Abstract
Background: Colorectal cancer counts as the third prevalent type of cancer and the fourth cause of death worldwide. The second-generation colon capsule endoscopy (CCE-2) is a new technology for the diagnosis of colon cancer. The aim of this review was to provide information on the diagnostic accuracy (diagnostic effectiveness) of the second-generation colon capsule endoscopy compared to colonoscopy for the diagnosis of colon cancer and disorders.
Methods: A systematic review of literature in PubMed, Scopus, Science Direct, and Cochrane Library and Iranian databases, such as MagIran, SID, Irandoc, the grey literature (via Google Scholar) was conducted on February 30, 2018. QUADAS-2 was used to assess the quality of the studies. MetaDiSc 2.0 software was used for the meta-analysis.
Results: In this review, 480 records were identified. Eight prospective cohort articles were included among which 7 included in the meta-analysis. For the diagnosis of colorectal polyps with a diameter of 6-10 mm, the pooled sensitivity and specificity were 84% (95% CI, 80% -88%) and 88% (95%CI, 85% -90%). For the diagnosis of 10 mm or bigger colorectal polyps, the pooled sensitivity and specificity were 84% (95% CI, 76%-89%) and 96% (95% CI, 94 %-97%). The sensitivity and specificity of the capsule in the detection of any size polyps were 93% (95% CI, 97%-84%) and 66% (95% CI, 48%-81%), respectively.
Conclusion: There is little evidence to show the accuracy of CCE-2. Nevertheless, this review showed that the second-generation colon capsule endoscopy has good accuracy in the detection of polyps and colorectal cancer among high- and middle-risk patients.
Keywords: Colon capsule endoscopy, Colonic polyp, Accuracy, CCE-2, Colonoscopy
↑ What is “already known” in this topic:
Colorectal polyps are usually diagnosed through colonoscopy, while colon capsule endoscopy is introduced during recent years for the same indication.
→ What this article adds:
Colon capsule endoscopy has better accuracy than colonoscopy in detecting colon polyps. When compared with colonoscopy, colon capsule endoscopy ends with a better experience in patients and can improve their satisfaction with the screening and diagnosis process.
Introduction
Colorectal cancer counts as the third prevalent type of cancer and is the fourth cause of death across the world (1-3). This type of cancer usually causes delayed symptoms; hence early diagnosis through regular screening programs are very critical (4). Screening of colorectal cancer can identify cancer, pre-cancerous polyps and other abnormal disorders. The earlier is cancer detected, the easier and less costly is the treatment (5). By screening, the detection of pre-cancerous polyps before the onset of the disease can lead to the treatment of cancer in the early stages and before it is transmitted to the other parts of the body. Therefore, the best method for cancer prevention is the early diagnosis and treatment of abnormal disorders and polyps (5, 6).
Among the available methods for diagnosis of colorectal disorders, colonoscopy is known as the standard test (7). Most of the published guidelines recommend one colonoscopy per 10 years of life among people aged 50 and over (8). But due to the complications that can be caused by colonoscopy, including bleeding and perforation, it is a painful and embarrassing process, and implementation of the instruction is less welcomed by patients (5, 7). The second-generation colon capsule endoscopy (CCE-2) is a new technology for the diagnosis of colon cancer, based on the development of previous technology and clinical experience with the use of a narrow intestinal endoscopy capsule. This technology is presented as a non-invasive substitution method for large bowel examination. When it is used, it does not require sedative and analgesic procedures (9). Considering the importance of fast and accurate diagnosis of polyps and colon cancer and the use of an appropriate and effective method, the aim of this review is to provide information on the diagnostic accuracy (diagnostic effectiveness) of the second-generation colon capsule endoscopy compared to colonoscopy for the diagnosis of colon cancer and disorders.
