Abstract
Background
Treatment of ST-elevation myocardial infarction (STEMI) in Canada is protocolized, and timely patient transfer can improve outcomes. Population-based processes of care in Canada for other cardiovascular conditions remain less clear. We aimed to describe the interhospital transfer of Canadian patients with acute cardiovascular disease.
Methods
We reviewed the Canadian Institute for Health Information Discharge Abstract Database for adult patients hospitalized with acute cardiovascular disease between 2013 and 2018. We compared patient characteristics and clinical outcomes based on transfer status (transferred, nontransferred) and presenting hospital (teaching, large community, medium community, and small community hospitals). The primary outcome of interest was in-hospital mortality.
Results
There were 476,753 patients with primary acute cardiovascular diagnoses, 48,579 (10.2%) of whom were transferred. Transferred patients were more frequently younger, male, and had fewer comorbidities. The most common diagnoses among transferred patients were non-STEMI (44.2%), STEMI (29.0%), and congestive heart failure (9.4%). Using teaching hospitals as a reference, transfer to large and medium community hospitals was associated with lower hospital mortality (adjusted odds ratio: 0.83, 95% confidence interval: 0.75-0.91 and 0.45, 95% confidence interval: 0.39-0.52, respectively).
Conclusions
Approximately 10% of patients with acute cardiovascular conditions are transferred to another hospital. Patient transfer may be associated with lower in-hospital mortality, with possible variability based on diagnosis, comorbidities, hospital of origin, and destination hospital. Further investigation into the optimization of care for patients with acute cardiovascular disease, including transfer practices, is warranted as regionalized care models continue to develop.
Résumé
Introduction
Au Canada, le traitement de l’infarctus du myocarde avec sus-décalage du segment ST (STEMI) découle d’un protocole qui prévoit au moment opportun le transfert des patients pour permettre d’améliorer les résultats cliniques. On n’en sait encore peu sur les processus de soins auprès de la population canadienne en ce qui concerne les autres maladies cardiovasculaires. Nous avions pour objectif de décrire les transferts interhospitaliers de patients canadiens atteints d’une maladie cardiovasculaire aiguë.
Méthodes
Nous avons passé en revue les résumés de la base de données de l’Institut canadien d’information sur la santé sur les congés des patients hospitalisés atteints d’une maladie cardiovasculaire aiguë entre 2013 et 2018. Nous avons comparé les caractéristiques des patients et les résultats cliniques en fonction du statut du transfert (patients transférés ou non transférés) et de l’hôpital de destination (hôpitaux d’enseignement, grands hôpitaux communautaires, hôpitaux communautaires moyens et petits hôpitaux communautaires). Le principal critère étudié était la mortalité intrahospitalière.
Résultats
Parmi les 476 753 patients qui avaient un diagnostic principal de maladie cardiovasculaire aiguë, 48 579 (10,2 %) ont été transférés. Les patients transférés étaient plus fréquemment jeunes, de sexe masculin, et avaient peu de comorbidités. Les diagnostics les plus fréquents parmi les patients transférés étaient les non-STEMI (44,2 %), les STEMI (29,0 %) et l’insuffisance cardiaque congestive (9,4 %). En utilisant comme référence les hôpitaux d’enseignement, les transferts vers de grands hôpitaux communautaires et des hôpitaux communautaires moyens étaient associés à une plus faible mortalité intrahospitalière (ratio d’incidence approché ajusté : 0,83, intervalle de confiance à 95 %, 0,75-0,91 et 0,45, intervalle de confiance à 95 %, 0,39-0,52, et ce, respectivement).
Conclusions
Approximativement 10 % des patients atteints d’une maladie cardiovasculaire aiguë sont transférés vers un autre hôpital. Le transfert des patients peut être associé à une plus faible mortalité intrahospitalière et montrer une variabilité en fonction du diagnostic, des comorbidités, de l’hôpital d’origine et de l’hôpital de destination. D’autres études liées à l’optimisation des soins des patients atteints d’une maladie cardiovasculaire aiguë, qui porteront de plus sur les pratiques de transfert, sont justifiées puisque l’élaboration de modèles de soins régionaux se poursuit.
Cardiovascular disease is the second leading cause of death among Canadians, and acute cardiovascular disease represents a leading reason for hospital admission and intensive care unit (ICU) resource utilization.1, 2, 3 Certain conditions, such as ST-elevation myocardial infarction (STEMI) and out-of-hospital cardiac arrest, require urgent treatment to prevent significant morbidity and mortality.4 Interhospital transfer protocols have been proposed for these time-sensitive conditions to provide timely diagnosis and treatment at centres with necessary resources.4, 5, 6 Regional systems of care for these specific conditions have been associated with improved patient outcomes.7,8 Patients presenting with other acute cardiovascular conditions also benefit from timely diagnosis and therapy; however, transfer practices for these patients are inconsistent and are often based on local practice and resource availability.
Among patients with acute cardiovascular conditions, clinical complexity is increasing due to advancing age, noncardiovascular comorbid illness, and critical care resource needs.9, 10, 11 The Canadian Cardiovascular Society (CCS) has proposed a geographic hub-and-spoke model of care to centralize resources at facilities that are best equipped to treat acute cardiovascular conditions.12 However, the current recommendations do not specify transfer criteria, including patient selection, timing, and destination hospital considerations. Moreover, there is significant interprovincial variability in cardiovascular intensive care unit (CICU) resource utilization and outcomes, suggesting that more standardized CICU admission and transfer criteria may be needed.13
Assessment of the current national transfer patterns and outcomes among Canadian patients with acute cardiovascular illness is a necessary first step toward defining better standards for national transfer criteria. The aim of this study was therefore to describe the characteristics and associated outcomes among transferred and nontransferred Canadian patients with acute cardiovascular conditions.
