Abstract
Spinal cord injury (SCI) often results in loss of the ability to keep the trunk erect and stable while seated. Functional neuromuscular stimulation (FNS) can cause muscles paralyzed by SCI to contract and assist with trunk stability. We have extended the results of a previously reported threshold-based controller for restoring upright posture using FNS in the sagittal plane to more challenging displacements of the trunk in the coronal plane. The system was applied to five individuals with mid-thoracic or higher SCI, and in all cases the control system successfully restored upright sitting. The potential of the control system to maintain posture in forward-sideways (diagonal) directions was also tested in three of the subjects. In all cases, the controller successfully restored posture to erect. Clinically, these results imply that a simple, threshold based control scheme can restore upright sitting from forward, lateral or diagonal leaning without a chest strap; and that removal of barriers to upper extremity interaction with the surrounding environment could potentially allow objects to be more readily retrieved from around the wheelchair. Technical performance of the system was assessed in terms of three variables: response time, recovery time and percent maximum deviation from erect. Overall response and recovery times varied widely among subjects in the coronal plane (415±213ms and 1381±883ms, respectively) and in the diagonal planes (530±230ms and 1800±820ms, respectively). Average response time was significantly lower (p < 0.05) than the recovery time in all cases. The percent maximum deviation from erect was of the order of 40% or less for 9 out of 10 cases in the coronal plane and 5 out of 6 cases in diagonal directions.
Keywords: Trunk Control, Seated Balance, Functional Neuromuscular Stimulation (FNS), Spinal Cord Injury (SCI), Self-Righting Control, Disturbance-Rejection Control
Introduction
Spinal Cord Injury (SCI) often results in paralysis of the muscles of the lower limbs and trunk, which can lead to the loss of functional control of seated posture and balance. Trunk control is the ability for people to maintain and direct motion of the torso with the muscles of the hips and trunk, and it is an essential ability that allows them to effectively interact with their environment with their upper extremities bilaterally without holding on to the wheelchair. Loss of trunk control and stability due to SCI or other movement disorders, usually requires the use of devices, straps, or voluntary effort by the arms to maintain trunk posture in a stable position. This often limits the functional area that can be explored in the upper-limb workspace [1]. Trunk instability is a major concern for individuals with SCI [2], which often results in a decline in the ability to undertake activities of daily living (ADLs) including wheelchair propulsion [3] and extended reaching tasks.
Functional Neuromuscular Stimulus (FNS) is a technique in which implanted or surface electrodes deliver current to the motor nerve which cause the muscles paralyzed by SCI to contract and produce force. A carefully planned coordinated recruitment of such activated muscles using engineering control methods could mitigate some of the issues listed above, and stabilize the trunk to improve seated posture and enable more functional interactions with objects in the environment [1,4,5].
Earlier studies on trunk postural control had extensively explored the strategies used by the intact central nervous system to maintain stability in erect seated postures [6,7]. In these studies, perturbation forces were applied to the trunks of seated able-bodied individuals and the various static and dynamic characteristic responses were captured and analyzed. In particular, important factors that affect stability such as intrinsic stiffness and damping for the intact system were determined and their implications to trunk stability examined. In other studies, musculoskeletal models were used to perform in-silico experiments to explore the potential impact of recruiting a variety of paralyzed muscles with FNS to support and maintain trunk posture statically in different planes around the workspace of the seated operator [8,9,10]. Overall, these studies provided the main tools to develop more advanced dynamic control systems that could be deployed in individuals with SCI and other movement disorders to maintain trunk posture in response to either internally generated or externally applied perturbations.
Studies directly involving individuals with SCI have explored the use of FNS to help maintain a stable seated posture [11,12], and in cases of loss of stability to restore the trunk to a desired posture [13,14]. However, these developments had concentrated on stability exclusively in the sagittal plane and the restoration of posture from deviations from upright in the forward direction only [15]. Similar issues associated with stability during lateral bending in the coronal plane or intermediate directions still need to be addressed [16].
The purpose of the current study was to explore the design and deployment of a disturbance-rejection closed-loop controller that uses FNS in individuals with SCI to restore upright posture, not only in the sagittal plane as explored in previous studies [13,14], but also in the coronal plane or diagonally between lateral and anterior-posterior directions. The results have implications for maintaining upright seated posture and balance during ADLs such as driving and wheelchair propulsion, as well as for the development of more advanced control systems in the future.
Methods
Study Participants
Five individuals paralyzed due to SCI at mid-thoracic or higher levels were recruited from a cohort of individuals with implanted neuroprostheses who were participants in other related studies in our laboratory [17,18,19,20]. The main inclusion criterion for all participants was absence of volitional trunk control; i.e. complete loss of voluntary function in the hip and trunk extensor and trunk lateral flexor muscles. Table 1 displays their clinical characteristics as well as the implanted muscles stimulated during the experiments. In particular, the main hip and trunk muscles activated for this study were: left (L) and right (R), lumbar Erector Spinae (ES), Quadratus Lumborum (QL), Gluteus Maximus (GX), posterior portion of Adductor Magnus (AM) and Semimembranosus (SM). For all volunteers, the nerves innervating the muscles listed in Table 1 were previously implanted with intramuscular [21], epimysial [22], or nerve cuff electrodes [23]. The implanted electrodes were connected to an eight-channel implanted receiver-stimulator [24] or 16-channel implanted stimulator-telemeter [25] that was controlled by an external control unit (ECU) via a close-coupled radio frequency link established by a transmitting coil placed on the skin over the pulse generator [26]. All participants had been using their systems with muscle activations set at customized low-level baseline values to stiffen their trunk just enough for day-to-day stabilization around the erect seated posture. Also, for each participant and for each muscle there exist maximum or high PW values which are those beyond which muscle force production did not increase, the subject felt some discomfort in breathing or sensation, or when the hardware limit of 250μs was reached. The values of the baseline and high PWs had been determined heuristically by trial-and-error and were pre-programmed into the ECU of each subject. All the participants signed approved institutional review board consent forms of the Louis Stokes Cleveland VA Medical Center before partaking in the study.
Table 1.
Summary of Clinical Characteristics of Participants in this Study
| Subject | Age | Gender | Height (cm) | Weight (kg) | Injury Level | AIS Grade†, | Time Post Injury * (y) | Time Post Implant * (y) | Muscles Stimulated |
|---|---|---|---|---|---|---|---|---|---|
| S-1 | 47 | F | 175.3 | 57.6 | C7 | B | 20.8 | 19.1 | ES, QL, GX, AM, SM |
| S-2 | 43 | F | 175.3 | 82.8 | T4 | A | 6.8 | 4.1 | ES, QL, GX, AM |
| S-3 | 28 | M | 185.4 | 52.6 | C5 | C | 7.9 | 2.5 | ES, QL, GX, AM,SM |
| S-4 | 51 | M | 188 | 81.6 | C5 | A | 29 | 8.2 | ES, QL, GX |
| S-5 | 50 | M | 172.7 | 71.7 | T3 | A | 2.3 | 1.5 | ES, QL, GX, AM |
Abbreviations: AM, posterior portion of Adductor Magnus; ES, lumbar Erector Spinae; GX, Gluteus Maximus; QL, Quadratus Lumborum; SM, Semimembranosus.
at time of testing
The American Spinal Injury Association Impairment Scale (AIS): A, motor and sensory complete; B, motor complete with sensory sparing; C, motor and sensory incomplete.
Experimental Setup
Figure 1 shows the experimental setup in which volunteers sat in their own wheelchairs in a suitable location in the laboratory. A custom wireless tilt sensor measuring 3cm × 1.5cm × 1cm consisting of a CC430F6137IRGC microcontroller (Texas Instruments, Dallas, TX) and a CMA3000-D01 accelerometer (VTI Technologies, Vantaa, Finland) was taped to the chest just below the manubrium of the sternum. The component of the acceleration due to gravity read by the sensor, which is a measure of trunk tilt in the sagittal and coronal planes, acted as a feedback control signal. The sensor signal was wirelessly transmitted at 40Hz to a MATLAB/Simulink program (The Mathworks, Natick, MA) executing in the xPC/Target real-time environment [27]. Control decisions based on the wireless sensor signals were conveyed to the ECU for transmission to the implanted pulse generator.
