Abstract
Background.
Anxiety symptoms gradually emerge during childhood and adolescence. Individual differences in behavioral inhibition (BI), an early-childhood temperament, may shape developmental paths through which these symptoms arise. Cross-sectional research suggests that level of early-childhood BI moderates associations between later anxiety symptoms and threat-related amygdala–prefrontal cortex (PFC) circuitry function. However, no study has characterized these associations longitudinally. Here, we tested whether level of early-childhood BI predicts distinct evolving associations between amygdala–PFC function and anxiety symptoms across development.
Methods.
Eighty-seven children previously assessed for BI level in early childhood provided data at ages 10 and/or 13 years, consisting of assessments of anxiety and an fMRI-based dot-probe task (including threat, happy, and neutral stimuli). Using linear-mixed-effects models, we investigated longitudinal changes in associations between anxiety symptoms and threat-related amygdala–PFC connectivity, as a function of early-childhood BI.
Results.
In children with a history of high early-childhood BI, anxiety symptoms became, with age, more negatively associated with right amygdala–left dorsolateral-PFC connectivity when attention was to be maintained on threat. In contrast, with age, low-BI children showed an increasingly positive anxiety–connectivity association during the same task condition. Behaviorally, at age 10, anxiety symptoms did not relate to fluctuations in attention bias (attention bias variability, ABV) in either group; by age 13, low-BI children showed a negative anxiety–ABV association, whereas high-BI children showed a positive anxiety–ABV association.
Conclusions.
Early-childhood BI levels predict distinct neurodevelopmental pathways to pediatric anxiety symptoms. These pathways involve distinct relations among brain function, behavior, and anxiety symptoms, which may inform diagnosis and treatment.
Keywords: Amygdala, anxiety, attention, behavioral inhibition, children, connectivity, developmental, fMRI, prefrontal cortex
Introduction
Anxiety symptoms gradually emerge during childhood and adolescence (Kessler et al., 2005a). Research on behavioral inhibition (BI), an early-childhood fearful temperament, suggests distinct neurodevelopmental paths through which these symptoms arise (Fox et al., 2001; Fox and Kalin, 2014; Pine and Fox, 2015). Specifically, in children with a history of high v. low BI, anxiety symptoms exhibit distinct associations with attention and associated function in amygdala–prefrontal cortex (PFC) circuitry (Hardee et al., 2013; Pine and Fox, 2015; White et al., 2017a). However, no brain imaging study tracks longitudinal associations between attention-related fronto-limbic circuitry function and anxiety symptoms as a function of early-childhood BI, precluding strong inferences on developmental trajectories. Here, we investigated longitudinal associations across peri-adolescence between anxiety symptoms and amygdala–PFC function during a threat-related attention task, in children assessed for BI in early childhood.
High relative to low BI manifests differently with age. In infancy and early childhood, BI manifests as increased reactivity to novelty and threat; in later childhood, it presents as reticence in social circumstances; and, in later life, it confers high risk for anxiety disorders (Fox et al., 2001; Hane et al., 2008; Pine and Fox, 2015). However, the association between early-childhood BI levels and later anxiety symptoms is modest, mixed at times (Caspi et al., 1996; Prior et al., 2000; Chronis-Tuscano et al., 2009; Lewis-Morrarty et al., 2012; Frenkel et al., 2015), and with evidence of moderation by cognitive factors (e.g. Barker et al., 2015; Frenkel et al., 2015; Buzzell et al., 2017). Thus, early-childhood BI levels may evolve into later anxiety symptomatology; however, the nature of this link is not yet understood.
Behavioral studies indicate that preferential allocation of attention to threat stimuli is one factor moderating this link (McDermott et al., 2009; White et al., 2011; Fox and Pine, 2012; Fox and Kalin, 2014; Perez-Edgar et al., 2014). Attention biases to threat relate to anxiety symptoms in youth and adults (Bar-Haim et al., 2007; Abend et al., 2018). In addition, threat biases moderate the association between early-childhood BI and later anxiety-related symptoms; specifically, children with a history of high v. low BI who display a threat bias also exhibit increased symptoms of anxiety (Perez-Edgar et al., 2010; Perez-Edgar et al., 2011; Cole et al., 2016; Morales et al., 2017; White et al., 2017a). Thus, while behaviorally inhibited temperament and attention biases to threat each predict anxiety symptoms independently, the interaction between these two factors further qualifies the conditions contributing to the development of anxiety. Moreover, these behavioral data suggest that attention biases may define two developmental pathways into later anxiety symptoms. In children with early BI, a bias toward threat identifies a particularly elevated risk, while in children without early BI, anxiety develops in the absence of such associations. Furthermore, the nature of the association between BI and attention bias changes with age, suggesting dynamic developmental interactions among early-childhood BI, attention to threat, and anxiety symptoms (White et al., 2017a). Delineating the developmental interplay among these factors informs our understanding of the emergence of pediatric anxiety symptoms (Pine and Fox, 2015).
Behavioral indices of attention allocation provide important, but limited, insight into mechanisms giving rise to anxiety, due to poor reliability of such indices and indirect relations between behavioral and neural correlates of attention (e.g. Price et al., 2015; White et al., 2016). Stronger insights may be gained through brain imaging, which yields reliable markers of threat-related attention processes (White et al., 2016). Previous studies identify amygdala–PFC functional connectivity, primarily with ventrolateral and dorsolateral PFC (DLPFC), during threat-related attention tasks as relating to anxiety-related symptoms (Monk et al., 2008; Hardee et al., 2013; Fu et al., 2017; White et al., 2017b). The amygdala and PFC constitute key nodes in a neural circuit influencing attention allocation to threats (LeDoux, 1996; Pine and Fox, 2015; Shechner and Bar-Haim, 2016). Variations in the interplay between these nodes may influence regulation of attention allocation to threat, contributing to the emergence of anxiety symptoms (Pine and Fox, 2015; LeDoux and Pine, 2016).
Amygdala–PFC circuitry undergoes substantial developmental change across childhood and adolescence. The plasticity of this circuitry may contribute to the evolving associations between threat-related attention and anxiety symptoms. Evidence suggests that the amygdala matures early (e.g. Thomas et al., 2001; Ulfig et al., 2003), whereas the PFC shows a more prolonged developmental trajectory (e.g. Monk et al., 2003; Gogtay et al., 2004). As such, the nature of functional connectivity between these regions may change with development in ways that influence effective PFC regulation of amygdala activity and its impact on attention allocation (e.g. Perlman and Pelphrey, 2011; Gee et al., 2013). In this developmental context, different levels of early-childhood BI, manifesting as a varying fearful temperament, may reflect early-onset differences in amygdala–PFC circuitry function that uniquely influence the emergence of anxiety symptoms as this circuitry matures (Perlman and Pelphrey, 2011; Birn et al., 2014; Casey et al., 2015; Pine and Fox, 2015).
Data from a small body of cross-sectional work using the dot-probe task support our hypotheses regarding dynamic relations among BI, anxiety, and attention-related amygdala–PFC connectivity. Two studies are particularly relevant. One examined clinically anxious and non-anxious youths aged 8–17 years. In this study, White et al. (2017b) observed that anxiety was related to positive amygdala–PFC connectivity when attention was maintained at a location previously occupied by a threat stimulus (so-called ‘threat-congruent’ trials). These findings suggest that perturbed amygdala–PFC connectivity manifests in anxiety during the processing of attended threats (White et al., 2017b). However, this study did not consider the influence of BI. The second study examined adults considerably older than in White et al. (2017b) but followed since infancy and classified with a history of high v. low BI (Hardee et al., 2013). In high- v. low-BI subjects, this study found stronger negative amygdala–PFC connectivity when processing threat stimuli, particularly in the presence of ongoing anxiety symptoms. Taken together, these studies related anxiety symptoms to perturbed amygdala–PFC connectivity and suggest that the age of the participant and the presence of BI influence relations this association. Specifically, the studies suggest a possible age-related shift in amygdala–PFC connectivity in anxiety symptoms from early positive to later negative connectivity (Hardee et al., 2013; White et al., 2017b). Critically, however, no longitudinal brain imaging study examines how early-childhood BI predicts distinct patterns of evolving associations between connectivity and anxiety symptoms. Thus, our hypotheses remain tentative.