Methods
The Canadian Institute for Health Information collects data for all acute care hospitalizations in Canadian provinces except Québec.14 The Discharge Abstract Database was reviewed from January 1, 2013, to December 31, 2018, for all patients with primary cardiovascular diagnoses as defined by any “I” diagnosis in the 10th revision of the International Statistical Classification of Diseases and Related Health Problems. Cardiogenic shock, which is an “R” code, was also included. Previously established cardiovascular and noncardiovascular comorbidities were also documented. The full list of primary diagnoses and cardiovascular comorbidities is provided in Supplemental Tables S1 and S2. All cardiovascular diagnoses not specifically listed in the tables were categorized as “other.” For each patient identified, data extracted included date of admission, age, gender, primary diagnosis, province, hospital of origin (deidentified), destination hospital (if transferred, deidentified), hospital type (teaching [TH], large community [LCH], medium community [MCH], and small community [SCH]), admission location (coronary care unit [CCU] or CICU, ICU, ward), preadmission comorbidities, secondary diagnoses, discharge date, length of stay (LOS), and discharge status (dead/alive). The hospital classification scheme was derived from the Canadian Institute for Health Information database and based on teaching status (“Teaching”) and/or hospital inpatient case load (Supplemental Table S3). Patients who remained at the destination hospital for less than 24 hours were excluded, as these patients were more likely to have been transferred for a diagnostic test or procedure within regional systems of care. Only the first transfer episode was included in the analysis. Outcomes were ascertained at the end of index hospitalization, even if subsequent transfers occurred.
Patient characteristics were compared between transferred patients and nontransferred patients using Wilcoxon and χ2 tests, as appropriate. Patients were further stratified by the most common primary diagnoses (STEMI, non–ST-elevation myocardial infarction [NSTEMI], and heart failure [HF], ventricular arrhythmia, cardiac arrest, atrial fibrillation/flutter, other), presenting hospital type (TH, LCH, MCH, and SCH), and by destination hospital type. Logistic regression models were used to examine the independent association between hospital size and in-hospital mortality, after adjusting for demographic variables and baseline comorbidities including demographic variables (age, gender), baseline comorbidities, diagnosis type, and in-hospital percutaneous coronary intervention or bypass grafting. All models used robust estimates of variance clustered by every hospital to account for hospital effect. Adjusted models were built using the backward stepwise selection method using a P value of 0.20 for removal. Hosmer-Lemeshow goodness of fit was used to examine the appropriateness of the fitted models. Statistical significance was set at P = 0.05, and all statistical tests were 2-sided. All statistical analyses were performed by a senior biostatistician with SAS statistical software (version 9.4; SAS Institute, Cary, NC).
Results
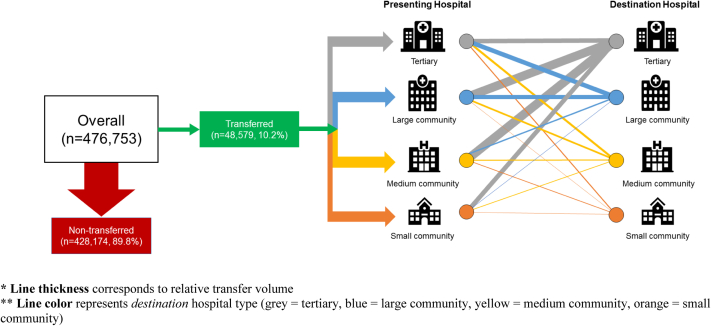
The dataset included 476,753 individual patient encounters. The most common primary diagnosis was HF (26.4%), followed by NSTEMI (23.8%) and “other” cardiovascular diagnoses (18.9%) (Supplemental Figure S1). There were 10.2% patients transferred to another hospital (Table 1). The most common diagnoses among transferred patients were NSTEMI (44.3%), STEMI (29.0%), and HF (9.4%). Figure 1 summarizes patient transfer status and distribution by hospital type. Compared with nontransferred patients, transferred patients were younger (median age, 67 years [interquartile range (IQR), 18] vs 74 years [IQR, 21], P < 0.001) and more often male (69.9% vs 57.9%, P < 0.001). Transferred patients had lower rates of all cardiovascular comorbidities and were more likely to be admitted to a CCU or ICU (30.1% vs 23.0% and 26.3% vs 11.8%, respectively, both P < 0.001). Nontransferred patients were more likely to be admitted to an acute care ward (58.5% vs 38.2%, P < 0.001). Patient transfer was associated with a shorter median hospital LOS (2 days [IQR, 5] vs 5 days [IQR, 6], P < 0.001) but a higher rate of 30-day readmission (6.0% vs 5.2%, P < 0.001; Supplemental Table S4). Similarly, transfer was associated with a longer total LOS up to 30 days post-admission patients (8 days [IQR, 13] vs 5 days [IQR, 7], P < 0.001). Patient transfer was associated with lower overall hospital mortality compared with nontransferred patients (6.7% vs 8.7%, P < 0.001). There were 7119 patients who were transferred twice. The majority of these patients (5875 of 7119; 82.5%) returned to the same hospital type (Supplemental Figure S2).