Figure 1:
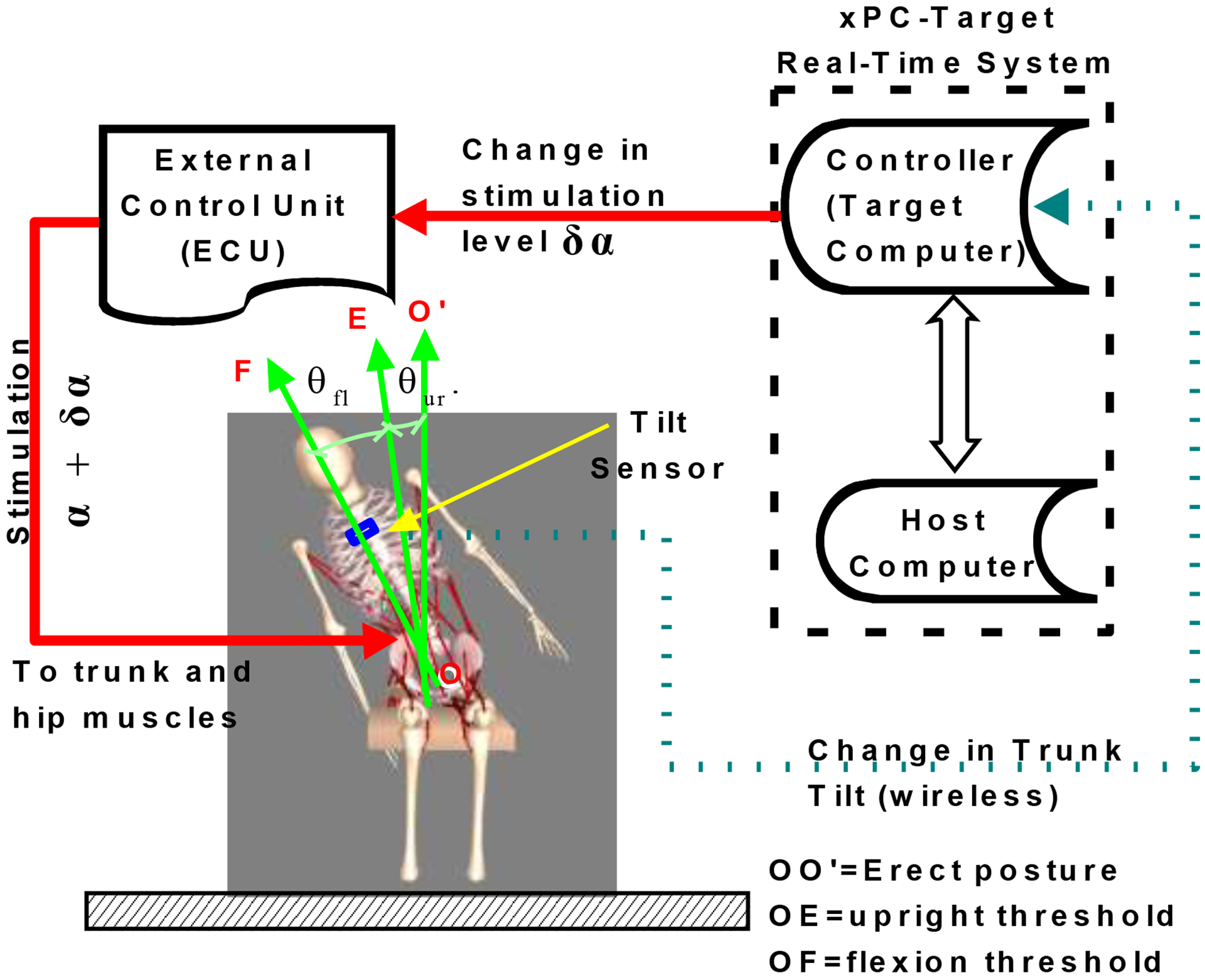
Schematic of trunk self-righting control system showing subject seated in work volume of motion capture cameras. Two computers, target and host, manage the real-time environment for the experiments. Green arrow OO’ represents erect pose. Settings for the thresholds are defined by green arrows OE (angle θur) for the upright and OF (angle θfl) for the flexion. Experiments were conducted with trunk tilt in the sagittal and coronal planes. The ECU controlled the delivery of stimulation to the implanted electrodes, which was modulated in real-time depending on the signal from the tilt sensor in the MATLAB xPC host-target environment.
Tilt Sensor Calibration
The feedback signal used for the controller was captured from the tilt sensor in the form of the acceleration due to gravity measured in g (within the ±2g range of the sensor). Simple calibration was used to estimate the trunk angle (in degs) from those readings. In the calibration experiments, completed just before the current set of experiments, an able-bodied subject wore the sensor at the same location as would be worn by SCI subjects and sat in a chair set in the measurement volume of a 16-camera motion capture system (Vicon Motion Systems Ltd., Oxford, UK). Reflective markers were placed on anatomical landmarks on the pelvis (anterior and posterior iliac spine, sacrum), arms (shoulder, upper arm, elbow, forearm, wrist, hand), the chair on which the subject sat, and a headband. From the erect posture, the subject leaned five times to the front and returned to erect and then the same number to the left and to the right and returned to erect. The motion capture data was measured at 100 Hz and the sensor data at 40 Hz. The marker coordinates from the motion capture were used to estimate the trunk flexion and bend angles. The sensor was calibrated by fitting a linear calibration lines between the sensor readings and the trunk angles using the sftool app and fit functions in the Curve Fitting Toolbox of MATLAB as follows:
| (1) |
| (2) |
In equations (1) and (2), , are the trunk angles in degrees; , the trunk tilt values obtained from the sensor reading in g; mS, mC the slopes and bS, bC the corresponding intercepts of the regression lines. Superscripts S and C stand for the sagittal and coronal planes respectively. Equation (2) was used for both tilting to the right and left away from erect in the coronal plane.
Setting Muscle Groups, Thresholds and Stimulation Parameters
Synergistic muscle groups specific to each direction of trunk deviation were recruited to restore upright posture. The main motions are the pure coronal plane movements Left (LF) and Right (RI) and the diagonal movements, Forward-Left (FL) and Forward-Right (FR) which are a combination of forward (sagittal) and sideways (coronal) plane movements. The original study design was for motion in the coronal plane only and conducted for all five subjects. Later diagonal motions were added to the study and only executed on three subjects (S1, S2 and S5) as the other two subjects (S3 and S4) were not available for additional data collection. Figure 2 shows the tilt sensor setup and the motions which would have an impact on the feedback loop as well as the muscles activated whenever the body moves in a given direction.
Figure 2:
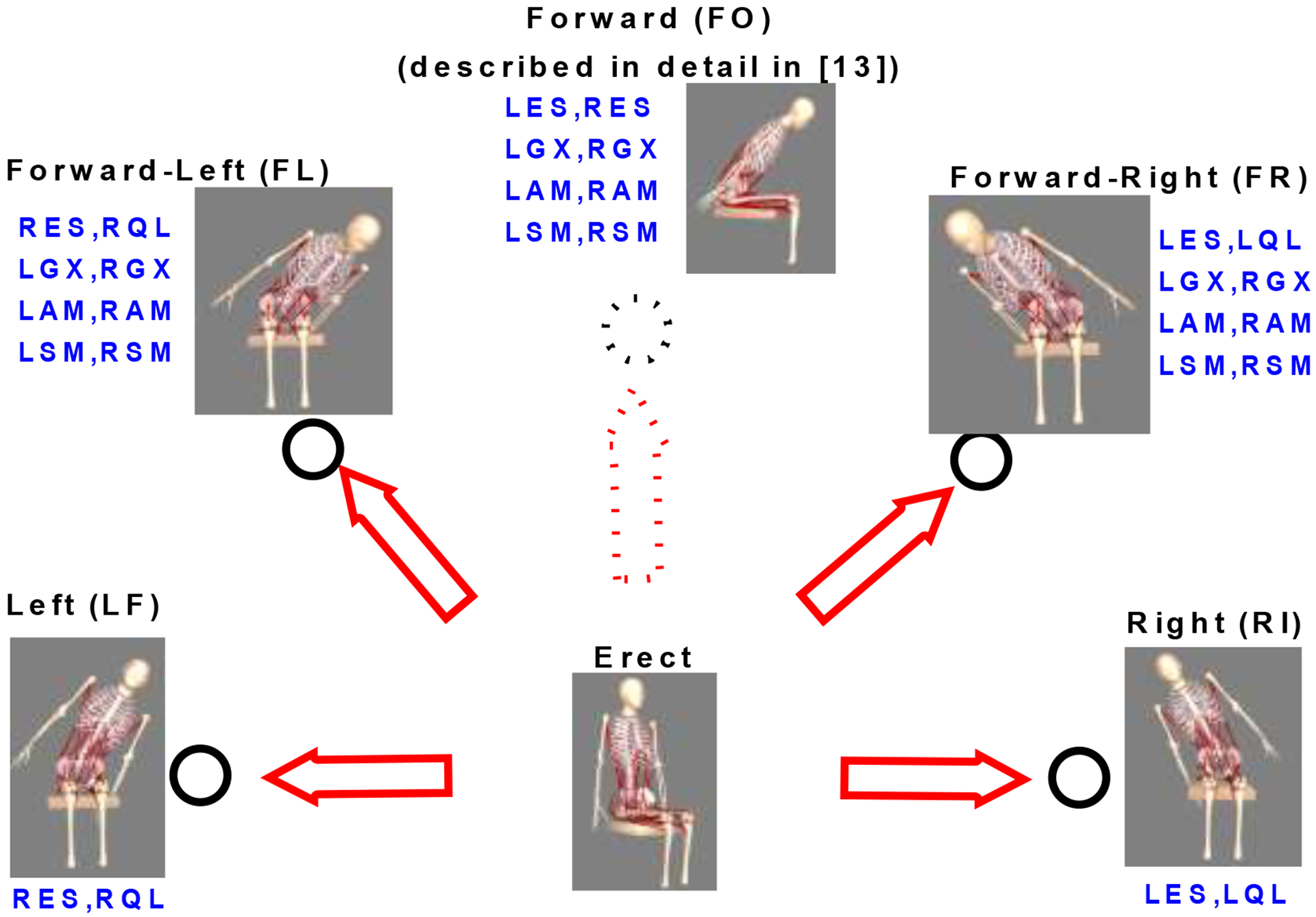
Trunk movement directions away from erect that were explored in this study - Left(LF), Right(RI), Forward-Left (FL), Forward-Right(FR). Forward (FO) direction is described in Murphy et al. [13]. The muscles activated in each direction are also shown; AM, posterior portion of Adductor Magnus; ES, lumbar Erector Spinae; GX, Gluteus Maximus; QL, Quadratus Lumborum; SM, Semimembranosus. Preceding L/R imply left and right sides respectively.
