPG |
| Liver function tests | MPG (Olofinjana and Taylor, 2005; Haro et al., 2006; Stroup et al., 2006) |
| Signs of chest infection | MPG |
Abbreviations: BAP, British Association of Psychopharmacology; BMI, body mass index; ECG, electrocardiogram; NICE, National Institute for Health and Care Excellence; MPG, Maudsley Prescribing Guidelines; TMAP, Texas Medication Algorithm Project.
aIf evidence of galactorrhoea/gynecomastia, menstrual disturbance, libido disturbance or erectile/ejaculatory disturbance in males
APA warned that the side effect risk is greater than for other adjunctive strategies and tolerability can be an issue (Nelson and Papakostas, 2009). RANZCP recommended close monitoring of side effects and stressed that these are of great concern, particularly for long-term therapy.
APA warned that AAPs can inhibit metabolism via CYP2D6, which results in decreased clearance of TCAs. CANMAT similarly cautioned against concomitant administration of cytochrome P450 inhibitors (Spina et al., 2012; Brandl et al., 2014). The CGP-S stated that there may be pharmacodynamic interactions between antipsychotics and TCAs. The MPG stressed that patients should be encouraged to have good physical mobility and stay well hydrated. General monitoring recommendations are included in Table 6. See individual guidelines for more detail and frequencies. The WFSBP, ICSI, NICE, and the CPG-S gave no general side effect or monitoring guidance for AAPs. Specific guidance can be seen in Tables 7–11.
General AAP Discontinuation Recommendations
The APA stated that it is not known for how long AAP treatment should be continued when effective. RANZCP advised that gradual withdrawal should be considered once a stable response is achieved, though in some cases ongoing use is required. NICE simply stated that the risk of experiencing withdrawal/discontinuation symptoms is higher in those taking other centrally acting medications (which includes antipsychotics).
The WFSBP cited 1 study in which quetiapine in maintenance was superior to placebo in preventing relapse or recurrence of MDD (Liebowitz et al., 2010) but advised that the negative effects on metabolic function, weight gain, and tardive dyskinesia (TD) should be considered (Gao et al., 2011), and other AAPs with positive results as acute adjunctive agents have not been evaluated as maintenance treatments.
The MPG stated that AAPs should never be stopped suddenly in relation to schizophrenia and psychosis but did not clearly make the same recommendation for TRD. They recommended discontinuation if the patient’s neutrophil count is <1.5*109/L, or if liver function tests indicate hepatitis or functional damage. If blood lipids or blood pressure are out of range, switching to another antipsychotic is advised. They also recommended switching drugs in cases of confirmed and symptomatic hyperprolactinemia, and discontinuation/switching if akathisia, weight gain, increase in plasma lipids, neuroleptic malignant syndrome, hyperglycemia, hyperprolactinemia, or sexual dysfunction are experienced. They advised that AAPs are probably safe to restart at the previous dose following a period of noncompliance.
Lithium
First-line lithium recommendations
The BAP recommended lithium as a first-line augmenter but cautioned that most studies augmented TCAs and the association with side effects. BAP discussed the lack of head-to-head comparisons, again mentioning the 2013 study in which quetiapine XR was at least as effective as lithium over 6 weeks (Bauer et al., 2013a), a randomized open comparison with lamotrigine in which lithium was nonsignificantly better (Schindler and Anghelescu, 2007a), and a meta-analysis of EU-licensed augmenters with similar response rates for lithium, quetiapine XR, and S-adenosyl-L-methionine, though S-adenosyl-L-methionine was significantly better compared directly with lithium (Turner et al., 2014). Finally, BAP discussed a blind-rated comparison with cognitive behavioral therapy (CBT) augmentation by Kennedy et al. (2003), which found a nonsignificant advantage of lithium at 8 weeks and 4 weeks post discontinuation.
The BAP highlighted the reduced risk of suicide associated with lithium compared with antidepressant monotherapy, which they claimed was supported by Level I evidence (not cited). They stated that modest, but reasonably sound evidence exists for lithium augmentation of an MAOI, referring to a meta-analysis of 10 small RCTs (Crossley and Bauer, 2007). A study in which lithium augmentation was used at stage 2 of a 4-step inpatient treatment program with a 59% response rate was also referred to (Birkenhäger et al., 2006), contrasting with the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study, in which just 16% of participants reached remission with lithium as a third-stage treatment. The BAP suggested this may be due to greater comorbidity rates, degree of resistance, and unknown adequacy of lithium treatment in STAR*D (Nierenberg et al., 2006). They also included a systematic review of 30 open-label and 10 placebo-controlled studies with relatively small sample sizes (Bauer et al., 2014). The BAP briefly discussed outcome prediction with lithium augmentation and highlighted the association between better outcomes and greater depressive symptomatology, significant weight loss, psychomotor retardation, a history of more than 3 depressive episodes, and a family history of major depression (not referenced).
The RANZCP guidelines also considered lithium augmentation to have Level I evidence, stating that it is widely used and supported and is more effective than placebo when used with TCAs, SSRIs, and other antidepressants (Bauer et al., 2000; Crossley and Bauer, 2007). Like BAP, RANZCP discussed predictors of lithium response, including a history of more than 3 prior depressive episodes and first-degree family history of bipolar or unipolar depression (Sugawara et al., 2010), as well as the predictive power of early response, stating that if no benefit is found within 7–10 days, alternative treatments should be considered (not referenced).
The WFSBP guidelines stated that there is evidence for use of lithium with a range of antidepressants, including TCAs (Joffe et al., 1993; Katona et al., 1995) and SSRIs (Katona et al., 1995; Baumann et al., 1996; Zullino and Baumann, 2001). A meta-analysis also mentioned by BAP was cited (Crossley and Bauer, 2007), as was the STAR*D comparison of T3 and lithium augmentation in which there was no significant difference in remission rates, but fewer side effects and dropouts for T3 (Nierenberg et al., 2006). WFSBP noted that lithium was effective in preventing suicide/suicide attempts, but it is not known if it has acute anti-suicide effects (Coppen et al., 1990; Thies-Flechtner et al., 1996; Tondo et al., 1997; Müller-Oerlinghausen, 1999; Schou, 2000; Guzzetta et al., 2007; Lauterbach et al., 2008; Cipriani et al., 2013).
The CPG-S recommended lithium as a first-line option, supported by Level B evidence, following nonresponse by the third or fourth week of antidepressant treatment, or at later stages in treatment, supported by Level C evidence. The CPG-S were predominantly reliant on the evidence and recommendations in the current NICE (2009) guidelines and stated that these data show significantly better response rates compared with placebo and nonsignificantly better remission rates. They also indicated that lithium was less well tolerated and highlighted the possibility of greater adverse effects when initiating lithium.
In addition to citing evidence included by NICE, the CPG-S considered 2 more recent articles: the STAR*D comparison of lithium and T3 augmentation (Nierenberg et al., 2006) and a multi-step algorithmic study, which by contrast reported that lithium augmentation had higher rates of remission than T3 (Gervasoni et al., 2009). Finally, a study that reported no difference in severity or response/remission between lithium and lamotrigine over 8 weeks was referenced (Schindler and Anghelescu, 2007a).
Other evidence considered by the CPG-S included the RCT comparison of lithium and CBT discussed by BAP (Kennedy et al., 2003), a systematic review of 11 studies in which antidepressant dose increase was no less effective than augmentation with either lithium or desipramine (Adli et al., 2005), and the 2007 comparison of lithium and quetiapine augmentation demonstrating no significant difference, also mentioned in relation to quetiapine by the CPG-S and APA (Dorée et al., 2007).
The MPG advised that lithium is well established and well supported in the literature and recommended by NICE, but there is poor tolerability at higher plasma levels, potential toxicity, and a need for plasma monitoring and specialist referral. They highlighted that it may not be effective in patients resistant to multiple treatments (Fava et al., 1994; Bauer and Dopfmer, 1999; Nierenberg et al., 2003, 2006; Crossley and Bauer, 2007). Again, STAR*D was mentioned, (Nierenberg et al., 2006) as well as the current BAP guidelines (Cleare et al., 2015), and the ongoing Lithium vs Quetiapine in Depression study which seeks to determine whether lithium or quetiapine is most effective when compared head-to-head over 12 months (Marwood et al., 2017). The MPG suggested the following characteristics may predict a better response to lithium: greater severity in depressive symptoms, psychomotor retardation, significant weight loss, family history of MDD, and personal history of at least 3 depressive episodes (Bauer et al., 2014). The MPG advised that treatment adherence should be included, and they also raised the potential protective effect against suicide, though the mechanism of this effect remains unknown (Cipriani et al., 2013).
Second-line recommendations
APA included lithium with Level II (moderate) confidence and stated that it is the most widely studied adjunctive treatment for this patient group, has the potential to reduce the risk of suicide, and has efficacy in preventing relapse (Austin et al., 1991; Bauer and Dopfmer, 1999; Cipriani et al., 2006; Crossley and Bauer, 2007). Again, the STAR*D comparison of lithium and T3 was discussed (Nierenberg et al., 2006). APA advised that the time from initiation to full response can range from a few days to 6 weeks.
CANMAT also recommended lithium as a second-line option, supported by Level II evidence. As one of the more recently published guidelines in this review, CANMAT discussed the network meta-analysis comparing the adjunctive effects of pharmacological augmenters with each other and placebo (Zhou et al., 2015). Only olanzapine, lithium, quetiapine, and T3 were more effective than placebo, and stronger efficacy was reported for the AAPs than for lithium and T3. CANMAT also referenced a 2014 systematic review that concluded that lithium was effective, though the sample sizes of the 10 included trials were small, as well as an additional meta-analysis of placebo-controlled RCTs demonstrating efficacy and the STAR*D comparison of lithium and T3 (Nierenberg et al., 2006; Bauer et al., 2014; Nelson et al., 2014).
Other recommendations
The ICSI recommended lithium augmentation of TCAs and advised caution when augmenting SSRIs in light of serotonin syndrome reports (not referenced). Seven placebo-controlled trials demonstrating the efficacy of lithium augmentation were mentioned, though not all were referenced (Delgado et al., 1988; Joffe et al., 1993; Katona et al., 1995; Baumann et al., 1996). The STAR*D comparison with T3 was also included (Nierenberg et al., 2006). The ICSI also noted studies in which increasing the antidepressant dose proved more effective than adding lithium (Fava et al., 1994; Perry et al., 1994).
NICE included lithium augmentation but did not specify the level of recommendation. They included 10 eligible RCTs, all of which were between 1 and 6 weeks in length and had been included in their previous guidelines (Zusky et al., 1988; Jensen et al., 1992; Joffe et al., 1993; Stein and Bernadt, 1993; Baumann et al., 1996; Shahal et al., 1996; Bloch et al., 1997; Cappiello et al., 1998; Januel et al., 2002; Nierenberg et al., 2006). NICE concluded that there was some evidence that lithium augmentation was effective in reducing the symptoms of depression and stressed that although lithium appears to be less acceptable than placebo, there was not enough evidence to determine whether this is due to the side-effect burden.
TMAP recommended lithium at stages 3 and 4 of their algorithm, making it a third- or fourth-line augmenter. They specifically recommended the use of lithium in addition to a TCA, but no evidence was cited to support these recommendations.
Dosage, monitoring, and adverse effects
Dosing, monitoring, and adverse effects recommendations for lithium are summarized in Table 12.
Table 12.
Lithium Monitoring and Dosing
| Guideline | Dose | Patient monitoring parameters | Specific side effects | Specific drug interactions |
|---|---|---|---|---|
| APA | Required blood level not confirmed | – | Caution with Parkinson’s as may worsen symptoms | – |
| BAP | Referred to a study in which plasma lithium levels of 0.6–1.2 mmol/L were more effective than those outside this range (Bauer et al., 2013a) and a meta-analysis in which most studies used 600–1200 mg/d (not clear recommendations; Crossley and Bauer, 2007) | – | – | – |
| CANMAT | 600–1200 mg/d, dosing 1-2x p/d, aiming for “therapeutic serum levels” (not specified) | – | – | – |
| CPG-S | – | – | – | – |
| ICSI | Referred to studies with usual dose of 300 mg/d administered 3 times during the day, serum levels >0.4 mmol/L (not a clear recommendation; Joffe et al., 1993; Katona et al., 1995; Baumann et al., 1996) | – | – | – |
| MPG | Plasma level 0.4–0.8 mmol/L, increased to 1.0 mmol/L if response suboptimal. 0.6 mmol/L minimum level for prophylaxis/maintenance. See guidelines for recommendations by lithium preparation and advice for impaired renal function. Lithium levels >0.8 mmol/L associated with higher risk of renal toxicity. Toxic effects occur at levels >1.5 mmol/L. Optimal plasma range less clear in unipolar than bipolar depression (Young, 2017). | Baseline: renal, thyroid, and cardiac function estimated GFR and TfTs as a minimum (Morriss and Benjamin, 2008). ECG in all patients with cardiovascular disease risk factors. Weight. Calcium levels desirable. Testing thyroid autoantibodies to aid elimination of hypothyroidism risk. Women advised to use contraception. Serum levels tested after 12 h in patients taking 1 daily dose at bedtime (Goodwin et al., 2016). For middle-aged women, TfTs monitored regularly in first year plus monitoring calcium levels (Livingstone and Rampes, 2006). Plasma lithium, eFGR, and TfTs every 6 mo in all patients and more regularly in special populations. Weight/ BMI monitoring. Calcium level monitoring desirable. Renal function monitored regularly in prolonged treatment (Aiff et al., 2015). | Mild GI upset, tremor, polyurea (Bowen et al., 1991; Ljubicic et al., 2008), thirst, polydipsia. Can cause diuresis and tolerance difficulties in patients with bladder disorder. Contraindicated in severe renal impairment (Gitlin, 1999; Lepkifker et al., 2004). Long-term use associated with impaired renal function, nephrogenic diabetes insipidus, nephrotic syndrome, and both reversible, irreversible kidney damage and increased risk of hypothyroidism (Johnston and Eagles, 1999; Frye et al., 2009; McKnight et al., 2012; Shine et al., 2015). Lithium associated with hyperparathyroidism (McKnight et al., 2012) and chronically elevated calcium levels associated with renal stones, osteoporosis, dyspepsia, hypertension, and renal impairment. May aggravate skin conditions, cause metallic taste, ankle edema, and weight gain. NMS sometimes seen (Gill et al., 2003). Impaired visual adaptation to dark (Metzner et al., 1993). Moderate caution advised in epilepsy as limited evidence of effect on seizures (Wickstrøm et al., 1980). Association with Ebsteins abnormality (though risk may be overestimated; McKnight et al., 2012; Diav-Citrin et al., 2014). Contraindicated in pregnancy. See guidelines for advice on toxicity symptoms and overdose. | Drugs altering renal sodium handling can precipitate lithium toxicity. Includes ACE inhibitors, thiazide diuretics, and NSAIDS. Rare reports of neurotoxicity with carbamazepine. Street drugs can be very toxic if taken erratically. Caffeine cautioned as may decrease lithium levels and withdrawal from caffeine may increase (Baethge et al., 2009). |
| NICE | Plasma levels 0.5–1.0 mmol/L considered therapeutic; toxicity may develop >1.5 mmol/L and can lead to death at 2.0 mmol/L | Baseline and at least every 6 mo: renal function, TFTs. Lithium levels at 1 wk and after every dose change until stable, then every 3 mo. ECG monitoring for patients with high risk of CVD/cardiac symptoms. NICE referred to their bipolar guidelines for further monitoring advice. | Range of cardiac effects: may be important in heart disease, elderly, high lithium levels, hypokalaemia, or those prescribed diuretics, hydroxyzine, and TCAs (Chong et al., 2001). Potential for “sick sinus” syndrome, first-degree heart block, ventricular ectopics, flattened T-waves, and increased QT dispersion, which are common and often subclinical (Reilly et al., 2000). Can also cause hypothyroidism and renal damage, among other effects. | Can interact with commonly prescribed drugs precipitating toxicity. See guidelines for advice on interactions with drugs used during surgery. |
| RANZCP | Trough plasma level (12 h after dosing) between 0.5 and 0.8mmol/L (Berghöfer et al., 2006; Malhi et al., 2011) | Baseline: FBC, renal function, thyroid function, and calcium levels. Check for pregnancy using hCG. At 6, 12, and 24 mo: renal function (urea, creatinine, electrolytes), endocrine (TSH, serum calcium, parathyroid hormones), serum lithium estimations (trough). Lithium should be carefully monitored due to potential for toxicity (Wilting et al., 2005; Lam et al., 2009). | Obesity, metabolic syndrome, hypertension, and diabetes. Small increased risk of fetal cardiac defects (Cohen et al., 1994; McKnight et al., 2012; Diav-Citrin et al., 2014). Recommend treatment with thyroid hormone if hypothyroidism occurs. Cautioned that long-term use associated with serious side effects (Van and Boer, 2006; Malhi et al., 2012). | – |
| TMAP | 300 mg/d titrated by 150 mg/d every 1/2 wk to target dose 600–900 mg/d. Max. based on serum levels considered with tolerability and response, aiming for 0.4–0.6 mmol/L 1–2 times p/d. | ECG at baseline and yearly as indicated. FBC baseline and yearly. TFTs at baseline, TSH every 6 mo/as indicated. BUN at baseline and as indicated (including creatine, glucose, and electrolytes). Urinalysis at baseline and as indicated. Pregnancy test if indicated. Lithium levels 1-wk post initiation, after each change in dose, and as clinically indicated. | Acne, acute renal dysfunction, cognition, diarrhea, dizziness, ECG changes, GI upset, hypothyroidism, nausea, polyuria, sedation, thirst, tremor, and weight gain | ACE inhibitors, caffeine, NSAIDs, osmotic diuretics, theophylline, and thiazide diuretics |
| WFSBP | Trough (12 h post) serum levels 0.6–0.8 mmol/L in acute and maintenance phase. 0.4–1.0 mmol/L may be appropriate depending on response and tolerability. Translates to approximately 900–1200/1500 mg/d lithium carbonate. Single daily dose may increase adherence and reduce side effects (Mosolov et al., 1997). XR formulation better tolerated. Acknowledged that optimal levels may vary (Schou, 1989; Malhi et al., 2011). Specific recommendations made for Asian and elderly populations. | Serum test no earlier than 5–7 days after first dose/changes in dose, 1–4/y, more if indicated. 1–2/y: TFTs, parathyroid function (e.g., blood calcium, and if this is elevated, parathyroid hormone), and renal function (eGFR, creatine; Schou et al., 1997; Livingstone and Rampes, 2006; American Psychiatric Association, 2010; Berger et al., 2013; Severus and Bauer, 2013; Bschor, 2014). Response assessed 2–4 wk post initiation. Monitor for response from 2 to 4 wk (Bschor et al., 2003). | Potential for serotonin syndrome when MAOI/other AD augmented with lithium. Reduced GFR, reduced urinary concentrating ability, polyurea and/or polydipsia, goiter and hypothyroidism, hyperparathyroidism, weight gain, GI symptoms, memory impairment or mental slowness (McKnight et al., 2012). Some patients receiving lithium for ≥10 y may develop rising creatine concentrations, but glomerular and tubular function more commonly affected (Bendz et al., 2010). Hand tremor. Side effects often dose dependent. See guidelines for guidance on how to counteract side effects. | – |
Abbreviations: –, not reported by guideline; AAPs, atypical antipsychotics; ACE, angiotensin-converting enzyme; AD, antidepressant; APA, American Psychiatric Association; BAP, British Association of Psychopharmacology; BUN, blood urea nitrogen; CANMAT, Canadian Network for Mood and Anxiety Disorders; CPG-S, Clinical Practice Guidelines in the Spanish NHS; CVD, cardiovascular disease; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; FBC, full blood count; GI, gastrointestinal; ICSI, Institute for Clinical Systems Improvement; MAOI, monoamine oxidase inhibitors; mg/d, milligrams per day; mmol/L, millimoles per litre; MPG, Maudsley Prescribing Guideline; NICE, National Institute for Health and Care Excellence; NMS, neuroleptic malignant syndrome; p/d, per day; NSAID, non-steroidal anti-inflammatory drug; QT, Q-T interval; RANZCP, Royal Australian and New Zealand College of Psychiatrists; TCAs, tricyclic antidepressants; TFTs, thyroid function tests; TMAP, Texas Medication Algorithm Project; TSH, thyroid stimulating hormone; WFSBP, World Federation of Societies of Biological Psychiatry; XR, extended release.
