Abstract
Mutations in the human HERC1 E3 ubiquitin ligase protein develop intellectual disability. The tambaleante (tbl) mouse carries a HERC1 mutation characterized by cerebellar ataxia due of adult cerebellar Purkinje cells death by extensive autophagy. Our previous studies demonstrated that both the neuromuscular junction and the peripheral nerve myelin sheaths are also affected in this mutant. Moreover, there are signs of dysregulated autophagy in the central nervous system in the tbl mouse, affecting spinal cord motor neurons, and pyramidal neurons of the neocortex and the hippocampal CA3 region. The tbl mutation affects associative learning, with absence of short- and long-term potentiation in the lateral amygdala, altered spinogenesis in their neurons, and a dramatic decrease in their glutamatergic input. To assess whether other brain areas engaged in learning processes might be affected by the tbl mutation, we have studied the tbl hippocampus using behavioral tests, ex vivo electrophysiological recordings, immunohistochemistry, the Golgi-Cox method and transmission electron microscopy. The tbl mice performed poorly in the novel-object recognition, T-maze and Morris water maze tests. In addition, there was a decrease in glutamatergic input while the GABAergic one remains unaltered in the hippocampal CA1 region of tbl mice, accompanied by changes in the dendritic spines, and signs of cellular damage. Moreover, the proportions of immature and mature neurons in the dentate gyrus of the tbl hippocampus differ relative to the control mice. Together, these observations demonstrate the important role of HERC1 in regulating synaptic activity during learning.
Keywords: autophagy, dendritic spines, glutamatergic input, hippocampus, LTP, synapses
Introduction
At the end of the 1980’s, the tambaleante (tbl) mutant mouse was described as a model of adult cerebellar ataxia, a phenotype caused by the nearly complete loss of cerebellar Purkinje cells (Wassef et al., 1987; Rossi et al., 1995). This Purkinje cell death was later proposed to be a model of autophagy (Dusart et al., 2006), and molecular studies identified the spontaneous Gly483Glu substitution in the RCC1 (Regulator of Chromosome Condensation 1) domain of the HERC1 ubiquitin ligase as the mutation that induces the overexpression of the protein responsible for this cell death (Mashimo et al., 2009).
HERC1 is an ubiquitin ligase of the HECT (Homologous to the E6-AP Carboxyl Terminus) family that belongs to the ubiquitin–proteasome system (UPS) (Sánchez-Tena et al., 2016; Schneider et al., 2018; García-Cano et al., 2019). Alterations to the UPS have been related to a variety of neurodegenerative disorders, such as Alzheimer’s, Huntington’s and Parkinson’s disease (de Vrij et al., 2004; Upadhya and Hegde, 2005; Rubinsztein, 2006; Hegde and Upadhya, 2007; van Tijn et al., 2012; Labbadia and Morimoto, 2015), as well as different types of spinal and muscular atrophy (Ramser et al., 2008; Rusmini et al., 2010, 2015; Deng et al., 2011; Dlamini et al., 2013; Rusmini et al., 2015). Furthermore, mutations in HECT E3 ligases have been linked to the pathogenesis of neuromuscular disorders, Parkinson’s disease and diseases of the autism spectrum, such as Angelman syndrome (for a review see Sánchez-Tena et al., 2016; Sluimer and Distel, 2018). In humans, mutations of HERC1 cause a polymorphic syndrome with (Nguyen et al., 2015) or without cerebellar affectation (Ortega-Recalde et al., 2015; Aggarwal et al., 2016; Hashimoto et al., 2016; Utine et al., 2017), yet always apparently associated with intellectual disability (Aggarwal et al., 2016) and in some cases related to the autism spectrum (Hashimoto et al., 2016; Utine et al., 2017). Furthermore, proteins with mutations in its RCC1-like domain (RLD) are involved in several other neuropathologies, such as juvenile amyotrophic lateral sclerosis 2 and X-linked retinitis pigmentosa (Mashimo et al., 2009).
HERC1 contains two RLD domains and the mutation carried by the tbl mice is in the N-terminal RLD domain (Mashimo et al., 2009). The N-terminal RLD domain may act as guanine nucleotide-release factor for ARF proteins (Sánchez-Tena et al., 2016) and by interacting with ARF/Rab GTPases, it influences intracellular vesicle trafficking (Sánchez-Tena et al., 2016). Moreover, HERC1 constitutes a ternary complex with clathrin and the heat shock protein, HSP70 (Rosa and Barbacid, 1997). Since clathrin mediated endocytosis is relevant for synaptic vesicle recycling (Rizzoli and Betz, 2005), alterations to the normal clathrin cycle could interfere with normal synaptic function.
The cerebellum is widely recognized as a key center for motor learning (for a review see Manto and Jissendi, 2012) and evidence is accruing that the cerebellum could play a pivotal role in non-motor learning (Lackey and Sillitoe, 2017). Damage to other brain areas as well as the cerebellum has been reported in several cerebellar mutant mice (see Porras-García et al., 2013) and these mutant mice display cerebellum related spatial learning alterations (for a review see Lalonde, 2002). In fact, we previously reported that the associative memory is also impaired in the adult tbl mice, impairment that is correlated to alterations of the dendritic spines on neurons in the lateral amygdala, and to the absence of short-term (STP) and long-term (LTP) potentiation in this nucleus (Pérez-Villegas et al., 2018). Thus, it is reasonable to hypothesize that tbl mutation could also affect the hippocampus both morphological and physiologically. Therefore, we have analyzed the tbl hippocampus using behavioral tests, histological methods, and electrophysiological ex vivo recordings to determine the extent and the physiological relevance of HERC1 ubiquitin ligase.
Materials and Methods
Animals
Tambaleante mice were obtained by breeding pairs of the tbl carrier mice, genotyping the offspring by PCR (Mashimo et al., 2009). Three to 4-month-old male tbl mice with a fully developed cerebellar ataxic phenotype and isogenic male control mice of the same age were used. The animals were handled in accordance with current Spanish and European legislation governing the use of experimental animals (RD 53/2013 - BOE 08/02/2013 and 2010/63/EU), and all experimental procedures were approved by the Pablo de Olavide University ethics committee and the Junta de Andalucía (Animal Health Service auth. # 13/06/2017/080).
Histological Procedures
Golgi-Cox Method and Dendritic Spine Analysis
Controls (n = 3) and tbl (n = 3) mutant mice were deeply anesthetized with an overdose of pentobarbital (80 mg/kg i.p.) and perfused intracardially with 4% paraformaldehyde (PFA) in phosphate buffer (PB 0.1M, pH 7.2–7.4). After dissection the brain was divided into two sagittal halves along the interhemispheric fissure and processed using a modified Golgi-Cox method (Bayram-Weston et al., 2016). Briefly, after 2 weeks in darkness in the Golgi-Cox solution (1.78% potassium dichromate, 1.78% mercuric chloride and 1.78% potassium chromate in distilled water) at room temperature (RT), the brain hemispheres were immersed for 24 h in 25% sucrose in Tris buffered saline (TBS, 0.1M pH 7.4). Sagittal frozen microtome sections (90 μm thick, Leitz) were obtained and collected in TBS. After a 5 min of treatment with 0.1% Triton X-100 in TBS, the sections were mounted on gelatin-coated slides and air-dried in the dark at RT. The sections were then rinsed in distilled water (1 min) and immersed in 25% ammonium hydroxide solution (Fluka, cat. 17093-1L), and after rinsing the sections in distilled water (1 min) they were transferred to Kodak Professional rapid fixer solution A for 20 min (Mychasiuk et al., 2013). After rinsing again with distilled water (1 min) the sections were dehydrated with ascending grades of ethanol (70%, 90% 1 min each, and 100% twice for 5 min each), cleared in xylene (2 min × 10 min) and mounted in DPX (Flores et al., 2005). Images were taken on a Zeiss Axioimager M1 microscope and the figures were prepared using Photoshop 8.0 software (Adobe®) with no additional correction.
Dendritic spines were analyzed as described by Pérez-Villegas et al. (2018). Briefly Z-stacks of completely filled, Golgi-Cox stained secondary dendrites of CA1 pyramidal neurons and dentate gyrus (DG) granule cells (optical section thickness = 0.5 μm) were visualized using a 100× oil-immersion objective with a numerical aperture of 1.74. The series of images were converted to RGB using the Fiji ImageJ software (W. Rasband, National Institutes of Health1) and then analyzed with the Reconstruct software2. Dendritic length, width and the length/width ratio were measured, and the spines were categorized according to steps 2 and 3 of the procedure in Risher et al. (2014). The data obtained were processed in Microsoft® Excel (steps 4–6 of the same authors).
Immunohistochemical Procedures and Quantification
Controls (n = 3) and tbl (n = 3) mice were used. Mice were deeply anesthetized with an overdose of pentobarbital (80 mg/kg i.p.) and perfused intracardially with 4% PFA in PB. After dissection, the brains were fixed overnight at 4°C in the same fixative and they were then immersed in 30% sucrose in PB at 4°C until they sank. Frozen coronal microtome sections (30 μm thick) were collected in PBS, and immunostained using the procedure reported by Pérez-Villegas et al. (2018). The primary antibodies used were: a rabbit polyclonal antiserum against calbindin (CaBP, 1:10,000, Swant, CB-38); a rabbit monoclonal antibody against caspase-3 (1:400, Thermo Fisher, #700182); a rabbit polyclonal antibody against cleaved caspase-3 (Asp175) (1:500, Cell Signaling, #9661); a goat polyclonal antiserum against doublecortin (DCX, 1:250, Santa Cruz, sc-8066); a mouse monoclonal antibody against the HuC/HuD neuronal proteins (1:200; Thermo Fisher, A-21271); a rabbit polyclonal antiserum against the SV2A presynaptic vesicle protein (1:200, Synaptic Systems, #119002); a mouse monoclonal antibody against the vesicular glutamate transporter 1 (VGLUT1, 1:100, Millipore, mab5502); and a rabbit polyclonal antiserum against glutamate decarboxylase 65 and 67 (GAD65-67, 1:500, Millipore, AB1511). The secondary antibodies used were: Alexa Fluor® 594 donkey-anti-goat (1:500, Invitrogen, A150132); Alexa Fluor® 594 donkey-anti-mouse (1:500, Invitrogen, A21203); and Alexa Fluor® 488 donkey-anti-rabbit (1:500, Invitrogen, A21206). The sections were counterstained with DAPI (1:5,000, Sigma, D9542) and images were acquired on an upright Zeiss Axioimager M1 microscope or on an upright Olympus FluoView 1000 confocal laser scanning microscope. The figures were prepared using the Photoshop 8.0 (Adobe®) software without additional corrections.
Immunoreactivity was quantified as indicated previously (Pérez-Villegas et al., 2018). Briefly, an alternating sequence of laser pulses was used to activate the different fluorescent probes during image acquisition. Images were acquired with a 60× oil-immersion objective at a numerical aperture of 1.42. Images from the hippocampal CA1 of control and tbl mice were obtained in the same session under similar conditions (laser intensities and photomultiplier voltages). Quantification of the fluorescent labeling density was performed offline with ImageJ and the size of the areas measured was determined automatically by defining outline masks based on the brightness thresholds from maximal projected confocal images. The control and tbl images of SV2A, VGLUT1, and GAD 65-67 expression in the CA1 area (38,725 μm2 of z-stacks made up of 9 slices each 0.5 μm thick) were captured as follows and expressed in arbitrary units: GAD 65–67: Green laser intensity 10%, with photomultiplier settings HV 760, Gain 1, Offset 8; VGLUT1: Argon laser intensity 10% with photomultiplier settings HV 773, Gain 1, Offset 5; SV2A: Green laser intensity 3.5%, with photomultiplier settings HV 680, Gain 1, Offset 30.
Transmission Electron Microscopy (TEM)
Control (n = 2) and tbl (n = 2) mice were deeply anesthetized with pentobarbital (80 mg/kg i.p.) and perfused intracardially with ice-cold 1% PFA, 1% glutaraldehyde and 0.02% CaCl2 fixative in PB. After dissection, the brains were stored overnight in the same fixative at 4°C and coronal slices (0.5–1 mm thick) of the brain were post-fixed in 2% OsO4 in PB, stained in block with 1% uranyl acetate in 70% ethanol, dehydrated and embedded in Durcupan (Fluka®). Ultrathin (50-70 nm) sections were obtained with a Leica UC6 ultramicrotome, collected in copper grids (150 and 300 mesh), and observed by TEM without counterstaining on a Zeiss Libra microscope at 80 kV (CITIUS, University of Seville).
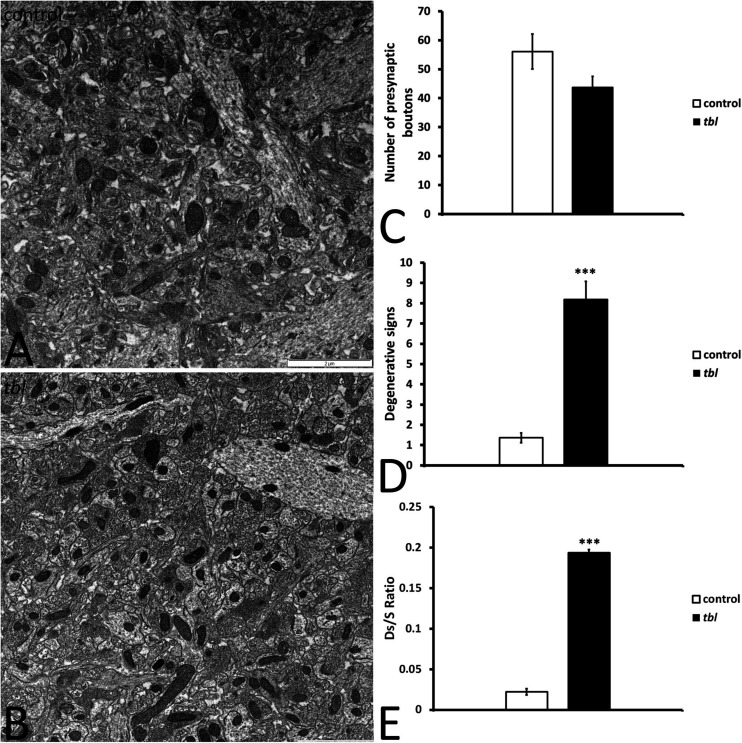
Mosaic 3 × 3 (85 μm2 area) or 4 × 4 (170 μm2 area) microphotographs were obtained with the multiple image acquisition application of the Olympus iTEM software®. Images were obtained from ultrathin sections of the middle tier of the CA1 stratum radiatum, with 3 μm between each field to be sure that all counted axospinous synapses were different. The presynaptic terminals counted had clear synaptic vesicles, and an evident pre- and postsynaptic density in the plane of the section. The areas analyzed for counting measured: (i) 1,678 μm2 control and 1,767 μm2 tbl CA1 to assess the degenerative presynaptic profiles (Figures 1–3); (ii) 1343.18 μm2 control and 1235.55 μm2 tbl CA1 to quantify the number of mitochondria within the presynaptic endings (Figures 2–4); and (iii) 1343.18 μm2 control and 1007.1504 μm2 tbl CA1 areas to evaluate the macular and perforated axospinous synapses (Figures 1–3). All counts were done with the Fiji ImageJ software (W. Rasband, National Institutes of Health3).
