Abstract
Background
Guidelines for surgical management of tetralogy of Fallot (TOF) are often based on low-quality evidence due to the many challenges of congenital heart disease: heterogeneous cardiac anatomy, consequences from surgical interventions arising years later, and scarcity of hard outcomes. The overarching goal of the Tetralogy of Fallot Research for Improvement of Valve replacement Intervention: A Bridge Across the Knowledge Gap (TRIVIA) study is to evaluate the long-term impact of the surgical management strategies in TOF. The specific objectives are: (1) to describe the long-term outcomes of TOF according to the native anatomy and the presence of genetic conditions, (2) to evaluate the long-term outcomes of surgical repair according to associated residual lesions, and (3) to evaluate the impact of paediatric pulmonary valve replacements on the long-term outcomes.
Methods
The TRIVIA study is a population-based cohort including all subjects with TOF in the province of Québec between 1980 and 2017. It links patient-level granular clinical data with long-term administrative health care data. We will evaluate mortality, cardiovascular interventions, and hospitalizations for adverse cardiovascular events using survival Cox models and marginal mean/rates models for recurrent events, respectively. Multivariate multilevel models will correct for potential confounders, and risk score matching will mitigate the potential of confounding by indication.
Results
The current TRIVIA cohort includes 1001 eligible subjects with TOF with complete lifelong follow-up for > 98%. The median follow-up is 17.1 years, totalling > 17,000 patient-years.
Conclusions
Universal health insurance data combined with granular clinical data enable the development of population-based cohorts, to which contemporary statistical methods are applied to address important research questions in congenital heart disease research.
Résumé
Contexte
Les lignes directrices pour la prise en charge chirurgicale de la tétralogie de Fallot (TdF) sont souvent basées sur des données de faible qualité en raison des nombreux défis posés par les maladies cardiaques congénitales : anatomie cardiaque hétérogène, répercussions des interventions chirurgicales survenant des années plus tard et rareté de conclusions solides. L'objectif principal de l’étude TRIVIA (Tetralogy of FallotResearch forImprovement ofValve replacementIntervention: ABridge Across the Knowledge Gap) est d'évaluer l'impact à long terme des stratégies de prise en charge chirurgicale dans les TdF. Les objectifs spécifiques sont les suivants : (1) décrire les issues à long terme de la TdF en fonction de l'anatomie native et de la présence de conditions génétiques, (2) évaluer les conséquences à long terme de la chirurgie réparatrice en fonction des lésions résiduelles associées, et (3) évaluer l'impact des remplacements valvulaires pulmonaires pédiatriques sur le pronostic à long terme.
Méthodes
L'étude TRIVIA est une étude de cohorte basée sur une population comprenant tous les sujets atteints de TdF dans la province du Québec entre 1980 et 2017. Elle fait le lien entre les données cliniques détaillées des patients et les données administratives des soins de santé à long terme. Nous évaluerons la mortalité, les interventions cardiovasculaires et les hospitalisations pour troubles cardiovasculaires en utilisant respectivement des analyses de survie par les modèles de Cox et des modèles de moyennes/taux marginaux pour les événements récurrents. Des modèles multivariés à plusieurs niveaux permettront de corriger les facteurs de confusion potentiels, et l'appariement par des scores de risque atténuera le potentiel de confusion par indication.
Résultats
La cohorte de l’étude TRIVIA comprend actuellement 1001 sujets admissibles atteints de TdF avec un suivi complet tout au long de la vie pour > 98 %. Le suivi médian est de 17,1 ans, pour un total de > 17 000 patients-années.
Conclusions
Les données de l'assurance maladie universelle combinées à des données cliniques détaillées permettent la mise en place de cohortes populationnelles, auxquelles les méthodes statistiques contemporaines pourront être appliquées afin de répondre aux questions importantes pour la recherche portant sur les maladies cardiaques congénitales.
Tetralogy of Fallot (TOF) is the most common cyanotic cardiac malformation with a prevalence of 0.2-0.5 cases per 1000 births.1, 2, 3 Although the postoperative mortality after correction of TOF has fallen below 2%,4,5 the long-term management is becoming increasingly complex. The generation of high-quality scientific evidence surrounding the management of TOF is stifled by the challenge of assessing the long-term effects of interventions in a highly heterogeneous population where hard outcomes are latent and scarce. The advent of innovative data science techniques compels us to generate empirically based knowledge with the overarching goal of improving the quality of evidence on which clinical decision making is based.
Rationale
Knowledge gaps in TOF
The surgical correction of TOF largely depends on the severity of stenosis and underdevelopment of the right ventricular outflow tract, whereas reintervention later in life is contingent on the long-term sequelae of this initial correction. The conundrum of the repair of “classic” TOF predominantly lies in the understanding of which residual lesions will be best tolerated in the long term: stenosis or regurgitation. Our appreciation of their long-term effect has been shifting, and valve-sparing procedures are now increasingly used over transannular patch techniques. This strategy has been associated with favourable outcomes when the pulmonary valve annulus is well developed6,7 but could result in significant residual pulmonary stenosis in patients with an underdeveloped valve.8, 9, 10 There have been studies on the short-term effect of both surgical strategies, but the long-term impact of their associated residual lesions remains elusive.