While seated in an erect posture with the muscle activation pulse widths (PWs) set at their clinically determined baseline levels, subjects were asked to use their upper extremities (UEs) to lean in the forward, left and right directions at small angular increments at a time, and relax and release all UE support after each trial lean. The upright threshold for each direction of lean was defined as the trunk tilt angle, measured with the tilt sensor, to which the subject could lean before beginning to fall (θur in Figure 1). In all cases a physical therapist spotted the subject so as to avoid falling. Thus the upright threshold defines the angle of trunk below which no additional stimulation over the baseline level is required to maintain that posture.
Similar to above, a set of trials were conducted to determine the flexion threshold. This threshold is defined as the angle of the trunk (θfl in Figure 1) beyond which application of the high stimulation PWs of all the muscles in the group for that direction working together would not be able to restore the trunk to upright. Considering that for most subjects their implanted muscles could restore their posture at relatively large angles away from erect, the flexion threshold was restricted to values that minimize frequent intervention by a spotter. In many situations, the high stimulation PWs were reduced to avoid spillover to unwanted and counterproductive muscle groups (e.g., abdominals or iliopsoas which flex the trunk and hips).
It should be noted that during the coronal (LF and RI) leaning movements, the flexion threshold in the forward direction was set at relatively small values (0 to 10°) to prevent the trunk from drifting off to the forward direction during the recovery phase by activating the forward-acting muscles.
In all experiments, the pulse amplitudes were nominally set at 20mA, except when reduced to mitigate undesired co-activation of the abdominal muscles, and were kept at constant levels throughout. Modulation of stimulation was achieved by altering PW between the stimulator limits of 0 and 250μs. The stimuli for all muscles were delivered at 30 Hz (33.3ms interpulse interval).
Experimental Procedure
Subjects remained seated in their wheelchairs and were kept in a stable upright seated position away from the backrest, using their UEs. To ensure quiet erect sitting stability, baseline stimulation was continuously applied. The subjects were then directed to use their UEs to initiate leaning of their trunk in a specified direction. Once the trunk started to fall under gravity, subjects were instructed to let go of any UE support. In the diagonal direction, targets were placed to help the subject remain consistent in the desired motion. Once the sensor detected that the tilt had crossed the set flexion threshold for the chosen direction, all the appropriate synergistic muscles intended to restore the trunk posture were maximally activated with the stimulation kept constant until the upright threshold was reached, at which point the stimulation was returned to the baseline levels. For the diagonal directions, the flexion thresholds for both directions must be crossed for the posture restoration to commence. Each trial consists of 2 to 3 repetitions in each direction; with a 5-minute rest between trials. The number of repetitions for all subjects varied between 10 and 15 for each direction; but more repetitions were taken to accommodate the potential of subjects accidentally using their upper extremities to balance themselves before the full cycle is completed. During each trial, a spotter provided support if there was a risk of the subject falling due to the controller not activating or the muscles not providing enough force for the motion. All such trials were eliminated in the subsequent analyses. The experiments generally took place in a period of 2–3 hours, with an additional hour required for setup and storage of the testing material.
Controller Design
The controller uses a wireless body-mounted accelerometer to estimate the angle at which a person’s trunk is leaning in order to modulate the level of stimulation directed to nerves innervating muscles of the trunk and hips. This change in stimulation leads to the return of upright sitting, preventing potentially injurious falls from the wheelchair or the necessity to use the upper extremities to regain upright posture and balance.
The x and z axes of the sensor taped to the sternum were oriented in the sagittal (pointing fore-aft) and coronal (pointing medial-lateral) planes, respectively, and the measured accelerations were processed to derive a static estimate of two dimensional trunk tilt [13]. This provided the feedback signal on the basis of which the controller makes a decision to change the activationα to the muscles via the on-off controller equations (3):
| (3) |
where is the stimulus PW delivered to muscle i when the flexion threshold is exceeded θfl is exceeded and is that delivered when the tilt angle falls below the upright threshold θur. This is also the same as the baseline stimulation pulse width. A State variable assists in defining the phase of movement. At the start of experiment its value is set to 1 and remains so with the subject in an erect posture and when the flexion threshold is not crossed. If flexion threshold is crossed, it is set to 2 and remains so until the upright threshold is crossed when it changes to the value of 1 again. Comparisons outside parentheses refer to movements in the RI direction, while those in parentheses are applicable to FO and LF movements. The appropriate unions of conditions in equation (3) apply to leans in the FL and FR directions.
Outcome Variables
Clinical outcome was assessed with respect to the system’s ability to restore posture in each direction for each subject in a binary fashion. The main technical outcome measures extracted from the experimental data were: (a) response time, (b) recovery time and (c) percent maximum deviation from erect. Figure 3 depicts these variables with respect to a typical profile of trunk bend angle as a function of time. The time between crossing the flexion threshold at the beginning of a lean and reversing direction when the lean is arrested is the response time, and the time following this reversal in direction until the upright threshold is reached defines the recovery time. A two-sample, one-tailed t-test was used to compare the relative magnitudes of the mean response and the mean recovery times for each subject and for all subjects combined. Statistical significance was tested at the 0.05 level. The percent maximum deviation from erect is a measure of the largest change in angle over the flexion threshold and is defined as [28]:
| (4) |
The quantities MD and RD in equation (4) are depicted in Figure 3.