Discontinuation
The discontinuation recommendations for lithium are summarized in Table 13.
Table 13.
Summary of Lithium Discontinuation Guidance
| Guideline | Discontinuation guidance |
|---|---|
| APA | If tolerated and effective, treatment should continue for at least an acute phase (typically 4–8 wk) and potentially beyond for relapse prevention. |
| BAP | Lithium should be maintained in combination with an antidepressant for at least 1 y to prevent relapse, though little available evidence in continuation phase. Routine use of lithium monotherapy in continuation phase not recommended. Supported their recommendations with conflicting evidence demonstrating that lithium had nonsignificant benefit over placebo or antidepressants for prophylaxis (Burgess et al., 2001; Cipriani et al., 2006), but lithium plus an antidepressant more effective than antidepressant monotherapy in preventing relapse in TRD patients who responded to lithium augmentation or ECT (Bauer et al., 2000; Sackeim et al., 2001). |
| CANMAT | – |
| CPG-S | – |
| ICSI | Care should be taken when discontinuing lithium as abrupt withdrawal associated with higher relapse rates |
| MPG | Advised against lithium monotherapy for prophylaxis. At least 2 y continuation in patients with ≥2 recent episodes with significant functional impairment, then reevaluate. If first episode, continue for 6–9 mo following remission. Following noncompliance, recommence at previous dose if adherence and tolerance good (Abou-Saleh et al., 2017a). Treatment should be indefinite if adherence good and treatment well tolerated, particularly if suspected bipolarity. Few data relating to lithium discontinuation in unipolar depression. Should be reduced slowly over at least 1 mo, avoiding incremental reductions >0.3 mmol/L in recurrent depression. Referred to work that proposed lithium should be used for prophylaxis in depression if had been 2 depressive episodes in 5 y or following 1 severe episode with strong suicide risk (Abou-Saleh et al., 2017b). |
| NICE | Responders with multiple historical relapses should remain on medication regardless of length of treatment pre-response. Insufficient evidence to determine effect beyond 2 y or if relevant to first-episode patients. If a medication to be stopped, it should be the augmenter not AD. Relapse likelihood lower if lithium continued (Prien et al., 1984). Not enough evidence to determine clinically important difference between continuing lithium as monotherapy or discontinuing both lithium and AD (i.e., placebo). |
| RANZCP | Care should be taken when discontinuing lithium as abrupt withdrawal associated with higher relapse rates (Bschor et al., 1999). Lithium monotherapy in maintenance phase could be considered if ADs not well tolerated (Cipriani et al., 2006). |
| TMAP | – |
| WFSBP | Continuation for at least 1 y if patient responds. Gradual withdrawal tapered over at least 3 mo if treatment has been >6 mo. If symptoms reoccur, maintenance dose of antidepressant and lithium should be resumed. Recommended administration for 2–4 wk and then monitor patient response (Bschor et al., 2003). Efficacy of lithium maintenance treatment well established (Coppen et al., 1990; Schou, 1997; Davis et al., 1999; Bauer et al., 2000; Paykel, 2001; Bschor et al., 2002). Evidence for lithium monotherapy in prophylaxis not sufficient (Souza and Goodwin, 1991; Burgess et al., 2001), but more recent meta-analysis showed efficacy of lithium when used for preventative purposes, though relative efficacy was not (Cipriani et al., 2006). Lithium + AD in continuation phase more beneficial than antidepressant + placebo, AD monotherapy, or lithium monotherapy (Kim et al., 1990; Bauer et al., 2000; Bschor et al., 2002). Stopping lithium can lead to relapse or recurrence. |
Abbreviations: –, not reported by guideline; AD, antidepressant; AAPs, atypical antipsychotics; APA, American Psychiatric Association; BAP, British Association of Psychopharmacology; CANMAT, Canadian Network for Mood and Anxiety Disorders; CPG-S, Clinical Practice Guidelines in the Spanish NHS; ECT, electroconvulsive therapy; ICSI, Institute for Clinical Systems Improvement; MPG, Maudsley Prescribing Guidelines; NICE, National Institute for Health and Care Excellence; RANZCP, Royal Australian and New Zealand College of Psychiatrists; TMAP, Texas Medication Algorithm Project; WFSBP, World Federation of Societies of Biological Psychiatry.
Anticonvulsant Mood Stabilizers
Second-line recommendations
BAP recommended lamotrigine as a second-line augmenter but stated that few studies support the use of anticonvulsants, citing trials showing limited efficacy (Barbee and Jamhour, 2002; Barbosa et al., 2003) and 1 study already referred to in which lamotrigine was comparable with lithium (Schindler and Anghelescu, 2007a). The MPG also listed lamotrigine as a second-line option, suggesting that it may be the best tolerated augmentation strategy, supported by 3 trials (Normann et al., 2002; Barbosa et al., 2003; Santos et al., 2008) and 1 retrospective chart review (Barbee and Jamhour, 2002).
Other recommendations
TMAP featured lamotrigine augmentation at stage 3a as an option for partial responders to stage 3. Lamotrigine was also included at stage 6 of TMAP, but no supporting evidence was cited.
APA advised that lamotrigine and other anticonvulsants such as carbamazepine and valproic acid may be beneficial in TRD but have not been extensively evaluated for this purpose (Cullen et al., 1991; Barbosa et al., 2003; Fava et al., 2006; Trivedi et al., 2006a; Schindler and Anghelescu, 2007b). APA also cautioned that anticonvulsant compounds may negatively impact mood (Schmitz, 2002), as some, including barbiturates and potentially vigabatrin, have been associated with an increased risk of depression (Levinson and Devinsky, 1999) supported by a statement from the US Food and Drug Administration (Kuehn, 2008).
Not recommended
NICE did not recommend lamotrigine, carbamazepine, or valproate as they considered the evidence inadequate at the time of publication (Hurley, 2002; Normann et al., 2002; Barbosa et al., 2003; Schindler and Anghelescu, 2007b; Santos et al., 2008) as did CANMAT (Zhou et al., 2015). NICE acknowledged that lamotrigine is generally well tolerated and has no major drug interactions and suggested that further trials are warranted given the existing evidence in epilepsy and bipolar disorder, though there is no evidence to suggest other anticonvulsants (e.g., gabapentin and topiramate) might be useful. There was just 1 open label study assessing valproate augmentation in unipolar depression at the time of publication (Davis et al., 1996) as existing evidence has indicated it is more effective in hypomanic rather than depressed states in bipolar disorder. NICE cited limited research examining carbamazepine in unipolar depression, with several open-label studies and 1 RCT showing benefit (Cullen et al., 1991; Ta Ketter, 1997; Dietrich and Emrich, 1998; Zhang et al., 2008). They also noted that many older patients responding to carbamazepine discontinued due to adverse effects (Cullen et al., 1991).
The CPG-S referred to the evidence base included by NICE for lamotrigine plus 2 additional RCTs (Nierenberg et al., 2006; Barbee et al., 2011) and stated that there is insufficient data to recommend augmentation with lamotrigine, carbamazepine, valproate, or topiramate.
ISCI, RANZCP, and WFSBP did not discuss augmentation with any mood stabilizer/anticonvulsant other than lithium for unipolar resistant depression.
Dosage
The MPG stated that 100, 200, or 400 mg/d has been used for adjunct lamotrigine, though this was not clearly a recommendation. TMAP offered the most comprehensive guidance for lamotrigine, recommending 25 mg/d for 2 weeks, increased to 50 mg/d for 2 weeks, then 100 mg/d for 1 week, with a maximum daily dose of 200 mg/d in the absence of enzyme inhibiting or inducing agents. TMAP offered slightly different guidance for patients taking carbamazepine or valproate in addition to lamotrigine. No other guidelines included dosage recommendations.
Side effects and monitoring
TMAP did not discuss side effects but recommended that renal function and hepatic function tests should be conducted prior to lamotrigine treatment and repeated yearly if indicated. A pregnancy test was also recommended if appropriate. TMAP stated that their recommendations were supported by Level B evidence but did not directly cite this work.
Discontinuation
BAP recommended that lamotrigine should be continued for up to 1 year after remission, with long-term treatment considered for higher risk patients. The MPG and TMAP did not offer specific withdrawal guidance, but as TMAP is algorithmic, further treatment is recommended should a patient not/partially respond. The MPG listed lamotrigine as a treatment that would require re-titration after 3 to 7 days of noncompliance. No other discontinuation guidance was provided for this group of treatments in unipolar depression.
Bupropion
Although licensed as an antidepressant in some countries, including the United States, bupropion is specifically listed as an augmenter in others and is therefore included as such in this review. The following section therefore only includes mention of bupropion as an antidepressant adjunct.
First-line recommendations
The MPG listed bupropion as a first-line augmenter for TRD, added to an SSRI. They stated that bupropion use as a “worthwhile” option is supported by STAR*D (Trivedi et al., 2006a) but highlighted that it is not licensed for use in depression in the United Kingdom (Fatemi et al., 1999; Pierre and Gitlin, 2000; Lam et al., 2004; Papakostas et al., 2006b; Zisook et al., 2010; Henssler et al., 2016).
TMAP initially recommended bupropion as an augmenter at stage 1A (for patients with a partial response to initial antidepressant treatment), then at stage 2A, stages 3, 3A, 4, and stages 6–8.
Second-line recommendations
CANMAT recommended bupropion as a second-line augmenter with Level 2 evidence, which included the network meta-analysis previously mentioned in which bupropion was not significantly more effective than placebo (Zhou et al., 2015).
Other recommendations
BAP listed bupropion as “other additions that could be considered” with level B evidence. APA stated that the addition of bupropion to an antidepressant is supported by limited evidence and clinical experience, citing a study in which an SSRI plus bupropion had better outcomes than SSRI or bupropion monotherapy, as per the APA (Lam et al., 2004). The BAP and APA also listed special populations in which bupropion may be an appropriate choice.
ICSI included bupropion in combination with an SSRI (recommendations not ranked). The STAR*D study was referenced (as in the MPG), indicating no significant difference between bupropion and buspirone augmentation of citalopram, though bupropion was better tolerated (Trivedi et al., 2006a). They also referred to several case series/case reports of bupropion augmentation that described beneficial results (Marshall and Liebowitz, 1996; Bodkin et al., 1997; Spier, 1998).
Not recommended
RANZCP and WFSBP did not recommend the use of bupropion as an augmentation (or combination) strategy for TRD, though both listed it as an antidepressant. WFSBP referenced the STAR*D comparison of bupropion and buspirone augmentation (Trivedi et al., 2006a), but in contrast to other guidelines, referred specifically to a secondary modified intention to treat analysis, including only participants with data from at least 1 follow-up visit, in which buspirone was less effective than bupropion (Bech et al., 2012).
The CPG-S and NICE did not include bupropion as an augmentation option. Both mentioned the STAR*D study in which buspirone and bupropion augmentation did not differ significantly in terms of efficacy (Trivedi et al., 2006b), though NICE highlighted that there was a greater reduction in self-rated depression severity for the bupropion group. NICE also noted that dropout due to side effects was lower in the bupropion group.
Dosage, side effects, and monitoring
See Table 14 for dosage, side effect, and monitoring recommendations for bupropion provided by the guidelines.
Table 14.
Bupropion Monitoring and Dosing
| Guideline | Dose | Patient monitoring parameters | Specific side effects | Specific drug interactions |
|---|---|---|---|---|
| APA | 150 mg/d titrated up to 300–450 mg/d (for IR and XR formulations). Suggested dosage in morning for activation purposes and to avoid insomnia. Advised taking after food or in divided doses if experiencing nausea/vomiting. | Blood pressure (association with hypertension) | Dry mouth, headaches, tremors, agitation, jitteriness, mild cognitive dysfunction, insomnia, and GI symptoms (Fava et al., 2005a). May improve symptoms of sleepiness and fatigue in some (Papakostas et al., 2006a). Can reduce seizure threshold and should be used with caution/avoided in those with preexisting seizure disorders/at risk of seizure. Bupropion should not be used in patients with anorexia nervosa or bulimia nervosa for this reason (American Psychiatric Association, 2006). Potential dopamine agonist effects may be beneficial for symptoms of Parkinson’s but could worsen psychosis. Relatively safe in overdose. As an antidepressant, bupropion does not have an indication for anxiety, and therefore clinicians may want to avoid use in these patients. Patients typically have weight loss or only minimal weight gain with bupropion; may be helpful for smoking cessation (Kleber et al., 2007; Hughes et al., 2014). Noted lack of data examining bupropion’s effect on pregnancy. Can help with sexual side effects of other ADs (Walker et al., 1993; Clayton et al., 2004). | Risk of seizure may be increased if bupropion prescribed with inhibitors of CYP 2D6, such as desipramine, sertraline, paroxetine, and fluoxetine, as this can increase blood levels of bupropion |
| BAP | No general dose recommendations but commented on a study (n = 3000) that reported better adherence with once-daily rather than twice-daily dosing (McLaughlin et al., 2007). | – | Insomnia/agitation, increased seizure potential, and QTc shortening (Castro et al., 2013a) | Moderate inhibitor of CYP2D6 (Spina et al., 2008), so advised seeking specialist advice if concerns about such interactions |
| CANMAT | 150–300 mg/d. Specific dosing advice for patients experiencing sexual dysfunction | – | – | – |
| CPG-S | Not recommended | |||
| ICSI | – | – | – | – |
| MPG | Up to 400 mg/d, or 300 mg for XR formulation. Specific advice for patients with renal impairment. Mentioned alternative formulations (e.g., buccal) but no dosing information (Almeida et al., 2015). | Blood pressure. Patients with renal impairment should be monitored for urinary retention, confusion, sedation, and postural hypotension, and suggested plasma level or ECG monitoring may prove useful. | Potential cardiac effects: slight increase in heart rate, increase in blood pressure that can sometimes be significant, rare postural hypotension, QTc shortening (although QTc prolongation has been reported in overdose; Paris and Saucier, 1998; Buckley and Faunce, 2003; Curry et al., 2005; Zhu et al., 2017), rare reports of arrhythmia in overdose. Potential for MI (Roose and Miyazaki, 2005; Dwoskin et al., 2006; Castro et al., 2013b; Eisenberg et al., 2013). Not indicated in patients with eating disorders, alcohol dependence, or seizure disorder. Can be pro-convulsive, particularly in IR formulation. Common adverse effects include: dizziness, taste changes, GI disturbance, concentration problems, tremor, and insomnia. Association between bupropion and prolactin, but single doses up to 100 mg do not appear to affect (Whiteman et al., 1982). Low risk of hyponatremia (Kate et al., 2013). Moderate toxicity in overdose: lowest dose likely to be fatal approximately 4.5 g, though there is evidence of a 13.5-g overdose that was not fatal (Zhu et al., 2016). Signs of overdose include tachycardia, arrhythmia, QTc and QRS prolongation, and seizures (Paris and Saucier, 1998; Buckley and Faunce, 2003; Curry et al., 2005; Mercerolle et al., 2008). | Caution advised when co-administered with medications known to induce or inhibit cytochrome CYP2B6 metabolism as clinical efficacy may be impacted. Administration alongside other drugs metabolised by this pathway should be avoided. |
| NICE | Not recommended | |||
| RANZCP | Not recommended | |||
| TMAP | 75–150 mg/d with max 400 mg/d for standard release, taken twice daily or 450 mg in XR formulation once daily. Both generic and branded (Wellbutrin) versions available. | Pregnancy test if indicated and monitoring for emergence of suicidal ideation/behavior | Constipation, dry mouth, headaches, insomnia, nausea, and seizures | Phenelzine, tranylcypromine, selegiline, carbamazepine, cyclosporine, linezolid, MAOIs, ritonavir, and TCAs also listed |
| WFSBP | Not recommended |
Abbreviations: –, not reported by guideline; ADs, antidepressants; APA, American Psychiatric Association; BAP, British Association of Psychopharmacology; CANMAT, Canadian Network for Mood and Anxiety Disorders; CBT, cognitive behavioural therapy; CPG-S, Clinical Practice Guidelines in the Spanish NHS; ICSI, Institute for Clinical Systems Improvement; IR, immediate release; MI, myocardial infarction; MOAI; monoamine oxidase inhibitors; MPG, Maudsley Prescribing Guidelines; NICE, National Institute for Health and Care Excellence; QT, Q-T interval; QRS, QRS complex; RANZCP, Royal Australian and New Zealand College of Psychiatrists; TCAs, tricyclic antidepressants; TMAP, Texas Medication Algorithm Project; WFSBP, World Federation of Societies of Biological Psychiatry; XR, extended release.
Discontinuation
The MPG advised that bupropion discontinuation symptoms, similar to those associated with SSRI use, have been documented in a few cases, but this was not specific to its use as an augmenter (Berigan and Harazin, 1999; Berigan, 2002). No other included guidelines included discontinuation advice for bupropion.
Buspirone
First-line recommendations
TMAP included buspirone augmentation at stage 1a and 2a of their algorithm, meaning it can be considered a first-line option. TMAP did not cite the evidence used.
Second-line recommendations
The MPG listed buspirone as a second-line augmenter when added to an SSRI. The STAR*D comparison with bupropion was again referenced (Trivedi et al., 2006b), though the MPG guarded that the need for a higher dose and poor tolerability are disadvantages (Appelberg et al., 2001; Trivedi et al., 2006b).
Other recommendations
The ICSI listed buspirone as an augmentation method for use in combination with an SSRI. They also referenced the STAR*D comparison with bupropion (Trivedi et al., 2006b) plus 2 case series/chart reviews reporting beneficial results (Bouwer and Stein, 1997; Dimitriou and Dimitriou, 1998).
APA recommended buspirone augmentation with its third level of confidence (recommended based on individual circumstances) for TRD patients with prominent features of anxiety and/or insomnia. A different STAR*D study was discussed in which CBT augmentation was reported to be as effective as buspirone add-on, bupropion add-on, or an antidepressant switch (Thase et al., 2007b). APA also noted the STAR*D comparison of buspirone and bupropion augmentation, and though both were associated with remission rates of approximately 30%, APA highlighted that bupropion was superior on a number of secondary outcomes (Trivedi et al., 2006b).
BAP included buspirone augmentation under “other additions that could be considered” (level B evidence). They stated that the evidence is less robust than for lithium, quetiapine, risperidone, and aripiprazole, and buspirone was not as well tolerated and marginally less effective than bupropion (Trivedi et al., 2006b). The BAP mentioned 2 other studies in which buspirone augmentation was not effective (Landen et al., 1998), though a beneficial effect was found in the more severely depressed patients (Appelberg et al., 2001).
Not recommended
The WFSBP guidelines did not recommend buspirone augmentation, referring to a narrative review by (Connolly and Thase, 2011) and 2 placebo-controlled RCTs (Landen et al., 1998; Appelberg et al., 2001). As discussed, they also referred to the STAR*D comparison with bupropion (Trivedi et al., 2006b), highlighting the modified intention to treat analysis in which buspirone was less effective (Bech et al., 2012).
Similarly, RANZCP advised that the evidence for buspirone augmentation is unconvincing, citing only the 2009 CANMAT guidelines (Lam et al., 2009). The current CANMAT guidelines briefly mentioned buspirone augmentation in relation to the network meta-analysis by Zhou and colleagues (2015), in which it was not more effective than placebo (Zhou et al., 2015).
NICE stated that there is insufficient evidence to recommend buspirone or determine if there is a clinically significant difference between SSRI monotherapy and buspirone augmentation. They advised that there was no evidence from double-blind, placebo-controlled studies at the time of publication. NICE also commented that at the time of writing, the United Kingdom did not have marketing authorization for this indication. Again, STAR*D was highlighted, as dropout was greater in patients taking buspirone compared with bupropion augmentation (Rush et al., 2003).