FIGURE 1.
Panels (A,B) illustrate the transmission electron mosaic microphotographs used for the quantitative analysis. The only signs of degeneration within presynaptic terminals were counted, as described in the preceding figure (Figures 10C,D). No differences were found in the number of presynaptic terminals between the control and tbl CA1 (C, p = 0.1027245). However, there were significant differences in the signs of degeneration in the tbl CA1 presynaptic endings (D, ***p = 0.000014), as well as in the presynaptic endings with degenerative signs/synapses ratio (E, *** p = 0.0000008) relative to the control CA1.
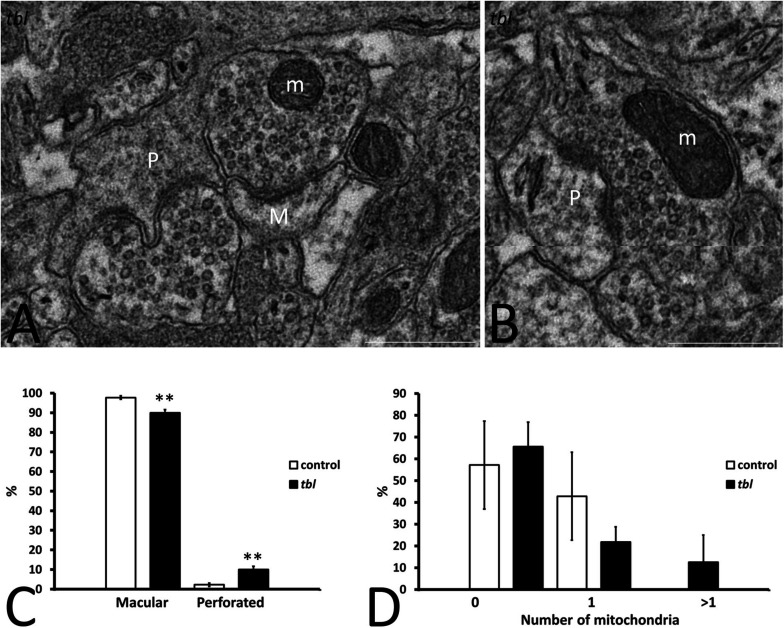
FIGURE 3.
Microphotographs of part of the mosaics of the tbl CA1 used for the quantitative analyses of axospinous synapses (A,B). A illustrates the criteria followed to assess the perforated (P) and non-perforated or macular (M) postsynaptic regions of axospinous synapses. Panel (B) shows a perforated synapse (P) whose presynaptic ending contains healthy mitochondria (m). Macular axospinous synapses were the most numerous in the control CA1 neuropil than in the tbl one (C, **p = 0.00202), while there were fewer perforated ones in the control CA1 neuropil than in tbl mice (C, **p = 0.00202). No significant differences were found in the number of perforated synapses with (D, 1 and >1) or without (D, 0) mitochondria within their presynaptic endings between the control and tbl CA1 neuropil (D, 0; p = 0.7218734; D, 1; p = 0.358118; D, >1; p = 0.350616). Bar = 0.5 μm (A,B).
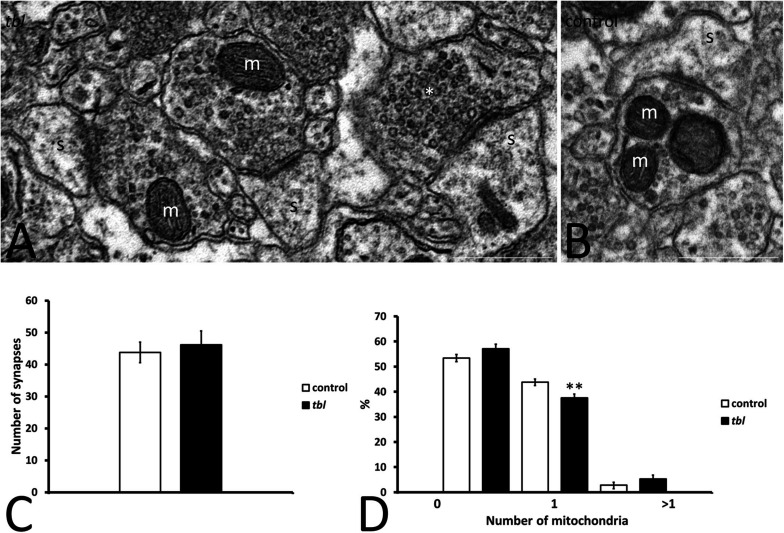
FIGURE 2.
Microphotographs of part of the mosaics of the control (B) and tbl (A) CA1 used for quantitative analyses illustrating axospinous synapses (s) without mitochondria in the presynaptic ending (A, asterisk), or those containing one (A, m) or more (B) mitochondria. No differences were found in the number of presynaptic terminals in the control and tbl CA1 (C, p = 0.6546196). The only statistically difference found was in the number of presynaptic endings with one mitochondrion, which was lower in the tbl than in the control CA1 (D, **p = 0.0073196): s, postsynaptic dendritic spine. Bars = 0.5 μm (A,B).
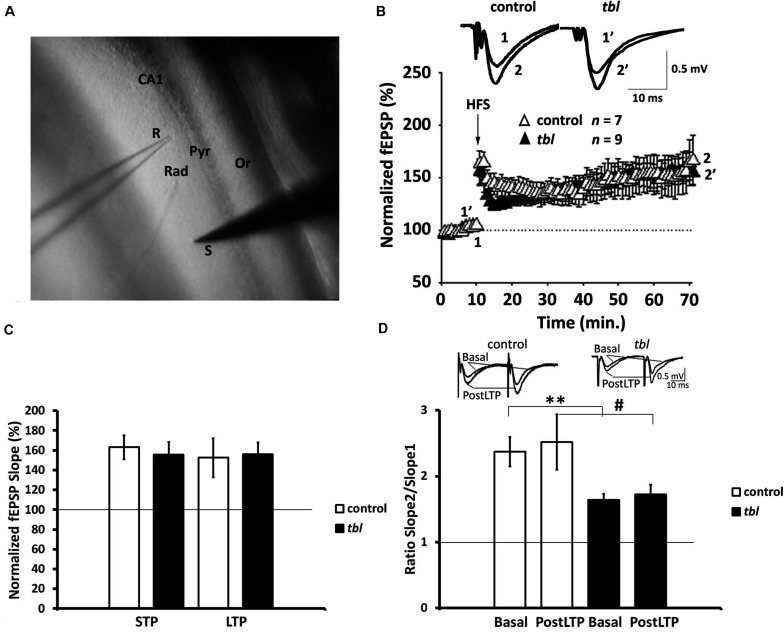
FIGURE 4.
Long-term potentiation (LTP) is preserved in the Schaffer collateral-CA1 synapses of tbl mutant mice. (A) Stimulation and recording electrode configuration: S, stimulation; R, recording; Rad, stratum radiatum; Pyr, pyramidal cell layer; Or, stratum oriens. (B) Time course of the LTP protocol on the slope of the fEPSP in slices of control (white triangles) and mutant mice (black triangles). Traces show the fEPSP before (1, 1′) and 60 min after (2, 2′) the LTP protocol was applied. The symbols represent the average responses plotted every 60 s for each genotype. After 10 min of control recordings, a HFS train was applied as indicated by the vertical arrow. The number of slices for each genotype used (n) is indicated. (C) LTP and STP did not differ between the genotypes (p > 0.1, in both LTP and STP). (D) Paired-pulse ratio (PPR, slope 2/slope 1) traces show the PPF of fEPSP in control and tbl mice during basal stimulation and 55–60 min. after the HFS protocol was applied. A reduction in basal stimulation between the genotypes is evident (**p < 0.01), a difference that is not statistically significant 55–60 min after the HFS protocol (postLTP: #p > 0.1).
Ex vivo Recordings
Slice Preparation
Coronal slices (350 μm thick) containing the hippocampus were prepared and maintained according to standard procedures (Negrete-Díaz et al., 2007; Andrade-Talavera et al., 2012). Briefly, animals were anesthetized with isofluorane (2%), decapitated, and their brain was removed and placed in ice-cold extracellular Ringer’s solution (R, in mM): 124 NaCl, 2.69 KCl, 1.25 KH2PO4, 2 MgSO4, 1.8 CaCl2, 26 NaHCO3, and 10 glucose (pH 7.2, 300 mOsm). Coronal vibratome slices were then maintained in continuously oxygenated extracellular solution for at least 1 h before use. All experiments were carried out at RT (23–26°C).
Electrophysiology
Field excitatory postsynaptic potentials (fEPSPs) were recorded with low-resistance glass pipettes filled with the external solution and situated in the CA1 region of the hippocampus (Arroyo-García et al., 2018). Potentials were evoked by applying electric pulses through monopolar electrodes placed in the stratum radiatum. Synaptic field potentials were elicited at a frequency of 0.2 Hz, and the slope of the recorded fEPSP was calculated and used as a measure of synaptic strength. After a stable fEPSP baseline period of 10 min, a LTP protocol was applied by stimulating Schaffer collateral fibers with a train of pulses at a frequency of 100 Hz during one second (HFS) at the same strength as the test stimulus. Post-stimulation recordings continued for 60 min and LTP was successfully induced when the average fEPSP slope size (measured 50–60 min after HFS) increased by at least 20% relative to the baseline (100%). STP was measured as the maximum slope (peak) after HFS stimulation. A 40 ms paired-pulse stimulation protocol was used for pair pulse ratio (PPR) analysis. The fEPSPs were recorded at 2 kHz using an Axopatch 200B (Molecular Devices) apparatus and they were acquired at 10 kHz. All measurements were performed and analyzed in a strictly blind manner, with the genotypes of the animals revealed only after the electrophysiological experiments and their evaluation were complete.
Data Analysis
Data were analyzed using the Clampfit software (Molecular Devices) and unless otherwise indicated, they are presented as the mean ± SEM obtained using the Student’s-t test. The last 10 min of recording was used to estimate the changes in synaptic efficacy compared to the baseline. To measure the PPR, the slope of the 2nd fEPSP was divided by the slope of the 1st fEPSP.
Behavioral Tests
Novel Object Recognition Test
Control (n = 5) and tbl (n = 5) mice were tested as described previously (Cubillos-Rojas et al., 2016). Mice were placed in a rectangular arena (55 cm × 40 cm × 40 cm) and two identical objects (A–A) were placed in the arena during the training phase (5 min). Short-term memory (STM) was assessed by comparing the amount of time spent exploring a novel object (B) relative to that spent exploring the familiar one (A). Twenty four hours after training, long-term memory (LTM) was tested by comparing the time spent by the mice exploring another novel object (A–C). The relative exploration of the novel objects was expressed as a discrimination index [DI = 5 (tnovel − tfamiliar)/(tnovel + tfamiliar)].
T-Maze Test
Exploratory memory was tested in a T-maze over three consecutive days (10 trials per day and mouse, n = 10). The trials for spontaneous alternation measurement were performed according to the protocol described by Deacon and Rawlins (2006). Briefly, the mice were confined to the arm they choose first for 30 s. Thereafter, all the doors and the central partition of the T-maze were removed, and the animals were left to choose freely between the two arms. Control mice performed each trial in less than 2 min but owing to their ataxia, the tbl mice took between 3 and 5 min to finish each trial.
Morris Water Maze Test
Spatial learning and memory were also tested in five control and five tbl mice using a procedure similar to that proposed earlier by Morris (1984). A circular pool, 100 cm in diameter and 45 cm high, was filled to a depth of 13 cm with water (23 ± 2°C) made opaque by the addition of 0.01% TiO2. Four arbitrary N, S, E, and W points divided the pool into four quadrants and a 7 cm diameter platform was hidden 1.5 cm below the water surface in the N quadrant. Each mouse performed 4 trials per day, with an inter-trial interval of 30 min, and in each trial (one trial per quadrant) the mice were placed in a different quadrant with their nose facing the pool wall. All the experimental sessions were recorded with a digital camera and the time spent to reach the platform (escape latency) was the main variable assessed, considering the maximum trial time as 90 s. In the training session (1st day) mice were first placed in the NE quadrant and if that they did not successfully reach the platform, they were manually guided to it. In the acquisition period (2nd to 5th days), mice were considered that have correctly found the platform when they remained on it for at least 10 s (5 s for tbl mice). The retention interval was 7 days and thus, on the 12th day after the beginning of the experiments the mice were subject to a trial similar to previous ones but in which the platform was removed. The time spent by the mice in the pool area where the platform should be located was measured.
The tbl mice have a low performance on motor test (see Mashimo et al., 2009; Porras-García et al., 2013; Bachiller et al., 2015). Therefore, we have measured the swimming speed of control and tbl mice before the onset of the test. As it would be expected, the swimming speed was faster in control (mean = 0.07 ± 0.01 m/seg) than in tbl (mean = 0.026 ± 0.002 m/seg) mice. However, this lowest speed did not impede that tbl mice successfully reached the platform.
Statistical Analysis
The statistical analyses of the data from behavioral tests and histological experiments were analyzed blind by EMP-V and MP-R. A two tailed Student’s t-test was used to compare the data from tbl and control mice. Any p-value less than 0.05 was considered significant, indicated as follows: ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. No significant values were indicated as follows: #p > 0.05.
