Abstract
Background
First Nations (FN) peoples in Canada face spiraling rates of type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD). Data on the extent of CVD risk-factor management in FN peoples with T2DM in Canada are scarce.
Methods
A T2DM registry with data from 7 FN communities in Canada was utilized to identify individuals eligible for primary and secondary CVD prevention. Proportions of individuals meeting clinical practice guideline–specified targets (hemoglobin A1c ≤7.0%; blood pressure ≤130/80 mm Hg; low-density lipoprotein ≤2 mmol/L) were calculated. Prescription of recommended cardioprotective medications (antithrombotic medication, lipid-lowering agents, renin-angiotensin-aldosterone system inhibitors, and beta-blockers) among those with CVD was assessed. χ2 tests were employed to evaluate differences between CVD prevention groups and sexes.
Results
Of the 2098 individuals in the registry, 18% had documented CVD (female: male = 1.12). Overall, <10% met all 3 clinical practice guideline targets. Attainment of hemoglobin A1c and blood pressure targets was comparable between primary and secondary CVD prevention groups, with<50% achieving targets. A greater proportion of the secondary prevention group met low-density lipoprotein targets compared to those without CVD (61.6% vs 40.9%, P < 0.01). In the secondary prevention group, beta-blockers were prescribed to only 20%, and <60% were prescribed antithrombotics, lipid-lowering medications, or agents targeting the renin-angiotensin-aldosterone system; <2% were prescribed medications from all 4 classes of cardioprotective medications.
Conclusions
Primary and secondary CVD prevention recommendations for individuals with T2DM are not being met for an alarmingly high proportion of FN peoples. These findings serve as an urgent call for proactive measures to reduce CVD events and related mortality in this high-risk population.
Résumé
Contexte
Au Canada, chez les membres des Premières Nations, le nombre de cas de diabète de type 2 (DT2) et de maladie cardiovasculaire (MCV) monte rapidement. On a peu de données sur l’importance de la prise en charge des facteurs de risque de MCV chez les membres des Premières Nations atteints de DT2 au Canada.
Méthodologie
Un registre du DT2 comportant des données sur sept communautés canadiennes des Premières Nations a été utilisé pour repérer les personnes admissibles à la prévention primaire et secondaire de la MCV. Les proportions de personnes qui avaient atteint les cibles énoncées dans les lignes directrices de pratique clinique (HbA1c ≤ 7,0 %; pression artérielle ≤ 130/80 mm Hg; lipoprotéines de basse densité ≤ 2 mmol/L) ont été calculées. La prescription des médicaments cardioprotecteurs recommandés (antithrombotiques, hypolipidémiants, inhibiteurs du système rénine-angiotensine-aldostérone et bêtabloquants) chez les patients présentant une MCV a été évaluée. Des tests du χ2 ont été employés pour évaluer les différences entre les groupes de prévention de la MCV et les sexes.
Résultats
Parmi les 2 098 personnes figurant au registre, 18 % présentaient une MCV documentée (rapport femmes:hommes = 1,12). Moins de 10 % de l’ensemble de ces personnes avaient atteint les trois cibles énoncées dans les lignes directrices de pratique clinique. Du point de vue de l’atteinte des cibles relatives à l’HbA1c et à la pression artérielle, les groupes de prévention primaire et secondaire étaient comparables, moins de 50 % des personnes ayant atteint ces cibles. Une plus grande proportion des individus du groupe de prévention secondaire que de ceux exempts de MCV avait atteint la cible relative aux lipoprotéines de basse densité (61,6 % vs 40,9 %, p < 0,01). Dans le groupe de prévention secondaire, des bêtabloquants avaient été prescrits chez seulement 20 % des sujets et des antithrombotiques, des hypolipidémiants ou des médicaments ciblant le système rénine-angiotensine-aldostérone avaient été prescrits chez moins de 60 % des sujets; des médicaments des quatre classes de médicaments cardioprotecteurs avaient été prescrits chez moins de 2 % des individus.
Conclusions
Les recommandations en matière de prévention primaire et secondaire de la MCV chez les personnes atteintes de DT2 ne sont pas suivies dans une proportion alarmante des membres des Premières Nations. Les constatations faites montrent qu’on doit de toute urgence prendre des mesures proactives pour réduire le nombre de cas de MCV et la mortalité connexe dans cette population à risque élevé.
Cardiovascular disease (CVD) is a leading cause of death globally; combined ischemic heart disease and stroke accounted for 15.2 million deaths in 2016.1 In Canada, CVD is second to cancer as the leading cause of death, accounting for just over 25% of all mortality.2 CVD mortality has decreased in Canada in association with trends in improvements in risk-factor modifications and medical treatments.3 It is currently unclear if this progress extends to the Indigenous peoples in Canada, for whom the burden of CVD continues to exceed that for non-Indigenous Canadians.4, 5, 6 The inaugural First Nations Regional Health Survey showed that First Nations (FN) adults aged 50-59 years had a prevalence of self-reported heart disease greater than two-fold that of the general Canadian population (11.5% vs 5.5%, respectively).7 In the Canadian Census Mortality and Cancer Follow-up Study (1991-2006), a 2.3-fold (95% confidence interval [CI] 2.1, 2.6) increase in risk of avoidable mortality from diseases of the circulatory system in FN women, and a 1.6-fold (95% CI 1.5, 1.8) higher risk in FN men, compared with non-Indigenous populations, was reported.8 A recent study documented a decline in incident cardiac events among FN peoples with type 2 diabetes mellitus (T2DM) in Ontario, but rates continued to be significantly higher than those in non-FN individuals.6
The imbalanced burden of CVD between Indigenous and non-Indigenous populations in Canada stems from the higher prevalence of modifiable CVD risk factors among Indigenous peoples, such as poor glucose control, obesity, dyslipidemia, hypertension, and tobacco use.9, 10, 11 These inequities are rooted in historical, political, and psychosocial factors resulting from drastic changes in lifestyles compounded by poor living conditions, and limited access to healthy foods and health care.12,13 Many Indigenous communities were hastily transitioned from physically demanding and time-consuming food procurement methods (hunting, fishing, gathering) to more sedentary lifestyles and increased consumption of processed foods.14 These changes are fueling an epidemic of cardiometabolic disease and pose important challenges to these communities.