The CPG-S guidelines did not include buspirone in their recommendations.
Dosage, monitoring, and adverse effects
See Table 15.
Table 15.
Buspirone Monitoring and Dosing
| Guideline | Dose | Monitoring | Specific side effects | Specific drug interactions |
|---|---|---|---|---|
| APA | – | – | Cautioned against augmenting MAOIs with buspirone, including reversible inhibitors of monoamine oxidase and selegiline, due to risk of serotonin syndrome (Sternbach, 1991; Boyer and Shannon, 2005; Stahl and Felker, 2008) | |
| BAP | – | – | – | – |
| CANMAT | Not recommended | |||
| CPG-S | Not recommended | |||
| ICSI | – | – | – | – |
| MPG | Up to 60 mg/d. Specific advice for renal impairment | Nausea, dizziness and headaches. Manufacturer contraindicates use in patients with severe renal impairment (Aronoff et al., 2007; Ashley and Currie, 2008). | ||
| NICE | Not recommended | |||
| RANZCP | Not recommended | |||
| TMAP | Starting dose 15 mg/d, increased every week by 15 mg/d until target dose of 20–60 mg/d (max 60 mg/d) reached. Dose should be divided and taken 2–3 times throughout day. | Pregnancy test as indicated. | Dizziness, drowsiness, headache and nausea | Alcohol, furazolidone, MAOIs, SNRIs, SSRIs, and grapefruit juice |
| WFSBP | Not recommended |
Abbreviations: –, not reported by guideline; APA, American Psychiatric Association; BAP, British Association of Psychopharmacology; CANMAT, Canadian Network for Mood and Anxiety Disorders; CPG-S, Clinical Practice Guidelines in the Spanish NHS; ICSI, Institute for Clinical Systems Improvement; MAOI, monoamine oxidase inhibitor; MPG, Maudsley Prescribing Guidelines; NICE, National Institute for Health and Care Excellence; RANZCP, Royal Australian and New Zealand College of Psychiatrists; SNRI, serotonin-norepinephrine reuptake inhibitors; SSRI, selective serotonin reuptake inhibitors; TMAP, Texas Medication Algorithm Project; WFSBP, World Federation of Societies of Biological Psychiatry.
Discontinuation
None of the included guidelines offered any discontinuation advice for buspirone augmentation.
Thyroid Hormones
First-line recommendations
TMAP recommended thyroid augmentation at both stage 1a and 2a of their algorithm. They did not cite the evidence to support these recommendations.
RANZCP included T3 as a Level I augmenter but noted that the results from clinical studies are inconsistent and thyroxine (T4) has been less widely assessed, though they found it reasonable to extrapolate the findings. RANZCP emphasized the mixed evidence for T3, highlighting a 2008 review (Cooper-Kazaz and Lerer, 2008), but advised that T3 augmentation should be used in those with and without subclinical hypothyroidism.
Second-line recommendations
CANMAT recommended T3 as a second-line augmenter supported by Level II evidence. As discussed, CANMAT referred to the network meta-analysis by Zhou et al. (2015) that reported T3 to be more effective than placebo. Despite this, they stated that there is insufficient evidence to recommend T3 as a first-line augmenter given that there have been no additional placebo-controlled RCTs since the 2008 review also mentioned by RANZCP, which identified only 2 (Cooper-Kazaz and Lerer, 2008). CANMAT also referred to the STAR*D trial (Nierenberg et al., 2006), noting that although there was no difference in remission rates between lithium and T3 augmentation, T3 was better tolerated.
WFSBP listed both T3 and T4 as second-line strategies but acknowledged that studies had largely focused on T3, and high-quality evidence remains sparse. WFSBP stated that the evidence base for T3 consisted of many case series and 13 prospective trials at the time of publication (9 open-label and 4 double-blind RCTs) and most augmented TCAs in TCA nonresponders (Joffe et al., 1993; Altshuler et al., 2001; Bauer and Whybrow, 2001). They noted that not all controlled double-blind studies significantly favored T3 vs placebo and highlighted the same meta-analysis as RANZCP (Aronson et al., 1996). They also mentioned the STAR*D comparison with lithium (Nierenberg et al., 2006). For T4, WFSBP reported a small number of studies (open-label) with response rates of approximately 50% for TRD patients using higher, supra-physiological doses (Bauer et al., 1998, 2002a).
The MPG listed T3 as a second-line option, mentioning evidence of good tolerability and citing STAR*D (Nierenberg et al., 2006). They highlighted that there are some negative studies (not referenced) and suggested that manufacturer monopoly may result in a high purchase cost for some counties.
APA recommended thyroid hormone augmentation with “moderate clinical confidence,” referring to evidence that it may increase antidepressant efficacy, again including STAR*D (Aronson et al., 1996; Nierenberg et al., 2006). APA did not mention T4 augmentation. BAP similarly recommended T3 as second-line (class B). BAP cited both the meta-analysis referenced by APA (Aronson et al., 1996) and the STAR*D study (Nierenberg et al., 2006) but stated that the meta-analysis included just 4 small RCTs, and while a significant improvement in HDRS scores was reported with T3, there was a nonsignificant difference in response rates. BAP also noted that lithium levels were not consistently monitored in STAR*D, and referenced 1 additional study, which although small, reported no difference between augmentation with T3, lithium, or placebo over 2 weeks (Joffe et al., 2006). BAP did not include T4 augmentation.
Other recommendations
ICSI included T3 augmentation as an option but did not specify the level of confidence. Again, STAR*D was referenced, indicating that T3 is better tolerated than lithium (Nierenberg et al., 2006), but the ICSI mentioned that placebo-controlled trials have found mixed results. There was no discussion of T4 augmentation.
Not recommended
NICE did not include T3 in their clinical practice recommendations due to a lack of evidence and advised that it should not be used routinely. They commented that existing studies predominantly assessed TCA augmentation (Altshuler et al., 2001), with STAR*D being the only evidence of efficacy with SSRIs at the time of publication (Nierenberg et al., 2006). NICE suggested that response rates have been variable across studies for thyroid hormone augmentation (Aronson et al., 1996) and referred to just 1 RCT in which there was a significant difference between T3 and placebo for response, but not the reduction of depressive symptoms (Joffe et al., 1993). NICE also noted 1 study in which T4 was inferior to T3 (Joffe and Singer, 1990) but made no recommendations for T4. NICE stated that there was no evidence regarding treatment acceptability for thyroid hormone augmentation, and thyroid hormones did not have UK marketing authorization for augmentation at the time of publication. They advised clinicians to obtain documented informed consent for this option.
Similarly, CPG-S (referring to NICE) advised there is not enough evidence to recommend augmentation with thyroid hormones. CPG-S drew on a study comparing a range of augmenters that found no significant difference between T3 and any other (Fang et al., 2011). Again the STAR*D study was referenced (Nierenberg et al., 2006) as was Joffe et al. (1993).
Dosage, side effects, and monitoring
See Table 16 for dosage, side effect, and monitoring recommendations for thyroid hormones provided by the guidelines.
Table 16.
Thyroid Hormone Dosage and Monitoring
| Guideline | Dose | Patient monitoring parameters | Specific side effects | Specific drug interactions |
|---|---|---|---|---|
| APA | 25 mcg/d increased to 50 mcg/d after ~1 wk if response inadequate | – | – | – |
| BAP | – | – | – | – |
| CANMAT | 25–50 mcg | – | – | – |
| CPG-S | Not recommended | Not recommended | Not recommended | Not recommended |
| ICSI | Usual doses of T3 vary between 25 and 50 mcg/d (Nelson, 2000) | – | – | – |
| MPG | 20–50 mcg/d but higher doses have been safely used | Clinical and biochemical TFT monitoring required. T3 augmentation would usually need specialist referral. | – | – |
| NICE | Not recommended | Not recommended | Not recommended | Not recommended |
| RANZCP | – | Monitored in same way as patients with Hypothyroidism: TSH, T3, and free T4 levels measured regularly (Rosenthal et al., 2011) | – | – |
| TMAP | – | Thyroid function test at baseline and as clinically indicated | Most common side effects are diarrhea, headache, irritability, nervousness, sweating, and tachycardia | Anticoagulants, hypoglycemics, oral contraceptives, TCAs |
| WFSBP | Advised that most studies dosed at 25–37.5 mcg/d and referred to Łojko and Rybakowski (2007) for T4 | – | Should be prescribed with caution due to potential adverse effects (not detailed) | – |
Abbreviations: –, not reported by guideline; APA, American Psychiatric Association; BAP, British Association of Psychopharmacology; CANMAT, Canadian Network for Mood and Anxiety Disorders; CPG-S, Clinical Practice Guidelines in the Spanish NHS; ICSI, Institute for Clinical Systems Improvement; MPG, Maudsley Prescribing Guidelines; NICE, National Institute for Health and Care Excellence; RANZCP, Royal Australian and New Zealand College of Psychiatrists; T3, triiodothyronine; T4, thyroxine; TCAs, tricyclic antidepressant; TMAP, Texas Medication Algorithm Project; TSH, thyroid stimulating hormone WFSBP, World Federation of Societies of Biological Psychiatry.
Discontinuation guidance
None of the guidelines included discontinuation advice for thyroid hormone augmentation.
Stimulants
Second-line recommendations
CANMAT recommended modafinil as a second-line adjunctive agent citing a meta-analysis of 4 MDD RCTs (Goss et al., 2013).
Other recommendations
APA recommended modafinil as an additional strategy for cases of antidepressant nonresponse but cautioned that there is less evidence for efficacy than for other treatments (evidence level III: recommended based on individual circumstances only). Despite this, APA highlighted modafinil as the best treatment for fatigue and hypersomnolence when combined with an SSRI, based on 4 studies (DeBattista et al., 2003a; Ninan et al., 2004; Dunlop et al., 2007; Fava et al., 2007). APA also advised that methylphenidate or dextroamphetamine may be useful (Masand et al., 1998; Lavretsky et al., 2006; Ravindran et al., 2008) but cautioned that not all clinical trials have shown benefits (Patkar et al., 2006).
The MGP featured modafinil in their “other reported treatments” section due to the lack of evidence supporting its use as an adjunctive treatment (Fawcett et al., 1991; DeBattista et al., 2004; Lavretsky et al., 2006; Taneja et al., 2007) and stated that other augmentation options are better supported, as the evidence for stimulants (methylphenidate, dexamfetamine, and lisdexamfetamine) is generally inconclusive and inadequate (Fawcett et al., 1991; Parker and Brotchie, 2010; Trivedi et al., 2013; Madhoo et al., 2014; Israel, 2015). In line with APA, the MPG recommended modafinil for patients experiencing fatigue but acknowledged that these effects are not clearly understood (DeBattista et al., 2003b; Fava et al., 2005b).
The guidance from BAP regarding modafinil augmentation is limited, as they stated that there is only preliminary evidence for its use over the short term and cited the same meta-analysis of 4 MDD RCTs as CANMAT (Goss et al., 2013). They concluded the evidence to be Level C (potentially including nonexperimental, descriptive studies). BAP also suggested that modafinil may improve sleepiness but, like the MPG stated that this effect is unclear and cited similar research to APA (Fava et al., 2007).
The ICSI recommended modafinil in addition to a TCA or SSRI, calling this a “jump-start response” and citing open-label studies demonstrating benefit for sleepiness and fatigue (Ninan et al., 2004; Schwartz et al., 2004). They highlighted the need for larger, higher quality RCTs to establish the benefit of stimulant augmentation (Fava et al., 2005b; Dunlop et al., 2007; Candy et al., 2008) and reported cases of sudden death, stroke, and myocardial infarction in adults taking doses recommended for attention deficit hyperactivity disorder. ICSI cautioned against prescribing in those with cardiac problems.
Not recommended
RANZCP stated that there was insufficient evidence of efficacy and so did not recommend stimulant augmentation, as did NICE. However, NICE referenced other sources for details about these strategies, including the 2002 WFSBP guidelines (Thase and Rush, 1997; Bauer et al., 2002b).
Neither modafinil nor any other stimulant featured in the CPG-S, TMAP, or WFSBP.
Dosage
The MGP and CANMAT recommended 100–400 mg/d of modafinil added to an antidepressant. APA recommended low doses of stimulants such as methylphenidate or dextroamphetamine, and the MPG advised 7.5–40 mg dexamfetamine or 50 mg of lisdexamfetamine when combined with an MAOI, 20–50 mg of lisdexamfetamine when combined with escitalopram, and 20–30mg of lisdexamfetamine when combined with another antidepressant. APA and BAP did not include dosage guidelines for stimulant augmentation.
Side effects and monitoring
Although APA did not explicitly advise on pre-prescription and monitoring tests for modafinil, they advised that clinicians should be aware of Stevens-Johnson syndrome and other cutaneous reactions, toxic epidermal necrolysis, drug rash with eosinophilia and systemic symptoms, and cytochrome P450 interactions. They also advised that modafinil can induce CYP 3A4 and render contraceptive and other medications metabolized through this route ineffective. APA also stated that stimulants should be added to MAOIs only with extreme caution (Feighner et al., 1985; Fawcett et al., 1991).
BAP recommended that modafinil should only be prescribed in specialist centers with careful monitoring, and the MPG cautioned that modafinil augmentation may worsen anxiety symptoms. No pre-prescribing or monitoring checks were reported for any other stimulant.
Discontinuation
The BAP, MPG, and CANMAT offered no discontinuation/withdrawal advice for modafinil. APA cited 1 study in which the effects of modafinil were maintained over 12 weeks (Fava et al., 2007), but it was not clear whether this was a recommended treatment length. The MPG included stimulants among the treatments that are “probably” safe to recommence at the previous dose following noncompliance.
Ketamine
Second-line recommendations
The MPG listed i.v. ketamine as a second-line augmenter and highlighted the potential for this to be superseded by its intranasal form in the near future (Daly et al., 2018). They also cited studies of intramuscular and subcutaneous administration, sublingual administration, and transmucosal routes (Lara et al., 2013; Nguyen et al., 2015; Loo et al., 2016). They discussed the very rapid response rates and high short-term remission rates and mentioned evidence of maintained response if repeated doses are administered (not referenced). The MPG stated that ketamine is usually well tolerated. However, the need for i.v. ketamine to be administered in hospital and poor availability were disadvantages.
Other recommendations
The ICSI listed ketamine infusion therapy a specialized treatment. They reviewed the evidence base for the antidepressant properties of ketamine (Aan Het Rot et al., 2012; Fond et al., 2014a) but stated that more research is required before recommendation of its use in standard clinical settings, especially studies evaluating long-term response and with larger sample sizes.
CANMAT recommend ketamine as an experimental adjunctive agent in an academic setting but echoed caution against adverse reactions, potential abuse, and its efficacy long term (Fond et al., 2014b; Serafini et al., 2014a; Coyle and Laws, 2015; Wan et al., 2015).
Not recommended
RANZCP, BAP, and WFSBP did not recommend ketamine augmentation. RANZCP and the BAP both considered a study by Murrough and colleagues (2013) as evidence for its success as a short-term treatment (Murrough et al., 2013) and highlighted potential adverse events and toxicity (Price et al., 2009; Zarate et al., 2012; Serafini et al., 2014b). The WFSBP recognized the growing interest in glutamatergic agents but concluded that there is insufficient evidence.
TMAP, APA, NICE, and CPG-S did not mention ketamine augmentation. NICE reasoned that the evidence base at the time of publication was too weak and clinical usage too low; however, they did refer readers to alternative sources should they wish to know more.
Dosage
The MPG listed a dose of 0.5 mg/kg over 40 minutes in its i.v. form. The BAP advised that optimal dosing was not yet established.
Monitoring and adverse effects
The MPG mentioned that cognitive side effects (e.g., confusion and dissociation) usually occur with ketamine augmentation and that it is associated with a temporary increase in blood pressure, tachycardia, and arrhythmias. The MPG acknowledged that the adverse effects associated with ketamine may be underestimated in the literature (Short et al., 2018), and advised a pre-treatment ECG (Aan het Rot et al., 2010). The ICSI and CANMAT did not offer side effect and monitoring advice.
Discontinuation
None of the included guidelines specifically discussed the discontinuation of ketamine augmentation, which remains relevant even in its i.v. form, as some of the studies discussed by the guidelines (e.g., the ICSI) included multiple doses (Szymkowicz et al., 2013).
Discussion
This review provides a comprehensive overview of treatment guidelines for pharmacological augmentation in unipolar depression, to aid clinicians in their provision of evidence-based care, support researchers in their identification of research needs, and guide the development of future guidelines and guideline updates.
Recommended Treatments
Lithium was the only agent to be recommended across all 10 guidelines and is the most widely studied pharmacological augmenter in patients who have not responded to 2 or more antidepressants (Strawbridge et al., 2019). None of the 4 North American guidelines recommended lithium as a first-line option, while 5 others did, indicating geographical differences in preference. Concerningly, the reason for this was not made apparent by the guidelines but may reflect the fact that lithium is not approved for use in unipolar TRD in the United States.
AAPs were also recommended by all 10 guidelines, though no single agent was clearly universally endorsed. Aripiprazole was the most widely recommended, broadly in line with existing meta-analytic evidence demonstrating aripiprazole to be the most widely studied AAP in TRD (Strawbridge et al., 2019) and to have the most robust evidence base, along with quetiapine (Zhou et al., 2015). All guidelines published from 2015 onwards (the date of the most recent AAP RCT included by Strawbridge et al., 2019) recommended aripiprazole as a first-line option, bar the ICSI who did not rank their recommendations. Risperidone, olanzapine, and quetiapine were also recommended by the majority, though just 1 RCT examining treatment efficacy was identified for each by Strawbridge et al. (2019). However, previous reviews have also identified atypical antipsychotics as the best supported pharmacological augmentation options for TRD (McIntyre et al., 2014).
Less consistency was found for all other augmenters. The BAP, MPG, TMAP, and APA made recommendations for anticonvulsant mood stabilizers (primarily lamotrigine), while NICE, CANMAT, and the CPG-S advised against their use due to a lack of evidence. This cannot be accounted for by variation in guideline publication date or licensing and instead indicates differences in evidence interpretation and perhaps guideline quality, though there was not a clear split according to AGREE II scores (Table 2). The ICSI, RANZCP, and WFSBP also failed to mention anticonvulsant mood stabilizers, and the CPG-S, TMAP, and WFSBP did not mention stimulants. While it is plausible that guidelines will have some differences in their interpretation of the evidence, stark inconsistencies reduce clarity and may mean that the treatment options available to a patient are somewhat dependent on the guideline used by their clinician rather than the full range of evidence based options available, which may in some cases be problematic. Clinicians may therefore wish to consult multiple guidelines and use this review to identify the most appropriate guidance for their needs.
There were clear differences in recommendations for bupropion and buspirone, some of which can be attributed to bupropion’s status as a licensed antidepressant in North America, but not Europe/Australasia. Most guidelines referred to the STAR*D comparison of bupropion and buspirone augmentation yet used it to support contrasting advice (e.g., the CPG-S and NICE did not recommend bupropion and buspirone, while the APA, MPG, and ICSI did) (Trivedi et al., 2006b). CANMAT was the only guideline to use the recent Zhou network analysis to support their recommendations despite the MPG and ICSI having later dates of publication. Clearly there is a need for greater alignment between guidelines in terms of their grading of available evidence to ensure consistent appraisal and transparent recommendations. The average AGREE II score for the Rigour of Development quality domain for guidelines in this review was 60/100, demonstrating considerable room for improvement across the board.
Thyroid hormone recommendations also varied despite all guidelines having the same RCT evidence base to draw on, as all RCT evidence in TRD was published prior to 2008 (Zhou et al., 2015; Strawbridge et al., 2019). Some differences may relate to variation in the point at which guidelines recommended augmentation, as (Strawbridge et al., 2019) reported just 1 RCT examining T3 in patients with 2 or more failed antidepressant treatment trials in their current episode of depression, while (Zhou et al., 2015) reported 5 studies in patients with at least 1 antidepressant treatment failure and reported it to be significantly more effective than placebo. Agreement between guidelines may therefore be limited by the absence of a universal definition for TRD (Fekadu et al., 2018). The included guidelines did employ differing criteria to indicate the use of augmentation, including differing resistance criteria (Table 3). This demonstrates the importance of the first domain of the AGREE II assessment: “Scope and Purpose,” which evaluates how well a guideline specifically describes the population to whom it applies. Proper attention to this domain during development should help to make clear whether discrepancies between recommendations are due to differences in evidence interpretation or differences in the population to which they are applicable (and therefore the relevant evidence base used). Scores on this domain had the highest mean score across included guidelines (Table 2), but there was still considerable variation, so improvements in this area can be made.
