Abstract
Background
Neural oscillations reflect rapidly changing brain excitability states. We have demonstrated previously with EEG-triggered transcranial magnetic stimulation (TMS) of human motor cortex that the positive vs. negative peak of the sensorimotor μ-oscillation reflect corticospinal low-vs. high-excitability states. In vitro experiments showed that induction of long-term depression (LTD) by low-frequency stimulation depends on the postsynaptic excitability state.
Objective/Hypothesis: We tested the hypothesis that induction of LTD-like corticospinal plasticity in humans by 1 Hz repetitive TMS (rTMS) is enhanced when rTMS is synchronized with the low-excitability state, but decreased or even shifted towards long-term (LTP)-like plasticity when synchronized with the high-excitability state.
Methods
We applied real-time EEG-triggered 1-Hz-rTMS (900 pulses) to the hand area of motor cortex in healthy subjects. In a randomized double-blind three-condition crossover design, pulses were synchronized to either the positive or negative peak of the sensorimotor μ-oscillation, or were applied at random phase (control). The amplitude of motor evoked potentials was recorded as an index of corticospinal excitability before and after 1-Hz-rTMS.
Results
1-Hz-rTMS at random phase resulted in a trend towards LTD-like corticospinal plasticity. RTMS in the positive peak condition (i.e., the low-excitability state) induced significant LTD-like plasticity. RTMS in the negative peak condition (i.e., the high-excitability state) showed a trend towards LTP-like plasticity, which was significantly different from the other two conditions.
Conclusion
The level of corticospinal depolarization reflected by phase of the μ-oscillation determines the degree of corticospinal plasticity induced by low-frequency rTMS, a finding that may guide future personalized therapeutic stimulation.
Keywords: TMS, EEG, Corticospinal excitability, LTD-Like plasticity, Brain-state-dependent stimulation, Brain oscillation
Highlights
-
•
Positive vs. negative phase of μ-rhythm are states of low vs. high excitability.
-
•
1-Hz-rTMS coupled to positive but not negative phase results in LTD-like plasticity.
-
•
Phase of μ-rhythm determines effect size of 1-Hz-rTMS induced plasticity.
Introduction
Repetitive transcranial magnetic stimulation (rTMS) can induce aftereffects in the human brain that outlast the period of stimulation and are thought to reflect long-term potentiation (LTP)- and long-term depression (LTD)-like synaptic plasticity [[1], [2], [3]]. Patterned protocols (e.g., intermittent or continuous theta burst stimulation, quadripulse stimulation with different pulse intervals) or regular low- or high-frequency rTMS are used to achieve up- or down-regulation of cortical excitability [[3], [4], [5]], however with high interindividual and intraindividual variability of outcomes, and low effect sizes [[6], [7], [8], [9], [10], [11]]. Indeed, induction of plasticity is not only dependent on the parameters of stimulation, but also on instantaneous excitability state of the stimulated neurons that is reflected by the level of postsynaptic depolarization. At the systems level, the phase of ongoing oscillations, as recorded by local field potentials or electroencephalography (EEG) reflects synchronized synaptic activity fluctuations of large neuronal populations, and these play a decisive role for the direction and magnitude of plasticity induction. In vitro, this has been shown in hippocampal slices for high-frequency bursts [12] as well as for single pulses [13], where the direction of aftereffects by an otherwise identical protocol was determined by the oscillatory phase during which the stimuli were applied.
Recently, this has also been demonstrated by real-time EEG-triggered TMS in the human [14]. The negative peak of the sensorimotor μ-rhythm as extracted with a reference-free Laplacian montage centered over EEG sensor C3 (following the nomenclature of the international 10–20 system [15]) corresponds to a corticospinal high-excitability state, thought to be generated by excitatory postsynaptic potentials (EPSPs) from activity of radially oriented pyramidal cells located on the crown of the pre- and/or postcentral gyrus [16] and projecting onto corticospinal cells of primary motor cortex located in the anterior wall of the central sulcus [17]. High-frequency rTMS with bursts of 100 Hz triplets, synchronized to this corticospinal high-excitability state led to LTP-like corticospinal plasticity, whereas the same burst stimulation during the low-excitability state (positive peak of the μ-rhythm) or a control condition (random phase of the μ-rhythm) had no significant effect [14].
These results are consistent with models of synaptic plasticity [18,19] that postulate the timing of presynaptic spiking (as evoked by the TMS pulses) relative to postsynaptic voltage (EPSPs, as measured by the EEG signal) is crucial for determining the degree and direction of induced synaptic plasticity.
One of the most frequently applied rTMS protocols is the near 1 Hz or low-frequency rTMS protocol, which typically induces LTD-like corticospinal plasticity. Described originally by Chen et al. [20], it was one of the first rTMS plasticity protocols to be characterized, and influential for the development of other rTMS protocols [21]. Currently, it is still one of the most widely used therapeutic protocols in clinical settings, e.g., by stimulating the contralesional hemisphere of stroke patients to support recovery during neurorehabilitation [22].
Here, we investigated the dependency of corticospinal plasticity induction on the phase of the ongoing sensorimotor μ-rhythm, applying 1 Hz rTMS triggered on real-time EEG-defined brain states. In accord with previous in vitro studies [23,24], we hypothesized that 1 Hz rTMS synchronized to the positive peak of the μ-rhythm, i.e., the corticospinal low-excitability state, will lead to strongest LTD-like plasticity, while synchronization to the negative peak, i.e., the high-excitability state, will result in attenuation of this LTD-like effect or even a switch towards LTP-like plasticity. If confirmed, this will elucidate further the physiological processes underlying rTMS plasticity induction, and this may lead to protocols in clinical settings with more reliable and stronger neuromodulatory and eventually therapeutic effects.