T2DM is a major risk factor for CVD, and these diseases share several modifiable risk factors and frequently coexist in patients. The First Nations Regional Health Survey (2008-2010) demonstrated that among individuals aged ≥ 55 years, those with T2DM reported higher proportions of stroke (10.4% vs 4.8%) and heart disease (29.1% vs 14.5%) than those without T2DM.15 T2DM prevalence may also be a greater risk factor for CVD in Indigenous women compared with men; the Strong Heart Study evaluated coronary heart disease in Indigenous Americans and found that T2DM was associated with greater than 2-fold higher prevalence rates (4.6 vs 1.8) in women compared with men.16 Given this elevated risk of CVD, there is a focus for primary prevention through a multifaceted approach including lifestyle changes (increased physical activity, optimized diet, and smoking cessation) as well as appropriate medications to attain glycemic, blood pressure (BP), and lipid targets to reduce CVD risk in individuals with T2DM.17 In individuals with T2DM and established CVD, secondary prevention recommendations are similar to those of primary prevention, with the addition of antithrombotic medications and use of antihyperglycemic agents with demonstrated cardiovascular outcome benefits to reduce the risk of subsequent cardiovascular events.17
Data on the extent of CVD risk-factor management and use of cardioprotective medications in Indigenous peoples in Canada with T2DM are scarce.6,18 The present analyses report modifiable risk-factor control in primary and secondary prevention of CVD, and prescription of cardioprotective medications for secondary prevention in a cross-section of FN peoples living on reserves across Canada.
Materials and Methods
Settings and individuals
Transformation of Indigenous Primary Healthcare Delivery (FORGE AHEAD [FA]) program, a Canadian, community-driven quality-improvement program to enhance T2DM care in FN communities, was initiated in 2013 and completed by 9 FN communities from across 5 provinces (British Columbia, Alberta, Manitoba, Quebec, Newfoundland and Labrador). Communities were recruited using a Web site and regional sharing of program information, and/or via personal communication by investigators who had preexisting partnerships with communities. There were 3 phases of the program: (i) national-level preparatory activities; (ii) development and implementation of community-driven quality-improvement interventions; and (iii) dissemination of results, which occurred within an 18-month period. Details of ethical approvals, governance, program components, and the knowledge translation plan have been published elsewhere.19,20 The program was implemented in 2 waves; the first began in October 2014, the second in June 2015. Communities from both waves were included in the current study.
Data collection
The FN Diabetes Surveillance System (FNDSS), a Web-based data collection tool developed by the Diabetes Alliance team at Western University,21 was a component of the FA program that allowed for the creation of a registry that enumerated all adults (aged ≥ 18 years) with T2DM in partnering communities. Local clinical data coordinators in each community using FNDSS (N = 8) were trained to populate the surveillance tool with demographic data, clinical measures, and prescription medications for each individual. Baseline values were identified as the latest value within the 12 months prior to starting the FA program in each community.19 The baseline data were examined in the analyses below. One community did not record baseline comorbidities, including CVD, as these data were not available in their electronic medical records. Individuals from this community were therefore excluded.
Outcome measures
Individuals were categorized into primary (no known CVD) and secondary (known CVD) CVD prevention groups. CVD status was determined by the presence or absence of ischemic heart disease and/or cerebrovascular disease diagnoses documented in FNDSS. Demographic data collected included age (years), sex, normal albuminuria (albumin-to-creatinine ratio ≤ 2 mg/mmol), estimated glomerular filtration rate (eGFR) ≥ 60 ml/min per 1.73 m2, body mass index ≤ 30 kg/m2, current smoking status, microvascular disease (neuropathy, nephropathy, and/or retinopathy), and hypertension diagnosis. Glycosylated hemoglobin (HbA1c), systolic and diastolic BP, and low-density lipoprotein (LDL) were also collected to assess management of cardiovascular risk factors per the Diabetes Canada Clinical Practice Guidelines.17
In individuals with CVD for which medication data were available, the presence or absence of the following was assessed: (i) antithrombotic medication (aspirin, clopidogrel, prasugrel, ticagrelor, and/or oral anticoagulant); (ii) lipid-lowering medication (statin and/or ezetimibe); (iii) medication targeting the renin-angiotensin-aldosterone system (RAAS); and (iv) beta-blockers. Additionally, individuals were categorized as receiving medications from all 4 classes of cardioprotective medications listed above.