Methods
Since the second generation of colon capsule endoscopy was produced in 2006, after the first generation of colon capsule endoscopy (10), a systematic review of the literature including databases and website including PubMed, Scopus, Science Direct and Cochrane Library, Iranian databases, such as MagIran, SID, Irandoc and the grey literature (via Google Scholar) was done from December 27, 2006 to February 30, 2018 (the strategy for each database is listed in Appendix 1). The Medical Subject Heading (MeSH) was used to construct the search strategy. In order to achieve the maximum number of available studies, in addition to the electronic search, hand searching was also carried out. To select the appropriate studies, two individuals independently reviewed the records and disagreements were discussed. Articles of conferences and papers other than original research were removed from our study. After completing the search, all of the published articles were transferred into EndNote X8 software, and then the duplicates were removed.
Inclusion criteria
Population: Individuals suspected of colon polyps with indication for second-generation colon capsule endoscopy and colonoscopy simultaneously.
Intervention
The second-generation colon capsule endoscopy Comparison: Colonoscopy Outcome: The desired outcome was the accuracy of the procedures in the detection of cancer and colon polyps (sensitivity and specificity and positive predictive value and negative predictive value).
Study Design
Diagnostic studies and all observational studies that performed to check the accuracy of the diagnostic tests of the two devices.
Exclusion criteria
Studies that investigated the treatment of diseases rather than colon cancer and colon polyps, along with studies that used other interventions (such as CCE-1) were excluded. Also, non-English language studies were not examined.
Quality assessment
Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) checklist (11) was used to examine the quality of the selected studies. Quality assessment criteria include patient selection bias, index test results bias, reference, and follow-up test results, and appropriate timing of tests. The table of the quality assessment of the studies is provided in Appendix 2.To assess the quality of the studies, two individuals independently reviewed the records and discussed the disagreement.
Data Extraction
To collect information from the included articles, a pre-designed checklist was used to extracted data. After completing, the table was checked by a second author. The data extraction table contained title, author, year of publication, journal name, setting of the study, study population, type of study, number of subjects studied, prevalence of polyps, level of bowel cleansing, outcomes (i.e., sensitivity or specificity), conclusion and the quality of the study. Then, based on the inclusion and exclusion criteria, the articles were selected and after studying the title of records, some of the irrelevant articles were excluded, also after checking the abstracts at this stage, a number of other articles were excluded. Moreover, based on the full text of the articles some of the articles were excluded. After these assessments, remaining records were critically reviewed on the basis of the checklist QUADAS-2. All of the studies reviewed by two individuals independently and the cases of disagreement were discussed.
Statistical Analysis
We used Cochran's Q test and I2 index to examine the heterogeneity of the studies. If there was any heterogeneity, the random model effect was applied to solve the heterogeneity problem. The heterogeneity was categorized in three levels (I2 <25%: low, 25% < I2 < 75%: moderate, and I2 >75%: high) (12, 13). The effectiveness of interventions was done through studying the consequences such as sensitivity and specificity. Using the same type of indicators extracted from the included studies, data pooling was done in MetaDiSc 2.0 software, which is usually used for the meta-analysis of diagnostic value studies. The hierarchical summary receiver operating characteristic (HSROC) model has been proposed in this study and the results were plotted using the Forest plot and SROC curves.
Results
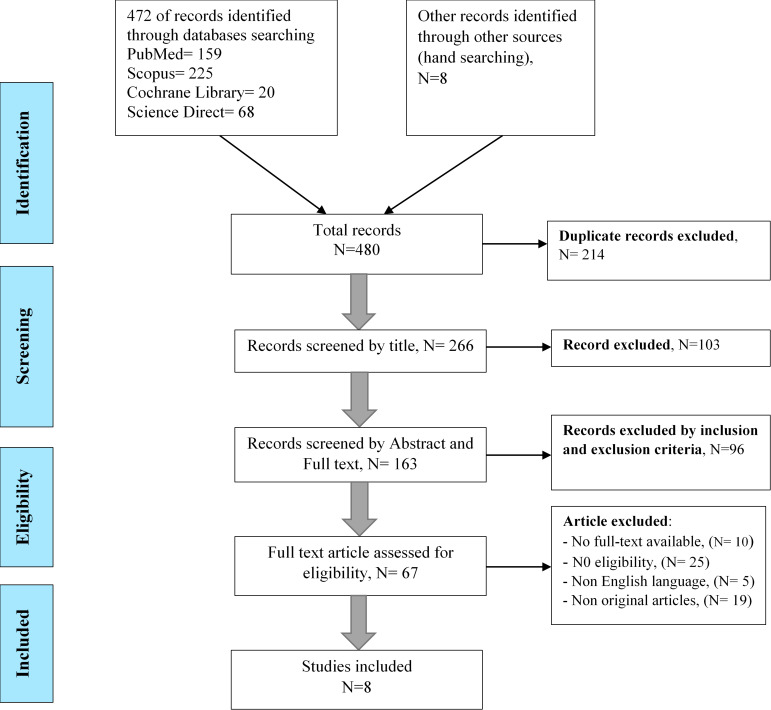
A total of 472 records were retrieved from the electronic databases and 8 articles were found through the hand searching.
