Abstract
Cancer stem cells (CSCs) have critical roles in tumor development, progression, and recurrence. They are responsible for current cancer treatment failure and remain questionable for the design and development of new therapeutic strategies. With this issue, medical imaging provides several clues for finding biological mechanisms and strategies to treat CSCs. This review aims to summarize current molecular imaging approaches for detecting CSCs. In addition, some promising issues for CSCs finding and explaining biological mechanisms have been addressed. Among the molecular imaging approaches, modalities including Magnetic resonance imaging (MRI) and positron emission tomography (PET) have the greatest roles and several new approaches such as optical imaging are in progress.
Keywords: Cancer stem cells, Molecular imaging, MRI, PET, Optical imaging
↑ What is “already known” in this topic:
Cancer stem cells (CSCs) are considered a major challenging concept in the field of cancer therapy. One of the novel and promising strategies that will revolutionize our understanding of CSCs theory is imaging technologies, providing new opportunities to locate the CSCs, and also evaluate the tumor biological processes involving CSCs.
→ What this article adds:
This study aimed to provide an overview of current molecular imaging approaches for detecting CSCs. In vivo CSCs imaging can provide opportunities to investigate therapeutic response or metastatic CSCs, tumor propagation and plasticity of CSCs at high resolution. To date, there is no best imaging modality to track CSCs in vivo.
Introduction
Despite recent advances in cancer treatment, cancer remains one of the most common leading causes of death worldwide (1, 2). Nowadays, although cancer is diagnosed and treated at earlier stages, some residual cancer cells, potentially resistant cells still exit after treatment and the same cells contribute to cancer recurrence. On the other hand, residual cancer cells are one possible cause of therapeutic failure (3, 4). These residual cells exhibit stem-like properties known as the cancer stem cells (CSCs), also called tumor-initiating cells (TICs) (3, 4). CSCs are considered a major challenging concept in the field of cancer therapy (4). Elimination of CSCs population, as a small subpopulation of cells within tumors, is essential to achieve stable, long-lasting remission, and cure of cancer (5).
In recent decades, many studies have been conducted on CSC theory to clarify understanding and eradication of CSCs (3-7). Of note, in-vivo understanding of the complex behavior of CSCs still remains largely a mystery. One of the novel and promising strategies that will revolutionize our understanding of CSCs theory is imaging technologies. Recent advances in preclinical and clinical imaging modalities have been provided new opportunities to locate the CSCs, and also evaluate the tumor biological processes involving CSCs. Accordingly, the aim of this review was to summarize recent studies on imaging techniques that have been utilized to monitor and visualize CSCs.
Cancer stem cells properties
CSCs are a rare subpopulation of tumor cells possessing similar characteristics as normal stem cells (6, 7). As suggested by recent studies, CSCs can self-renew and have the pluripotent capacity (8). There are several unique characteristics for CSCs, including self-renewal ability, capability to develop into multiple lineages, potential to proliferate extensively, being rare subpopulation of cells, radioresistance, chemoresistance, and promoting invasion and metastatic activity (4, 6, 9, 10).
Initially, CSCs were discovered in hematological cancer (11). In 1994, it proved that the CD34+/CD38− (as cell surface markers) cells from acute myeloid leukemia (AML) patients can induce hematopoietic malignancy in NOD/SCID mice (11). Subsequently, studies have identified CSCs in a variety of solid tumors, including breast cancer, brain cancer, colon cancer, pancreas cancer, lung cancer, ovarian cancer, prostate cancer, melanoma, and glioblastoma (7, 12-18).
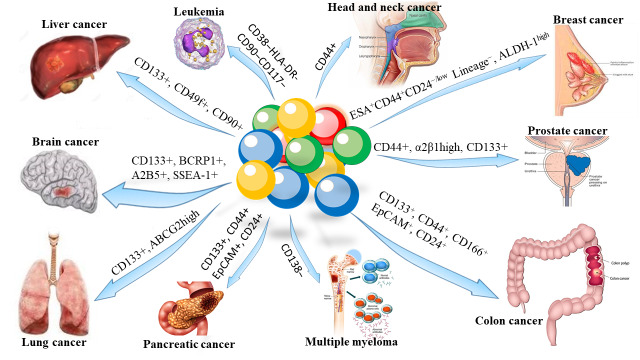
Distinct and specific cell surface biomarker phenotypes have used to isolate and distinguish CSCs from other cancer cells and normal stem cells. Nowadays, fluorescence-activated cell sorting (FACS) based on cell surface biomarkers or intracellular molecules is known as the main method for identifying CSCs (6). There are various CSCs markers such as CD44, CD24, CD90, CD133, epithelial-specific antigen (ESA), and aldehyde dehydrogenase1 (ALDH1) that express in a specific cancer type (6, 7, 12, 13, 19), as shown in Figure 1.
Fig. 1.