Statistical analyses
All statistical analyses were conducted using R, version 3.5.1 (R Foundation for Statistical Computing). Proportions of individuals meeting specified targets (HbA1c ≤ 7.0%; BP ≤ 130/80 mm Hg, LDL ≤ 2.0 mmol/L), means of clinical measures (HbA1c; systolic BP; diastolic BP; LDL), and prescriptions for each class of cardioprotective medications were calculated for individuals with available data for each category. Comparisons between primary and secondary CVD prevention groups employed 2-tailed t tests to compare means, and χ2 tests to compare proportions. Analyses were also conducted stratified by sex.
Results
Cohort demographics
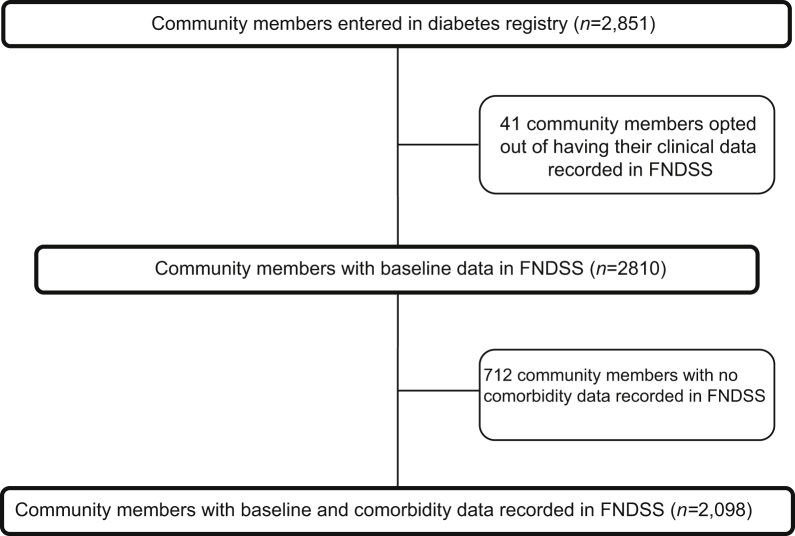
From the overall FA baseline cohort (N = 2098; 55.3% female) of FN individuals with T2DM living on reserves, CVD (ischemic heart disease and/or cerebrovascular disease) was documented in 17.9% (N = 376; Fig. 1). A total of 15.3% (n = 177) of women and 21.3% of men (n = 199) in the cohort had CVD diagnoses recorded. Among those with CVD, a greater proportion of women had been diagnosed with cerebrovascular disease (20.9% vs 13.6%), compared with men, but this difference was not statistically significant (Supplemental Table S1).
Figure 1.
Participant flow diagram. FNDSS, First Nations Diabetes Surveillance System.
Individuals with CVD were older than those without (69.2 vs 58.7 years, P < 0.01; Table 1), and almost half of the CVD cohort had been diagnosed with T2DM for over a decade, compared to less than a quarter of those without CVD. In individuals with CVD, women were older than men (70.9 vs 67.6 years, P = 0.02) and had a longer mean duration of T2DM than men with CVD (13.0 years [standard deviation: 8.4] vs 10.2 years [standard deviation: 7.0]; Supplemental Table S1). These differences were not seen in the primary prevention group.
Table 1.
Baseline characteristics of First Nations individuals with type 2 diabetes from communities participating in the Transformation of Indigenous Primary Healthcare Delivery (FORGE AHEAD) program, stratified by presence or absence of cardiovascular disease diagnosis
| Characteristic | No known cardiovascular disease | Known cardiovascular disease | P |
|---|---|---|---|
| Age (y), M (SD) | 58.7 (14.2) n = 1722 | 69.2 (13.2) n = 376 | < 0.01 |
| Diabetes mellitus diagnosed > 10 years ago | 22.0 (378) n = 1722 | 47.3 (178) n = 376 | < 0.01 |
| Estimated glomerular filtration rate ≥ 60 ml/min per 1.73 m2 | 86.1 (298) n = 346 | 74.0 (157) n = 212 | < 0.01 |
| Albumin-to-creatinine ratio ≤ 2.00 mg/mmol | 57.2 (281) n = 491 | 54.7 (116) n = 104 | 0.54 |
| Body mass index ≤ 30 kg/m2 | 34.0 (109) n = 321 | 32.5 (37) n = 114 | 0.77 |
| Microvascular disease | 5.6 (96) n = 1722 | 23.1 (87) n = 376 | < 0.01 |
| Diagnosed hypertension | 50.6 (550) n = 1722 | 81.0 (294) n = 376 | < 0.01 |
| Current smoker | 57.6 (329) n = 571 | 48.1 (90) n = 187 | 0.03 |
Values are % (n), unless otherwise indicated. n = indicates total n with data available.
M, mean; SD, standard deviation.
Fewer individuals with CVD had an eGFR ≥ 60 ml/min per 1.73 m2, compared to individuals without CVD (74.0% vs 86.1%, P < 0.01; Table 1). In individuals with CVD, a smaller proportion of women had an eGFR ≥60 ml/min per 1.73 m2, compared to men (65.5% vs 84.8%, P < 0.01; Supplemental Table S1). Over half of the cohort had an albumin-to-creatinine ratio ≤ 2.0 mg/mmol, and roughly two-thirds were obese in both the primary and secondary CVD prevention groups, with no difference between sexes.