Here, we used a prospective longitudinal design to examine whether high v. low early-childhood BI predicts distinct neurodevelopmental pathways to anxiety symptoms. To this end, we documented longitudinal associations between anxiety symptoms and threat-related amygdala–PFC connectivity in a sample of children who were previously assessed for BI from infancy through early childhood. Anxiety symptoms and amygdala–PFC connectivity were both measured at ages 10 and 13 years, allowing us to examine associations among these factors using longitudinal data during a critical developmental period when anxiety symptoms typically increase (Kessler et al., 2005a, 2005b). We hypothesized that high v. low early-childhood BI predicts distinct associations between anxiety symptoms and threat-related amygdala–PFC function over time. Given previous cross-sectional findings in distinct age groups, we predicted age-related changes in the relations among BI, anxiety, and amygdala–PFC connectivity. We specifically predicted that, among high-BI children, anxiety symptoms relate to positive amygdala–PFC connectivity, indicative of increased engagement by threat. However, we expected this relationship to attenuate with age, as this circuitry matures (Gee et al., 2013; Hardee et al., 2013; White et al., 2017b).
Methods and materials
Participants
Participants were drawn from a larger community cohort of 291 developmentally healthy children selected at 4 months of age based on criteria for negative and positive reactivity to novelty (Fox et al., 2001), for a longitudinal study on the temperament of BI (see details in Hane et al. (2008)). Individuals were assessed at ages 2 and 3 years for levels of BI (see below). See Supplementary material for information about inclusion/exclusion criteria and diagnoses. In terms of behavioral data, associations between BI, anxiety symptoms, and threat bias at ages 5 and 7 years were previously reported for this full sample (White et al., 2017a). No previous study reports on the brain imaging data in the current study.
A total of 107 participants meeting inclusion criteria provided data for the current study. Data were collected at two time points: around age 10 (M = 10.51 years, S.D. = 0.43) and age 13 (M = 13.04, S.D. = 0.65) years, providing time-points before and after the median age-of-onset of anxiety disorders in the general population (11 years; Kessler et al., 2005a). Eight participants who provided data at age 10 were excluded from analyses (five performed the attention task with sub-threshold accuracy, two aborted, one had excessive head motion during scanning). Twelve participants who provided data at age 13 were excluded (two for sub-threshold accuracy, three aborted, seven for data collection technical issues). Thus, the final sample consisted of data provided by 87 participants (81%). Of those, 61 participants provided data at age 10 (36 females) and 64 provided data at age 13 (37 females), for a total of 125 scans (see Table 1 for demographic details). Thirty-eight participants provided data at both time-points. To provide more generalizable group effects, data from all 87 participants were used in analyses (see below). Study procedures were approved by the National Institute of Mental Health and University of Maryland Institutional Review Boards. Informed consent and assent were obtained from parents and youth, respectively.
Table 1.
Sample demographics, clinical indices, and performance on the dot-probe task, by group and time-point of data collection
| Age 10a | Age 13b | Total | ||||
|---|---|---|---|---|---|---|
| Low BI n = 28 | High BI n = 33 | Low BI n = 29 | High BI n = 35 | Low BI n = 39 | High BI n = 48 | |
| Demographics | ||||||
| Sex (% female) | 57.1% | 60.6% | 59.3% | 56.8% | 58.2% | 58.6% |
| Age (years) | 10.42 (0.40) | 10.58 (0.45) | 13.09 (0.68) | 13.00 (0.64) | 11.72 (1.47) | 11.93 (1.42) |
| IQ | 114.40 (12.57) | 115.85 (12.40) | 115.26 (13.76) | 117.81 (13.35) | 114.82 (13.05) | 116.89 (12.86) |
| Clinical indices | ||||||
| BI scores | −0.59 (0.42) | 0.55 (0.49) | −0.56 (0.48) | 0.49 (0.46) | −0.58 (0.45) | 0.52 (0.47) |
| SCARED | 18.29 (9.39) | 14.67 (7.80) | 11.24 (7.68) | 10.32 (6.72) | 14.83 (9.22) | 12.37 (7.52) |
| Current anxiety dx (n) | 1 | 3 | 5 | 4 | 6 | 7 |
| Dot-probe performance | ||||||
| Threat bias (ms) | 0.38 (30.31) | 6.54 (31.18) | 9.47 (21.94) | 12.00 (37.83) | 4.85 (27.85) | 9.42 (34.02) |
| Happy bias (ms) | 9.50 (30.74) | −0.25 (30.74) | −1.45 (26.17) | 7.73 (36.00) | 4.13 (29.87) | 3.97 (32.62) |
| ABV threat | 0.047 (0.020) | 0.048 (0.021) | 0.046 (0.016) | 0.048 (0.019) | 0.046 (0.018) | 0.048 (0.020) |
| ABV happy | 0.041 (0.015) | 0.050 (0.014) | 0.052 (0.021) | 0.048 (0.020) | 0.046 (0.019) | 0.049 (0.017) |
BI, behavioral inhibition; SCARED, Screen for Childhood Anxiety Related Emotional Disorders (total scores); dx, diagnosis; ABV, attention bias variability.
At age 10 and 13, BI scores significantly differed between the low- and high-BI groups, whereas the other factors did not (see text).
n = 23 provided data only at age 10.
n = 26 provided data only at age 13.
Behavioral inhibition assessment
BI was assessed in the larger cohort (N = 291) when children were 2 and 3 years of age. Laboratory assessments and parent reports were combined into BI composite scores that were then standardized across the full cohort, and median-split into low- and high-BI samples that were used in several studies on developmental trajectories as a function of early-childhood BI (Fox et al., 2001; Lahat et al., 2014; Lamm et al., 2014; Buzzell et al., 2017; Lahat et al., 2018). For comparability with these previous reports, we retained participants’ original categorical group allocation in the current study (see Table 1); additional analyses using BI as a continuous measure are reported in Supplementary material. At each time-point, the low- and high-BI groups differed in mean BI composite score (the age 10 and 13 samples were not identical since not all participants contributed data at both time-points), ps < 0.001, but not in age, sex, IQ, attention bias indices (see below), or the Screen for Childhood Anxiety Related Emotional Disorders (SCARED) scores (or their change over time), ps > 0.11, ensuring that early-childhood BI differentiated the groups.
Current anxiety symptom severity
Current anxiety symptom severity was measured within 6 weeks of each scan using SCARED (Birmaher et al., 1997, 1999), a 41-item child- and parent-report measure of anxiety symptomology. Cronbach’s α for youth and parent measures at each time-point were >0.90. Total scores were calculated by summing all item-level scores. Total scores from each parent–youth dyad were averaged (Guyer et al., 2008; Michalska et al., 2017; Shechner et al., 2017) to create a mean score reflecting current symptom severity for each participant.