Surface biomarkers of cancer stem cells (CSCs)
CSCs are resistant to traditional radiotherapy and chemotherapy (4). Radioresistance mechanisms of CSCs can be attributed to activation of DNA repair, overexpression of reactive oxygen species (ROS) scavenger proteins, activation of cell cycle checkpoint proteins, activation of anti-apoptotic pathways, and protection by microenvironmental niche (4, 20). Recently, Ghaffari and colleagues have reviewed new physical approaches to eradicate CSCs (4).
Molecular imaging
Molecular imaging (MI) is a novel promising noninvasive strategy that can provide the ability of quantitative measurement and visualization of the function of biological and cellular processes in vivo (21, 22). In contrast to anatomical imaging that is useful for disease or cancer diagnosis, surgical guidance/follow-up, and treatment monitoring, MI can improve specificity of cancer screening and early diagnosis and personalized treatment without invasive biopsies or surgical procedures (21). MI permits real-time tracking and monitoring of biological, physiological, and pathological processes in vivo. Cancer biomarker-based MI is an effective strategy for cancer diagnosis.
There are several preclinical and clinical MI approaches such as positron emission tomography (PET), single photon emission computed tomography (SPECT), magnetic resonance imaging (MRI), ultrasound, and optical imaging (OI) (21). Table 1 summarizes the pros and cons of MI modalities. Currently, PET, SPECT, MRI, and ultrasound have clinical use, whereas OI modalities such as bioluminescence imaging (BLI), intravital microscopy, fluorescence-mediated tomography (FMT) do not have clinical use, and are in progress.
Table 1. General characteristics of molecular imaging modalities .
| Imaging modality | Type of signal | Quantitative | Spatial resolution | Penetration depth |
Common Contrast agents/ Readout |
Real-time imaging |
Whole-body imaging |
Data acquisition time | Cost |
| Nuclear medicine (SPECT and PET) | Gamma-ray | Yes | 0.3-2mm | No limit |
PET: 18F, 68Ga, 64Cu, etc. SPECT: 99mTc, 123I, etc. |
No | Yes | Min | High |
| MRI | Radiofrequency waves | Yes | 50-250μm | No limit | Gd3+, SPIO, USPIO, etc. | No | Yes | Min to Hrs | High |
| Ultrasound | High-frequency sound waves (>20KHz) | Yes | 30-500μm | Several cm | Contrast Microbubbles | Yes | No | Sec to Min | Low |
| Optical | Visible light or near infrared | No, except FMT | 1-5mm | ≤ 1 cm |
Fluorescent molecules & dyes, Light absorbing nanoparticles |
Yes | No | Sec to Min | Low |
FMT: Fluorescence-mediated tomography; SPIO: Superparamagnetic iron oxide; USPIO: Ultra-small superparamagnetic iron oxide
Cancer stem cells imaging
CSCs imaging is a new approach that can identify the mechanisms of resistance of CSCs to treatment, response to therapy, and predict the outcome of therapy, as well as prognosis. On the other hand, MI can explore CSCs biology (23). Various imaging modalities have been investigated for in vivo imaging of CSCs such as PET, SPECT, MRI, and OI modalities, including BLI, FMT, and near-infrared (NIR) fluorescence reflectance imaging, and have shown interesting results for in vivo imaging of CSCs. Taken all together, in vivo imaging of CSCs results in applying a more personalized treatment planning regimen. The specificity and sensitivity of molecular probes targeting genes or proteins that have a great role in cancer growth and progression can be key factors that determine the accuracy of cancer prediction.
To our knowledge, there are few studies conducted to track CSCs with MI modalities, as shown in Table 2. The application of the MI in CSCs is discussed in the following. Table 2 outlines MI in tracking and monitoring CSCs.
Table 2. A summary of recent studies on tracking various cancer stem cells (CSCs) by molecular imaging modalities .