In Figure 1, the screening process is presented in accordance with the PRISMA standard. The revealed articles were evaluated for inclusion/exclusion criteria independently by two researchers. No study was omitted after the quality assessment and all the selected studies were entered into the final analysis.
Fig. 1.
The process of screening articles according to the PRISMA standard
Study Features
Eight prospective cohort articles (14-21) were included. All studies were written in English. The total number of participants was 1238, of whom 611 were men and 627 women. The largest sample size was 689 (17), while the smallest study included only 23 participants (18). The average age of participants in the studies ranged from 49.8 (20) to 62.5 (19). The level of bowel cleaning was appropriate in all studies. A summary of the characteristics of the included studies is shown in Table 1.
Table 1. Characteristics of the included studies reporting the diagnostic accuracy of the second-generation colon capsule endoscopy .
| Study | Country | Population | Index test | Gold standard |
|
Parodi et al. 2018 (13) |
Italy, France |
Mean age 57 y (range 26-82); 97 female, 80 male |
CCE-2* | OC** |
|
Igawa et al. 2017 (14) |
Japan |
Mean age 59 y 6 female, 24 male |
CCE-2 | OC |
|
Douglas R Morgan et al. 2016 (15) |
USA |
Mean age 60.2 y (range 32-70); 27 female, 23 male |
CCE-2 | CC |
|
Douglas K. Rex et al. 2015 (16) |
USA, Israel |
Mean age 57 y 386 female, 303 male |
CCE-2 | CC*** |
|
Alexander F Hagel, et al. 2014 (17) |
Germany |
Mean age 51 y (range 24–75 y); 10 female, 14 male |
CCE-2 | CC |
|
Holleran et al. 2014 (18) |
Ireland |
Mean age 62.5 y (SD 5.8 y); 28 female, 34 male |
CCE-2 | OC |
|
Spada et al. 2011 (19) |
Europe |
Mean age 60 y (SD 9 y); 45 female, 72 male |
CCE-2 | CC |
|
Eliakim et al. 2009 (20) |
Israel |
Mean age 49.8 y (range 18–57 y); 33 female, 65male |
CCE-2 | CC |
*Colon Capsule Endoscopy-2, **Optical Colonoscopy, *** Conventional Colonoscopy
Diagnostic Accuracy of second-generation colon capsule endoscopy
In all studies, the second-generation colon capsule endoscopy was the intervention test. In three studies (14, 15, 19), the reference test was an optical colonoscopy, and in the remaining (16-18, 20, 21), conventional colonoscopy was the reference test. Colonoscopy was performed while the operators and physicians were blind to the results of the capsule test. Five studies reported the sensitivity and specificity of the endoscopic colon to detect colorectal polyps in dimensions of at least 6 ≤ mm and 10 ≤ mm in each patient (14, 16, 17, 20, 21). Two papers also reported the sensitivity and specificity of the capsule in the diagnosis of colorectal polyps at any size (18, 19). Since one of the papers (15) intended to diagnose a specific type of tumor, which was incompatible with the other papers, the related results are reported separately and narratively. The polyp-matching algorithms differed between the included studies which are summarized in Table 2.