Diagnoses of microvascular disease (23.1% vs 5.6%, P < 0.01) and hypertension (81.0% vs 50.6%, P < 0.15) were more prevalent in individuals with CVD compared to individuals without CVD (Table 1). Notably, a larger proportion in the primary prevention group were current smokers, compared to the secondary prevention group (57.6 % vs 48.1% P = 0.03). In the primary prevention group, a larger proportion of men were current smokers (63.7% vs 52.6%, P < 0.01) and had been diagnosed with hypertension (56.0% vs 46.4%, P < 0.01) compared to women (Supplemental Table S1).
Cardiometabolic risk factors
Overall, less than 50% of all individuals met HbA1c and BP targets (Table 2). A larger proportion of women with CVD met HbA1c targets, compared to men (Supplemental Table S2). BP targets were met by a greater number of women compared to men in both the CVD and no CVD groups. Among individuals with CVD, approximately two-thirds of individuals met LDL targets, whereas approximately 40% of those without CVD had an LDL ≤2 mmol/L. Less than 10% of the cohort achieved all 3 guideline-recommended targets, and there was no significant difference between the primary and secondary prevention groups (6.8% vs 8.8%, P = 0.33) or between sexes. Women with CVD had the highest proportion of individuals meeting all 3 targets, which was significantly higher than for women without CVD (11.0% vs 7.5%, P < 0.01).
Table 2.
Proportion of First Nations individuals with type 2 diabetes from communities participating in the Transformation of Indigenous Primary Healthcare Delivery (FORGE AHEAD) program attaining cardiometabolic targets∗ stratified by presence or absence of cardiovascular disease diagnosis
| Target | Noknown cardiovascular disease | Known cardiovascular disease | P |
|---|---|---|---|
| Hemoglobin A1c ≤ 7.00% | 48.4 (445) n = 919 | 45.4 (133) n = 293 | 0.37 |
| Blood pressure ≤ 130/80 mm Hg | 30.0 (289) n = 961 | 33.0 (104) n = 315 | 0.33 |
| Low-density lipoprotein ≤ 2.00 mmol/L | 40.9 (303) n = 741 | 61.6 (165) n = 268 | < 0.01 |
| All 3 on target | 6.8 (41) n = 604 | 8.7 (21) n = 241 | 0.33 |
Values are % (n), unless otherwise indicated. n = indicates total n with data available.
Diabetes Canada Clinical Practice Guideline recommended targets.17
Mean clinical risk factor measures were all above guideline-recommended thresholds, except for mean diastolic BP in both groups, and LDL in individuals with CVD diagnoses (Table 3). Individuals with CVD had a lower mean diastolic BP and LDL level compared to those without CVD; otherwise, mean clinical risk factor measures were comparable between the 2 groups. There were no significant differences in the means of clinical measures between sexes, aside from a small but statistically significant difference in systolic and diastolic BP in individuals without CVD, and in diastolic BP in those with CVD (Supplemental Table S3).
Table 3.
Median and mean diabetes-related clinical measures for First Nations individuals with type 2 diabetes from communities participating in the Transformation of Indigenous Primary Healthcare Delivery (FORGE AHEAD), stratified by presence or absence of cardiovascular disease diagnosis
| Measure | No known cardiovascular disease |
Known cardiovascular disease |
P∗ | ||
|---|---|---|---|---|---|
| Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | ||
| Hemoglobin A1c, % | 7.1 (6.3, 9.0) n = 919 | 7.9 (2.6) n = 919 | 7.2 (6.4, 8.4) n = 293 | 7.9 (4.7) n = 293 | 0.37 |
| Systolic blood pressure, mm Hg | 133.0 (122.0, 146.0) n = 961 | 134.9 (17.5) n = 961 | 133.0 (120.5,149.0) | 136.5 (20.3) n = 315 | 0.33 |
| Diastolic blood pressure, mm Hg | 78.0 (71.0, 84.0) n = 741 | 77.7 (10.3) n = 741 | 72.0 (65.0, 80.0) | 72.0 (10.3) n = 268 | < 0.01 |
| Low-density lipoprotein, mmol/L | 2.3 (1.7, 3.0) n = 604 | 2.4 (1.0) n = 604 | 1.80 (1.5, 2.3) | 2.0 (0.8) n = 241 | < 0.01 |
n = indicates total n with data available.
IQR, interquartile range; SD, standard deviation.
For 2-tailed t test comparing means.
Cardioprotective medications in secondary prevention
Medication data were available for 43.6% (n = 164) of individuals with CVD. Among individuals with CVD, those for whom medication data were available were younger (62.0 vs 74.7 years, P < 0.01), had a lower proportion of microvascular disease diagnoses (12.8% vs 31.1%, P < 0.01), and had a smaller proportion of smokers (28.6% vs 88.5%, P < 0.01) compared to those for whom medication data were not available (Supplemental Table S4). Moreover, the cohort with medication data available had a greater proportion of ischemic heart disease (95.7% vs 89.6, P = 0.03) and less cerebrovascular disease (9.8% vs 22.6%, P < 0.01) compared to those without medication data.
Among individuals with CVD and prescription data available, <60% were prescribed an antithrombotic, lipid-lowering medication or agent targeting RAAS, and beta-blockers were only prescribed to 20% of these individuals (Table 4). Overall, <2% of these individuals had a drug from each of the 4 classes of recommended cardioprotective medication for secondary prevention prescribed. There were no significant differences between sexes.
Table 4.
