Abstract
Background
A core outcome set for studies on cardiac disease in pregnancy is being developed. Incorporating perspectives of patients and health care providers (HCPs) is an essential step in developing this core outcome set, and eliciting these outcomes is the objective of this study.
Methods
We interviewed pregnant women with heart disease, family members, and HCPs, until data saturation was attained. Participants were asked to share experiences and perspectives, and comment on outcomes they deemed important. Interviews were recorded and transcribed verbatim, and interpretive analysis was used to translate experiences into measurable outcomes. These were classified under 5 core outcome areas, based on a taxonomy of outcomes for medical research. A comparison of the distribution of outcomes within outcome areas, between patients and HCPs, and between interviews and published literature is presented.
Results
We obtained 17 outcomes from 13 patients and 3 family members, mostly related to general wellness of the baby, congenital anomalies, mental health, and health care delivery; and 45 outcomes from 10 HCPs, which were mostly clinical. Outcomes in published literature when compared with participant interviews put greater emphasis on clinical outcomes (94% vs 76.5%, P = 0.03) and limited emphasis on life impact (0% vs 17.6%, P < 0.001).
Conclusions
Although clinical outcomes are the main focus of published research in heart disease and pregnancy, patients and HCPs emphasize the importance of outcomes related to general maternal and fetal well-being and life impact, which are seldom reported. Including these outcomes in future studies is essential to facilitating patient-centred care for pregnant women with cardiac disease.
Résumé
Contexte
Les auteurs s'emploient actuellement à établir un ensemble de paramètres de base aux fins des études sur la cardiopathie durant la grossesse. L’intégration des points de vue des patientes et des professionnels de la santé constitue une étape essentielle à l’élaboration de cet ensemble de paramètres de base; c’est là l’objectif de l’étude présentée ici.
Méthodologie
Nous avons interviewé des femmes enceintes atteintes d’une cardiopathie, des membres de leur famille et des professionnels de la santé jusqu’à ce que le seuil de saturation des données soit atteint. Les participants étaient invités à faire part de leur vécu et de leurs points de vue, et à fournir des commentaires quant aux paramètres qu’ils estimaient importants. Les entrevues ont été enregistrées puis transcrites mot à mot; nous avons ensuite utilisé une analyse interprétative pour traduire les expériences relatées en paramètres mesurables. Ces expériences ont été regroupées en cinq grandes catégories, en fonction d’une taxonomie des résultats mesurés dans le domaine de la recherche médicale. Nous comparons ici la répartition des paramètres dans les différentes catégories entre patientes et professionnels de la santé, et entre résultats des entrevues et littérature médicale publiée.
Résultats
Nous avons cerné 17 paramètres auprès de 13 patientes et trois membres de leur famille, généralement associés au bien-être du bébé, aux anomalies congénitales, à la santé mentale et à la prestation des soins de santé, ainsi que 45 paramètres auprès de 10 professionnels de la santé, principalement de nature clinique. Les paramètres publiés dans la littérature médicale sont quant à eux plus axés sur les résultats cliniques que les paramètres dégagés à l’issue des entrevues (94 % vs 76,5 %, p = 0,03) et moins sur les répercussions sur la qualité de vie (0 % vs 17,6 %, p < 0,001).
Conclusions
Bien que les études publiées sur la cardiopathie et la grossesse soient principalement axées sur les résultats cliniques, les patientes et les professionnels de la santé ont fait ressortir l’importance des paramètres liés au bien-être général de la mère et du fœtus et aux répercussions sur leur qualité de vie, dont font peu souvent état les études publiées. Il est essentiel d’inclure ces paramètres dans les futures études pour favoriser des soins centrés sur les besoins des femmes enceintes atteintes d’une cardiopathie.
