Abstract
Background
The Hearts in Rhythm Organization (HiRO) is a team of Canadian inherited heart rhythm and cardiomyopathy experts, genetic counsellors, nurses, researchers, patients, and families dedicated to the detection of inherited arrhythmias and cardiomyopathies, provision of best therapies, and protection from the tragedy of sudden cardiac arrest.
Methods
Recently, existing disease-specific registries were merged into the expanded National HiRO Registry, creating a single common data set for patients and families with inherited conditions that put them at risk for sudden death in Canada. Eligible patients are invited to participate in the registry and optional biobank from 20 specialized cardiogenetics clinics across Canada.
Results
Currently, there are 4700 participants enrolled in the National HiRO Registry, with an average of 593 participants enrolled annually over the past 5 years. The capacity to enable knowledge translation of research findings is built into HiRO’s organizational infrastructure, with 3 additional working groups (HiRO Clinical Care Committee, HiRO Active Communities Committee, and HiRO Annual Symposium Committee), supporting the organization’s current goals and priorities as set alongside patient partners.
Conclusion
The National HiRO Registry aims to be an integrated research platform to which researchers can pose novel research questions leading to a better understanding, detection, and clinical care of those living with inherited heart rhythm and cardiomyopathy conditions and ultimately to prevent sudden cardiac death.
Résumé
Contexte
La Hearts in Rhythm Organization (HiRO) est une équipe d’experts canadiens en matière de rythmes cardiaques et de cardiomyopathies héréditaires, de conseillers en génétique, d’infirmières, de chercheurs, de patients et de familles qui se consacrent à la détection des arythmies et des cardiomyopathies héréditaires, à la mise en place des meilleures thérapies et à la protection contre la tragédie que représente une mort subite d’origine cardiaque.
Méthodes
Récemment, les registres existants relatifs à des maladies spécifiques ont été fusionnés en un registre national élargi de l’HiRO, créant ainsi un ensemble de données commun unique à destination des patients et leurs familles, atteints de maladies héréditaires, qui sont à risque de mort subite au Canada. Les patients admissibles sont invités à s’associer au registre et à la biobanque facultative regroupant 20 cliniques spécialisées en cardiogénétique au Canada.
Résultats
Actuellement, 4 700 participants sont inscrits au registre national de l’HiRO, avec une moyenne de 593 participants inscrits chaque année au cours des cinq dernières années. La capacité à favoriser l’application des connaissances issues de la recherche fait partie de la structure organisationnelle de l’HiRO, avec trois groupes de travail supplémentaires (comité des soins cliniques de l’HiRO, comité des communautés cctives de l’HiRO et comité du symposium annuel de l’HiRO), soutenant les objectifs et les priorités actuels de l’organisation tels qu’ils ont été fixés en partenariat avec les patients.
Conclusion
Le registre national de l’HiRO vise à devenir une plateforme de recherche intégrée au sein de laquelle les chercheurs peuvent exposer des questions de recherche inédites permettant de mieux comprendre, détecter et soigner les personnes atteintes de troubles du rythme cardiaque et de cardiomyopathie héréditaires et, à terme, de prévenir la mort subite d’origine cardiaque.
Inherited heart rhythm and cardiomyopathy conditions are estimated to affect 1/200 Canadians, with the most common conditions being hypertrophic (HCM) and arrhythmogenic cardiomyopathies, Brugada syndrome, and long QT syndrome (LQTS).1, 2, 3, 4 Given that in most of these disorders, symptoms occur in a minority of potentially affected individuals (5%-20%), many living with these conditions are unaware of their increased risk of sudden cardiac death (SCD). Ultimately, deaths due to inherited cardiac conditions contribute to a proportion of the approximately 30,000 Canadians who die each year due to sudden cardiac arrest, with deaths due an inherited cause more likely to affect Canadians at a young age.5,6 Once detected, treatments for these conditions are often simple, inexpensive, and highly effective at reducing SCD risk.7,8 This highlights the importance of detecting at-risk individuals with family screening, and recognition of that risk leading to expert directed prevention strategies.
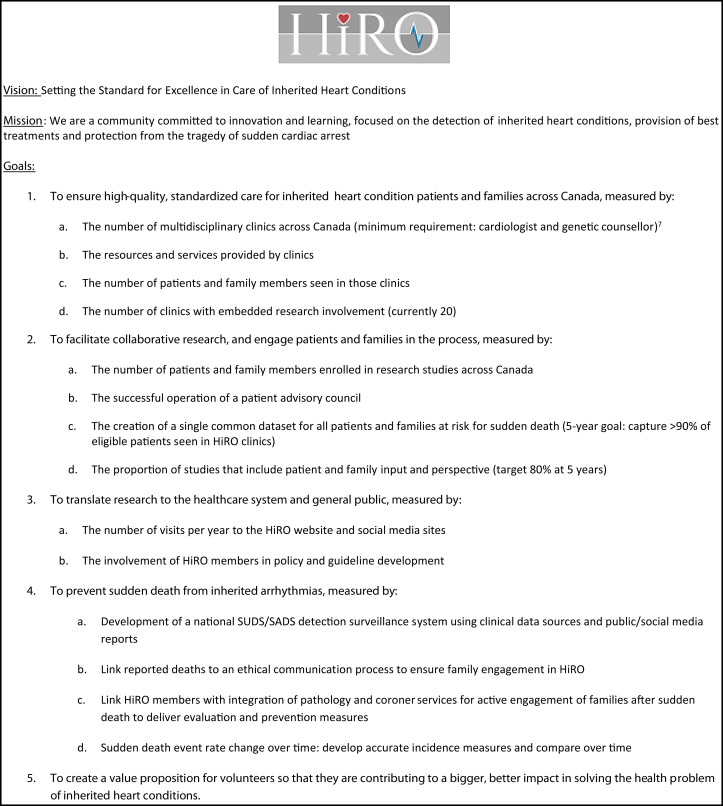
The Hearts in Rhythm Organization (HiRO, pronounced hero, https://heartsinrhythm.ca) is a team of Canadian inherited heart rhythm and cardiomyopathy experts, genetic counsellors, nurses, researchers, patients, and families dedicated to improving the awareness and detection of inherited heart rhythm disorders and cardiomyopathies. This organization was founded by the Canadian Genetic Heart Rhythm group, an existing collaboration of Canadian clinician-investigators working together on 3 national research registries, including the Cardiac Arrest Survivors with Preserved Ejection Fraction Registry (CASPER),9, 10, 11, 12 the Canadian Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC) Registry,13 and the National Long QT Syndrome Registry (LQTS).14, 15, 16 In 2016, this group of investigators expanded their annual research meeting to include knowledge users, extending invitations to health care professionals and trainees from Canada’s inherited heart rhythm and cardiomyopathy clinics, as well as patients and families living with these disorders. HiRO has since grown into a comprehensive national team, integrating clinical excellence, research, education, and patient engagement to identify genetic causes of SCD and devise effective screening and prevention systems. The vision for HiRO is to set the standard for excellence in care of inherited heart conditions (Fig. 1).