Proportion of First Nations individuals with type 2 diabetes and cardiovascular disease prescribed medications appropriate for secondary prevention for cardiovascular disease from communities participating in the Transformation of Indigenous Primary Healthcare Delivery (FORGE AHEAD) program
| Medication category | Individuals with cardiovascular disease (Total n = 164) | Women with cardiovascular disease (Total n = 73) | Men with cardiovascular disease (Total n = 91) | P |
|---|---|---|---|---|
| Aspirin or other antithrombotic | 59.2 (97) | 54.8 (40) | 62.6 (57) | 0.31 |
| Statin or other lipid-lowering medication | 48.2 (79) | 52.1 (38) | 45.1 (41) | 0.37 |
| Renin-angiotensin-aldosterone system inhibitor | 57.3 (94) | 56.2 (41) | 58.2 (53) | 0.79 |
| Beta-blocker | 20.1 (33) | 16.4 (12) | 23.1 (21) | 0.29 |
| All 4 classes of medications | 1.8 (3) | 2.7 (2) | 1.1 (1) | 0.44 |
Values are % (n), unless otherwise indicated.
Discussion
In a cross section of FN peoples with T2DM living on a reserve, a population at high risk of CVD, suboptimal management of CVD risk factors in individuals for both primary and secondary CVD prevention was demonstrated. Less than half met glycemic targets; only a third had adequately controlled BP; and in individuals without CVD, less than half met LDL targets. Overall, <10% of the cohort met all 3 clinical targets. Additionally, among individuals with documented CVD and available medication data (43.6% of the CVD cohort), <2% were prescribed all 4 classes of cardioprotective medications. These results highlight a significant care gap in preventing and managing CVD in FN individuals with T2DM in Canada.
Nearly a decade ago, the Canadian First Nations Diabetes Clinical Management and Epidemiologic study (CIRCLE), a cross-sectional study documenting the clinical management of T2DM and related complications in FN peoples in Canada, revealed major care gaps in 19 FN communities.22 In the current study, a smaller proportion of our cohort was meeting BP targets (30.7% vs 34.7%), compared to the population in the CIRCLE study; however, we demonstrate a roughly 10% increase in the proportion of FN individuals with T2DM meeting glycemic (47.7% vs 38.9%) and LDL (46.4% vs 35.2%) targets, compared to that in the CIRCLE study. Although this increase illustrates some small improvement in risk-factor management over time, significant disparities continue to exist between FN populations and the general Canadian population. In a cross-section of T2DM patients (<10% Indigenous) followed by primary care physicians in Canada, the Diabetes Mellitus Status in Canada (DM-SCAN) study documented comparable, if not slightly higher, proportions of attainment of guideline-recommended targets in patients with and without coronary artery disease (CAD).23 Akin to the findings in the current study, Grenier and colleagues23 found that LDL targets were more likely to be met in patients with CAD than in those without CAD (66.0% vs 54.5%, P < 0.01), and there was no difference between groups in meeting HbA1c targets (48.5% vs 50.5%, P = 0.24). Divergent from the findings of Grenier and colleagues indicating that patients with CAD were more likely to achieve BP targets (39.1% vs 35.8%, P = 0.05), individuals with CVD in our study were not more likely to have attained BP targets (33.0% vs 30.1%, P = 0.33). Moreover, patients with CAD were more likely to meet all 3 recommended targets, compared to those without CAD (15.4% vs 12.0%, P < 0.01) in the DM-SCAN study. In our cross section of FN individuals, not only was there no difference in attaining all 3 targets between the CVD and no CVD groups (8.8% vs 6.8%, P = 0.33), but the proportion of individuals meeting all 3 targets was roughly half that in the general Canadian population in the DM-SCAN study. The importance of targeting not only glycemic but also cardiovascular risk factors was underlined in Steno-2 study, a randomized controlled trial that showed that multifactorial, target-driven treatment of cardiovascular risk factors in patients with T2DM and microalbuminuria reduced cardiovascular events and mortality.24 Moreover, in a cohort of 26,636 patients with T2DM, Nichols and colleagues showed that patients who met HbA1c, BP, and LDL targets had lower rates of hospitalization for CVD compared to those who did not meet targets or met only HbA1c targets.25
Smoking is an important risk factor for CVD. Higher rates of smoking in Indigenous populations, compared to the general population, have been well documented and acknowledged as a significant public health issue, yet this disparity remains.26 Over half of the individuals in our baseline cohort with smoking status documented were active smokers. This percentage is significantly higher than the ∼10% reported in the general population with T2DM from the DM-SCAN study.23 The proportion of smokers in the current study is also higher than the ∼40% documented in Indigenous communities in the Study of Health Assessment and Risk in Ethnic Groups and Aboriginal Peoples (SHARE-AP) and the CIRCLE study over a decade ago.10,22 The proportion of active smokers in FA communities was greater in FN men than women. Notably, the global population-attributable fraction of CVD for men with T2DM is 4-fold higher than that in women with T2DM.27 Reassuringly, smoking cessation is accompanied by a reduction in risk of CVD, in comparison to ongoing smoking, and its effects are similar in both sexes.28 Programs to promote smoking cessation among Indigenous adults are imperative to curb CVD rates.29 Through the Canadian government’s FN and Inuit Component of the Federal Tobacco Control Strategy, 16 smoking-cessation projects in Indigenous communities have been funded, but their impact has yet to be reported.30