Dot-probe task
Considerable research applies the dot-probe task to measure attention biases to threat-related stimuli (MacLeod et al., 1986; Bar-Haim et al., 2007; Van Bockstaele et al., 2014; Abend et al., 2018), yielding reliable amygdala–PFC connectivity measures (White et al., 2016). To test for specificity of findings to threat and given previous findings in BI regarding bias to positive-valence stimuli (Perez-Edgar et al., 2010; Shechner et al., 2012), the task included angry and happy faces. At each time-point, participants completed the same version of the fMRI dot-probe task (Supplementary material). Briefly, in each trial (online Supplementary Fig. S1), a pair of angry and neutral, happy and neutral, or two neutral faces was presented. A probe then replaced one of the faces (counterbalanced across emotions). Thus, five trial types were presented: angry-congruent (AC, 48 trials), angry-incongruent (AI, 48 trials), happy-congruent (HC, 48 trials), happy-incongruent (HI, 48 trials), and neutral (N, 96 trials; neutral–neutral trials). These conditions enabled us to isolate neural and behavioral responses to threat-related and positive stimuli.
Imaging data acquisition and analysis
fMRI acquisition and preprocessing
Neuroimaging data were collected on a 3 T General Electric scanner (Waukesha, Wisconsin, USA), using a 32-channel head coil. Blood oxygen level-dependent (BOLD) signal was measured by echoplanar imaging at 2.5 × 2.5 × 3.0 mm voxel resolution. Data were preprocessed and analyzed using AFNI (Cox, 1996). See online Supplementary material for additional details.
Analyses
Based on previous findings (Monk et al., 2008; Hardee et al., 2013; White et al., 2016; White et al., 2017b), primary analyses focused on task-related amygdala–PFC functional connectivity. Individual-level general linear models (GLMs) included regressors for correct trials across the five task conditions (AC, AI, HC, HI, N) and nuisance regressors. To identify task-specific differences in functional connectivity, we used generalized psychophysiological interaction (gPPI; McLaren et al., 2012), with anatomically defined right and left amygdala seeds (Hardee et al., 2013; White et al., 2017b). In addition, GLMs were also created using the same regressors to test secondary analyses of changes in mean BOLD signal activation. Estimated βs, one for each regressor, were generated at the individual-subject level and submitted to group-level analyses.
Of note, some participants provided data only at one time-point (see Table 1 for breakdown by BI group). Absence of data was not associated with BI group, SCARED scores, age, or gender, all ps > 0.27. To provide generalizable results, prior research indicates the importance of basing longitudinal analyses on all participants providing data at any time-point (Matta et al., 2017). Therefore, group-level analyses were conducted on all participants contributing at least one useable data-point (N = 87) using linear mixed-effects (LME) models through AFNI’s 3dLME program (Chen et al., 2013). LME overcomes missing data in longitudinal designs, yielding more reliable effect estimates than complete-case analyses (Donders et al., 2006; Chen et al., 2013; Matta et al., 2017). Group (Low BI, High BI) served as a between-subjects factor. Condition (AC, AI, HC, HI, N) served as a within-subject factor, and Anxiety (SCARED score, collected around the time of each scan and mean-centered separately for each scan) served as a continuous, between-subjects factor. Data for the Condition and Anxiety factors were collected at each of the two time-points; thus, reflecting the longitudinal design of the study, a fourth variable, Time (Age 10, Age 13), was included as a within-subject factor reflecting the longitudinal design of the study. Finally, in addition to these fixed-effects variables and their interactions, random effects for intercept and SCARED scores across participants were included in the LME model.
Across all analyses, significant clusters were identified using an initial voxel-wise threshold of p < 0.005. Based on previous findings and our focus on amygdala–PFC connectivity (White et al., 2016; White et al., 2017b), we applied a mask encompassing all gray-matter PFC voxels with y ⩾ 0 coordinates. Using AFNI’s 3dClustSim tool, which assumes a non-Gaussian auto-correlation smoothing function (Cox et al., 2017) in light of Eklund et al. (2016), we calculated a cluster-wise threshold size of 734 mm3 reflecting a family-wise error rate of α = 0.05 (based on 10 000 Monte-Carlo simulations). Group maps were also thresholded to include only voxels for which 90% of participants had valid data (White et al., 2016; Stoddard et al., 2017; White et al., 2017b). Within each significant cluster identified in group-level analyses, estimated βs for each individual participant were extracted for further analysis as described below.
Our primary hypothesis was that the association between current anxiety symptoms and threat-related amygdala–PFC functional connectivity changes with development, and that early-childhood BI moderates this developmental effect. This hypothesis was tested via gPPI analyses, one each for the left and right amygdala seeds. In each analysis, gPPI β estimates were submitted to an LME model testing the omnibus statistics for the Group × Anxiety × Condition × Time interaction, with the Time factor representing the two time-points (ages 10 and 13) at which anxiety and fMRI task data were collected. Effects within significant clusters identified by this four-way interaction were interpreted by decomposing the interaction into lower level interactions, and then into effect estimates of two-variable associations (b slope coefficients) tested using χ2 likelihood ratio, all within the omnibus LME models (online Supplementary Fig. S2). As secondary analyses, we examined the omnibus effect on mean BOLD signal activation using the same analytic plan. In addition to threat-related processing, prior behavioral studies also document existing, but weaker, associations among attention biases to positive stimuli, anxiety, and BI (Perez-Edgar et al., 2010; Shechner et al., 2012; White et al., 2017a); as such, we also report effects on task conditions involving happy faces.
Behavioral data analysis
To complement the analyses of imaging data, two types of behavioral measures indexing attentional processes were analyzed: attention bias and attention bias variability (ABV) scores (Bar-Haim et al., 2007; Shechner and Bar-Haim, 2016).
Attention bias scores
Attention biases to threat-related stimuli have been associated with anxiety, including in children and adolescents (Bar-Haim et al., 2007; Van Bockstaele et al., 2014; Abend et al., 2018). Here, we computed attention bias scores to threat and to positive stimuli (see online supplementary material). An analytic approach similar to that used for the imaging data was applied to bias scores, with scores submitted to an LME model testing the omnibus effect for the Group (Low BI, High BI) × Anxiety (SCARED scores) × Condition (Threat, Happy) × Time (Age 10, Age 13) interaction.
ABV scores
ABV measures within-session temporal variability and fluctuation in attention biases, taken to reflect a loss of attentional control and stability, and has been shown to exhibit stronger reliability than attention bias scores (Iacoviello et al., 2014; Naim et al., 2015; Price et al., 2015; Zvielli et al., 2015; Shechner and Bar-Haim, 2016). ABV scores to threat and to positive stimuli were calculated in accord with previous studies (see online Supplementary material), and then submitted to an LME model testing the Group × Anxiety × Condition (Threat, Happy) × Time interaction.
Statistical analyses were conducted using AFNI, the nlme and phia packages in R (Pinheiro and Bates, 2000), and SPSS 23. All statistical tests were two-sided; significance threshold was set to α ⩽ 0.05.
Results
Imaging analyses
Right amygdala functional connectivity
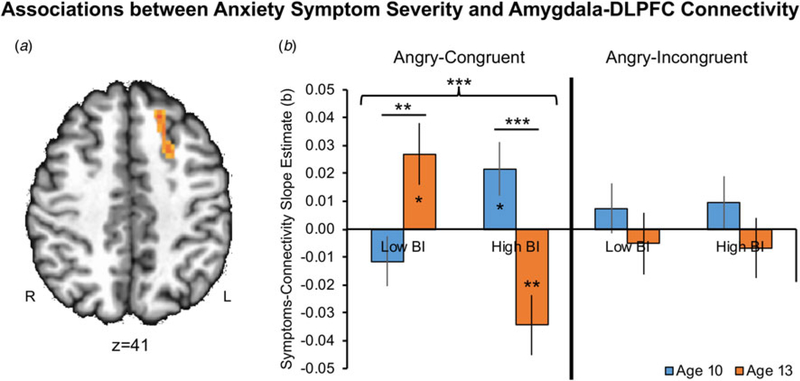
For right amygdala functional connectivity, a significant omnibus Group × Anxiety × Condition × Time interaction emerged in left DLPFC [LPI peak coordinates: [−19,21,39], k = 57, 890 mm3, F(4,500) = 6.26, p = 0.0001; Figure 1a]. To identify the task conditions contributing to this interaction effect, we next tested the Group × Anxiety × Time interaction effect separately within each of the five conditions (online Supplementary Fig. S3). For conciseness, and in line with our primary hypothesis focusing specifically on threat-related processing, Fig. 1b presents the decomposition of this interaction effect for the AC and AI conditions, which differ by requiring attention to either be maintained on, or shifted away from, threat, respectively. The Group × Anxiety × Time interaction was significant only for the AC condition, F(1,32) = 21.04, p = 0.0001, Fig. 1b (left). See online Supplementary material for non-significant results in the remaining four conditions.