In contemporary cohorts, a third of patients with TOF have severe pulmonary regurgitation (PR) after their corrective surgery.2 The chronic effect of severe PR leads to a downward spiral of right ventricle dilatation, increased risk of biventricular dysfunction, decreased exercise tolerance, life-threatening arrhythmias, and sudden death.2,11, 12, 13 Consequently, pulmonary valve replacement (PVR) has become the most common reintervention in patients with repaired TOF in the hope that timely intervention would normalize ventricular volumes and improve heart function, quality of life, and survival.14 It has been suggested that there is a right ventricle dilatation threshold over which right ventricular remodelling is unlikely to occur after PVR.15, 16, 17 This has led to PVR being offered earlier, notably to asymptomatic patients. Over the last decade, the prevalence of PVR has more than tripled and the mean age at first PVR has decreased from 35 to 20 years. Paediatric PVR is now performed in 10%-15% of children with repaired TOF.18, 19, 20, 21 To this day, the long-term benefits of PVR in asymptomatic individuals, more so in children and adolescents, have yet to be clearly demonstrated.
Methodological challenges in TOF
Recent reviews have highlighted the paucity of high-quality scientific evidence for PVR’s efficacy and timing in patients with repaired TOF.2,22, 23, 24 With the lack of frequent hard outcomes and heterogeneity of cardiac native anatomy, the amount of resources required to conduct adequately powered prospective trials with sufficient follow-up is often insurmountable. Hence, published guidelines for PVR mostly rely on low-quality evidence. Published guidelines for PVR mostly rely on expert opinions and are restricted to an adult population.12,25, 26, 27, 28, 29
The choice of a study design should be one that allows an important research question to be addressed in a way that optimizes feasibility, validity, and precision.30 For decades, researchers and clinicians automatically ranked prospective trials high in the hierarchy of the scientific evidence paradigm, because their straightforward study design alleviates the need to decipher complex causal structures.31, 32, 33 In parallel, the increasing availability of high-quality observational data and the advances in analytical methods now allow for better precision and increased validity through the reduction of previously unaccounted biases. Experts have been calling for a paradigm shift that would place less emphasis on the study design and more on precision and validity.34,35 We believe that the specificities and challenges of congenital heart disease (CHD) research call for such innovations to generate valid and precise results with yet feasible study designs and analytical approaches.
Here, we describe the Tetralogy of Fallot Research for Improvement of Valve replacement Intervention: A Bridge Across the Knowledge Gap (TRIVIA) cohort. We are leveraging the current Canadian universal health care system potentials by proposing the linkage of clinical and administrative data to create a long-term population-based TOF cohort with lifetime follow-up. We will discuss why this approach has the potential of producing high-quality scientific evidence in CHD research and how the traditional caveats of retrospective designs may be overcome with contemporary statistical methods.
Study objectives
The overarching goal of the TRIVIA study is to evaluate the long-term impact of the different surgical management and interventional strategies in TOF. We have set 3 specific study objectives:
-
(1)
To describe the long-term outcomes of TOF according to the native cardiac anatomic subtype and the presence of a genetic syndrome.
-
(2)
To evaluate the long-term outcomes of the strategies for surgical repair of TOF according to their associated residual lesions.
-
(3)
To evaluate the impact of a paediatric PVR on the long-term outcomes by first identifying the long-term risk factors of adverse clinical outcomes in adolescents and then determining the impact of paediatric PVR on long-term outcomes according to the pre-PVR risk factors.
Methods
Study design and overview
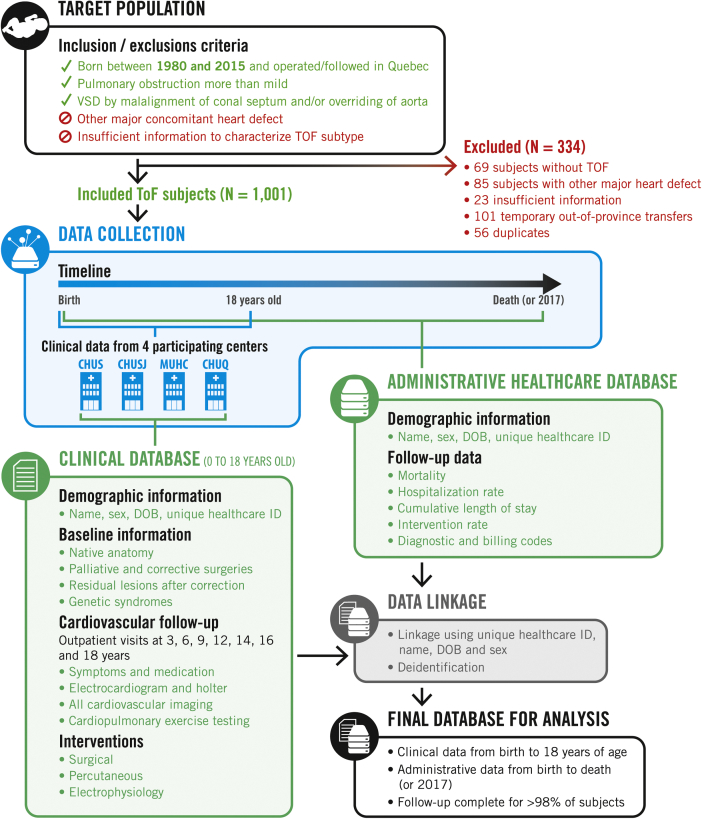
The TRIVIA study is a population-based multicentre longitudinal retrospective cohort study. We included subjects from all 4 tertiary paediatric cardiology centres in Québec (Centre Hospitalier Universitaire [CHU] Sherbrooke, CHU Sainte-Justine, CHU Québec, and Montreal Children’s Hospital). All patients with TOF in the province of Québec are followed in either of these centres until they reach 18 years of age. This makes it possible to create a populational cohort including all patients with TOF in Québec. The cohort is composed of retrospective clinical data from birth to 18 years of age enriched with more > 35 years of health care administrative data. This enables a complete follow-up from birth to death or 2017. The study flowchart is presented in Figure 1.