Results
Analysis of the Dendritic Spines on CA1 Pyramidal Neurons and DG Granule Cells
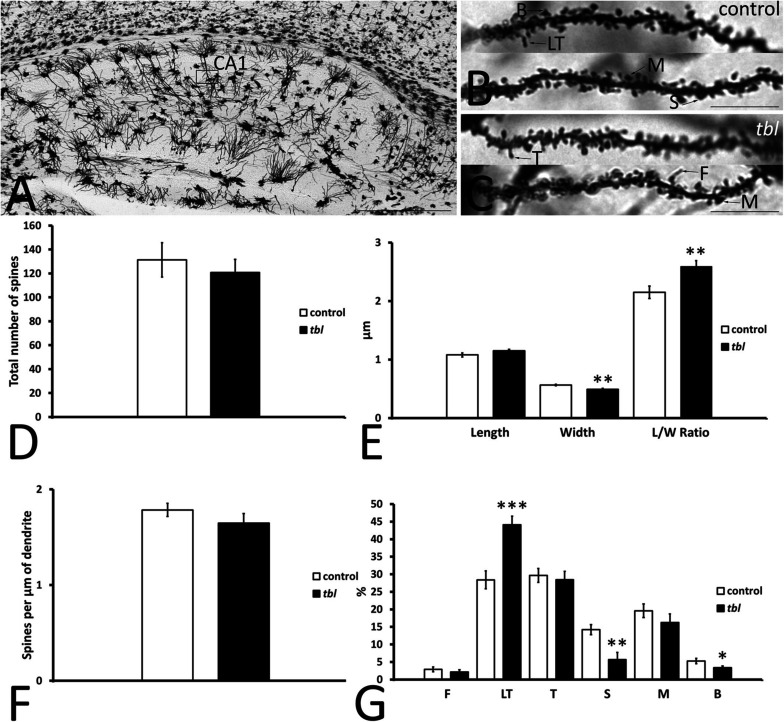
CA1 pyramidal neurons are easily distinguished by their triangular or pyramidal soma within the pyramidal cell layer, from which several basal and one or two primary dendrites arise. Primary dendrites and their branches spread through the stratum radiatum and the stratum lacunosum-moleculare where they receive distinct inputs (i.e., Schaffer collateral from CA3 pyramidal neurons and entorhinal afferents, respectively) (Amaral and Lavenex, 2007). Here we analyzed segments of the secondary dendrites of pyramidal neurons placed within the stratum radiatum, approximately at the same level at which the electrophysiological recordings were obtained (Figure 5A, open rectangle, see also Figure 4A). Dendrite segments 15–20 μm long that were completely filled by the Golgi-Cox metallic mercuric deposit were analyzed (Figures 5B,C), using previously described criteria to categorize and quantify the dendritic spines (Pérez-Villegas et al., 2018). Dendritic spines counts were made over a total dendrite length of 519.18 μm in control and 517.43 μm in tbl neurons. There was a slight yet not significant decrease in the number of spines counted (Figure 5D; control mean = 131.29 ± 14.35 vs. tbl mean = 120.88 ± 10.80; p = 0.29), and in the spines density on tbl pyramidal dendrites relative to the controls (Figure 5F; p = 0.14). However, there was a significant decrease in the spine width in the tbl pyramidal neurons relative to the control spines (Figure 5E; p < 0.01), which was responsible for the significant increase in the spine length/width ratio (Figure 5E; p < 0.01). Indeed, this shift was coincident with the significant decrease in the stubby and branched mature forms of tbl spines (Figure 5G; p < 0.01 and p < 0.05, respectively). Decreases that were accompanied by a significant increase in the immature long thin forms of spines on tbl pyramidal dendrites relative to controls (Figure 5G; p < 0.001).
FIGURE 5.
Microphotographs of parasagittal sections through a Golgi-Cox impregnated hippocampus (A–C). (B–C) Illustrate the fully filled dendritic segments used to count and categorize the spines. (D–G) Graphical representations of the number of spines counted (D), the differences in spine length (E), spine width (E), the spine length/width ratio (E, L/W Ratio) and in the density of spines (F) in the CA1 of control and tbl mice. The proportions of the dendritic spine types counted are also shown (G). The smaller width of the spines and the higher length/wide ratio indicate the mainly immature spines in the tbl CA1. The most mature spines, mushroom spines (G, M) seem to be less abundant in the tbl CA1, although these values are not statistically significant, whereas a significant decrease in the number of mature stubby and branched spines is evident in the tbl mice (G, S and B). In addition, there is a significant increase in the number of immature thin long spines (G, LT) in the tbl CA1. The asterisks indicate significant differences in the Student’s t-test: **p = 0.002978 (E, width); **p = 0.005185 (E, L/W ratio); ***p = 0.0003845 (G, LT); **p = 0.002825 (G, S); *p = 0.0290842 (G, B). CA1, cornu ammonis 1; F, filopodia; LT, long thin spines; T, thin spines; M, mushroom spines; S, stubby spines; B, branched spines. Bars = 2mm (A), and 10 μm (B,C).
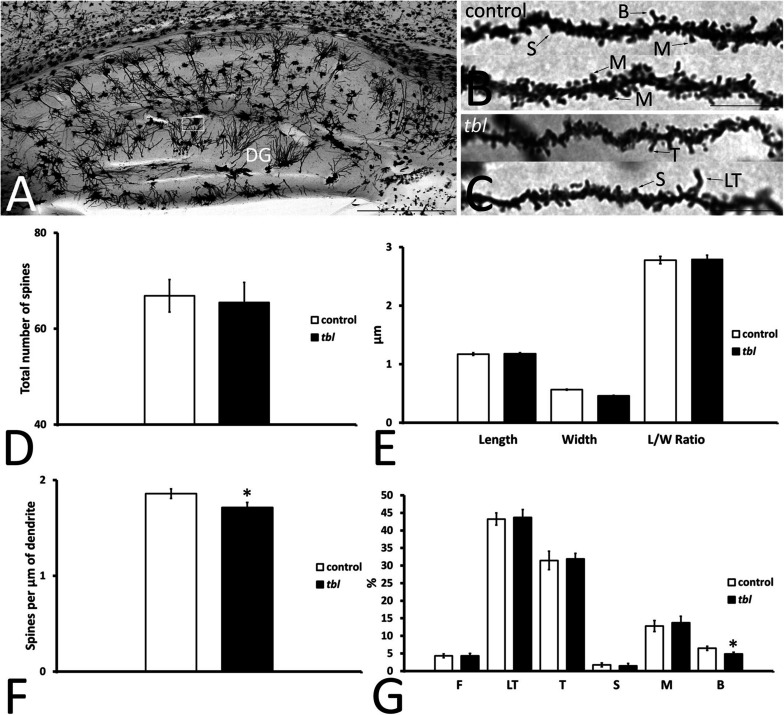
Granule cells are the projection neurons in the DG and their axons mainly end as mossy fibers to the CA3 field of the hippocampus (Amaral and Lavenex, 2007). Their dendrites arise from an ovoid cell body, spreading through the molecular layer and establishing dendritic trees in the suprapyramidal blade (dorsal leaf of Desmond and Levy, 1985) of the molecular layer, which are larger than those in the infrapyramidal blade (ventral leaf in Desmond and Levy, 1985). Here we analyzed the dendritic spines on granule cells in the outer third of the suprapyramidal blade (Figure 6A), which almost exclusively receives afferents from the perforant pathway (see figures 802 and 809 from Ramón y Cajal, 1904). Like the CA1 dendritic spines, 15–20 μm length segments of completely impregnated dendrites were considered (Figures 6B,C), analyzing total dendrite lengths of 544.99 μm for control and 503.12 μm for tbl mice. The number (Figure 6D; p = 0.80), the length and width, and the length/width ratio values (Figure 6E; p = 0.80, p = 0.78, and p = 0.87, respectively) were very similar between the control and the tbl mice, although the density of spines was lower on granule cell dendrites in the tbl DG than on control dendrites (Figure 6F; p < 0.05). Furthermore, while very long thin spines were observed on tbl granule cell dendrites (Figure 6C), the only significant difference among the different types of spines on granule cell dendrites was a decrease in the number of mature branched spines in the tbl mice relative to the controls (Figure 6G; p < 0.05).
FIGURE 6.
Microphotographs of parasagittal sections through the Golgi-Cox impregnated hippocampus (A). (B–C) Show the completely impregnated dendritic segments of granule cells from the suprapyramidal blade of the dentate gyrus [DG: box in (A) represents the area used to count and categorize the spines]. (D–G) Graphical representation of differences in the number of spines (D), the spine length (E), in spine width (E), the spine length/width ratio (E, L/W ratio), and the density of spines (F) in the DG of control and tbl mice. The proportions of the dendritic spine types counted are shown in (G). No significant differences were found in the number (D; p = 0.7971753), length (E; p = 0.8019998) width (E; p = 0.7770199), and length/width ratio (E; p = 0.8729458) between tbl and control mice. However, the density of dendritic spines was lower in the tbl DG than in the controls (F). The only significant differences in the type of dendritic spines was found in the branched spines, of which there were slightly fewer on tbl granule cell dendrites with respect to the controls (G). The asterisks indicate significant differences in the Student’s t-test: *p = 0.0271162 (F); *p = 0.0418517 (G, B). DG, dentate gyrus; F, filopodia; LT, long thin spines; T, thin spines; M, mushroom spines; S, stubby spines; B, branched spines. Bar = 2 mm (A), and 10 μm (B,C).
Immunohistochemistry
The Main Synaptic Vesicle Populations
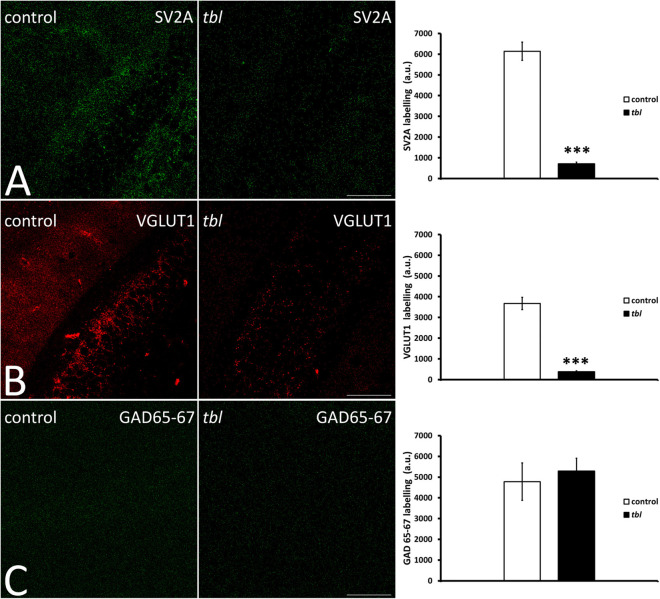
Quantitative analysis of confocal laser microscopy images from the hippocampus demonstrated significantly weaker punctate immunolabelling of the integral membrane protein of presynaptic vesicles SV2A, and that of VGLUT1 considered to be a marker of glutamatergic synaptic vesicles (Figures 7A,B), in the tbl CA1 than in the control CA1, labeling fewer vesicles (Figures 7A,B; SV2A, p < 0.001; and VGLUT1, p < 0.001). By contrast, no significant differences in the intensity of presynaptic GABAergic GAD 65-67 immunoreactivity were found (Figure 7C; p = 0.39) between control and tbl CA1.
FIGURE 7.
Laser confocal microphotographs (A–C) of coronal sections through the CA1 field of the hippocampus. (A–C) Z stacks of 9 slices (0.5 μm thick) illustrating the distribution of SV2A (A), VGLUT1 (B), and GAD 65–67 (C) immunoreactive synaptic vesicles in the control and tbl mice. Graphs showing the number of synaptic vesicles identified by SV2A immunoreactivity (A), the synaptic glutamatergic vesicles identified by VGLUT1 expression (B), and the GABAergic endings identified with the GAD 65–67 antibody (C). The tbl CA1 possesses significantly fewer vesicles expressing SV2A (A, ***p = 4.0179E–14) and VGLUT1 (B, ***p = 1.68366E–12) as presynaptic markers, while no difference was found in GAD 65-67 expression (C, p = 0.3280439). Bar = 100 μm (A–C).
Immature vs. Mature Neurons in the Dentate Gyrus
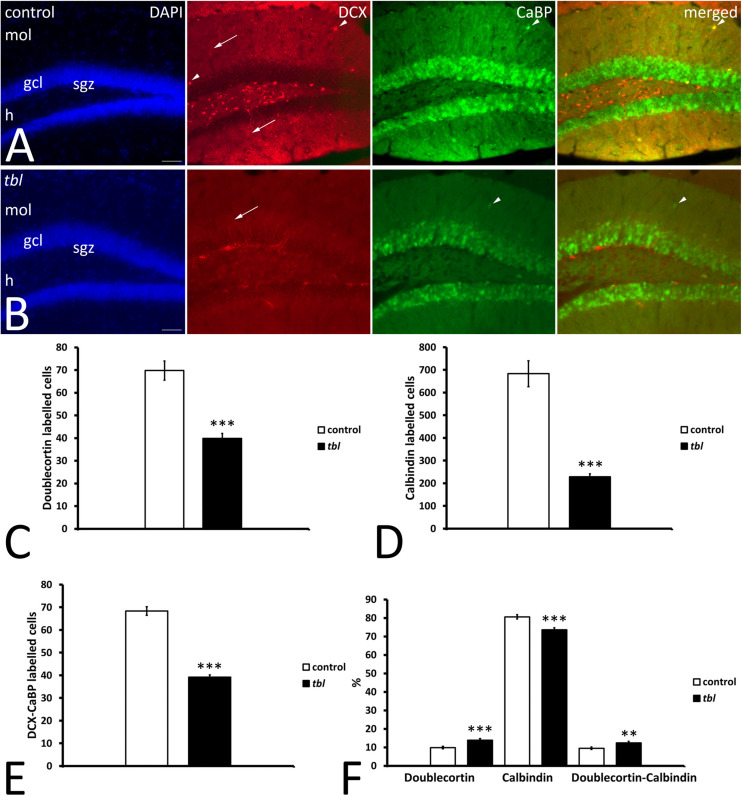
Learning and memory are related to adult hippocampal neurogenesis (AHN) (Snyder, 2019). Therefore, to assess the possible effect of the HERC1 mutation on the maturation of postnatally born DG neurons, we assessed AHN using DCX and CaBP antibodies as markers of immature and mature neurons, respectively (Radic et al., 2017). DCX immunoreactive cells were mainly located in the subgranular zone of both the control and tbl DG (Figure 8). While some scattered DCX labeled cell bodies were also observed throughout the granule cells layer and the molecular layer of the control DG (Figure 8), they were virtually absent at these locations in the tbl DG. CaBP immunoreactive cell bodies were located within the granule cell layer, albeit with some exceptions (Figure 8). The qualitative observation of more CaBP immunoreactive cells in the DG of control mice relative to tbl ones was confirmed by the quantitative analysis. The counts of labeled cells made in 50 microphotographs from control and tbl mice, having each microphotograph an area of 356116.46 μm2 and containing the crest of the DG (“V” region), indicated that the number of DCX (Figure 8C; p < 0.001) and CaBP (Figure 8D; p < 0.001) labeled neurons was consistently higher in control than in the tbl DG. There was also a significant difference in the number of double-labeled cells between the control and tbl DG (Figure 8E; p < 0.001). However, when comparing the different cell types, there was a higher proportion of immature DCX-labeled neurons in the tbl DG relative to the control DG (Figure 8F; p < 0.001). Variation was also evident in the percentage of DCX and DCX/CaBP labeled cells, which was higher in the tbl DG than in the controls (Figure 8F; p < 0.001; and p < 0.01, respectively). Conversely, the percentage of CaBP expressing cells was significantly lower in the tbl DG (Figure 8F; p < 0.001). Hence, the tbl DG seems to possess fewer mature neurons than the control DG.