| Molecular imaging technique | Imaging agent | Biomarker | Type of cancer | Reference |
| PET | 125I | CD133 | Colon | [25] |
| 64Cu-NOTA | AC133 | Brain | [26] | |
| 18F-FDG | K19 | Hepatocelluar carcinoma | [27] | |
| 64Cu-ATSM | CD133 | Colon | [28] | |
| MRI | Ferritin heavy chain | CD44+/CD24− | Breast | [38] |
| HA-MNCs | CD44 | Breast | [42] | |
| APTEDB-TCL-SPIONs | EDB-FN | Breast | [43] | |
| Dox@APTEDB-TCL-SPIONs | EDB-FN | Breast | [44] | |
| NIR-FMT | Antibody AC133.1 | CD133 | CD133-overexpressing glioblastoma | [48] |
| NIR-FMT | Antibody AC133 | CD133 | Orthotopic glioblastoma model | [26] |
| Intravital microscopy | Yellow fluorescent protein | CD133 | Human glioblastoma | [49] |
|
NIR-fluorescence Imaging |
NIRSHs | CD44 | Gastric | [52] |
| BLI | Optical bifusion reporter genes | CD44 | Breast | [53] |
| Fluorescence imaging | Fluorescent protein (ZsGreen) | 26S proteasome | Human glioma and breast | [54] |
| MRI/SPECT/NIR-fluorescence imaging |
CD44 antibody conjugated SWCNTs tagged with SPIONs and either Gallium-67 or Vivotag-S750 fluorophores |
CD44 | Breast | [57] |
| MRI/ fluorescence imaging | Fe3O4@PEI@Cy5.5@PEG@HCBP-1 NPs | HCBP-1+ | Lung | [58] |
| MRI/ fluorescence imaging | Anti-CD133 mAb-nano-MSN | CD133 | Glioblastoma | [59] |
PET: Positron emission modality; MRI: Magnetic resonance imaging; NIR-FMT: Near-infrared fluorescence-mediated tomography; BLI: Bioluminescence imaging; SPECT: Single positron emission computed tomography; Cu-ATSM: Copper labeled diacetyl-bis(N4-methylthiosemicarbazone); 18F-FDG: 18F-fluorodeoxyglucose; HA-MNCs: Hyaluronan-modified magnetic nanoclusters; APTEDB-TCL-SPIONs: Extra domain B of fibronectin--specific peptide-conjugated thermally cross-linked Superparamagnetic iron oxide nanoparticles; Dox@APTEDB-TCL-SPIONs: Doxorubicin (Dox)-loaded APTEDB-TCL-SPIONs; NIRSHs: NIR-sensitive supramolecular hydrogels; SWCNTs: Single-walled carbon nanotubes
Positron Emission Tomography
PET imaging is a high sensitive modality compared with other traditional imaging techniques. It also provides noninvasive, real-time tracking in vivo, as well as quantitative data (21). PET with 18F-fluorodeoxyglucose (18F-FDG) analysis is considered as a promising and emerging clinical tool for cancer diagnosis and staging, as well as monitoring of response to treatment (24). Few studies have been evaluated the application of PET for CSCs imaging, as observable in Table 2. CD133, a membrane protein, is considered as a CSCs marker in a wide variety of tumors. A recent study by Jin et al. has examined the potential of in vivo radionuclide imaging of CSCs using Iodine-125 (125I)- labeled ANC9C5, anti-CD133 antibody in human colon carcinoma HCT116 xenograft–bearing mice model. Data from their study revealed that the expression of CD133 was reflected by biodistribution and intratumoral distribution of 125I- labeled ANC9C5. Radioiodinated anti-CD133 monoclonal antibody (mAb) ANC9C5 in comparison with the control antibody showed nearly a 2-fold higher tumor uptake. In addition, intratumoral distribution of 125I- labeled ANC9C5 and expression of CD133 had a good overlap on autoradiography. As a consequence of that study, radio-immuno-targeting of CSCs using PET is possible (25). In another study, AC133 (an epitope of the second extracellular loop of CD133) was detected by PET imaging (26). Gaedicke et al. developed clinically relevant tracers that can provide highly sensitive and high-resolution monitoring of AC133+ glioblastoma CSCs in both subcutaneous and intracerebral xenograft tumors using PET and NIR fluorescence molecular tomography (FMT). MicroPET with 64Cu-NOTA-AC133 mAb can clearly image s.c. xenografts containing AC133+ CSCs, and can also obtain accurate and high-resolution images of small brain tumor lesions (2-3 mm in size) (26).
Recently, keratin 19 (K19) is identified as a novel hepatocellular carcinoma (HCC)-CSC marker (27). Since 18F-FDG-PET is an effective imaging modality for predicting postoperative outcome in HCC, it can be utilized to track K19+ CSCs in HCC. For these purposes, the expression of K19 and glucose transporter-1 (GLUT1) was examined in human HCC surgical specimens. A significant correlation was found between the expression of K19 and GLUT1 expression and FDG accumulation in HCC patients. 18F-FDG uptake in K19+ HCC cells was significantly higher than in K19-cells. Furthermore, K19 regulates the accumulation of 18F-FDG through TGFβ/Smad signaling pathways, including Sp1 and downstream target GLUT1. Data indicate the 18F-FDG-PET is a novel approach for identifying K19 expression in HCC tissues (27).
64Cu-diacetyl-bis (N4-methylthiosemicarbazone) (64Cu-ATSM) is also reported to be an imaging agent for PET, which can target hypoxic tumors. 64Cu-ATSM has a high accumulation in regions of CD133+ high expression, and can then kill such regions by radiation. As a result, it can reduce the percentage of CD133+ cells. Taken together, 64Cu-ATSM not only is PET imaging agent, but it also is a potential agent for internal radiotherapy (28).
Magnetic Resonance Imaging
Without question, nowadays, MRI is an important clinical tool in disease or cancer diagnosis. MRI as a noninvasive imaging modality, has high spatial resolution, and uses non-ionizing radiation (21, 29, 30). MRI can provide morphological and pathophysiological information in living subjects (29). Recent advances in new MR methods resulted in using MRI in the field of MI that evaluates specific cellular or subcellular events. Owing to the ability of MRI with contrast agents (CAs) for labeling cells, dynamic assessment of cell migration into target tissues is provided (31). MRI is capable to detect single cells in both stem cell studies and cancer cell tracking studies (32). Ultra-small superparamagnetic iron oxide (USPIO) and superparamagnetic iron oxide (SPIO) are two most important MR labels that are currently used to track cells (30). Contrast with conventional paramagnetic gadolinium-based CAs, USPIO and SPIO have low toxicity, subnanomolar-range detection limits, and higher contrast enhancement (33, 34).