It is estimated that 1%-4% of all pregnancies are complicated by congenital or acquired cardiac disease.1 Despite advances in the management of these pregnancies, cardiac disease remains a significant cause of maternal and perinatal morbidity and mortality in both high- and low-income countries.2 In the past 2 decades, there has been a surge of well-designed prospective studies to address unanswered questions relating to the care of these women, with a view to developing appropriate guidelines. We conducted a systematic review, to determine variations in outcome reporting in studies on cardiac disease and pregnancy, and to determine the distribution of these outcomes by the 5 core outcome areas—mortality, clinical/physiological, life impact/functioning, resource use, and adverse events—as classified by a recently published taxonomy of medical outcomes.3 This systematic review identified 148 distinct outcomes, reported in 409 prospective studies on pregnancy and heart disease, published between 1980 and 2018 (unpublished data, 2020). Not only were we able to show considerable variation with regard to outcome reporting and measurement in these studies, but also that most reported outcomes were in the “clinical/physiological” and “adverse effects” core outcome areas (n = 139, 94%), with little-to-no emphasis on outcomes related to resource use (n = 3, 2%) or life impact/functioning (n = 0). In fact, patient-reported outcomes (PROs), defined as “any report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else,”4 were seldom reported in studies on pregnancy and cardiac disease.
International initiatives such as Core Outcome Measures in Effectiveness Trials (COMET) have proposed the development of core outcome sets—a minimum standardized set of outcomes obtained through input from patients and other stakeholders involved in their care—as a solution to this problem of outcome reporting in clinical trials.5 A key feature of core outcome sets is the incorporation of patient and stakeholder input in determining what outcomes should be measured in future clinical trials. The development of a core outcome set for studies on cardiac disease in pregnancy (COSCarP) is currently underway.6 This 5-step process aims to arrive at consensus from a group of international stakeholders on a final set of outcomes that should be reported on all future studies on cardiac disease and pregnancy. The systematic review alluded to earlier is the first of these steps and represents outcomes considered important to researchers in the field. The second step involves the elicitation of outcomes from patients and stakeholders. Although a systematic review summarizing the qualitative research on the topic identified 11 studies highlighting the experiences and perceptions of women with cardiac disease in pregnancy, none of these were aimed at eliciting outcomes deemed important to these patients during pregnancy and the postpartum period.7 We therefore conducted this study with the primary aim of eliciting outcomes from pregnant women with cardiac disease, their family members and health care professionals (HCPs) involved in their care, to inform the development of COSCarP, and inform future research in the area. Our study’s secondary aims were to compare and contrast outcomes considered important by pregnant women and family members vs HCPs, and those obtained from this study, with those previously reported in the literature.
Methods
Study participants and recruitment
This study was approved by the Mount Sinai Hospital Research Ethics Board (REB#18-0126-E). Between July and August 2018, pregnant women with congenital or acquired cardiac disease and their family members were recruited from the cardiac disease in pregnancy clinic of the Special Pregnancy Program at Mount Sinai Hospital in Toronto, Canada. Patients were eligible to participate if they had a congenital or acquired cardiac condition, as identified from their medical records. Family members most involved in medical decision making, who attended the clinic appointments, were also invited to participate in interviews. Proficiency in English was a requirement for both patients and family members to participate in interviews. Patient lists were reviewed before the clinic visit, and all patients who met the eligibility criteria were directly approached in clinic, by a study investigator, not involved in the clinical care of the patients. The investigator explained the basis of the study and answered any questions they may have had, and then allowed them and their family members to decide whether they would like to participate. No direct invites to eligible patients were sent out. Patients and their family members who expressed interest in participating in the study were given the option of being interviewed either in person or over the telephone. HCPs were contacted through e-mail, via contact lists assembled by the study investigators. HCPs were eligible for inclusion if they cared for pregnant women with cardiac disease, in a clinical context. Sampling was purposive to ensure diverse representation of patients in terms of the nature of the cardiac condition,8 and the inclusion of HCPs from different countries and of various clinical disciplines involved in the care of pregnant patients with cardiac disease. Decisions with regard to sampling were made by the study’s lead investigator. Recruitment was continued until data saturation—the point at which no new outcomes were identified in 2 successive interviews—was attained.