FIGURE 8.
Microphotographs of coronal sections through the dentate gyrus of control (A) and tbl mice (B) illustrating DCX and CaBP immunoreactivity. DCX labeled cells occupy the subgranular zone, spreading their dendrites through the granule cell layer toward the molecular layer (arrows), while CaBP immunoreactive cells lie within the granule cell layer. A few labeled cells can be detected in the molecular layer (arrowheads). (C–F) Graphical representation of the number of DCX (C), CaBP (D), and DCX-CaBP (E) labeled cells, and the proportions of single and double-labeled cells (F). The dentate gyrus of control mice possesses significantly more DCX, CaBP, and DCX-CaBP labeled cells than in tbl mice (C, ***p = 2.0063654E–08; D, ***p = 2.387923 E–10; E, ***p = 1.665851E–12). However, the percentages of single and double labeled cells indicate that tbl mice possess significantly fewer calbindin immunoreactive neurons in the dentate gyrus than the controls (F, ***p = 0.0000242). By contrast, a higher proportion of DCX and DCX-CaBP labeled cells is found in the tbl dentate gyrus than in the controls (F, ***p = 0.0004682; and **p = 0.0060857, respectively), gcl, granule cells layer; h, hilus; mol, molecular layer; sgz, subgranular zone. Bar = 50 μm (A,B).
Our previous analysis on the immunohistochemical expression of the autophagy markers Beclin 1, LC3 and p62 in the CA3 field of the hippocampus showed that although the immunoreactivity of these proteins appeared to be stronger in the tbl than in control mice, almost all of these differences were not statistically significant (Ruiz et al., 2016). In neurodegenerative processes, the autophagy-apoptosis relationship is a mechanism potentially provoking neuronal cell death (Lee and Gao, 2009; Grishchuk et al., 2011; Napoletano et al., 2018). Furthermore, it was recently postulated that caspase-3 was less strongly activated by the pharmacologically attenuation of dysregulated autophagy in 6-OHDA treated cultured neurons (Chung et al., 2018). This autophagy-apoptosis link led us to study the distribution of caspase-3 and cleaved caspase-3 in the DG. Caspase-3 immunoreactive cells were randomly distributed throughout the DG, being most abundant in the polymorphic cell layer or hilus (Figure 9). Two qualitative differences stand out in caspase-3 immunoreactivity between control and tbl mice. First, the number and intensity of caspase-3 labeling was apparently greater in the tbl DG (Figures 9B,C) than in the control DG (Figure 9A). Secondly, while all caspase-3 positive cells were also immunoreactive to the neuronal marker HuC/HuD in the tbl DG (Figures 9B,C), some caspase-3 labeled cells did not express these neuronal proteins in control mice (Figure 9A). Cell counting ratified these qualitative differences and hence, there were significantly more cells expressing caspase-3 in the tbl than in the control DG (401.05 ± 46.82 and 172.56 ± 14.04, respectively; p < 0.001), as was the case for the number of neurons expressing caspase-3 in the DG (Figure 9G; p < 0.001). Similar results were found when observing the localization of cleaved caspase-3 in DG neurons (Figures 9D–F), and the number of double labeled neurons (Figure 9F) was significantly lower in the control than in the tbl DG (Figure 9H; p < 0.001).
FIGURE 9.
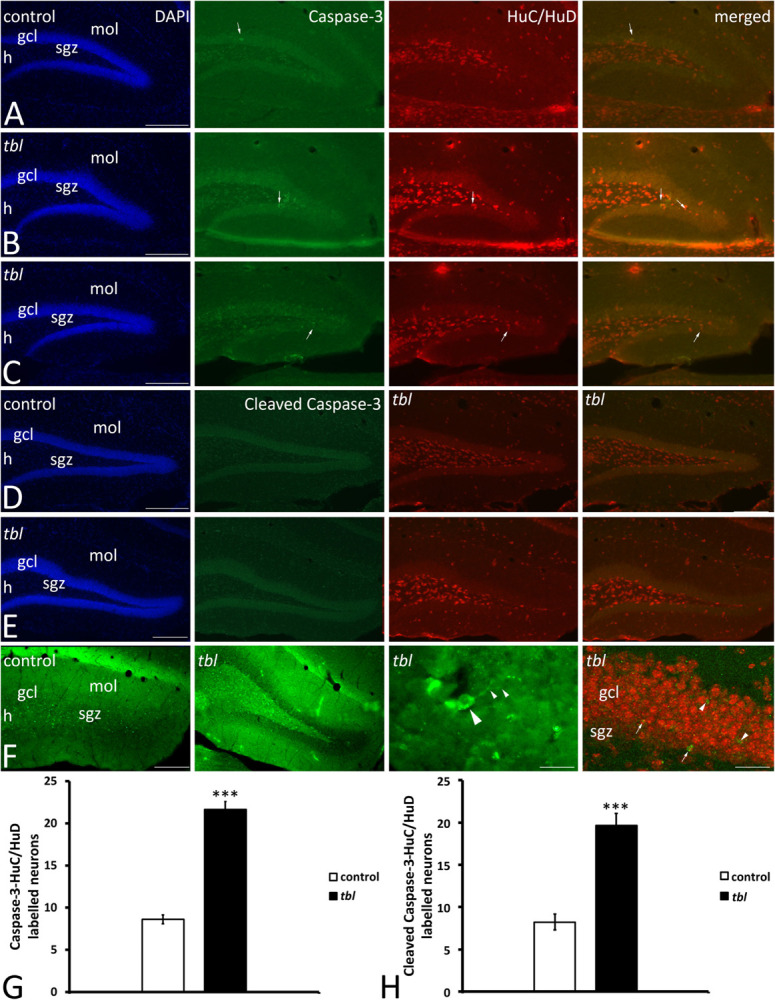
Microphotographs of coronal sections through the rostral (A,B) and middle (C–F) regions of the dentate gyrus of control (A,C,E) and tbl mice (B,D,F) to illustrate the immunoreactivity to caspase 3 and the neuronal marker proteins HuC/HuD. Almost all caspase-3 immunoreactive cells are located within the hilus, the subgranular zone and the granule cell layer, while HuC/HuD also labels interneurons located in the molecular layer. In the control DG it is possible to find caspase-3 labeled cells that do not co-express HuC/HuD (A,C, arrows), whereas in the tbl DG, caspase-3 immunoreactive cells also are labeled by the HuC/HuD antibody (B,D, arrows). Quantitative analysis demonstrates that the tbl DG has significantly more caspase-3-HuC/HuD (G, ***p = 1.902245E–12) and cleaved caspase-3-HuC/HuD double labeled neurons than the controls (H, ***p = 5.524E–06). gcl, granule cells layer; h, hilus; mol, molecular layer; sgz, subgranular zone. Bar = 200 μm (A–F), and 30 and 60 μm (F).
In summary, present results strongly suggest that rather than alterations to the generation and differentiation of newly born cells in the DG, mature neuronal cell loss is greater in the tbl mutant mouse DG.
Transmission Electron Microscopy (TEM)
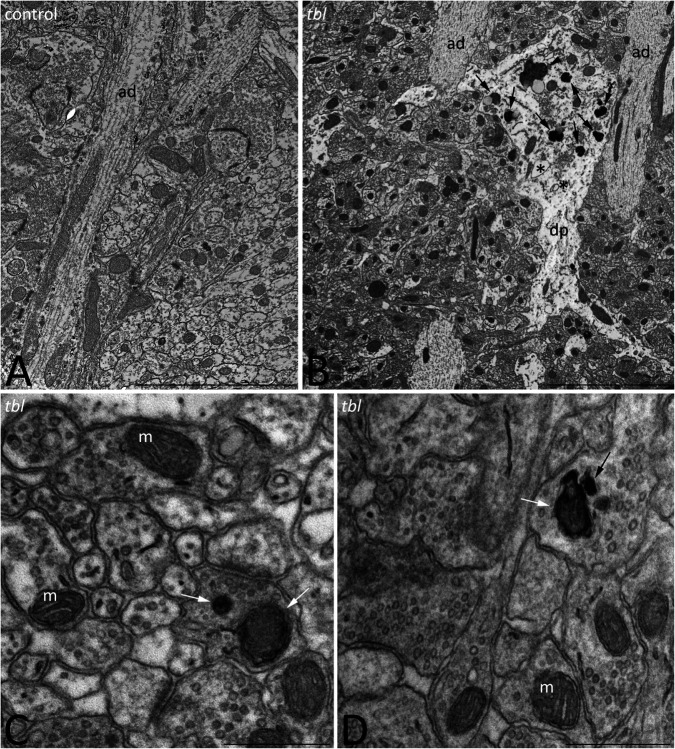
We previously described that pyramidal neurons of the CA3 field show signs of autophagy in the tbl hippocampus (Ruiz et al., 2016). Here, ultrastructural studies of the mid-regions of the stratum radiatum of the tbl CA1 (the same level at which electrophysiological recordings were made) identified signs of cell damage within the neuropil. Degenerative profiles containing large vacuoles with or without double membranes, dilated endoplasmic reticulum cisterns and lysosomes in different phases of maturity were evident, along with apical dendrites with a normal aspect (Figure 10B). Some of these lysosomes possessed a disorganized internal crest that resembled mitochondrial debris, mimicking mitolysosomes (Figure 10B). Mature mitophagosomes were also observed in axonal varicosities and presynaptic endings (Figures 10C,D), while no signs of evident damage were found in the cytoplasm of the postsynaptic CA1 dendritic spines of mutant mice. Quantification confirmed the significant differences for the signs of degeneration in presynaptic endings in the tbl stratum radiatum neuropil (Figure 1D; p < 0.001; and, Figure 1E; p < 0.001), while no significant differences between tbl and control mice were found in either the number of presynaptic endings (Figure 1C; p = 0.10) or in the size of neuropil area analyzed (control mean = 152.56 ± 10.38 vs. tbl mean = 160,63 ± 30.31; p = 0.81). Similarly, there were no differences in the ratio of the number of presynaptic endings relative to their area in μm2 (control mean = 0.37 ± 0.027 vs. tbl mean = 0.31 ± 0.029; p = 0.20), which would justify any errors in the differences in degeneration. Therefore, these observations reinforce and extend the earlier observations of damage (Ruiz et al., 2016) to the presynaptic endings in the CA1 of the tbl hippocampus.
FIGURE 10.
Transmission electron microphotographs through the stratum radiatum in the hippocampal CA1 region of control (A) and tbl (B–D) mice. Degenerative profiles (B, dp) whose cytoplasm is filled with altered vacuoles (B, asterisks), numerous lysosomes and necrotic debris (B, arrows), and mitolysosomes (B, arrowhead) are evident in the tbl mutant CA1. Mitophagic vacuoles and dark debris are often observed in axons and presynaptic terminals throughout the tbl CA1 neuropil (C,D, arrows). ad, apical dendrite; m, mitochondria. Bar = 5 μm (A,B), and 0.5 μm (C,D).
The degree of presynaptic activity has been related to the presence of one or more mitochondria within the presynaptic terminal (Smith et al., 2016). Therefore, we counted the axospinous synapses with or without mitochondria at the presynaptic terminals in mosaic images of the control and tbl CA1 (control area mean = 149.24 ± 14.47 vs. tbl area mean = 137.28 ± 16.12; p = 0.59). Axospinous synapses were identified according the criteria of Peters et al. (1976), and the most abundant were those presynaptic endings possessing none (Figure 2A) or one healthy mitochondria (Figure 2A) per section, with synapses containing two or more mitochondria observed less frequently (Figure 2B). The number of axospinous synapses was similar in the control (394; mean = 43.78 ± 3.21) and tbl DG (416; mean = 46.22 ± 4.28; Figure 2C, p = 0.65). This quantitative analysis ratified that performed previously in the rat hippocampus by Shepherd and Harris (1998), with almost 50% of presynaptic endings in the control and tbl CA1 are devoid of mitochondria (Figure 2D). The difference in the number of presynaptic endings without mitochondria or with two or more mitochondria was slightly greater in the tbl than in control CA1 axospinous synapses, although these changes were not significant (Figure 2D; p = 0.12 and, p = 0.20, respectively). Notwithstanding, the decrease in the number of presynaptic endings possessing one mitochondrion in tbl CA1 axospinous synapses was significant when considered relative to their increase in the control CA1 (Figure 2D; p < 0.01).