In addition to appropriate risk-factor management, the ability of cardioprotective medications to improve health outcomes in individuals with CVD has long been established.3 Regrettably, the proportion of FN individuals with T2DM and CVD receiving antithrombotics was 7.8% less than that in the general population of Canada, in the DM-SCAN study (59.2% vs 67.0%), and statins, RAAS inhibitors, and beta-blockers were prescribed 39.9% (48.2% vs 88.1%), 31.7% ( 57.3% vs 89.0%), and 26.8% (20.1% vs 46.9%) less, respectively.22 In contrast, a recent study found that in FN peoples aged ≥65 years with T2DM, living in Ontario, >60% had prescriptions for each class of cardioprotective medication.6 These higher proportions may be due to restricting the analyses to an older population with increased drug coverage. Of note, our study did not include any communities from Ontario, and among our cohort with CVD, those with medication data available were younger than those for whom we did not have this information. The low rates of medical management of CVD in the high-risk population represented in the current study are especially concerning given studies showing low rates of revascularization and worse outcomes in FN populations; in a cohort of patients undergoing coronary angiography in Alberta, FN individuals were less likely to receive coronary angiography within 24 hours of an acute myocardial infarct (odds ratio 0.7; 95% CI 0.6, 0.9) and had higher mortality (hazard ratio 1.4; 95% CI 1.1, 1.8) after angiography compared to non-FN individuals.31 In Ontario, revascularization rates in FN peoples recently have been shown to be increasing.6
In the current study, FN women with CVD have better glycemic and BP control than men, which is in contrast to a recent review of non-Indigenous populations.32 Al-Salameh and colleagues also reported higher LDL levels and an underuse of aspirin in women with T2DM, compared to men.32 Additionally, several studies in the general population have suggested that adherence to statins, antihypertensive drugs, and insulin is lower in women than in men.33, 34, 35 Although we did not address adherence, the better glycemic and BP control in women with CVD in the FA cohort could suggest greater adherence to medication and lifestyle changes in FN women, compared to men, which is in contrast to findings among non-Indigenous populations.
Disparities in CVD management in Indigenous communities are rooted in barriers to access to medications and services due to fractured health care delivery.36 Medical mistrust has also been reported frequently in Indigenous studies and needs to be addressed to deliver optimal care.37,38 Recently, Anand and colleagues demonstrated that within FN communities across Canada, communities with greater trust among community members, higher socioeconomic status, higher social support, and higher education had lower rates of CVD risk factors.39 Poorer medication literacy also positions Indigenous peoples at a disadvantage in receiving care; in an urban Indigenous health centre, Smylie and colleagues found that individuals with or at high risk of CVD had low CVD medication knowledge, placing them at risk for medication errors.9 Encouragingly, implementation of an education program, which was delivered by a trained Indigenous nurse and included a tablet application, pill card, and booklet, was demonstrated to a significantly improve medication knowledge.9
This study has some limitations. Surveillance health data regarding Indigenous peoples in Canada are substandard, largely due to the failure to engage Indigenous peoples in partnerships to govern and manage their data.40 FNDSS, a tool developed by working directly with communities, was used to collect the data in this study; however, further refinements are needed to best suit community needs and resources. In this study using FNDSS, we did not have complete data for all individuals, and we only considered individuals for whom the variable of interest was recorded; <50% of participants with CVD had medication data available, and only ∼20% had BMI data recorded. This finding in part highlights the inconsistency in documentation of medications and conditions, a common gap found in many Indigenous communities and one of the drivers for establishing a disease registry. Modifications are currently underway to improve the usability of FNDSS and allow communities to interpret/use their own health information, as they deem appropriate.41 Although we had medication data for only 43.6% of the CVD cohort, those for whom we did have prescription data were younger, had less microvascular disease, and included more nonsmokers, suggesting that this cohort was “healthier” than those without medication data; this could bias our data on the proportion of FN individuals with T2DM and CVD receiving appropriate medication for secondary prevention. Additionally, CVD diagnoses were based on chart review by local clinical data coordinators, but we did not have information on how these diagnoses were achieved. Any diagnoses or labs not available in the community chart could not be entered in the FNDSS database. Further, the data were collected prior to the addition of sodium–glucose cotransporter-2 inhibitors and glucagon-like peptide–1 receptor agonist to the Non-Insured Health Benefits (NIHB) Program, through which many FN individuals receive prescription medications. We therefore could not assess if these diabetes medications with proven CVD outcome benefits, which should be a key prescription option for this high-risk population moving forward, were being prescribed. In spite of these limitations, it is clear that there continues to be a substantial gap in CVD prevention in FN in Canada.
Conclusions
A disheartening proportion of FN peoples with T2DM living on a reserve are not attaining targets for modifiable CVD risk factors. This study underscores areas for which there is need for greater CVD risk-factor control and highlights that Indigenous communities need to be supported to develop CVD prevention and management interventions tailored to their cultural, social, historical, and geographic contexts to improve CVD outcomes.
Funding Sources
This work was supported by the Canadian Institutes of Health Research (funding reference numbers #MCO 117675, #297910, and #PME-133824), a grant from AstraZeneca Canada Inc., and the Lawson Foundation.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: Ethics approval for the FORGE AHEAD Program was granted by Western University Health Sciences Research Ethics Board (#103895, approved June 17, 2013), the Health Research Ethics Board of Alberta (CHC-14-0054, approved December 1, 2014), the Cree Board of Health and Social Services of James Bay (#2014-DSP-03, approved October 2, 2014), Mi’kmaw Ethics Watch, Unama’ki College, Cape Breton University (approved January 29, 2014), and Mi’kmaq Confederacy Ethics Review Committee, Prince Edward Island (approved March 14, 2014).