Figure 1.
The Hearts in Rhythm Organization (HiRO) vision, mission, and goals. SADS, sudden arrhythmogenic death syndrome; SUDS, sudden unexplained death syndrome.
Methods
Registry recruitment
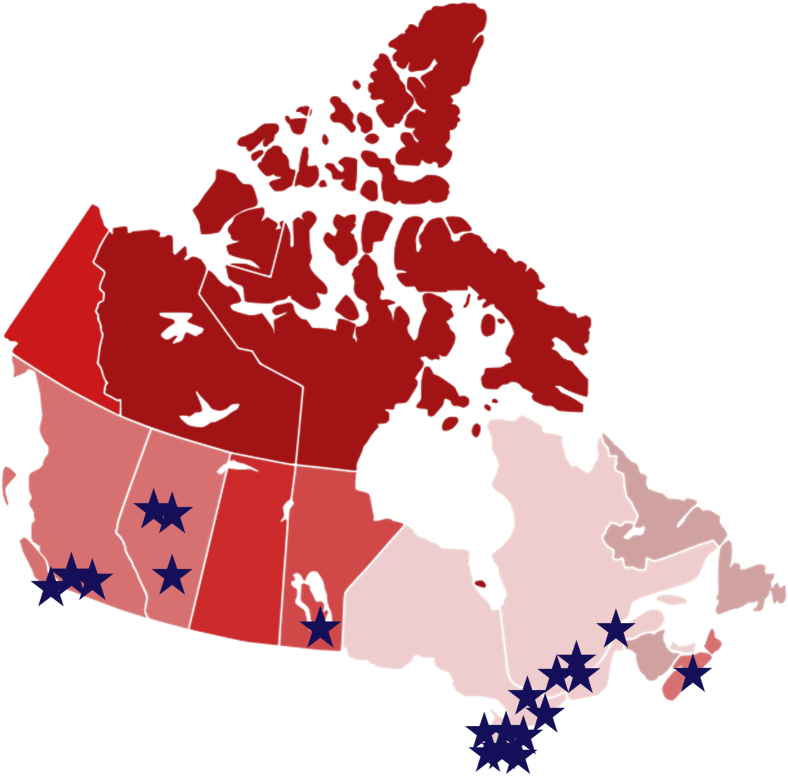
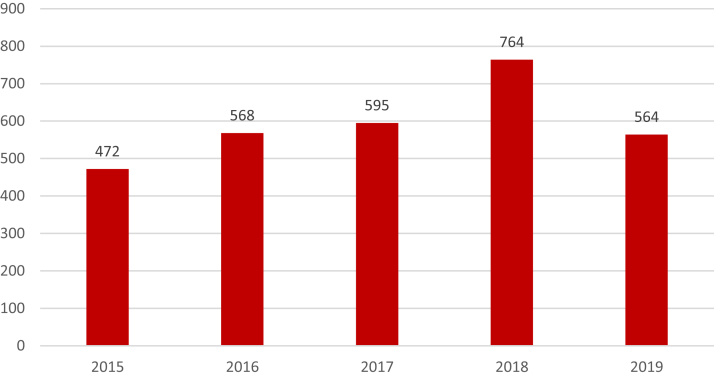
Central to HiRO’s vision is the National HiRO Registry, a comprehensive research registry that recently merged the existing CASPER, National ARVC, and National LQTS registries into 1 standardized protocol with expanded inclusion criteria and unified research objectives. Patients can participate in the National HiRO Registry from 20 specialized cardiogenetics clinics across Canada (Fig. 2).17 Eligible patients are invited to participate in the registry and optional biobank (Table 1). Any patients with a known inherited heart rhythm or cardiomyopathy condition, or an unexplained cardiac arrest, and their first-degree family members are eligible if willing to sign consent to share their health care information.18 Sudden death cases are also included in the registry postmortem with the consent of the estate executor. Dilated cardiomyopathy without a known genetic variant or positive family history, aortopathies, and familial hypercholesterolemia cases are excluded. Participants are able to provide consent to be contacted for future research opportunities (Supplemental Appendix S1). Currently, there are 4700 participants enrolled in the National HiRO Registry, with an average of 593 participants enrolled annually over the past 5 years (Fig. 3).
Figure 2.
National Hearts in Rhythm Organization (HiRO) Registry participating centres.
Table 1.