Based on the structure of the postsynaptic density, two types of asymmetric axospinous synapses were observed in cortical and other brain areas: (i) non-perforated synapses (also named macular) with a single, continuous postsynaptic density (Figure 3A); and, (ii) perforated synapses in which the postsynaptic membrane density is divided by a small spinule (Calverley and Jones, 1987) (Figures 3A,B). Perforated synapses have been related to both plastic (Carlin and Siekevitz, 1983) and degenerative changes (Adams and Jones, 1982) in dendritic spines. In accordance with previous quantifications (Calverley and Jones, 1987), perforated synapses were always less numerous than macular synapsis in both control and tbl mice. No differences were found in the total number of axospinous synapses (control 175; mean = 25 ± 2.903 vs. tbl 268; mean = 33.5 ± 5.35; p = 0.20) or in the synapse densities in the CA1 neuropil (control mean = 0.196 ± 0.036 vs. tbl mean = 0.29 ± 0.063; p = 0.23). However, the number of non-perforated and perforated synapses between control and tbl DG varied, and there was a noticeable increase in perforated synapses in the tbl CA1 relative to the controls (control mean = 0.571 ± 0.02 vs. tbl mean = 3.375 ± 0.68; p < 0.01). In addition, the proportion of either type of synapse was significantly different between control and tbl DG. Thus, the increase in perforated synapses in tbl (Figure 3C; p < 0.01) was accompanied by an increase in the percentage of non-perforated synapses in the control CA1 (Figure 3C; p < 0.01). When we compare the presynaptic endings with or without mitochondria, no significant differences were found between the control and tbl mice CA1 (without mitochondria; p = 0.72; with one mitochondrion; p = 0.36; and with two or more mitochondria; p = 0.35; Figure 3D).
CA1 Hippocampus Slice Recordings
The synaptic plasticity in both control and tbl mice were characterized by recording the fEPSPs evoked in CA1 hippocampal neurons (Figure 4A) by stimulation of Schaffer’s collaterals in the stratum radiatum of the brain slices (Figure 4A) (Andrade-Talavera et al., 2016; Arroyo-García et al., 2018). We used an HFS protocol to induce LTP, which induced clear STP and robust LTP in both control and tbl mice. The magnitude of potentiation (LTP) was expressed as the mean percentage of the fEPSP slope with respect to the baseline (set at 100%), and when quantified 50–60 min after stimulation it reached 152.6 ± 19.7% of the baseline in control mice vs. 155.4 ± 12.2% of the baseline in tbl mice (Figures 4B,C; p > 0.1). Likewise, STP was measured as the maximum slope found after HFS and it was 163.1 ± 12.2% vs. 155.8 ± 12.7% in control mice and tbl mice respectively (Figures 4B,C; p > 0.1).
In addition, the PPR was studied (the slope of the 2nd fEPSP divided by the slope of the 1st fEPSP slope) after LTP was induced by the HFS protocol (PostLTP), although it did not show differences in control (2.52 ± 0.42%) and tbl mice (1.73 ± 0.15%) (Figure 4D; p > 0.1), indicating there were no changes in the probability of release due the induction of LTP. However, while no differences in the magnitude of LTP and STP were found between control and tbl mice, and postsynaptic expression of this LTP was apparent in both groups of mice, paired-pulse facilitation PPF data indicated a clear difference in basal synaptic transmission between control and tbl mice. Thus, basal PPF was weaker in tbl than in control mice (tbl: 1.63 ± 0.09% vs. 2.37 ± 0.33% in control mice (Figure 4D; p < 0.01), indicating that a mechanism involved in PPF is at least partially impaired in tbl mice.
Hence, whereas synaptic transmission in the CA1 seems to be affected in tbl mice (PPF is reduced), this change in release does not affect the induction or expression of LTP in tbl mice, and both normal LTP and STP can be induced in these mice.
Behavioral Studies
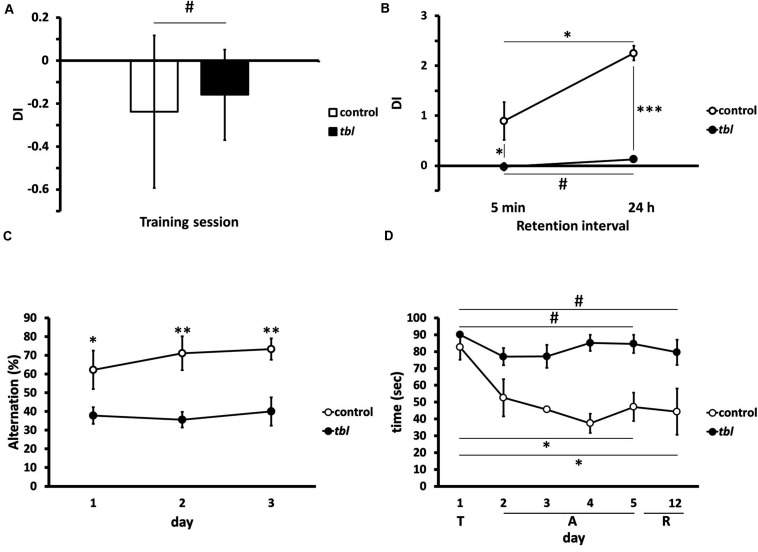
We used three tests to determine the effect of the tbl mutation on hippocampal memory tasks (for a review see Morris, 2007). In the novel-object recognition memory there was no difference between control and tbl mice during the training period (Figure 11A; p = 0.43), although there were significant differences in both STM (tested 5 min after training) and LTM (tested 24 h after training) with control mice displaying enhanced exploratory preference of the new objects relative to tbl mice (Figure 11B; p < 0.05 for STM; and, p < 0.001 for LTM). Moreover, there was also a significant difference between STM and LTM indicative of learning in control mice during the test (Figure 11B; p < 0.05).
FIGURE 11.
Graphical representations of the novel-object recognition (A,B), the spontaneous alternation (C) and the Morris water maze (D) tests. Note the impaired recognition memory in tbl mice (B), expressed as a Discrimination Index (A,B, DI), with respect to the controls at both short (B, 5 min, *p = 0.0337565) and long (B, 24 h, ***p = 0.000017) retention intervals after the training session (A, #p = 0.428051). The differences in spontaneous alternation between control and tbl mice (C) clearly shows the loss of working memory in tbl mice (C, day 1 *p = 0.0406423; day 2 **p = 0.0058376; and, day 3 **p = 0.0047633). In the spatial reference memory task, and irrespective of the swimming speed of each group, control mice consistently decreased their escape latencies over the days (D, day 5 vs. day 1 *p = 0.0169509; and, day 6 vs. day 1 *p = 0.0459339), whereas tbl mice do not (D, day 5 vs. day 1 #p = 0.2113249; and, day 6 vs. day 1 #p = 0.1499300). T, training session; A, acquisition period; R, retention interval.
Differences in the spontaneous alternation between control and tbl mice were significant from the first (Figure 11C; p < 0.05) to the last day of the experiment in which mice were tested (Figure 11C; p < 0.01 the 2nd day; and, p < 0.01 the 3rd day). Thus, while control mice increased their spontaneous alternation over time (62 ± 10.30% the 1st day; 71% 9.02 ± the 2nd day; and, 73 ± 5.67% the 3rd day), this was not the case for tbl mice that retained a spontaneous alternation below 50% (38 ± 4.44% the 1st day; 36 ± 4.16% the 2nd day; and, 40 ± 7.53% the 3rd day).
There were also differences in spatial learning and memory between tbl and control mice when analyzed in the Morris water maze test in. Thus, while control mice quickly learned to reach the hidden platform during the acquisition period (Figure 11D; p < 0.05), the escape latency to reach the platform did not shorten in tbl mice (Figure 11D; p = 0.211). Furthermore, there were clear differences in the escape latencies between control and tbl mice (p < 0.05) and the retention interval (p < 0.05); however, it could be argued that these differences were due to the faster swimming speed of control regarding tbl mice (see above in section “Morris Water Maze Test”), rather than the demonstration of damage of learning and spatial memory in tbl mutants. Notwithstanding the different swimming speed, the absence of a clear shortening in the latency of tbl mice along the test (Figure 11D; p = 0.211), clearly indicates that spatial learning and LTM were impaired by the tbl mutation.
Discussion
The behavioral results presented here show that memory and learning processes involving the hippocampus (for a review see Morris, 2007) are impaired in tbl mice, which is perhaps not surprising given that several spontaneous mutations affecting cerebellar Purkinje cells also induce hippocampal damage (e.g., leaner, reeler, staggerer, and weaver mutant mice) (for a review see Grüsser-Cornehls and Bäurle, 2001; Porras-García et al., 2013). Moreover, ataxic mutant mice with primary cerebellar atrophy show altered spatial orientation and spontaneous alterations, while mice in which the cerebellum was not involved in ataxia did not show hippocampal-related learning difficulties (Lalonde, 2002). However, none of the cerebellar mutations with complete Purkinje cells loss produced evident hippocampal damage and the anomalous performance of these mice in hippocampal tasks was correlated to a putative alteration in the influence of the cerebellum on the cerebral cortex (Lalonde, 2002). As reported previously for the lateral amygdala (Pérez-Villegas et al., 2018), the alterations to dendritic spines observed here in the hippocampal CA1 and DG regions of tbl mice include signs of degeneration in the presynaptic terminal of CA1 axospinous synapses. By contrast, the weaker glutamatergic input to the CA1 in the presence of unaltered GABAergic input would justify the behavioral impairment, although unlike the lateral amygdala no significant alterations to STP and LTP were found in the tbl CA1.
From their seminal description by Ramón y Cajal (1888), dendritic spines were associated with neuronal communication. Through TEM, spines were related to excitatory synaptic transmission (Gray, 1959), and after the development of in vitro experimental methods, dendritic spines were consistently linked to learning and memory due to the plastic changes associated to LTP and/or LTD (for a review see Yuste and Bonhoeffer, 2001; Matsuzaki et al., 2004; Bailey et al., 2015; Grienberger et al., 2015; Segal, 2017). Three main morphological types of spines have been classified: mushroom, thin and stubby (Peters and Kaiserman-Abramof, 1969). Of these, the larger mushroom spines have been defined as stable spines and linked to memory, while stubby spines are the most abundant during postnatal development (Rochefort and Konnerth, 2012), and they have been classified as immature spines (Bourne and Harris, 2007). However, recent time-lapse super-resolution stimulated emission depletion studies demonstrated that stubby spines are mushroom spines whose neck is not so easy to visualize in conventional Golgi and EM analyses (Tønnesen et al., 2014), implying that stubby spines can be considered here as mature spines. Thin spines are silent spines, targets for the induction of LTP and hence, implicated in the ability to learn (Kasai et al., 2010). Immature long thin spines and filopodia are most abundant during synaptogenesis (Dunaevsky et al., 1999) and they finally evolve into mature spines (Segal, 2017). Variations in the number of spines and alterations in the spine morphology have been found in intellectual disability syndromes (IDs) (Levenga and Willemsen, 2012), in most of which a loss of spines is frequently coupled to a relative increase in immature spines, as in Patau’s and Down’s syndrome (Marin-Padilla, 1974, 1976; Purpura, 1975). However, while not all IDs are correlated with an evident decrease in spine number, they are related with a relative increase in immature spines, as occurs in autism spectrum disorders like the X-linked IDs (Levenga and Willemsen, 2012). Thus, studies on the hippocampus of the Fmr1-null mice, the most common murine model of Fragile X syndrome, did not provide clear information relative to the number, density, and maturity of the dendritic spines (Jawaid et al., 2018). Furthermore, studies of several mouse models of Rett syndrome also produced conflicting data on the number and density of hippocampal spines (Xu et al., 2014).
The dendritic spine counting performed here identified two different panoramas. There is an increase in the length of spines in the tbl CA1, which is correlated with an increase in immature long thin spines, and is coincident with a decrease in the stubby and branched mature spines, while the total number of spines and their density remains unchanged relative to the control CA1. Conversely, there is a significant decrease in spine density in the tbl relative to the control DG, principally due to a loss of mature branched spines. These changes are accompanied by differences in the proportions of the different morphologies in the postsynaptic regions of the spines. Four categories of chemical synapses have been reported in the nervous system based on their invaginations (Petralia et al., 2018). Of these, the simple axospinous synapse possesses a straight single postsynaptic density and it corresponds to those defined as non-perforated (Calverley and Jones, 1987) or macular (Marrone and Petit, 2002) synapses. Category 1 synapses correspond to the perforated synapses in which a small spinule devoid of a postsynaptic density protrudes into the presynaptic ending (Calverley and Jones, 1987). Perforated synapses have been defined as large stabilized mushroom spines that are involved in memory and learning as these spines are enhanced after LTP dependent learning (Marrone and Petit, 2002; Petralia et al., 2018). Furthermore, strengthening glutamatergic innervation induces the addition of AMPA receptors within the postsynaptic area (Park, 2018; Buonarati et al., 2019) that is responsible for the increase in postsynaptic density size (for review see Marrone and Petit, 2002; Yuste, 2010; Segal, 2017).
A clear decrease in perforated synapses in the CA1 region of Fmr1 null mice coincided with their anomalous behavior (Jawaid et al., 2018), yet in the CA1 of tbl mice a significant increase in the number of perforated synapses was concomitant with a decrease of simple or macular synapses. The presence of more perforated synapses in the CA1 of tbl mice that perform poorly in learning and memory tests may be surprising, although the presence of perforated synapses has also been related to aging (Adams and Jones, 1982), reactive synaptogenesis after CA1 denervation (Marrone and Petit, 2002), and experimental dopamine depletion in the striatum (Anaya-Martínez et al., 2014). Furthermore, the permanence or increase due to de novo spinogenesis has been related to chronic denervation or long-term deprivation of the appropriate presynaptic inputs (Sotelo, 1978; Zuo et al., 2005). An increase in the number of immature spines was recently reported in the lateral amygdala of tbl mutant mice, even that of anomalous somatic spines (Pérez-Villegas et al., 2018). This increase in immature spines is associated with a dramatic decrease in glutamatergic inputs to this nucleus, yet no changes in NMDA and AMPA receptors were evident (Pérez-Villegas et al., 2018). Similar results were observed in the tbl CA1 region, where the decrease in glutamatergic synaptic vesicles contrasted with the stable NMDA (GluN1R) and AMPA (GluA1R) receptor subunit expression (data not shown). In addition, our TEM study adds new information regarding the presynaptic elements of axospinous synapses in the tbl CA1 neuropil, demonstrating significant signs of autophagy-mitophagy and a significant decrease in the number of macular synapses whose presynaptic terminals possess one mitochondrion. Mitochondria play a pivotal role in normal synaptic physiology, where they are responsible for glutamate synthesis (Waagepetersen et al., 2003), and mitochondrial ATP is essential for SV dynamics (Sudhof and Rizo, 2011). Indeed, synaptic terminals possessing mitochondria have fewer vesicles under conditions of LTP (reflecting an increase in neurotransmitter exocytosis), when postsynaptic spines show signs of sustained synaptic plasticity compared to those on presynaptic terminals in which mitochondria are absent (Smith et al., 2016). Thus, the decrease in the number of synapses containing mitochondria, together with the existence of mitophagy and evidence of degeneration in tbl presynaptic terminals, would at least partially explain the behavioral impairment of this mutant due to a loss of synaptic efficacy. Moreover, our studies on tbl hippocampal cultured neurons (Montes-Fernández et al., 2020) demonstrate a reduction in the ready releasable pool (RRP) and the resting pool (RP) of synaptic vesicles. A reduction in the RRP has also been described in the tbl neuromuscular junction, even before the onset of the ataxic syndrome (Bachiller et al., 2015), and in our cultures this coincided with a decrease in the total number of synaptic vesicles, a weaker clathrin expression and the absence of interactions between clathrin and mutated RLD1 (Montes-Fernández et al., 2020). Hence, the HERC1 mutation alters normal synaptic vesicles dynamics in presynaptic terminals.