By contrast, differences between ketamine recommendations can largely be explained by differing dates of guideline publication. The MPG is both the most recently published and the only guideline to recommend (i.v.) ketamine as second line. CANMAT and the ICSI, published between 2016 and 2018, recommended ketamine as a specialist/experimental treatment. TMAP, the APA, NICE, and the CPG-S did not mention ketamine at all, but only 1 of the studies cited by the more recent guidelines predates their development (Price et al., 2009). This is with the exception of the CPG-S (2014), which broadly made recommendations in line with the NICE (2009) guidelines. Intranasal esketamine has now been approved by the US Food and Drug Administration (FDA, 2019) and European Medicines Agency (Mahase, 2019), and we can therefore expect its inclusion in future treatment guidelines. However, its role in the treatment paradigm for TRD patients remains to be established, with some healthcare payers and providers having concerns about efficacy, treatment costs, and logistics around administration. Indeed, no European Health Technology Assessment agencies have approved esketamine, unlike several US payers, and therefore differences are expected in future US vs European treatment guidelines. For example, NICE have very recently stated that they do not currently recommend its use due to uncertainties over its clinical and cost effectiveness, though their appraisal remains in progress (NICE, 2020).
This raises 2 important issues. First, the importance of regular guideline updates. Although it is not possible for any guideline to remain completely up to date, regular updates to the core guidance are clearly necessary. For example, the current full NICE guidance for MDD remains the 2009 version included in this review, though additional advice such as the decision not to recommend esketamine is made via their website. This does allow the provision of guidance in line with the latest developments in research and licensing but relies on users seeking this additional information out rather than simply being able to use their current full edition. Of course a balance has to be struck between updating the full guideline regularly and providing interim updates, but it is arguable that in the case of NICE (along with other guidelines in this review, such as the widely used APA guidelines), the interval since the last full update is too long given the subsequent advances in research and drug approval. A negative consequence of this may be the underuse of new and potentially more efficacious or tolerable treatments in clinical practice. Secondly, the decision by NICE not to recommend esketamine at present in contrast with US payers and insurers highlights the relevance of guideline affiliation and local context. NICE is the payer for the publicly funded National Health Service (NHS) in the UK, and therefore consideration of cost effectiveness plays a large role in their decision-making and their guidance is of primary utility to NHS clinicians. This may not be the case for guidelines produced by other governing bodies, and therefore recommendations may differ. This in itself is justifiable, providing that guidelines explicitly state the intended context for their use and the weighting of factors such as cost effectiveness and research evidence in their decision-making. We suggest this is not explicit enough at present.
Other Recommendations
The greatest discrepancy between guidelines was in their monitoring and discontinuation advice, particularly for lithium, in which regular monitoring is of the utmost importance to ensure patient safety. This has the potential to be not only misleading but also potentially dangerous, as guidelines commonly fail to clarify whether their recommendations are comprehensive or provide a brief summary and might account for some of the substandard monitoring reported in clinical practice. According to a recent review, only approximately 50% of lithium serum levels were within the recommended range for patients with bipolar disorder in 1 NHS trust, while adherence to safety and monitoring for recommended renal and thyroid tests was between 21% and 39% (Nikolova et al., 2018). Another review stated that only 60% of clinical trials and audits met safety monitoring standards for the prescription of lithium in the United Kingdom, and none fully met the recommendations (Aubry et al., 2017). Similarly, monitoring rates are suboptimal for AAPs (predominantly reviewed in patients with schizophrenia-spectrum disorders; Mitchell et al., 2012). The role of guidelines should be to support the highest standard of patient care, but instead our findings suggest that poor monitoring could be partially attributable to discrepancies between treatment guidelines, as unclear guidance has previously been cited as a problem leading to inconsistent care and may contribute to clinical uncertainty and subsequent treatment underutilization (Hollingshead et al., 2015). A recent consensus statement pertaining to antidepressant adverse events confirmed the need for adequate risk assessment and safety monitoring when initiating pharmacological treatment for depression (Dodd et al., 2018), and so this should be an essential part of any treatment guideline, particularly where polypharmacy is concerned. In this review, the MPG guidelines had the most detailed monitoring advice, but as it is not freely available online usability is somewhat limited.
Quality Assessment
As mentioned, some inconsistencies may be accounted for by differences in guideline quality. All eligible guidelines were assessed using the AGREE II tool (supplementary Table 1). Nine did not meet the cut-off for inclusion, scoring lowest on “rigor of development,” “editorial independence,” and “applicability,” which relate to the likely barriers and facilitators to implementation and resource implications. The link between guideline quality and discrepant recommendations is best demonstrated by TMAP, which had the lowest mean score across quality domains and often made recommendations at odds with other guidelines. TMAP was also the only guideline to consistently fail to cite the evidence on which their recommendations were based and were the only commercially funded guideline (though the ICSI received sponsorship from private member medical groups). Adherence to a set of general guideline development rules, and the use of a consistent evidence grading system such as the recent WFSBP guidelines on how to grade treatment evidence for guideline development (Hasan et al., 2019), may improve the overall quality of treatment guidelines and support greater alignment between future recommendations irrespective of funding source or stakeholder involvement.
Limitations
Despite the comprehensive nature of this review, there are some limitations. A range of possible reasons for inconsistencies has been discussed, including differences in evidence interpretation, but it is also possible that the licensing and availability of medications around the world may have contributed more extensively than discussed. Thus, some indication of the degree to which local drug licensing and availability have influenced the recommendations made would be valuable in future guidelines.
It is also important to acknowledge that recommendations can only be as good as the evidence on which they are based. The Strawbridge meta-analysis clearly demonstrates the paucity of RCT evidence for pharmacological augmenters in patients who have failed to respond to 2 antidepressants (Strawbridge et al., 2019), the point at which many of guidelines recommended augmentation (Table 3). There is also a need, acknowledged by some, for more head-to-head studies with a longer term follow-up, potentially improving consistency across guidelines and more comprehensive discontinuation advice. Further, patients in a clinical trial may not truly reflect the wider population, limiting the generalizability of the results and demonstrating the need for more pragmatic and naturalistic RCTs examining real-world efficacy. This would ensure that guidelines are able to accurately meet the needs of their target population.
There is also the potential issue of publication bias, as null trials may not be considered when formulating recommendations. Recent publication of null results in high-profile journals, such as antidepressant augmentation with metyrapone for TRD (McAllister-Williams et al., 2016), supports progress in this area. Finally, our review only included guidelines available in English, and therefore it is possible that some widely used publications have not been considered. Additionally, recommendations relevant to specific subpopulations or specialist groups (e.g., pregnant women or older patients) and adjunctive agents that were not listed as first or second-line by at least 1 of the included guidelines were not reviewed, which would be of benefit.
Future Directions
It is clear from the findings of this review that changes are needed to the way in which treatment guidelines are developed to ensure that they support clinicians and facilitate the provision of high-quality evidence-based care. The number of available guidelines and the apparent variation in their quality is cause for concern. The collective requirement by publishing bodies for the application of a guideline development tool, equivalent to the Vancouver Recommendations provided by the International Committee of Medical Journal Editors (ICMJE, 2019) or the widely used Consolidated Standards of Reporting Trials statement for RCTs (Schulz et al., 2010), would be of great benefit. The quality domains outlined in the AGREE II recommendations (Brouwers et al., 2010) provide a strong basis for this, but consistent application to psychiatric guidelines has not been achieved and additional consideration of need is warranted. This review includes guidelines from around the world that have the potential to be beneficial given global differences in treatment cost, availability, and acceptability. However, such considerations were not clearly reflected in the recommendations, and therefore the need for such a range of publications from several sources is questionable. The expectation for developers to use a tool such as the AGREE II and explicitly state the unfulfilled need their publication meets would ensure that published guidelines are both necessary and of good quality.
Conclusions
To our knowledge, this is by far the most comprehensive review of augmentation treatment guidelines for unipolar depression. Although some differences are inevitable given the limited evidence base and the somewhat subjective nature of balancing factors such as efficacy and tolerability, greater consistency could be achieved by the standardization of development. This review will therefore aid future guideline development in this field and beyond as well as serve as a useful tool to comprehensively summarize the treatment recommendations for clinicians and researchers working in unipolar resistant depression.
Supplementary Material
Acknowledgments
This paper represents independent research funded in part by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care. The funder had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Statement of Interest
In the last 3 years, A.J.C. has received honoraria for speaking from Lundbeck; honoraria for consulting from Allergan, Livanova, Janssen, and Lundbeck; and sponsorship for attending an academic conference from Janssen. A.H.Y. has received honoraria for speaking from Astra Zeneca, Lundbeck, Eli Lilly, Sunovion; honoraria for consulting from Allergan, Livanova and Lundbeck, Sunovion, Janssen; and research grant support from Janssen in the last 3 years. R.Z. provides private psychiatric services at The London Depression Institute; is an honorary PI at D’OR Institute for Research & Education, Rio de Janeiro, a not-for-profit organization; on the Advisory Board for Science, a US not-for-profit organization; has consulted for Fortress Biotech; is a co-investigator on a Livanova-funded study; has received speaker honoraria for medical symposia and educational activities from Lundbeck and Janssen; and collaborates with Janssen, EMIS PLC, and Alloc Modulo Ltd. L.M. is an employee of COMPASS Pathways Ltd. No other authors have conflicts of interest to declare. It is also worth noting that A.J.C. is the lead author of the British Association of Psychopharmacology (BAP) guidelines included in this review and A.H.Y. is an author of the included Maudsley Prescribing Guidelines (MPG), on which A.J.C. is acknowledged for his contributions. Neither A.H.Y. nor A.J.C. received financial benefits for their involvement in writing these guidelines. A.H.Y. and A.J.C. were not involved in the quality review of the guidelines in this manuscript: this was conducted by authors R.T., L.M., E.O., V.D.A., S.M., and B.V.
References
- Aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ (2010) Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry 67:139–145. [DOI] [PubMed] [Google Scholar]
- Aan Het Rot M, Zarate CA Jr, Charney DS, Mathew SJ (2012) Ketamine for depression: where do we go from here? Biol Psychiatry 72:537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Saleh MT, Müller-Oerlinghausen B, Coppen AJ (2017) Lithium in the episode and suicide prophylaxis and in augmenting strategies in patients with unipolar depression. Int J Bipolar Disord 5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adli M, Baethge C, Heinz A, Langlitz N, Bauer M (2005) Is dose escalation of antidepressants a rational strategy after a medium-dose treatment has failed? A systematic review. Eur Arch Psychiatry Clin Neurosci 255:387–400. [DOI] [PubMed] [Google Scholar]
- Agid O, Seeman P, Kapur S (2006) The “delayed onset” of antipsychotic action–an idea whose time has come and gone. J Psychiatry Neurosci 31:93–100. [PMC free article] [PubMed] [Google Scholar]
- Aiff H, Attman PO, Aurell M, Bendz H, Ramsauer B, Schön S, Svedlund J (2015) Effects of 10 to 30 years of lithium treatment on kidney function. J Psychopharmacol 29:608–614. [DOI] [PubMed] [Google Scholar]
- Almeida NMG, Lima R, Alves TFR, Rebelo M de A, Severino P, Chaud MV(2015) A novel dosage form for buccal administration of bupropion. Braz J Pharm Sci 51:91–100. [Google Scholar]
- Altshuler LL, Bauer M, Frye MA, Gitlin MJ, Mintz J, Szuba MP, Leight KL, Whybrow PC (2001) Does thyroid supplementation accelerate tricyclic antidepressant response? A review and meta-analysis of the literature. Am J Psychiatry 158:1617–1622. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2006) Treatment of patients with eating disorders, third edition. American Psychiatric Association. Am J Psychiatry 163:4–54. [PubMed] [Google Scholar]
- American Psychiatric Association (APA) (2010) Practice guideline for the treatment of patients with major depressive disorder. 3rd ed. Available at: https://psychiatryonline.org/guidelines. Accessed January 22, 2019. [Google Scholar]
- Andersen SW, Clemow DB, Corya SA (2005) Long-term weight gain in patients treated with open-label olanzapine in combination with fluoxetine for major depressive disorder. J Clin Psychiatry 66:1468–1476. [DOI] [PubMed] [Google Scholar]
- Appelberg BG, Syvälahti EK, Koskinen TE, Mehtonen OP, Muhonen TT, Naukkarinen HH (2001) Patients with severe depression may benefit from buspirone augmentation of selective serotonin reuptake inhibitors: results from a placebo-controlled, randomized, double-blind, placebo wash-in study. J Clin Psychiatry 62:448–452. [DOI] [PubMed] [Google Scholar]
- Aronoff GR, Bennett WM, Berns J, Brier M, Kasbekar N, Mueller B, Pasko D, Smoyer W(2007) Drug prescribing in renal failure: dosing guidelines for adults. 5th ed. Philadelphia, PA: American College of Physicians Philadelphia. [Google Scholar]
- Aronson R, Offman HJ, Joffe RT, Naylor CD (1996) Triiodothyronine augmentation in the treatment of refractory depression. A meta-analysis. Arch Gen Psychiatry 53:842–848. [DOI] [PubMed] [Google Scholar]
- Ashley C, Currie A (2008) The renal drug handbook. Abingdon, UK: Radcliffe Publishing. [Google Scholar]
- Atasoy N, Erdogan A, Yalug I, Ozturk U, Konuk N, Atik L, Ustundag Y (2007) A review of liver function tests during treatment with atypical antipsychotic drugs: a chart review study. Prog Neuropsychopharmacol Biol Psychiatry 31:1255–1260. [DOI] [PubMed] [Google Scholar]
- Atmaca M, Kuloglu M, Tezcan E (2005) A new atypical antipsychotic: quetiapine-induced sexual dysfunctions. Int J Impot Res 17:201–203. [DOI] [PubMed] [Google Scholar]
- Aubry RE, Scott L, Cassidy E (2017) Lithium monitoring patterns in the United Kingdom and Ireland: can shared care agreements play a role in improving monitoring quality? A systematic review. Ir J Psychol Med 34:127–140. [DOI] [PubMed] [Google Scholar]
- Austin MP, Souza FG, Goodwin GM (1991) Lithium augmentation in antidepressant-resistant patients. A quantitative analysis. Br J Psychiatry 159:510–514. [DOI] [PubMed] [Google Scholar]
- Baethge C, Tondo L, Lepri B, Baldessarini RJ (2009) Coffee and cigarette use: association with suicidal acts in 352 Sardinian bipolar disorder patients. Bipolar Disord 11:494–503. [DOI] [PubMed] [Google Scholar]
- Baldessarini RJ, Cohen BM, Teicher MH (1988) Significance of neuroleptic dose and plasma level in the pharmacological treatment of psychoses. Arch Gen Psychiatry 45:79–91. [DOI] [PubMed] [Google Scholar]
- Baldwin D, Mayers A (2003) Sexual side-effects of antidepressant and antipsychotic drugs. Adv Psychiatr Treat 9:202–210. [Google Scholar]
- Barbee JG, Jamhour NJ (2002) Lamotrigine as an augmentation agent in treatment-resistant depression. J Clin Psychiatry 63:737–741. [DOI] [PubMed] [Google Scholar]
- Barbee JG, Thompson TR, Jamhour NJ, Stewart JW, Conrad EJ, Reimherr FW, Thompson PM, Shelton RC (2011) A double-blind placebo-controlled trial of lamotrigine as an antidepressant augmentation agent in treatment-refractory unipolar depression. J Clin Psychiatry 72:1405–1412. [DOI] [PubMed] [Google Scholar]
- Barbosa L, Berk M, Vorster M (2003) A double-blind, randomized, placebo-controlled trial of augmentation with lamotrigine or placebo in patients concomitantly treated with fluoxetine for resistant major depressive episodes. J Clin Psychiatry 64:403–407. [DOI] [PubMed] [Google Scholar]
- Barbui C, Conti V, Cipriani A (2014) Antipsychotic drug exposure and risk of venous thromboembolism: a systematic review and meta-analysis of observational studies. Drug Saf 37:79–90. [DOI] [PubMed] [Google Scholar]
- Bauer M, Adli M, Ricken R, Severus E, Pilhatsch M (2014) Role of lithium augmentation in the management of major depressive disorder. CNS Drugs 28:331–342. [DOI] [PubMed] [Google Scholar]
- Bauer M, Baur H, Berghöfer A, Ströhle A, Hellweg R, Müller-Oerlinghausen B, Baumgartner A (2002a) Effects of supraphysiological thyroxine administration in healthy controls and patients with depressive disorders. J Affect Disord 68:285–294. [DOI] [PubMed] [Google Scholar]
- Bauer M, Bschor T, Kunz D, Berghöfer A, Ströhle A, Müller-Oerlinghausen B (2000) Double-blind, placebo-controlled trial of the use of lithium to augment antidepressant medication in continuation treatment of unipolar major depression. Am J Psychiatry 157:1429–1435. [DOI] [PubMed] [Google Scholar]
- Bauer M, Dell’osso L, Kasper S, Pitchot W, Dencker Vansvik E, Köhler J, Jørgensen L, Montgomery SA (2013a) Extended-release quetiapine fumarate (quetiapine XR) monotherapy and quetiapine XR or lithium as add-on to antidepressants in patients with treatment-resistant major depressive disorder. J Affect Disord 151:209–219. [DOI] [PubMed] [Google Scholar]
- Bauer M, Dopfmer S (1999) Lithium augmentation in treatment-resistant depression: meta-analysis of placebo-controlled studies. J Clin Psychopharmacol 19:427–434. [DOI] [PubMed] [Google Scholar]
- Bauer M, El-Khalili N, Datto C, Szamosi J, Eriksson H (2010) A pooled analysis of two randomised, placebo-controlled studies of extended release quetiapine fumarate adjunctive to antidepressant therapy in patients with major depressive disorder. J Affect Disord 127:19–30. [DOI] [PubMed] [Google Scholar]
- Bauer M, Hellweg R, Gräf KJ, Baumgartner A (1998) Treatment of refractory depression with high-dose thyroxine. Neuropsychopharmacology 18:444–455. [DOI] [PubMed] [Google Scholar]
- Bauer M, Pfennig A, Severus E, Whybrow PC, Angst J, Möller HJ; World Federation of Societies of Biological Psychiatry. Task Force on Unipolar Depressive Disorders (2013b) World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: update 2013 on the acute and continuation treatment of unipolar depressive disorders. World J Biol Psychiatry 14:334–385. [DOI] [PubMed] [Google Scholar]
- Bauer M, Severus E, Köhler S, Whybrow PC, Angst J, Möller HJ; Wfsbp Task Force on Treatment Guidelines for Unipolar Depressive Disorders (2015) World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders. part 2: maintenance treatment of major depressive disorder-update 2015. World J Biol Psychiatry 16:76–95. [DOI] [PubMed] [Google Scholar]
- Bauer M, Whybrow PC (2001) Thyroid hormone, neural tissue and mood modulation. World J Biol Psychiatry 2:59–69. [DOI] [PubMed] [Google Scholar]
- Bauer M, Whybrow PC, Angst J, Versiani M, Möller HJ; World Federation of Societies Biological Psychiatry Task Force on Treatment Guidelines for Unipolar Depressive Disorders (2002b) World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: acute and continuation treatment of major depressive disorder. World J Biol Psychiatry 3:5–43. [DOI] [PubMed] [Google Scholar]
- Baumann P, Nil R, Souche A, Montaldi S, Baettig D, Lambert S, Uehlinger C, Kasas A, Amey M, Jonzier-Perey M (1996) A double-blind, placebo-controlled study of citalopram with and without lithium in the treatment of therapy-resistant depressive patients: a clinical, pharmacokinetic, and pharmacogenetic investigation. J Clin Psychopharmacol 16:307–314. [DOI] [PubMed] [Google Scholar]
- Bayes AJ, Parker GB (2018) Comparison of guidelines for the treatment of unipolar depression: a focus on pharmacotherapy and neurostimulation. Acta Psychiatr Scand 137:459–471. [DOI] [PubMed] [Google Scholar]
- Bech P, Fava M, Trivedi MH, Wisniewski SR, Rush AJ (2012) Outcomes on the pharmacopsychometric triangle in bupropion-SR vs. buspirone augmentation of citalopram in the STAR*D trial. Acta Psychiatr Scand 125:342–348. [DOI] [PubMed] [Google Scholar]
- Bendz H, Schön S, Attman PO, Aurell M (2010) Renal failure occurs in chronic lithium treatment but is uncommon. Kidney Int 77:219–224. [DOI] [PubMed] [Google Scholar]
- Berger M, Riedel M, Tomova N, Obermeier M, Seemüller F, Dittmann S, Moeller HJ, Severus E (2013) Do current screening recommendations allow for early detection of lithium-induced hyperparathyroidism in patients with bipolar disorder? Int J Bipolar Disord 1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghöfer A, Grof P, Müller-Oerlinghausen B (2006) Recommendations for the safe use of lithium. In: Lithium in neuropsychiatry (Bauer M, Grof P, Muller-Oerlinghausen B, eds.) pp 463–484. London: CRC Press. [Google Scholar]
- Berigan TR. (2002) Bupropion-associated withdrawal symptoms revisited: a case report. Prim Care Companion J Clin Psychiatry 4:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berigan TR, Harazin JS (1999) Bupropion-associated withdrawal symptoms: a case report. Prim Care Companion J Clin Psychiatry 1:50–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Marcus RN, Swanink R, McQuade RD, Carson WH, Corey-Lisle PK, Khan A (2007) The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry 68:843–853. [DOI] [PubMed] [Google Scholar]
- Birkenhäger TK, van den Broek WW, Moleman P, Bruijn JA (2006) Outcome of a 4-step treatment algorithm for depressed inpatients. J Clin Psychiatry 67:1266–1271. [DOI] [PubMed] [Google Scholar]
- Bloch M, Schwartzman Y, Bonne O, Lerer B (1997) Concurrent treatment of nonresistant major depression with desipramine and lithium: a double-blind, placebo-controlled study. J Clin Psychopharmacol 17:44–48. [DOI] [PubMed] [Google Scholar]
- Bobes J, Garc A-Portilla MP, Rejas J, Hern Ndez G, Garcia-Garcia M, Rico-Villademoros F, Porras A (2003) Frequency of sexual dysfunction and other reproductive side-effects in patients with schizophrenia treated with risperidone, olanzapine, quetiapine, or haloperidol: the results of the EIRE study. J Sex Marital Ther 29:125–147. [DOI] [PubMed] [Google Scholar]
- Bodkin JA, Lasser RA, Wines JD Jr, Gardner DM, Baldessarini RJ (1997) Combining serotonin reuptake inhibitors and bupropion in partial responders to antidepressant monotherapy. J Clin Psychiatry 58:137–145. [DOI] [PubMed] [Google Scholar]
- Bouwer C, Stein DJ (1997) Buspirone is an effective augmenting agent of serotonin selective re-uptake inhibitors in severe treatment-refractory depression. S Afr Med J 87:534–547. [PubMed] [Google Scholar]
- Bowen RC, Grof P, Grof E (1991) Less frequent lithium administration and lower urine volume. Am J Psychiatry 148:189–192. [DOI] [PubMed] [Google Scholar]
- Boyer EW, Shannon M (2005) The serotonin syndrome. N Engl J Med 352:1112–1120. [DOI] [PubMed] [Google Scholar]
- Brandl EJ, Tiwari AK, Zhou X, Deluce J, Kennedy JL, Müller DJ, Richter MA (2014) Influence of CYP2D6 and CYP2C19 gene variants on antidepressant response in obsessive-compulsive disorder. Pharmacogenomics J 14:176–181. [DOI] [PubMed] [Google Scholar]
- Brosseau L, Rahman P, Toupin-April K, Poitras S, King J, De Angelis G, Loew L, Casimiro L, Paterson G, McEwan J (2014) A systematic critical appraisal for non-pharmacological management of osteoarthritis using the appraisal of guidelines research and evaluation II instrument. PLoS ONE 9 Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3888378/Accessed January 18, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna SE, Littlejohns P, Makarski J, Zitzelsberger L; AGREE Next Steps Consortium (2010) AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ 182:E839–E842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bschor T. (2014) Lithium in the treatment of major depressive disorder. Drugs 74:855–862. [DOI] [PubMed] [Google Scholar]
- Bschor T, Kunz D, Bauer M (1999) Successful lithium augmentation: how long should lithium be continued. Paper presented in XI World Congress of Psychiatry; August 6 -11, 1999; Hamburg, Germany.