Table 1.
Characteristics and outcomes based on transfer status
| Transferred (n = 48,579) | Nontransferred (n = 428,174) | |
|---|---|---|
| Demographics | ||
| Age (y), median (IQR) | 67 (18) | 74 (21) |
| Male (%) | 69.9 | 57.9 |
| Care location, n (%) | ||
| CICU or CCU | 14,615 (30.1) | 98,627 (23.0) |
| ICU | 12,788 (26.3) | 53,748 (11.8) |
| Acute care ward | 18,549 (38.2) | 250,564 (58.5) |
| Unknown | 2627 (5.4) | 25,235 (5.9) |
| Primary diagnosis, n (%) | ||
| Heart failure | 4586 (9.4) | 121,328 (28.3) |
| NSTEMI | 21,496 (44.2) | 91,935 (21.5) |
| STEMI | 14,107 (29.0) | 49,153 (11.5) |
| Ventricular arrhythmia | 1423 (2.9) | 7736 (1.8) |
| Cardiac arrest | 614 (1.3) | 5854 (1.4) |
| Atrial fibrillation/flutter | 1847 (3.8) | 67,066 (15.7) |
| Other | 4506 (9.3) | 85,642 (20.0) |
| Comorbidities, n (%) | ||
| Hypertension | 1365 (2.8) | 38,066 (8.9) |
| Diabetes | 1933 (4.0) | 45,016 (10.5) |
| Dyslipidemia | 243 (0.5) | 6223 (1.5) |
| Myocardial infarction | 960 (2.0) | 18,097 (4.2) |
| Heart failure | 766 (1.6) | 12,304 (2.9) |
| Percutaneous coronary intervention | 82 (0.2) | 1937 (0.5) |
| Coronary artery bypass | 110 (0.2) | 2629 (0.6) |
| Atrial fibrillation | 1579 (3.3) | 44,263 (10.3) |
| Cerebrovascular disease | 97 (0.2) | 2122 (0.5) |
| Peripheral vascular disease | 119 (0.2) | 2830 (0.7) |
| COPD | 378 (0.8) | 13,842 (3.2) |
| Chronic kidney disease | 327 (0.7) | 8016 (1.9) |
| Dementia | 39 (0.1) | 3749 (0.9) |
| Cancer | 101 (0.2) | 4136 (1.0) |
| Outcomes | ||
| Hospital length of stay (LOS, d), (median, IQR) | 2 (5) | 5 (6) |
| LOS from admission to day 30, median (IQR) | 8 (13) | 5 (7) |
| 30-day readmission, n (%) | 2914 (6.0) | 22,360 (5.2) |
| Hospital mortality, n (%) | 3273 (6.7) | 37,257 (8.7) |
CCU, coronary care unit; CICU, cardiac intensive care unit; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; IQR, interquartile range; LOS, length of stay; NSTEMI, non–ST-segment elevation myocardial infarction; STEMI, ST-elevation myocardial infarction.
Figure 1.
Patient transfer status and distribution by hospital type. Line thickness corresponds to relative transfer volume. Line color represents destination hospital type (gray = tertiary, blue = large community, yellow = medium community, orange = small community).
Outcomes—presenting hospital
The majority of transferred patients presented initially to an LCH (33.0%), followed by a TH (27.4%), MCH (26.8%), and SCH (12.9%) (Supplemental Table S5). Presentation to an LCH was associated with a longer median hospital LOS (3 days [IQR 6]), P < 0.0001) compared with the other presenting hospital types (Table 2). Presentation to an LCH was associated with the longest total LOS from admission until day 30 (median, 9 days [IQR, 13]) and the highest rate of 30-day readmission (6.4%). SCH presentation was associated with the shortest LOS from admission until day 30 (median, 7 days [IQR, 10]), P < 0.0001). MCH presentation was associated with the lowest rate 30-day readmission rate (5.7%, P = 0.04). MCH presentation was associated with the lowest unadjusted hospital mortality (6.1%), followed by LCH (6.8%), TH (7.0%), and SCH (7.5%, all P < 0.0001).
Table 2.
Outcomes of overall population based on transfer status, as a function of presenting hospital type
| Teaching (n = 13,292) | Large community (n = 16,038) | Medium community (n = 12,996) | Small community (n = 6253) | P value | |
|---|---|---|---|---|---|
| Hospital LOS, mean (SD) | 2 (6) | 3 (6) | 2 (4) | 2 (3) | < 0.0001 |
| Hospital mortality | 925 (7.0) | 1083 (6.8) | 796 (6.1) | 469 (7.5) | < 0.0001 |
| 30-day readmission | 780 (5.9) | 1030 (6.4) | 738 (5.7) | 366 (5.9) | 0.04 |
| LOS from admission to day 30, median (IQR) | 7 (16) | 9 (13) | 8 (14) | 7 (10) | < 0.0001 |
IQR, interquartile range; LOS, length of stay; SD, standard deviation.