Fig. 1.
Associations between current anxiety symptoms and right amygdala–left DLPFC functional connectivity at ages 10 and 13 for the AC and AI task conditions and per BI group (Low BI, High BI). Each bar represents the slope estimate between SCARED scores and PPI β estimates for the respective group, task condition, and age. Asterisks within bars indicate slope estimates significantly different from 0; asterisks between bars indicate significant differences between slope estimates. Error bars indicate one standard error of the slope estimate. DLPFC, dorsolateral prefrontal cortex; SCARED, Screen for Child Anxiety Related Disorders; AC, angry-congruent; AI, angry-incongruent; BI, behavioral inhibition; PPI, psychophysiological interaction; *p < 0.05, **p < 0.01, ***p < 0.001.
Next, within the AC condition (Fig. 1b, left), we examined the longitudinal nature of changes in associations between anxiety symptoms and amygdala–DLPFC connectivity (Anxiety × Time interaction), as a function of BI group. The Anxiety × Time interaction was significant in the low-BI group, F(1,15) = 11.04, p = 0.005. Follow-up correlations indicated no significant association between anxiety symptoms and amygdala–DLPFC connectivity at age 10, b = −0.015, p = 0.20. However, there was a significant positive association between these variables at age 13, b = 0.027, p = 0.015. In the high-BI group, the Anxiety × Time interaction effect also was significant, F(1,17) = 10.53, p = 0.005. However, follow-up tests indicated a different pattern from the low-BI group, with a significant positive association between anxiety symptoms and amygdala–DLPFC connectivity at age 10, b = 0.022, p = 0.024, but a negative association at age 13, b = −0.034, p = 0.001. Thus, children with a history of low v. high BI exhibited distinct longitudinal patterns of association between anxiety symptoms and amygdala–DLPFC connectivity.
A series of separate auxiliary analyses testing the omnibus effect are reported in online Supplementary material. The first analysis aimed to verify the validity of the LME model (Matta et al., 2017) by considering only participants who provided data at both time-points (n = 38). A second analysis used BI as a continuous variable. A third exploratory analysis added sex as a fifth factor which was considered as a nuisance variable in the model. These analyses yielded left DLPFC clusters in full or partial overlap with the DLPFC cluster reported for the primary analysis, albeit with a smaller cluster extent. A fourth auxiliary analysis contrasted brain function between participants who provided data at one time-point v. those who provided data at both time-points, but found no significant differences between these groups.
Additional lower order interaction effects emerged within the model tested in the primary analysis on all participants (Table 2). Of note, a significant Group × Anxiety × Condition interaction emerged in bilateral superior frontal gyrus (SFG) and right dorsomedial PFC (dmPFC). Decomposition of this interaction revealed a similar pattern across clusters (online Supplementary Fig. S4). Specifically, in the HC condition, the low-BI group exhibited a negative association between connectivity and anxiety symptoms ( ps < 0.005), whereas the high-BI group showed a positive association between these factors ( ps < 0.025). This suggests a time-invariant group difference in amygdala–PFC connectivity when processing happy faces.
Table 2.
Results of two functional connectivity analyses using right and left amygdala seeds
| Peak Talairach coordinates | Cluster size (mm3; k) | Peak location | Brodmann area | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Right amygdala seed | ||||||
| Group × Anxiety × Condition × Time | ||||||
| −19 | 21 | 39 | 890; 57 | L dorsolateral PFC | L BA8 | |
| Group × Anxiety × Condition | ||||||
| 11 | 64 | 11 | 5671; 363 | R superior frontal gyrus | R BA10 | |
| −34 | 49 | 16 | 2656; 170 | L superior frontal gyrus | L BA10 | |
| 1 | 34 | 36 | 1359; 87 | R dorsomedial PFC | R BA8 | |
| Group × Anxiety × Time | ||||||
| 4 | 36 | 4 | 1218; 78 | R anterior cingulate | R BA32 | |
| Group × Anxiety | ||||||
| −51 | 21 | 16 | 2015; 129 | L inferior frontal gyrus | L BA45 | |
| Time | ||||||
| −24 | 29 | 41 | 3015; 193 | L medial frontal gyrus | L BA8 | |
| 29 | 6 | −4 | 1296; 83 | L superior frontal gyrus | L BA10 | |
| Left amygdala seed | ||||||
| Group × Anxiety × Time | ||||||
| −54 | 26 | 19 | 984; 63 | L inferior frontal gyrus | L BA45 | |
| Anxiety × Time | ||||||
| −46 | 31 | 16 | 3656; 234 | L inferior frontal gyrus | L BA46 | |
| 36 | 36 | 9 | 1062; 68 | R inferior frontal gyrus | R BA46 | |
| Group × Time | ||||||
| −6 | 24 | 56 | 1171; 75 | L superior frontal gyrus | L BA6 | |
| Time | ||||||
| 9 | 26 | 46 | 6687; 428 | R medial frontal gyrus | R BA8 | |
| 49 | 4 | 19 | 1203; 77 | R inferior frontal gyrus | R BA44 | |
| −21 | 6 | 44 | 984; 63 | L middle frontal gyrus | L BA24 | |
| 34 | 39 | 9 | 937; 60 | R middle frontal gyrus | R BA10 | |
L, left; R, right; PFC, prefrontal cortex; BA, Brodmann area.
Presented are all significant clusters that emerged for all effects tested within the two linear mixed-effects models.
Initial voxel-wise threshold was p = 0.005; cluster size threshold was 734 mm3 (reflecting a family-wise error rate of α = 0.05; k = 56).
Left amygdala functional connectivity
For left amygdala functional connectivity, the hypothesized omnibus Group × Anxiety × Condition × Time interaction did not yield significant clusters. Lower order interaction effects emerged primarily in bilateral inferior and medial frontal gyri (Table 2), but did not include a task-condition term.
Functional activation
No significant clusters emerged for the Group × Anxiety × Condition × Time interaction conducted on PFC activation β estimates. See online Supplementary Table S1 for all clusters identified in lower order effects. In addition, no significant clusters emerged for the Group × Anxiety × Condition × Time interaction in either right or left amygdala, even when cluster extent threshold for these regions was reduced to two contiguous voxels.
Behavioral analyses
Attention bias scores
There was no significant main or interaction effect when testing the Group × Anxiety × Condition × Time effect on attention bias scores, all Fs < 1.52, ps > 0.22.
