Abstract
Background
Optimising HIV pre-exposure prophylaxis (PrEP) provision requires insight into preferences of PrEP regimens and PrEP discontinuation. We assessed regimen switching and discontinuation and their determinants among men who have sex with men (MSM) participating in the Amsterdam PrEP demonstration project.
Methods
Between 3-August-2015 and 31-May-2016, we enrolled MSM (n = 374) and TGP (n = 2) in a prospective, longitudinal study. Participants could choose between daily or event-driven PrEP regimens at enrolment and every 3 months. We assessed transition intensities (TI) and determinants of switching (i) between regimens, and (ii) from either regimen to discontinuing PrEP using a multi-state Markov model. PrEP discontinuation was defined as formally stopping study participation or having no study visit for ≥6 months.
Findings
Of 367 analysed participants, 73·3% chose daily and 26·7% event-driven PrEP at enrolment. Median follow-up was 3·1 years (IQR 2·9–3·2). 121 participants switched their PrEP regimen at least once (cumulative probability 34·2%, 95% CI 29·4–39·6), with 90 switches from event-driven to daily PrEP (TI 0·35/PY, 95% CI 0·29–0·44) and 113 switches from daily to event-driven PrEP (TI 0·16/PY, 95% CI 0·13–0·20). Switching from event-driven to daily PrEP was associated with younger age, not reporting sex with HIV-positive partners, chemsex, and sexual compulsivity. Switching from daily to event-driven PrEP were associated with younger age and lower sexual satisfaction. 67 participants discontinued PrEP (cumulative probability 17·7%, 95% CI 14·1–22·2), with no difference between regimens: event-driven (n = 23, TI 0·08/PY, 95% CI 0·05–0·13) and daily PrEP (n = 44, TI 0·06/PY, 95% CI 0·04–0·08). Discontinuing daily PrEP was associated with younger age, fewer casual partners, and higher number of condomless anal sex acts with casual partners.
Interpretation
Switching between PrEP regimens was common, while going from event-driven to daily PrEP use was associated with certain sexual-related determinants (i.e. chemsex, sexual compulsivity, no known HIV-positive partners). PrEP discontinuation rates were low and independent of regimens. PrEP care should consider the reasons for choice and switch of regimen and involve education on safely switching or discontinuing PrEP, especially among younger MSM.
Funding
ZonMw, H-TEAM, RIVM, GGD research funds, Aidsfonds, Amsterdam Diner Foundation, Gilead Sciences, Gilead Sciences Europe Ltd, Janssen Pharmaceuticals, MAC AIDS Fund, ViiV Healthcare.
Keywords: Pre-exposure prophylaxis, HIV, Prevention, Sexual behaviour, Men who have sex with men, Event-driven
Research in Context.
Evidence before this study
A search of PubMed and conference databases from the International AIDS Society Conference and Conference on Retroviruses and Opportunistic Infections until 25 March 2020 revealed that most PrEP studies have focused on daily PrEP only, likely because event-driven PrEP was included much later for clinical use in the majority of PrEP guidelines and some countries adopted event-driven PrEP only very recently. No long-term longitudinal study has quantitatively addressed the frequency and determinants of PrEP regimen switches and no data on long-term PrEP retention rates and reasons for PrEP discontinuation are available for settings in which both daily and event-driven PrEP were used. Studies addressing daily PrEP retention showed that participants who discontinue PrEP were more likely to be younger, identify as black, and report decreased HIV risk as a reason for discontinuing PrEP.
Added value of this study
We report on the frequency and determinants of switching between PrEP regimens and on PrEP retention and determinants of discontinuing daily and event-driven PrEP regimens. Our results show that regimen switches were common during the first three years of PrEP use and that overall retention was relatively high at 82%, with no difference between regimens. We observed that younger MSM were more likely to switch between regimens and more likely to discontinue daily PrEP and that certain sexual behaviours, in particular those related to HIV risk, were substantial drivers of regimen switches and discontinuations. In addition, more than half of those discontinuing PrEP reported having CAS with casual partners in the three months prior to their last study visit.
Implications of all the available evidence
PrEP care should focus on time-varying individual needs and preferences, and PrEP care providers should consider the PrEP user's patterns of sexual behaviour in order to facilitate suitable regimen choices. As some PrEP users may be at risk of HIV acquisition after discontinuing PrEP, PrEP counselling should include a discussion of all available risk reduction strategies, and should include education on how to safely start, switch and stop PrEP regimens. The information obtained regarding younger users and their higher rates of discontinuation helps inform public health specialists that this group may require additional, intensified and tailored campaigns promoting PrEP retention.