With regard to CSCs imaging, several groups of researchers have been investigated the feasibility of detecting CSCs with MRI. As demonstrated in studies, SPIO can successfully detect and track glioblastoma CSCs in vitro (35). In vivo tracking and imaging cells of interest using MRI are performed by two approaches. In the first method, CA is utilized as a labeling or targeting agent. As reported in several studies, SPIO nanoparticles (SPIONs) have been recently employed as CAs for tracking and imaging various cells owing to their high relaxivity (31, 36). SPIONs functionalized with targeting ligands, such as monoclonal antibodies, peptides and nucleotide conjugation are recognized as useful CAs for molecular imaging (37). When SPIONs are placed in the magnetic field, T2 signal intensities significantly reduced (38). However, it should be noted that MRI signal intensity change is determined by the amount of USPIO and SPIO. Therefore, using SPIONs is not suitable for the long-term imaging of cells because the CAs become diluted by cell proliferation (38). On the other hand, SPIONs distribute to CSCs daughter cells, and also these nanoparticles can be absorbed by macrophages. Therefore, above-mentioned mechanisms can reduce the sensitivity and specificity of the signal (39). To overcome these limitations, recently, Choi et al. used the MRI reporter gene ferritin to noninvasively track breast CSCs (BCSCs). The overexpression of ferritin resulted in increasing of cell iron uptake, producing low signal intensities in MRI during cell division (38). MRI can be used for studying dynamic processes, such as the migration and invasion of cells over an extended period. Furthermore, this can provide temporal and spatial information for anti-cancer treatment effects on a specific cell population. The number of cancer cells in deep tissues can be quantified by calculating R2* (=1/T2*) values from T2* mapping of MRI images (40, 41). In Choi et al. study, BCSCs transduced with ferritin heavy chain (FTH) and enhanced green fluorescence protein (EGFP) dual reporter genes were transplanted into NOD/SCID mice to permit noninvasive monitoring BCSC-derived populations during tumor growth and to show tumor responses after docetaxel chemotherapy. Both in vitro and in vivo studies have showed a significant difference in MRI signal intensities (R2* values) between BCSCs and FTH-BCSCs. As revealed in histological analysis, areas showing high R2* values in docetaxel-treated FTH-BCSC tumors by MRI contained EGFP+/FTH+ viable cell populations with high percentages of CD44+/CD24− cells. In the light of these results, ferritin-based MRI can be used as a noninvasive method to monitor viable cell populations in tumors after chemotherapy (38).
In addition, Lim et al. have introduced hyaluronan-modified magnetic nanoclusters (HA-MNCs) as another labeling technique targeting CSCs for in vitro and in vivo studying of CD44-overexpressing breast cancer using MRI. CD44, a cell surface glycoprotein, is a major surface receptor for HA, an immune-neutral polysaccharide. Signal intensity on the T2-weighted MR images of cells treated with HA-MNCs was significantly reduced, demonstrating as an effective MR CA. HA-MNCs have good biocompatibility and excellent capability for targeted diagnosis of CD44-overexpressing breast cancer (42).
It has been demonstrated that extra domain B of fibronectin (EDB-FN) can be utilized as a putative biomarker of BCSCs (43). Previous study by Sun et al. showed the feasibility of detecting BCSCs with MRI using EDB-FN-specific peptide (APTEDB)-conjugated thermally cross-linked (APTEDB-TCL)-SPIONs (43). In another study by same group, theranostic ability of doxorubicin (Dox)-loaded APTEDB-TCL-SPIONs (Dox@APTEDB-TCL-SPIONs) in a BCSC xenograft mouse model was evaluated. The results indicated that Dox@APTEDB-TCL-SPIONs can selectively target BCSCs in vivo. Moreover, Dox@APTEDB-TCL-SPIONs can detect BCSCs within tumors by targeting EDB-FN-expressing cells (44).
Optical imaging
Optical imaging (OI) is a new and highly versatile modality for noninvasive in vivo MI, and plays a vital role in molecular and cellular imaging (21). OI techniques are cheap in comparison with other imaging modalities. The most important concerns in imaging CSCs using OI can be attributed to choosing reporter signal and imaging modalities because CSCs constitute only a small percentage of population of tumor cells (45).
Bioluminescence signal emitted from cells although has low sensitivity and specificity, it can identify tumor growth, regression, and metastases (45). BLI can monitor molecular and physiological processes in real time such as cell survival and gene expression (46). In order to obtain an optical signal from subpopulation of cells it needs to select a reporter signal. Luciferase reporter plasmid is known as one of the main specific reporter signal, and is high sensitive to measure biologic activity in growing tumor (47).