Table 2. Summarized of polyp-matching between the included studies .
| Study | Diagnostic accuracy data reported | Sensitivity (95% confidence) | Specificity (95% confidence) |
| Parodi (2018) | Polyp 6≤ | 91% (71.9 - 96.1) | 88% (81.5 – 93) |
| Polyp 10≤ | 89% (71.9 – 96.1) | 95% (89.8 – 97.3) | |
| Morgan (2016) | Polyp 6≤ | 93% (66 – 99.7) | 80% ( 62.5 – 90.9) |
| Polyp 10≤ | 100% (56.1 – 100) | 93% (79.9 – 98.2) | |
| Rex (2015) | Polyp 6≤ | 81% (82-90) | 93% (92-96) |
| Polyp 10≤ | 80% (77-92) | 97% (96–99) | |
| Holleran (2014) | Polyp in any size | 95% (81–99) | 65% (44–83) |
| Hagel (2014) | Polyp in any size | 90.9% (85–100) | 67.6% (35–98) |
| Spada (2011) | Polyp 6≤ | 84% (74–95) | 64% (52-76) |
| Polyp 10≤ | 88% (76–99) | 95% (90–100) | |
| Eliakim (2009) | Polyp 6≤ | 89% (70–97) | 76% (72-78) |
| Polyp 10≤ | 88% (56–98) | 89% (86-90) |
Meta-analysis results
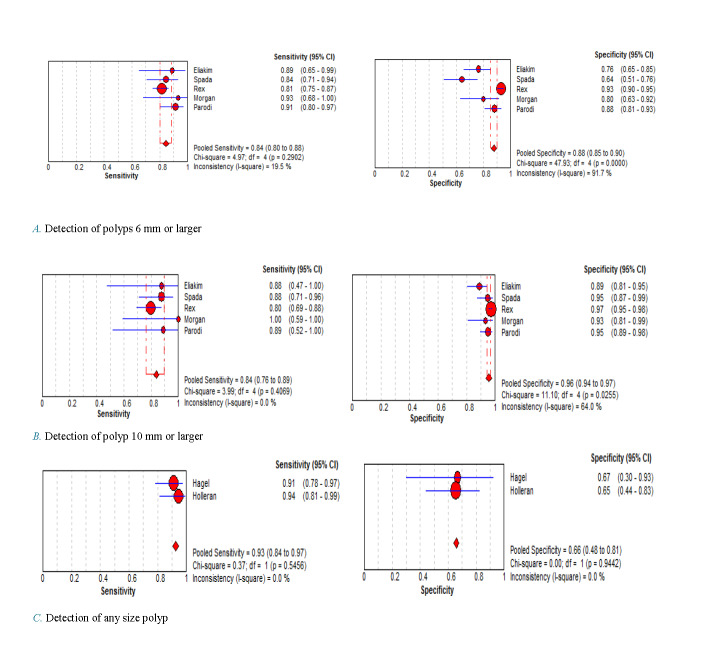
For the diagnosis of colorectal polyps with a diameter of 6 mm or larger, the pooled sensitivity and specificity were 84% (95% CI, 80%-88%) and 88% (CI 95%, 85%-90%) respectively (Fig. 2 A). The I2 coefficient for the heterogeneity of sensitivity was 19.5% (p=0.29), which shows no heterogeneity. However, the heterogeneity of the specificity was 91% (p<0.001), which means obvious significant heterogeneity.
Fig. 2.
Forest plots of the sensitivity and specificity of second-generation colon capsule endoscopy in the detection of colorectal polyps of varying sizes
For the diagnosis of 10 mm or larger colorectal polyps, as (Fig. 2 B) demonstrates, the pooled sensitivity was 84% (95% CI, 76% -89%) and the specificity was 96% (95% CI, 94% -97%). The I2 coefficient for the heterogeneity of sensitivity was 0.0% and for specificity 64%. The p-value for sensitivity and specificity was 0.40 and 0.02 respectively, which indicate significant and moderate to high heterogeneity in the specificity results.
The sensitivity and specificity of the capsule in the detection of any size of polyp were 93% (95% CI, 84%-97%) and 66% (95% CI, 48%-81%), respectively (Fig. 2 C). The I2 heterogeneity coefficient for both sensitivity and characteristic was zero percent (p = 0.54 and 0.94 respectively) which indicate no heterogeneity.