Figure 3:
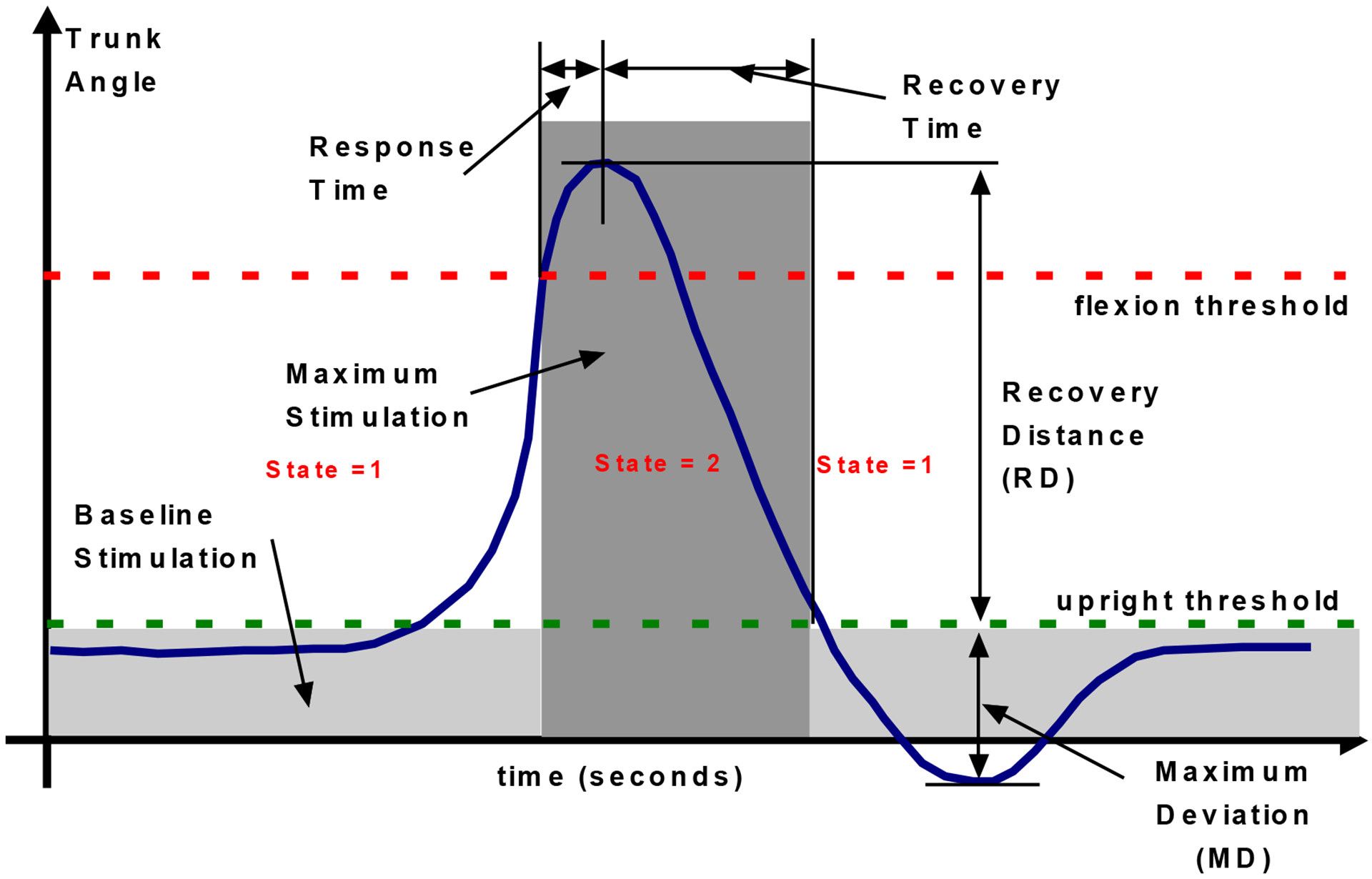
Controller operation. Blue trace shows typical profile of changes in trunk angle over time with the main control performance parameters indicated. A state variable keeps information on status of phase of motion.
Results
Muscle Stimulation Parameters
The baseline and high stimulation PWs for each subject and for each muscle are listed in Table 2. The baseline stimulation used during regular erect sitting varied between 0–80μs with most values being closer to 10μs for most muscles. On the other hand, the stimulation during the active section of the controller varied between 40 and 250μs depending on the subject and muscle.
Table 2:
Stimulation pulse-widths for each subject. The ‘Baseline’ values were those used for steady state erect sitting while the ‘High’ values were the maximum PWs applied whenever the trunk crossed the flexion threshold in all directions. A dash (−) entry implies that electrodes were not implanted to activate the muscle in that subject.
| Muscle | Side | S-1 | S-2 | S-3 | S-4 | S-5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | High | Baseline | High | Baseline | High | Baseline | High | Baseline | High | ||
| Erector Spinae | Right | 25 | 100 | 19 | 250 | 0 | 100 | 29 | 205 | 10 | 150 |
| Left | 25 | 250 | 4 | 150 | 0 | 70 | 12 | 205 | 8 | 150 | |
| Quadratus Lumborum | Right | 25 | 250 | 5 | 150 | 0 | 250 | 105 | 250 | 80 | 250 |
| Left | 35 | 50 | 5 | 120 | 0 | 90 | 10 | 175 | 8 | 250 | |
| Iliopsoas | Right | - | - | 0 | 125 | - | - | - | - | 8 | 250 |
| Left | - | - | 0 | 105 | - | - | - | - | 1 | 250 | |
| Gluteus Maximus | Right | 35 | 250 | 5 | 250 | 0 | 60 | 20 | 250 | 3 | 250 |
| Left | 40 | 250 | 3 | 250 | 0 | 60 | 1 | 250 | 8 | 250 | |
| Semimembranosus | Right | 12 | 250 | - | - | 6 | 45 | - | - | - | - |
| Left | 40 | 250 | - | - | - | - | - | - | - | - | |
| Posterior Adductors | Right | 25 | 250 | 5 | 250 | 2 | 40 | - | - | 1 | 250 |
| Left | 25 | 200 | 2 | 250 | 7 | 150 | - | - | 15 | 250 | |
Tilt sensor calibration
The values (with 95% confidence bounds) of the slope and intercept in equation (1) were mS = −172 (−184.1, −160) and bS 146.9 (138.6, 155.2). The goodness of fit reported an R-square of 0.971 with a RMSE of 3.97 degs. The corresponding values in equation (2) were mC = −135 (−142, −128) and bC = 134.6 (127.3, 142) with the goodness of fit reporting an R-square of 0.992 with a RMSE of 4.02 degs.
Flexion and Upright Thresholds
The thresholds are displayed in Table 3 for all subjects and for each direction of lean. The original data were captured from the sensor output which was recorded in acceleration (g) values. The values in Table 3 were the sensor readings converted to degrees using the linear calibration equations (1) and (2).
Table 3.
Flexion and upright threshold values for each of the five subjects and the four directions examined in the current study. Left and flexion trunk leaning were defined to have negative angles, while right and extension movements produced positive trunk angles. A dash (−) entry implies that the subject was not tested in that direction. For the diagonal movements, there were two sets of thresholds - one for the forward (FO) direction and the second for the left (LF) or right (RI) directions.
| Direction | Left | Right | Forward-Left | Forward-Right | ||||
|---|---|---|---|---|---|---|---|---|
| Subject | Upright (deg) | Flexion (deg) | Upright (deg) | Flexion (deg) | Upright (deg) | Flexion (deg) | Upright (deg) | Flexion (deg) |
| S-1 | 0 | −20 | 0 | 16 | −15 (FO) −5 (LF) |
−43 (FO) −30 (LF) |
−10 (FO) 0 (RI) |
−40 (FO) 13 (RI) |
| S-2 | 0 | −15 | 5 | 33 | −5 (FO) 0 (LF) |
−30 (FO) −27 (LF) |
−10 (FO) 0 (RI) |
−54 (FO) 18 (RI) |
| S-3 | 0 | −11 | 9 | 25 | - | - | - | - |
| S-4 | 0 | −14 | 10 | 21 | - | - | - | - |
| S-5 | 8 | −28 | 0 | 16 | −12 (FO) 0 (LF) |
−40 (FO) −30 (LF) |
0 (FO) 0 (RI) |
−45 (FO) 45 (RI) |
Overall, from Table 3, the upright thresholds vary between 0 and 10 degrees and the flexion thresholds vary between 11 and 33 degrees in absolute value in the coronal plane. Across all subjects and directions, the values were larger (up to 45 degrees) in the diagonal (combined forward and side bending) movements mainly because of the ability to lean further in the forward direction.
Changes in trunk angle profiles
The results of the self-righting controller with subjects leaning left and right in the coronal plane are shown in Figure 4 for each of the five subjects recruited for the study. From these figures, it can be seen that the left leaning trials all have similar motions in which the subjects’ trunk bend angle initially increased continuously from the erect posture close to 0 degrees until the flexion threshold was reached. Immediately thereafter the movement was arrested, and the trunk bend angle started to decrease. The decrease continued until the upright threshold was reached, at which point the high stimulation was deactivated, and the subject eventually resumed a near erect posture and used their UEs to support themselves and initiate the next repetition of the movement. This same pattern followed in the right lean direction except the subjects’ trunk bend angle increased from its erect value before decreasing again to return close to erect when stimulation was deactivated.