Currently, fluorescence imaging is considered as the best OI technique for imaging CSCs. Due to sensitive detectors and the intensity and stability of fluorescence signal, imaging of fluorescent cells in vivo at high resolutions has been provided. The application of multiple fluorophores at the same time can be a useful method for imaging biological features of CSCs. There are several factors that should be considered in choosing OI devices for studying CSCs, including penetration depth in biological tissue, imaging time points, and using multispectral unmixing (45). Recently, several studies have been investigated imaging CSCs using OI, as summarized in Table 2. Tsurumi et al. using near-infrared fluorescence molecular tomography (NIR-FMT) indicated that the feasibility of non-invasive antibody-based in vivo imaging of tumor-associated CD133 (AC133) on mouse subcutaneous xenograft models. In addition, CD133 antibody-based tumor targeting was effective. A low signal to noise ratio was found in cells expressing CD133 at endogenous levels (48). In another study, it has demonstrated that in vivo CD133 imaging using fluorescence labelled-mAb can also be utilized to an orthotopic glioblastoma model. The Alexa 680-labeled AC133 mAb revealed a remarkable high in vivo fluorescence signal in the tumor region in comparison with the Alexa 680-labeled isotype control antibody (26).
Intravital microscopy is an optical imaging technique that can visualize CSCs with a proper resolution. In addition, it has several benefits such as deeper penetration, minimal image distortion, signal quantification, and three-dimensional image reconstruction (49, 50). According to this, Lathia et al. showed that CSCs growth can be seen in vivo through lentivirus-transduced fluorescence-labeled CSCs (CD133+ cells) (49). Moreover, intravital microscopy may be used to investigate CSCs plasticity (51).
A novel NIR-sensitive molecular imaging probe based on hydrogel complexes has recently developed that can visualize CD44-expressing gastric CSCs. NIR-sensitive supramolecular hydrogels (NIRSHs, Cy5.5- conjugated polyethyleneimine/hyaluronic acid polyplexes) were fabricated by polyplexing in an aqueous medium, and showed good water-stability, biocompatibility, and specificity to CD44 (52).
Application of reporter genes is another technique for studying CSCs in vivo. Dual-function bioluminescence imaging-fluorescent reporter constructs (Luc2 fused with eGFP coding sequence) in BCSCs were recently applied. Luc2 sequence was used for whole body tracking by bioluminescence imaging (BLI) while EGFP was employed for intravital imaging and ex vivo analysis. With BLI, as few as 10 CD44+ cells of stably labeled BCSCs could be tracked in vivo, that provides studies of early tumor growth and spontaneous metastasis (53). In addition to surface protein markers, in vivo tracking of CSCs can also performed by proteasome activity (54). A recent study has found a reduction of 26S proteasome activity in CSCs. According to this, in vivo CSCs tracking in human glioma and breast cancer was provided. Human glioma and breast cancer cells were engineered to stably express ZsGreen-ornithine decarboxylase, a fluorescence fusion protein that accumulates in cells in the absence of 26S proteasome activity. In vivo tracking of ZsGreen positive cells was successfully performed by fluorescence imaging. The results of the study suggested that reduced 26S proteasome activity can be a property of CSCs (54).
Multimodal imaging
As stated earlier, there are several MI techniques such as MRI, PET, and OI. Each imaging modality has its own strengths and limitations. The main goal of multimodality imaging is to combine best characteristics of separate imaging modalities. Recently, several studies have developed multimodality imaging probe for studying CSCs. FDG-PET/CT is known as a standard clinical practice and main diagnostic tool to distinguish cancer cells from normal cells (55). Studies have been shown that patients with ovarian clear cell carcinoma (CCC) have a low mean maximal standardized uptake value of FDG in comparison with patients with serous adenocarcinoma or endometrioid adenocarcinoma in FDG-PET/CT, reflecting glutaminolysis of its CSC-like properties (55).
The ability of NPs as CAs or carriers to efficiently intensify signals in various imaging modalities has demonstrated. Accordingly, incorporation of NPs in multimodality imaging techniques have been investigated, and multimodality nanoprobes were developed (56). Regarding the role of multimodality imaging in tracking CSCs, CD44 antibody-conjugated single-walled carbon nanotubes (SWCNTs) were recently developed as a biocompatible multimodality nanoprobes. CD44 antibody-conjugated SWCNTs and their conjugation with various imaging tracers i.e., SPION, Gallium-67 or Vivotag-S750 NIR fluorophores allowed noninvasive tracking and monitoring of BCSCs using MRI, SPECT, and NIR-fluorescence imaging (57).
Zhou et al. synthesized a multifunctional peptide-fluorescent-magnetic nanocomposites (Fe3O4@PEI@ Cy5.5@PEG@HCBP-1 NPs) to recognize the lung CSCs specifically, and enrich the HCBP-1 positive CSCs from H460 tumor xenografts effectively (58). Their results showed that this agent can be used for fluorescent and MR imaging of lung CSCs in tumor xenografts. A novel smart immunomagnetic nanosensor (anti-CD133 mAb-conjugated nanoscale magnetic sensor (mAb-nano-MSN)) is introduced for MI of the targeted glioblastoma CSCs. This fabricated immunomagnetic nanosensor can be monitored real-time in the targeted glioblastoma CSCs as a fluorescence nanoprobe and distinguished CAs for MRI (59).