Optimal sensitivity and specificity point for the diagnosis of polyps
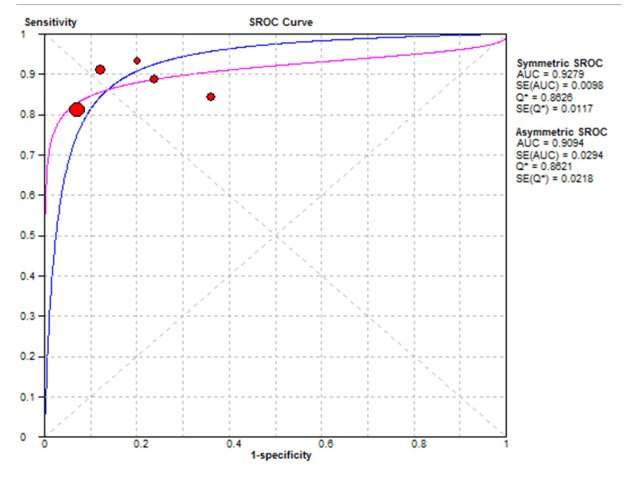
As the SROC curve in Figure 3 shows, the optimal sensitivity and specificity in detecting a polyp of 6 mm or larger is about 87%. This means that the correct diagnosis occurs when 87% of patients are correctly diagnosed, and 87% of healthy people are also diagnosed as non-patients.
Fig. 3.
The SROC curve of the endoscopic colon in the detection of 6 mm and larger polyps
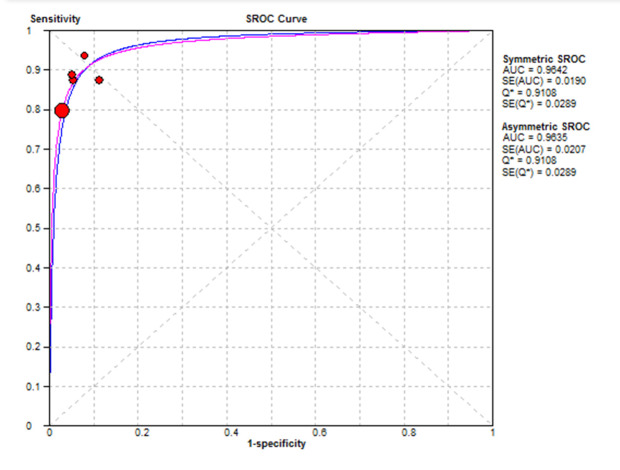
The SROC curve in Figure 4 shows that the optimal sensitivity and specificity in detecting a 10 mm or larger polyp is about 91%. This means that the correct diagnosis is made when 91% of patients are correctly diagnosed and 91% of healthy people are also diagnosed as non-patient. Since the number of pooled studies is less than 10, there was no possibility of reviewing the publication bias (22).
Fig. 4.
The SROC curve of the endoscopic colon in the detection of 10 mm and larger polyps
As we mentioned previously, some findings across the papers could not get pooled through meta-analysis, which is reported separately as follows:
Parodi et al. (14) recognized the sensitivity of the capsule for the diagnosis of patients with 6-10 mm and 10 mm and larger polyp adenoma 95% (95% CI, 83.5%-98.6%) and 99.9% (95% CI, 72.2%- 97.5%). They reported a specificity of 80.3% (95% CI, 72.8% -86.1%) and 92.3% (95% CI, 95% -87% -88%), for those sizes respectively. In this study, the sensitivity of polyp-based capsule for the detection of adenomatous polyps of 6 mm or smaller, 6-10 mm and ones larger than 10 mm were 29.5% (95% CI, 19.6% -41.97%), 84.4% (95% CI, 73.9% -91.4%) and 87.5% (95% CI, 71.9% -95%) respectively.
Igawa et al. (15) reported the sensitivity of 81% and specificity of 100% for the capsule in detecting large tumors with positive predictive value and negative predictive values of 100% and 69%, respectively. Sensitivity and specificity of the capsule for detecting LST-G were 71% and 100%, respectively, and positive predictive value and negative predictive values were 100% and 92%, respectively. Sensitivity and specificity of the capsule for detecting LST-NG were 86% and 100%, respectively, and positive predictive value and negative predictive value were 100% and 89%, respectively.