On multivariable analysis, after adjusting for clinical and demographic factors and using TH as a reference, presentation to an MCH was associated with decreased hospital mortality (odds ratio [OR]: 0.69, 95% confidence interval [CI]: 0.61-0.78) but not presentation to an LCH (OR: 0.95, 95% CI: 0.86-1.06) or SCH (OR: 0.98, 95% CI: 0.86-1.12; Table 3). Among patients with HF and atrial fibrillation, presentation to an SCH was associated with increased mortality (OR: 1.44, 95% CI: 1.11-1.87 and OR: 2.41, 95% CI: 1.23-4.78, respectively). Among patients with STEMI, presentation to an LCH or MCH was associated with decreased hospital mortality (OR: 0.74, 95% CI: 0.60-0.91 and OR: 0.64, 95% CI: 0.50-0.82, respectively). Among patients with NSTEMI, presenting to an MCH was associated with decreased hospital mortality (OR: 0.64, 95% CI: 0.47-0.87).
Table 3.
Adjusted OR for in-hospital mortality based on presenting hospital type (reference: teaching hospitals)
| Admission diagnosis | Teaching (n = 13,292) | Large community (n = 16,038) | Medium community (n = 12,996) | Small community (n = 6253) |
|---|---|---|---|---|
| All patients | 13,292 | 16,038 | 12,996 | 6253 |
| Adjusted OR (95% CI) | REF | 0.95 (0.86, 1.06) | 0.69 (0.61, 0.78) | 0.98 (0.86, 1.12) |
| Heart failure | 1133 | 1378 | 1125 | 950 |
| Adjusted OR (95% CI) | REF | 1.07 (0.85, 1.35) | 0.96 (0.74, 1.24) | 1.44 (1.11, 1.87) |
| All ACS (STEMI/NSTEMI) | 9683 | 11,998 | 9730 | 4192 |
| Adjusted OR (95% CI) | REF | 0.95 (0.81, 1.10) | 0.68 (0.57, 0.83) | 0.85 (0.68, 1.05) |
| STEMI | 3040 | 7629 | 7203 | 3624 |
| Adjusted OR (95% CI) | REF | 0.74 (0.60, 0.91) | 0.64 (0.50, 0.82) | 0.83 (0.64, 1.08) |
| NSTEMI | 6643 | 4369 | 2527 | 568 |
| Adjusted OR (95% CI) | REF | 1.16 (0.94, 1.45) | 0.64 (0.47, 0.87) | 0.66 (0.43, 1.04) |
| Ventricular arrhythmia | 239 | 616 | 449 | 119 |
| Adjusted OR (95% CI) | REF | 0.93 (0.48, 1.81) | 0.56 (0.24, 1.30) | 0.43 (0.11, 1.61) |
| Cardiac arrest | 124 | 284 | 177 | 29 |
| Adjusted OR (95% CI) | REF | 0.65 (0.37, 1.14) | 0.72 (0.38, 1.34) | 0.76 (0.27, 2.13) |
| Atrial fibrillation | 253 | 387 | 568 | 639 |
| Adjusted OR (95% CI) | REF | 1.54 (0.78, 3.06) | 1.59 (0.78, 3.24) | 2.41 (1.23, 4.78) |
| Other | 1860 | 1375 | 947 | 324 |
| Adjusted OR (95% CI) | REF | 0.87 (0.70, 1.08) | 0.54 (0.41, 0.72) | 0.60 (0.40, 0.90) |
Models were adjusted for demographic variables (age, gender), baseline comorbidities, diagnosis type, and in-hospital percutaneous coronary intervention or bypass grafting.
Bold represents statistically significant results.
ACS, acute coronary syndrome; CI, confidence interval; NSTEMI, non–ST-segment elevation myocardial infarction; OR, odds ratio; STEMI, ST-elevation myocardial infarction.
Outcomes—destination hospital
The most common destination hospitals among transferred patients were TH (59.0%), followed by LCH (23.9%), MCH (13.0%), and SCH (4.7%). Figure 1 summarizes the relative transfer volumes to each destination hospital type. Transfer to a TH and SCH was associated with higher median LOS compared with LCH and MCH (6 days [IQR, 8] and 6 days [IQR, 11] vs 3 days [IQR, 5] and 3 days [IQR, 6], respectively, all P < 0.0001). The median total LOS from admission to day 30 was lowest in patients transferred to LCH (4 days [IQR, 8]), followed by MCH (5 days [IQR, 9]), TH (11 days [IQR, 13]), and SCH (11 days [IQR, 18], all P < 0.0001). The rate of readmission within 30 days was lowest for patients transferred to TH (5.1%), followed by MCH (6.5%), SCH (7.1%), and LCH (7.7%, all P < 0.0001). Transfer to an MCH was associated with the lowest unadjusted hospital mortality (4.4%), and transfer to an SCH was associated with the highest hospital mortality (10.9%, both P < 0.0001).
On multivariable analysis, after adjusting for clinical and demographic factors and using TH as a reference, transfer to an LCH and MCH was associated with decreased hospital mortality (OR: 0.83, 95% CI: 0.75-0.91 and OR: 0.45, 95% CI: 0.0.39-0.52, respectively; Table 4). Transfer to an SCH was associated with increased hospital mortality (OR: 1.58, 95% CI: 1.11-2.26). Among patients with STEMI and NSTEMI, transfer to an LCH and MCH was associated with reduced hospital mortality (OR: 0.25, 95% CI: 0.19-0.33 and OR: 0.08, 95% CI: 0.05-0.13 for STEMI, and OR: 0.74, 95% CI: 0.55-0.98 and OR: 0.32, 95% CI: 0.19-0.54 for NSTEMI). Transfer to an LCH was associated with decreased mortality in patients with ventricular arrhythmias (OR: 0.25, 95% CI: 0.06-0.94).
Table 4.