Figure 1.
Study flowchart. CHUQ, Centre Hospitalier Universitaire de Québec; CHUS, Centre Hospitalier Universitaire de Sherbrooke; CHUSJ, Centre Hospitalier Universitaire Sainte-Justine; DOB, date of birth; ID, identifier; MUHC, McGill University Health Center; TOF, tetralogy of Fallot.
Patient population
All subjects with TOF born between 1980 and 2015 and followed in one of the 4 participating centres were included. We defined TOF as having a ventricular septal defect with an anterior malalignment of the conal septum or an aorta overriding the interventricular septum, combined with an obstruction of the pulmonary outflow tract (Doppler gradient > 20 mm Hg). Subjects with other anatomic variants of TOF such as pulmonary atresia with ventricular septal defect and the absent pulmonary valve syndrome were also included. Subjects were identified by medical records services using the International Classification of Disease (ICD) code 9th and 10th revisions and by interrogation of echocardiography databases. Potential subjects’ charts were manually reviewed. Subjects with a concomitant severe heart defect (eg, auriculoventricular septal defect) or with missing information on native anatomy were excluded.
Outcomes
The principal outcomes are: (1) all-cause mortality, (2) unplanned hospitalizations, and (3) cardiovascular interventions. Hospitalizations will be restricted to unplanned hospitalizations for adverse cardiac events (eg, endocarditis, arrhythmias, heart failure, etc.) using ICD-9 and ICD-10 and will exclude hospitalizations for elective procedures. Cardiovascular interventions (including all surgical, percutaneous, and electrophysiology) will be selected using physicians’ and surgeons’ billing codes, and the proportion of each type of intervention will be described.
Clinical data collection
Clinical data were manually abstracted from electronic and paper patients’ charts, including stored archived charts, using a standardized form on the data capture tool REDCap.36 Baseline clinical data included information on demographics, native cardiac anatomy, detailed information on palliative and corrective procedures, associated residual lesions of interventions, and genetic testing. We collected follow-up data at 3, 6, 9, 12, 14, 16, and 18 years of age. This included all interventions, surgical procedures, cardiovascular imaging, symptoms, medication, electrocardiograms, exercise tests, and Holter monitoring.
Health care administrative data collection
Health care administrative data were extracted from the Québec Congenital Heart Disease database.1,37 This database was developed and validated by the McGill Adult Unit for Congenital Heart Disease Excellence (MAUDE unit) at McGill University. It comprises billing data from the Régie de l’Assurance Maladie du Québec (single payer for the government universally insured population of Québec), discharge diagnostic, and procedural data from the Ministry of Health (all hospitalizations and procedures performed in Québec). This database captures information on all CHD subjects in Québec between 1982 and 2017.
Data linkage and ethical considerations
The clinical and administrative databases were linked by a unique health care identification number attributed at birth. Data linkage was further validated using the subjects’ name, sex, and date of birth. The resulting linked dataset was then striped from identifiers, and only deidentified data are used for analysis.
The project has been approved by the research ethics board of each participating institution and by the Commission d’Accès à l’Information du Québec (the governing entity regulating access to governmental data in Québec). Because the final cohort consists of retrospective deidentified data, individual informed consent was waived.
Statistical analysis plan
Objective 1: long-term prognosis of patients with TOF
The aim is to establish robust population-based estimates of long-term survival and morbidity to improve counselling for fetal and neonatal diagnosis. We will evaluate mortality using a Cox multivariate model. Hospitalizations and interventions will be evaluated with a marginal mean/rates model for recurrent events, which is an extension of the Cox survival analysis for modelling of the number of events over time. Outcomes will be computed from birth until death or 2017. We will include the following predictive variables: the subtype of TOF (“classic” pulmonary stenosis TOF or TOF with pulmonary atresia) and the presence of a genetic syndrome. We will compute estimates of mortality and morbidity with 95% confidence intervals for each clinical profile, adjusted for the surgical era.
Objective 2: evaluation of long-term outcomes according to surgical correction strategies of classic TOF
The aim is to compare long-term outcomes of patients undergoing transannular patch and valve-sparing (VS) and more specifically, patients with a significant right ventricular outflow track obstruction for which a decision between transannular patch and VS is difficult (ie, choice between free PR or residual pulmonary stenosis). Therefore, the variables of interest will combine information on surgical procedures and residual lesions at the first postoperative clinical follow-up. Specifically, we will assess 3 study groups: (1) transannular patch, (2) valve sparing without significant residual lesions, and (3) valve sparing with significant residual stenosis. The time at risk will span from the first postoperative day after surgical correction until death or 2017. We will use the same outcomes and models mentioned in objective 1, except that models will include random effects for multilevel adjustment of clustering variables such as surgical centre and era. Models will also include potential confounders such as age at surgical correction, the presence of hypoplastic branch pulmonary arteries, and the presence of genetic syndromes.