Based on Rex et al. (17) the sensitivity and specificity for detecting lesions larger than 6 mm were 88% and 82%, repectively. The sensitivity and specificity for lesions up to 10 mm were 92% and 95%, respectively.
Holleran et al. (19) reported a sensitivity of 89% and specificity of 96% for detecting large lesions.
Spada et al. (20) reported a sensitivity of 90% (95% CI, 80% -99%) and specificity of 64% (95% CI, 52% -76%) for the capsule in the diagnosis of neoplastic polyps larger than 6 mm. The sensitivity and specificity for 10 mm polyps were 93% (95% CI, 84% -100%) and 95% (CI 95%, 90% -100%), respectively.
Discussion
The aim of this review was to provide information on the diagnostic accuracy (diagnostic effectiveness) of the second-generation colon capsule endoscopy compared to colonoscopy for the diagnosis of colon cancer and disorders. According to the evidence, endoscopy of the colon using the second-generation colon capsule endoscopy has a good sensitivity in the identification of patients with colorectal polyps. The sensitivity reported in the reviewed studies ranged 81 to 93% for 6–10 mm polyps and 80% to 100% for polyps of 10 mm and larger sizes. The specificity for these two sizes of polyps was 64-93% and 89-97%, respectively (14, 16-21). According to our findings, for the diagnosis of colorectal polyps with a diameter of 6 mm or larger, the pooled sensitivity and specificity were 84% (95% CI, 80% -88%) and 88% (CI 95%, 85%-90%), respectively. Also, for the diagnosis of 10 mm or larger colorectal polyps, the pooled sensitivity was 84% (95% CI, 76%-89%) and the specificity was 96% (95% CI, 94%-97%). The sensitivity is above the 50% cut-off, defined by the American Cancer Society as an acceptable test for screening purposes (6).
The sensitivity and specificity results showed heterogeneity, which may be due to the difference in the amount of bowel preparation across different studies; proper bowel preparation can facilitate the diagnosis of polyps through colonic endoscopy. In the endoscopic process, the colon requires more preparation compared to colonoscopy. Some authors have suggested that, given the low number of false positives, the use of colon capsule endoscopy can reduce the number of unwanted colonoscopies (19). However, a high level of false-negative outcomes may result in late detection of colorectal cancer, which may cause a particular concern among patients with a high risk of colorectal cancer.
Limitations
This study was limited to the observational design in which the possibility of bias is unknown. Nevertheless, conducting randomized trials for the comparison of colonoscopy and CCE-2 may not be that feasible. Since we did not have access to grey literature databases in Iran, this was one of the limitations of the study too.
Conclusion
This review showed that the second-generation colon capsule endoscopy has a good accuracy in the detection of polyps and colorectal cancer among high- and middle-risk patients. It can be used as a pilot test for a specific population in Iran. Using this technology, we can increase patients’ satisfaction and improve their quality of life. However, the CCE-2 lacks the ability to perform biopsy or remove the polyps, which can be done by conventional colonoscopy.
Acknowledgments
This article is a part of an MS thesis, supported by Kerman University of Medical Sciences with an approval number of IR.KMU.REC.1397.110 issued by Kerman University of Medical Sciences.
Conflict of Interests
The authors declare that they have no competing interests.