Figure 4:
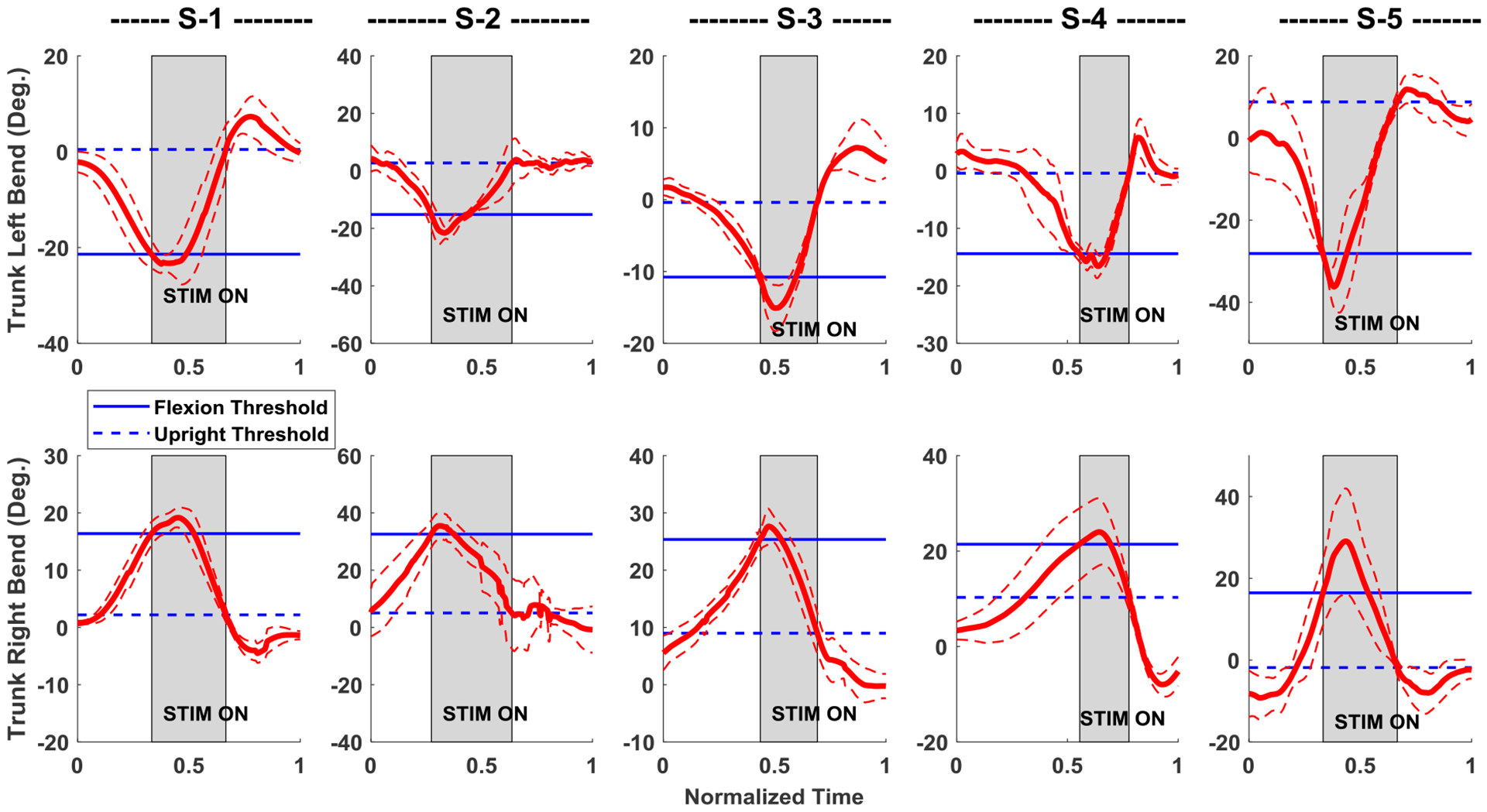
Average motion of five subjects S-1 to S-5, leaning in either direction in the coronal plane while seated with the controller on. Thick red lines are the mean, and thin dashed red lines are error bands representing one standard deviation from the mean of the trunk angle; all calculated using the 10–15 repetitions for each direction. Left and right trunk lean have negative and positive measures respectively. The high stimulation (gray shaded area) turns on when the trunk bend angle crosses the blue solid (flexion threshold) line and turns off when the bend angle crosses the blue dashed (upright threshold) line.
The joint angles during diagonal leaning between the sagittal and coronal planes (forward-left and forward-right) for the three subjects tested (S-1, S-2, and S-5) are shown in Figure 5 for both directions (FL and FR). The trunk pitch components varied between −40 and −60 degrees across the three subjects between the two directions. Trunk bend angles varied between −30 and −43 degrees in the FL direction and between 17 and 42 degrees in the FR direction.
Figure 5:
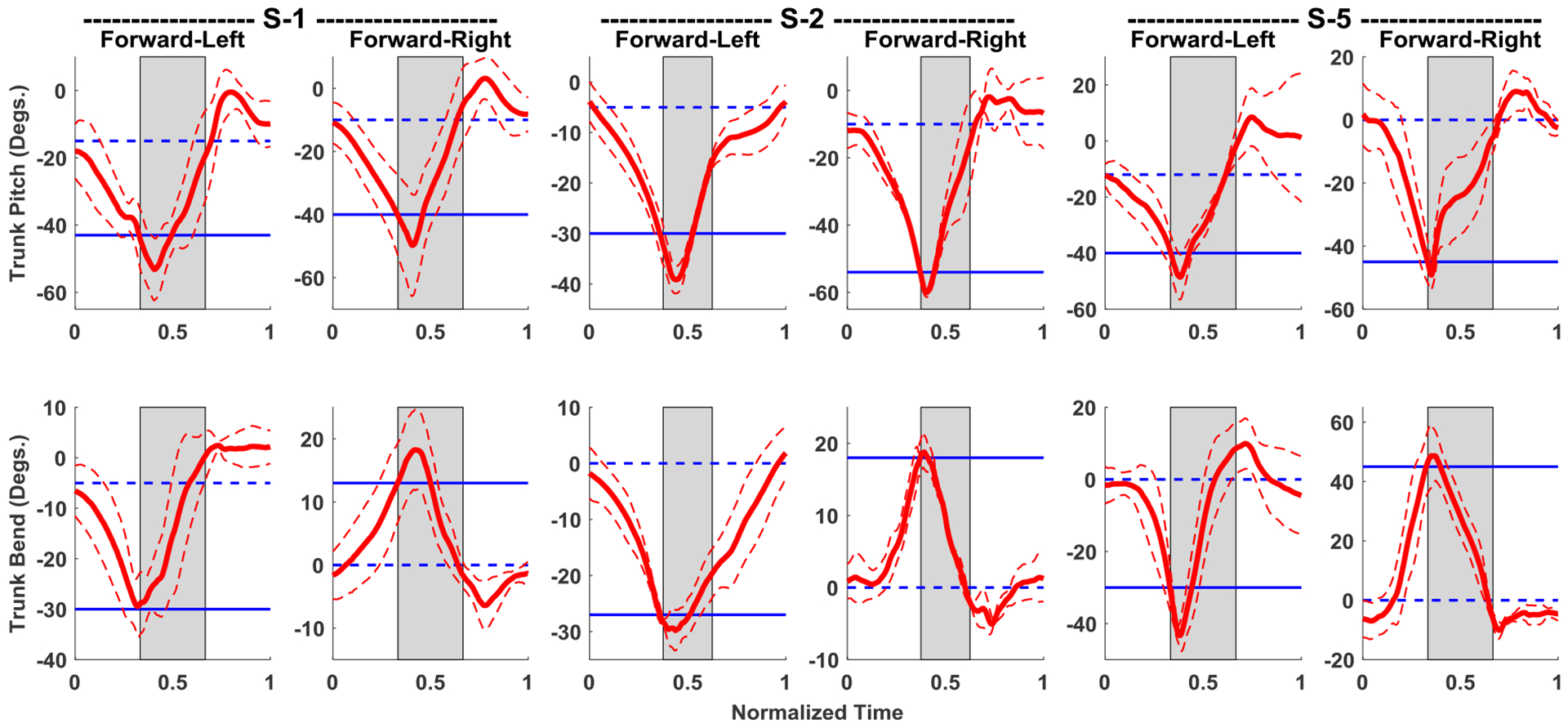
Mean joint angle data for diagonal (Forward-Left and Forward-Right), while controller is active, for three subjects S-1, S-2 and S-5. Thick red lines are the mean, and thin dashed red lines are error bands representing one standard deviation from the mean of the trunk angle; all calculated using the 10–15 repetitions for each direction. Left and right trunk lean have negative and positive measures respectively. The high stimulation (gray shaded area) turns on when the trunk bend angle crosses the blue solid (flexion threshold) line and turns off when the bend angle crosses the blue dashed (upright threshold) line.