Interview process
The COSCarP project is being conducted under the auspices of the Outcome Reporting in Obstetric Studies (OROS) group,9 an international collaboration led by the University of Toronto, comprising clinicians, qualitative researchers, graduate students, medical students, and research assistants committed to the cause of ensuring standardization and comprehensiveness of outcome reporting in obstetric studies. The OROS group is currently overseeing the development of 9 core outcome sets, while training graduate and medical students in various aspects of core outcome set development, including the conduct of stakeholder interviews. So as to avoid the problem of power balance between patients and physicians, interviews for this study were conducted by the medical student, with no role in the clinical care of the patient. This student who is part of the OROS group has not only received instruction on the conduct of interviews, pertinent to the elicitation of outcomes, but also worked under the supervision of experts and fellow graduate students who are proficient in the conduct of interviews and in interpretive analysis. She was also involved in the development of the study protocol and interview guides,6 and co-authored a meta-synthesis of interviews on patients with obesity.10
At the commencement of each interview, the interviewer explained the objective and nature of the study, and obtained verbal (phone interviews) or written (in-person interviews) consent. An optional questionnaire was provided to participants along with the consent form, to obtain information on age, parity, ethnicity, household income, and other demographic information, which could have influenced responses. Interviews were scheduled to last 60 minutes and were audio-recorded for later analysis.
Interviews were conducted in a semistructured format. The interviewer followed a written script specific to either patients/family members or HCPs with several open-ended questions. The interview guides for patients and care providers are presented as Supplemental Methods S1 and S2. Although the interviews had a semistructured format with a focus on eliciting outcomes related to their care, we encouraged participants to talk freely about their experiences and perspectives, acknowledging that the concept of outcomes can be difficult to explain and discuss with those who do not work and think in a health intervention context.11,12 However, the interviewer was trained to redirect and focus the participant on eliciting outcomes when deemed necessary, and to ensure that the participant focussed on both individuals of the mother-fetus dyad.13 Interviews were audio-recorded and transcribed verbatim, by a medical transcriptionist.
Analysis
Our analytical methodology was derived from interpretive evidence synthesis methods14 and relied on our ability to recognize and extract cardiology- and pregnancy-related outcomes. Although thematic analysis offers insight into experiences and perspectives, it does not enable the elicitation of potentially measurable outcomes that would inform the development of a core outcome set or inform future (quantitative) clinical research. Because this was the primary intent of this study, we performed interpretive analysis, wherein we translated experiences and perspectives obtained through interviews into outcomes as previously described.12 A single reviewer (CH) analyzed the transcribed interviews, identifying outcomes, if directly reported, and using interpretive analysis to extract outcomes from the experiences or perspective shared by participants, particularly with the patients and family member interviews. Where there was uncertainty surrounding whether or not a section of an interview could be accurately translated into outcomes, this was discussed with a second reviewer (RDD). Outcomes were grouped into maternal vs fetal/neonatal outcomes and further, into 1 of 5 broad core outcome areas—mortality, clinical/physiological, functioning/life impact, resource use, and adverse events based on a recently published taxonomy for outcomes in medical research.3
Results
Participants
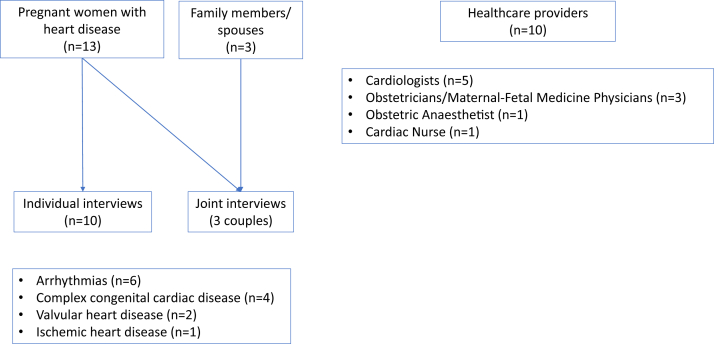
The study flowchart is presented in Figure 1. We interviewed 13 pregnant women, 10 of whom were interviewed individually and 3 along with their family members (all of whom were spouses). The HCPs represented Canada, the Netherlands, the United Kingdom, and the United States. The demographic characteristics of all participants are presented in Table 1.