- Bschor T, Berghöfer A, Ströhle A, Kunz D, Adli M, Müller-Oerlinghausen B, Bauer M (2002) How long should the lithium augmentation strategy be maintained? A 1-year follow-up of a placebo-controlled study in unipolar refractory major depression. J Clin Psychopharmacol 22:427–430. [DOI] [PubMed] [Google Scholar]
- Bschor T, Lewitzka U, Sasse J, Adli M, Köberle U, Bauer M (2003) Lithium augmentation in treatment-resistant depression: clinical evidence, serotonergic and endocrine mechanisms. Pharmacopsychiatry 36(Suppl 3):S230–S234. [DOI] [PubMed] [Google Scholar]
- Buckley NA, Faunce TA (2003) ‘Atypical’ antidepressants in overdose: clinical considerations with respect to safety. Drug Saf 26:539–551. [DOI] [PubMed] [Google Scholar]
- Burgess S, Geddes J, Hawton K, Townsend E, Jamison K, Goodwin G(2001) Lithium for maintenance treatment of mood disorders. Cochrane Database Syst Rev, CD003013. [DOI] [PubMed] [Google Scholar]
- Byerly MJ, Lescouflair E, Weber MT, Bugno RM, Fisher R, Carmody T, Varghese F, Rush AJ (2004) An open-label trial of quetiapine for antipsychotic-induced sexual dysfunction. J Sex Marital Ther 30:325–332. [DOI] [PubMed] [Google Scholar]
- Candy M, Jones L, Williams R, Tookman A, King M (2008) Psychostimulants for depression. Cochrane Database Syst Rev: CD006722. [DOI] [PubMed] [Google Scholar]
- Cappiello A, McDougle CJ, Delgado PL, Malison RT, Jatlow P, Charney DS, Heninger GR, Price LH (1998) Lithium and desipramine versus desipramine alone in the treatment of severe major depression: a preliminary study. Int Clin Psychopharmacol 13:191–198. [DOI] [PubMed] [Google Scholar]
- Castro VM, Clements CC, Murphy SN, Gainer VS, Fava M, Weilburg JB, Erb JL, Churchill SE, Kohane IS, Iosifescu DV, Smoller JW, Perlis RH (2013a) QT interval and antidepressant use: a cross sectional study of electronic health records. BMJ 346:f288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaggar PS, Shaw SM, Williams SG (2011) Effect of antipsychotic medications on glucose and lipid levels. J Clin Pharmacol 51:631–638. [DOI] [PubMed] [Google Scholar]
- Chen CY, Lin TY, Wang CC, Shuai HA (2011) Improvement of serum prolactin and sexual function after switching to aripiprazole from risperidone in schizophrenia: a case series. Psychiatry Clin Neurosci 65:95–97. [DOI] [PubMed] [Google Scholar]
- Chong SA, Mythily, Mahendran R (2001) Cardiac effects of psychotropic drugs. Ann Acad Med Singapore 30:625–631. [PubMed] [Google Scholar]
- Cipriani A, Smith K, Burgess S, Carney S, Goodwin G, Geddes J (2006) Lithium versus antidepressants in the long-term treatment of unipolar affective disorder. Cochrane Database Syst Rev: CD003492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani A, Hawton K, Stockton S, Geddes JR (2013) Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. Centre for Reviews and Dissemination (UK) https://www.ncbi.nlm.nih.gov/books/NBK153537/Accessed January 27, 2019. [DOI] [PubMed] [Google Scholar]
- Citrome L, Macher JP, Salazar DE, Mallikaarjun S, Boulton DW (2007) Pharmacokinetics of aripiprazole and concomitant carbamazepine. J Clin Psychopharmacol 27:279–283. [DOI] [PubMed] [Google Scholar]
- Clayton AH, Warnock JK, Kornstein SG, Pinkerton R, Sheldon-Keller A, McGarvey EL (2004) A placebo-controlled trial of bupropion SR as an antidote for selective serotonin reuptake inhibitor-induced sexual dysfunction. J Clin Psychiatry 65:62–67. [DOI] [PubMed] [Google Scholar]
- Cleare A, Pariante CM, Young AH, Anderson IM, Christmas D, Cowen PJ, Dickens C, Ferrier IN, Geddes J, Gilbody S, Haddad PM, Katona C, Lewis G, Malizia A, McAllister-Williams RH, Ramchandani P, Scott J, Taylor D, Uher R; Members of the Consensus Meeting (2015) Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2008 British Association for Psychopharmacology guidelines. J Psychopharmacol 29:459–525. [DOI] [PubMed] [Google Scholar]
- Cohen LS, Friedman JM, Jefferson JW, Johnson EM, Weiner ML (1994) A reevaluation of risk of in utero exposure to lithium. JAMA 271:146–150. [PubMed] [Google Scholar]
- Connolly KR, Thase ME (2011) If at first you don’t succeed: a review of the evidence for antidepressant augmentation, combination and switching strategies. Drugs 71:43–64. [DOI] [PubMed] [Google Scholar]
- Cooper-Kazaz R, Lerer B (2008) Efficacy and safety of triiodothyronine supplementation in patients with major depressive disorder treated with specific serotonin reuptake inhibitors. Int J Neuropsychopharmacol 11:685–699. [DOI] [PubMed] [Google Scholar]
- Coppen A, Standish-Barry H, Bailey J, Houston G, Silcocks P, Hermon C (1990) Long-term lithium and mortality. Lancet 335:1347. [DOI] [PubMed] [Google Scholar]
- Corya SA, Williamson D, Sanger TM, Briggs SD, Case M, Tollefson G (2006) A randomized, double-blind comparison of olanzapine/fluoxetine combination, olanzapine, fluoxetine, and venlafaxine in treatment-resistant depression. Depress Anxiety 23:364–372. [DOI] [PubMed] [Google Scholar]
- Coyle CM, Laws KR (2015) The use of ketamine as an antidepressant: a systematic review and meta-analysis. Hum Psychopharmacol 30:152–163. [DOI] [PubMed] [Google Scholar]
- Crawford P. (2002) Interactions between antiepileptic drugs and hormonal contraception. CNS Drugs 16:263–272. [DOI] [PubMed] [Google Scholar]
- Crossley NA, Bauer M (2007) Acceleration and augmentation of antidepressants with lithium for depressive disorders: two meta-analyses of randomized, placebo-controlled trials. J Clin Psychiatry 68:935–940. [DOI] [PubMed] [Google Scholar]
- Cullen M, Mitchell P, Brodaty H, Boyce P, Parker G, Hickie I, Wilhelm K (1991) Carbamazepine for treatment-resistant melancholia. J Clin Psychiatry 52:472–476. [PubMed] [Google Scholar]
- Curry SC, Kashani JS, LoVecchio F, Holubek W (2005) Intraventricular conduction delay after bupropion overdose. J Emerg Med 29:299–305. [DOI] [PubMed] [Google Scholar]
- Daly EJ, Singh JB, Fedgchin M, Cooper K, Lim P, Shelton RC, Thase ME, Winokur A, Van Nueten L, Manji H, Drevets WC (2018) Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry 75:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Guarda LA, Allen LG (2017) Liver injury associated with quetiapine: an illustrative case report. J Clin Psychopharmacol 37:623–625. [DOI] [PubMed] [Google Scholar]
- Datapharm Communications Ltd (2017) Electronic medicines compendium Available at: https://www.medicines.org.uk/emc/. Accessed October 10, 2019.
- Davis JM, Janicak PG, Hogan DM (1999) Mood stabilizers in the prevention of recurrent affective disorders: a meta-analysis. Acta Psychiatr Scand 100:406–417. [DOI] [PubMed] [Google Scholar]
- Davis LL, Kabel D, Patel D, Choate AD, Foslien-Nash C, Gurguis GN, Kramer GL, Petty F (1996) Valproate as an antidepressant in major depressive disorder. Psychopharmacol Bull 32:647–652. [PubMed] [Google Scholar]
- DeBattista C, Doghramji K, Menza MA, Rosenthal MH, Fieve RR; Modafinil in Depression Study Group (2003a) Adjunct modafinil for the short-term treatment of fatigue and sleepiness in patients with major depressive disorder: a preliminary double-blind, placebo-controlled study. J Clin Psychiatry 64:1057–1064. [DOI] [PubMed] [Google Scholar]
- DeBattista C, Doghramji K, Menza MA, Rosenthal MH, Fieve RR; Modafinil in Depression Study Group (2003b) Adjunct modafinil for the short-term treatment of fatigue and sleepiness in patients with major depressive disorder: a preliminary double-blind, placebo-controlled study. J Clin Psychiatry 64:1057–1064. [DOI] [PubMed] [Google Scholar]
- DeBattista C, Lembke A, Solvason HB, Ghebremichael R, Poirier J (2004) A prospective trial of modafinil as an adjunctive treatment of major depression. J Clin Psychopharmacol 24:87–90. [DOI] [PubMed] [Google Scholar]
- de Leon J, Wynn G, Sandson NB (2010) The pharmacokinetics of paliperidone versus risperidone. Psychosomatics 51:80–88. [DOI] [PubMed] [Google Scholar]
- Delgado PL, Price LH, Charney DS, Heninger GR (1988) Efficacy of fluvoxamine in treatment-refractory depression. J Affect Disord 15:55–60. [DOI] [PubMed] [Google Scholar]
- Diav-Citrin O, Shechtman S, Tahover E, Finkel-Pekarsky V, Arnon J, Kennedy D, Erebara A, Einarson A, Ornoy A (2014) Pregnancy outcome following in utero exposure to lithium: a prospective, comparative, observational study. Am J Psychiatry 171:785–794. [DOI] [PubMed] [Google Scholar]
- Dietrich DE, Emrich HM (1998) The use of anticonvulsants to augment antidepressant medication. J Clin Psychiatry 59(Suppl 5):51–58; discussion 59. [PubMed] [Google Scholar]
- Dimitriou EC, Dimitriou CE (1998) Buspirone augmentation of antidepressant therapy. J Clin Psychopharmacol 18:465–469. [DOI] [PubMed] [Google Scholar]
- Dodd S, Mitchell PB, Bauer M, Yatham L, Young AH, Kennedy SH, Williams L, Suppes T, Lopez Jaramillo C, Trivedi MH, Fava M, Rush AJ, McIntyre RS, Thase ME, Lam RW, Severus E, Kasper S, Berk M (2018) Monitoring for antidepressant-associated adverse events in the treatment of patients with major depressive disorder: an international consensus statement. World J Biol Psychiatry 19:330–348. [DOI] [PubMed] [Google Scholar]
- Dorée JP, Des Rosiers J, Lew V, Gendron A, Elie R, Stip E, Tourjman SV (2007) Quetiapine augmentation of treatment-resistant depression: a comparison with lithium. Curr Med Res Opin 23:333–341. [DOI] [PubMed] [Google Scholar]
- Dossenbach M, Dyachkova Y, Pirildar S, Anders M, Khalil A, Araszkiewicz A, Shakhnovich T, Akram A, Pecenak J, McBride M, Treuer T (2006) Effects of atypical and typical antipsychotic treatments on sexual function in patients with schizophrenia: 12-month results from the Intercontinental Schizophrenia Outpatient Health Outcomes (IC-SOHO) study. Eur Psychiatry 21:251–258. [DOI] [PubMed] [Google Scholar]
- Dubé S, Tollefson GD, Thase ME, Briggs SD, Van Campen LE, Case M, Tohen M (2007) Onset of antidepressant effect of olanzapine and olanzapine/fluoxetine combination in bipolar depression. Bipolar Disord 9:618–627. [DOI] [PubMed] [Google Scholar]
- Dunlop BW, Crits-Christoph P, Evans DL, Hirschowitz J, Solvason HB, Rickels K, Garlow SJ, Gallop RJ, Ninan PT (2007) Coadministration of modafinil and a selective serotonin reuptake inhibitor from the initiation of treatment of major depressive disorder with fatigue and sleepiness: a double-blind, placebo-controlled study. J Clin Psychopharmacol 27:614–619. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Rauhut AS, King-Pospisil KA, Bardo MT (2006) Review of the pharmacology and clinical profile of bupropion, an antidepressant and tobacco use cessation agent. CNS Drug Rev 12:178–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg MJ, Grandi SM, Gervais A, O’Loughlin J, Paradis G, Rinfret S, Sarrafzadegan N, Sharma S, Lauzon C, Yadav R, Pilote L; ZESCA Investigators (2013) Bupropion for smoking cessation in patients hospitalized with acute myocardial infarction: a randomized, placebo-controlled trial. J Am Coll Cardiol 61:524–532. [DOI] [PubMed] [Google Scholar]
- El-Khalili N, Joyce M, Atkinson S, Buynak RJ, Datto C, Lindgren P, Eriksson H (2010) Extended-release quetiapine fumarate (quetiapine XR) as adjunctive therapy in major depressive disorder (MDD) in patients with an inadequate response to ongoing antidepressant treatment: a multicentre, randomized, double-blind, placebo-controlled study. Int J Neuropsychopharmacol 13:917–932. [DOI] [PubMed] [Google Scholar]
- Fabrazzo M, Perris F, Monteleone P, Esposito G, Catapano F, Maj M (2012) Aripiprazole augmentation strategy in clomipramine-resistant depressive patients: an open preliminary study. Eur Neuropsychopharmacol 22:132–136. [DOI] [PubMed] [Google Scholar]
- Fang Y, Yuan C, Xu Y, Chen J, Wu Z, Cao L, Yi Z, Hong W, Wang Y, Jiang K, Cui X, Calabrese JR, Gao K; OPERATION Study Team (2011) A pilot study of the efficacy and safety of paroxetine augmented with risperidone, valproate, buspirone, trazodone, or thyroid hormone in adult Chinese patients with treatment-resistant major depression. J Clin Psychopharmacol 31:638–642. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Emamian ES, Kist DA (1999) Venlafaxine and bupropion combination therapy in a case of treatment-resistant depression. Ann Pharmacother 33:701–703. [DOI] [PubMed] [Google Scholar]
- Fava M, Davidson KG (1996) Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am 19:179–200. [DOI] [PubMed] [Google Scholar]
- Fava M, Mischoulon D, Iosifescu D, Witte J, Pencina M, Flynn M, Harper L, Levy M, Rickels K, Pollack M (2012) A double-blind, placebo-controlled study of aripiprazole adjunctive to antidepressant therapy among depressed outpatients with inadequate response to prior antidepressant therapy (ADAPT-A Study). Psychother Psychosom 81:87–97. [DOI] [PubMed] [Google Scholar]
- Fava M, Rosenbaum JF, McGrath PJ, Stewart JW, Amsterdam JD, Quitkin FM (1994) Lithium and tricyclic augmentation of fluoxetine treatment for resistant major depression: a double-blind, controlled study. Am J Psychiatry 151:1372–1374. [DOI] [PubMed] [Google Scholar]
- Fava M, Rush AJ, Thase ME, Clayton A, Stahl SM, Pradko JF, Johnston JA (2005a) 15 years of clinical experience with bupropion HCl: from bupropion to bupropion SR to bupropion XL. Prim Care Companion J Clin Psychiatry 7:106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M, Rush AJ, Wisniewski SR, Nierenberg AA, Alpert JE, McGrath PJ, Thase ME, Warden D, Biggs M, Luther JF, Niederehe G, Ritz L, Trivedi MH (2006) A comparison of mirtazapine and nortriptyline following two consecutive failed medication treatments for depressed outpatients: a STAR*D report. Am J Psychiatry 163:1161–1172. [DOI] [PubMed] [Google Scholar]
- Fava M, Thase ME, DeBattista C (2005b) A multicenter, placebo-controlled study of modafinil augmentation in partial responders to selective serotonin reuptake inhibitors with persistent fatigue and sleepiness. J Clin Psychiatry 66:85–93. [DOI] [PubMed] [Google Scholar]
- Fava M, Thase ME, DeBattista C, Doghramji K, Arora S, Hughes RJ (2007) Modafinil augmentation of selective serotonin reuptake inhibitor therapy in MDD partial responders with persistent fatigue and sleepiness. Ann Clin Psychiatry 19:153–159. [DOI] [PubMed] [Google Scholar]
- Fawcett J, Kravitz HM, Zajecka JM, Schaff MR (1991) CNS stimulant potentiation of monoamine oxidase inhibitors in treatment-refractory depression. J Clin Psychopharmacol 11:127–132. [PubMed] [Google Scholar]
- FDA (2019) February 12, 2019: Joint Meeting of the Psychopharmacologic Drugs Advisory Committee (PDAC) and the Drug Safety and Risk Management (DSaRM) Advisory Committee - 02/12/2019 - 02/12/2019. FDA; https://www.fda.gov/advisory-committees/february-12-2019-joint-meeting-psychopharmacologic-drugs-advisory-committee-pdac-and-drug-safety-and. Accessed April 17, 2020. [Google Scholar]
- Feighner JP, Herbstein J, Damlouji N (1985) Combined MAOI, TCA, and direct stimulant therapy of treatment-resistant depression. J Clin Psychiatry 46:206–209. [PubMed] [Google Scholar]
- Fekadu A, Wooderson SC, Markopoulo K, Donaldson C, Papadopoulos A, Cleare AJ (2009) What happens to patients with treatment-resistant depression? A systematic review of medium to long term outcome studies. J Affect Disord 116:4–11. [DOI] [PubMed] [Google Scholar]
- Fekadu A, Donocik JG, Cleare AJ (2018) Standardisation framework for the Maudsley staging method for treatment resistance in depression. BMC Psychiatry 18:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fond G, Loundou A, Rabu C, Macgregor A, Lançon C, Brittner M, Micoulaud-Franchi JA, Richieri R, Courtet P, Abbar M, Roger M, Leboyer M, Boyer L (2014a) Ketamine administration in depressive disorders: a systematic review and meta-analysis. Psychopharmacology (Berl) 231:3663–3676. [DOI] [PubMed] [Google Scholar]
- Fond G, Loundou A, Rabu C, Macgregor A, Lançon C, Brittner M, Micoulaud-Franchi JA, Richieri R, Courtet P, Abbar M, Roger M, Leboyer M, Boyer L (2014b) Ketamine administration in depressive disorders: a systematic review and meta-analysis. Psychopharmacology (Berl) 231:3663–3676. [DOI] [PubMed] [Google Scholar]
- Frye MA, Yatham L, Ketter TA, Goldberg J, Suppes T, Calabrese JR, Bowden CL, Bourne E, Bahn RS, Adams B (2009) Depressive relapse during lithium treatment associated with increased serum thyroid-stimulating hormone: results from two placebo-controlled bipolar I maintenance studies. Acta Psychiatr Scand 120:10–13. [DOI] [PubMed] [Google Scholar]
- Gao K, Kemp DE, Fein E, Wang Z, Fang Y, Ganocy SJ, Calabrese JR (2011) Number needed to treat to harm for discontinuation due to adverse events in the treatment of bipolar depression, major depressive disorder, and generalized anxiety disorder with atypical antipsychotics. J Clin Psychiatry 72:1063–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garakani A, Martinez JM, Marcus S, Weaver J, Rickels K, Fava M, Hirschowitz J (2008) A randomized, double-blind, and placebo-controlled trial of quetiapine augmentation of fluoxetine in major depressive disorder. Int Clin Psychopharmacol 23:269–275. [DOI] [PubMed] [Google Scholar]
- Gervasoni N, Aubry JM, Gex-Fabry M, Bertschy G, Bondolfi G (2009) Is there a place for tricyclic antidepressants and subsequent augmentation strategies in obtaining remission for patients with treatment resistant depression? Pharmacol Res 59:202–206. [DOI] [PubMed] [Google Scholar]
- Gill J, Singh H, Nugent K (2003) Acute lithium intoxication and neuroleptic malignant syndrome. Pharmacotherapy 23:811–815. [DOI] [PubMed] [Google Scholar]
- Gitlin M. (1999) Lithium and the kidney: an updated review. Drug Saf 20:231–243. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, et al. (2016) Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol 30:495–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss AJ, Kaser M, Costafreda SG, Sahakian BJ, Fu CH (2013) Modafinil augmentation therapy in unipolar and bipolar depression: a systematic review and meta-analysis of randomized controlled trials. J Clin Psychiatry 74:1101–1107. [DOI] [PubMed] [Google Scholar]