Adjusted mortality based on destination hospital type (reference: teaching hospitals)
| Admission diagnosis | Teaching (n = 28,653) | Large community (n = 11,593) | Medium community (n = 6073) | Small community (n = 2260) |
|---|---|---|---|---|
| All patients | 28,653 | 11,593 | 6073 | 2260 |
| Adjusted OR (95% CI) | REF | 0.83 (0.75, 0.91) | 0.45 (0.39, 0.52) | 1.93 (1.64, 2.28)) |
| Heart failure | 1164 | 468 | 331 | 468 |
| Adjusted OR (95% CI) | REF | 1.08 (0.79, 1.48) | 1.06 (0.71, 1.56) | 1.58 (1.11, 2.26) |
| All ACS (STEMI/NSTEMI) | 12,468 | 7444 | 3941 | 750 |
| Adjusted OR (95% CI) | REF | 0.45 (0.37, 0.54) | 0.15 (0.11, 0.22) | 1.38 (0.89, 2.14) |
| NSTEMI | 8798 | 2290 | 1480 | 370 |
| Adjusted OR (95% CI) | REF | 0.74 (0.55, 0.98) | 0.32 (0.19, 0.54) | 1.83 (0.98, 3.42) |
| STEMI | 3670 | 5154 | 2461 | 380 |
| Adjusted OR (95% CI) | REF | 0.25 (0.19, 0.33) | 0.08 (0.05, 0.13) | 0.67 (0.36, 1.26) |
| Ventricular arrhythmia | 1229 | 120 | 47 | 7 |
| Adjusted OR (95% CI) | REF | 0.25 (0.06, 0.94) | 0.50 (0.13, 1.93) | NE |
| Cardiac arrest | 147 | 53 | 9 | 3 |
| Adjusted OR (95% CI) | REF | 1.18 (0.43, 3.23) | 8.42 (1.09, 64.90) | 54.31 (2.26-) |
| Atrial fibrillation | 861 | 228 | 152 | 134 |
| Adjusted OR (95% CI) | REF | 1.01 (0.27, 3.87) | 2.91 (0.83, 10.20) | 2.77 (0.71, 10.77) |
| Other | 12,284 | 3280 | 1593 | 914 |
| Adjusted OR (95% CI) | REF | 1.12 (0.98, 1.27) | 0.66 (0.54, 0.80) | 1.50 (1.20, 1.88) |
Models were adjusted for demographic variables (age, gender), baseline comorbidities, diagnosis type, and in-hospital percutaneous coronary intervention or bypass grafting.
Bold represents statistically significant results.
ACS, acute coronary syndrome; CI, confidence interval; NSTEMI, non–ST-segment elevation myocardial infarction; OR, odds ratio; STEMI, ST-elevation myocardial infarction.
Outcomes—common diagnoses
Characteristics and outcomes of transferred patients with the most common admission diagnoses (HF, NSTEMI, and STEMI) are summarized in Table 5. Patients with cardiac arrest were the most likely to be admitted to an ICU or CCU and had the highest hospital mortality (29.8%). Patients with HF and “other” diagnoses were older than those with other diagnoses (median age, 73 years); the second-highest rate of hospital mortality was among patients with HF (17.5%). Characteristics of transferred patients by age, sex, and primary diagnosis are summarized in Supplemental Table S6.
Table 5.
Characteristics and outcomes of transferred patients based on admission diagnosis
| Heart failure (n = 4586) | NSTEMI (n = 21,496) | STEMI (n = 14,107) | Ventricular arrhythmia (n = 1423) | Cardiac arrest (n = 614) | AF (n = 1847) | Other (n = 4506) | |
|---|---|---|---|---|---|---|---|
| Demographics | |||||||
| Age (y), median (IQR) | 73 (64, 81) | 67 (58, 75) | 63 (55, 73) | 66 (57, 74) | 64 (53, 72) | 71 (62, 79) | 73 (63, 80) |
| Male, n (%) | 2771 (60.4) | 15,399 (71.6) | 10,305 (73.0) | 1081 (76.0) | 455 (74.1) | 1091 (59.1) | 2844 (63.1) |
| Care location, n (%) | |||||||
| CICU or CCU | 546 (11.9) | 3760 (17.5) | 8783 (62.3) | 309 (21.7) | 68 (11.1) | 150 (8.1) | 999 (22.2) |
| ICU | 905 (19.7) | 5956 (27.7) | 3143 (22.3) | 652 (45.8) | 387 (63.0) | 479 (25.9) | 1266 (28.1) |
| Acute care ward | 2921 (63.7) | 10,273 (47.8) | 1961 (13.9) | 384 (27.0) | 145 (23.6) | 1113 (60.3) | 1752 (38.9) |
| Unknown | 214 (4.7) | 1507 (7.0) | 220 (1.6) | 78 (5.5) | 14 (2.3) | 105 (5.7) | 489 (10.9) |
| Outcomes | |||||||
| Hospital LOS, mean ± SD | 8.1 ± 10.8 | 4.75 ± 7.4 | 2.25 ± 3.7 | 4.7 ± 5.6 | 5.1 ± 7.0 | 4.8 ± 5.9 | 10.3 ± 12.8 |
| Hospital mortality, n (%) | 804 (17.5) | 881 (4.1) | 576 (4.1) | 68 (4.8) | 183 (9.8) | 137 (7.4) | 624 (13.8) |
| 30-day readmission, n (%) | 313 (6.8) | 1275 (5.9) | 908 (6.4) | 83 (5.8) | 19 (3.1) | 135 (7.3 | 181 (4.0) |
| LOS from admission to day 30, mean ± SD | 17.3 ± 9.3 | 11.1 ± 8.3 | 6.7 ± 6.6 | 12.6 ± 7.3 | 13.7 ± 9.3 | 12.0 ± 8.4 | 17.1 ± 10.0 |
AF, atrial fibrillation; CCU, coronary care unit; CICU, cardiac intensive care unit; ICU, intensive care unit; IQR, interquartile range; LOS, length of stay; NSTEMI, non–ST-segment elevation myocardial infarction; SD, standard deviation; STEMI, ST-elevation myocardial infarction.