Material and methods
Participants
The study was performed in accordance with the Declaration of Helsinki and approved by the local ethics committee at the medical faculty of the University of Tübingen (716/2014BO2). Experiments were conducted in conformity with current TMS safety guidelines [25], and participants gave their written informed consent prior to enrolment. Eighteen right-handed healthy young adults (7 male, mean age (±1 SD): 24.2 ± 3.3 years, age range: 19–29 years, mean laterality score (±1 SD) in the Edinburgh handedness inventory [26]: 0.91 ± 0.15) without history of neurological or psychiatric disease or usage of CNS active drugs were recruited from a pool of pre-screened participants. Twelve of the 18 subjects (66.7%) were newly recruited subjects that had never participated in any other experiment performed in our lab before (aside from the pre-screening session). The other 6 subjects participated in one or several of our previous studies [14,27,30,41,42]. All subjects had an anatomical cranial MRI scan for neuronavigation. The only inclusion requirement from our pre-screened pool was the presence of a single spectral EEG peak in the 9–13 Hz alpha band measured over sensorimotor area, with a signal-to-noise ratio (SNR) after removal of fitted 1/f noise > 5 dB, since sufficient SNR is a requirement for adequate phase estimation of the phase-detection algorithm [[27], [28], [29]]. In addition, participants had to meet the following criteria, which were tested in the first experimental session (‘screening session’), prior to the actual plasticity experiments: (1) resting motor threshold (RMT) of first dorsal interosseus (FDI) muscle 62.5% of maximum stimulator output (MSO), allowing for exploration of a stimulation intensity range of up to 160% RMT (160% × 62.5% = 100% MSO); (2) average peak-to-peak amplitude of the resting-state μ-oscillation signal ≥ 3 μV during triggering (see below). This criterion ensured sufficient accuracy of our phase-trigger algorithm since, unlike the phase-triggered experiments performed in our group previously [14,27,30], no μ-power threshold was applied for stimulation in the plasticity experiments in order to achieve a stimulation rate as close as possible to 1 Hz; (3) To ensure that subjects showed a μ-rhythm-dependent phase stimulation effect with the positive peak being the low excitability state and the negative peak being the high excitability state [14], the median MEP elicited by TMS pulses triggered at the negative peak had to be larger than the median MEP at the positive peak (no statistical test was performed to screen for this criterion). Two participants were excluded because they did not meet the RMT criterion. Three further subjects were excluded due to low-amplitude μ-oscillation signal, two of them also failed the phase effect criterion. Another subject was excluded, because the phase effect criterion was not met. This resulted in a final sample size of 12 participants. A single session in one subject was repeated, due to local headache resulting from pressure of the coil held by the fixation device. Otherwise, procedures were tolerated without any adverse effects.
EEG and EMG recordings
A combined EEG-TMS set-up was used to synchronize stimulation pulses with the target phase of sensorimotor μ-rhythm. Scalp EEG was recorded from a 64-channel TMS-compatible Ag/AgCl sintered ring electrode cap (EasyCap GmbH, Germany) in the International 10–20 system arrangement [15]. Electrodes were prepared by mild skin abrasion utilizing an abrasive gel (Nuprep Skin Prep Gel, Weaver and Company, USA) followed by application of conductive gel (Electrode Cream, GE Medical Systems, USA) until the desired impedance of <5 kΩ was attained. EMG was recorded from the voluntarily relaxed right FDI muscle with adhesive hydrogel electrodes (Kendall, Covidien, Ireland) in a bipolar belly-tendon montage. EEG and EMG were recorded simultaneously with a 24-bit biosignal amplifier (NeurOne Tesla with Digital Out Option, Bittium Biosignals Ltd., Finland) at a sample rate of 5 kHz, in DC mode, with a 1.25 kHz lowpass anti-aliasing filter.
TMS set-up
A passively cooled TMS figure-of-eight coil (PMD70-pCool, 70 mm winding diameter) connected to a magnetic stimulator (Research 100, MAG & More GmbH, Germany) was used, configured to deliver biphasic single cosine cycle pulses with 160 μs period, such that the major second component of the induced electric field was oriented from lateral-posterior to medial-anterior, i.e., orthogonal to the central sulcus. Each TMS pulse was triggered through an external trigger input from the real-time computer system. For determining RMT and the motor evoked potential (MEP) input-output (IO)-curve, stimulation intensity was set by an analog control signal between 0 and 5 V corresponding to 0–100% MSO through an analog output port interface (UEI PD2-MF-64-500/16L, United Electronic Instruments, USA) enabling automated adjustment of stimulation intensity. Stimulation was applied to the hand representation of left primary motor cortex (M1). The motor hot spot was identified as the coil position and orientation resulting consistently in maximum MEP amplitudes [31]. RMT was defined as the lowest intensity that elicited MEPs with a peak-to-peak amplitude of ≥50 μV in at least 5 out of 10 trials [32], and determined by an automated algorithm. A stereoscopic neuronavigation system (Localite GmbH, Sankt Augustin, Germany) with individual MRI scans of each subject was used to maintain a consistent coil position over the motor hot spot within and between sessions. The coil was held in position using a mechanical arm, with 3D coil position monitored continuously throughout the experiment and corrected manually if necessary.
Real-time EEG-triggered TMS
The real-time processing system used in this study is described in detail in Zrenner et al. [14]. Briefly, an algorithm implemented in Simulink Real-Time (Mathworks Ltd, USA, R2016a) was used for real-time EEG data processing and to trigger TMS pulses. Sensorimotor μ-rhythm was extracted by computing a surface Laplacian montage centered over left sensorimotor cortex comprising of EEG channel C3 referenced to the average of four surrounding channels (CP1, CP5, FC1, FC5) (Fig. 1a) [33]. Data was down-sampled from 5 kHz to 1 kHz by averaging 5 samples, and a sliding window of data of 500 ms width was used to compute estimates of instantaneous phase. The signal window was band-pass filtered (finite impulse response filter with order 128 and pass band 9–13 Hz), the last 64 ms were discarded because of edge artifacts and then forward predicted by an autoregressive model (Yule-Walker, order 30) for 128 ms. Instantaneous phase was determined using the Hilbert transform. In addition, the power spectrum was calculated from a sliding window of 1024 samples using Hann-windowed FFT and integrating spectral power in the 9–13 Hz frequency band (for a detailed discussion on optimal filter parameters please see Refs. [29]). However, for the rTMS intervention in the plasticity experiments, no power threshold was implemented. The stimulator was triggered at the desired phase closest to (before or after) 1000 ms after the previous trigger, to achieve a median trigger frequency of 1 Hz.
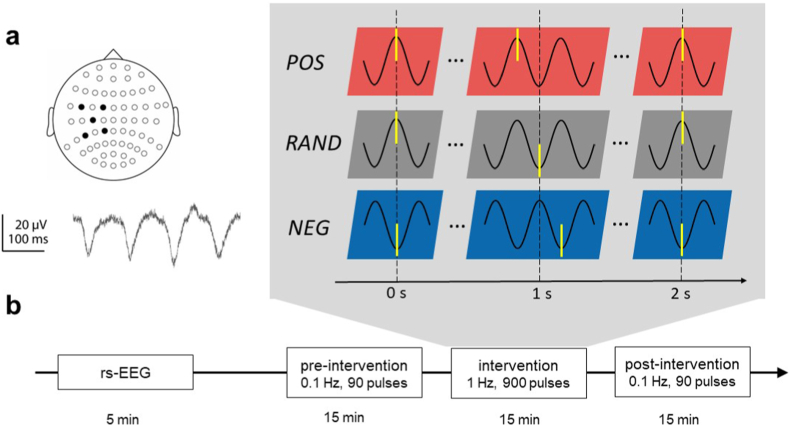
Fig. 1.