- Guzzetta F, Tondo L, Centorrino F, Baldessarini RJ (2007) Lithium treatment reduces suicide risk in recurrent major depressive disorder. J Clin Psychiatry 68:380–383. [DOI] [PubMed] [Google Scholar]
- Haddad PM, Anderson IM (2002) Antipsychotic-related QTc prolongation, torsade de pointes and sudden death. Drugs 62:1649–1671. [DOI] [PubMed] [Google Scholar]
- Haro JM, Novick D, Suarez D, Alonso J, Lépine JP, Ratcliffe M; SOHO Study Group (2006) Remission and relapse in the outpatient care of schizophrenia: three-year results from the Schizophrenia Outpatient Health Outcomes study. J Clin Psychopharmacol 26:571–578. [DOI] [PubMed] [Google Scholar]
- Hasan A, Bandelow B, Yatham LN, Berk M, Falkai P, Möller HJ, Kasper S; WFSBP Guideline Task Force Chairs (2019) WFSBP guidelines on how to grade treatment evidence for clinical guideline development. World J Biol Psychiatry 20:2–16. [DOI] [PubMed] [Google Scholar]
- Hellerstein DJ, Batchelder S, Hyler S, Arnaout B, Corpuz V, Coram L, Weiss G (2008) Aripiprazole as an adjunctive treatment for refractory unipolar depression. Prog Neuropsychopharmacol Biol Psychiatry 32:744–750. [DOI] [PubMed] [Google Scholar]
- Hennessy S, Bilker WB, Knauss JS, Margolis DJ, Kimmel SE, Reynolds RF, Glasser DB, Morrison MF, Strom BL (2002) Cardiac arrest and ventricular arrhythmia in patients taking antipsychotic drugs: cohort study using administrative data. BMJ 325:1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henssler J, Bschor T, Baethge C (2016) Combining antidepressants in acute treatment of depression: a meta-analysis of 38 studies including 4511 patients. Can J Psychiatry 61:29–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann-Eßer W, Siering U, Neugebauer EAM, Brockhaus AC, McGauran N, Eikermann M (2018) Guideline appraisal with AGREE II: online survey of the potential influence of AGREE II items on overall assessment of guideline quality and recommendation for use. BMC Health Serv Res 18:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead NA, Meints S, Middleton SK, Free CA, Hirsh AT (2015) Examining influential factors in providers’ chronic pain treatment decisions: a comparison of physicians and medical students. BMC Med Educ 15:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Stead LF, Hartmann-Boyce J, Cahill K, Lancaster T (2014) Antidepressants for smoking cessation. Cochrane Database Syst Rev CD000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley SC. (2002) Lamotrigine update and its use in mood disorders. Ann Pharmacother 36:860–873. [DOI] [PubMed] [Google Scholar]
- ICMJE (2019) International Committee of Medical Journal Editors | Recommendations Available at: http://www.icmje.org/recommendations/. Accessed April 23, 2020.
- Institute of Medicine (U.S.) , Graham R, eds. (2011) Clinical practice guidelines we can trust. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- Israel JA. (2015) Combining stimulants and monoamine oxidase inhibitors: a reexamination of the literature and a report of a new treatment combination. Prim Care Companion CNS Disord 17 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4805402/. Accessed May 31, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Januel D, Massot O, Poirier MF, Olié JP, Fillion G (2002) Interaction of lithium with 5-HT(1B) receptors in depressed unipolar patients treated with clomipramine and lithium versus clomipramine and placebo: preliminary results. Psychiatry Res 111:117–124. [DOI] [PubMed] [Google Scholar]
- Jensen HV, et al. (1992) Combining nortriptyline and lithium in elderly depressed patients: a double-blind, placebo-controlled study. Lithium 3:259–262. [Google Scholar]
- Joffe RT, Singer W (1990) A comparison of triiodothyronine and thyroxine in the potentiation of tricyclic antidepressants. Psychiatry Res 32:241–251. [DOI] [PubMed] [Google Scholar]
- Joffe RT, Singer W, Levitt AJ, MacDonald C (1993) A placebo-controlled comparison of lithium and triiodothyronine augmentation of tricyclic antidepressants in unipolar refractory depression. Arch Gen Psychiatry 50:387–393. [DOI] [PubMed] [Google Scholar]
- Joffe RT, Sokolov ST, Levitt AJ (2006) Lithium and triiodothyronine augmentation of antidepressants. Can J Psychiatry 51:791–793. [DOI] [PubMed] [Google Scholar]
- Johnston AM, Eagles JM (1999) Lithium-associated clinical hypothyroidism. Prevalence and risk factors. Br J Psychiatry 175:336–339. [DOI] [PubMed] [Google Scholar]
- Jon DI, Kim DH, Seo HJ, Kwon YJ, Kim MD, Yang JC, Suh HS, Min KJ, Pae CU, Bahk WM (2013) Augmentation of aripiprazole for depressed patients with an inadequate response to antidepressant treatment: a 6-week prospective, open-label, multicenter study. Clin Neuropharmacol 36:157–161. [DOI] [PubMed] [Google Scholar]
- Kate N, Grover S, Kumar S, Modi M (2013) Bupropion-induced hyponatremia. Gen Hosp Psychiatry 35:681.e11–681.e12. [DOI] [PubMed] [Google Scholar]
- Katona CL, Abou-Saleh MT, Harrison DA, Nairac BA, Edwards DR, Lock T, Burns RA, Robertson MM (1995) Placebo-controlled trial of lithium augmentation of fluoxetine and lofepramine. Br J Psychiatry 166:80–86. [DOI] [PubMed] [Google Scholar]
- Kawai N, Baba A, Suzuki T (2002) Risperidone failed to improve polydipsia-hyponatremia of the schizophrenic patients. Psychiatry Clin Neurosci 56:107–110. [DOI] [PubMed] [Google Scholar]
- Keitner GI, Garlow SJ, Ryan CE, Ninan PT, Solomon DA, Nemeroff CB, Keller MB (2009) A randomized, placebo-controlled trial of risperidone augmentation for patients with difficult-to-treat unipolar, non-psychotic major depression. J Psychiatr Res 43:205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DL, Conley RR (2006) A randomized double-blind 12-week study of quetiapine, risperidone or fluphenazine on sexual functioning in people with schizophrenia. Psychoneuroendocrinology 31:340–346. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Segal ZV, Cohen NL, Levitan RD, Gemar M, Bagby RM (2003) Lithium carbonate versus cognitive therapy as sequential combination treatment strategies in partial responders to antidepressant medication: an exploratory trial. J Clin Psychiatry 64:439–444. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Lam RW, McIntyre RS, Tourjman SV, Bhat V, Blier P, Hasnain M, Jollant F, Levitt AJ, MacQueen GM, McInerney SJ, McIntosh D, Milev RV, Müller DJ, Parikh SV, Pearson NL, Ravindran AV, Uher R; CANMAT Depression Work Group (2016) Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 3. Pharmacological treatments. Can J Psychiatry 61:540–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HR, Delva NJ, Lawson JS (1990) Prophylactic medication for unipolar depressive illness: the place of lithium carbonate in combination with antidepressant medication. Can J Psychiatry 35:107–114. [DOI] [PubMed] [Google Scholar]
- Kleber HD, et al. ; Work Group on Substance Use Disorders; American Psychiatric Association; Steering Committee on Practice Guidelines (2007) Treatment of patients with substance use disorders, second edition. American Psychiatric Association. Am J Psychiatry 164:5–123. [PubMed] [Google Scholar]
- Knegtering R, Castelein S, Bous H, Van Der Linde J, Bruggeman R, Kluiter H, van den Bosch RJ (2004) A randomized open-label study of the impact of quetiapine versus risperidone on sexual functioning. J Clin Psychopharmacol 24:56–61. [DOI] [PubMed] [Google Scholar]
- Komossa K, Depping AM, Gaudchau A, Kissling W, Leucht S (2010) Second-generation antipsychotics for major depressive disorder and dysthymia. Cochrane Database Syst Rev: CD008121. [DOI] [PubMed] [Google Scholar]
- Kuehn BM. (2008) FDA warns of adverse events linked to smoking cessation drug and antiepileptics. JAMA 299:1121–1122. [DOI] [PubMed] [Google Scholar]
- Lam RW, Hossie H, Solomons K, Yatham LN (2004) Citalopram and bupropion-SR: combining versus switching in patients with treatment-resistant depression. J Clin Psychiatry 65:337–340. [PubMed] [Google Scholar]
- Lam RW, Kennedy SH, Grigoriadis S, McIntyre RS, Milev R, Ramasubbu R, Parikh SV, Patten SB, Ravindran AV; Canadian Network for Mood and Anxiety Treatments (CANMAT) (2009) Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults. III. Pharmacotherapy. J Affect Disord 117(Suppl 1):S26–S43. [DOI] [PubMed] [Google Scholar]
- Landén M, Björling G, Agren H, Fahlén T (1998) A randomized, double-blind, placebo-controlled trial of buspirone in combination with an SSRI in patients with treatment-refractory depression. J Clin Psychiatry 59:664–668. [PubMed] [Google Scholar]
- Lara DR, Bisol LW, Munari LR (2013) Antidepressant, mood stabilizing and procognitive effects of very low dose sublingual ketamine in refractory unipolar and bipolar depression. Int J Neuropsychopharmacol 16:2111–2117. [DOI] [PubMed] [Google Scholar]
- Lauterbach E, Felber W, Müller-Oerlinghausen B, Ahrens B, Bronisch T, Meyer T, Kilb B, Lewitzka U, Hawellek B, Quante A, Richter K, Broocks A, Hohagen F (2008) Adjunctive lithium treatment in the prevention of suicidal behaviour in depressive disorders: a randomised, placebo-controlled, 1-year trial. Acta Psychiatr Scand 118:469–479. [DOI] [PubMed] [Google Scholar]
- Lavretsky H, Park S, Siddarth P, Kumar A, Reynolds CF 3rd (2006) Methylphenidate-enhanced antidepressant response to citalopram in the elderly: a double-blind, placebo-controlled pilot trial. Am J Geriatr Psychiatry 14:181–185. [DOI] [PubMed] [Google Scholar]
- Lepkifker E, Sverdlik A, Iancu I, Ziv R, Segev S, Kotler M (2004) Renal insufficiency in long-term lithium treatment. J Clin Psychiatry 65:850–856. [DOI] [PubMed] [Google Scholar]
- Leucht S, Busch R, Hamann J, Kissling W, Kane JM (2005) Early-onset hypothesis of antipsychotic drug action: a hypothesis tested, confirmed and extended. Biol Psychiatry 57:1543–1549. [DOI] [PubMed] [Google Scholar]
- Leucht S, Komossa K, Rummel-Kluge C, Corves C, Hunger H, Schmid F, Asenjo Lobos C, Schwarz S, Davis JM (2009) A meta-analysis of head-to-head comparisons of second-generation antipsychotics in the treatment of schizophrenia. Am J Psychiatry 166:152–163. [DOI] [PubMed] [Google Scholar]
- Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, Samara M, Barbui C, Engel RR, Geddes JR, Kissling W, Stapf MP, Lässig B, Salanti G, Davis JM (2013) Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 382:951–962. [DOI] [PubMed] [Google Scholar]
- Levinson DF, Devinsky O (1999) Psychiatric adverse events during vigabatrin therapy. Neurology 53:1503–1511. [DOI] [PubMed] [Google Scholar]
- Liebowitz M, Lam RW, Lepola U, Datto C, Sweitzer D, Eriksson H (2010) Efficacy and tolerability of extended release quetiapine fumarate monotherapy as maintenance treatment of major depressive disorder: a randomized, placebo-controlled trial. Depress Anxiety 27:964–976. [DOI] [PubMed] [Google Scholar]
- Liperoti R, Gambassi G, Lapane KL, Chiang C, Pedone C, Mor V, Bernabei R (2005) Conventional and atypical antipsychotics and the risk of hospitalization for ventricular arrhythmias or cardiac arrest. Arch Intern Med 165:696–701. [DOI] [PubMed] [Google Scholar]
- Littrell KH, Johnson CG, Littrell SH, Peabody CD (1997) Effects of olanzapine on polydipsia and intermittent hyponatremia. J Clin Psychiatry 58:549. [DOI] [PubMed] [Google Scholar]
- Livingstone C, Rampes H (2006) Lithium: a review of its metabolic adverse effects. J Psychopharmacol 20:347–355. [DOI] [PubMed] [Google Scholar]
- Ljubicic D, Letica-Crepulja M, Vitezic D, Bistrovic IL, Ljubicic R (2008) Lithium treatments: single and multiple daily dosing. Can J Psychiatry 53:323–331. [DOI] [PubMed] [Google Scholar]
- Łojko D, Rybakowski JK (2007) L-thyroxine augmentation of serotonergic antidepressants in female patients with refractory depression. J Affect Disord 103:253–256. [DOI] [PubMed] [Google Scholar]
- Loo CK, Gálvez V, O’Keefe E, Mitchell PB, Hadzi-Pavlovic D, Leyden J, Harper S, Somogyi AA, Lai R, Weickert CS, Glue P (2016) Placebo-controlled pilot trial testing dose titration and intravenous, intramuscular and subcutaneous routes for ketamine in depression. Acta Psychiatr Scand 134:48–56. [DOI] [PubMed] [Google Scholar]
- Luan S, Wan H, Wang S, Li H, Zhang B (2017) Efficacy and safety of olanzapine/fluoxetine combination in the treatment of treatment-resistant depression: a meta-analysis of randomized controlled trials. Neuropsychiatr Dis Treat 13:609–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhoo M, Keefe RS, Roth RM, Sambunaris A, Wu J, Trivedi MH, Anderson CS, Lasser R (2014) Lisdexamfetamine dimesylate augmentation in adults with persistent executive dysfunction after partial or full remission of major depressive disorder. Neuropsychopharmacology 39:1388–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahase E. (2019) Esketamine is approved in Europe for treating resistant major depressive disorder. BMJ 367 https://www.bmj.com/content/367/bmj.l7069. Accessed April 17, 2020. [DOI] [PubMed] [Google Scholar]
- Mahmoud RA, Pandina GJ, Turkoz I, Kosik-Gonzalez C, Canuso CM, Kujawa MJ, Gharabawi-Garibaldi GM (2007) Risperidone for treatment-refractory major depressive disorder: a randomized trial. Ann Intern Med 147:593–602. [DOI] [PubMed] [Google Scholar]
- Malhi GS, Tanious M, Gershon S (2011) The lithiumeter: a measured approach. Bipolar Disord 13:219–226. [DOI] [PubMed] [Google Scholar]
- Malhi GS, Tanious M, Das P, Berk M (2012) The science and practice of lithium therapy. Aust N Z J Psychiatry 46:192–211. [DOI] [PubMed] [Google Scholar]
- Malhi GS, Bassett D, Boyce P, Bryant R, Fitzgerald PB, Fritz K, Hopwood M, Lyndon B, Mulder R, Murray G, Porter R, Singh AB (2015) Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders. Aust N Z J Psychiatry 49:1087–1206. [DOI] [PubMed] [Google Scholar]
- Mallikaarjun S, Shoaf SE, Boulton DW, Bramer SL (2008) Effects of hepatic or renal impairment on the pharmacokinetics of aripiprazole. Clin Pharmacokinet 47:533–542. [DOI] [PubMed] [Google Scholar]
- Maneeton N, Maneeton B, Srisurapanont M, Martin SD (2012) Quetiapine monotherapy in acute phase for major depressive disorder: a meta-analysis of randomized, placebo-controlled trials. BMC Psychiatry 12:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus RN, McQuade RD, Carson WH, Hennicken D, Fava M, Simon JS, Trivedi MH, Thase ME, Berman RM (2008) The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a second multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol 28:156–165. [DOI] [PubMed] [Google Scholar]
- Marshall RD, Liebowitz MR (1996) Paroxetine/bupropion combination treatment for refractory depression. J Clin Psychopharmacol 16:80–81. [DOI] [PubMed] [Google Scholar]
- Marwick KF, Taylor M, Walker SW (2012) Antipsychotics and abnormal liver function tests: systematic review. Clin Neuropharmacol 35:244–253. [DOI] [PubMed] [Google Scholar]
- Marwood L, Taylor R, Goldsmith K, Romeo R, Holland R, Pickles A, Hutchinson J, Dietch D, Cipriani A, Nair R, Attenburrow MJ, Young AH, Geddes J, McAllister-Williams RH, Cleare AJ (2017) Study protocol for a randomised pragmatic trial comparing the clinical and cost effectiveness of lithium and quetiapine augmentation in treatment resistant depression (the LQD study). BMC Psychiatry 17:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masand PS, Anand VS, Tanquary JF (1998) Psychostimulant augmentation of second generation antidepressants: a case series. Depress Anxiety 7:89–91. [DOI] [PubMed] [Google Scholar]
- McAllister-Williams RH, Anderson IM, Finkelmeyer A, Gallagher P, Grunze HC, Haddad PM, Hughes T, Lloyd AJ, Mamasoula C, McColl E, Pearce S, Siddiqi N, Sinha BN, Steen N, Wainwright J, Winter FH, Ferrier IN, Watson S; ADD Study Team (2016) Antidepressant augmentation with metyrapone for treatment-resistant depression (the ADD study): a double-blind, randomised, placebo-controlled trial. Lancet Psychiatry 3:117–127. [DOI] [PubMed] [Google Scholar]
- McIntyre A, Gendron A, McIntyre A (2007) Quetiapine adjunct to selective serotonin reuptake inhibitors or venlafaxine in patients with major depression, comorbid anxiety, and residual depressive symptoms: a randomized, placebo-controlled pilot study. Depress Anxiety 24:487–494. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Filteau MJ, Martin L, Patry S, Carvalho A, Cha DS, Barakat M, Miguelez M (2014) Treatment-resistant depression: definitions, review of the evidence, and algorithmic approach. J Affect Disord 156:1–7. [DOI] [PubMed] [Google Scholar]
- McKnight RF, Adida M, Budge K, Stockton S, Goodwin GM, Geddes JR (2012) Lithium toxicity profile: a systematic review and meta-analysis. Lancet 379:721–728. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Hogue SL, Stang PE (2007) Once-daily bupropion associated with improved patient adherence compared with twice-daily bupropion in treatment of depression. Am J Ther 14:221–225. [DOI] [PubMed] [Google Scholar]
- Mercerolle M, Denooz R, Lachâtre G, Charlier C (2008) A fatal case of bupropion (Zyban) overdose. J Anal Toxicol 32:192–196. [DOI] [PubMed] [Google Scholar]
- Metzner JL, Dentino AN, Godard SL, Hay DP, Hay L, Linnoila M (1993) Impairment in driving and psychiatric illness. J Neuropsychiatry Clin Neurosci 5:211–220. [DOI] [PubMed] [Google Scholar]
- Meulendijks D, Mannesse CK, Jansen PA, van Marum RJ, Egberts TC (2010) Antipsychotic-induced hyponatraemia: a systematic review of the published evidence. Drug Saf 33:101–114. [DOI] [PubMed] [Google Scholar]
- Ministry of Health, Social Services and Equality. Galician Agency for Health, Technology Assessment (avalia-t), Working Group of the Clinical Practice Guideline on the Management of Depression in Adults (2014) Clinical practice guideline on the management of depression in adults Available at: http://content.guidelinecentral.com/guideline/get/pdf/2560. Accessed January 22, 2019.