Figure 1.
Study flowchart.
Table 1.
Characteristics of participants
| Pregnant women with cardiac disease (n = 13)∗ | |
|---|---|
| Age | 34 ± 4 y (range, 26-40 y) |
| Employment | Employed full time (n = 7, 53.8%) Self-employed (n = 2, 15.4%) Employed part time (n = 1, 7.7%) Homemaker (n = 1, 7.7%) |
| Education | Postgraduate degree (n = 2, 15.4%) Undergraduate degree (n = 5, 38.5%) College diploma (n = 6, 46.2%) |
| Ethnicity | Caucasian (n = 8, 61.5%) South Asian (n = 2, 15.4%) East Asian (n = 1, 7.7%) African or Afro-Caribbean (n = 2, 15.4%) |
| Gestational age | 29 ± 7 wk (range, 16-37 wk) |
| Cardiac disease | Complex congenital heart disease (n = 4, 3.1%) Valvular heart disease (n = 2, 15.4%) Electrical heart disease (n = 6, 46.2%) Ischaemic heart disease (n = 1, 7.7%) |
| Health care providers (n = 10) | |
| Role | Cardiologist (n = 5, 50%) Obstetrician/maternal-fetal medicine physician (n = 3, 33.3%) Anaesthesiologist (n = 1, 10%) Cardiac nurse practitioner (n = 1, 10%) |
| Region, Country | Toronto, Ontario, Canada (n = 5, 50%) London, Ontario, Canada (n = 1, 10%) Vancouver, British Colombia, Canada (n = 1, 10%) California, USA (n = 1, 10%) Amsterdam, The Netherlands (n = 1, 10%) London, UK (n = 1, 10%) |
Ten pregnant women with cardiac disease participated in individual interviews and 3 in shared interviews with their spouses.
Interpretive analysis
In keeping with our study’s primary aim, our primary analysis involved translating quotes, anecdotes, experiences, and perspectives into measurable outcomes. Although the analytical process was identical for both HCP and patient/family member interviews, the latter relied quite heavily on interpretive analysis, because the concept of “outcomes” was less easily understood. Some quotes explicitly contained measurable outcomes and were easily translated. For example, “It’s still a big issue … just kind of like overwhelming anxiety sometimes, I am worrying constantly…,” which was translated to “maternal anxiety,” and “there was like a bit of concern when my son was born that I may have passed my condition on to him,” which was translated to “genetic transmission of maternal condition.” In some other cases, the outcome had to be deduced. For example, “I think once I gave birth I kind of fell off the radar … I had my 6-week appointment, but I feel like aftercare is kind-of dismissed” was translated to “maternal follow-up after childbirth,” whereas “I kind-of debated on taking the beta blockers after (the doctor) mentioned that the baby might be smaller” and “to have any type of medication in my system that will somehow go in utero to my child or, in any way affect his or her development and, while the baby would be exposed to a certain amount of it… it would be a very small amount but even that I am not comfortable with and, if I can do anything else before this I, I really see this as a last resort” were translated to “effect of medication on fetal development.” Not all experiences from these interviews could be translated into outcomes. Through this process, 17 unique outcomes were retrieved from the patient and family member interviews. HCP interviews, in contrast, seldom required the use of interpretive analysis and provided 45 unique outcomes. All these outcomes, taxonomized by core outcome areas, are presented in Table 2.
Table 2.