ABV scores
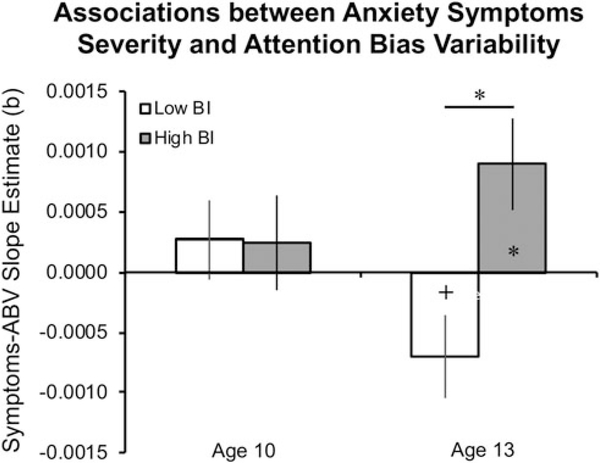
There was no significant Group × Anxiety × Condition × Time interaction on ABV scores, F(1,149) = 0.19, p = 0.67. However, the Group × Anxiety × Time interaction effect tested within the model was significant, F(1,149) = 5.34, p = 0.022 (Fig. 2). This finding indicates that early-childhood BI moderated the change in anxiety–ABV association over development and across both emotional expressions. Follow-up tests indicated no difference in anxiety–ABV associations between the BI groups at age 10, F(1,57) = 0.05, p = 0.83. In contrast, a significant group difference in anxiety–ABV associations emerged by age 13, F(1,60) = 6.64, p = 0.012, with the low-BI group showing a trend toward a negative association between anxiety and ABV, b = −0.0007, p = 0.073, while the high-BI group exhibited a significant positive association, b = 0.0009, p = 0.019. These results suggest that with age, anxiety symptoms relate to increased attentional stability (lower ABV) among individuals with a history of low BI, and to decreased attentional stability (greater ABV) among individuals with a history of high BI.
Fig. 2.
Associations between current anxiety symptom severity and ABV scores at ages 10 and 13, in the low-BI and high-BI groups. Each bar represents the slope estimate between SCARED scores and ABV scores (averaged across angry-neutral and happy-neutral trials) collected at the same age. Asterisks within bars indicate slope estimates significantly different from 0; asterisks between bars indicate significant differences between slope estimates. Error bars indicate one standard error of the slope estimate. ABV, attention bias variability; BI, behavioral inhibition; SCARED, Screen for Child Anxiety Related Disorders; *p < 0.05, +p < 0.10.
Discussion
The current study tested the hypothesis that level of early-childhood BI predicts distinct patterns of associations between anxiety symptoms and attention-related amygdala–PFC circuitry function across development. Consistent with our hypothesis, evidence for two distinct developmental trajectories emerged from the primary neural and behavioral findings. As children with a history of high BI were getting older, anxiety symptoms became more negatively correlated with DLPFC–amygdala connectivity when processing salient, proximal threats; the opposite developmental pattern was observed in low-BI children. Furthermore, on task behavior, a history of high BI predicted a negative association between anxiety symptoms and attentional stability that emerged by age 13, while the opposite pattern was observed in low-BI children. Together, these findings suggest that different early-childhood BI levels predict distinct neurodevelopmental anxiety trajectories.
This study builds on previous work that finds amygdala–PFC connectivity to reliably index threat-related attention orienting (White et al., 2016), which, in turn, relates consistently to anxiety symptoms (Monk et al., 2008; Hardee et al., 2013; Birn et al., 2014; Price et al., 2016; White et al., 2017b). Indeed, the longitudinal associations in the current study emerged specifically in threat-related amygdala–DLPFC connectivity. Such findings are consistent with previous reports of associations between brain activity specifically in the AC condition and anxiety-related phenotypes (Britton et al., 2012; Thai et al., 2016; White et al., 2017b), as well as age-related changes in patterns of amygdala–PFC connectivity (Gee et al., 2013; Wu et al., 2016). Taken together, the findings highlight the importance of developmental changes in brain functions engaged when processing attended, as opposed to unattended, threats (Cisler and Koster, 2010). These developmental changes unfold differently based on a child’s early-life levels of BI and their level of anxiety at the time of scanning. As such, this study extends previous cross-sectional work on the association between anxiety symptoms and neural circuitry function by illuminating distinct developmental trajectories through which these associations arise.
Specifically, these two developmental pathways differ based on early-childhood BI levels and the nature of subsequent associations between amygdala–DLPFC connectivity and anxiety symptoms. PFC–amygdala circuitry has been implicated in emotion regulation and attention control, especially as related to anxiety symptoms (Bishop, 2007; Bishop, 2008; Kim et al., 2011; Etkin et al., 2015). Evidence indicates that this circuitry undergoes substantial maturation during childhood and adolescence (Thomas et al., 2001; Ulfig et al., 2003; Gogtay et al., 2004), causing developmental shifts in PFC regulation of amygdala activity (e.g. Perlman and Pelphrey, 2011; Gee et al., 2013; Wu et al., 2016). Varying levels of BI may reflect early-onset differences in amygdala–PFC circuitry function, which become more pronounced as this circuitry matures in peri-adolescence, and differentially relate to the emergence of anxiety symptoms (Bishop, 2008). Specifically, one developmental pathway is associated with high early-childhood BI. We found that this temperamental pattern of increased fear responses (Fox et al., 2000; Fox et al., 2005; Hane et al., 2008) predicted a pattern in which increases in anxiety symptoms unfold with age in association with decreasing threat-related DLPFC–amygdala connectivity and, at a task-behavior level, attentional stability. A high-BI temperament may therefore indicate an early-emerging deficiency in the capacity to regulate bottom-up attention capture by threats, manifesting as increased behavioral and physiological fear responses throughout childhood (Fox et al., 2005; Bishop, 2007; Birn et al., 2014). As PFC–amygdala circuitry matures, increased anxiety may relate to decreased functional ‘cross-talk’ between these nodes and to decreased attentional stability. As high BI has been shown to confer risk for anxiety disorders later in development (e.g. Frenkel et al., 2015), high-BI children in the current sample are expected to manifest steadily increasing levels of anxiety symptoms over time, coupled with decreasing DLPFC–amygdala connectivity in the AC condition.
Of note, while prior work shows that high BI confers risk for anxiety, the magnitude of this reported association is only moderate, and is not always observed (Caspi et al., 1996; Lewis-Morrarty et al., 2012; Frenkel et al., 2015; White et al., 2017a); indeed, the low- and high-BI groups here manifested equivalent levels of anxiety symptom severity. As a result, many children with low levels of BI may also develop anxiety symptoms. Our findings suggest that, in these children, anxiety symptoms arise through a second developmental pathway, distinct from that for children with a history of high BI. This second trajectory is characterized by reduced fear responses in early childhood as well as increasingly positive associations between anxiety symptoms and both threat-related DLPFC–amygdala connectivity and, at a task-behavior level, attentional stability. A history of low BI may reflect a pattern of amygdala–PFC function associated behaviorally with reduced fear reactivity, potentially reflecting early-emerging, enhanced cortical regulation of bottom-up processing of threat stimuli (Bishop, 2007). With development, however, some individuals may exhibit excessive dominance of top-down influence, and diminished phasic amygdala responses, manifesting as increased amygdala–DLPFC coupling during attention allocation to threat. Tonic and indiscriminate amygdala activation has been associated with diminished sensitivity to the associability of environmental cues, and to inefficient deployment of attentional resources toward them, potentially contributing to the emergence of anxiety (Grupe and Nitschke, 2013). Further research is needed to directly link this maladaptive processing pattern to the emergence of anxiety symptoms.
The relatively low levels of anxiety symptoms at both time-points do constrain these conclusions. However, prior research suggests that BI and sub-clinical anxiety symptoms represent two of the strongest risk factors in childhood for later anxiety disorders (Beesdo et al., 2009). As such, clinical relevance arises from the demonstration of dynamic relationships between these two important risk factors. Findings suggest that these risk factors predict changes in functioning within a circuit previously linked to clinical disorders. This demonstration might shape views of risk factors and their influence on the brain. Consideration should be given to shifting emphasis from static views of childhood risk to more developmental perspectives, emphasizing dynamic interplay among risk factors as drivers of brain development.