Conclusion and future perspectives
In vivo monitoring and tracking of CSCs can result in achieving novel therapeutic approaches, improving radiation oncology outcomes. In vivo CSCs imaging can provide opportunities to investigate therapeutic response or metastatic CSCs, tumor propagation and plasticity of CSCs at high resolution. To date, there is no best imaging modality to track CSCs in vivo. However, PET and MRI currently are useful modality for investigating therapeutic response or metastatic CSCs, and also OI techniques can be suitable modality for evaluating tumor propagation and plasticity of CSCs. CSCs is known as an important cause of local recurrences and distant metastases, thus in vivo imaging of CSCs can be a useful tool to detect these events at the early steps.
Currently, PET, MRI, and OI are promising MI modalities that can be used for clinical application of CSCs detection. Advances in CSC-specific tracers, CAs, and imaging modalities with high resolution will result in improving MI for CSCs detection.
Conflict of Interests
The authors declare that they have no competing interests.
Cite this article as: Ghaffari1 H, Atashzar MR, Abdollahi H. Molecular imaging in tracking cancer stem cells: A review. Med J Islam Repub Iran. 2020 (3 Aug);34:90. https://doi.org/10.34171/mjiri.34.90
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Atashzar MR, Baharlou R, Karami J, Abdollahi H, Rezaei R, Pourramezan F. et al. Cancer stem cells: A review from origin to therapeutic implications. J Cell Physiol. 2020;235:790–803. doi: 10.1002/jcp.29044. [DOI] [PubMed] [Google Scholar]
- 3.Ayob AZ, Ramasamy TS. Cancer stem cells as key drivers of tumour progression. J Biomed Sci. 2018;25:20. doi: 10.1186/s12929-018-0426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghaffari H, Beik J, Talebi A, Mahdavi SR, Abdollahi H. New physical approaches to treat cancer stem cells: a review. Clin Transl Oncol. 2018;20:1502–21. doi: 10.1007/s12094-018-1896-2. [DOI] [PubMed] [Google Scholar]
- 5.Al-Hajj M, Becker MW, Wicha M, Weissman I, Clarke MF. Therapeutic implications of cancer stem cells. Curr Opin Genet Dev. 2004;14:43–7. doi: 10.1016/j.gde.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Chen K, Huang YH, Chen JL. Understanding and targeting cancer stem cells: therapeutic implications and challenges. Acta Pharmacol Sin. 2013;34:732. doi: 10.1038/aps.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu Z, Pestell TG, Lisanti MP, Pestell RG. Cancer stem cells. Int J Biochem Cell Biol. 2012;44:2144–51. doi: 10.1016/j.biocel.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 9.Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med. 2007;58:267–84. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- 10.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–51. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 11.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J. et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–8. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 12.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J. et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–8. [PubMed] [Google Scholar]
- 14.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S. et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–35. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 15.O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–10. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 16.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–51. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 17.Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connolly D, Foster R. et al. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proc Natl Acad Sci U S A. 2006;103:11154–9. doi: 10.1073/pnas.0603672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S. et al. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65:9328–37. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- 19.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M. et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–67. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krause M, Dubrovska A, Linge A, Baumann M. Cancer stem cells: Radioresistance, prediction of radiotherapy outcome and specific targets for combined treatments. Adv Drug Deliv Rev. 2017;109:63–73. doi: 10.1016/j.addr.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Pysz MA, Gambhir SS, Willmann JK. Molecular imaging: current status and emerging strategies. Clin Radiol. 2010;65:500–16. doi: 10.1016/j.crad.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mankoff DA. A definition of molecular imaging. J Nucl Med. 2007;48:18n, 21n. [PubMed] [Google Scholar]