The tambaleante mutation lies at the RLD1 of HERC1 (Mashimo et al., 2009). RLDs interact with ARF/Rab GTPases, and they have been implicated in intracellular membrane trafficking and clathrin dynamics (Sánchez-Tena et al., 2016). Therefore, the mutated HERC1 protein dysregulates clathrin coating and affects the endocytic pathway of synaptic vesicle recycling (Montes-Fernández et al., 2020), provoking a decrease in the RRP and RP, which explains the loss of glutamatergic synaptic vesicles in the tbl CA1. In addition, Rab endosomes are involved in sustaining mitochondria in axons, and the Rab7a mutation alters mitochondrial physiology and the anterograde axonal transport of mitochondria, contributing to Charcot Marie-Tooth disease (Cioni et al., 2019). Although a more in depth analysis of mitochondrial dynamics is needed in tbl neurons, the possibility exists that alterations to the interactions between Rab proteins and mutated HERC1 could alter late endosome-mitochondrial relationships; inducing mitochondrial dysfunction and eliciting the anomalous mitophagy that disrupts neuronal homeostasis (Martinez-Vicente, 2017). The E3 ligases in the UPS have been implicated in the maintenance of spine size, density and number, through regulation of AMPA receptor expression, or of NMDA receptor and RhoA activity (Hamilton and Zito, 2013; Mertz et al., 2015; Hamilton et al., 2017). Indeed, spinogenesis is also dampened when UPS activity is blocked (Hamilton and Zito, 2013). In our previous study on the lateral amygdala of tbl mice (Pérez-Villegas et al., 2018) we suggested that the increase in immature dendritic spines could reflect the increase in proteasome activity potentially caused by HERC1 overexpression (Mashimo et al., 2009). However, the absence of differences in the main glutamate receptors and the lack of evident signs of damage in the postsynaptic sites of axospinous synapses, together with the presence of presynaptic mitophagy, the smaller size of the RRP and RP of synaptic vesicles (Montes-Fernández et al., 2020, and present results), and the fewer presynaptic endings containing one mitochondrion, lead us to hypothesize that changes to spines in the tbl hippocampus could be a secondary effect. These changes may reflect the dysregulation of the normal synaptic transmission necessary for the formation and maintenance of dendritic spines (Yuste and Bonhoeffer, 2004; Turrigiano, 2008) rather than primary damage to the postsynaptic spine itself provoked by the mutation.
Autophagy plays a key role in neuronal homeostasis; it is essential for memory (Glatigny et al., 2019) and its dysfunction has been related to several neurological disorders (Ghavani et al., 2014; Lim and Yue, 2015; Nikoletopoulou et al., 2015). HERC1 overexpression elicits Purkinje cell death in association with extensive autophagy (Mashimo et al., 2009), as well as affecting other central nervous system neuronal populations (Ruiz et al., 2016). The data presented here extend previous data reported by Ruiz et al. (2016) on the hippocampal damage caused by the HERC1 mutation, demonstrating that the tbl DG possesses fewer postnatal mature CaBP hippocampal neurons, while new or immature DCX expressing cell populations seem not to be affected. Hence, the dysfunction in autophagy does not affect AHN. The regulation of apoptotic and necrotic cell death by autophagy is essential for homeostasis (Napoletano et al., 2018), the dysregulation of which has been implicated in several neurodegenerative diseases (Menzies et al., 2017; Alfaro et al., 2019). Indeed, recent evidence suggests that dysregulated apoptosis itself causes further cell death and apoptotic neuronal cell death (Chung et al., 2018). The increase in the caspase-3 and cleaved caspase-3 labeled neurons in the tbl DG is consistent with this proposal, opening the possibility that some cell death in the hippocampus may be driven by the tbl mutation, although less evident than in the cerebellum. AHN is implicated in learning and memory (Snyder, 2019), and an effect of the HERC1 mutation on such events cannot be ruled out. However, the scant evidence of largescale neuronal loss in the hippocampus compared to that seen in the tbl cerebellum cannot explain the behavioral impairments shown by tbl mice in this study. However, additional experiments using bromodeoxyuridine labeling and TUNEL staining to determine the exact rate of AHN and cell death will help us to define the role of HERC1 overproduction on adult neuronal renewal in the tbl DG.
It is possible that dysregulated macroautophagy-mitophagy occurs at the afferent axonal endings of the tbl CA1 and there is evidence that autophagy influences homeostasis of the presynaptic machinery (Vijayan and Verstreken, 2017; Lüningschrör and Sendtner, 2018). Thus, studies in the Lurcher mutant mouse demonstrated that Purkinje cell axons react earlier and more strongly to autophagy than the Purkinje cell bodies (Yue, 2007). Furthermore, in Lurcher mice lacking the Atg7 gene, which encodes the autophagy-related protein 7, the axonal degeneration occurs before to and independently of Purkinje cell death (Yue et al., 2008). In fact, it is well established that the greatest number of neuronal autophagosomes accumulates in axon terminals (Maday and Holzbaur, 2014) and that axonal degeneration after injury is prevented by autophagy (He et al., 2016). Of the proteins involved in presynaptic autophagy, at least two would be affected by the tbl mutation. Firstly, the interaction of HERC1 with Rab GTPases (Sánchez-Tena et al., 2016; García-Cano et al., 2019), and among them Rab26 GTPase, is related to autophagy of the presynaptic machinery in the oldest or damaged synaptic vesicles (Binotti et al., 2015). Secondly, the mammalian target of rapamycin complex 1 (mTORC1) plays a key role in autophagy (Shimobayashi and Hall, 2014) and enhances autophagy and induces a loss of synaptic vesicles (Hernandez et al., 2012), when is inhibited. As mTORC1 activity is dampened by the tbl mutation (Mashimo et al., 2009; Bachiller et al., 2018), this decrease could explain the signs of autophagy and the reduction in the number of synaptic vesicles at the neuromuscular junction (Bachiller et al., 2015), in the lateral amygdala (Pérez-Villegas et al., 2018), in cultured hippocampal neurons (Montes-Fernández et al., 2020), and hippocampal CA1 presynaptic endings of tbl mice (present results).
Glutamatergic transmission plays a key role in LTP mechanism, and in the structural and molecular changes associated with learning (He et al., 2012). Postsynaptic glutamate receptors are thought to be essential for LTP induced spinogenesis (Yuste and Bonhoeffer, 2001; Lamprecht and LeDoux, 2004; Yuste, 2013; Rogerson et al., 2014) and LTP is frequently linked to the increase in the number of AMPARs in the postsynaptic membrane after NMDAR activation even in the CA1 region of the hippocampus (Kasai et al., 2010; Rogerson et al., 2014; Grienberger et al., 2015; Park, 2018; Buonarati et al., 2019). However, as was the case in the lateral amygdala (Pérez-Villegas et al., 2018), quantitative analysis of the tbl CA1 region did not demonstrate changes in the distribution of the GluN1 or GluA1 subunits and the differences in spine morphology cannot be related to alterations of the ionotropic glutamate receptors. As such, the similar number of ionotropic glutamate receptors contrasts with the reduction in the number of synaptic vesicles and in the VGLUT1 expression in the tbl CA1.
From our results, the altered learning in tbl mice seems to be parallel to a decrease in presynaptic glutamatergic input rather than to alterations in postsynaptic glutamatergic transmission. Indeed, we found electrophysiological differences in basal synaptic transmission in tbl mice when compared to control mice, with a lower PPF in tbl mice. Hence, the decrease in VGLUT1 expression and the lower number of vesicles seems to alter basal synaptic transmission at the level of the PPF in fEPSP recordings. Interestingly, these changes in presynaptic activity do not affect the STP (at 100 Hz) or LTP, indicating that STP or LTP do not account for the learning and memory deficits observed in tbl mice. As differences in PPF are observed (two pulses at 50 Hz), it is possible that while synapses in control and mutant mice share the same characteristics at high stimulation frequencies (above 50 Hz), these synapses behave differently at lower and less persistent stimulation frequencies, accounting for the learning and memory deficits observed in tbl mice. Although future ex vivo experiments will explain these differences, our study on cultured hippocampal neurons indicates that there are consistently more FM-143 de-stained synaptic vesicles in cultured neurons from control than tbl mice when were stimulated with 20 Hz trains after 40 and 700 action potentials. (Montes-Fernández et al., 2020).
In conclusion, the HERC1 mutation impairs hippocampal learning and memory in tbl mice. This learning deficit correlates with a decrease in the number of glutamatergic synaptic vesicles, signs of autophagy-mitophagy in presynaptic endings and alterations to basal synaptic efficacy despite STP and LTP remain unaffected. Therefore, the HERC1 E3 ligase protein, like other E3 ligase proteins, could contribute to the regulation of postsynaptic dendritic spinogenesis and to the homeostasis of presynaptic terminals. However, whether these alterations are due to the mutated RLD1 domain interfering with normal synaptic vesicles dynamics (Montes-Fernández et al., 2020) and/or altering the late-endosome-mitochondria relationship or to alterations in the proteostasis dysregulated by macroautophagy, or a combination of them remains unclear. Nevertheless, the data presented allow us to propose this tbl mutation as another model to study presynaptic homeostasis and its role in the maintenance of postsynaptic spines as a correlate for learning and memory processes.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
The animal study was reviewed and approved by Pablo de Olavide University ethics committee and the Junta de Andalucía (Animal Health Service auth. # 13/06/2017/080). Written informed consent was obtained from the owners for the participation of their animals in this study.
Author Contributions
JA: conceptualization, writing – original draft, and supervision. EP-V, MP-R, JN-D, GAT, and JA: methodology. EP-V, MP-R, JN-D, and JA: data acquisition and analysis. EP-V, MP-R, JN-D, RR, JR, GAT, AR-M, and JA: review and Editing. RR, JR, GdT, and AR-M: funding acquisition. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are indebted to Dr. A. M. Carrion for his assistance with the mouse genotyping. The technical assistance of J. L. Ribas, F. J. García-Reyes, and M. Sánchez-Enciso is greatly appreciated, and we acknowledge the assistance of Dr. M. Sefton in the preparation of this manuscript.
Funding. This work was funded by the following grants: EP-V (DGICYT BFU2011-27207 and Spanish Junta de Andalucía CTS-2257); JN-D (CONACYT postdoctoral scholarship); MP-R (Ph.D. Fellowship from the Plan Propio UPO and by a Ph.D. Fellowship from the Basque Country Government); RR (Spanish Junta de Andalucía BIO-113 and SAF2015-64171-R); JR (MINECO-AEI/FEDER, UE BFU2016-80295-R); GAT (Spanish Junta de Andalucía BIO-209 and MINECO-AEI/FEDER, UE BFU2015-64536-R); AR-M (MINECO-AEI/FEDER, UE BFU2015-68655-P and the Junta de Andalucía P11-CVI-7290); and JA (Spanish Junta de Andalucía BIO-122 and MINECO-AEI/FEDER, UE PID2019-109569GB-I00).