The current results point to developmental changes in threat-related DLPFC–amygdala connectivity as a potential factor dissociating phenocopies of pediatric anxiety symptoms, i.e. subtypes of anxiety symptoms with distinct psychobiological mechanisms. Identifying distinct phenocopies of anxiety symptoms may potentially promote more accurate diagnosis and effective treatment. While the groups studied here manifested equivalent levels of anxiety symptom severity, our results suggest that symptoms differentially related to neural and behavioral threat-processing profiles. Phenotyping according to pathophysiological mechanisms, rather than symptom-based diagnostic categories, guides the development of more precisely targeted novel treatments (Insel et al., 2010; Cuthbert, 2015; Shanmugan et al., 2016). For example, procedures aiming to enhance attention stability (Naim et al., 2015; Shechner and Bar-Haim, 2016) may alleviate anxiety symptoms in children with a history of high, but not low, BI. Similarly, treatments developed to modulate neural connectivity patterns (Paret et al., 2016; Nicholson et al., 2017) could consider BI history in determining application parameters and which patients are most likely to respond.
This study identified developmental trajectories characterized by distinct longitudinal patterns of associations between threat-related amygdala–DLPFC connectivity and anxiety symptoms. Of note, other lower order interactions revealed associations between anxiety symptoms and amygdala–PFC connectivity that did not change with age. These associations emerged primarily in the HC condition, with low BI predicting a negative association between anxiety and amygdala–SFG and –dmPFC connectivity, in contrast to high BI, which predicted the opposite pattern. While most research on attention biases in anxiety focuses on threat processing, previous findings in BI note consistent associations between anxiety and attention biases to positive stimuli (Perez-Edgar et al., 2010; Shechner et al., 2012; White et al., 2017a). The current results extend these findings. Thus, at age 10, BI levels predict a similar association between anxiety and connectivity when subjects maintain attention on either positive or threat stimuli, suggesting general reactivity to emotional stimuli as a function of BI. By age 13, however, these associations change for threat stimuli only, suggesting that anxiety symptoms may track more closely with maturational changes in circuitry underlying threat processing (Gee et al., 2013; Casey et al., 2015).
Several limitations of this study should be considered. First, the sample size was modest. Second, there were missing data due to attrition, often related to unavoidable factors such as use of dental hardware in puberty. Third, mean anxiety levels were generally sub-clinical, and did not differ between low- and high-BI groups. While our longitudinal design attempted to capture the emergence of pediatric anxiety symptoms by placing data collection points before and after the median age of anxiety symptoms onset in the general population (age 11 years; Kessler et al., 2005a), symptoms typically peak later in adolescence (Beesdo et al., 2009). As such, our findings suggest distinct trajectories from risk factors (Fox et al., 2001; Frenkel et al., 2015; White et al., 2017a) to symptoms which may fully manifest clinically only later in development. Future studies may consider data collection during later adolescence when anxiety symptoms are expected to manifest more severely. Finally, a broader limitation to this field of research is the use of neutral faces as contrasts to emotional faces, as anxious individuals have been shown to process neutral faces differently than healthy controls, both behaviorally and neurally (Filkowski and Haas, 2017). Although prior research reveals attentional biases in anxiety using such faces (e.g. Abend et al., 2018), the field may benefit from establishing other types of baseline emotion conditions (Filkowski and Haas, 2017).
These limitations are offset by several strengths. Because the study presents longitudinal data collected over 10 years, many key variables represent within-subject factors, which generally possess more statistical power than between-subjects factors. Furthermore, we applied an LME statistical approach in all analyses; this approach is considered more valid than complete-case approaches which introduce selection biases. Finally, anxiety–connectivity associations emerged in terms of individual differences, i.e. continuous associations spanning the range of anxiety symptoms, and as such may be more generalizable than group mean differences. Of note, BI effects emerged more strongly when a categorical operationalization of this factor was used in line with previous work, potentially indicative of qualitative as well as quantitative differences between BI levels.
In conclusion, this longitudinal study provides evidence for two distinct neurodevelopmental pathways that relate levels of early-childhood BI to pediatric anxiety symptoms. These pathways highlight longitudinal interactions among BI and anxiety symptoms in relation to neural activity during threat-related attention allocation. As such, these findings extend our understanding of the pathophysiology of pediatric anxiety and help guide the development of targeted treatments.
Supplementary Material
Acknowledgements.
We thank the participants and families, as well as the staff of the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health.
Financial support. This research was supported (in part) by the NIMH Intramural Research Program (ZIAMH002781-15, NCT00018057; RA, CS, LWK, CF, KK, SPH, BEB, EL, DSP) and NIH grant U01MH093349 (NAF).
Footnotes
Conflict of interest. None.
Ethical standards. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Supplementary material. The supplementary material for this article can be found at https://doi.org/10.1017/S0033291718003999.
References
- Abend R, De Voogd EL, Salemink E, Wiers RW, Perez-Edgar K, Fitzgerald A, White LK, Salum GA, He J, Silverman WK, Pettit JW, Pine DS and Bar-Haim Y (2018) Association between attention bias to threat and anxiety symptoms in children and adolescents. Depression and Anxiety 35, 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ and Van Ijzendoorn MH (2007) Threat-related attentional bias in anxious and non-anxious individuals: a meta-analytic study. Psychological Bulletin 133, 1–24. [DOI] [PubMed] [Google Scholar]
- Barker TV, Reeb-Sutherland B, Degnan KA, Walker OL, Chronis-Tuscano A, Henderson HA, Pine DS and Fox NA (2015) Contextual startle responses moderate the relation between behavioral inhibition and anxiety in middle childhood. Psychophysiology 52, 1544–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesdo K, Knappe S and Pine DS (2009) Anxiety and anxiety disorders in children and adolescents: developmental issues and implications for DSM-V. Psychiatric Clinics of North America 32, 483–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J and Neer SM (1997) The screen for child anxiety related emotional disorders (SCARED): scale construction and psychometric characteristics. Journal of the American Academy of Child and Adolescent Psychiatry 36, 545–553. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Brent DA, Chiappetta L, Bridge J, Monga S and Baugher M (1999) Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a replication study. Journal of the American Academy of Child and Adolescent Psychiatry 38, 1230–1236. [DOI] [PubMed] [Google Scholar]
- Birn RM, Shackman AJ, Oler JA, Williams LE, Mcfarlin DR, Rogers GM, Shelton SE, Alexander AL, Pine DS, Slattery MJ, Davidson RJ, Fox AS and Kalin NH (2014) Evolutionarily conserved prefrontal-amygdalar dysfunction in early-life anxiety. Molecular Psychiatry 19, 915–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SJ (2007) Neurocognitive mechanisms of anxiety: an integrative account. Trends in Cognitive Sciences 11, 307–316. [DOI] [PubMed] [Google Scholar]
- Bishop SJ (2008) Neural mechanisms underlying selective attention to threat. Annals of the New York Academy of Sciences 1129, 141–152. [DOI] [PubMed] [Google Scholar]