Outcome variables
Figure 6(a) displays the averages of the coronal plane response and recovery times with error bars for both the left and right directions of lean for all five subjects tested (five trials each). The equivalent values for the diagonal plane movements are shown in Figure 6(b). The overall mean response times for left and right lean are 465±275ms and 360±140ms respectively, while the means of the recovery times are 1630±990ms and 1130±785ms respectively. The average response time is significantly lower (p < 0.05) than the recovery time in all cases, including the mean of all subjects, when using a two-sample, one-tailed t-test. For the diagonal movements, the overall mean response times for forward left and forward right lean are 520±220ms and 550±260ms respectively, while the means of the recovery times are 1780±970ms and 1800±740ms, respectively. Figure 7(a) depicts the average percent maximum deviation from erect for five subjects in the coronal plane experiments. Overall, all five subjects had percent maximum deviation from erect at or below 40% in both directions, except for S-4 who had a mean percent maximum deviation from erect that exceeded 100% in experiments involving falls to the right hand side. The equivalent percent maximum deviation from erect values for movement in the diagonal directions is given in Figure 7(b) for three subjects. Five out of six (three subjects in two directions) values were on the order of 40% or below, the lone exception being for subject S-5 who had a value around 60% in trunk pitch for movement in the forward-left direction.
Figure 6:
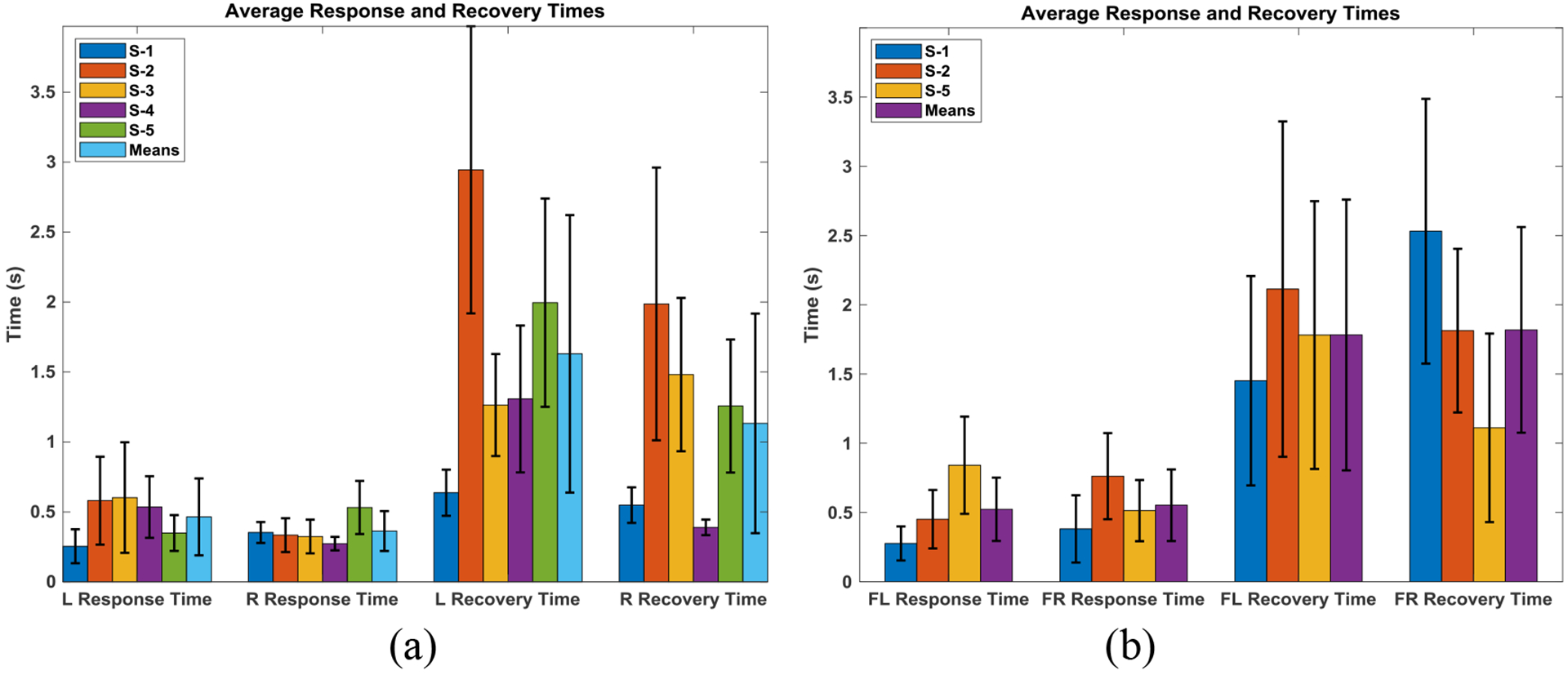
Mean response and recovery times with error bars (one standard deviation) of ten to fifteen repetitions in the left and right direction of lean in the coronal plane with the controller active for all five subjects (a) and the equivalent values for diagonal plane (forward left, FL, and forward right, FR) action for three of the five subjects (b).
Figure 7:
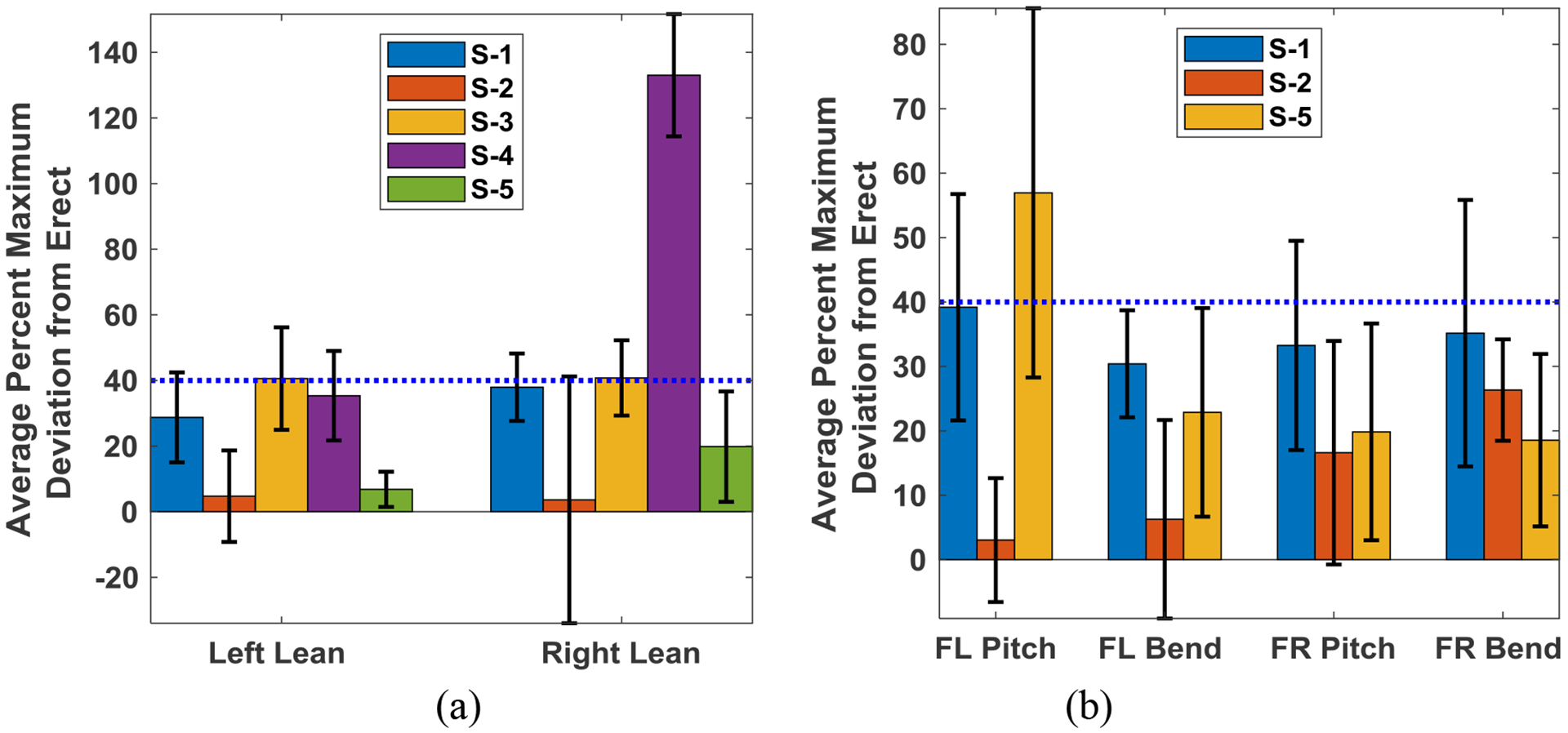
Average (+/− SD) percent maximum deviation from erect for leans in the left and right directions for five subjects with error bars (a) and the equivalent values for diagonal plane (Forward Left, FL, and Forward Right, FR) action for three subjects (b). Dashed blue lines are 40% average percent maximum deviation from erect.