References
- Adams I., Jones D. G. (1982). Synaptic remodelling and astrocytic hypertrophy in rat cerebral cortex from early to late adulthood. Neurobiol. Aging 3 179–186. 10.1016/0197-4580(82)90038-0 [DOI] [PubMed] [Google Scholar]
- Aggarwal S., Bhowmik A. D., Ramprasad V. L., Murugan S., Dalal A. (2016). A splice site mutation in HERC1 leads to syndromic intellectual disability with macrocephaly and facial dysmorphism: further delineation of the phenotypic spectrum. Am. J. Med. Genet. A 170 1868–1873. 10.1002/ajmg.a.37654 [DOI] [PubMed] [Google Scholar]
- Alfaro I. E., Albornoz A., Molina A., Moreno J., Cordero K., Criollo A., et al. (2019). Chaperone mediated autophagy in the crosstalk of neurodegenerative diseases and metabolic disorders. Front. Endocrin. 9:778. 10.3389/fendo.2018.00778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral D., Lavenex P. (2007). “Hippocampal neuroanatomy,” in The Hippocampus Book, eds Andersen P., Morris R., Amaral D., Bliss T., O’Keefe J. (New York: Oxford University Press; ), 37–114. 10.1093/acprof:oso/9780195100273.003.0003 [DOI] [Google Scholar]
- Anaya-Martínez V., Gutiérrez-Valdez A. L., Ordoñez-Librado J. L., Montiel-Flores E., Sánchez-Betancourt J., Sánchez Vazquez, et al. (2014). The presence of perforated synapses in the striatum after dopamine depletion, is this a sign of maladaptative brain plasticity? Microscopy 63 427–435. 10.1093/jmicro/dfu032 [DOI] [PubMed] [Google Scholar]
- Andrade-Talavera Y., Duque-Feria P., Negrete-Díaz J. V., Sihra T. S., Flores G., Rodríguez-Moreno A. (2012). Presynaptic kainate receptor-mediated facilitation of glutamate release involves Ca2+ -calmodulin at mossy fiber-CA3 synapses. J. Neurochem. 122 891–899. 10.1111/j.1471-4159.2012.07844.x [DOI] [PubMed] [Google Scholar]
- Andrade-Talavera Y., Duque-Feria P., Paulsen O., Rodríguez-Moreno A. (2016). Presynaptic spike timing-dependent long-term depression in the mouse hippocampus. Cer. Cort. 26 411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo-García L., Vázquez-Roque R. A., Díaz A., Treviño S., De La Cruz F., Flores G., et al. (2018). The effects of non-selective dopamine receptor activation by apomorphine in the mouse hippocampus. Mol. Neurobiol. 55 8625–8636. 10.1007/s12035-018-0991-2 [DOI] [PubMed] [Google Scholar]
- Bachiller S., Roca-Ceballos M. A., García-Domínguez I., Pérez-Villegas E. M., Martos-Carmona D., Pérez-Castro M. Á, et al. (2018). HERC1 ubiquitin ligase is required for normal axonal myelination in the peripheral nervous system. Mol Neurobiol. 55 8856–8868. 10.1007/s12035-018-1021-0 [DOI] [PubMed] [Google Scholar]
- Bachiller S., Rybkina T., Porras-García E., Pérez-Villegas E., Tabares L., Armengol J. A., et al. (2015). The HERC1 E3 ubiquitin ligase is essential for normal development and for neurotransmission at the mouse neuromuscular junction. Cell. Mol. Life Sci. 72 2961–2971. 10.1007/s00018-015-1878-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey C. H., Kandel E. R., Harris K. M. (2015). Structural components of synaptic plasticity and memory consolidation. Cold Spring Harbor Perspect. Biol. 7:a021758 10.11.1/cshperspect.a021758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayram-Weston Z., Olsen E., Harrison D. J., Dunnet S. B. (2016). Optimising Golgi-Cox staining for use with perfusion-fixed brain tissues validated in the zQ175 mouse model of Huntington’s disease. J. Neurosci. Meth. 65 81–88. 10.1016/j.jneumeth.2015.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binotti B., Pavlos N. J., Riedel D., Wenzel D., Vorbrüggen G., Schalk A. M., et al. (2015). The GTPase Rab26 links synaptic vesicles to the autophagy pathway. Elife 4:e05597. 10.7554/eLife.05597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne J., Harris K. M. (2007). Do spines learn to be mushroom spines that remember? Curr. Op. Neurobiol. 17 81–386. 10.1016/jconb.2007.04.009 [DOI] [PubMed] [Google Scholar]
- Buonarati O. R., Hammes E. A., Watson J. F., Greger I. H., Hell J. W. (2019). Mechanisms of postsynaptic localization of AMPA-type glutamate receptors and their regulation during long-term potentiation. Sci. Sign. 12 eaar6889. 10.1126/scisignal.aar6889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calverley R. K. S., Jones D. G. (1987). A serial-section study of perforated synapses in rat neocortex. Cell Tiss. Res. 247 565–572. 10.1007/bf00215750 [DOI] [PubMed] [Google Scholar]
- Carlin R. K., Siekevitz P. (1983). Plasticity in the central nervous system: do synapses divide? Proc. Natl. Acad. Sci. U.S.A. 80 3517–3521. 10.1073/pnas.80.11.3517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y., Lee J., Jung S., Lee Y., Cho J. W., Oh Y. J. (2018). Dysregulated autophagy contributes to caspase-dependent neuronal apoptosis. Cell Death Dis. 9:1189. 10.1038/s41419-018-1229-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioni J. M., Lin J. Q., Holtermann A. V., Koppers M., Jakobs M. A. H., Azizi A., et al. (2019). Late endosomes act as mRNA translation platforms and sustain mitochondria in axons. Cell 176 56–72. 10.1016/j.cell.2018.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillos-Rojas M., Schneider T., Hadjebi O., Pedrazza L., de Oliveira J. R., Langa F., et al. (2016). The HERC2 ubiquitin ligase is essential for embryonic development and regulates motor coordination. Oncotarget 7 56083–56106. 10.18632/oncotarget.11270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vrij F. M., Fischer D. F., van Leeuwen F. W., Hol E. M. (2004). Protein quality control in Alzheimer’s disease by the ubiquitin proteasome system. Prog. Neurobiol. 74 249–270. 10.1016/j.pneurobio.2004.10.001 [DOI] [PubMed] [Google Scholar]
- Deacon R. M. J., Rawlins J. N. P. (2006). T-maze alternation in the rodent. Nat. Protoc. 1 7–12. 10.1038/nprot.2006.2 [DOI] [PubMed] [Google Scholar]
- Deng H. X., Chen W., Hong S. T., Boycott K. M., Gorrie G. H., Siddique N., et al. (2011). Mutations in UBQLN2 cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia. Nature 477 211–215. 10.1038/nature10353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond N. L., Levy W. B. (1985). Granule cell dendritic spine density in the rat hippocampus varies with spine shape and location. Neurosci. Lett. 54 219–224. 10.1016/s0304-3940(85)80082-3 [DOI] [PubMed] [Google Scholar]
- Dlamini N., Josifova D. J., Paine S. M., Wraige E., Pitt M., Murphy A. J., et al. (2013). Clinical and neuropathological features of X-linked spinal muscular atrophy (SMAX2) associated with a novel mutation in the UBA1 gene. Neuromusc. Disord. 23 391–398. 10.1016/j.nmd.2013.02.001 [DOI] [PubMed] [Google Scholar]
- Dunaevsky A., Tashiro S., Majewska A., Mason C., Yuste R. (1999). Developmental regulation of spine motility in the mammalian central nervous system. Proc. Natl. Acad. Sci. U.S.A. 96 13438–13443. 10.1073/pnas.96.23.13438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusart I., Guenet J. L., Sotelo C. (2006). Purkinje cell death: differences between developmental cell death and neurodegenerative death in mutant mice. Cerebellum 5 163–173. 10.1080/14734220600699373 [DOI] [PubMed] [Google Scholar]
- Flores G., Alquicer G., Silva-Gómez A. B., Zaldívar G., Stewart J., Quirion R., et al. (2005). Alterations in dendritic morphology of prefrontal cortical and nucleus accumbens neurons in post-pubertal rats after neonatal excitotoxic lesions of the ventral hippocampus. Neuroscience 133 463–470. 10.1016/j.neuroscience.2005.02.021 [DOI] [PubMed] [Google Scholar]
- García-Cano J., Martinez-Martinez A., Sala-Gaston J., Pedrazza L., Rosa J. L. (2019). HERCing: structural and functional relevance of the large HERC ubiquitin ligases. Front. Physiol. 10:1014. 10.3389/fphys.2019.01014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghavani S., Shojaei S., Yeganeh B., Ande S. R., Jangamreddy J. R., Mehrpour M., et al. (2014). Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog. Neurobiol. 112 24–49. [DOI] [PubMed] [Google Scholar]
- Glatigny M., Moriceau S., Rivagorda M., Ramos-Brossier M., Nascimbeni A. C., Lante F., et al. (2019). Autophagy is required for memory formation and reverses age-related memory decline. Curr. Biol. 29 435.e–448.e. 10.1016/j.cub.2018.12.021 [DOI] [PubMed] [Google Scholar]
- Gray E. G. (1959). Axo-somatic and axo-dendritic synapses of the cerebral cortex: an electron microscope study. J. Anat. 93 420–433. [PMC free article] [PubMed] [Google Scholar]
- Grienberger C., Chen X., Konnerth A. (2015). Dendritic function in vivo. Trends Neurosci. 38 45–54. 10.1016/j.tins.2014.11.002 [DOI] [PubMed] [Google Scholar]
- Grishchuk Y., Ginet V., Truttmann A. C., Clarke P. G., Puyal J. (2011). Beclin 1-independent autophagy contributes to apoptosis in cortical neurons. Autophagy 7 1115–1131. 10.4161/auto.7.10.16608 [DOI] [PubMed] [Google Scholar]
- Grüsser-Cornehls U., Bäurle J. (2001). Mutant mice as a model for cerebellar ataxia. Prog. Neurobiol. 63 489–540. 10.1016/s0301-0082(00)00024-1 [DOI] [PubMed] [Google Scholar]
- Hamilton A. M., Lambert J. T., Parajuli L. K., Vivas O., Park D. K., Stein I. S., et al. (2017). A dual role for the RhoGEF Ephexin5 in regulation of dendritic spine outgrowth. Mol. Cell. Neurosci. 80 66–74. 10.1016/j.mcn.2017.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton A. M., Zito K. (2013). Breaking it down: the ubiquitin proteasome system in neuronal morphogenesis. Neur. Plast. 2013:196848. 10.1155/2013/196848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto R., Nakazawa T., Tsurusaki Y., Yasuda Y., Nagayasu K., Matsumura K., et al. (2016). Whole-exome sequencing and neurite outgrowth analysis in autism spectrum disorder. J. Hum. Genet. 61 199–206. 10.1038/jhg.2015.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H., Mahnke A. H., Doyle S., Fan N., Wang C. C., Hall B. J., et al. (2012). Neurodevelopmental role for VGLUT2 in pyramidal neuron plasticity, dendritic refinement, and in spatial learning. J. Neurosci. 32 15886–15901. 10.1523/JNEUROSCI.4505-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M., Ding Y., Chu C., Tang J., Xiao Q., Luo Z. G. (2016). Autophagy induction stabilizes microtubules and promotes axon regeneration after spinal cord injury. Proc. Natl. Acad. Sci. U.S.A. 113 11324–11329. 10.1073/pnas.1611282113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde A. N., Upadhya S. C. (2007). The ubiquitin-proteasome pathway in health and disease of the nervous system. Trends Neurosci. 30 587–595. 10.1016/j.tins.2007.08.005 [DOI] [PubMed] [Google Scholar]
- Hernandez D., Torres C. A., Setlik W., Cebrián C., Mosharov E. V., Tang G., et al. (2012). Regulation of presynaptic neurotransmission by macroautophagy. Neuron 74 277–284. 10.1016/j.neuron.2012.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawaid S., Kidd G. J., Wang J., Swetlik C., Dutta R., Trapp B. D. (2018). Alterations in CA1 hippocampal synapses in a mouse model of fragile X syndrome. Glia 66 789–780. 10.1002/glia.23284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Fukuda M., Watanabe S., Hayashi-Takagi A., Noguchi J. (2010). Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci. 33 121–129. 10.1016/j.tins.2010.01.001 [DOI] [PubMed] [Google Scholar]
- Labbadia J., Morimoto R. I. (2015). The biology of proteostasis in aging and disease. Ann. Rev. Biochem. 84 435–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackey E. P., Sillitoe R. V. (2017). “Motor circuit abnormalities during cerebellar development,” in Development Of The Cerebellum From Molecular Aspects To Diseases, ed. Marzban H. (New York, NY: Springer International Publishing AG; ), 105–127. 10.1007/978-3-319-59749-2_6 [DOI] [Google Scholar]
- Lalonde R. (2002). The neurobiological basis of spontaneous alternation. Neurosci. Biobehav. Rev. 26 91–104. 10.1016/s0149-7634(01)00041-0 [DOI] [PubMed] [Google Scholar]
- Lamprecht R., LeDoux J. (2004). Structural plasticity and memory. Nat. Rev. Neurosci. 5 45–54. [DOI] [PubMed] [Google Scholar]
- Lee J. A., Gao F. B. (2009). Inhibition of autophagy induction delays neuronal cell loss caused by dysfunctional ESCRT-III in frontotemporal dementia. J. Neurosci. 29 8506–8511. 10.1523/jneurosci.0924-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenga J., Willemsen R. (2012). Perturbation of dendritic protrusions in intellectual disability. Prog. Brain Res. 197 153–158. 10.1016/b978-0-444-54299-1.00008-x [DOI] [PubMed] [Google Scholar]
- Lim J., Yue Z. (2015). Neuronal aggregates: formation, clearance, and spreading. Dev. Cell 32 491–501. 10.1016/j.devcel.2015.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüningschrör P., Sendtner M. (2018). Autophagy in the presynaptic compartment. Curr. Op. Neurobio. 51 0–85. [DOI] [PubMed] [Google Scholar]
- Maday S., Holzbaur E. L. (2014). Autophagosome biogenesis in primary neurons follows an ordered and spatially regulated pathway. Dev. Cell 30 71–85. 10.1016/j.devcel.2014.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manto M. U., Jissendi P. (2012). Cerebellum: links between development, developmental disorders and motor learning. Front. Neuroanat. 6:1. 10.3389/fnana.2012.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Padilla M. (1974). Structural organization of the cerebral cortex (motor area) in human chromosomal aberrations. A Golgi study. I. D1 (13-15) trisomy, Patau syndrome. Brain Res. 66 375–391. 10.1016/0006-8993(74)90054-7 [DOI] [PubMed] [Google Scholar]
- Marin-Padilla M. (1976). Pyramidal cell abnormalities in the motor cortex of a child with Down’s syndrome. A Golgi study. J. Comp. Neurol. 167 63–81. 10.1002/cne.901670105 [DOI] [PubMed] [Google Scholar]
- Marrone D. F., Petit T. L. (2002). The role of synaptic morphology in neural plasticity: Structural interactions underlying synaptic power. Brain Res. Rev. 38 291–308. 10.1016/s0165-0173(01)00147-3 [DOI] [PubMed] [Google Scholar]