National Hearts in Rhythm Organization (HiRO) Registry inclusion and exclusion criteria
| HiRO Registry inclusion criteria | HiRO Registry exclusion criteria |
|---|---|
|
Inherited arrhythmias (IA): long QT syndrome (LQT), short QT syndrome (SQT), catecholaminergic polymorphic ventricular tachycardia (CPVT), Brugada (BrS), arrhythmogenic right ventricular cardiomyopathy (ARVC), familial cardiac conduction disease (FCCD) Inherited cardiomyopathies (ICM): hypertrophic cardiomyopathy (HCM), Mendelian dilated cardiomyopathy (DCM) including lamin and phosopholambin (LMNA and PLN), and left ventricular noncompaction (LVNC) Unexplained cardiac arrest syndromes: including early repolarization (ER), idiopathic ventricular fibrillation (IVF), short coupled IVF (SCIF), polymorphic ventricular tachycardia not otherwise diagnosed (PMVT, NYD), sudden arrhythmic death syndromes (SADS), and malignant mitral valve prolapse (MVP) Deceased cases of sudden cardiac death: suspicious for an inherited arrhythmia/cardiomyopathy condition Carriers of a pathogenic or likely-pathogenic variant in an IA- or ICM-related gene not otherwise meeting criteria Unaffected first- and second-degree relatives of anyone meeting the above criteria |
Known sarcoidosis Mitral valve prolapse unless unexplained cardiac arrest or syncope with documented PMVT Heart failure/nonfamilial dilated cardiomyopathy without a positive family history of affected FDRs or SDRs Aortopathies including Marfan syndrome, Ehlers Danlos, familial thoracic aortic aneurysm, and dissection Neuromuscular disease Familial hypercholesterolemia |
FDR, first-degree relative; SDR, second-degree relative.
Figure 3.
Annual enrollment of participants in the National Hearts in Rhythm Organization (HiRO) Registry since 2015.
Biobank samples
Participants in the National HiRO Registry are also invited to provide a blood sample to be stored. Both whole blood for DNA extraction and serum for biomarker studies are now collected, although samples collected before 2019 predominantly stored DNA only.
All biobank samples collected as part of an active substudy are stored in a national core biobank. Biobank samples drawn on participants not enrolled in an active substudy may be stored at local sites or in the national biobank at the local investigators’ discretion.
Investigators requesting stored biobank samples for genetic studies will be solicited to return genetic data for inclusion in the HiRO Registry. Over time, the HiRO Registry aims to generate a genomic sequencing databank for future studies as an alternative to using stored samples.
Novel genetic variants identified as part of a study using HiRO biobank samples will be classified by the study team according to the 2015 American College of Medical Genetics and Association for Molecular Pathology standards and guidelines for the interpretation of sequence variants.19 Any variant classified as pathogenic or likely pathogenic in a gene associated with an inherited heart rhythm or cardiomyopathy condition will be returned to local investigators to be shared with the study participant. An ethics board approved process for results disclosure is in place. Incidental or secondary genetic findings not related to cardiac health are not returned to participants. A genetic counsellor is available as part of the HiRO research team to facilitate in conveying results of future studies using HiRO samples.
Data collection
Following the informed consent process, a core baseline data set is collected for all National HiRO Registry participants, including clinical demographics and health history, family history, clinical genetic testing results, and a list of cardiac testing performed. A baseline electrocardiogram (ECG) is also collected, with a preferred XML file format. If available, the variant call files or binary alignment map files are requested from the clinical genetic testing laboratory for inclusion in the registry. This allows clinical genetic testing results to be reanalyzed over time. Each participant is assigned a working diagnosis and strength according to study specific definitions (Table 2).9 This core data set was developed with the primary goal of facilitating case finding for future research studies (Supplemental Appendix S2).
Table 2.
National Hearts in Rhythm Organization (HiRO) Registry criteria for assigning working diagnosis
| Strength = Definite | Strength = Probable | Strength = Possible | |
|---|---|---|---|
| LQTS7 | LQTS risk score ≥ 3.5 in the absence of a secondary cause for QT prolongation and/or Unequivocally pathogenic variant in one of the LQTS genes and/or QTc ≥ 500 ms in repeated 12-lead electrocardiogram (ECG) in the absence of a secondary cause for QT prolongation |
QTc between 480 and 499 ms in repeated 12-lead ECGs in a patient with unexplained syncope in the absence of a secondary cause for QT prolongation and in the absence of a pathogenic variant | LQTS risk score 2.0-3.5 in the presence of a family history of definite LQTS that is genotype negative or when genetic testing has not been performed |
| Acquired LQTS (aLQTS) | LQTS risk score ≥ 3.5 in the presence of a secondary cause of QT prolongation and/or QTc ≥ 500 ms in the presence of a secondary cause of QT prolongation |
QTc between 480 and 499 ms in a patient with unexplained syncope in the presence of a secondary cause of QT prolongation | NA |
| BrS7 | ST elevation with type 1 morphology ≥ 2 mm in ≥ 1 of the right precordial leads V1-V2 positioned in the 4th, 3rd, or 2nd intercostal spaces, either spontaneously or after provocative drug test with IV class 1 drugs | Unequivocal pathogenic variant in SCN5A leading to decreased Nav1.5 function in the presence of family history of definite BrS or in the context of a molecular autopsy | ST elevation with type 2 morphology and provocative testing has not been performed in presence of family history of definite BrS |
| CPVT7 | Structurally normal heart, normal ECG, and unexplained exercise or catecholamine-induced bidirectional VT or PVCs or VT in an individual younger than 40 y and/or Presence of an unequivocal pathogenic variant and/or Family members of a CPVT index case with a normal heart who manifest exercise-induced premature ventricular contractions or bidirectional/polymorphic VT |
Structurally normal heart, normal ECG, and unexplained exercise or catecholamine-induced bidirectional VT or PVCs) or VT in an individual older than 40 y | NA |
| SQTS7 | QTc < 330 ms | QTc < 360 ms and one or more of:
|
Unequivocal pathogenic variant carrier with a QTc ≥ 360 ms |
| ERS7 | J-point elevation ≥ 1 mm in ≥ 2 contiguous inferior and/or lateral leads of a 12-lead in a patient resuscitated from otherwise unexplained VF or polymorphic VT | SCD victim with a negative autopsy and medical chart review, with a previous ECG demonstrating J-point elevation ≥ 1 mm in ≥ 2 contiguous inferior and/or lateral leads of a standard 12-lead ECG | NA |
| SCVF (Steinberg) | Short-coupled PVCs (RR < 350 ms) triggering polymorphic VT/VF, where other known electrical and myocardial diseases have been excluded | Resuscitated ventricular fibrillation and documented recurrent short-coupled PVCs (< 350 ms) without demonstration of PVC-triggered VT/VF, where other known electrical and myocardial diseases have been excluded | NA |
| UCA/IVF7,20,21 | Resuscitated cardiac arrest from a shockable rhythm, where known etiologies have been excluded, using cardiac imaging, stress/epinephrine, and procainamide testing | Resuscitated cardiac arrest from a shockable rhythm, where known etiologies have been partially excluded | NA |
| SADS | Sudden cardiac death with negative toxicology and normal autopsy including cardiac pathology expertise, not otherwise fulfilling diagnostic criteria of specific syndromes | Sudden cardiac death with negative toxicology and normal autopsy without cardiac pathology expertise or with nondiagnostic cardiac abnormalities, not otherwise fulfilling diagnostic criteria of specific syndromes | Sudden cardiac death below age 40 in an otherwise healthy individual with incomplete postmortem assessment (autopsy and toxicology) |
| Polymorphic VT | Syncope with documented polymorphic VT without cardiac arrest, where known etiologies have been excluded | NA | NA |
| HCM22 | Wall thickness ≥ 15 mm (z-score ≥ 2 in children) in 1 or more LV myocardial segments that is not explained solely by loading conditions (eg, SBP > 160), excluding isolated basal septal hypertrophy in the elderly and/or Wall thickness ≥ 13 mm in first-degree relatives of patients with definite HCM or with a pathogenic variant |
NA | Wall thickness 13-14 mm in one or more LV myocardial segments that is not explained solely by loading conditions (eg, SBP > 160), excluding isolated basal septal hypertrophy in the elderly, in the absence of first-degree relatives of patients with definite HCM |
| DCM | LV systolic dysfunction (LVEF < 50%) AND enlargement, that is not explained by abnormal loading conditions, coronary artery disease, or a recent cardiac arrest | NA | NA |
| ARVC23 | Task Force criteria: 2 major or 1 major and 2 minor criteria or 4 minor from different categories | Task Force criteria: 1 major and 1 minor or 3 minor criteria from different categories | Task Force criteria: 1 major or 2 minor criteria from different categories |
| LVNC | LVNC diagnosed by TTE or CMR | NA | NA |
| UCM | Unclassified cardiomyopathy: presence of cardiomyopathy not fulfilling diagnostic criteria for the 4 other entities, eg, presence of significant fibrosis on magnetic resonance. Describe clinical findings in comments | UCA/SCD with a pathogenic or likely pathogenic variant in a cardiomyopathy gene but no cardiomyopathy phenotype | NA |
| Myocarditis24,25 | Endomyocardial biopsy-confirmed myocarditis (Dallas criteria) | Clinically suspected myocarditis according to published criteria including CMR evidence, in the absence of an endomyocardial biopsy | Clinically suspected myocarditis according to published criteria in the absence of cardiac magnetic resonance imaging and endomyocardial biopsy |
| Coronary spasm26 | Evidence of angina in the absence of fixed coronary artery stenosis > 50% AND Transient ischemic ECG changes during the spontaneous episodes and/or a positive acetylcholine/ergonovine test showing evidence of > 90% coronary vasoconstriction |
Polymorphic VT/VF in the absence of fixed coronary artery stenosis > 50% or another etiology AND a positive acetylcholine/ergonovine test showing evidence of > 90% coronary vasoconstriction | Evidence of nitrate-responsive angina in the absence of transient ischaemic ECG changes and coronary artery spasm |
| Malignant mitral valve prolapse syndrome27 | Presence of bileaflet mitral valve prolapse in a patient with otherwise unexplained polymorphic VT/VF with frequent complex PVCs thought to originate from the papillary muscle | Presence of bileaflet mitral valve prolapse in a patient with otherwise unexplained polymorphic VT/VF in the absence of frequent PVCs originating from the papillary muscle OR Presence of single leaflet mitral valve prolapse in a patient with otherwise unexplained polymorphic VT/VF and evidence of myocardial fibrosis |
Presence of single leaflet mitral valve prolapse in a patient with otherwise unexplained polymorphic VT/VF, without myocardial fibrosis or without assessment for myocardial fibrosis |
| Pause-dependent VT/VF | Polymorphic VT/VF in the context of severe bradycardia with recurrent documented PVCs/VT after pauses | NA | NA |
| Unaffected/normal | Family member negative for known familial mutation with normal cardiac investigations | Family member with normal cardiac investigations where genetic testing is negative in proband or unavailable OR Family member negative for known familial mutation with borderline cardiac investigations not otherwise fulfilling diagnostic criteria |
NA |
| Unclassified genetic variant carrier | Phenotypically unaffected carrier of a pathogenic or likely pathogenic variant not otherwise fitting other diagnostic criteria | Phenotypically unaffected carrier of a variant(s) of unknown significance not otherwise fitting other diagnostic criteria | NA |
ARVC, arrhythmogenic right ventricular cardiomyopathy; BrS, Brugada syndrome; CMR, cardiovascular magnetic resonance imaging; CPVT, catecholaminergic polymorphic ventricular tachycardia; DCM, dilated cardiomyopathy; ERS, early repolarization syndrome; HCM, hypertrophic cardiomyopathy; IV, intravenous; IVF, idiopathic ventricular fibrillation; LQTS, long QT syndrome; LVEF, left ventricular ejection fraction; LVNC, left ventricular noncompaction; PVC, premature ventricular contraction; SADS, sudden arrhythmogenic death syndrome; SBP, systemic blood pressure; SCD, sudden cardiac death; SCVF, short coupled ventricular fibrillation; SQTS, short QT syndrome; TTE, transthoracic echocardiogram; UCA, unexplained cardiac arrest; UCM, unclassified cardiomyopathy; VF, ventricular fibrillation; VT, ventricular tachycardia.