Objective 3: long-term impact of paediatric PVR
The aim is to compare the long-term outcomes of an aggressive prevention approach of paediatric PVR (< 18 years of age) with a more conservative strategy without intervention before adulthood. We will include all subjects with corrected TOF excluding those who received a right ventricle to pulmonary artery conduit or those with pulmonary atresia. Subjects will be divided into 2 groups (paediatric-PVR or no-PVR) and will be followed from the first postoperative day until death or 2017. Subjects will be censored if a PVR is performed after 18 years of age. We will evaluate the following outcomes: hospitalization and intervention rates, using the models described in objective 2. To reduce the potential of confounding by indication, patients undergoing paediatric PVR will be matched to patients in the no-PVR group in a 1:5 ratio using a risk score. This risk score is analogous to the propensity score but will represent the theoretical preoperative risk of developing the outcome of interest.38, 39, 40 It will be calculated using clinical pre-PVR variables available during the paediatric follow-up for both study groups.
Validation of statistical models
We will assess the potential of collinearity between predictors before model construction. The proportional risk postulate will be verified for the Cox multivariate model, and a stratified Cox model will be used if the postulate is violated. The adequacy of risk score matching for objective 3 will be validated by comparing the distribution of risk between groups. All models will be validated for normality, homoscedasticity, and linearity of residuals.
Multiple imputation for missing data
We will use multiple imputation for variables with > 5% of missing values. Variables with > 40% of missing values will be excluded from the analysis.41 We will use the PROC MI module of SAS version 9.4 with the fully conditional specification method for arbitrary missing patterns to produce 50 imputed databases.42 The imputation model will include every covariable of interest. The analyses will be performed in parallel in each of the resulting databases and estimates will be aggregated with SAS PROC MiAnalyze to obtain final results. We will verify the missing completely at random (MCAR) pattern assumption with Little’s test and sensitivity analysis using the pattern mixture approach.43
Results
A total of 1335 potential subjects were identified. Of those, 334 subjects were excluded (see details in Fig. 1). Of the 1001 subjects with TOF, we were able to link clinical and administrative data in 936 subjects (93.5%). For the subjects without a possible linkage with clinical and administrative data, outcomes data were nonetheless available for 53 subjects who died before reaching adulthood. Complete follow-up data were thus available for 989 subjects (98.8%). The median follow-up was 17.1 years (Q25-Q75 = 7.3-26.7) for a total follow-up duration of > 17,000 patient-years.
This cohort offers ample statistical power to answer a wide variety of research questions. The smallest sample size will be obtained for the third objective. If we consider that approximately 80% of subjects have classic TOF and suppose an approximately 10% paediatric PVR incidence with an annualized hospitalization rate of 0.4 ± 0.15,44,45 the statistical power (β) to detect a difference of hospitalization rate of ≥ 0.1 hospitalization per patient-year between groups is > 95% at α = 0.05.
Discussion
To practice evidence-based medicine, as explained by Sackett in his seminal article of 1996,46 is to integrate clinical expertise and the best external scientific evidence available to make a sound clinical decision. The quality of scientific evidence should be based on the confidence that one has on the validity of the conclusions drawn from a specific study. Such validity is multifaceted and encompasses a long list of potential biases. As many others before us, we advocate here for a shift in perspective of what is considered a robust evidence. We urge researchers and clinicians to consider the adequacy of the methods in relation to the research question over that of a simple and sometimes misleading stratification of quality of evidence based on the study design.30,34,35 Let us discuss how retrospective population-based cohorts like the TRIVIA study can be used as an alternative to the prospective research design to produce valid and robust scientific evidence in CHD research.
Benefits of population-based cohorts
The benefits of population-based cohorts include ample statistical power—both in terms of the number of subjects and length of the follow-up—and reduction of selection bias, whereas contemporary statistical methods can mitigate the conventional caveats of retrospective study designs. As children undergoing interventions have a long life expectancy and will endure the long-term chronic effect of residual lesions, we must resist the temptation of measuring success of interventions on the short-term survival and complication rates. A sobering example of this is provided by the scientific literature on PVR in corrected TOF: the risks and benefits balance appeared favourable for the first decade after PVR, but longer-term studies have dampened our enthusiasm by failing to show unequivocal benefits.17,47, 48, 49 Retrospective study designs leveraging the existing and available data allow the capturing of long-term outcomes, which often cannot realistically be achieved by prospective designs.
Furthermore, the Canadian setting, with its universal health care insurance, enables the creation of population-based cohorts, therefore avoiding selection and referral biases and providing a true denominator. This improves internal and external validity and provides robust real-world evidence in an uncontrolled setting to shed light on the true effect of interventions, all the while taking into account even the patients lost to clinical follow-up.50
The linkage of granular clinical data with that of extensive administrative follow-up data is a significant strength. Investigators have often had to compromise between longer term but less precise administrative data and granular clinical data but with shorter follow-up and potential selection and referral bias.1,37,49 The use of clinical data for long-term follow-up could provide highly comprehensive data, but is challenging because a significant proportion of patients with TOF are transferred to other institutions for their adult care. On the other hand, the extraction of outcomes from an administrative data source allows for long-term-follow-up of nearly all subjects with low sample attrition. To our knowledge, the TRIVIA cohort is the first to combine precise cardiovascular phenotype, longitudinal assessment of cardiac morphology and function, and long-term systematic administrative data over a large unselected population of patients with TOF.