Experimental design. a μ-rhythm was derived using a 5-channel (black dots) Laplace transform centered on EEG sensor C3 (top), an example raw data trace is shown at the bottom. b Plasticity sessions started with a 5 min resting-state EEG (rs-EEG) to test the accuracy of our phase triggering algorithm. Thereafter, each plasticity session contained a block of 90 single TMS pulses at a rate of 0.1 Hz before and after the rTMS intervention block, with TMS pulses applied irrespective of the EEG signal (“open loop”). For the intervention block, a double-blind, randomized crossover design was applied, so that each participant received 900 pulses of ∼1 Hz rTMS in the positive peak condition (POS), 1 Hz rTMS in the random phase (RAND, irrespective of μ-rhythm) or ∼1 Hz rTMS in the negative peak condition (NEG). Time points of stimulation are indicated schematically by yellow bars. TMS was applied to the hand representation of left primary motor cortex. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Experimental sessions
Each of the 12 subjects underwent four experimental sessions. Sessions were spaced apart by at least 72 h to prevent carryover effects. Subject were seated comfortably in an adjustable chair and instructed to fixate a cross approximately 1 m in front of them during data recording. Head position was stabilized by a vacuum neck support pillow (Vacuform, B. u.W. Schmidt GmbH, Germany).
Screening session
Before the three 1 Hz rTMS plasticity-induction sessions, one screening session was performed to check the inclusion criteria described above and to determine SI50%max. This session consisted of four blocks: (1) 5 min eyes open resting-state (rs)-EEG was obtained to validate the accuracy of our phase trigger algorithm [14]. (2) RMT was determined as described above. (3) A recruitment curve was established as described earlier in Schaworonkow et al. [27], and SI50%max was determined as the stimulation intensity resulting in median MEP amplitude of 50% of maximum MEP amplitude. (4) The effect of μ-rhythm phase on MEP amplitude was explored in each subject by applying 100 TMS pulses each in the random phase, negative peak and positive peak conditions in a randomized order. The μ-power threshold for stimulation in this screening session was adjusted manually, targeting a median interstimulus interval (ISI) of 2.5 s. Across participants, an ISI of 2.42 s was achieved with an interquartile range (IQR) of 0.47 s.
Plasticity sessions
Three experimental sessions (‘plasticity sessions’) were conducted in a double-blind, randomized crossover design (Fig. 1b), consisting of 5 min rs-EEG recording and a 45 min plasticity block. To measure the effect of the intervention on corticospinal excitability, 90 TMS pulses were applied open-loop (i.e., non-informed by EEG data of μ-rhythm phase) at a rate of 0.1 Hz before and after the rTMS block. The intervention block consisted of 900 TMS pulses at approximately 1 Hz with each pulse applied, in three different sessions, either at the (1) positive peak, (2) random phase, or (3) negative peak of ongoing μ-rhythm. Stimulation intensity was set to SI50%max as determined in the screening session. Across participants, this resulted in mean stimulation intensity (±1 SD) of 123 4% RMT. This relatively high rTMS intensity of SI50%max was chosen based on the following considerations: (1) The 1 Hz rTMS-induced LTD-like plasticity effect depends on stimulation intensity, with higher stimulation intensities leading to a more pronounced LTD-like effect [[34], [35], [36]]. (2) The brain-state-dependent stimulation effect on MEP amplitude also depends on stimulation intensity, with lower intensities leading to a greater relative effect [27]. (3) The greatest absolute phase effect was observed at SI50%max [27] and a robust 1 Hz LTD-like effect is described for this stimulation intensity as well [20,34,36,37].
Data analysis and statistics
Data was analyzed with Matlab (Mathworks Ltd, USA, R2019a). Circular statistics was performed for analysis of real-time phase targeting accuracy, applying formulas adapted from the CircStat Matlab toolbox [38], using circular standard deviation (()) as a measure of angular spread.
MEP trials with muscle activity in the 100 ms prior to the TMS pulse were discarded with a threshold criterion (max-min amplitude > 40 μV). The median portion of trials discarded was 4.0% (IQR 1.3–11.2). Peak-to-peak MEP amplitudes were determined within the interval of 20–50 ms after the TMS pulse.
Absolute MEP amplitudes were log transformed for statistical analysis. Significance testing was performed using IBM SPSS Statistics v.26. Normality distribution was tested by Shapiro-Wilk test. Normally distributed data with more than two intervention groups measuring the same subjects within groups was tested by repeated-measurements analysis of variance (rmANOVA), with the main effects of Phase (three levels: positive peak, random phase, negative peak) and Time (two levels: pre-intervention, post-intervention). Mauchly’s test was used to test for violation of sphericity. If sphericity could not be assumed, Greenhouse-Geisser correction was applied. In case of significant rmANOVA, post-hoc paired, two-tailed t-tests were applied. Non-normally distributed data with more than two intervention groups measuring the same subjects within groups were tested by Friedman Test. A significance threshold of p < 0.05 was used.
Results
Phase-dependent excitability
RmANOVA confirmed a significant effect of Phase on MEP amplitude (F2,22 = 4.54, p = 0.02) (Fig. 2) in the 12 subjects included in this study. Mean (±1 SEM) normalized MEP amplitude was 93.9 ± 3.1% in the positive peak condition (p = 0.32 vs. random phase; p = 0.04 vs. negative peak), 98.2 ± 1.7% in the random phase condition (p = 0.03 vs. negative peak, all paired two-sided t-tests), and 108.0 ± 3.0% in the negative peak condition. MEP amplitude in the negative peak condition was on average 14.1 5.8% (mean ± 1 SEM) larger compared to the positive peak condition, in line with results from our previous study [14]. However, this finding was to be expected, since larger MEPs in the negative compared to the positive peak condition was an inclusion criterion of this study.
Fig. 2.