Outcomes elicited from participant interviews
| Core outcome area | Pregnant women and family members (n = 13) | Health care providers (n = 10) |
|---|---|---|
| Mortality/survival |
|
|
| Physiological/clinical outcomes | ||
| Cardiac |
|
|
| Congenital, familial, and genetic |
|
|
| Pregnancy, puerperium, and perinatal |
|
|
| Other clinical outcomes |
|
|
| Adverse events/effects |
|
|
| Functioning | Emotional functioning/well-being(15) Stress/anxiety/mental health/fatigue (n = 5)Global quality of life(16)Quality of life/maintaining day-to-day functioning in pregnancy (n = 2)Delivery of care(17) Appropriate health care management (through medications, follow-up, continuity of care) (n = 4) | Physical functioning(42) Long-term maternal health (n = 2) (43) Safety/suitability for subsequent pregnancy (n = 1) Role functioning (44) Symptom control and capacity to raise a child (n = 1) |
| Resource use |
|
|
In keeping with our secondary aims, we first compared core outcome areas represented by interviews with patients and family members vs HCPs. The outcomes most frequently reported by the 13 sets of interviews involving patients and family members were related to general health and well-being of fetus/baby (n = 13, 100%), congenital defects in baby (n = 6, 46%), stress/anxiety/mental health/fatigue (n = 5, 38%), and appropriate health care management, which included medications, follow-up, and continuity of care (n = 4, 31%). Although outcomes generated through individual patient interviews and joint interviews with family members were identical, family members (spouses) expressed a greater concern for maternal health over fetal health. In contrast, the most commonly reported outcomes by the 10 HCPs were thromboembolism (n = 10, 100%), arrhythmias (n = 9, 90%), new-onset or worsening heart failure (n = 9, 90%), prematurity and associated complications including cerebral palsy or respiratory difficulties (n = 9, 90%), maternal mortality (n = 8, 80%), and mode of delivery (n = 6, 60%). No appreciable differences were noted in the outcomes provided from HCPs of different geographic origins, nor were there appreciable differences in outcomes from the various medical specialties, apart from a greater emphasis on the respective field of specialization.
We finally compared the outcomes obtained through this study, with those obtained from our systematic review of published studies (unpublished data, 2020). As shown in Table 3, none of the 148 identified in the systematic review of clinical studies were related to the core outcome area of “life impact,” whereas 132 of 148 (94%) were related to the clinical outcomes and adverse events and 3 of 148 (2%) were related to resource use. In contrast, 13 of 17 (76.5%) of the outcomes obtained from the interviews were clinical or adverse event–related outcomes, 3 of 17 (17.6%) were related to life impact, and none were related to resource use.
Table 3.
Proportion of reported outcomes by core outcome area in health care user interviews, health care provider interviews, and published literature
| Core outcome areas | Pregnant woman and family members (n = 17 outcomes) | Health care providers (n = 45 outcomes) | Systematic review of published studies (n = 148 outcomes) | P value |
|---|---|---|---|---|
| Mortality and survival | n = 1 (5.9%) | n = 3 (6.7%) | n = 6 (4%) | > 0.99 |
| Clinical outcomes and adverse events | n = 13 (76.5%) | n = 33 (73.4%) | n = 139 (94%) | 0.03 |
| Functioning/life impact | n = 3 (17.6%) | n = 3 (6.7%) | n = 0 (0%) | < 0.0001 |
| Resource use | n = 0 (0%) | n = 1 (2.2%) | n = 3 (2%) | > 0.99 |
Discussion
This study, conducted as part of developing a core outcome set for studies on cardiac disease in pregnancy, identified 17 unique outcomes reported by patients and family members, and 45 outcomes identified by HCPs. These outcomes represented all 5 core outcome areas, which include mortality/survival, clinical/physiological, adverse events, resource use, and life impact/functioning, the last area of which is not represented in published research on heart disease and pregnancy. In addition to providing patient- and stakeholder-reported outcomes for the purpose of developing a core outcome set and informing future research, this study highlights the importance of including PROs in studies on pregnancy and heart disease, to truly reflect benefits and harms or interventions, from a patient-centric perspective.