Limitations and mitigation strategies
Information bias is an inherent limitation of retrospective designs in which clinical data are manually abstracted from medical charts. We used a systematic and structured electronic form to extract clinical data from patients’ chart. Studies with a similar research setting and abstraction methods have shown that the validity and fidelity of manually collected clinical data in an electronic database are > 95%.51, 52, 53 To evaluate the validity of the collected data, we will cross-validate both clinical and administrative datasets to compute an agreement percentage, for variables available in both data sources. Missing data and attrition can also significantly hamper internal validity and generalization of results. To address this, we first combined 2 complementary sources of data, one of which contains populational governmental data that capture follow-up data, even for patients lost to clinical follow-up. Such systematically collected administrative health care data have been shown to provide a high degree of validity and robustness.54, 55, 56 Because clinical data are prone to a higher proportion of missing values, we will use contemporary Bayesian imputation methods that are widely recognized for their robustness and statistical validity.57, 58, 59 In particular, some patients with TOF will be followed in outreach clinics for which clinical data may not be available. The proportion of such patients is difficult to estimate, but cross-referencing clinical and billing data will be used to assess its magnitude. Nevertheless, we expected that most critical clinical variable will be available and imputation methods will be used for missing follow-up data.
Finally, retrospective designs provide less control on potential confounders. We will use multivariate multilevel models that will reduce the influence of potential differential distribution of confounders between groups to isolate the effect of the variables of interest and account for the covariance attributable to clustering variables such as surgical era and hospital centres. These models have shown high reliability in various simulation studies.60,61 Moreover, the use of correction score matching (eg, risk score) has been shown to be the most efficient method to reduce the potential of confounding by indication, over stratification, inverse-probability adjustment, and simple multivariate integration of preoperative factors.62, 63, 64 Some simulation studies reported a correction of the bias by nearly 99%, demonstrating the robustness and validity of this approach.63 Overall, it has been shown that these contemporary methodological approaches combined with innovative data collection schemes produce estimates with precision and validity that are equivalent to controlled prospective trials.65,66 Nevertheless, it is possible that residual confounding may be present, especially for surgical era and indication bias.
Conclusions
With the TRIVIA cohort, we seize the opportunity to link population-based clinical and universal health care administrative data to build a real-life unselected cohort that provides detailed cardiovascular assessment and long-term follow-up at low cost. We emphasize the feasibility and validity of this type of research in a Canadian setting as a result of accessible population-based administrative data and the use of modern statistical approaches. This will produce much-needed scientific evidence in the surgical management of TOF such as long-term evaluation of initial surgical correction and PVR, allowing the scaling of survival against the risk of reinterventions and adverse cardiovascular events. Results from this study will help to guide decision making of surgical management of TOF. Specifically, we will evaluate the long-term impact of the more aggressive approach of VS resulting in significant residual pulmonary stenosis and help identify which patients would benefit from an early PVR according to their clinical profile during adolescence.
Although the current protocol targets TOF, the TRIVIA cohort serves as a test case for creating population-based cohorts of subjects with CHD, keeping in mind that many of the methodological challenges are similar to other complex CHDs. The future lies on the continued enrichments of these cohorts with prospective accumulation of clinical data as well as with nested studies that bring in granular patient-reported outcomes. Harnessing the benefits of sharing data from multiple nested designs on the same cohort is our best hope of making more informed clinical decisions to ultimately improve quality of life and decreasing morbidity.
Acknowledgements
The authors would like to thank Jaime Colavincenzo, Harrison Saulnier, Vincent Hamilton, Steffany Poupart, Marianne McCarthy, Émilie Ma, Zhuyin Xu, and Sarah-Maria Sfairy for their valuable contribution to clinical data collection. The authors would also like to acknowledge the helpful and thorough advice of Liming Guo and Aihua Liu regarding administrative data cleaning and management.
Funding Sources
This project is funded by the Canadian Institute of Health Research (grant #PJT148812), the Fondation des Étoiles, the Fonds Bobeau Coeur, and Fondation du CHU Sainte-Justine. SB receives a PhD scholarship from the Fonds de Recherche du Québec – Santé. FD and AM receive salary support from the Fonds de Recherche du Québec – Santé.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: This study adheres to the guidelines of the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans.
See page 668 for disclosure information.