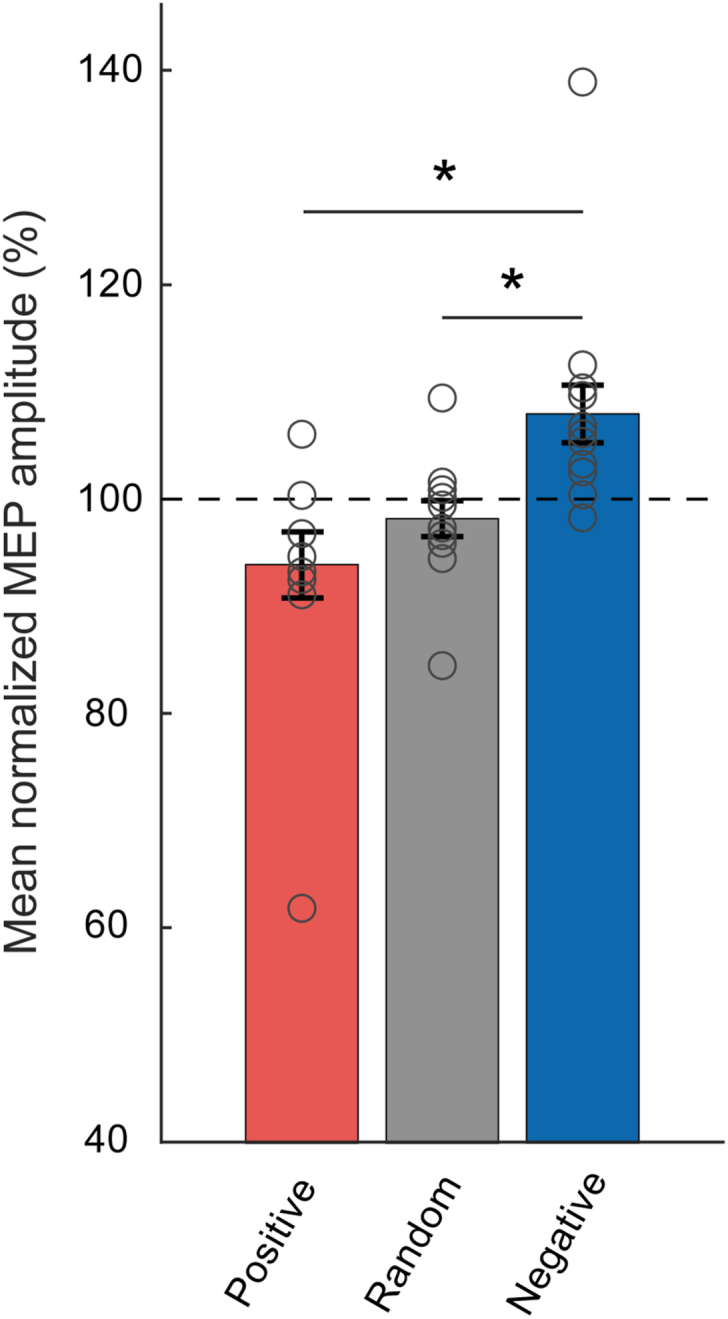
Excitability data. Bars represent mean ± 1 SEM (n = 12) of the MEP amplitude in the positive peak (red), at random phase (grey), and negative peak (blue) phase conditions of the ongoing μ-rhythm, normalized to the mean of all three conditions. Individual data are shown by circles. ∗p < 0.05. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Real-time phase targeting accuracy
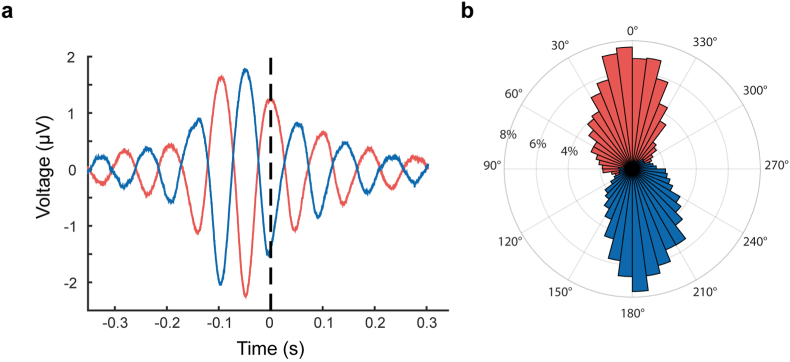
We analyzed the accuracy of our trigger algorithm in the rs-EEG prior to 1 Hz rTMS plasticity induction, using markers generated by the real-time system without triggering the stimulator. This enabled us to compare the triggers for positive and negative peaks of the μ-rhythm with the actual “true” phase, which we could determine from the C3 Laplacian transformed EEG signal with standard non-causal methods (band-pass filter and Hilbert transform), as this signal was not affected by any stimulus-related artifacts (Fig. 3a). 200 non-stimulating EEG phase-triggered markers were recorded per subject and phase condition before each plasticity measurement. The average peri-stimulus C3 Laplacian transformed EEG signal across all participants was calculated (without applying any filters) to visualize the average oscillation (Fig. 3a). The mean phase angle (±1 circular SD) across all participants was 13.9 ± 54.4° for the positive peak and −163.1 ± 54.9° for the negative peak (Fig. 3b).
Fig. 3.
Phase accuracy tested in the triggered but non-stimulated rs-EEG. a Mean C3-centered Laplace-transformed EEG signal for negative peak (blue) and positive peak (red) across all real-time triggered but non-stimulated trials of all subjects (n = 12). Dashed vertical line represents time point of the trigger. b Binned distribution (7.5° per bin, 2% frequency steps) of actual phase angle at the time of trigger of non-stimulated trials as determined by Hilbert transformation of the band-pass filtered segment of data before and after each marker. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Due to the TMS-induced artifact, phase targeting accuracy could not be calculated using the same method in the rTMS intervention conditions. However, the pre-stimulus average C3-Laplace-transformed EEG signal (which was used by the real-time system to estimate phase and trigger the stimulator) is shown without applying any filtering or post-processing in Fig. 4a (900 epochs per subject per condition, 12 subjects). We also performed spectral analysis of the rs-EEG. The results showed an alpha component typically of 9–11 Hz (Supplementary Material, Fig. S1).
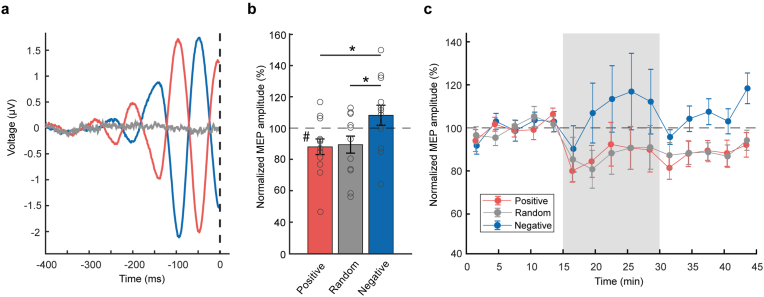
Fig. 4.