- 23.Abdollahi H. SU‐E‐I‐39: Molecular Image Guided Cancer Stem Cells Therapy. Med Phys. 2014;41:138–9. [Google Scholar]
- 24.Shen B, Huang T, Sun Y, Jin Z, Li XF. Revisit 18F-fluorodeoxyglucose oncology positron emission tomography: "systems molecular imaging" of glucose metabolism. Oncotarget. 2017;8:43536–42. doi: 10.18632/oncotarget.16647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin ZH, Sogawa C, Furukawa T, Saito Y, Aung W, Fujibayashi Y. et al. Basic studies on radioimmunotargeting of CD133-positive HCT116 cancer stem cells. Mol Imaging. 2012;11:445–50. [PubMed] [Google Scholar]
- 26.Gaedicke S, Braun F, Prasad S, Machein M, Firat E, Hettich M. et al. Noninvasive positron emission tomography and fluorescence imaging of CD133+ tumor stem cells. Proc Natl Acad Sci USA. 2014;111:E692–E701. doi: 10.1073/pnas.1314189111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawai T, Yasuchika K, Ishii T, Katayama H, Yoshitoshi EY, Ogiso S. et al. Keratin 19, a Cancer Stem Cell Marker in Human Hepatocellular Carcinoma. Clin Cancer Res. 2015;21:3081–91. doi: 10.1158/1078-0432.CCR-14-1936. [DOI] [PubMed] [Google Scholar]
- 28.Yoshii Y, Furukawa T, Kiyono Y, Watanabe R, Mori T, Yoshii H. et al. Internal radiotherapy with copper-64-diacetyl-bis (N4-methylthiosemicarbazone) reduces CD133+ highly tumorigenic cells and metastatic ability of mouse colon carcinoma. Nucl Med Biol. 2011;38:151–7. doi: 10.1016/j.nucmedbio.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Kalimuthu S, Jeong JH, Oh JM, Ahn BC. Drug Discovery by Molecular Imaging and Monitoring Therapy Response in Lymphoma. Int J Mol Sci. 2017:18. doi: 10.3390/ijms18081639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yahyapour R, Farhood B, Graily G, Rezaeyan A, Rezapoor S, Abdollahi H. et al. Stem Cell Tracing Through MR Molecular Imaging. Tissue Eng Regen Med. 2018;15:249–61. doi: 10.1007/s13770-017-0112-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu W, Frank JA. Detection and quantification of magnetically labeled cells by cellular MRI. Eur J Radiol. 2009;70:258–64. doi: 10.1016/j.ejrad.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heyn C, Ronald JA, Mackenzie LT, MacDonald IC, Chambers AF, Rutt BK. et al. In vivo magnetic resonance imaging of single cells in mouse brain with optical validation. Magn Reson Med. 2006;55:23–9. doi: 10.1002/mrm.20747. [DOI] [PubMed] [Google Scholar]
- 33.Arbab AS, Bashaw LA, Miller BR, Jordan EK, Lewis BK, Kalish H. et al. Characterization of biophysical and metabolic properties of cells labeled with superparamagnetic iron oxide nanoparticles and transfection agent for cellular MR imaging. Radiology. 2003;229:838–46. doi: 10.1148/radiol.2293021215. [DOI] [PubMed] [Google Scholar]
- 34.Leuschner C, Kumar CS, Hansel W, Soboyejo W, Zhou J, Hormes J. LHRH-conjugated magnetic iron oxide nanoparticles for detection of breast cancer metastases. Breast Cancer Res Treat. 2006;99:163–76. doi: 10.1007/s10549-006-9199-7. [DOI] [PubMed] [Google Scholar]
- 35.Wang X, Wei F, Liu A, Wang L, Wang JC, Ren L. et al. Cancer stem cell labeling using poly(L-lysine)-modified iron oxide nanoparticles. Biomaterials. 2012;33:3719–32. doi: 10.1016/j.biomaterials.2012.01.058. [DOI] [PubMed] [Google Scholar]
- 36.Long CM, Bulte JWM. In vivo tracking of cellular therapeutics using magnetic resonance imaging. Expert Opin Biol Ther. 2009;9:293–306. doi: 10.1517/14712590802715723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Artemov D, Mori N, Okollie B, Bhujwalla ZM. MR molecular imaging of the Her-2/neu receptor in breast cancer cells using targeted iron oxide nanoparticles. Magn Reson Med. 2003;49:403–8. doi: 10.1002/mrm.10406. [DOI] [PubMed] [Google Scholar]
- 38.Choi Y, Kim HS, Cho KW, Lee KM, Yi YJ, Eun SJ. et al. Noninvasive identification of viable cell populations in docetaxel-treated breast tumors using ferritin-based magnetic resonance imaging. PloS one. 2013;8:e52931. doi: 10.1371/journal.pone.0052931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heryanto YD, Achmad A, Taketomi-Takahashi A, Tsushima Y. In vivo molecular imaging of cancer stem cells. Am J Nucl Med Mol Imaging. 2014;5:14–26. [PMC free article] [PubMed] [Google Scholar]
- 40.Choi SH, Cho HR, Kim HS, Kim YH, Kang KW, Kim H. et al. Imaging and quantification of metastatic melanoma cells in lymph nodes with a ferritin MR reporter in living mice. NMR Biomed. 2012;25:737–45. doi: 10.1002/nbm.1788. [DOI] [PubMed] [Google Scholar]