- Britton JC, Bar-Haim Y, Carver FW, Holroyd T, Norcross MA, Detloff A, Leibenluft E, Ernst M and Pine DS (2012) Isolating neural components of threat bias in pediatric anxiety. Journal of Child Psychology and Psychiatry 53, 678–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzell GA, Troller-Renfree SV, Barker TV, Bowman LC, Chronis-Tuscano A, Henderson HA, Kagan J, Pine DS and Fox NA (2017) A neurobehavioral mechanism linking behaviorally inhibited temperament and later adolescent social anxiety. Journal of the American Academy of Child and Adolescent Psychiatry 56, 1097–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Glatt CE and Lee FS (2015) Treating the developing versus developed brain: translating preclinical mouse and human studies. Neuron 86, 1358–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Newman DL and Silva PA (1996) Behavioral observations at age 3 years predict adult psychiatric disorders – longitudinal evidence from a birth cohort. Archives of General Psychiatry 53, 1033–1039. [DOI] [PubMed] [Google Scholar]
- Chen G, Saad ZS, Britton JC, Pine DS and Cox RW (2013) Linear mixed-effects modeling approach to FMRI group analysis. Neuroimage 73, 176–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronis-Tuscano A, Degnan KA, Pine DS, Perez-Edgar K, Henderson HA, Diaz Y, Raggi VL and Fox NA (2009) Stable early maternal report of behavioral inhibition predicts lifetime social anxiety disorder in adolescence. Journal of the American Academy of Child and Adolescent Psychiatry 48, 928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM and Koster EH (2010) Mechanisms of attentional biases towards threat in anxiety disorders: an integrative review. Clinical Psychology Review 30, 203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole CE, Zapp DJ, Fettig NB and Perez-Edgar K (2016) Impact of attention biases to threat and effortful control on individual variations in negative affect and social withdrawal in very young children. Journal of Experimental Child Psychology 141, 210–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW (1996) AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research 29, 162–173. [DOI] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RC and Taylor PA (2017) FMRI clustering in AFNI: false-positive rates redux. Brain Connectivity 7, 152–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN (2015) Research domain criteria: toward future psychiatric nosologies. Dialogues in Clinical Neuroscience 17, 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donders AR, Van Der Heijden GJ, Stijnen T and Moons KG (2006) Review: a gentle introduction to imputation of missing values. Journal of Clinical Epidemiology 59, 1087–1091. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE and Knutsson H (2016) Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences of the United States of America 113, 7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Buchel C and Gross JJ (2015) The neural bases of emotion regulation. Nature Reviews Neuroscience 16, 693–700. [DOI] [PubMed] [Google Scholar]
- Filkowski MM and Haas BW (2017) Rethinking the use of neutral faces as a baseline in fMRI neuroimaging studies of axis-I psychiatric disorders. Journal of Neuroimaging 27, 281–291. [DOI] [PubMed] [Google Scholar]
- Fox AS and Kalin NH (2014) A translational neuroscience approach to understanding the development of social anxiety disorder and its pathophysiology. American Journal of Psychiatry 171, 1162–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA and Pine DS (2012) Temperament and the emergence of anxiety disorders. Journal of the American Academy of Child and Adolescent Psychiatry 51, 125–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA, Schmidt LA and Henderson HA (2000) Developmental psychophysiology: conceptual and methodological perspectives In T CJ, Tassinary LG and Berntson GG (eds), Handbook of Psychophysiology, 2nd Edn. Cambridge: Cambridge University Press. [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD and Schmidt LA (2001) Continuity and discontinuity of behavioral inhibition and exuberance: psychophysiological and behavioral influences across the first four years of life. Child Development 72, 1–21. [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE and Ghera MM (2005) Behavioral inhibition: linking biology and behavior within a developmental framework. Annual Review of Psychology 56, 235–262. [DOI] [PubMed] [Google Scholar]
- Frenkel TI, Fox NA, Pine DS, Walker OL, Degnan KA and Chronis-Tuscano A (2015) Early childhood behavioral inhibition, adult psychopathology and the buffering effects of adolescent social networks: a twenty-year prospective study. Journal of Child Psychology and Psychiatry 56, 1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Taber-Thomas BC and Perez-Edgar K (2017) Frontolimbic functioning during threat-related attention: relations to early behavioral inhibition and anxiety in children. Biological Psychology 122, 98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, Hare TA, Bookheimer SY and Tottenham N (2013) A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. Journal of Neuroscience 33, 4584–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent III TF, Herman DH, Clasen LS, Toga AW, Rapoport JL and Thompson PM (2004) Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the USA 101, 8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe DW and Nitschke JB (2013) Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nature Reviews Neuroscience 14, 488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Lau JY, Mcclure-Tone EB, Parrish J, Shiffrin ND, Reynolds RC, Chen G, Blair RJ, Leibenluft E, Fox NA, Ernst M, Pine DS and Nelson EE (2008) Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Archives of General Psychiatry 65, 1303–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hane AA, Fox NA, Henderson HA and Marshall PJ (2008) Behavioral reactivity and approach-withdrawal bias in infancy. Developmental Psychology 44, 1491–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardee JE, Benson BE, Bar-Haim Y, Mogg K, Bradley BP, Chen G, Britton JC, Ernst M, Fox NA, Pine DS and Perez-Edgar K (2013) Patterns of neural connectivity during an attention bias task moderate associations between early childhood temperament and internalizing symptoms in young adulthood. Biological Psychiatry 74, 273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoviello BM, Wu G, Abend R, Murrough JW, Feder A, Fruchter E, Levinstein Y, Wald I, Bailey CR, Pine DS, Neumeister A, Bar-Haim Y and Charney DS (2014) Attention bias variability and symptoms of posttraumatic stress disorder. Journal of Traumatic Stress 27, 232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C and Wang P (2010) Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. American Journal of Psychiatry 167, 748–751. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR and Walters EE (2005a) Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Archives of General Psychiatry 62, 593–602. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR and Walters EE (2005b) Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the national comorbidity survey replication. Archives of General Psychiatry 62, 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN and Whalen PJ (2011) The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behavioural Brain Research 223, 403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahat A, Lamm C, Chronis-Tuscano A, Pine DS, Henderson HA and Fox NA (2014) Early behavioral inhibition and increased error monitoring predict later social phobia symptoms in childhood. Journal of the American Academy of Child & Adolescent Psychiatry 53, 447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahat A, Benson BE, Pine DS, Fox NA and Ernst M (2018) Neural responses to reward in childhood: relations to early behavioral inhibition and social anxiety. Social Cognitive and Affective Neuroscience 13, 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Walker OL, Degnan KA, Henderson HA, Pine DS, Mcdermott JM and Fox NA (2014) Cognitive control moderates early childhood temperament in predicting social behavior in 7-year-old children: an ERP study. Developmental Science 17, 667–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledoux JE (1996) The Emotional Brain: The Mysterious Underpinnings of Emotional Life. New York: Simon & Schuster. [Google Scholar]
- Ledoux JE and Pine DS (2016) Using neuroscience to help understand fear and anxiety: a two-system framework. American Journal of Psychiatry 173, 1083–1093. [DOI] [PubMed] [Google Scholar]
- Lewis-Morrarty E, Degnan KA, Chronis-Tuscano A, Rubin KH, Cheah CSL, Pine DS, Henderon HA and Fox NA (2012) Maternal overcontrol moderates the association between early childhood behavioral inhibition and adolescent social anxiety symptoms. Journal of Abnormal Child Psychology 40, 1363–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod C, Mathews A and Tata P (1986) Attentional bias in emotional disorders. Journal of Abnormal Psychology 95, 15–20. [DOI] [PubMed] [Google Scholar]