Plasticity experiment data. a Mean pre-stimulus C3-Hjorth EEG signal for all stimuli in the rTMS intervention period for positive peak (red), random phase (grey) and negative peak condition (blue), approximately 900 trials per condition and subject, across all 12 participants. The dashed vertical line represents the time of the TMS pulse. b Mean ± 1 SEM (n = 12) post-intervention MEP amplitude normalized to mean pre-intervention MEP amplitude in the three phase conditions. Circles indicate individual data. ∗p = 0.02 (two-sided two-tailed t-tests), #p < 0.05 (one-sided two-tailed t-test, indicating difference from 100%). c Mean MEP amplitude for each Phase condition, binned in 3 min segments. RTMS intervention period is indicated by the grey area. Data is normalized to the mean pre-intervention MEP amplitude of each participant and Phase condition (100%, black dashed line). Error bars indicate ±1 SEM. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Phase-dependent plasticity
The mean absolute MEP amplitudes (±1 SEM) were 1.93 ± 0.25 mV (positive peak), 1.64 ± 0.21 mV (random phase) and 1.69 ± 0.27 mV (negative peak) for the pre-intervention period. RmANOVA did not show an effect of Phase on absolute pre-intervention MEP amplitude (F2,22 = 0.49, p = 0.62). Therefore, there were no differences in pre-intervention MEP amplitudes between Phase conditions that could have accounted for the observed (see below) differential rTMS effects on corticospinal plasticity. Individual absolute pre- and post-intervention MEP amplitude data (in mV) are shown in the Supplementary Material (Fig. S2).
RmANOVA of the absolute MEP amplitude data (Fig. S2) did not yield significant effects of Phase (F2,22 = 0.31, p = 0.74) or Time (F1,11 = 2.67, p = 0.13) but a significant Phase × Time interaction (F1,11 = 3.85, p = 0.04). Post hoc paired t-tests revealed a significant decrease of MEP amplitude post-intervention vs. pre-intervention (effect of Time) for the positive peak condition (p = 0.04), but not for the random phase (p = 0.07) and negative peak conditions (p = 0.41).
For assessing further the effects of Phase, post-intervention MEP amplitudes were normalized to the corresponding pre-intervention values of each subject and Phase condition. RmANOVA showed a significant effect of Phase (F2,22 = 5.19, p = 0.01). This was explained by significant post hoc differences in the positive peak (mean ± 1 SEM, 88.0 ± 5.0%, p = 0.02) and random phase conditions (89.1 ± 5.5%, p = 0.02) compared to the negative peak condition (108.2 ± 6.4%) (Fig. 4b). One-sided two-tailed t-tests confirmed a significant change of normalized MEP amplitude from 100% for the positive peak condition (p = 0.04) but not for the random phase (p = 0.09) and negative peak conditions (p = 0.25) (Fig. 4b).
MEP amplitudes normalized to mean pre-intervention before, during and after the 1 Hz rTMS interventions are visualized over time in Fig. 4c showing means ± 1 SEM in 3-min bins (18 data points per bin pre- and post-intervention, 180 data points per bin during the intervention). During the phase-synchronized 1 Hz rTMS intervention period, MEPs showed a larger amplitude in the negative peak condition as compared to the positive peak and random phase condition, due to the negative peak condition representing a high-excitability state [14]: Positive peak (mean ± 1 SEM) 90.6 ± 11.0%, random phase 90.3 ± 10.3%, negative peak 114.1 ± 18.3%, all data normalized to pre-intervention. The rmANOVA indicated that these effects of Phase were not significant (F1.27,13.99 = 3.05, p = 0.10).
Interstimulus interval (ISI) analysis
Since the trigger intervals in the intervention period were dependent on the ongoing μ-rhythm for the positive peak and negative peak condition (cf. Fig. 1b), the frequency was not exactly 1 Hz and there was some jitter. We therefore analyzed the median ISIs of every subject in the intervention period for all three groups. Positive peak stimulation resulted in a median ISI (IQR) of 999 (60) ms, random phase stimulation in 997 (1) ms, and negative peak stimulation in 997 (48) ms. Friedman Test did not show a significant effect of Phase (Χ2(2) = 0.50, p = 0.78).
Discussion
This EEG-TMS study demonstrates for the first time, to the best of our knowledge, that induction of LTD-like corticospinal plasticity in human motor cortex depends on the state of corticospinal excitability, as indexed by the phase of the ongoing μ-oscillation. Similar LTD-like plasticity effects were induced by low-frequency stimulation synchronized with the low-excitability state (positive peak of the μ-oscillation) and by random phase stimulation, while otherwise identical rTMS synchronized with the high-excitability state (negative peak of the μ-oscillation) resulted in a trend towards LTP-like plasticity, significantly different from the other two conditions. These findings substantiate the notion that stimulation-induced brain plasticity is determined not only by parameters of the stimulation protocol, such as frequency, intensity, number of pulses and pattern of stimulation, but also by the current state of the brain at the time of stimulation.
Physiology
Induction of LTD-like corticospinal plasticity by low-frequency (1 Hz) rTMS occurs at the level of motor cortex because late I-waves but not the I1-wave or the D-wave are selectively suppressed in association with the depression of MEP amplitude [39]. Late I-waves indicate synaptic activation of the corticospinal cells by EPSPs, most likely through circuits of excitatory interneurons [40]. Therefore, LTD-like plasticity induced by low-frequency rTMS can be viewed as a model of long-term depression of interneuronal circuitry projecting with excitatory synapses onto corticospinal cells.
We have shown consistently in several studies (with only minor overlap of subjects between studies), that MEPs are larger if the TMS pulse is applied at the time of the negative peak of the sensorimotor μ-oscillation compared to the positive peak [14,27,30,41,42]. Therefore, the negative peak of the μ-rhythm is a more excitable state of M1 output neurons. This higher excitability might be induced by pulsatile excitatory inputs, received by these neurons at the instant of the negative EEG peak, rendering their postsynaptic potential towards stronger depolarization. Indeed, EPSPs at the apical dendrites (superficial layers) of radially oriented pyramidal cells underlying the EEG montage are considered to be a major contributor to the negative deflection of the overlying surface EEG [16,43]. Yet, the precise mechanisms of these relationships remain elusive at the moment and need to be addressed in further research work.