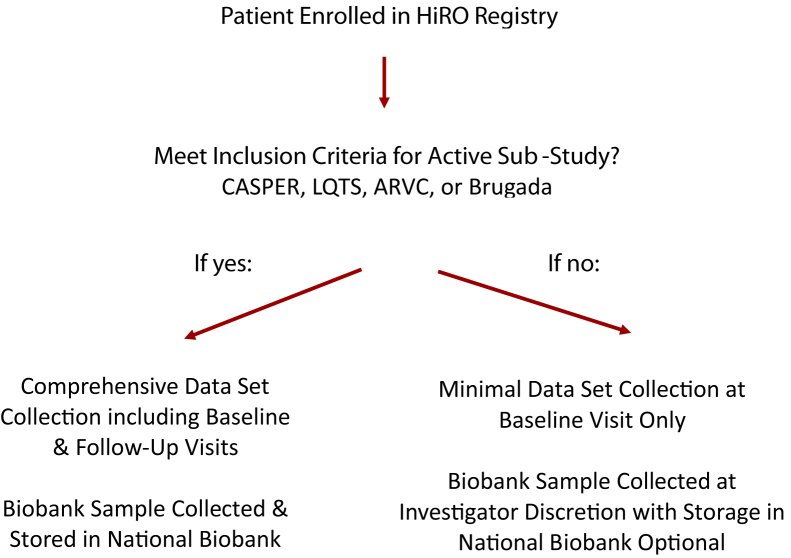
A more expansive data set, including results of clinical cardiac investigations, medication history, disease-specific variables, and ongoing follow-up data, is collected for cases that meet active substudy inclusion criteria (Fig. 4). Currently, active substudies include the existing CASPER, National ARVC Registry, National LQTS Registry as well as the newly created National Brugada Registry. Inclusion criteria for each of these substudy registries can be found in Table 3. All CASPER substudy participants with an unexplained cardiac arrest whose underlying etiology remains unexplained after systematic clinical evaluation have case data included in the “Role of Electrophysiology Testing in Survivors of Unexplained Cardiac Arrest” (EPS ARREST) registry (clinicaltrials.gov: NCT03079414), evaluating the role of the invasive electrophysiology study in this patient population. Funding to create a National HCM Registry and Biobank has been recently obtained and will be available as of April 2020. In addition to the current HiRO Registry enrolling sites, the HCM registry and Biobank will also include specialized HCM clinics.
Figure 4.
Data set collection for the National Hearts in Rhythm Organization (HiRO) Registry and HiRO Registry substudies. ARVC, Arrhythmogenic Right Ventricular Cardiomyopathy; CASPER, Cardiac Arrest Survivors with Preserved Ejection Fraction Registry; LQTS, Long QT Syndrome Registry.
Table 3.
Inclusion criteria for Hearts in Rhythm Organization (HiRO) substudies with comprehensive data collection and longitudinal follow-up
| HiRO Registry substudies | Inclusion criteria | Exclusion criteria |
|---|---|---|
| CASPER1 |
|
|
| National ARVC Registry2 |
|
|
| National LQTS Registry |
|
|
| National Brugada Registry |
|
|
ARVC, arrhythmogenic right ventricular cardiomyopathy; CASPER, Cardiac Arrest Survivors with Preserved Ejection Fraction Registry; ECG, electrocardiogram; IV, intravenous; LQTS, long QT syndrome; LVEF, left ventricular ejection fraction; TFC, task force criteria.
The National HiRO Registry uses a custom research data gathering, management, and reporting system known as Pedigree Pro (PDG) to facilitate electronic data capture, generate reports, and track biobank samples for all enrolled participants. PDG is entirely web-based and accessed online through a virtual private cloud server hosted on Canada’s installation of Amazon Web Service. No personal identifying information is included in the data system, with all documents deidentified by the site research coordinator before being uploaded into the data system. Cases entered in the HiRO Registry are assigned a unique study identifier that is recorded alongside the participant’s name on a master list decoder sheet (.ods file), with all family cases enrolled across Canada linked via an assigned family identifier. PDG offers investigator sites the ability to display and search identified data contained on the master decoder sheet by loading the master decoder sheet into the PDG interface. The data system can read information contained on the file and allow an investigator site user to search the system by patient name without the personal identifiers of the participants being compromised. However, this information is not included in the data system and does not leave the investigator's research office. Each biobank sample is also coded with a unique barcode, which is scanned directly into the system, linking the sample to the case data in addition to tracking the sample's location. Participating research investigator centres can access data and generate reports for their local participants. Access to the entire HiRO data system is restricted to the HiRO coordinating centre and the HiRO steering committee.
In addition to online data entry, PDG supports the upload of clinical documents in multiple file formats, allowing for diagnostic tests to be uploaded directly into the system. This enables a core lab approach to test interpretation, with cardiac test results being entered in the data system by study team members not involved in the clinical care of the participant. Custom tools for online test reading, such as electronic calipers for ECG reading, are built-in directly to PDG. Furthermore, the data system allows the upload of file formats that contain certain variables stored as metadata, including ECGs as XML files and echocardiogram or magnetic resonance imaging files as DICOM images. When these files are uploaded to PDG, any data variables collected in the National HiRO Registry are extracted from metadata and automatically populated, eliminating the need for manual data entry. Furthermore, ECGs as XML files have the advantage of automated, client-side deidentification, removing the need for manual deidentification before data system upload.