- Martinez-Vicente M. (2017). Neuronal mitophagy in neurodegenerative diseases. Front. Mol. Neurosci. 10:64. 10.3389/fnmol.2017.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashimo T., Hadjebi O., Amair-Pinedo F., Tsurumi T., Langa F., Serikawa T., et al. (2009). Progressive Purkinje cell degeneration in tambaleante mutant mice is a consequence of a missense mutation in HERC1 E3 ubiquitin ligase. PLoS Genet. 5:e1000784. 10.1371/journal.pgen.1000784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M., Honkura N., Ellis-Davies G. C., Kasai H. (2004). Structural basis of long-term potentiation in single dendritic spines. Nature 429 761–766. 10.1038/nature02617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies F. M., Fleming A., Caricasole A., Bento C. F., Andrews S. P., Ashkenazi A., et al. (2017). Autophagy and neurodegeneration: pathogenic mechanisms and therapeutic opportunities. Neuron 93 1015–1034. 10.1016/j.neuron.2017.01.022 [DOI] [PubMed] [Google Scholar]
- Mertz J., Tan H., Pagala V., Bai B., Chen P. C., Li Y., et al. (2015). Sequential elution interactome analysis of the mind bomb 1 ubiquitin ligase reveals a novel role in dendritic spine outgrowth. Mol. Cell. Prot. 14 1898–1910. 10.1074/mcp.M114.045898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montes-Fernández M. A., Pérez-Villegas E. M., Garcia-Gonzalo F. R., Pedrazza L., Rosa J. L., Alvarez, et al. (2020). The HERC1 ubiquitin ligase regulates presynaptic membrane dynamics of central synapses. Sci. Rep. 10:12057 10.1038/s41598-020-68970-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. (1984). Developments of water-maze procedure for studying spatial learning in the rat. J. Neurosci. Meth. 11 47–60. 10.1016/0165-0270(84)90007-4 [DOI] [PubMed] [Google Scholar]
- Morris R. (2007). “Theories of hippocampal function,” in The Hippocampus Book, eds Andersen P., Morris R., Amaral D., Bliss T., O’Keefe J. (New York, NY: Oxford University Press; ), 581–713. 10.1093/acprof:oso/9780195100273.003.0013 [DOI] [Google Scholar]
- Mychasiuk R., Gibb R., Kolb B. (2013). Visualizing the effects of a positive early experience, tactile stimulation, on dendritic morphology and synaptic connectivity with Golgi-Cox staining. J. Vis. Exp. 79:e50694. 10.3791/50694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoletano F., Baron O., Vandenabeele P., Mollereau B., Fanto M. (2018). Intersection between regulated cell death and autophagy. Trends Cell Biol. 148 1–16. 10.1016/j.tcb.2018.12.007 [DOI] [PubMed] [Google Scholar]
- Negrete-Díaz J. V., Sihra T. S., Delgado-García J. M., Rodríguez-Moreno A. (2007). Kainate receptor-mediated presynaptic inhibition converges with presynaptic inhibition mediated by Group II mGluRs and long-term depression at the hippocampal mossy fiber-CA3 synapse. J. Neu. Transm. 114 1425–1431. 10.1007/s00702-007-0750-4 [DOI] [PubMed] [Google Scholar]
- Nguyen L. S., Schneider T., Rio M., Moutton S., Siquier-Pernet K., Verny F., et al. (2015). A nonsense Q4 variant in HERC1 is associated with intellectual disability, megalencephaly, thick corpus callosum and cerebellar atrophy. Eur. J. Hum. Genet 24 455–458. 10.1038/ejhg.2015.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikoletopoulou V., Papandreou M.-E., Tavermarakis V. (2015). Autophagy in the physiology and pathology of the central nervous system. Cell Death Diff. 22 398–407. 10.1038/cdd.2014.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Recalde O., Beltrán O. I., Gálvez J. M., Palma-Montero A., Restrepo C. M., Mateus H. E., et al. (2015). Biallelic HERC1 mutations in a syndromic form of overgrowth and intellectual disability. Clin. Genet. 88 e1–e3. 10.1111/cge.12634 [DOI] [PubMed] [Google Scholar]
- Park M. (2018). AMPA receptor trafficking for postsynaptic potentiation. Front. Cell. Neurosci. 12:61. 10.3389/fncel.2018.00361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Villegas E. M., Negrete-Díaz J. V., Porras-García M. E., Ruiz R., Carrión A. M., Rodríguez-Moreno A., et al. (2018). Mutation of the HERC 1 ubiquitin ligase impairs associative learning in the lateral amygdala. Mol. Neurobiol. 55 1157–1168. 10.1007/s12035-016-0371-8 [DOI] [PubMed] [Google Scholar]
- Peters A., Kaiserman-Abramof I. R. (1969). The small pyramidal neuron of the rat cerebral cortex. The synapses upon dendritic spines. Z. Zellfors. Mikrosk. Anat. 100 487–506. 10.1007/bf00344370 [DOI] [PubMed] [Google Scholar]
- Peters A., Palay S. L., Webster H. (1976). The Fine Structure Of The Nervous System: The Neurons And Supporting Cells. New York, NY: W.B. Saunders Co. [Google Scholar]
- Petralia R. S., Wang Y.-X., Mattson M. P., Yao P. J. (2018). Invaginating structures in mammalian synapses. Front. Synap. Neurosci. 10:4. 10.3389/fnsyn.2018.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porras-García M. E., Ruiz R., Pérez-Villegas E. M., Armengol J. A. (2013). Motor learning of mice lacking cerebellar Purkinje cells. Front. Neuroanat. 7:4 10.3389/fnana 2013.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purpura D. P. (1975). Normal and aberrant neuronal development in the cerebral cortex of human fetus and young infant. UCLA Forum Med. Sci. 18 141–169. 10.1016/b978-0-12-139050-1.50014-8 [DOI] [PubMed] [Google Scholar]
- Radic T., Frieß L., Vijikumar A., Jungenitz T., Deller T., Schwarzacher S. W. (2017). Differential postnatal expression of neuronal maturation markers in the dentate gyrus of mice and rats. Front. Neuroanat. 11:104. 10.3389/fnana.2017.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramón y Cajal S. (1888). Estructura de los centros nerviosos de las aves. Rev. Trim. Histol. Norm. Patol. 1 1–10. [Google Scholar]
- Ramón y Cajal S. (1904). Textura Del Sistema Nervioso Del Hombre Y Los Vertebrados, Vol. III. Madrid: Imprenta y Librería Nicolás Moya, 1028–1042. [Google Scholar]
- Ramser J., Ahearn M. E., Lenski C., Yariz K. O., Hellebrand H., von Rhein M., et al. (2008). Rare missense and synonymous variants in UBE1 are associated with X-linked infantile spinal muscular atrophy. Am. J. Hum. Genet. 82 188–193. 10.1016/j.ajhg.2007.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risher W. C., Ustunkaya T., Singh Alvarado J., Eroglu C. (2014). Rapid Golgi analysis method for efficient and unbiased classification of dendritic spines. PLoS One 9:e107591. 10.3791/50694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzoli S. O., Betz W. J. (2005). Synaptic vesicle pools. Nat. Rev. Neuroci. 6 57–69. [DOI] [PubMed] [Google Scholar]
- Rochefort N. L., Konnerth A. (2012). Dendritic spines: from structure to in vivo function. EMBO Rep. 13 699–708. 10.1038/embor.2012.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogerson T., Cai D. J., Frank A., Sano Y., Shobe J., Lopez-Aranda M. F., et al. (2014). Synaptic tagging during memory allocation. Nat. Rev. Neurosci. 15 157–169. 10.1038/nrn3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa J. L., Barbacid M. (1997). A giant protein that stimulates guanine nucleotide exchange on ARF1 and Rab proteins forms a cytosolic ternary complex with clathrin and Hsp70. Oncogene 15 1–6. 10.1038/sj.onc.1201170 [DOI] [PubMed] [Google Scholar]
- Rossi F., Jankovski A., Sotelo C. (1995). Target neuron controls the integrity of afferent axon phenotype: a study on the Purkinje cell-climbing fiber system in cerebellar mutant mice. J. Neurosci. 15 2040–2056. 10.1523/jneurosci.15-03-02040.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein D. C. (2006). The roles of intracellular protein-degradation pathways in neurodegeneration. Nature 443 780–786. 10.1038/nature05291 [DOI] [PubMed] [Google Scholar]
- Ruiz R., Pérez-Villegas E. M., Bachiller S., Rosa J. L., Armengol J. A. (2016). HERC 1 ubiquitin ligase mutation affects neocortical, CA3 hippocampal and spinal cord projection neurons: an ultrastructural study. Front. Neuroanat. 10:42. 10.3389/fnana.2016.00042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusmini P., Bolzoni E., Crippa V., Onesto E., Sau D., Galbiati M., et al. (2010). Proteasomal and autophagic degradative activities in spinal and bulbar muscular atrophy. Neurobiol. Dis. 40 361–369. 10.1016/j.nbd.2010.06.016 [DOI] [PubMed] [Google Scholar]
- Rusmini P., Polanco M. J., Cristofani R., Cicardi M. E., Meroni M., Galbiati M., et al. (2015). Aberrant autophagic response in the muscle of a knock-in mouse model of spinal and bulbar muscular atrophy. Sci. Rep. 5:15174. 10.1038/srep15174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Tena S., Cubillos-Rojas M., Schneider T., Rosa J. L. (2016). Functional and pathological relevance of HERC family proteins: a decade later. Cell. Mol. Life Sci. 73 1955–1968. 10.1007/s00018-016-2139-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider T., Martinez-Martinez A., Cubillos-Rojas M., Bartrons R., Ventura F., Rosa J. L. (2018). The E3 ubiquitin ligase HERC1 controls the ERK signaling pathway targeting C-RAF for degradation. Oncotarget 9 31531–31548. 10.18632/oncotarget.25847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M. (2017). Dendritic spines: morphological building blocks of memory. Neurobiol Learn. Mem. 138 3–9. 10.1016/j.nlm.2016.06.007 [DOI] [PubMed] [Google Scholar]
- Shepherd G. M., Harris K. M. (1998). Three-dimensional structure and composition of CA3-CA1 axons in rat hippocampal slices: implications for presynaptic connectivity and compartmentalization. J. Neurosci. 18 8300–8310. 10.1523/jneurosci.18-20-08300.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimobayashi M., Hall M. N. (2014). Making new contacts: the mTOR network in metabolism and signalling crosstalk. Nat. Rev. Mol. Cell Biol. 15 155–162. 10.1038/nrm3757 [DOI] [PubMed] [Google Scholar]
- Sluimer J., Distel B. (2018). Regulating the human HECT E3 ligases. Cell. Mol. Life Sci. 75 3121–3141. 10.1007/s00018-018-2848-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. L., Bourne J. N., Cao G., Chirillo M. A., Ostroff L. E., Watson D. J., et al. (2016). Mitochondrial support of persistent presynaptic vesicle mobilization with age-dependent synaptic growth after LTP. eLife 5:e15275. 10.7554/eLife.15275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder J. S. (2019). Recalibrating the relevance of adult neurogenesis. Trends Neurosci. S0166–S2236. 10.1016/j.tins.2018.12.001 [DOI] [PubMed] [Google Scholar]
- Sotelo C. (1978). Purkinje cell ontogeny: formation and maintenance of spines. Prog. Brain Res. 48 149–168. 10.1016/s0079-6123(08)61021-3 [DOI] [PubMed] [Google Scholar]
- Sudhof T. C., Rizo J. (2011). Synaptic vesicle exocytosis. Cold Spring H arb. Persp. Biol. 3:a005637. 10.1101/cshperspect.a005637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tønnesen J., Katona G., Rózsa B., Nägerl U. V. (2014). Spine neck plasticity regulates compartmentalization of synapses. Nat. Neurosci. 17 678–685. 10.1038/nn.3682 [DOI] [PubMed] [Google Scholar]
- Turrigiano G. G. (2008). The self-tuning neuron: synaptic scaling of excitatory synapses. Cell 135 422–435. 10.1016/j.cell.2008.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhya S. C., Hegde A. N. (2005). Ubiquitin-proteasome pathway components as therapeutic targets for CNS maladies. Curr. Pharm. Des. 11 3807–3828. 10.2174/138161205774580651 [DOI] [PubMed] [Google Scholar]
- Utine G. E., Taşkıran E. Z., Koşukcu C., Karaosmanoğlu B., Güleray N., Doğan ÖA., et al. (2017). HERC1 mutations in idiopathic intellectual disability. Eur. J. Med. Genet. 60 279–283. 10.1016/j.ejmg.2017.03.007 [DOI] [PubMed] [Google Scholar]
- van Tijn P., Dennissen F. J., Gentier R. J., Hobo B., Hermes D., Steinbusch H. W., et al. (2012). Mutant ubiquitin decreases amyloid β plaque formation in a transgenic mouse model of Alzheimer’s disease. Neurochem. Int. 61 739–748. 10.1016/j.neuint.2012.07.007 [DOI] [PubMed] [Google Scholar]
- Vijayan V., Verstreken P. (2017). Autophagy in the presynaptic compartment in health and disease. J. Cell Biol. 216 1895–1906. 10.1083/jcb.201611113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waagepetersen H. S., Sonnewald U., Schousboe A. (2003). Compartmentation of glutamine, glutamate, and GABA metabolism in neurons and astrocytes: functional implications. Neuroscientist 9 398–403. 10.1177/1073858403254006 [DOI] [PubMed] [Google Scholar]
- Wassef M., Sotelo C., Cholley B., Brehier A., Thomasset M. (1987). Cerebellar mutations affecting the postnatal survival of Purkinje cells in the mouse disclose a longitudinal pattern of differentially sensitive cells. Dev. Biol. 124 379–389. 10.1016/0012-1606(87)90490-8 [DOI] [PubMed] [Google Scholar]
- Xu X., Miller E. C., Pozzo-Miller L. (2014). Dendritic spines dysgenesis in Rett syndrome. Front. Neuroanat. 8:97. 10.3389/fnana.2014.00097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Z. (2007). Regulation of neuronal autophagy in axon. Autophagy 3 139–141. 10.4161/auto.3602 [DOI] [PubMed] [Google Scholar]
- Yue Z., Wang Q. J., Komatsu M. (2008). Neuronal autophagy: going the distance to the axon. Autophagy 4 94–96. 10.4161/auto.5202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R. (2010). Dendritic Spines. Cambridge, MA: The MIT press. [Google Scholar]
- Yuste R. (2013). Electrical compartmentalization in dendritic spines. Ann. Rev. Neurosci. 36 429–449. 10.1146/annurev-neuro-062111-150455 [DOI] [PubMed] [Google Scholar]
- Yuste R., Bonhoeffer T. (2001). Morphological changes in dendritic spines associated with long-term synaptic plasticity. Ann. Rev. Neurosci. 24 1071–1089. 10.1146/annurev.neuro.24.1.1071 [DOI] [PubMed] [Google Scholar]
- Yuste R., Bonhoeffer T. (2004). Genesis of dendritic spines: insights from ultrastructural and imaging studies. Nat. Rev. Neurosci. 5 24–34. 10.1038/nrn1300 [DOI] [PubMed] [Google Scholar]
- Zuo Y., Yang G., Gan W. B. (2005). Long-term sensory deprivation prevents dendritic spine loss in primary somatosensory cortex. Nature 436 261–265. 10.1038/nature03715 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.