In vitro experiments on rat hippocampal slices demonstrated that induction of LTD by low-frequency stimulation was reliably possible by slight postsynaptic depolarization achieved by current injection, whereas the same low-frequency stimulation resulted in LTP if paired with a stronger level of postsynaptic depolarization [24]. Similarly, high-frequency or burst stimulation protocols in various in vitro preparations resulted in LTP if paired with a high level of postsynaptic depolarization but in LTD if paired with a lower level of depolarization or hyperpolarization [23,44]. This is also similar to our previous observation that 100 Hz burst rTMS induced LTP-like corticospinal excitability only when synchronized with the negative peak of the ongoing μ-oscillation, i.e., a putatively high level of postsynaptic depolarization of the corticospinal cells [14].
One puzzling finding is that, for the 1 Hz rTMS plasticity effect, the reduction of amplitude in the positive peak condition differed only slightly from that of the random phase condition. This finding is analogous to Zrenner et al. [14], where high-frequency burst rTMS at the negative peak of the μ-oscillation resulted in LTP-like corticospinal plasticity, whereas stimulation at random phase and the positive peak did not result in significant plasticity. One possible explanation is a non-linear dependence of plasticity induction on the level of postsynaptic depolarization, as observed in slice experiments [44,45] and supported by recent computer models [18], suggesting that LTP requires a distinctly stronger postsynaptic depolarization compared to LTD. Another explanation for the similarity between random phase and positive peak conditions could be, that the negative peak lasts for only a relatively brief proportion of the μ-oscillation, owing to the typical asymmetric arciform shape of the μ-rhythm [46,47], with the negative peak sharper and shorter compared to the positive peak (see Fig. 1a). Therefore, in the random phase condition, the different length of the two phases will lead to an increased probability of triggering TMS pulses at the low excitability broad positive “peak”. This could also explain, why the MEP amplitudes in the random phase condition were closer to the positive peak than negative peak condition (cf. Fig. 2).
LTD-like corticospinal plasticity in the random phase condition resulted in a non-significant trend (p = 0.07) of MEP amplitude depression. This finding contrasts at first sight with the seemingly robust induction of LTD-like plasticity by open-loop 1 Hz rTMS in the literature [34]. However, the aftereffects of low-frequency stimulation are influenced by many factors: these include stimulus intensity [[34], [35], [36]], number of stimuli [48], coil orientation [49] and pulse wave form [50]. These stimulation factors, that go far beyond the simplistic concept of pure frequency-dependence of rTMS-induced plasticity effects, may contribute, in concert with a multitude of biological factors [51], to the well-known inter- and intraindividual variability, that has also been described for low-frequency rTMS [52,53]. Therefore, our non-significant finding of LTD-like plasticity after 1 Hz rTMS in the random phase condition is not surprising. Large inter-subject variability and, on average, nil effects of rTMS plasticity protocols also have been reported in several other recent studies [[6], [7], [8], [9], [10], [11]].
Limitations
MEP amplitudes during the 1 Hz rTMS intervention were larger for the negative peak condition compared to the other two conditions (see Fig. 4c). Although this difference was not significant (see Results), this suggests that the three Phase conditions did not only differ in the level of postsynaptic depolarization, but also in the proportion of corticospinal cells that reached firing threshold by the TMS pulse. However, it is very unlikely that these differences in MEP amplitude during the intervention explain the differential effects of the phase of the μ-oscillation on rTMS-induced corticospinal plasticity: The magnitude of the 1 Hz rTMS-induced LTD-like effect depends on stimulation intensity, with higher intensities leading to more pronounced effects [[34], [35], [36]]. Therefore, larger MEP amplitudes during the intervention would have been expected to lead to a more pronounced LTD-like effect. As a consequence, the observed differential effect of the phase of the μ-oscillation on LTD-like corticospinal plasticity was likely underestimated. Another limitation of our study is that, due to the requirement of a good SNR of the μ-oscillation, the EEG-TMS method can be applied only to the fraction of subjects satisfying this criterion [29]. Therefore, it is unclear to what extent our findings would be generalizable to a non-selected population. Finally, the duration of the observed differential effect of μ-oscillation phase on 1 Hz rTMS-induced LTD-like plasticity is unclear as we recorded only for 15 min post-intervention.
Implications
In conclusion, we have shown a dependency of low-frequency rTMS-induced corticospinal plasticity on the instantaneous phase of the ongoing μ-oscillation. Induction of LTD-like plasticity by low-frequency stimulation was reversed to an LTP-like trend, significantly different from the other two stimulation conditions, if the TMS pulses were synchronized with the negative peak of the μ-oscillation, a state of high postsynaptic depolarization. Findings substantiate the view that induction of synaptic plasticity in human cortex does not only depend on the parameters of the stimulation protocol but also on the brain state at the time of stimulation. The novel real-time EEG-TMS technology may be used to develop brain-state-dependent personalized stimulation protocols for plasticity induction that are characterized by reduced inter-subject variability and larger effect size, features that likely will turn out beneficial for rTMS treatment of brain disorders.
Credit author statement
Authors: David Baur (DB), Dragana Galevska (DG), Sara Hussain (SH), Leonardo G Cohen (LGC), Ulf Ziemann (UZ), Christoph Zrenner (CZ)
Conception or design of the work: DB, UZ, CZ
Data collection: DB, DG
Data analysis and interpretation: DB, DG, SH, LGC, UZ, CZ
Drafting the article: DB, CZ
Critical revision of the article: UZ
Final approval of the version to be published: DB, DG, SH, LGC, UZ, CZ
Declaration of competing interest
CZ is coordinator of and partially funded through an EXIST Transfer of Research grant by the German Federal Ministry for Economic Affairs and Energy (grant 03EFJBW169). The goal of this grant is the commercialization of a real-time EEG analysis device through a spin-off start-up to enable therapeutic brain-oscillation synchronized stimulation, sync2brain GmbH, of which he is a shareholder. UZ has received grants Bristol Myers Squibb, Janssen Pharmaceutica NV, Servier, Biogen Idec GmbH, and personal fees from Bayer Vital GmbH, Pfizer GmbH, CorTec GmbH, all outside of this work. All other authors have no conflicts of interest to disclose.
Acknowledgements
DB acknowledges support from the Junior Clinician Scientist Program of the Faculty of Medicine, University of Tübingen (grant 437-0-0). CZ acknowledges support from the Clinician Scientist Program of the Faculty of Medicine, University of Tübingen (grant 391-0-0). UZ acknowledges support from the German Research Foundation (grant ZI 542/7-1). Furthermore, this project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No 810377). SH acknowledges support from the NINDS Intramural Competitive Fellowship Program.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.brs.2020.09.005.