Studies specifically aimed at eliciting outcomes from patients and stakeholders involved in their care are now being widely seen as an integral part of developing core outcome sets, to inform future research. This is especially true for obstetric conditions and has been the focus of the OROS group.9 Meta-syntheses of qualitative studies, which are conducted to elicit experiences and perceptions on varying aspects of care, and not with the primary intent of obtaining outcomes for purposes of informing future research, are not ideal for obtaining PROs.10 Patient and stakeholder interviews which enable researchers to obtain first-hand information from those directly affected by a condition and proposed interventions, and about how they would prioritize outcomes while making medical decisions, have therefore been the focus of recent publications.15, 16, 17, 18 While conducting these interviews, in keeping with the primary intention of eliciting outcomes, attention must be paid to the use of a semistructured interview guide and the liberal use of prompts to redirect patients and family members to focus on outcomes. Interpretive analysis, wherein quotes, anecdotes, experiences, and perceptions are translated into measurable outcomes, forms an integral part of the research process, especially for patient interviews, as the concept of outcomes can be difficult to explain and discuss with those who do not work and think in a health intervention context.11,12
In our study, we found that although both groups of participants considered clinical/physiological outcomes (which included adverse events) related to their cardiac condition and pregnancy, importantly, they also commented on the importance of outcomes related to life impact and functioning, such as physical, emotional, and role functioning, as well as global quality of life and care delivery. In general, patients and family members favoured holistic health outcomes over specific clinical outcomes. Family members tended to show a greater preference for maternal health over fetal health, when compared with pregnant women, who prioritized the health of their babies equally, if not over, their own health. HCPs seemed to put a greater emphasis on maternal mortality, mode of delivery, adverse effects of treatment, and resource use, whereas the primary focus of patients and family members was the general well-being and health of the baby, mental health, and avoiding congenital malformations. Between HCPs, although there seemed to be consensus on outcomes they considered important, each specialist (cardiologist vs obstetrician vs anaesthetist) emphasized outcomes related to their field of expertise. These differences highlight how patients, family members, and stakeholders differ in how they prioritize outcomes, all of which should ideally be represented in clinical research.
An important finding is that published studies tend to emphasize mostly clinical/physiological outcomes (including adverse events), with 94% of the 148 unique outcomes identified in our systematic review, belonging to this core outcome area (unpublished data, 2020). Thus outcomes considered important by patients and stakeholders, belonging to the other core areas, especially life impact/functioning and resource use, are underrepresented in published research. For example, outcomes related to stress, anxiety, and mental health, reported as important by 5 of 13 patients, and quality of life, reported by 2 of 13 patients and 1 HCP, were not reported in a single one of the 409 studies on heart disease and pregnancy, included in the systematic review. Incorporating these patient- and stakeholder-reported outcomes in future research on pregnancy in cardiac disease is vital in this era of patient-centred care, and is the focus of COSCarP, which aims to ensure that outcomes reported in studies on cardiac diseases in pregnancy are not just restricted to treating cardiac pathology and improving specific pregnancy outcomes but include the psychosocial and functional dimensions of health and not just the absence of infirmity.
This paper has several strengths and implications for future research. This is the first study to focus on eliciting from pregnant women with cardiac disease, their family members and care providers, outcomes that they consider important, and which should therefore be measured in clinical trials. Health service users included not only a diverse group of patients, in terms of their demographics, and nature of heart disease, but also family members, adding an additional dimension to the health service user perspective. HCPs similarly included cardiologists, obstetricians, maternal-fetal medicine physicians, an anaesthesiologist, and a specialist nurse, with expertise in the management of pregnant women with heart disease, and represented 4 countries in Europe and North America. As this study was designed specifically to elicit outcomes, our choice of using interpretive analysis have further strengthened the focus and elicitation of outcomes. Having previously conducted a systematic review on this topic, we were able to draw comparisons between outcomes considered important by 3 important stakeholder groups—patients and clinicians in this study vs researchers in the systematic review, and to show how published studies do not report on core outcome areas such as life impact, functioning, mental health, and delivery of care considered important by patients with cardiac disease.