Discussion
Using a large national database, our results emphasize existing evidence that acute cardiovascular conditions are a common reason for presentation to Canadian hospitals, with an average of almost 100,000 patient encounters per year. We found that approximately 10% of patients admitted for acute cardiovascular conditions are transferred to another centre. Transferred patients are younger, have fewer medical comorbidities, and lower overall mortality compared with nontransferred patients. Patients with NSTEMI and STEMI are most likely to be transferred. Most transferred patients are treated at TH and require significant critical care resources as demonstrated by the frequent rates of transfer to CCUs. Using patients transferred to a TH as a reference, transfer to an LCH and MCH was associated with the lowest hospital mortality, whereas transfer to an SCH was associated with the highest hospital mortality. Interestingly, the incidence of hospital mortality among transferred patients with HF was 15%-20% across all presenting hospital types, suggesting that these patients are at particularly high risk of adverse outcomes.
Interhospital transfer for patients with acute coronary syndromes (ACS), especially STEMI, is encouraged when appropriate criteria exist;15 however, the evidence for patients with conditions other than ACS is limited. In a large retrospective cohort of American Medicare beneficiaries with common cardiac and noncardiac medical conditions, Mueller et al.16 demonstrated that less than 2% of overall patients are transferred. Importantly, the 3 most common diagnoses among transferred patients were acute cardiac conditions (ACS, HF, and arrhythmia), and together comprised 50% of the total number of transferred patients.16 Similar to our findings, transferred patients with ACS had lower short-term mortality compared with nontransferred patients, and patients with HF had higher mortality. Our study showed that transfer was approximately 5 times more common in Canada, reflecting an important difference in care patterns between nations, which may be partly explained by a higher concentration of advanced diagnostic and therapeutic cardiac care (eg, cardiac catheterization and cardiac surgery) at specific Canadian centres. Need for higher-level procedures is likely a leading reason for patient transfer to TH and LCH settings.
Transferred patients are younger, more often male, and have less comorbid illness. Whether this reflects an implicit bias in referral patterns or is reflective of actual patient needs is uncertain. Age limits may be used as a cutoff for access to certain higher-level therapies, such as mechanical circulatory support, but this likely represents a small proportion of overall transferred patients. Patients with more comorbid illness may be considered “unsalvageable” and not transferred as a result. There may be a role in establishing standardized, bias-free criteria for transfer within referral networks to address these discrepancies.
The association with higher hospital mortality among TH and SCH patients reported in our study is consistent with previous reports. A study of patients with NSTEMI in British Columbia demonstrated similar 1-year mortality between patients admitted to TH when compared with community hospitals, with increased rates of readmission due to ACS or death in patients originally admitted to TH.3 The reasons for these findings are unclear, and several explanations were proposed: (1) referral bias, with sicker patients being transferred to TH, and (2) higher rates of coronary artery bypass grafting among community hospital patients, which likely reduced the incidence of repeat revascularization.17 In addition, Hassan et al.18 previously showed that patients outside of urban areas had less access to percutaneous coronary interventions and bypass surgery, with longer wait times for these therapies, which may partially explain the increased incidence of mortality in SCH settings.
Our study suggests that HF was associated with high rates of hospital mortality—approximately 15%-20% depending on the hospital type—which is higher than in previously studied cohorts. HF is the most common admission diagnosis to acute care Canadian hospitals in our study and the third most common among transferred patients. A recent study of contemporary North American tertiary and quaternary CICUs showed that HF is the second most common reason for admission after ACS but represents the longest LOS and most frequent indication for CICU therapies.19 Moreover, HF was the second leading cause of CICU mortality, with an incidence of 12.0%. In a large retrospective analysis of 3.2 million HF hospitalizations in the United States between 2005 and 2015, all of which were patients 65 years and older, Wadhera et al.20 showed that 30-day mortality ranged from 7.7% to 9.5%. Among hospitalized patients with HF in Ontario, 30-day mortality was approximately 11%.21 Another review of data from Canadian CICUs showed that overall hospital mortality among patients with HF exceeds 20%, with significant interprovincial variability and a higher incidence of mortality at TH compared with other hospital types.22 Importantly, transferred patients were excluded from these analyses. In our study, outcomes among patients with HF are markedly worse than STEMI and NSTEMI. Patients with HF are being transferred for critical care support and therapies, as evidenced by the frequent admission to CCUs among transferred patients with HF, and these transferred patients likely have more severe illness as described above. Given that HF is a common clinical presentation that is increasing in frequency and represents a significant cause of morbidity, mortality, and CICU resource utilization, these findings should underscore existing efforts to identify patients at high risk of decompensation who are most likely to benefit from advanced CICU care.23,24
Despite the high prevalence of acute cardiovascular illness, there is little evidence to guide transfer practices for patients with conditions other than STEMI and NSTEMI with high-risk features.4,25 Our findings add to the evidence base in several important ways. First, we demonstrate that acute cardiovascular presentations other than ACS are common across all hospital types. Second, we showed that patients are infrequently transferred to higher level centres with approximately 10% of patients overall being referred. Our finding that transferred patients were younger, had less comorbid illness, and had lower hospital mortality than nontransferred patients suggests that transferred patients may represent a more “salvageable” patient population. Despite this, patient outcomes were often worse at TH, both for overall and for specific diagnoses. There are several potential explanations for this finding: the effect may largely be attributable to referral bias, with patients who are more critically ill referred for transfer, often later in their clinical course, and when therapeutic options may be limited. Furthermore, patients with less critical illness may be directed to LCH or MCH, which may explain why hospital mortality for many of the studied diagnoses is lower at these hospital types when compared with TH.