- Mitchell AJ, Delaffon V, Vancampfort D, Correll CU, De Hert M (2012) Guideline concordant monitoring of metabolic risk in people treated with antipsychotic medication: systematic review and meta-analysis of screening practices. Psychol Med 42:125–147. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Plos Med 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montejo González AL, Rico-Villademoros F, Tafalla M, Majadas S; Spanish Working Group for the Study of Psychotropic-Related Sexual Dysfunction (2005) A 6-month prospective observational study on the effects of quetiapine on sexual functioning. J Clin Psychopharmacol 25:533–538. [DOI] [PubMed] [Google Scholar]
- Montgomery JH, Tekell JL (2003) Adjunctive quetiapine treatment of the polydipsia, intermittent hyponatremia, and psychosis syndrome: a case report. J Clin Psychiatry 64:339–341. [DOI] [PubMed] [Google Scholar]
- Montgomery S, Dell’Osso L, Kasper S, Pitchot E, Dencker-Vansvik E, Kohler J, Jorgensen L, Bauer M (2010) P01-75 - Quetiapine XR or lithium combination with antidepressants in treatment resistant depression. Eur Psychiatry 25:296. [Google Scholar]
- Morriss R, Benjamin B (2008) Lithium and eGFR: a new routinely available tool for the prevention of chronic kidney disease. Br J Psychiatry 193:93–95. [DOI] [PubMed] [Google Scholar]
- Mosolov SN, Kuzavkova MV, Uzbekov MG, Misionzhhnik EIu, Ryzhov AM (1997) A comparative clinico-pharmacokinetic study of the use of equivalent daily doses of lithium carbonate and Contemnol. Zh Nevrol Psikhiatr Im SS Korsakova 97:30–33. [PubMed] [Google Scholar]
- Müller-Oerlinghausen B. (1999) Evidence report: treatment of depression - newer pharmacotherapies. Agency for Health Care Policy and Research (AHCPR). Pharmacopsychiatry 32:230–231. [PubMed] [Google Scholar]
- Murray-Thomas T, Jones ME, Patel D, Brunner E, Shatapathy CC, Motsko S, Van Staa TP (2013) Risk of mortality (including sudden cardiac death) and major cardiovascular events in atypical and typical antipsychotic users: a study with the general practice research database. Cardiovasc Psychiatry Neurol 2013:247486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, Collins KA, Mathew SJ, Charney DS, Iosifescu DV (2013) Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry 74:250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Health and Clinical Excellence (NICE) (2009) The treatment and management of depression in adults (Updated Edition). https://www.nice.org.uk/guidance/cg90/chapter/1-guidance. Accessed November 30, 2018. [Google Scholar]
- Nelson JC. (2000) Augmentation strategies in depression 2000. J Clin Psychiatry 61(Suppl 2):13–19. [PubMed] [Google Scholar]
- Nelson JC, Baumann P, Delucchi K, Joffe R, Katona C (2014) A systematic review and meta-analysis of lithium augmentation of tricyclic and second generation antidepressants in major depression. J Affect Disord 168:269–275. [DOI] [PubMed] [Google Scholar]
- Nelson JC, Papakostas GI (2009) Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry 166:980–991. [DOI] [PubMed] [Google Scholar]
- Nguyen L, Marshalek PJ, Weaver CB, Cramer KJ, Pollard SE, Matsumoto RR (2015) Off-label use of transmucosal ketamine as a rapid-acting antidepressant: a retrospective chart review. Neuropsychiatr Dis Treat 11:2667–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICE (2020) Nasal spray medicine for treatment-resistant depression not recommended by NICE | News and features | News.National Institute for Health and Care Excellence; https://www.nice.org.uk/news/article/nasal-spray-medicine-for-treatment-resistant-depression-not-recommended-by-nice. Accessed April 3, 2020. [Google Scholar]
- Nierenberg AA, Papakostas GI, Petersen T, Montoya HD, Worthington JJ, Tedlow J, Alpert JE, Fava M (2003) Lithium augmentation of nortriptyline for subjects resistant to multiple antidepressants. J Clin Psychopharmacol 23:92–95. [DOI] [PubMed] [Google Scholar]
- Nierenberg AA, Fava M, Trivedi MH, Wisniewski SR, Thase ME, McGrath PJ, Alpert JE, Warden D, Luther JF, Niederehe G, Lebowitz B, Shores-Wilson K, Rush AJ (2006) A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry 163:1519–1530; quiz 1665. [DOI] [PubMed] [Google Scholar]
- Nikolova VL, Pattanaseri K, Hidalgo-Mazzei D, Taylor D, Young AH (2018) Is lithium monitoring NICE? Lithium monitoring in a UK secondary care setting. J Psychopharmacol 32:408–415. [DOI] [PubMed] [Google Scholar]
- Ninan PT, Hassman HA, Glass SJ, McManus FC (2004) Adjunctive modafinil at initiation of treatment with a selective serotonin reuptake inhibitor enhances the degree and onset of therapeutic effects in patients with major depressive disorder and fatigue. J Clin Psychiatry 65:414–420. [DOI] [PubMed] [Google Scholar]
- Normann C, Hummel B, Schärer LO, Hörn M, Grunze H, Walden J (2002) Lamotrigine as adjunct to paroxetine in acute depression: a placebo-controlled, double-blind study. J Clin Psychiatry 63:337–344. [DOI] [PubMed] [Google Scholar]
- Olofinjana B, Taylor D (2005) Antipsychotic drugs – information and choice: a patient survey. Psychiatr Bull 29:369–371. [Google Scholar]
- Osborn DP, Levy G, Nazareth I, Petersen I, Islam A, King MB (2007) Relative risk of cardiovascular and cancer mortality in people with severe mental illness from the United Kingdom’s General Practice Rsearch Database. Arch Gen Psychiatry 64:242–249. [DOI] [PubMed] [Google Scholar]
- Ostroff RB, Nelson JC (1999) Risperidone augmentation of selective serotonin reuptake inhibitors in major depression. J Clin Psychiatry 60:256–259. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Petersen TJ, Kinrys G, Burns AM, Worthington JJ, Alpert JE, Fava M, Nierenberg AA (2005) Aripiprazole augmentation of selective serotonin reuptake inhibitors for treatment-resistant major depressive disorder. J Clin Psychiatry 66:1326–1330. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Nutt DJ, Hallett LA, Tucker VL, Krishen A, Fava M (2006a) Resolution of sleepiness and fatigue in major depressive disorder: a comparison of bupropion and the selective serotonin reuptake inhibitors. Biol Psychiatry 60:1350–1355. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Worthington JJ 3rd, Iosifescu DV, Kinrys G, Burns AM, Fisher LB, Homberger CH, Mischoulon D, Fava M (2006b) The combination of duloxetine and bupropion for treatment-resistant major depressive disorder. Depress Anxiety 23:178–181. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Shelton RC, Smith J, Fava M (2007) Augmentation of antidepressants with atypical antipsychotic medications for treatment-resistant major depressive disorder: a meta-analysis. J Clin Psychiatry 68:826–831. [DOI] [PubMed] [Google Scholar]
- Paris PA, Saucier JR (1998) ECG conduction delays associated with massive bupropion overdose. J Toxicol Clin Toxicol 36:595–598. [DOI] [PubMed] [Google Scholar]
- Parker G, Brotchie H (2010) Do the old psychostimulant drugs have a role in managing treatment-resistant depression? Acta Psychiatr Scand 121:308–314. [DOI] [PubMed] [Google Scholar]
- Patkar AA, Masand PS, Pae CU, Peindl K, Hooper-Wood C, Mannelli P, Ciccone P (2006) A randomized, double-blind, placebo-controlled trial of augmentation with an extended release formulation of methylphenidate in outpatients with treatment-resistant depression. J Clin Psychopharmacol 26:653–656. [DOI] [PubMed] [Google Scholar]
- Patsalos PN, Fröscher W, Pisani F, van Rijn CM (2002) The importance of drug interactions in epilepsy therapy. Epilepsia 43:365–385. [DOI] [PubMed] [Google Scholar]
- Paykel ES. (2001) Continuation and maintenance therapy in depression. Br Med Bull 57:145–159. [DOI] [PubMed] [Google Scholar]
- Peluso MJ, Lewis SW, Barnes TR, Jones PB (2012) Extrapyramidal motor side-effects of first- and second-generation antipsychotic drugs. Br J Psychiatry 200:387–392. [DOI] [PubMed] [Google Scholar]
- Perez-Iglesias R, Mata I, Pelayo-Teran JM, Amado JA, Garcia-Unzueta MT, Berja A, Martinez-Garcia O, Vazquez-Barquero JL, Crespo-Facorro B (2009) Glucose and lipid disturbances after 1 year of antipsychotic treatment in a drug-naïve population. Schizophr Res 107:115–121. [DOI] [PubMed] [Google Scholar]
- Perry PJ, Zeilmann C, Arndt S (1994) Tricyclic antidepressant concentrations in plasma: an estimate of their sensitivity and specificity as a predictor of response. J Clin Psychopharmacol 14:230–240. [PubMed] [Google Scholar]
- Philip NS, Carpenter LL, Tyrka AR, Price LH (2008) Augmentation of antidepressants with atypical antipsychotics: a review of the current literature. J Psychiatr Pract 14:34–44. [DOI] [PubMed] [Google Scholar]
- Pierre JM, Gitlin MJ (2000) Bupropion-tranylcypromine combination for treatment-refractory depression. J Clin Psychiatry 61:450–451. [DOI] [PubMed] [Google Scholar]
- Preskorn SH. (2012) Clinically important differences in the pharmacokinetics of the ten newer “atypical” antipsychotics: part 1. J Psychiatr Pract 18:199–204. [DOI] [PubMed] [Google Scholar]
- Price RB, Nock MK, Charney DS, Mathew SJ (2009) Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry 66:522–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prien RF, Kupfer DJ, Mansky PA, Small JG, Tuason VB, Voss CB, Johnson WE (1984) Drug therapy in the prevention of recurrences in unipolar and bipolar affective disorders. Report of the NIMH Collaborative Study Group comparing lithium carbonate, imipramine, and a lithium carbonate-imipramine combination. Arch Gen Psychiatry 41:1096–1104. [DOI] [PubMed] [Google Scholar]
- Rapaport MH, Gharabawi GM, Canuso CM, Mahmoud RA, Keller MB, Bossie CA, Turkoz I, Lasser RA, Loescher A, Bouhours P, Dunbar F, Nemeroff CB (2006) Effects of risperidone augmentation in patients with treatment-resistant depression: results of open-label treatment followed by double-blind continuation. Neuropsychopharmacology 31:2505–2513. [DOI] [PubMed] [Google Scholar]
- Ravindran AV, Kennedy SH, O’Donovan MC, Fallu A, Camacho F, Binder CE (2008) Osmotic-release oral system methylphenidate augmentation of antidepressant monotherapy in major depressive disorder: results of a double-blind, randomized, placebo-controlled trial. J Clin Psychiatry 69:87–94. [DOI] [PubMed] [Google Scholar]
- Ray WA, Meredith S, Thapa PB, Meador KG, Hall K, Murray KT (2001) Antipsychotics and the risk of sudden cardiac death. Arch Gen Psychiatry 58:1161–1167. [DOI] [PubMed] [Google Scholar]
- Reeves H, Batra S, May RS, Zhang R, Dahl DC, Li X (2008) Efficacy of risperidone augmentation to antidepressants in the management of suicidality in major depressive disorder: a randomized, double-blind, placebo-controlled pilot study. J Clin Psychiatry 69:1228–1236. [DOI] [PubMed] [Google Scholar]
- Reilly JG, Ayis SA, Ferrier IN, Jones SJ, Thomas SH (2000) QTc-interval abnormalities and psychotropic drug therapy in psychiatric patients. Lancet 355:1048–1052. [DOI] [PubMed] [Google Scholar]
- Reilly JG, Ayis SA, Ferrier IN, Jones SJ, Thomas SH (2002) Thioridazine and sudden unexplained death in psychiatric in-patients. Br J Psychiatry 180:515–522. [DOI] [PubMed] [Google Scholar]
- Remington G, Kwon J, Collins A, Laporte D, Mann S, Christensen B (2007) The use of electronic monitoring (MEMS) to evaluate antipsychotic compliance in outpatients with schizophrenia. Schizophr Res 90:229–237. [DOI] [PubMed] [Google Scholar]
- Robinson DG, Schooler NR, Correll CU, John M, Kurian BT, Marcy P, Miller AL, Pipes R, Trivedi MH, Kane JM (2018) Psychopharmacological treatment in the RAISE-ETP study: outcomes of a manual and computer decision support system based intervention. Am J Psychiatry 175:169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roose SP, Miyazaki M (2005) Pharmacologic treatment of depression in patients with heart disease. Psychosom Med 67(Suppl 1):S54–S57. [DOI] [PubMed] [Google Scholar]
- Rosenthal LJ, Goldner WS, O’Reardon JP (2011) T3 augmentation in major depressive disorder: safety considerations. Am J Psychiatry 168:1035–1040. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH (1996) The inventory of depressive symptomatology (IDS): psychometric properties. Psychol Med 26:477–486. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi M, Fava M (2003) Depression, IV: STAR*D treatment trial for depression. Am J Psychiatry 160:237. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M (2006) Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 163:1905–1917. [DOI] [PubMed] [Google Scholar]
- Sackeim HA, Haskett RF, Mulsant BH, Thase ME, Mann JJ, Pettinati HM, Greenberg RM, Crowe RR, Cooper TB, Prudic J (2001) Continuation pharmacotherapy in the prevention of relapse following electroconvulsive therapy: a randomized controlled trial. JAMA 285:1299–1307. [DOI] [PubMed] [Google Scholar]
- Santos MA, Rocha FL, Hara C (2008) Efficacy and safety of antidepressant augmentation with lamotrigine in patients with treatment-resistant depression: a randomized, placebo-controlled, double-blind study. Prim Care Companion J Clin Psychiatry 10:187–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler F, Anghelescu IG (2007a) Lithium versus lamotrigine augmentation in treatment resistant unipolar depression: a randomized, open-label study. Int Clin Psychopharmacol 22:179–182. [DOI] [PubMed] [Google Scholar]
- Schindler F, Anghelescu IG (2007b) Lithium versus lamotrigine augmentation in treatment resistant unipolar depression: a randomized, open-label study. Int Clin Psychopharmacol 22:179–182. [DOI] [PubMed] [Google Scholar]
- Schmitz B. (2002) Antidepressant drugs: indications and guidelines for use in epilepsy. Epilepsia 43(Suppl 2):14–18. [DOI] [PubMed] [Google Scholar]
- Schou M. (1989) Lithium prophylaxis: myths and realities. Am J Psychiatry 146:573–576. [DOI] [PubMed] [Google Scholar]
- Schou M. (1997) Forty years of lithium treatment. Arch Gen Psychiatry 54:9–13; discussion 14. [DOI] [PubMed] [Google Scholar]
- Schou M. (2000) Suicidal behavior and prophylactic lithium treatment of major mood disorders: a review of reviews. Suicide Life Threat Behav 30:289–293. [PubMed] [Google Scholar]
- Schulz KF, Altman DG, Moher D; CONSORT Group (2010) CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Bmj 340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz TL, Azhar N, Cole K, Hopkins G, Nihalani N, Simionescu M, Husain J, Jones N (2004) An open-label study of adjunctive modafinil in patients with sedation related to serotonergic antidepressant therapy. J Clin Psychiatry 65:1223–1227. [DOI] [PubMed] [Google Scholar]
- Scottish Intercollegiate Guidelines Network (SIGN) (2012) Methodology checklist 2: controlled trials [Internet] http://www.sign.ac.uk. https://www.sign.ac.uk/checklists-and-notes.html. Accessed October 11, 2018.