Despite these strengths, our study had a number of limitations that are important to acknowledge. The first is that for purposes of this paper, we did not conduct thematic analysis to further explore the patient experience and the reasons behind their perspectives. This was intentional, but this was also not required, because a meta-synthesis of (thematically analyzed) qualitative studies on experiences of pregnant women with cardiac diseases has been recently published.7 Second, although we gathered international representation for HCPs, patients and their family members were all recruited from a multidisciplinary clinic in Toronto, Canada. We acknowledge that although Toronto is among the most multicultural cities in the world, and our centre serves a large proportion of women from diverse socioeconomic, ethnic, and other demographic backgrounds, Canada is still a resource-rich setting and it is possible that in resource-poor settings, outcomes and experiences may be different for patients and their family members. This was done for purposes of practicality, to get an in-depth idea of what patient and stakeholder outcomes might have been missed in published studies. However, this study is not the only source for incorporating patient perspectives into the core outcome set, as we intend to recruit patients globally for an online Delphi survey, in the near future, which will enable patients and HCPs from around to the world to add to the long list of outcomes that we have obtained through the systematic review and these interviews. Third, despite the use of interpretive analysis, some participant experiences were difficult to convert into measurable outcomes and were therefore placed under the most appropriate core outcome area, which may have diluted or oversimplified the participant’s original statement. Another limitation is that patient-centred care research typically should include patients at all stages of research including design, analysis, interpretation, and knowledge translation. Although researchers did not include patients in the design aspect of the COSCarP study, this far, patients will be involved in the planning, interpretation, and analysis of subsequent steps, which include the Delphi process and a consensus meeting. Finally, an important concern about any form of qualitative research is reflexivity, the contextual intersecting relationships between the participants and themselves.19 During the process of planning and conduct of the study, the researchers were aware of their own positions and how this could influence the study and results. The interview guide was designed with the help of qualitative researchers, with only contextual input from the COSCarP investigators. Interviews were audio-recorded and transcribed verbatim, by an independent, professional medical transcriptionist. Transcripts were analyzed by the researcher who conducted the interviews. COSCarP investigators were not involved in their conduct or analysis, except for minimal involvement by the senior author in case of uncertainties. These measures notwithstanding, we acknowledge that some interpretation of data that have been drawn may be influenced by the views and bias of the researchers.
Conclusion
Through the conduct of in-depth interviews with 13 pregnant persons with cardiac disease, 3 family members, and 10 HCPs, this study identified 17 unique PROs and 45 outcomes reported by HCPs involved in their care. Although the specific outcomes differed between patients and HCPs, both groups reported as important outcomes related to life impact, in addition to mortality, adverse events, and clinical outcomes, which is the focus of published research in the field. It is important that future studies on pregnancy and cardiac disease report on outcomes considered important by patients and relevant stakeholders. To facilitate this, COSCarP is in the process of arriving at international consensus on a minimum standardized list of outcomes that must be reported in all studies on pregnancy and cardiac disease, and these interviews will go a long way in ensuring that patient- and stakeholder-reported outcomes are considered in the final core outcome set.
Acknowledgements
The authors would like to acknowledge all participants—patients, family members, and health care professionals, Amanda Urquhart for her assistance with transcribing the interviews, members of the OROS lab for their input in the development of the interview guide and study design, the COSCarP investigators for their input into various steps of developing the core outcome set, and Dr Rizwana Ashraf for her editorial assistance.
Funding Sources
This project was made possible through the Tolnai grant, Mount Sinai Hospital, Toronto, Canada, awarded to R.D.D. in June 2018.
Disclosures
R.D.D. has received speaking honoraria and research grants from Ferring, Canada, for projects on induction of labour, which are not related to COSCarP. C.H. has no conflicts of interest to disclose.
Footnotes
Ethics Statement: This study was approved by the Mount Sinai Hospital Research Ethics Board (REB#18-0126-E) and has adhered to relevant ethical guidelines.
See page 460 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2020.05.010.