- 41.Townson JL, Ramadan SS, Simedrea C, Rutt BK, MacDonald IC, Foster PJ. et al. Three-Dimensional Imaging and Quantification of Both Solitary Cells and Metastases in Whole Mouse Liver by Magnetic Resonance Imaging. Cancer Res. 2009;69:8326. doi: 10.1158/0008-5472.CAN-09-1496. [DOI] [PubMed] [Google Scholar]
- 42.Lim E-K, Kim H-O, Jang E, Park J, Lee K, Suh J-S. et al. Hyaluronan-modified magnetic nanoclusters for detection of CD44-overexpressing breast cancer by MR imaging. Biomaterials. 2011;32:7941–50. doi: 10.1016/j.biomaterials.2011.06.077. [DOI] [PubMed] [Google Scholar]
- 43.Sun Y, Kim HS, Park J, Li M, Tian L, Choi Y. et al. MRI of breast tumor initiating cells using the extra domain-B of fibronectin targeting nanoparticles. Theranostics. 2014;4:845–57. doi: 10.7150/thno.8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun Y, Kim HS, Kang S, Piao YJ, Jon S, Moon WK. Magnetic Resonance Imaging-Guided Drug Delivery to Breast Cancer Stem-Like Cells. Adv Healthc Mater. 2018;7:e1800266. doi: 10.1002/adhm.201800266. [DOI] [PubMed] [Google Scholar]
- 45.Hart LS, El-Deiry WS. Invincible, but not invisible: imaging approaches toward in vivo detection of cancer stem cells. J Clin Oncol. 2008;26:2901–10. doi: 10.1200/JCO.2008.16.9573. [DOI] [PubMed] [Google Scholar]
- 46.de Almeida PE, van Rappard JRM, Wu JC. In vivo bioluminescence for tracking cell fate and function. Am J Physiol Heart Circ Physiol. 2011;301:H663–H71. doi: 10.1152/ajpheart.00337.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sweeney TJ, Mailander V, Tucker AA, Olomu AB, Zhang W, Cao Y. et al. Visualizing the kinetics of tumor-cell clearance in living animals. Proc Natl Acad Sci U S A. 1999;96:12044–9. doi: 10.1073/pnas.96.21.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsurumi C, Esser N, Firat E, Gaedicke S, Follo M, Behe M. et al. Non-invasive in vivo imaging of tumor-associated CD133/prominin. PloS One. 2010;5:e15605. doi: 10.1371/journal.pone.0015605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lathia JD, Gallagher J, Myers JT, Li M, Vasanji A, McLendon RE. et al. Direct in vivo evidence for tumor propagation by glioblastoma cancer stem cells. PloS One. 2011;6:e24807. doi: 10.1371/journal.pone.0024807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ustione A, Piston DW. A simple introduction to multiphoton microscopy. J Microsc. 2011;243:221–6. doi: 10.1111/j.1365-2818.2011.03532.x. [DOI] [PubMed] [Google Scholar]
- 51.Zomer A, Ellenbroek SI, Ritsma L, Beerling E, Vrisekoop N, Van Rheenen J. Intravital imaging of cancer stem cell plasticity in mammary tumors. Stem Cells. 2013;31:602–6. doi: 10.1002/stem.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park J, Ku M, Kim E, Park Y, Hong Y, Haam S. et al. CD44-specific supramolecular hydrogels for fluorescence molecular imaging of stem-like gastric cancer cells. Integr Biol (Camb) 2013;5:669–72. doi: 10.1039/c3ib20203h. [DOI] [PubMed] [Google Scholar]
- 53.Liu H, Patel MR, Prescher JA, Patsialou A, Qian D, Lin J. et al. Cancer stem cells from human breast tumors are involved in spontaneous metastases in orthotopic mouse models. Proc Natl Acad Sci U S A. 2010;107:18115–20. doi: 10.1073/pnas.1006732107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vlashi E, Kim K, Lagadec C, Donna LD, McDonald JT, Eghbali M. et al. In vivo imaging, tracking, and targeting of cancer stem cells. J Natl Cancer Inst. 2009;101:350–9. doi: 10.1093/jnci/djn509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sato M, Kawana K, Adachi K, Fujimoto A, Taguchi A, Fujikawa T. et al. Low uptake of fluorodeoxyglucose in positron emission tomography/computed tomography in ovarian clear cell carcinoma may reflect glutaminolysis of its cancer stem cell-like properties. Oncol Rep. 2017;37:1883–8. doi: 10.3892/or.2017.5398. [DOI] [PubMed] [Google Scholar]
- 56.Li X, Zhang XN, Li XD, Chang J. Multimodality imaging in nanomedicine and nanotheranostics. Cancer Biol Med. 2016;13:339–48. doi: 10.20892/j.issn.2095-3941.2016.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Al Faraj A, Shaik AS, Al Sayed B, Halwani R, Al Jammaz I. Specific targeting and noninvasive imaging of breast cancer stem cells using single-walled carbon nanotubes as novel multimodality nanoprobes. Nanomedicine (Lond) 2016;11:31–46. doi: 10.2217/nnm.15.182. [DOI] [PubMed] [Google Scholar]
- 58.Zhou X, Chen L, Wang A, Ma Y, Zhang H, Zhu Y. Multifunctional fluorescent magnetic nanoparticles for lung cancer stem cells research. Biointerfaces. 2015;134:431–9. doi: 10.1016/j.colsurfb.2015.07.030. [DOI] [PubMed] [Google Scholar]
- 59.Wang X, Li B, Li R, Yang Y, Zhang H, Tian B. et al. Anti-CD133 monoclonal antibody conjugated immunomagnetic nanosensor for molecular imaging of targeted cancer stem cells. Sens Actuators B. 2018;255:3447–57. [Google Scholar]