Cardiovascular professional societies, including the CCS, have identified a growing need for comprehensive CICU care and have outlined recommendations for organizational, staffing, and training among CICUs.12,26 The major CCS recommendations include a 3-tier classification of CICUs based on patient acuity and available resources.12 In short, level 1 CICUs are ideally suited to provide comprehensive care to patients with the most acute cardiovascular and noncardiovascular pathology. Most level 1 CICUs are located in urban, tertiary, and quaternary-care academic medical centres. The CCS has proposed a geographic hub-and-spoke model to centralize the care of the most acute patients to centres with optimal experience and staffing. However, the model does not specify any patient- or centre-specific transfer criteria. Although these data do not take into account the LOS at presenting hospitals before patient transfer, delays in transferring patients may have affected patient outcomes. Therefore, our results, as well as previous research demonstrating significant interprovincial variability in CICU admission practices, should prompt efforts to clarify transfer criteria that can accommodate both local practice and national standards for care to optimize patient outcomes at the regional and national level.
Limitations
Although our study aimed to understand national care patterns among patients with cardiovascular disease, there are several limitations. First, these results are based on retrospective data and are purely correlational, without patient-level data (including disease severity) available for adjustment; any association between transfer status and patient outcomes requires further prospective study. Second, we do not have clinical data, including therapies provided and markers of illness severity, which would have added details to clinical decisions made regarding transfer (vs nontransfer) and destination hospital selection. Third, our analysis of transferred patients was limited to those who spent over 24 hours at the destination hospital. This was designed to exclude patients who were transferred for procedures and subsequently returned to their original hospital, such as those with ACS who were transferred for percutaneous coronary interventions and were returned to the referring hospital shortly following afterward. It is possible, however, that transferred patients who died within 24 hours of arrival were unintentionally excluded from analysis, which would underestimate mortality data from TH and LCH (the 2 predominant destination hospitals). Fourth, transferred patients had to survive until hospital transfer. There were likely patients in the nontransferred group who died at the presenting hospital where transfer might have been indicated had they survived. Thus, there may be a potential survivor bias when comparing outcomes between transferred and nontransferred patients. Fifth, only the initial transfer during index hospitalization was included in the analysis. Subsequent interhospital transfers, such as back to the initial referring hospital, may impact outcomes. Thus, we ascertained outcomes at the end of index hospitalization regardless of the number of transfers. Lastly, our data reflect national transfer practice patterns and do not allow discrimination at a regional or provincial level, which remains to be studied. Moreover, these data represent a consolidation of patient data over a 5-year period, during which time new evidence may have led to improvements in standards of cardiac care. Further investigation into the effect of these optimizations on patient outcomes over time, if any, should be defined in future research.
Conclusions
Acute cardiovascular conditions are common reasons for hospital admission across Canada. Patients are infrequently transferred, and there is significant variability in transfer practices based on patient factors, presenting and destination hospital, and patient acuity. Diagnosis-specific outcomes vary significantly amongst transferred patients, and further prospective study is needed. Certain conditions, such as HF, may be associated with worse outcomes than previously reported. Further efforts to identify high-risk patients should be undertaken, with the goal of improving transfer criteria, particularly for patients with conditions other than ACS.
Acknowledgements
The authors would like to thank the staff at the Canadian Institute for Health Information for their assistance with data collection and preparation.
Funding Sources
The authors have no funding sources to declare.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: The research reported adheres to relevant ethical guidelines.
See page 545 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2020.07.003.