Appendix 1
Appendix 1. Literature Search Strategies
Search date:February 30, 2018
Databases searched: PubMed, Scopus, Cochrane Library, Science Direct
Search Strategy:
Pubmed
(((colonoscopy[MeSH Terms] OR colonoscop*[Title/Abstract] OR sigmoidoscopy[MeSH Terms] OR sigmoidoscop*[Title/Abstract]) AND ("second generation colon capsule endoscopy"[Title/Abstract] OR "colon capsule endoscopy"[Title/Abstract] OR "capsule colonoscopy"[Title/Abstract] OR "CCE-2"[Title/Abstract] OR "PCC-2"[Title/Abstract] OR "colon capsule"[Title/Abstract] OR "PCCE-2"[Title/Abstract] OR "pillcam-2"[Title/Abstract])))
Scopus
TITLE-ABS-KEY ("second generation colon capsule endoscopy") OR TITLE-ABS-KEY ("PCC2") OR TITLE-ABS-KEY ("CCE2") OR TITLE-ABS-KEY ("pillcam 2") OR TITLE-ABS-KEY ("colon capsule") OR TITLE-ABS-KEY ("colon capsule endoscopy") OR TITLE-ABS-KEY ("capsule colonoscopy") AND TITLE-ABS-KEY (colonoscopy) OR TITLE-ABS-KEY (sigmoidoscopy)
Cochrane Library
("second generation colon capsule endoscopy”: ti,ab,kw or "PCC2":ti,ab,kw or "CCE2":ti,ab,kw or "colon capsule":ti,ab,kw or "colon capsule endoscopy":ti,ab,kw or "capsule colonoscopy":ti,ab,kw) and ("colonoscopy":ti,ab,kw or "sigmoidoscopy":ti,ab,kw)
Science Direct
TITLE-ABSTR-KEY(colonoscop*) or TITLE-ABSTR-KEY(sigmoidoscop*) and TITLE-ABSTR-KEY ("second generation colon capsule endoscopy") or TITLE-ABSTR-KEY(CCE2) or TITLE-ABSTR-KEY(PCC2) or TITLE-ABSTR-KEY ("colon capsule") or TITLE-ABSTR-KEY (pillcam 2) or TITLE-ABSTR-KEY ("colon capsule endoscopy") or TITLE-ABSTR-KEY ("capsule colonoscopy").
Appendix 2
Appendix 2. Quality Assessment
The quality of the studies was evaluated by the researcher. In all 8 studies, there was enough information to create a two by two table of the index test results, and these eight studies entered the final stage.
Risk of Bias for studies of Second-generation Colon Capsule Endoscopy (QUADAS-2) .
| Author, Year | Risk of Bias | |||
| Patient Selection | Index Test | Reference Standard | Flow and Timing | |
| Eliakim (2009) | High a | Low | Low | High b |
| Spada (2011) | High a | Low | Low | High b |
| Hagel (2014) | High a | Low | Low | High b |
| Holleran (2014) | Low | Low | Low | Low |
| Rex (201) | Unclear | Low | Low | High b |
| Morgan (2016) | Unclear | Low | Low | High b |
| Igawa (2017) | Low | Low | Low | High b |
| Parodi (2018) | Low | Low | Low | Low |
Abbreviations: QUADAS-2, Quality Assessment of Diagnostic Accuracy Studies.
aPatients were not selected randomly or consecutively.
bNot all patients were included in the analysis.
Cite this article as: Alihosseini S, Aryankhesal A, Sabermahani A. Second-generation colon capsule endoscopy for detection of colorectal polyps: A meta-analysis. Med J Islam Repub Iran. 2020 (16 Jul);34:81. https://doi.org/10.34171/mjiri.34.81
References
- 1.Dolatkhah R, Somi MH, Bonyadi MJ, Asvadi Kermani I, Farassati F, Dastgiri S. Colorectal cancer in Iran: molecular epidemiology and screening strategies. J Cancer Epidemiol. 2015:2015. doi: 10.1155/2015/643020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Organization WH. Health in 2015: from MDGs to SDGs. Geneva: World Health Organization. 2015.
- 3. Ramazani Daryasari R, Nade Ali F, Modirian M, Arjomandpour M, Salavati F, Fazeli M, et al. National Comprehensive Cancer Control Program. Non-communicable Disease Center, Cancer Department, Ministry of Health and Medical Education (2011)(2011).
- 4.Kasper DL, Fauci AS, Hauser SL. Harrison’s Principles of Internal Medicine, 16th Edition. Neurology. 2005;64(8):1488–9. [Google Scholar]
- 5. Pourdastgardan R. Gastrointestinal cancers. Tehran: Green book; 2014.