- Matta TH, Flournoy JC and Byrne ML (2017) Making an unknown unknown a known unknown: missing data in longitudinal neuroimaging studies. Developmental Cognitive Neuroscience 33, 83–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcdermott JM, Perez-Edgar K, Henderson HA, Chronis-Tuscano A, Pine DS and Fox NA (2009) A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biological Psychiatry 65, 445–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mclaren DG, Ries ML, Xu G and Johnson SC (2012) A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage 61, 1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalska KJ, Machlin L, Moroney E, Lowet DS, Hettema JM, Roberson-Nay R, Averbeck BB, Brotman MA, Nelson EE, Leibenluft E and Pine DS (2017) Anxiety symptoms and children’s eye gaze during fear learning. Journal of Child Psychology and Psychiatry 58, 1276–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Mcclure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, Charney DS, Ernst M and Pine DS (2003) Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage 20, 420–428. [DOI] [PubMed] [Google Scholar]
- Monk CS, Telzer EH, Mogg K, Bradley BP, Mai XQ, Louro HMC, Chen G, Mcclure-Tone EB, Ernst M and Pine DS (2008) Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Archives of General Psychiatry 65, 568–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales S, Taber-Thomas BC and Pérez-Edgar KE (2017). Patterns of attention to threat across tasks in behaviorally inhibited children at risk for anxiety. Developmental Science 20, e12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naim R, Abend R, Wald I, Eldar S, Levi O, Fruchter E, Ginat K, Halpern P, Sipos ML, Adler AB, Bliese PD, Quartana PJ, Pine DS and Bar-Haim Y (2015) Threat-Related attention bias variability and posttraumatic stress. American Journal of Psychiatry 172, 1242–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson AA, Rabellino D, Densmore M, Frewen PA, Paret C, Kluetsch R, Schmahl C, Theberge J, Neufeld RW, Mckinnon MC, Reiss J, Jetly R and Lanius RA (2017) The neurobiology of emotion regulation in posttraumatic stress disorder: amygdala downregulation via real-time fMRI neurofeedback. Human Brain Mapping 38, 541–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paret C, Ruf M, Gerchen MF, Kluetsch R, Demirakca T, Jungkunz M, Bertsch K, Schmahl C and Ende G (2016) fMRI neurofeedback of amygdala response to aversive stimuli enhances prefrontal-limbic brain connectivity. Neuroimage 125, 182–188. [DOI] [PubMed] [Google Scholar]
- Perez-Edgar K, Bar-Haim Y, Mcdermott JM, Chronis-Tuscano A, Pine DS and Fox NA (2010) Attention biases to threat and behavioral inhibition in early childhood shape adolescent social withdrawal. Emotion 10, 349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Edgar K, Reeb-Sutherland BC, Mcdermott JM, White LK, Henderson HA, Degnan KA, Hane AA, Pine DS and Fox NA (2011) Attention biases to threat link behavioral inhibition to social withdrawal over time in very young children. Journal of Abnormal Child Psychology 39, 885–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Edgar K, Taber-Thomas B, Auday E and Morales S (2014) Temperament and attention as core mechanisms in the early emergence of anxiety. Children and Emotion: New Insights into Developmental Affective Science 26, 42–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman SB and Pelphrey KA (2011) Developing connections for affective regulation: age-related changes in emotional brain connectivity. Journal of Experimental Child Psychology 108, 607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS and Fox NA (2015) Childhood antecedents and risk for adult mental disorders. Annual Review of Psychology 66, 459–485. [DOI] [PubMed] [Google Scholar]
- Pinheiro JC and Bates DM (2000) Mixed-effects Models in S and S-PLUS. New York: Springer. [Google Scholar]
- Price RB, Kuckertz JM, Siegle GJ, Ladouceur CD, Silk JS, Ryan ND, Dahl RE and Amir N (2015) Empirical recommendations for improving the stability of the dot-probe task in clinical research. Psychological Assessment 27, 365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Allen KB, Silk JS, Ladouceur CD, Ryan ND, Dahl RE, Forbes EE and Siegle GJ (2016) Vigilance in the laboratory predicts avoidance in the real world: a dimensional analysis of neural, behavioral, and ecological momentary data in anxious youth. Developmental Cognitive Neuroscience 19, 128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior M, Smart D, Sanson A and Oberklaid F (2000) Does shy-inhibited temperament in childhood lead to anxiety problems in adolescence? Journal of the American Academy of Child and Adolescent Psychiatry 39, 461–468. [DOI] [PubMed] [Google Scholar]
- Shanmugan S, Wolf DH, Calkins ME, Moore TM, Ruparel K, Hopson RD, Vandekar SN, Roalf DR, Elliott MA, Jackson C, Gennatas ED, Leibenluft E, Pine DS, Shinohara RT, Hakonarson H, Gur RC, Gur RE and Satterthwaite TD (2016) Common and dissociable mechanisms of executive system dysfunction across psychiatric disorders in youth. American Journal of Psychiatry 173, 517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechner T and Bar-Haim Y (2016) Threat monitoring and attention-bias modification in anxiety and stress-related disorders. Current Directions in Psychological Science 25, 431–437. [Google Scholar]
- Shechner T, Britton JC, Perez-Edgar K, Bar-Haim Y, Ernst M, Fox NA, Leibenluft E and Pine DS (2012) Attention biases, anxiety, and development: toward or away from threats or rewards? Depression and Anxiety 29, 282–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechner T, Jarcho JM, Wong S, Leibenluft E, Pine DS and Nelson EE (2017) Threats, rewards, and attention deployment in anxious youth and adults: an eye tracking study. Biological Psychology 122, 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard J, Tseng WL, Kim P, Chen G, Yi J, Donahue L, Brotman MA, Towbin KE, Pine DS and Leibenluft E (2017) Association of irritability and anxiety with the neural mechanisms of implicit face emotion processing in youths with psychopathology. JAMA Psychiatry 74, 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai N, Taber-Thomas BC and Perez-Edgar KE (2016) Neural correlates of attention biases, behavioral inhibition, and social anxiety in children: an ERP study. Developmental Cognitive Neuroscience 19, 200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Whalen PJ, Eccard CH, Dahl RE, Ryan ND and Casey BJ (2001) Amygdala response to facial expressions in children and adults. Biological Psychiatry 49, 309–316. [DOI] [PubMed] [Google Scholar]
- Ulfig N, Setzer M and Bohl J (2003) Ontogeny of the human amygdala. Annals of the New York Academy of Sciences 985, 22–33. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele B, Verschuere B, Tibboel H, De Houwer J, Crombez G and Koster EH (2014) A review of current evidence for the causal impact of attentional bias on fear and anxiety. Psychological Bulletin 140, 682–721. [DOI] [PubMed] [Google Scholar]
- White LK, Mcdermott JM, Degnan KA, Henderson HA and Fox NA (2011) Behavioral inhibition and anxiety: the moderating roles of inhibitory control and attention shifting. Journal of Abnormal Child Psychology 39, 735–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White LK, Britton JC, Sequeira S, Ronkin EG, Chen G, Bar-Haim Y, Shechner T, Ernst M, Fox NA, Leibenluft E and Pine DS (2016) Behavioral and neural stability of attention bias to threat in healthy adolescents. Neuroimage 136, 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White LK, Degnan KA, Henderson HA, Perez-Edgar K, Walker OL, Shechner T, Leibenluft E, Bar-Haim Y, Pine DS and Fox NA (2017a) Developmental relations among behavioral inhibition, anxiety, and attention biases to threat and positive information. Child Development 88, 141–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White LK, Sequeira S, Britton JC, Brotman MA, Gold AL, Berman E, Towbin K, Abend R, Fox NA, Bar-Haim Y, Leibenluft E and Pine DS (2017b) Complementary features of attention bias modification therapy and cognitive-behavioral therapy in pediatric anxiety disorders. American Journal of Psychiatry 174, 775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MJ, Kujawa A, Lu LH, Fitzgerald DA, Klumpp H, Fitzgerald KD, Monk CS and Phan KL (2016) Age-related changes in amygdala-frontal connectivity during emotional face processing from childhood into young adulthood. Human Brain Mapping 37, 1684–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvielli A, Bernstein A and Koster EH (2015) Temporal dynamics of attentional bias. Clinical Psychological Science 3, 772–788. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.