References
- 1.Marelli A.J., Ionescu-Ittu R., Mackie A.S. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation. 2014;130:749–756. doi: 10.1161/CIRCULATIONAHA.113.008396. [DOI] [PubMed] [Google Scholar]
- 2.Apitz C., Webb G.D., Redington A.N. Tetralogy of Fallot. Lancet. 2009;374:1462–1471. doi: 10.1016/S0140-6736(09)60657-7. [DOI] [PubMed] [Google Scholar]
- 3.Egbe A., Uppu S., Lee S., Ho D., Srivastava S. Changing prevalence of severe congenital heart disease: a population-based study. Pediatr Cardiol. 2014;35:1232–1238. doi: 10.1007/s00246-014-0921-7. [DOI] [PubMed] [Google Scholar]
- 4.Khairy P., Aboulhosn J., Gurvitz M.Z. Arrhythmia burden in adults with surgically repaired tetralogy of Fallot: a multi-institutional study. Circulation. 2010;122:868–875. doi: 10.1161/CIRCULATIONAHA.109.928481. [DOI] [PubMed] [Google Scholar]
- 5.Marelli A., Beauchesne L., Mital S., Therrien J., Silversides C.K. Canadian Cardiovascular Society 2009 Consensus Conference on the management of adults with congenital heart disease: introduction. Can J Cardiol. 2010;26:e65–e69. doi: 10.1016/s0828-282x(10)70353-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexiou C., Mahmoud H., Al-Khaddour A. Outcome after repair of tetralogy of Fallot in the first year of life. Ann Thorac Surg. 2001;71:494–500. doi: 10.1016/s0003-4975(00)02444-9. [DOI] [PubMed] [Google Scholar]
- 7.Boni L., Garcia E., Galletti L. Current strategies in tetralogy of Fallot repair: pulmonary valve sparing and evolution of right ventricle/left ventricle pressures ratio. Eur J Cardiothorac Surg. 2009;35:885–889. doi: 10.1016/j.ejcts.2009.01.016. discussion 889-90. [DOI] [PubMed] [Google Scholar]
- 8.Awori M.N., Mehta N.P., Mitema F.O., Kebba N. Optimal use of Z-scores to preserve the pulmonary valve annulus during repair of tetralogy of Fallot. World J Pediatr Congenit Heart Surg. 2018;9:285–288. doi: 10.1177/2150135118757991. [DOI] [PubMed] [Google Scholar]
- 9.Hoashi T., Kagisaki K., Meng Y. Long-term outcomes after definitive repair for tetralogy of Fallot with preservation of the pulmonary valve annulus. J Thorac Cardiovasc Surg. 2014;148:802–808. doi: 10.1016/j.jtcvs.2014.06.008. discussion 808-9. [DOI] [PubMed] [Google Scholar]
- 10.Gellis L., Banka P., Marshall A., Emani S., Porras D. Transcatheter balloon dilation for recurrent right ventricular outflow tract obstruction following valve-sparing repair of tetralogy of Fallot. Catheter Cardiovasc Interv. 2015;86:692–700. doi: 10.1002/ccd.25930. [DOI] [PubMed] [Google Scholar]
- 11.Geva T., Sandweiss B.M., Gauvreau K., Lock J.E., Powell A.J. Factors associated with impaired clinical status in long-term survivors of tetralogy of Fallot repair evaluated by magnetic resonance imaging. J Am Coll Cardiol. 2004;43:1068–1074. doi: 10.1016/j.jacc.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 12.Baumgartner H., Bonhoeffer P., De Groot N.M. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010) Eur Heart J. 2010;31:2915–2957. doi: 10.1093/eurheartj/ehq249. [DOI] [PubMed] [Google Scholar]
- 13.Gatzoulis M.A., Balaji S., Webber S.A. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet. 2000;356:975–981. doi: 10.1016/S0140-6736(00)02714-8. [DOI] [PubMed] [Google Scholar]
- 14.Oechslin E.N., Harrison D.A., Harris L. Reoperation in adults with repair of tetralogy of Fallot: indications and outcomes. J Thorac Cardiovasc Surg. 1999;118:245–251. doi: 10.1016/S0022-5223(99)70214-X. [DOI] [PubMed] [Google Scholar]
- 15.Therrien J., Siu S.C., McLaughlin P.R. Pulmonary valve replacement in adults late after repair of tetralogy of Fallot: are we operating too late? J Am Coll Cardiol. 2000;36:1670–1675. doi: 10.1016/s0735-1097(00)00930-x. [DOI] [PubMed] [Google Scholar]
- 16.Oosterhof T., van Straten A., Vliegen H.W. Preoperative thresholds for pulmonary valve replacement in patients with corrected tetralogy of Fallot using cardiovascular magnetic resonance. Circulation. 2007;116:545–551. doi: 10.1161/CIRCULATIONAHA.106.659664. [DOI] [PubMed] [Google Scholar]
- 17.Dave H.H., Buechel E.R., Dodge-Khatami A. Early insertion of a pulmonary valve for chronic regurgitation helps restoration of ventricular dimensions. Ann Thorac Surg. 2005;80:1615–1620. doi: 10.1016/j.athoracsur.2005.04.058. discussion 1620-1. [DOI] [PubMed] [Google Scholar]
- 18.Hickey E.J., Veldtman G., Bradley T.J. Functional health status in adult survivors of operative repair of tetralogy of Fallot. Am J Cardiol. 2012;109:873–880. doi: 10.1016/j.amjcard.2011.10.051. [DOI] [PubMed] [Google Scholar]
- 19.Gengsakul A., Harris L., Bradley T.J. The impact of pulmonary valve replacement after tetralogy of Fallot repair: a matched comparison. Eur J Cardiothorac Surg. 2007;32:462–468. doi: 10.1016/j.ejcts.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 20.de Ruijter F.T., Weenink I., Hitchcock F.J., Meijboom E.J., Bennink G.B. Right ventricular dysfunction and pulmonary valve replacement after correction of tetralogy of Fallot. Ann Thorac Surg. 2002;73:1794–1800. doi: 10.1016/s0003-4975(02)03586-5. discussion 1800. [DOI] [PubMed] [Google Scholar]