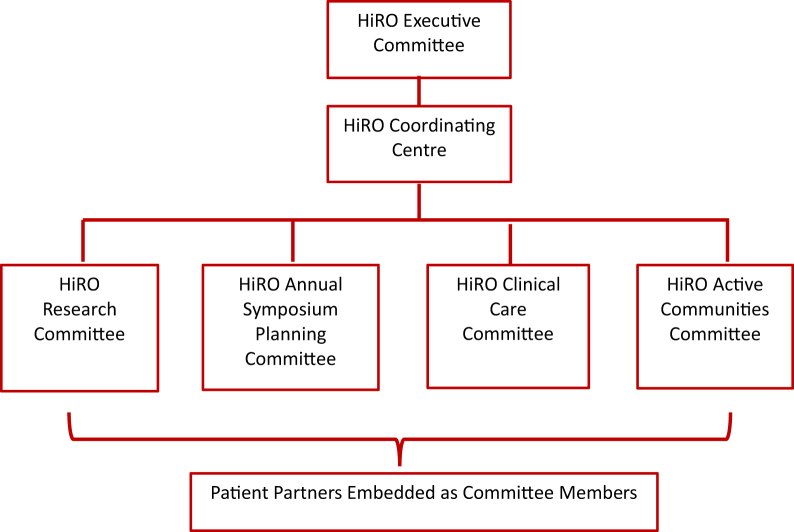
HiRO organizational structure
Overseen by the HiRO Executive Committee, HiRO’s organizational structure is currently composed of 4 multidisciplinary working groups, which align with the organizations current goals and priorities as set alongside HiRO patient partners (Fig. 5). These working groups are supported by the HiRO Coordinating Centre, providing infrastructure support by organizing meetings, facilitating communication between groups, and overseeing the HiRO website and social media accounts (https://heartsinrhythm.ca, @HeartsInRhythm).
Figure 5.
The Hearts in Rhythm Organization (HiRO) organizational structure.
HiRO Research Committee: The National HiRO Registry
The HiRO Research Committee’s primary mandate is to oversee design, recruitment, data collection, and study proposal review for the National HiRO Registry. The recent combination of the existing CASPER, ARVC, LQTS national registries and novel Brugada registry into 1 umbrella registry will allow for improved resource utilization, more efficient knowledge translation efforts, and a better capacity to answer novel research questions, particularly for genetic conditions in which phenotypes may vary. Overall, the National HiRO Registry aims to involve a majority of Canadian inherited heart rhythm and cardiomyopathy patients in clinical research, to develop a large case registry of participants at risk of SCD due to genetic conditions, as well as their unaffected first-degree relatives. The primary study objectives of the National HiRO Registry are to:
-
(a)
Better characterize the natural history and disease progression of inherited heart rhythm and cardiomyopathy patients by identifying cardiovascular and genetic risk factors for life-threatening arrhythmia.
-
(b)
Establish genotype-phenotype correlations to compare cases with known disease-causing genetic variants, evaluate novel gene modifiers, and discover genetic mechanisms of disease by harnessing stored biobank samples.
-
(c)
Develop risk model outputs and surveillance systems to prevent SCD from inherited heart rhythm and cardiomyopathy conditions.
-
(d)
Develop new care pathways, including improved guidance for prevention and treatment of inherited heart rhythm and cardiomyopathy conditions in addition to facilitating knowledge translation.
HiRO Clinical Care Committee
The HiRO Clinical Care Committee is composed of cardiac genetic counsellors, nurses, physicians, and patient/family partners working together to standardize and improve delivery of care across provinces for those living with inherited heart rhythm and cardiomyopathy conditions. A primary goal of the clinical care committee is to ensure that HiRO research findings are incorporated into future diagnostic and care guidelines. The HiRO clinic care committee publishes a quarterly newsletter sharing the practices and structure of different Canadian clinics, as well as highlighting new research publications that may impact clinical care practices.28 Additional initiatives have included the creation of Provincial Inherited Heart Rhythm Disorder eConsult programs in Ontario and British Columbia, allowing family physicians and other specialists to ask patient-specific clinical questions to the HiRO specialists in both provinces via a secure online portal.29 Members of the HiRO clinical care committee have also compiled a clinical care toolkit to share patient resources, letter templates, triage guidelines, and other clinical tools amongst the provincial cardiogenetic clinics (Supplemental Appendix S3).30
HiRO Active Communities
The HiRO Active Communities team is composed of heart rhythm and sports cardiology specialists, researchers, patients/families, and community advocates striving to improve the safety of Canadians at risk of sudden cardiac arrest in their local communities. Members of this committee are actively working with provincial government agencies to advocate for improved public screening and resuscitation measures, including introducing legislation mandating cardiopulmonary resuscitation and automated external defibrillator training in schools. In addition, members of the HiRO Active Communities team are collaborating with other Canadian organizations including Cardiac Arrest Response and Education, Canadian Cardiovascular Safety in Sport Network, Heart and Stroke, Canadian Heart Rhythm Society, and Cardiac Arrhythmia Network of Canada to ensure that the needs and opinions of inherited heart rhythm and cardiomyopathy patients and families are taken into consideration when working to improve the safety of Canadian communities.
HiRO Annual Symposium Committee
The HiRO Annual Symposium Committee is responsible for the planning and facilitation of the annual HiRO Symposium, a meeting bringing together stakeholders from across Canada to review the ongoing HiRO initiatives and relevant clinical updates, and set priorities for the following year. Beginning in 2016, the HiRO has held 4 annual symposia, bringing together Canada’s inherited heart rhythm and cardiomyopathy care teams, researchers, patients, and families for a 2-day meeting. These symposia focus on group discussions to identify differences in care delivery models between provinces, share recent research findings, and identify patient priorities for future awareness goals and research studies. Alongside the HiRO Symposium, a public forum is held annually to engage the local community, with patients, health care providers, students, and community media invited to attend. Past public forums have also included partnership with Heart and Stroke to facilitate community cardiopulmonary resuscitation and automated external defibrillator training opportunities.
Discussion
The National HiRO Registry aims to be an integrated research platform to which Canadian researchers can pose novel research questions leading to a better understanding, detection, and clinical care of those living with inherited heart rhythm and cardiomyopathy conditions and ultimately to prevent SCD in unsuspecting healthy individuals. Although there are many international registries collecting data on the similar patient populations, the majority focus only on participants and family members of those who have experienced a cardiac arrest or sudden death (eg, Danish Cardiac Arrest registry), or focus on patients with a single disease seen at large tertiary referral centres (eg, International LQTS Registry).31 Both of these registry designs may represent a more severely affected cohort than the general population. By broadening the inclusion criteria of the National HiRO Registry to include multiple phenotypes, this registry will be better equipped to take a genotype-first approach when designing studies, providing researchers the opportunity to identify the different phenotypes present in patients with similar genetic changes, which is a current limitation in disease-specific registries. This national platform also aims to enable identification of participants for future clinical trials and establish itself as a valuable data source in the international inherited heart rhythm and cardiomyopathy research community. Furthermore, creation of the HiRO working groups brings stakeholders in each province together, allowing for a national concerted effort on awareness initiatives, community advocacy, improved clinical care, and prevention of sudden cardiac arrest.