- 6.Levin B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J. et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570–95. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Sung JJ, Ng SC, Chan FK, Chiu HM, Kim HS, Matsuda T. et al. An updated Asia Pacific Consensus Recommendations on colorectal cancer screening. Gut. 2015;64(1):121–32. doi: 10.1136/gutjnl-2013-306503. [DOI] [PubMed] [Google Scholar]
- 8.Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology Guidelines for Colorectal Cancer Screening 2008. Am J Gastroenterol. 2009;104(3):739–50. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 9.Spada C, Pennazio M, Hassan C, Riccioni ME, Costamagna G. Colon capsule endoscopy. G Ital Endos Dig. 2011;34(2):103–8. [Google Scholar]
- 10.Hosoe N, Matsuoka K, Naganuma M, Ida Y, Ishibashi Y, Kimura K. et al. Applicability of second-generation colon capsule endoscope to ulcerative colitis: a clinical feasibility study. J Gastroenterol Hepatol. 2013;28(7):1174–9. doi: 10.1111/jgh.12203. [DOI] [PubMed] [Google Scholar]
- 11.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB. et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–36. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 12.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ades AE, Lu G, Higgins JP. The interpretation of random-effects meta-analysis in decision models. Med Decis Making. 2005;25(6):646–54. doi: 10.1177/0272989X05282643. [DOI] [PubMed] [Google Scholar]
- 14.Parodi A, Vanbiervliet G, Hassan C, Hebuterne X, De Ceglie A, Filiberti RA. et al. Colon capsule endoscopy to screen for colorectal neoplasia in those with family histories of colorectal cancer. Gastrointest Endosc. 2018;87(3):695–704. doi: 10.1016/j.gie.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 15.Igawa A, Oka S, Tanaka S, Otani I, Kunihara S, Chayama K. Evaluation for the Clinical Efficacy of Colon Capsule Endoscopy in the Detection of Laterally Spreading Tumors. Digestion. 2017;95(1):43–8. doi: 10.1159/000452367. [DOI] [PubMed] [Google Scholar]
- 16.Morgan DR, Malik PR, Romeo DP, Rex DK. Initial US evaluation of second-generation capsule colonoscopy for detecting colon polyps. BMJ Open Gastroenterol. 2016;3(1):e000089. doi: 10.1136/bmjgast-2016-000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rex DK, Adler SN, Aisenberg J, Burch WCJr, Carretero C, Chowers Y. et al. Accuracy of capsule colonoscopy in detecting colorectal polyps in a screening population. Gastroenterology. 2015;148(5):948–57 e2. doi: 10.1053/j.gastro.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 18.Hagel AF, Gabele E, Raithel M, Hagel WH, Albrecht H, de Rossi TM. et al. Colon capsule endoscopy: detection of colonic polyps compared with conventional colonoscopy and visualization of extracolonic pathologies. Can J Gastroenterol Hepatol. 2014;28(2):77–82. doi: 10.1155/2014/691785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holleran G, Leen R, O'Morain C, McNamara D. Colon capsule endoscopy as possible filter test for colonoscopy selection in a screening population with positive fecal immunology. Endoscopy. 2014;46(6):473–8. doi: 10.1055/s-0034-1365402. [DOI] [PubMed] [Google Scholar]
- 20.Spada C, Hassan C, Munoz-Navas M, Neuhaus H, Deviere J, Fockens P. et al. Second-generation colon capsule endoscopy compared with colonoscopy. Gastrointest Endosc. 2011;74(3):581–9 e1. doi: 10.1016/j.gie.2011.03.1125. [DOI] [PubMed] [Google Scholar]
- 21.Eliakim R, Yassin K, Niv Y, Metzger Y, Lachter J, Gal E. et al. Prospective multicenter performance evaluation of the second-generation colon capsule compared with colonoscopy. Endoscopy. 2009;41(12):1026–31. doi: 10.1055/s-0029-1215360. [DOI] [PubMed] [Google Scholar]
- 22. Higgins J, Green S. The Cochrane collaboration, Cochrane Handbook for SystematicReviews of Intervention v 5.1.0. 2011, http://handbook-5-1.cochrane.org/.