Discussion
Seated stability is an important function for individuals with various levels of SCI. Stability of seated postures ensures safety during the execution of various ADLs such as driving, wheelchair propulsion, manipulating objects, transfers, etc. The study presented here is the first to examine the potential of ensuring a stable erect posture in the coronal and diagonal planes using feedback control of neural stimulation. The system consistently returned all subjects to an upright sitting posture automatically and without use of the upper extremities after extreme lateral and diagonal leans. The laboratory-based results of this simple threshold-based stimulation control system may have numerous benefits such as preventing potentially injurious falls, obviating the need for restrictive chest belts and straps, or allowing objects to be retrieved from around the wheelchair. The utility of the system, or others like it, remains to be determined under real world conditions in the home and community environments.
Response and recovery times have been identified as an important measure for restoration of stable seated posture with FNS [13]. The response times observed with the threshold-based system were relatively large, possibly due to the electromechanical delay (EMD) between the onset of muscle electrical activity (as measured by EMG) and measurable tension output from the muscle [29,30]. Generally, EMD is estimated between 30 and 100 ms [29] for intact human muscle. In isometric experiments using surface electrodes on quadriceps muscles of able-bodied individuals, EMD values of 14 to 105 ms were obtained [31]. In the current trunk study with stimulation of paralyzed muscles, the response time (around 500ms) was larger than the typical EMD. This large difference could be explained by the additional delay in the computation of control decisions that occurs between when the sensor determines the crossing of the flexion threshold to the time when stimulus command reaches the electrodes, the intrinsic delay in the excitation-contraction coupling of muscle force production (that is the time between issuing a stimulus pulse to the nerve and the paralyzed muscle beginning to contract), and the effort required to overcome the inertia in halting and reversing the momentum of the trunk. This large delay is important to take into account in the design of more advanced and sophisticated control systems for restoring trunk stability with FNS in individuals with SCI as it could affect their stability and performance [32,33,34]. Similarly, recovery time is highly related to the rise time variable used to describe the swiftness of response of control systems [28]. In situations where posture needs to be restored rapidly, such as in falls, it is important that upright sitting is restored as rapidly as possible [15]. Recovery times were relatively short in this study, but were also affected by the contractile properties of paralyzed muscle activated by neural stimulation as well as the weight and body proportions of the individual subject. It is also observed that there is a smaller inter-subject variation in the recovery time in the right side which may associated with the dominant handedness of the subjects as most of the subjects declared that they were predominantly right handed.
Percent maximum deviation from erect is similar to the maximum percent overshoot that is commonly used as a transient response characteristic of feedback control systems [28] and is a measure of the degree of intrinsic damping in the system. Generally, it is desirable to keep the damping ratio to around 0.4 to 0.8 to avoid sluggish response (high value) or oscillatory response (low value); which, for second order systems, implies maximum percent overshoot of the order of 1.5% – 25% [28]. The values of percent maximum deviation from erect in the current study were also relatively large, but on the order of 40% or less except for two conditions out of the 16 tested (five subjects leaning in two lateral directions, and three subjects leaning in two directions diagonally). This indicates that the trunk systems of individuals with SCI utilizing FNS have a fairly low damping ratio on the order of 0.3, which falls just outside the desirable minimum of 0.4 for linear second order systems. However, the trunk system may not exactly behave as a second order system [28].
In the majority of the outcome measures we examined, our study indicated significant benefits of the feedback controller over baseline stimulation; which is unable to restore erect posture once the upright threshold is exceeded without upper extremity effort. The main exception where the closed-loop feedback controller did not produce significant change in the outcome measures was in the case of subject S-4. This may be because this subject had only one hip extensor muscle implanted and in addition has significant trunk weakness due to his injury level (C5, AIS A). For individuals with higher level injuries it may be beneficial to recruit additional hip and trunk muscles to assist with return to upright postures.
There were a number of limitations to the current study that would warrant examination in the future. First, all the synergistic muscles for a given plane were recruited to act simultaneously. A more detailed control paradigm should determine a more suitable order and amount of recruitment to be harnessed from each muscle in order to optimize the outcomes. A second limitation is that trunk axial rotation was ignored in the analyses, which focused primarily on lateral bending and forward flexion/extension. Future studies should consider including this important movement as it may have special repercussions for deploying the trunk in the diagonal planes. Another limitation is that only a small subset of possible leaning postures was examined in the current study, so future work should concentrate on generalizing the system for any arbitrary direction. Finally, the results reported were for trials captured in a single session on a single day. Replicating the results with more subjects and repeating the experiments on several days or sessions to determine the reproducibility of the results will be examined in future studies. While muscle fatigue could be considered a major issue for FNS systems, all the subjects in the current study continuously exercised and used their systems at home for at least 18 months before testing. In addition, the primary muscles used in this study of coronal plane movements were postural muscles in the trunk which have more fatigue resistance than muscles in the upper and lower extremities [35]. Another limitation is that in order to enable them to initiate the lean for the next repetition, the subjects were instructed to use their upper extremities to re-stabilize themselves as soon as a repetition in a trial was completed based on their perception of assuming close to erect posture. This resulted in them not attaining a steady state condition between repetitions, but rather expedited the return to the initial condition required to begin the next trial. Including control of movement in the opposite direction by stimulating contralateral muscles would have required for more time between repetitions and prolonged the testing sessions.
Overall, the results of the current work indicate the potential for feedback self-righting control to restore unstable seated postures in the coronal plane in individuals with SCI using FNS, as was previously demonstrated for restoring erect postures in the sagittal plane [13]. A major long term goal of our work is to enable neuroprostheses users to maintain seated balance hands-free from a support device at any task-dependent posture desired, thus allowing them to explore a wider volume of the workspace around them as they undertake ADLs. While this is far from being achieved, this study represents a contribution toward advancing the development and deployment of self-righting control systems that may be clinically useful and extend the intrinsic capabilities of seated wheelchair users with SCI.
Conclusions
We have designed and deployed a real-time self-righting feedback controller for restoration of trunk posture in the coronal and diagonal planes in individuals with various levels of SCI using FNS of their paralyzed muscles. The controller was tested in five individuals with cervical and thoracic level SCI who had been implanted with various FNS systems for seated trunk stability. The results showed that the self-righting feedback controller successfully restored posture to erect in all the subjects from trunk angles that were impossible to restore with baseline stimulation alone automatically and without use of the arms. Other controller performance measures indicate the efficacy of the controller in terms of the time required to successfully restore the trunk to erect posture. The ability to restore the trunk to erect from fairly large angles of lean indicates the future potential for users of seated balance neuroprostheses to deploy their trunk to larger regions around their wheelchairs, thus enabling them to undertake ADLs in ways that would be impossible without a feedback control system.
Highlights.
Self-righting controller using neuromuscular stimulation restores erect trunk posture.
Controller worked for individuals paralyzed by various levels of spinal cord injury.
External control unit used accelerometer-measured trunk tilt to regulate stimulation.
One chest-worn sensor corrected posture deviations in coronal and diagonal planes.
Controller assessed by response time, recovery time and percentage maximum deviation.
Acknowledgements
The authors would like to acknowledge the contributions of our study participants, the Motion Study Laboratory at the Louis Stokes Cleveland Veterans Affairs Medical Center, and the Cleveland APT Center.
Funding
This material was based on work supported in part by the National Institutes of Health (Grant 1R01NS101043-01) and the Department of Defense, SCIR Program (Grant W81XWH-17-1-0240).
List of Abbreviations
- SCI
Spinal Cord Injury
- ADLs
activities of daily living
- FNS
Functional Neuromuscular Stimulation
- PW
Pulse Width
- UE
Upper extremities
- ECU
External Control Unit
- AM
Posterior portion of Adductor Magnus
- ES
Lumbar Erector Spinae
- GX
Gluteus Maximus
- QL
Quadratus Lumborum
- SM
Semimembranosus
- L
Left (referring to side of body)
- R
Right (referring to side of body)
- FO
Forward (referring to direction of motion)
- LF
Left (referring to direction of motion)
- RI
Right (referring to direction of motion)
- EMD
Electromechanical Delay
- AIS
American Spinal Injury Association Impairment Scale
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests: All authors declare no competing interests in this work.
Ethical approval: The research was approved by VA IRB #07101-H36.