Supplementary Material
References
- 1.Elkayam U., Goland S., Pieper P.G., Silversides C.K. High-risk cardiac disease in pregnancy: part I. J Am Coll Cardiol. 2016;68:396–410. doi: 10.1016/j.jacc.2016.05.048. [DOI] [PubMed] [Google Scholar]
- 2.Knight M., Nair M., Tuffnell D. MBRRACE-UK, National Perinatal Epidemiology Unit, University of Oxford; Oxford, UK: 2017. Saving Lives, Improving Mothers’ Care—Lessons Learned to Inform Maternity Care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2013–15. [Google Scholar]
- 3.Dodd S., Clarke M., Becker L. A taxonomy has been developed for outcomes in medical research to help improve knowledge discovery. J Clin Epidemiol. 2018;96:84–92. doi: 10.1016/j.jclinepi.2017.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirkham J.J., Davis K., Altman D.G. Core outcome set-STAndards for Development: the COS-STAD recommendations. PLoS Med. 2017;14 doi: 10.1371/journal.pmed.1002447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williamson P.R., Altman D.G., Bagley H. The COMET Handbook: version 1.0. Trials. 2017;18(Suppl 3):280. doi: 10.1186/s13063-017-1978-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Souza R., Hall C., Sermer M., Siu S., Silversides C. Development of a core outcome set for studies on cardiac disease in pregnancy (COSCarP): a study protocol. Trials. 2020;21:300. doi: 10.1186/s13063-020-04233-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dawson A.J., Krastev Y., Parsonage W.A. Experiences of women with cardiac disease in pregnancy: a systematic review and metasynthesis. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2018-022755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Souza R., Sermer M., Silversides C.K. Pregnancy in women with congenital heart disease. Obstet Med. 2015;8:18–25. doi: 10.1177/1753495X14568055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Souza R., OROS Investigators Outcome Reporting in Obstetric Studies (OROS) Project. 2019. https://www.obgyn.utoronto.ca/oros-project Available at:
- 10.Dadouch R., Hall C., Du Mont J., D’Souza R., OROS investigators Obesity in pregnancy—patient-reported outcomes in qualitative research: a systematic review. J Obstet Gynaecol Can. 2020;42:1001–1011. doi: 10.1016/j.jogc.2019.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Keeley T., Williamson P., Callery P. The use of qualitative methods to inform Delphi surveys in core outcome set development. Trials. 2016;17:230. doi: 10.1186/s13063-016-1356-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaufman J., Ryan R., Hill S. Qualitative focus groups with stakeholders identify new potential outcomes related to vaccination communication. PloS One. 2018;13 doi: 10.1371/journal.pone.0201145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D'Souza R., Shah P.S., Sander B. Clinical decision analysis in perinatology. Acta Obstet Gynecol Scand. 2018;97:491–499. doi: 10.1111/aogs.13264. [DOI] [PubMed] [Google Scholar]
- 14.Pope C., Mays N., Popay J. Open University Press; New York: 2007. Synthesizing Qualitative and Quantitative Health Evidence: A Guide to Methods. [Google Scholar]
- 15.Dadouch R., Faheim M., Juando-Prats C. Development of a core outcome set for studies on obesity in pregnant patients (COSSOPP): a study protocol. Trials. 2018;19:655. doi: 10.1186/s13063-018-3029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duffy J.M.N., Hirsch M., Ziebland S., McManus R.J. International Collaboration to Harmonise Outcomes in Pre-eclampsia (iHOPE). Methodological decisions influence the identification of potential core outcomes in pre-eclampsia related studies: a sensitivity analysis informing the development of guidelines for future core outcome set developers. BJOG. 2019;126:1482–1490. doi: 10.1111/1471-0528.15892. [DOI] [PubMed] [Google Scholar]
- 17.King A., D’Souza R., Teshler L., N S, Malinowski A. The development of a core outcome set for studies on perinatal venous thromboembolism (COSPVenTE): a study protocol. BMJ Open. 2020;10:e034017. doi: 10.1136/bmjopen-2019-034017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Souza R., Villani L., Hall C. Core outcome set for studies on pregnant women with vasa previa (COVasP): a study protocol. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2019-034018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dodgson J.E. Reflexivity in qualitative research. J Hum Lact. 2019;35:220–222. doi: 10.1177/0890334419830990. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.