Appendix A. Supplementary data
References
- 1.Huang Y.Z., Lu M.K., Antal A., Classen J., Nitsche M., Ziemann U. Plasticity induced by non-invasive transcranial brain stimulation: a position paper. Clin Neurophysiol. 2017;128(11):2318–2329. doi: 10.1016/j.clinph.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55(2):187–199. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 3.Ziemann U., Paulus W., Nitsche M.A., Pascual-Leone A., Byblow W.D., Berardelli A. Consensus: motor cortex plasticity protocols. Brain Stimul. 2008;1(3):164–182. doi: 10.1016/j.brs.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Hamada M., Ugawa Y. Quadripulse stimulation-a new patterned rTMS. Restor Neurol Neurosci. 2010;28(4):419–424. doi: 10.3233/RNN-2010-0564. [DOI] [PubMed] [Google Scholar]
- 5.Suppa A., Huang Y.Z., Funke K., Ridding M.C., Cheeran B., Di Lazzaro V. Ten years of theta burst stimulation in humans: established knowledge, unknowns and prospects. Brain Stimul. 2016;9:323–335. doi: 10.1016/j.brs.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Hamada M., Murase N., Hasan A., Balaratnam M., Rothwell J.C. vol. 23. Cerebral cortex; New York, NY: 1991. The role of interneuron networks in driving human motor cortical plasticity; pp. 1593–1605. 2013, 7. [DOI] [PubMed] [Google Scholar]
- 7.Lopez-Alonso V., Cheeran B., Rio-Rodriguez D., Fernandez-Del-Olmo M. Inter-individual variability in response to non-invasive brain stimulation paradigms. Brain Stimul. 2014;7(3):372–380. doi: 10.1016/j.brs.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Hordacre B., Goldsworthy M.R., Vallence A.M., Darvishi S., Moezzi B., Hamada M. Variability in neural excitability and plasticity induction in the human cortex: a brain stimulation study. Brain Stimul. 2017;10(3):588–595. doi: 10.1016/j.brs.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Jannati A., Block G., Oberman L.M., Rotenberg A., Pascual-Leone A. Interindividual variability in response to continuous theta-burst stimulation in healthy adults. Clin Neurophysiol. 2017;128(11):2268–2278. doi: 10.1016/j.clinph.2017.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sasaki T., Kodama S., Togashi N., Shirota Y., Sugiyama Y., Tokushige S.I. The intensity of continuous theta burst stimulation, but not the waveform used to elicit motor evoked potentials, influences its outcome in the human motor cortex. Brain Stimul. 2018;11:400–410. doi: 10.1016/j.brs.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Rocchi L., Ibáñez J., Benussi A., Hannah R., Rawji V., Casula E. Variability and predictors of response to continuous theta burst stimulation: a TMS-EEG study. Front Neurosci. 2018;12 doi: 10.3389/fnins.2018.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huerta P.T., Lisman J.E. Bidirectional synaptic plasticity induced by a single burst during cholinergic theta oscillation in CA1 in vitro. Neuron. 1995;15(5):1053–1063. doi: 10.1016/0896-6273(95)90094-2. [DOI] [PubMed] [Google Scholar]
- 13.Huerta P.T., Lisman J.E. Heightened synaptic plasticity of hippocampal CA1 neurons during a cholinergically induced rhythmic state. Nature. 1993;364(6439):723–725. doi: 10.1038/364723a0. [DOI] [PubMed] [Google Scholar]
- 14.Zrenner C., Desideri D., Belardinelli P., Ziemann U. Real-time EEG-defined excitability states determine efficacy of TMS-induced plasticity in human motor cortex. Brain Stimul. 2018;11:374–389. doi: 10.1016/j.brs.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 15.Seeck M., Koessler L., Bast T., Leijten F., Michel C., Baumgartner C. The standardized EEG electrode array of the IFCN. Clin Neurophysiol : Off J Int Feder Clinc Neurophysiol. 2017;128(10):2070–2077. doi: 10.1016/j.clinph.2017.06.254. [DOI] [PubMed] [Google Scholar]
- 16.Kirschstein T., Kohling R. What is the source of the EEG? Clin EEG Neurosci. 2009;40(3):146–149. doi: 10.1177/155005940904000305. [DOI] [PubMed] [Google Scholar]
- 17.DeFelipe J., Conley M., Jones E.G. Long-range focal collateralization of axons arising from corticocortical cells in monkey sensory-motor cortex. J Neurosci. 1986;6(12):3749–3766. doi: 10.1523/JNEUROSCI.06-12-03749.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clopath C., Gerstner W. Voltage and spike timing interact in stdp - a unified model. Front Synaptic Neurosci. 2010;2:25. doi: 10.3389/fnsyn.2010.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gjorgjieva J., Clopath C., Audet J., Pfister J.P. A triplet spike-timing-dependent plasticity model generalizes the Bienenstock-Cooper-Munro rule to higher-order spatiotemporal correlations. Proc. Natl. Acad. Sci. U.S.A. 2011;108(48):19383–19388. doi: 10.1073/pnas.1105933108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen R., Classen J., Gerloff C., Celnik P., Wassermann E.M., Hallett M. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48(5):1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- 21.Ziemann U. Thirty years of transcranial magnetic stimulation: where do we stand? Exp Brain Res. 2017;235(4):973–984. doi: 10.1007/s00221-016-4865-4. [DOI] [PubMed] [Google Scholar]
- 22.Lefaucheur J.-P., Aleman A., Baeken C., Benninger D.H., Brunelin J., Di Lazzaro V. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014–2018) Clin Neurophysiol. 2020;131(2):474–528. doi: 10.1016/j.clinph.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Stanton P.K., Sejnowski T.J. Associative long-term depression in the hippocampus induced by hebbian covariance. Nature. 1989;339(6221):215–218. doi: 10.1038/339215a0. [DOI] [PubMed] [Google Scholar]
- 24.Lin J.H., Way L.J., Gean P.W. Pairing of pre- and postsynaptic activities in hippocampal CA1 neurons induces long-term modifications of NMDA receptor-mediated synaptic potential. Brain Res. 1993;603(1):117–120. doi: 10.1016/0006-8993(93)91306-d. [DOI] [PubMed] [Google Scholar]