See page 553 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2020.07.004.
Supplementary Material
References
- 1.World Health Organization The top 10 causes of death. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death Available at: Accessed August 28, 2020.
- 2.Statistics Canada The 10 leading causes of death. https://www150.statcan.gc.ca/n1/pub/82-625-x/2014001/article/11896-eng.htm Available at: Accessed August 28, 2020.
- 3.Wijeysundera H.C., Machado M., Farahati F. Association of temporal trends in risk factors and treatment uptake with coronary heart disease mortality, 1994-2005. JAMA. 2010;303:1841–1847. doi: 10.1001/jama.2010.580. [DOI] [PubMed] [Google Scholar]
- 4.Tobe S.W., Maar M., Roy M.A., Warburton D.E.R. Preventing cardiovascular and renal disease in Canada’s aboriginal populations. Can J Cardiol. 2015;31:1124–1129. doi: 10.1016/j.cjca.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 5.Reading J. Confronting the growing crisis of cardiovascular disease and heart health among aboriginal peoples in Canada. Can J Cardiol. 2015;31:1077–1080. doi: 10.1016/j.cjca.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 6.Chu A., Han L., Roifman I. Trends in cardiovascular care and event rates among First Nations and other people with diabetes in Ontario, Canada, 1996–2015. Can Med Assoc J. 2019;191:E1291. doi: 10.1503/cmaj.190899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.First Nations Information Governance Centre . First Nations Centre; Ottawa, Canada: 2005. First Nations Regional Longitudinal Health Survey (RHS) 2002/03: Results for Adults, Youth and Children Living in First Nations Communities. [Google Scholar]
- 8.Park J., Tjepkema M., Goedhuis N., Pennock J. Avoidable mortality among First Nations adults in Canada: A cohort analysis. Health Rep. 2015;26:10–16. [PubMed] [Google Scholar]
- 9.Smylie J., O’Brien K., Xavier C.G. Primary care intervention to address cardiovascular disease medication health literacy among Indigenous peoples: Canadian results of a pre-post-design study. Can J Public Health. 2018;109:117–127. doi: 10.17269/s41997-018-0034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anand S.S., Yusuf S., Jacobs R. Risk factors, atherosclerosis, and cardiovascular disease among aboriginal people in Canada: the Study of Health Assessment and Risk Evaluation in Aboriginal Peoples (SHARE-AP) Lancet. 2001;358:1147–1153. doi: 10.1016/s0140-6736(01)06255-9. [DOI] [PubMed] [Google Scholar]
- 11.Yusuf S., Reddy S., Ounpuu S., Anand S. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104:2746–2753. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 12.Gracey M., King M. Indigenous health part 1: determinants and disease patterns. Lancet. 2009;374:65–75. doi: 10.1016/S0140-6736(09)60914-4. [DOI] [PubMed] [Google Scholar]
- 13.Daniel M., Lekkas P., Cargo M. Environments and cardiometabolic diseases in aboriginal populations. Heart, Lung Circ. 2010;19:306–315. doi: 10.1016/j.hlc.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Kuhnlein H.V., Receveur O. Dietary change and traditional food systems of indigenous peoples. Annu Rev Nutr. 1996;16:417–442. doi: 10.1146/annurev.nu.16.070196.002221. [DOI] [PubMed] [Google Scholar]
- 15.First Nations Information Governance Centre (FNIGC) FNIGC; 2012. First Nations Regional Health Survey (RHS) 2008/10: National Report on Adults, Youth and Children Living in First Nations Communities. [Google Scholar]
- 16.Howard B.V., Lee E.T., Fabsitz R.R. Diabetes and coronary heart disease in American Indians: the Strong Heart Study. Diabetes. 1996;45(suppl 3):S6–S13. doi: 10.2337/diab.45.3.s6. [DOI] [PubMed] [Google Scholar]
- 17.Stone J.A., Houlden R.L., Lin P., Udell J.A., Verma S., Diabetes Canada Clinical Practice Guidelines Expert Committee Cardiovascular protection in people with diabetes. Can J Diabetes. 2018;42(suppl 1):S162–S169. doi: 10.1016/j.jcjd.2017.10.024. [DOI] [PubMed] [Google Scholar]
- 18.Gasevic D., Ross E.S., Lear S.A. Ethnic differences in cardiovascular disease risk factors: a systematic review of North American evidence. Can J Cardiol. 2015;31:1169–1179. doi: 10.1016/j.cjca.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 19.Naqshbandi Hayward M., Paquette-Warren J., Harris S.B., Team F.A.P. Developing community-driven quality improvement initiatives to enhance chronic disease care in Indigenous communities in Canada: the FORGE AHEAD program protocol. Health Res Policy Syst. 2016;14:55. doi: 10.1186/s12961-016-0127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naqshbandi Hayward M., Pace R., Zaran H. Closing the indigenous health gap in Canada: results from the TransFORmation of IndiGEnous PrimAry HEAlthcare Delivery (FORGE AHEAD) Program. Diabetes Res Clin Pract. 2020;162:108066. doi: 10.1016/j.diabres.2020.108066. [DOI] [PubMed] [Google Scholar]
- 21.Hayward M.N., Harris S.B., Caruso R. Evaluation of a web-based diabetes surveillance system for first nations. CanJ Diabetes. 2012;36:S33. [Google Scholar]