- 21.Egbe A.C., Vallabhajosyula S., Connolly H.M. Trends and outcomes of pulmonary valve replacement in tetralogy of Fallot. Int J Cardiol. 2020;299:136–139. doi: 10.1016/j.ijcard.2019.07.063. [DOI] [PubMed] [Google Scholar]
- 22.Marelli A. The future of adult congenital heart disease research: precision health services delivery for the next decade. Can J Cardiol. 2019;35:1609–1619. doi: 10.1016/j.cjca.2019.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Weiss J.B., Grant A., Marelli A. Assessment of electronic health information system use and need in US adult congenital heart disease centers. Congenit Heart Dis. 2011;6:134–138. doi: 10.1111/j.1747-0803.2011.00498.x. [DOI] [PubMed] [Google Scholar]
- 24.Villafane J., Feinstein J.A., Jenkins K.J. Hot topics in tetralogy of Fallot. J Am Coll Cardiol. 2013;62:2155–2166. doi: 10.1016/j.jacc.2013.07.100. [DOI] [PubMed] [Google Scholar]
- 25.Warnes C.A., Williams R.G., Bashore T.M. ACC/AHA 2008 Guidelines for the Management of Adults with Congenital Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease) Circulation. 2008;118:e714–833. doi: 10.1161/CIRCULATIONAHA.108.190690. [DOI] [PubMed] [Google Scholar]
- 26.Silversides C.K., Kiess M., Beauchesne L. Canadian Cardiovascular Society 2009 Consensus Conference on the management of adults with congenital heart disease: outflow tract obstruction, coarctation of the aorta, tetralogy of Fallot, Ebstein anomaly and Marfan's syndrome. Can J Cardiol. 2010;26:e80–97. doi: 10.1016/s0828-282x(10)70355-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geva T. Repaired tetralogy of Fallot: the roles of cardiovascular magnetic resonance in evaluating pathophysiology and for pulmonary valve replacement decision support. J Cardiovasc Magn Reson. 2011;13:9. doi: 10.1186/1532-429X-13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stout K.K., Daniels C.J., Aboulhosn J.A. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e698–800. doi: 10.1161/CIR.0000000000000603. [DOI] [PubMed] [Google Scholar]
- 29.Fanaroff A.C., Califf R.M., Windecker S., Smith S.C., Jr., Lopes R.D. Levels of evidence supporting American College of Cardiology/American Heart Association and European Society of Cardiology Guidelines, 2008-2018. JAMA. 2019;321:1069–1080. doi: 10.1001/jama.2019.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sackett D.L., Wennberg J.E. Choosing the best research design for each question. BMJ. 1997;315:1636. doi: 10.1136/bmj.315.7123.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryan A., Duignan S., Kenny D., McMahon C.J. Decision making in paediatric cardiology. Are we prone to heuristics, biases and traps? Pediatr Cardiol. 2018;39:160–167. doi: 10.1007/s00246-017-1742-2. [DOI] [PubMed] [Google Scholar]
- 32.Deaton A., Cartwright N. Understanding and misunderstanding randomized controlled trials. Soc Sci Med. 2018;210:2–21. doi: 10.1016/j.socscimed.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones D.S., Podolsky S.H. The history and fate of the gold standard. Lancet. 2015;385:1502–1503. doi: 10.1016/S0140-6736(15)60742-5. [DOI] [PubMed] [Google Scholar]
- 34.Murad M.H., Asi N., Alsawas M., Alahdab F. New evidence pyramid. Evid Based Med. 2016;21:125–127. doi: 10.1136/ebmed-2016-110401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seshia S.S., Young G.B. The evidence-based medicine paradigm: where are we 20 years later? Part 1. Can J Neurol Sci. 2013;40:465–474. doi: 10.1017/s0317167100014542. [DOI] [PubMed] [Google Scholar]
- 36.Harris P.A., Taylor R., Thielke R. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marelli A.J., Mackie A.S., Ionescu-Ittu R., Rahme E., Pilote L. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation. 2007;115:163–172. doi: 10.1161/CIRCULATIONAHA.106.627224. [DOI] [PubMed] [Google Scholar]
- 38.Rosenbaum P.R., Rubin D.B. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39:33–38. [Google Scholar]
- 39.Rosenbaum P.R., Rubin D.B. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 40.Rothman K.J., Greenland S., Lash T.L. Lippincott Williams & Wilkins; Philadelphia, PA: 2008. Modern Epidemiology. [Google Scholar]
- 41.Madley-Dowd P., Hughes R., Tilling K., Heron J. The proportion of missing data should not be used to guide decisions on multiple imputation. J Clin Epidemiol. 2019;110:63–73. doi: 10.1016/j.jclinepi.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16:219–242. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 43.Little R.J., Wang Y. Pattern-mixture models for multivariate incomplete data with covariates. Biometrics. 1996;52:98–111. [PubMed] [Google Scholar]
- 44.Cedars A., Benjamin L., Vyhmeister R. Contemporary hospitalization rate among adults with complex congenital heart disease. World J Pediatr Congenit Heart Surg. 2016;7:334–343. doi: 10.1177/2150135116639541. [DOI] [PubMed] [Google Scholar]