- 25.Rossi S., Hallett M., Rossini P.M., Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 27.Schaworonkow N., Triesch J., Ziemann U., Zrenner C. EEG-triggered TMS reveals stronger brain state-dependent modulation of motor evoked potentials at weaker stimulation intensities. Brain Stimul. 2019;12(1):110–118. doi: 10.1016/j.brs.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 28.Nikulin V.V., Brismar T. Phase synchronization between alpha and beta oscillations in the human electroencephalogram. Neuroscience. 2006;137(2):647–657. doi: 10.1016/j.neuroscience.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 29.Zrenner C., Galevska D., Nieminen J.O., Baur D., Stefanou M.I., Ziemann U. The shaky ground truth of real-time phase estimation. Neuroimage. 2020:116761. doi: 10.1016/j.neuroimage.2020.116761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bergmann T.O., Lieb A., Zrenner C., Ziemann U. Pulsed facilitation of corticospinal excitability by the sensorimotor mu-alpha rhythm. J Neurosci. 2019;39(50):10034–10043. doi: 10.1523/JNEUROSCI.1730-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rossini P.M., Burke D., Chen R., Cohen L.G., Daskalakis Z., Di Iorio R. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol. 2015;126(6):1071–1107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Groppa S., Oliviero A., Eisen A., Quartarone A., Cohen L.G., Mall V. A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol. 2012;123(5):858–882. doi: 10.1016/j.clinph.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hjorth B. An on-line transformation of EEG scalp potentials into orthogonal source derivations. Electroencephalogr Clin Neurophysiol. 1975;39(5):526–530. doi: 10.1016/0013-4694(75)90056-5. [DOI] [PubMed] [Google Scholar]
- 34.Fitzgerald P.B., Fountain S., Daskalakis Z.J. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117(12):2584–2596. doi: 10.1016/j.clinph.2006.06.712. [DOI] [PubMed] [Google Scholar]
- 35.Fitzgerald P.B., Brown T.L., Daskalakis Z.J., Chen R., Kulkarni J. Intensity-dependent effects of 1 Hz rTMS on human corticospinal excitability. Clin Neurophysiol. 2002;113(7):1136–1141. doi: 10.1016/s1388-2457(02)00145-1. [DOI] [PubMed] [Google Scholar]
- 36.Lang N., Harms J., Weyh T., Lemon R.N., Paulus W., Rothwell J.C. Stimulus intensity and coil characteristics influence the efficacy of rTMS to suppress cortical excitability. Clin Neurophysiol. 2006;117(10):2292–2301. doi: 10.1016/j.clinph.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 37.Heide G., Witte O.W., Ziemann U. Physiology of modulation of motor cortex excitability by low-frequency suprathreshold repetitive transcranial magnetic stimulation. Exp Brain Res. 2006;171(1):26–34. doi: 10.1007/s00221-005-0262-0. [DOI] [PubMed] [Google Scholar]
- 38.Berens P. CircStat: a MATLAB toolbox for circular statistics. J Stat Software. 2009;31(10):1–21. [Google Scholar]
- 39.Di Lazzaro V., Pilato F., Dileone M., Profice P., Oliviero A., Mazzone P. Low-frequency repetitive transcranial magnetic stimulation suppresses specific excitatory circuits in the human motor cortex. J Physiol. 2008;586(18):4481–4487. doi: 10.1113/jphysiol.2008.159558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ziemann U. I-waves in motor cortex revisited. Exp Brain Res. 2020;238:1601–1610. doi: 10.1007/s00221-020-05764-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schaworonkow N., Caldana Gordon P., Belardinelli P., Ziemann U., Bergmann T.O., Zrenner C. Mu-rhythm extracted with personalized EEG filters correlates with corticospinal excitability in real-time phase-triggered EEG-TMS. Front Neurosci. 2018;12:954. doi: 10.3389/fnins.2018.00954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stefanou M.I., Desideri D., Belardinelli P., Zrenner C., Ziemann U. Phase synchronicity of mu-rhythm determines efficacy of interhemispheric communication between human motor cortices. J Neurosci. 2018;38(49):10525–10534. doi: 10.1523/JNEUROSCI.1470-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brienza M., Mecarelli O. Neurophysiological basis of EEG. In: Mecarelli O., editor. Clinical electroencephalography. Springer International Publishing; Cham: 2019. pp. 9–21. [Google Scholar]
- 44.Artola A., Brocher S., Singer W. Different voltage-dependent thresholds for inducing long-term depression and long-term potentiation in slices of rat visual cortex. Nature. 1990;347(6288):69–72. doi: 10.1038/347069a0. [DOI] [PubMed] [Google Scholar]
- 45.Artola A., Singer W. Long-term depression of excitatory synaptic transmission and its relationship to long-term potentiation. Trends Neurosci. 1993;16(11):480–487. doi: 10.1016/0166-2236(93)90081-v. [DOI] [PubMed] [Google Scholar]
- 46.Hari R., Salmelin R. Human cortical oscillations: a neuromagnetic view through the skull. Trends Neurosci. 1997;20(1):44–49. doi: 10.1016/S0166-2236(96)10065-5. [DOI] [PubMed] [Google Scholar]
- 47.Tiihonen J., Kajola M., Hari R. Magnetic mu rhythm in man. Neuroscience. 1989;32(3):793–800. doi: 10.1016/0306-4522(89)90299-6. [DOI] [PubMed] [Google Scholar]
- 48.Touge T., Gerschlager W., Brown P., Rothwell J.C. Are the after-effects of low-frequency rTMS on motor cortex excitability due to changes in the efficacy of cortical synapses? Clin Neurophysiol. 2001;112(11):2138–2145. doi: 10.1016/s1388-2457(01)00651-4. [DOI] [PubMed] [Google Scholar]
- 49.Sommer M., Norden C., Schmack L., Rothkegel H., Lang N., Paulus W. Opposite optimal current flow directions for induction of neuroplasticity and excitation threshold in the human motor cortex. Brain Stimul. 2013;6(3):363–370. doi: 10.1016/j.brs.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 50.Sommer M., Lang N., Tergau F., Paulus W. Neuronal tissue polarization induced by repetitive transcranial magnetic stimulation? Neuroreport. 2002;13(6):809–811. doi: 10.1097/00001756-200205070-00015. [DOI] [PubMed] [Google Scholar]
- 51.Ridding M.C., Ziemann U. Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J Physiol. 2010;588(13):2291–2304. doi: 10.1113/jphysiol.2010.190314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maeda F., Keenan J.P., Tormos J.M., Topka H., Pascual-Leone A. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp Brain Res. 2000;133(4):425–430. doi: 10.1007/s002210000432. [DOI] [PubMed] [Google Scholar]
- 53.Maeda F., Keenan J.P., Tormos J.M., Topka H., Pascual-Leone A. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2000;111(5):800–805. doi: 10.1016/s1388-2457(99)00323-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.