Knowledge Translation
In addition to primary research objectives, knowledge translation initiatives are a critical component of HiRO to both improve awareness and ensure that research findings are appropriately incorporated into clinical care, benefiting participants and other patients. The capacity to enable knowledge translation of research findings is built into HiRO’s organizational infrastructure (Fig. 5), with the 3 additional HiRO working groups supporting ongoing efforts. An established e-mail network between study investigators and clinicians allows complex case discussion, and rapid dissemination of study results from the National HiRO Registry directly to clinical care teams across Canada. Furthermore, HiRO has a strong online presence, with the HiRO website (https://heartsinrhythm.ca), Twitter (@HeartsInRhythm), and Facebook accounts becoming an international resource for staying current on the latest cardiogenetic research. Finally, the annual HiRO Symposium has created an ongoing venue to discuss practice changes based on emerging data.
Limitations
Recruitment of participants in the National HiRO Registry is currently limited to patients referred to one of the 20 speciality centres participating in the National HiRO Registry, and the diagnostic strategy and outcomes may not reflect patients seen at smaller community centres. Evolution of the collected data set and bio samples over time has resulted in some newly added data variables and bio sample types being unavailable on participants historically enrolled in the CASPER, National LQTS, and National ARVC registries. Several sites and geographies are underrepresented in the registry; however, opportunities for collaboration and sharing case findings with all Canadian centres are ongoing.
Conclusion
The HiRO is a national community focused on the detection of inherited arrhythmias and cardiomyopathies, provision of best therapies, and protection from the tragedy of sudden cardiac arrest. Recently, existing disease-specific registries were merged into the expanded National HiRO Registry, creating a single common data set for patients and families with inherited conditions that put them at risk for sudden death in Canada. This national research program is well supported by the network of specialized inherited heart rhythm centres and active working groups to facilitate the translation of research finding to the health care system and general public.
Acknowledgements
We are especially grateful for the commitment of the HiRO research professionals who are responsible for conducting the research program according to ethical guidelines, and committed to recruiting HiRO Registry participants across Canada. We are equally grateful for the HiRO research participants who willingly share their experiences and health care information to advance our understanding of inherited causes of sudden death, cardiac arrest, heart rhythm conditions, and cardiomyopathies. Lastly, the HiRO acknowledges our HiRO patient partners, Debbie and Steven Boyd, Per and Ulla Brunes, Amanda Cook, Paule and Glenn Corneil, Debbie and Alan Corrance, David Fowlie, Paul Kennedy, Claire MacEwing, Darrell and Margaret Porubanec, Robin Thay, and Vickie Pynn for their guidance, support, and partnership.
Funding Sources
The study is supported by the Canadian Institutes of Health Research (Hearts in Rhythm Organization, A.D.K., Principal Investigator, RN380020-406814; HCM registry and biobank, R.T.., Principal Investigator, RN402587-428321). A.D.K. receives support from the Sauder Family and Heart and Stroke Foundation Chair in Cardiology (Vancouver, BC), the Paul Brunes Chair in Heart Rhythm Disorders (Vancouver, BC), the Paul Albrechtsen Foundation (Winnipeg, MB), the Thay Long QT Syndrome Fund (Edmonton, AB), and Darrell and Margaret Porubanec (Kelowna, BC). J.C.-T., R.T., and M.T. receive support from the Philippa and Marvin Carsley Chair (Montreal, QC). R.T. is a Clinical Research Scholar of the Fonds de la Recherche du Québec – Santé.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: The National HiRO Registry has been approved by the University of British Columbia-Providence Health Care (UBC-PHC) Research Ethics Board (H19-01358).
See page 661 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2020.05.006.
Supplementary Material
References
- 1.Maron B.J., Gardin J.M., Flack J.M. Prevalence of hypertrophic cardiomyopathy in a general population of young adults: echocardiographic analysis of 4111 subjects in the CARDIA study. Circulation. 1995;92:785–789. doi: 10.1161/01.cir.92.4.785. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz P.J., Stramba-Badiale M., Crotti L. Prevalence of the congenital long QT syndrome. Circulation. 2009;120:1761. doi: 10.1161/CIRCULATIONAHA.109.863209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peters S., Trümmel M., Meyners W. Prevalence of right ventricular dysplasia-cardiomyopathy in a non-referral hospital. Int J Cardiol. 2004;97:499–501. doi: 10.1016/j.ijcard.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 4.Vutthikraivit W., Rattanawong P., Putthapiban P. Worldwide prevalence of Brugada syndrome: a systematic review and meta-analysis. Acta Cardiol Sin. 2018;34:267. doi: 10.6515/ACS.201805_34(3).20180302B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fishman G.I., Chugh S.S., DiMarco J.P. Sudden cardiac death prediction and prevention: report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation. 2010;122:2335–2348. doi: 10.1161/CIRCULATIONAHA.110.976092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pilmer C.M., Porter B., Kirsh J.A. Scope and nature of sudden cardiac death before age 40 in Ontario: a report from the cardiac death advisory committee of the office of the chief coroner. Heart Rhythm. 2013;10:517–523. doi: 10.1016/j.hrthm.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Priori S.G., Wilde A.A., Horie M. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10:1932–1963. doi: 10.1016/j.hrthm.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Janzen M.L., Cheung C., Sanatani S. Cost analysis of patients referred for inherited heart rhythm disorder evaluation. Can J Cardiol. 2017;33:814–821. doi: 10.1016/j.cjca.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Krahn A.D., Healey J.S., Chauhan V. Systematic assessment of patient with unexplained cardiac arrest: Cardiac Arrest Survivors with Preserved Ejection Fraction Registry (CASPER) Circulation. 2009;120:278–285. doi: 10.1161/CIRCULATIONAHA.109.853143. [DOI] [PubMed] [Google Scholar]