Supplementary Material
References
- 1.Healthcare Cost and Utilization Project (HCUP). HCUP Statistical Briefs—Hospitalizations Overview. 2018. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb_hospoverview.jsp Available at: [PubMed]
- 2.Statistics Canada Table 102-0561—Leading causes of death, total population, by age group and sex, Canada, annual, CANSIM (database) 2018. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310039401 Available at:
- 3.Sedlak T.L., Gao M., Lee M., Humphries K.H., Cairns J.A. Outcomes and transfer patterns for first non-ST-elevation myocardial infarction (NSTEMI): comparisons between community and tertiary care hospitals. Can J Cardiol. 2014;30:1562–1569. doi: 10.1016/j.cjca.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Wong G.C., Welsford M., Ainsworth C. 2019 Canadian Cardiovascular Society/Canadian Association of Interventional Cardiology Guidelines on the acute management of ST-elevation myocardial infarction: focused update on regionalization and reperfusion. Can J Cardiol. 2019;35:107–132. doi: 10.1016/j.cjca.2018.11.031. [DOI] [PubMed] [Google Scholar]
- 5.Nichol G., Aufderheide T.P., Eigel B. Regional systems of care for out-of-hospital cardiac arrest a policy statement from the American Heart Association. Circulation. 2010;121:709–729. doi: 10.1161/CIR.0b013e3181cdb7db. [DOI] [PubMed] [Google Scholar]
- 6.Wong G.C., van Diepen S., Ainsworth C. Canadian Cardiovascular Society/Canadian Cardiovascular Critical Care Society/Canadian Association of Interventional Cardiology position statement on the optimal care of the postarrest patient. Can J Cardiol. 2017;33:1–16. doi: 10.1016/j.cjca.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 7.Henry T.D., Sharkey S.W., Burke M.N. A regional system to provide timely access to percutaneous coronary intervention for ST-elevation myocardial infarction. Circulation. 2007;116:721–728. doi: 10.1161/CIRCULATIONAHA.107.694141. [DOI] [PubMed] [Google Scholar]
- 8.Le May M.R., So D.Y., Dionne R. A citywide protocol for primary PCI in ST-segment elevation myocardial infarction. N Eng J Med. 2008;358:231–240. doi: 10.1056/NEJMoa073102. [DOI] [PubMed] [Google Scholar]
- 9.Sinha S.S., Sjoding M.W., Sukul D. Changes in primary noncardiac diagnoses over time among elderly cardiac intensive care unit patients in the United States. Circ Cardiovasc Qual Outcomes. 2017;10 doi: 10.1161/CIRCOUTCOMES.117.003616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldfarb M., van Diepen S., Liszkowski M. Noncardiovascular disease and critical care delivery in contemporary cardiac and medical intensive care units. J Intensive Care Med. 2019;34:537–543. doi: 10.1177/0885066617741873. [DOI] [PubMed] [Google Scholar]
- 11.Berg D.D., Bohula E.A., van Diepen S. Epidemiology of shock in contemporary cardiac intensive care units. Circ Cardiovasc Qual Outcomes. 2019;12 doi: 10.1161/CIRCOUTCOMES.119.005618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le May M., van Diepen S., Liszkowski M. From coronary care units to cardiac intensive care units: recommendations for organizational, staffing, and educational transformation. Can J Cardiol. 2016;32:1204–1213. doi: 10.1016/j.cjca.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 13.van Diepen S., Bakal J.A., Lin M. Variation in critical care unit admission rates and outcomes for patients with acute coronary syndromes or heart failure among high- and low-volume cardiac hospitals. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.114.001708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canadian Institute for Health Information Discharge Abstract Database metadata. 2019. https://www.cihi.ca/en/discharge-abstract-database-metadata Available at:
- 15.O’Gara P.T., Kushner F.G., Ascheim D.D. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction. Circulation. 2013;127:e362–425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- 16.Mueller S., Zheng J., Orav E.J.P., Schnipper J.L. Inter-hospital transfer and patient outcomes: a retrospective cohort study. BMJ Qual Saf. 2019;28:e1. doi: 10.1136/bmjqs-2018-008087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alter D.A., Tu J.V., Austin P.C., Naylor C.D. Waiting times, revascularization modality, and outcomes after acute myocardial infarction at hospitals with and without on-site revascularization facilities in Canada. J Am Coll Cardiol. 2003;42:410–419. doi: 10.1016/s0735-1097(03)00640-5. [DOI] [PubMed] [Google Scholar]
- 18.Hassan A., Pearce N.J., Mathers J. The effect of place of residence on access to invasive cardiac services following acute myocardial infarction. Can J Cardiol. 2009;25:207–212. doi: 10.1016/s0828-282x(09)70062-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bohula E.A., Katz J.N., van Diepen S. Demographics, care patterns, and outcomes of patients admitted to cardiac intensive care units: The Critical Care Cardiology Trials Network Prospective North American Multicenter Registry of Cardiac Critical Illness. JAMA Cardiol. 2019;4:928–935. doi: 10.1001/jamacardio.2019.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wadhera R.K., Joynt Maddox K.E., Wasfy J.H. Association of the hospital readmissions reduction program with mortality among Medicare beneficiaries hospitalized for heart failure, acute myocardial infarction, and pneumonia. JAMA. 2018;320:2542–2552. doi: 10.1001/jama.2018.19232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donio P.J., Freitas C., Austin P.C. Comparison of readmission and death among patients with cardiac disease in Northern vs Southern Ontario. Can J Cardiol. 2019;35:341–351. doi: 10.1016/j.cjca.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 22.van Diepen S., Lin M., Ezekowitz J.A. Interprovincial differences in Canadian coronary care unit resource use and outcomes. Can J Cardiol. 2017;33:166–169. doi: 10.1016/j.cjca.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Verma S., Kaul P., Lin M. Patients with acute coronary syndromes and heart failure admitted to critical care units in teaching hospitals have lower observed inhospital mortality and 30-day readmission rates. Circulation. 2016;134:A15864. [Google Scholar]
- 24.Lee D.S., Ezekowitz J.A. Risk stratification in acute heart failure. Can J Cardiol. 2014;30:312–319. doi: 10.1016/j.cjca.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki N., Lee J., Park S. Development and validation of an acute heart failure-specific mortality predictive model based on administrative data. Can J Cardiol. 2013;29:1055–1061. doi: 10.1016/j.cjca.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 26.Amsterdam E.A., Wenger N.K., Brindis R.G. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes. J Am Coll Cardiol. 2014;64:e139–228. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.