- 45.Hickey E.J., Veldtman G., Bradley T.J. Late risk of outcomes for adults with repaired tetralogy of Fallot from an inception cohort spanning four decades. Eur J Cardiothorac Surg. 2009;35:156–164. doi: 10.1016/j.ejcts.2008.06.050. discussion 164. [DOI] [PubMed] [Google Scholar]
- 46.Sackett D.L., Rosenberg W.M., Gray J.A., Haynes R.B., Richardson W.S. Evidence based medicine: what it is and what it isn't. BMJ. 1996;312:71–72. doi: 10.1136/bmj.312.7023.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferraz Cavalcanti P.E., Sa M.P., Santos C.A. Pulmonary valve replacement after operative repair of tetralogy of Fallot: meta-analysis and meta-regression of 3,118 patients from 48 studies. J Am Coll Cardiol. 2013;62:2227–2243. doi: 10.1016/j.jacc.2013.04.107. [DOI] [PubMed] [Google Scholar]
- 48.Hallbergson A., Gauvreau K., Powell A.J., Geva T. Right ventricular remodeling after pulmonary valve replacement: early gains, late losses. Ann Thorac Surg. 2015;99:660–666. doi: 10.1016/j.athoracsur.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 49.Dobbels B., Herregods M.C., Troost E. Early versus late pulmonary valve replacement in patients with transannular patch-repaired tetralogy of Fallot. Interact Cardiovasc Thorac Surg. 2017;25:427–433. doi: 10.1093/icvts/ivx118. [DOI] [PubMed] [Google Scholar]
- 50.Blonde L., Khunti K., Harris S.B., Meizinger C., Skolnik N.S. Interpretation and impact of real-world clinical data for the practicing clinician. Adv Ther. 2018;35:1763–1774. doi: 10.1007/s12325-018-0805-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han J., Chen K., Fang L. Improving the efficacy of the data entry process for clinical research with a natural language processing-driven medical information extraction system: quantitative field research. JMIR Med Inform. 2019;7 doi: 10.2196/13331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hong M.K., Yao H.H., Pedersen J.S. Error rates in a clinical data repository: lessons from the transition to electronic data transfer—a descriptive study. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2012-002406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walther B., Hossin S., Townend J. Comparison of electronic data capture (EDC) with the standard data capture method for clinical trial data. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mackie A.S., Ionescu-Ittu R., Pilote L., Rahme E., Marelli A.J. Hospital readmissions in children with congenital heart disease: a population-based study. Am Heart J. 2008;155:577–584. doi: 10.1016/j.ahj.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 55.Mackie A.S., Pilote L., Ionescu-Ittu R., Rahme E., Marelli A.J. Health care resource utilization in adults with congenital heart disease. Am J Cardiol. 2007;99:839–843. doi: 10.1016/j.amjcard.2006.10.054. [DOI] [PubMed] [Google Scholar]
- 56.Bouchardy J., Therrien J., Pilote L. Atrial arrhythmias in adults with congenital heart disease. Circulation. 2009;120:1679–1686. doi: 10.1161/CIRCULATIONAHA.109.866319. [DOI] [PubMed] [Google Scholar]
- 57.Chen Q., Williams S.Z., Liu Y., Chihuri S.T., Li G. Multiple imputation of missing marijuana data in the Fatality Analysis Reporting System using a Bayesian multilevel model. Accid Anal Prev. 2018;120:262–269. doi: 10.1016/j.aap.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 58.Pedersen A.B., Mikkelsen E.M., Cronin-Fenton D. Missing data and multiple imputation in clinical epidemiological research. Clin Epidemiol. 2017;9:157–166. doi: 10.2147/CLEP.S129785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rubin D.B. John Wiley & Sons; Hoboken, NJ: 2004. Multiple Imputation for Nonresponse in Surveys. [Google Scholar]
- 60.Greenland S. When should epidemiologic regressions use random coefficients? Biometrics. 2000;56:915–921. doi: 10.1111/j.0006-341x.2000.00915.x. [DOI] [PubMed] [Google Scholar]
- 61.Witte J.S., Greenland S. Simulation study of hierarchical regression. Stat Med. 1996;15:1161–1170. doi: 10.1002/(SICI)1097-0258(19960615)15:11<1161::AID-SIM221>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 62.Austin P.C. The relative ability of different propensity score methods to balance measured covariates between treated and untreated subjects in observational studies. Med Decis Making. 2009;29:661–677. doi: 10.1177/0272989X09341755. [DOI] [PubMed] [Google Scholar]
- 63.Austin P.C., Grootendorst P., Anderson G.M. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med. 2007;26:734–753. doi: 10.1002/sim.2580. [DOI] [PubMed] [Google Scholar]
- 64.Rosenbaum P.R., Rubin D.B. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. 1984;79:516–524. [Google Scholar]
- 65.Concato J., Shah N., Horwitz R.I. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000;342:1887–1892. doi: 10.1056/NEJM200006223422507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Golder S., Loke Y.K., Bland M. Meta-analyses of adverse effects data derived from randomised controlled trials as compared to observational studies: methodological overview. PLoS Med. 2011;8 doi: 10.1371/journal.pmed.1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]