- 10.Steinberg C., Padfield G.J., Champagne J. Cardiac abnormalities in first-degree relatives of unexplained cardiac arrest victims: a report from the Cardiac Arrest Survivors with Preserved Ejection Fraction Registry. Circ Arrhythm Electrophysiol. 2016;9 doi: 10.1161/CIRCEP.115.004274. [DOI] [PubMed] [Google Scholar]
- 11.Herman A.R., Cheung C., Gerull B. Outcome of apparently unexplained cardiac arrest: results from investigation and follow-up of the prospective cardiac arrest survivors with preserved ejection fraction registry. Circ Arrhythm Electrophysiol. 2016;9 doi: 10.1161/CIRCEP.115.003619. [DOI] [PubMed] [Google Scholar]
- 12.Mellor G., Laksman Z.W., Tadros R. Genetic testing in the evaluation of unexplained cardiac arrest: from the CASPER (Cardiac Arrest Survivors with Preserved Ejection Fraction Registry) Circ Cardiovasc Genet. 2017;10 doi: 10.1161/CIRCGENETICS.116.001686. [DOI] [PubMed] [Google Scholar]
- 13.Krahn A.D., Healey J.S., Gerull B. The Canadian arrhythmogenic right ventricular cardiomyopathy registry: rationale, design, and preliminary recruitment. Can J Cardiol. 2016;32:1396–1401. doi: 10.1016/j.cjca.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Roberts J.D., Krahn A.D., Ackerman M.J. Loss-of-function KCNE2 variants: true monogenic culprits of long-QT syndrome or proarrhythmic variants requiring secondary provocation? Circ Arrhythm Electrophysiol. 2017;10 doi: 10.1161/CIRCEP.117.005282. [DOI] [PubMed] [Google Scholar]
- 15.Mellor G.J., Panwar P., Lee A.K. Type 8 long QT syndrome: pathogenic variants in CACNA1C-encoded Cav1. 2 cluster in STAC protein binding site. Europace. 2019;21:1725–1732. doi: 10.1093/europace/euz215. [DOI] [PubMed] [Google Scholar]
- 16.Roberts J.D., Asaki S.Y., Mazzanti A. An international multi-center evaluation of type 5 long QT syndrome: a low penetrant primary arrhythmic condition. Circulation. 2020;141:429–439. doi: 10.1161/CIRCULATIONAHA.119.043114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Hearts in Rhythm Organization Website “Research Team.”. https://hiro.heartsinrhythm.ca/research_team Available at:
- 18.Roberts J.D., Gollob M.H., Young C. Bundle branch re-entrant ventricular tachycardia: novel genetic mechanisms in a life-threatening arrhythmia. JACC Clin Electrophysiol. 2017;3:276–288. doi: 10.1016/j.jacep.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Richards S., Aziz N., Bale S. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–423. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Somani R., Krahn A.D., Healey J.S. Procainamide infusion in the evaluation of unexplained cardiac arrest: from the Cardiac Arrest Survivors with Preserved Ejection Fraction Registry (CASPER) Heart Rhythm. 2014;11:1047–1054. doi: 10.1016/j.hrthm.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 21.Krahn A.D., Healey J.S., Chauhan V.S. Epinephrine infusion in the evaluation of unexplained cardiac arrest and familial sudden death: from the cardiac arrest survivors with preserved Ejection Fraction Registry. Circ Arrhythm Electrophysiol. 2012;5:933–940. doi: 10.1161/CIRCEP.112.973230. [DOI] [PubMed] [Google Scholar]
- 22.Authors/Task Force Members. Elliott P.M., Anastasakis A., Borger M.A. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC) Eur Heart J. 2014;35:2733–2779. doi: 10.1093/eurheartj/ehu284. [DOI] [PubMed] [Google Scholar]
- 23.Marcus F.I., McKenna W.J., Sherrill D. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010;121:1533–1541. doi: 10.1161/CIRCULATIONAHA.108.840827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caforio A.L., Pankuweit S., Arbustini E. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648. doi: 10.1093/eurheartj/eht210. [DOI] [PubMed] [Google Scholar]
- 25.Friedrich M.G., Sechtem U., Schulz-Menger J. Cardiovascular magnetic resonance in myocarditis: a JACC White Paper. J Am Coll Cardiol. 2009;53:1475–1487. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beltrame J.F., Crea F., Kaski J.C. Coronary Vasomotion Disorders International Study Group (COVADIS): international standardization of diagnostic criteria for vasospastic angina. Eur Heart J. 2017;38:2565–2568. doi: 10.1093/eurheartj/ehv351. [DOI] [PubMed] [Google Scholar]
- 27.Sriram C.S., Syed F.F., Ferguson M.E. Malignant bileaflet mitral valve prolapse syndrome in patients with otherwise idiopathic out-of-hospital cardiac arrest. J Am Coll Cardiol. 2013;62:222–230. doi: 10.1016/j.jacc.2013.02.060. [DOI] [PubMed] [Google Scholar]
- 28.The Hearts in Rhythm Organization Website “Find a Clinic.”. https://hiro.heartsinrhythm.ca/find_a_clinic Available at:
- 29.Cranston L. Improving Access to Inherited Heart Rhythm Disorder Experts Through eConsult: Innovation New Programs in Canada. EP Lab Digest. July 2019 https://www.eplabdigest.com/improving-access-inherited-heart-rhythm-disorder-experts-through-econsult-innovative-new-programs-canada Available at: Accessed January 30, 2020. [Google Scholar]
- 30.The Hearts in Rhythm Organization Website “Toolkit.”. https://hiro.heartsinrhythm.ca/toolkit Available at:
- 31.Paratz E.D., Rowsell L., Zentner D. Cardiac arrest and sudden cardiac death registries: a systematic review of global coverage. Open Heart. 2020;7 doi: 10.1136/openhrt-2019-001195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.