- 22.Harris S.B., Naqshbandi M., Bhattacharyya O. Major gaps in diabetes clinical care among Canada’s First Nations: results of the CIRCLE study. Diabetes Res Clin Pract. 2011;92:272–279. doi: 10.1016/j.diabres.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Grenier J., Leiter L.A., Langer A. Glycaemic control and cardiovascular risk factor management in patients with diabetes with and without coronary artery disease: insights from the diabetes mellitus status in Canada survey. Eur Heart J Qual Care Clin Outcomes. 2016;2:277–284. doi: 10.1093/ehjqcco/qcw013. [DOI] [PubMed] [Google Scholar]
- 24.Gaede P., Oellgaard J., Carstensen B. Years of life gained by multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: 21 years follow-up on the Steno-2 randomised trial. Diabetologia. 2016;59:2298–2307. doi: 10.1007/s00125-016-4065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nichols G.A., Joshua-Gotlib S., Parasuraman S. Independent contribution of A1C, systolic blood pressure, and LDL cholesterol control to risk of cardiovascular disease hospitalizations in type 2 diabetes: an observational cohort study. J Gen Intern Med. 2013;28:691–697. doi: 10.1007/s11606-012-2320-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gionet L., Roshanafshar S. Statistics Canada; Ottawa: 2013. Select Health Indicators of First Nations People Living Off Reserve, Métis and Inuit. [Google Scholar]
- 27.Pan A., Wang Y., Talaei M., Hu F.B. Relation of smoking with total mortality and cardiovascular events among patients with diabetes mellitus: a meta-analysis and systematic review. Circulation. 2015;132:1795–1804. doi: 10.1161/CIRCULATIONAHA.115.017926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blomster J.I., Woodward M., Zoungas S. The harms of smoking and benefits of smoking cessation in women compared with men with type 2 diabetes: an observational analysis of the ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation) trial. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2015-009668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chamberlain C., Perlen S., Brennan S. Evidence for a comprehensive approach to Aboriginal tobacco control to maintain the decline in smoking: an overview of reviews among Indigenous peoples. Syst Rev. 2017;6:135. doi: 10.1186/s13643-017-0520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Health Canada First Nations and Inuit component of the federal tobacco control strategy. 2020. https://www.canada.ca/en/health-canada/services/publications/healthy-living/canada-tobacco-strategy.html Available at: Accessed August 28, 2020.
- 31.Bresee L.C., Knudtson M.L., Zhang J. Likelihood of coronary angiography among First Nations patients with acute myocardial infarction. CMAJ. 2014;186:E372–E380. doi: 10.1503/cmaj.131667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Salameh A., Chanson P., Bucher S., Ringa V., Becquemont L. Cardiovascular disease in type 2 diabetes: a review of sex-related differences in predisposition and prevention. Mayo Clin Proc. 2019;94:287–308. doi: 10.1016/j.mayocp.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Woodward M., Peters S.A., Huxley R.R. Diabetes and the female disadvantage. Women’s Health (Lond) 2015;11:833–839. doi: 10.2217/whe.15.67. [DOI] [PubMed] [Google Scholar]
- 34.Lewey J., Shrank W.H., Bowry A.D. Gender and racial disparities in adherence to statin therapy: a meta-analysis. Am Heart J. 2013;165:665–678. doi: 10.1016/j.ahj.2013.02.011. 678.e1. [DOI] [PubMed] [Google Scholar]
- 35.Gupta P., Patel P., Strauch B. Risk factors for nonadherence to antihypertensive treatment. Hypertension. 2017;69:1113–1120. doi: 10.1161/HYPERTENSIONAHA.116.08729. [DOI] [PubMed] [Google Scholar]
- 36.Harris S.B., Tompkins J.W., TeHiwi B. Call to action: a new path for improving diabetes care for Indigenous peoples, a global review. Diabetes Res Clin Pract. 2017;123:120–133. doi: 10.1016/j.diabres.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 37.Jacklin K.M., Henderson R.I., Green M.E. Health care experiences of Indigenous people living with type 2 diabetes in Canada. CMAJ. 2017;189:E106–E112. doi: 10.1503/cmaj.161098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Auger M., Howell T., Gomes T. Moving toward holistic wellness, empowerment and self-determination for Indigenous peoples in Canada: Can traditional Indigenous health care practices increase ownership over health and health care decisions? Can J Public Health. 2016;107:e393–e398. doi: 10.17269/CJPH.107.5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anand S.S., Abonyi S., Arbour L. Explaining the variability in cardiovascular risk factors among First Nations communities in Canada: a population-based study. Lancet Planet Health. 2019;3:e511–e520. doi: 10.1016/S2542-5196(19)30237-2. [DOI] [PubMed] [Google Scholar]
- 40.Smylie J., Firestone M. Back to the basics: identifying and addressing underlying challenges in achieving high quality and relevant health statistics for indigenous populations in Canada. Stat J IAOS. 2015;31:67–87. doi: 10.3233/SJI-150864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pace R., Zaran H., Harris S. Morressier; Busan, South Korea: 2019. Implementation and evaluation of a diabetes registry in First Nation communities in Canada from the FORGE AHEAD study. International Diabetes Federation Congress. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.