- Serafini G, Howland RH, Rovedi F, Girardi P, Amore M (2014a) The role of ketamine in treatment-resistant depression: a systematic review. Curr Neuropharmacol 12:444–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini G, Howland RH, Rovedi F, Girardi P, Amore M (2014b) The role of ketamine in treatment-resistant depression: a systematic review. Curr Neuropharmacol 12:444–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serretti A, Chiesa A (2011) Sexual side effects of pharmacological treatment of psychiatric diseases. Clin Pharmacol Ther 89:142–147. [DOI] [PubMed] [Google Scholar]
- Severus E, Bauer M (2013) Managing the risk of lithium-induced nephropathy in the long-term treatment of patients with recurrent affective disorders. BMC Med 11:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahal B, Piel E, Mecz L, Kremer I, Klein E (1996) Lack of advantage for imipramine combined with lithium versus imipramine alone in the treatment of major depression–a double-blind controlled study. Biol Psychiatry 40:1181–1183. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Tollefson GD, Tohen M, Stahl S, Gannon KS, Jacobs TG, Buras WR, Bymaster FP, Zhang W, Spencer KA, Feldman PD, Meltzer HY (2001) A novel augmentation strategy for treating resistant major depression. Am J Psychiatry 158:131–134. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Williamson DJ, Corya SA, Sanger TM, Van Campen LE, Case M, Briggs SD, Tollefson GD (2005) Olanzapine/fluoxetine combination for treatment-resistant depression: a controlled study of SSRI and nortriptyline resistance. J Clin Psychiatry 66:1289–1297. [DOI] [PubMed] [Google Scholar]
- Shine B, McKnight RF, Leaver L, Geddes JR (2015) Long-term effects of lithium on renal, thyroid, and parathyroid function: a retrospective analysis of laboratory data. Lancet 386:461–468. [DOI] [PubMed] [Google Scholar]
- Short B, Fong J, Galvez V, Shelker W, Loo CK (2018) Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry 5:65–78. [DOI] [PubMed] [Google Scholar]
- Simon JS, Nemeroff CB (2005) Aripiprazole augmentation of antidepressants for the treatment of partially responding and nonresponding patients with major depressive disorder. J Clin Psychiatry 66:1216–1220. [DOI] [PubMed] [Google Scholar]
- Smith RC, Lindenmayer JP, Hu Q, Kelly E, Viviano TF, Cornwell J, Vaidhyanathaswamy S, Marcovina S, Davis JM (2010) Effects of olanzapine and risperidone on lipid metabolism in chronic schizophrenic patients with long-term antipsychotic treatment: a randomized five month study. Schizophr Res 120:204–209. [DOI] [PubMed] [Google Scholar]
- Song ZW, Liu XB, Li YD (2007) Venlafaxine combined with low-dose risperidone for treatment resistant depression. J Clin Rehab Tis Eng Res11. [Google Scholar]
- Souza FG, Goodwin GM (1991) Lithium treatment and prophylaxis in unipolar depression: a meta-analysis. Br J Psychiatry 158:666–675. [DOI] [PubMed] [Google Scholar]
- Spielmans GI, Berman MI, Linardatos E, Rosenlicht NZ, Perry A, Tsai AC (2013) Adjunctive atypical antipsychotic treatment for major depressive disorder: a meta-analysis of depression, quality of life, and safety outcomes. Plos Med 10:e1001403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spier SA. (1998) Use of bupropion with SRIs and venlafaxine. Depress Anxiety 7:73–75. [PubMed] [Google Scholar]
- Spina E, Perucca E (2002) Clinical significance of pharmacokinetic interactions between antiepileptic and psychotropic drugs. Epilepsia 43(Suppl 2):37–44. [DOI] [PubMed] [Google Scholar]
- Spina E, Santoro V, D’Arrigo C (2008) Clinically relevant pharmacokinetic drug interactions with second-generation antidepressants: an update. Clin Ther 30:1206–1227. [DOI] [PubMed] [Google Scholar]
- Spina E, Trifirò G, Caraci F (2012) Clinically significant drug interactions with newer antidepressants. CNS Drugs 26:39–67. [DOI] [PubMed] [Google Scholar]
- Stahl SM, Felker A (2008) Monoamine oxidase inhibitors: a modern guide to an unrequited class of antidepressants. CNS Spectr 13:855–870. [DOI] [PubMed] [Google Scholar]
- Stein G, Bernadt M (1993) Lithium augmentation therapy in tricyclic-resistant depression. A controlled trial using lithium in low and normal doses. Br J Psychiatry 162:634–640. [DOI] [PubMed] [Google Scholar]
- Sternbach H. (1991) The serotonin syndrome. Am J Psychiatry 148:705–713. [DOI] [PubMed] [Google Scholar]
- Stoll AL, Haura G (2000) Tranylcypromine plus risperidone for treatment-refractory major depression. J Clin Psychopharmacol 20:495–496. [DOI] [PubMed] [Google Scholar]
- Straus SM, Bleumink GS, Dieleman JP, van der Lei J, ‘t Jong GW, Kingma JH, Sturkenboom MC, Stricker BH (2004) Antipsychotics and the risk of sudden cardiac death. Arch Intern Med 164:1293–1297. [DOI] [PubMed] [Google Scholar]
- Strawbridge R, Carter B, Marwood L, Bandelow B, Tsapekos D, Nikolova VL, Taylor R, Mantingh T, de Angel V, Patrick F, Cleare AJ, Young AH (2019) Augmentation therapies for treatment-resistant depression: systematic review and meta-analysis. Br J Psychiatry 214:42–51. [DOI] [PubMed] [Google Scholar]
- Stroup TS, Lieberman JA, McEvoy JP, Swartz MS, Davis SM, Rosenheck RA, Perkins DO, Keefe RS, Davis CE, Severe J, Hsiao JK; CATIE Investigators (2006) Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatry 163:611–622. [DOI] [PubMed] [Google Scholar]
- Stroup TS, Lieberman JA, McEvoy JP, Davis SM, Swartz MS, Keefe RS, Miller AL, Rosenheck RA, Hsiao JK; CATIE Investigators (2009) Results of phase 3 of the CATIE schizophrenia trial. Schizophr Res 107:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subotnik KL, Casaus LR, Ventura J, Luo JS, Hellemann GS, Gretchen-Doorly D, Marder S, Nuechterlein KH (2015) Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry 72:822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suehs BT, Argo TR, Bendele SD, Crismon ML, Trivedi MH, Kurian B(2008) Texas medication algorithm project procedural manual: major depressive disorder algorithms. Austin, TX: The Texas Department of State Health Services. [Google Scholar]
- Sugawara H, Sakamoto K, Harada T, Ishigooka J (2010) Predictors of efficacy in lithium augmentation for treatment-resistant depression. J Affect Disord 125:165–168. [DOI] [PubMed] [Google Scholar]
- Szymkowicz SM, Finnegan N, Dale RM (2013) A 12-month naturalistic observation of three patients receiving repeat intravenous ketamine infusions for their treatment-resistant depression. J Affect Disord 147:416–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ta Ketter P. (1997) Addition of monoamine oxidase inhibitors to carbamazepine: preliminary evidence of safety and antidepressant efficacy in treatment-resistant depression. Year B Psychiatry Appl Ment Health. Available at: https://insights.ovid.com/year-book-psychiatry-applied-mental-health/ybps/1997/00/100/addition-monoamine-oxidase-inhibitors/23/00062674. Accessed February 5, 2019. [PubMed] [Google Scholar]
- Takahashi H, Kamata M, Yoshida K, Higuchi H, Ishigooka J (2008) Augmentation with olanzapine in TCA-refractory depression with melancholic features: a consecutive case series. Hum Psychopharmacol 23:217–220. [DOI] [PubMed] [Google Scholar]
- Taneja I, Haman K, Shelton RC, Robertson D (2007) A randomized, double-blind, crossover trial of modafinil on mood. J Clin Psychopharmacol 27:76–79. [DOI] [PubMed] [Google Scholar]
- Taylor DM, Paton C, Kapur S(2015) The Maudsley prescribing guidelines in psychiatry, 12th Edition. Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- Taylor D, Barnes T, Young A (2018) The Maudsley prescribing guidelines in psychiatry. 13th ed. Hoboken, NJ: Wiley-Blackwell. [Google Scholar]
- Taylor RW, Marwood L, Greer B, Strawbridge R, Cleare AJ (2019) Predictors of response to augmentation treatment in patients with treatment-resistant depression: a systematic review. J Psychopharmacol (Oxf) 33:1323–1339. doi: 10.1177/0269881119872194. [DOI] [PubMed] [Google Scholar]
- Thase ME, Corya SA, Osuntokun O, Case M, Henley DB, Sanger TM, Watson SB, Dubé S (2007a) A randomized, double-blind comparison of olanzapine/fluoxetine combination, olanzapine, and fluoxetine in treatment-resistant major depressive disorder. J Clin Psychiatry 68:224–236. [DOI] [PubMed] [Google Scholar]
- Thase ME, Friedman ES, Biggs MM, Wisniewski SR, Trivedi MH, Luther JF, Fava M, Nierenberg AA, McGrath PJ, Warden D, Niederehe G, Hollon SD, Rush AJ (2007b) Cognitive therapy versus medication in augmentation and switch strategies as second-step treatments: a STAR*D report. Am J Psychiatry 164:739–752. [DOI] [PubMed] [Google Scholar]
- Thase ME, Rush AJ (1997) When at first you don’t succeed: sequential strategies for antidepressant nonresponders. J Clin Psychiatry 58(Suppl 13):23–29. [PubMed] [Google Scholar]
- Thase ME, Youakim JM, Skuban A, Hobart M, Augustine C, Zhang P, McQuade RD, Carson WH, Nyilas M, Sanchez R, Eriksson H (2015a) Efficacy and safety of adjunctive brexpiprazole 2 mg in major depressive disorder: a phase 3, randomized, placebo-controlled study in patients with inadequate response to antidepressants. J Clin Psychiatry 76:1224–1231. [DOI] [PubMed] [Google Scholar]
- Thase ME, Youakim JM, Skuban A, Hobart M, Zhang P, McQuade RD, Nyilas M, Carson WH, Sanchez R, Eriksson H (2015b) Adjunctive brexpiprazole 1 and 3 mg for patients with major depressive disorder following inadequate response to antidepressants: a phase 3, randomized, double-blind study. J Clin Psychiatry 76:1232–1240. [DOI] [PubMed] [Google Scholar]
- Thies-Flechtner K, Müller-Oerlinghausen B, Seibert W, Walther A, Greil W (1996) Effect of prophylactic treatment on suicide risk in patients with major affective disorders. Data from a randomized prospective trial. Pharmacopsychiatry 29:103–107. [DOI] [PubMed] [Google Scholar]
- Tohen M, McDonnell DP, Case M, Kanba S, Ha K, Fang YR, Katagiri H, Gomez JC (2012) Randomised, double-blind, placebo-controlled study of olanzapine in patients with bipolar I depression. Br J Psychiatry 201:376–382. [DOI] [PubMed] [Google Scholar]
- Tondo L, Jamison KR, Baldessarini RJ (1997) Effect of lithium maintenance on suicidal behavior in major mood disorders. Ann N Y Acad Sci 836:339–351. [DOI] [PubMed] [Google Scholar]
- Trangle M, Gursky J, Haight R, Hardwig J, Hinnenkamp T, Kessler D, Mack N, Myszkowski M (2016) Adult Depression in Primary Care. Bloomington, MN: Institute for Clinical Systems Improvement.
- Trivedi MH, Fava M, Wisniewski SR, Thase ME, Quitkin F, Warden D, Ritz L, Nierenberg AA, Lebowitz BD, Biggs MM, Luther JF, Shores-Wilson K, Rush AJ; STAR*D Study Team (2006a) Medication augmentation after the failure of SSRIs for depression. N Engl J Med 354:1243–1252. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Fava M, Wisniewski SR, Thase ME, Quitkin F, Warden D, Ritz L, Nierenberg AA, Lebowitz BD, Biggs MM, Luther JF, Shores-Wilson K, Rush AJ; STAR*D Study Team (2006b) Medication augmentation after the failure of SSRIs for depression. N Engl J Med 354:1243–1252. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Thase ME, Osuntokun O, Henley DB, Case M, Watson SB, Campbell GM, Corya SA (2009) An integrated analysis of olanzapine/fluoxetine combination in clinical trials of treatment-resistant depression. J Clin Psychiatry 70:387–396. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Cutler AJ, Richards C, Lasser R, Geibel BB, Gao J, Sambunaris A, Patkar AA (2013) A randomized controlled trial of the efficacy and safety of lisdexamfetamine dimesylate as augmentation therapy in adults with residual symptoms of major depressive disorder after treatment with escitalopram. J Clin Psychiatry 74:802–809. [DOI] [PubMed] [Google Scholar]
- Truven Health Analytics (2018) Micromedix software product http://truvenhealth.com/products/micromedex. Accessed October 10, 2019.
- Turner P, Kantaria R, Young AH (2014) A systematic review and meta-analysis of the evidence base for add-on treatment for patients with major depressive disorder who have not responded to antidepressant treatment: a European perspective. J Psychopharmacol 28:85–98. [DOI] [PubMed] [Google Scholar]
- van Gerven HA, Boer WH (2006) Chronic renal function disorders during lithium use. Ned Tijdschr Geneeskd 150:1715–1718. [PubMed] [Google Scholar]
- Verdolini N, Hidalgo-Mazzei D, Murru A, Pacchiarotti I, Samalin L, Young AH, Vieta E, Carvalho AF (2018) Mixed states in bipolar and major depressive disorders: systematic review and quality appraisal of guidelines. Acta Psychiatr Scand 138:196–222. [DOI] [PubMed] [Google Scholar]
- Vieta E, Bauer M, Montgomery S, McIntyre RS, Szamosi J, Earley WR, Eriksson H (2013) Pooled analysis of sustained response rates for extended release quetiapine fumarate as monotherapy or adjunct to antidepressant therapy in patients with major depressive disorder. J Affect Disord 150:639–643. [DOI] [PubMed] [Google Scholar]
- Vrignaud L, Aouille J, Mallaret M, Durrieu G, Jonville-Béra AP (2014) Hypersexuality associated with aripiprazole: a new case and review of the literature. Therapie 69:525–527. [DOI] [PubMed] [Google Scholar]
- Walker PW, Cole JO, Gardner EA, Hughes AR, Johnston JA, Batey SR, Lineberry CG (1993) Improvement in fluoxetine-associated sexual dysfunction in patients switched to bupropion. J Clin Psychiatry 54:459–465. [PubMed] [Google Scholar]
- Wan LB, Levitch CF, Perez AM, Brallier JW, Iosifescu DV, Chang LC, Foulkes A, Mathew SJ, Charney DS, Murrough JW (2015) Ketamine safety and tolerability in clinical trials for treatment-resistant depression. J Clin Psychiatry 76:247–252. [DOI] [PubMed] [Google Scholar]
- Wen XJ, Wang LM, Liu ZL, Huang A, Liu YY, Hu JY (2014) Meta-analysis on the efficacy and tolerability of the augmentation of antidepressants with atypical antipsychotics in patients with major depressive disorder. Braz J Med Biol Res 47:605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman PD, Peck AW, Fowle AS, Smith P (1982) Bupropion fails to affect plasma prolactin and growth hormone in normal subjects. Br J Clin Pharmacol 13:743–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickstrøm E, Amrein R, Haefelfinger P, Hartmann D (1980) Pharmacokinetic and clinical observations on prolonged administration of flunitrazepam. Eur J Clin Pharmacol 17:189–196. [DOI] [PubMed] [Google Scholar]
- Wilting I, Movig KL, Moolenaar M, Hekster YA, Brouwers JR, Heerdink ER, Nolen WA, Egberts AC (2005) Drug-drug interactions as a determinant of elevated lithium serum levels in daily clinical practice. Bipolar Disord 7:274–280. [DOI] [PubMed] [Google Scholar]
- Wooderson SC, Fekadu A, Markopoulou K, Rane LJ, Poon L, Juruena MF, Strawbridge R, Cleare AJ (2014) Long-term symptomatic and functional outcome following an intensive inpatient multidisciplinary intervention for treatment-resistant affective disorders. J Affect Disord 166:334–342. [DOI] [PubMed] [Google Scholar]
- Wright BM, Eiland EH 3rd, Lorenz R (2013) Augmentation with atypical antipsychotics for depression: a review of evidence-based support from the medical literature. Pharmacotherapy 33:344–359. [DOI] [PubMed] [Google Scholar]
- Yoshimura R, Umene-Nakano W, Ueda N, Ikenouchi-Sugita A, Hori H, Nakamura J (2008) Addition of risperidone to sertraline improves sertraline-resistant refractory depression without influencing plasma concentrations of sertraline and desmethylsertraline. Hum Psychopharmacol 23:707–713. [DOI] [PubMed] [Google Scholar]
- Yoshimura R, Kishi T, Hori H, Ikenouchi-Sugita A, Katsuki A, Umene-Nakano W, Iwata N, Nakamura J (2012) Comparison of the efficacy between paroxetine and sertraline augmented with aripiprazole in patients with refractory major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 39:355–357. [DOI] [PubMed] [Google Scholar]
- Young AH. (2017) Lithium for long-term treatment of unipolar depression. Lancet Psychiatry 4:511–512. [DOI] [PubMed] [Google Scholar]
- Zarate CA Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, Selter J, Marquardt CA, Liberty V, Luckenbaugh DA (2012) Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry 71:939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JP, Gallego JA, Robinson DG, Malhotra AK, Kane JM, Correll CU (2013) Efficacy and safety of individual second-generation vs. first-generation antipsychotics in first-episode psychosis: a systematic review and meta-analysis. Int J Neuropsychopharmacol 16:1205–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Dong L, Shao F, Tan X, Ying K (2011) Antipsychotics and venous thromboembolism risk: a meta-analysis. Pharmacopsychiatry 44:183–188. [DOI] [PubMed] [Google Scholar]
- Zhang ZJ, Tan QR, Tong Y, Li Q, Kang WH, Zhen XC, Post RM (2008) The effectiveness of carbamazepine in unipolar depression: a double-blind, randomized, placebo-controlled study. J Affect Disord 109:91–97. [DOI] [PubMed] [Google Scholar]
- Zhou X, Ravindran AV, Qin B, Del Giovane C, Li Q, Bauer M, Liu Y, Fang Y, da Silva T, Zhang Y, Fang L, Wang X, Xie P (2015) Comparative efficacy, acceptability, and tolerability of augmentation agents in treatment-resistant depression: systematic review and network meta-analysis. J Clin Psychiatry 76:e487–e498. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Kolawole T, Jimenez XF (2016) Atypical findings in massive bupropion overdose: a case report and discussion of psychopharmacologic issues. J Psychiatr Pract 22:405–409. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Krause M, Huhn M, Rothe P, Schneider-Thoma J, Chaimani A, Li C, Davis JM, Leucht S (2017) Antipsychotic drugs for the acute treatment of patients with a first episode of schizophrenia: a systematic review with pairwise and network meta-analyses. Lancet Psychiatry 4:694–705. [DOI] [PubMed] [Google Scholar]
- Zisook S, Kasckow JW, Lanouette NM, Golshan S, Fellows I, Vahia I, Mohamed S, Rao S (2010) Augmentation with citalopram for suicidal ideation in middle-aged and older outpatients with schizophrenia and schizoaffective disorder who have subthreshold depressive symptoms: a randomized controlled trial. J Clin Psychiatry 71:915–922. [DOI] [PubMed] [Google Scholar]
- Zullino D, Baumann P (2001) Lithium augmentation in depressive patients not responding to selective serotonin reuptake inhibitors. Pharmacopsychiatry 34:119–127. [DOI] [PubMed] [Google Scholar]
- Zusky PM, Biederman J, Rosenbaum JF, Manschreck TC, Gross CC, Weilberg JB, Gastfriend DR (1988) Adjunct low dose lithium carbonate in treatment-resistant depression: a placebo-controlled study. J Clin Psychopharmacol 8:120–124. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.