References
- [1].Kukke SN, Triolo RJ. The effects of trunk stimulation on bimanual seated workspace. IEEE Trans Neural Syst Reha- bil Eng. 2004;12(2):177–85. [DOI] [PubMed] [Google Scholar]
- [2].Anderson KD. Targeting Recovery: Priorities of the Spinal Cord-Injured Population. Journal of Neurotrauma 2004; 21(10): 1371–1383. [DOI] [PubMed] [Google Scholar]
- [3].Kulig K, Newsman CJ, et al. The effect of level of spinal cord injury on shoulder joint kinetics during manual wheelchair propulsion. Clinical Biomechanics, 2001; 16: 744–751. [DOI] [PubMed] [Google Scholar]
- [4].Park ES, Park CI, Lee HJ, Cho YS. The effect of electrical stimulation on the trunk control in young children with spastic diplegic cerebral palsy. J Korean Med Sci 2001; 16(3): 347–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Vanoncini M, Holderbaum W, Andrews BJ. Electrical Stimulation for trunk control in paraplegia: A feasibility study. Control Engineering Practice 2012; 20(12): 1247–1258. [Google Scholar]
- [6].Thrasher TA, Sin VW, Masani K, Vette AH, Craven BC, Popovic MR. Responses of the trunk to multidirectional perturbations during unsupported sitting. J Appl Biomech. 2010; 26:332–40. [DOI] [PubMed] [Google Scholar]
- [7].Vette AH, Masani K, Wu N, Popovic MR. Multidirectional quantification of trunk stiffness and damping during unloaded natural sitting. Med Eng Phys. 2014; 36(1):102–9. [DOI] [PubMed] [Google Scholar]
- [8].Wilkenfeld A, Triolo RJ, Audu ML: Feasibility of Functional Electrical Stimulation for Control of Seated Posture after Spinal Cord Injury: A Simulation Study. Journal of Rehabilitation Research and Development 2006; 43:139–152. [DOI] [PubMed] [Google Scholar]
- [9].Lambrecht JM, Audu ML, Triolo RJ, Kirsch RF: Musculoskeletal model of trunk and hips for development of seated-posture-control neuroprosthesis. Journal of Rehabilitation Research and Development 2009; 46:515–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Vette AH, Yoshida T, Thrasher TA, Masani K, Popovic MR. A comprehensive three-dimensional dynamic model of the human head and trunk for estimating lumbar and cervical joint torques and forces from upper body kinematics. Med Eng Phys. 2012; 34(5):640–9. [DOI] [PubMed] [Google Scholar]
- [11].Triolo RJ., Boggs L, Miller ME, Nemunaitis G Nagy J and Bailey SN Implanted Electrical Stimulation of the Trunk for Seated Postural Stability and Function After Cervical Spinal Cord Injury: A Single Case Study. Archives of Physical Medicine and Rehabilitation. 2009; 9:340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Triolo RJ., Bailey SN, Miller ME, Lombardo LM and Audu ML. Effects of Stimulating Hip and Trunk Muscles on Seated Stability, Posture, and Reach After Spinal Cord Injury. Archives of Physical Medicine and Rehabilitation. 2013; 94:1766–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Murphy JO, Audu ML, Lombardo LM, Foglyano KM, Triolo RJ. Feasibility of closed-loop controller for righting seated posture after spinal cord injury. J Rehabil Res Dev. 2014; 51(5):747–760. [DOI] [PubMed] [Google Scholar]
- [14].Audu ML., Lombardo LM., Schnellenberger JR., Foglyano KM, Miller ME. and Triolo RJ. A neuroprosthesis for control of seated balance after spinal cord injury. Journal of NeuroEngineering and Rehabilitation. 2015; 12(8):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Armstrong KL, Lombardo LM, Foglyano KM, Audu ML and Triolo RJ. Automatic application of neural stimulation during wheelchair propulsion after SCI enhances recovery of upright sitting from destabilizing events. Journal of NeuroEngineering and Rehabilitation, 2018; 15:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kamper D, Barin K, Parnianpour M, Reger S, Weed H. Preliminary investigation of the lateral postural stability of spinal cord-injured individuals subjected to dynamic perturbations. International Medical Society of Paraplegia, 2009; 27: 40–46 [DOI] [PubMed] [Google Scholar]
- [17].Triolo RJ, Bailey SN, Lombardo LM, Miller ME, Foglyano K, Audu ML. Effects of intramuscular trunk stimulation on manual wheelchair propulsion mechanics in 6 subjects with spinal cord injury. Archives of physical medicine and rehabilitation. 2013; 94(10):1997–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Crawford A, Armstrong K, Loparo K, Audu ML, Triolo RJ. Detecting destabilizing wheelchair conditions for maintaining seated posture. Disability and Rehabilitation: Assistive Technology. 2018; 13(2):178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Armstrong KL, Lombardo LM, Foglyano KM, Audu ML, Triolo RJ. Automatic application of neural stimulation during wheelchair propulsion after SCI enhances recovery of upright sitting from destabilizing events. Journal of neuroengineering and rehabilitation, 2018;15(1): Article ID 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Triolo RJ, Bailey SN, Miller ME, Rohde LM, Anderson JS, Davis JA, Abbas JJ, DiPonio LA, Forrest GP, Gater DR, et al. Longitudinal performance of a surgically implanted neuroprosthesis for lower extremity exercise, standing, and transfers after spinal cord injury. Arch Phys Med Rehabil. 2012; 93:896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Memberg WD, Peckham PH, Keith MW. A surgically-implanted intramuscular electrode for an implantable neuromuscular stimulation system. IEEE Trans Biomed Eng. 1994; 2:80–91. [Google Scholar]
- [22].Akers JM, Peckham PH, Keith MW, Merritt K. Tissue response to chronically stimulated implanted epimysial and intramuscular electrodes. IEEE Trans Rehabil Eng. 1996; 5:207–220. [DOI] [PubMed] [Google Scholar]
- [23].Fisher L, Tyler D, Triolo R. Optimization of selective stimulation parameters for multi-contact electrodes. JNeuroeng Rehabil. 2013; 10(1):Article ID 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Smith B, Peckham PH, Keith MW, Roscoe DD. An externally powered, multichannel implantable stimulator for versatile control of paralyzed muscle. IEEE Trans Biomed Eng. 1987; 34:499–508. [DOI] [PubMed] [Google Scholar]
- [25].Smith B, Tang Z, Johnson MW, Pourmehdi S, Gazdik MM, Buckett JR, Peckham PH. An externally powered, multichannel, implantable stimulator-telemeter for control of paralyzed muscle. IEEE Trans Biomed Eng 1998; 45:463–475. [DOI] [PubMed] [Google Scholar]
- [26].Trier SC, Buckett JR, Campean A, Miller ME, Montague FW, Vrabec TL, Weisgarber JA (2001) A Modular External Control Unit for Functional Electrical Stimulation In: Proceedings of the 6th Annual Conference of the International Functional Electrical Stimulation Society. International Functional Electrical Stimulation Society, Cleveland, pp 109. [Google Scholar]
- [27].(2007) xPC Target Selecting Hardware Guide. The MathWorks Inc; Available via Mathworks. https://www.mathworks.com/tagteam/37937_xpc_target_selecting_hardware_guide.pdf [Google Scholar]
- [28].Ogata K, Modern Control Engineering (5th Edition), Prentice-Hall, Inc., Upper Saddle River, NJ 07458. [Google Scholar]
- [29].Cavanagh PR and Komi PV: Electromechanical Delay in Human Skeletal Muscle Under Concentric and Eccentric Contractions, Eur. J. Appl. Physiol 1979; 42:159–163. [DOI] [PubMed] [Google Scholar]
- [30].Sharma N, Gregory C and Dixon WE: Predictor-based compensation for electromechanical delay during neuromuscular electrical stimulation. IEEE Trans. Neural Syst. Rehabil. Eng 2011; 19:601–611. [DOI] [PubMed] [Google Scholar]
- [31].Bouillon F: Measure, modeling and compensation of fatigue-induced delay during neuromuscular electrical stimulation. MS Thesis, University of Florida; 2013. [Google Scholar]
- [32].Ai B, Sentis L, Paine N, Han S, Mok A and Fok CL. Stability and performance analysis of time-delayed actuator control systems. Journal of Dynamic Systems, Measurement and Control, Transactions of the ASME. 2016; 138(5). 10.1115/1.4032461. [DOI] [Google Scholar]
- [33].Vette AH, Masani K and Popovic MR: Neural-mechanical feedback control scheme generates physiological ankle torque fluctuation during quiet stance. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2010; 18:86–95. [DOI] [PubMed] [Google Scholar]
- [34].Vette AH, Masani K and Popovic MR. Time delay from muscle activation to torque generation during quiet stance: Implications for closed-loop control via FES. Biomedizinische Technik. 2008; 53(Suppl. 1):423–425. [Google Scholar]
- [35].Ng JK, Richardson CA, Kippers V, Parnianpour M. Relationship between muscle fiber composition and functional capacity of back muscles in healthy subjects and patients with back pain. Journal of Orthopaedic & Sports Physical Therapy. 1998; 27(6):389–402. [DOI] [PubMed] [Google Scholar]