Alt-text: Unlabelled box
1. Introduction
Oral pre-exposure prophylaxis (PrEP) with tenofovir disoproxil fumarate and emtricitabine is an effective measure to prevent human immunodeficiency virus (HIV) acquisition [1]. Two modalities of PrEP have been evaluated in randomised clinical trials and are currently included in international guidelines: PrEP taken every 24 h (i.e. daily) and PrEP taken before and after sexual contact (i.e. event-driven) [2].
Considerable uptake of this biomedical intervention has been observed in mostly men who have sex with men (MSM). Recent studies conducted in this population have shown that there is substantial variation in whether MSM prefer to initiate daily over event-driven PrEP [[3], [4], [5]–6]. An individual's choice of regimen over time is also likely to fluctuate with changing sexual and social contexts [7]. To date, reasons for changing PrEP regimens can only be inferred from single studies assessing hypothetical preferences after initiating PrEP [8] or qualitative analysis [9], while no long-term longitudinal study on this issue has been conducted.
Understanding why individuals switch PrEP regimens is important for several reasons. First, PrEP users may not be aware or informed of the regimen most suitable to them. If they are poorly informed about regimen options and the possibility of switching to an alternative regimen, adherence to PrEP could be compromised [10]. Second, frequent switching could be a proxy for dynamic changes in behaviour, including sexual behaviours carrying a risk for HIV acquisition. On the one hand, PrEP users could be quite capable in estimating their evolving HIV risk or understanding when they are able to better adhere to a given regimen [9], thus switching could indicate self-efficacious PrEP use. On the other hand, maintaining sufficient PrEP coverage of at-risk sex acts could be challenging for some individuals switching regimens, who may be in need of additional adherence counselling. Finally, daily PrEP use is associated with more CAS acts with casual partners and higher incidence of bacterial STIs than event-driven PrEP [11], making choice of PrEP regimen a useful tool to assess risk of HIV and other STI.
In addition, retention in PrEP care is important for prevention and early detection of HIV, safety monitoring, adherence and sexual behaviour counselling, and testing for other sexually transmitted infections (STI). Despite its importance, long-term retention rates and characteristics of those who are not retained are understudied in real-life settings. The few studies addressing retention have shown that those who discontinue PrEP or are not retained in PrEP care are usually younger [12,13] and have a decreased perceived risk of HIV [12,14]. However, median follow-up time was generally one year or shorter in these studies. Moreover, sexual behaviours differ between users of daily and event-driven PrEP [11], hence discontinuing PrEP due to perceived HIV risk could also be distinct between regimens.
We therefore studied the frequency and determinants of switching and discontinuing daily and event-driven PrEP among MSM from the Amsterdam PrEP (AMPrEP) demonstration project in the Netherlands [3].
2. Methods
2.1. Study design and participants
AMPrEP is a prospective, open-label demonstration study conducted at the STI clinic of the Public Health Service of Amsterdam, the Netherlands. Study design and procedures have been described in detail previously [3]. Briefly, HIV-negative MSM and transgender persons aged 18 years or older who had sex with men were eligible for inclusion if they reported one of the following in the 6 months prior to inclusion: condomless anal sex (CAS) with casual partners, at least one bacterial STI, use of post-exposure prophylaxis after a sexual risk incident or an HIV-positive sexual partner with detectable viral load [3]. Enrolment took place between 3 August 2015 and 31 May 2016 and follow-up is ongoing. All participants provided written informed consent.
The study was approved by the ethics board of the Academic Medical Center (Amsterdam, the Netherlands; NL49504·018·14) and is registered with the Netherlands Trial Registry, number NL5302. The study protocol is available online (https://www.ggd.amsterdam.nl/infectieziekten/soa-hiv-sense/prep/amprep/). AMPrEP is part of the HIV Transmission Elimination AMsterdam (H-TEAM) Initiative, a multidisciplinary and integrative approach to stop the urban HIV epidemic.
2.2. Procedures
Eligible participants self-selected either daily or event-driven PrEP (both free-of-charge) at inclusion after receiving objective information about both regimens from the study clinician. At each study visit, satisfaction with the chosen PrEP regimen was discussed and participants were given the opportunity to switch regimen [3]. Daily PrEP consisted of a single tablet containing tenofovir disoproxil fumarate 245 mg combined with emtricitabine 200 mg, taken once per day. Event-driven PrEP consisted of two tablets taken between 24 h and 2 h before sexual intercourse, followed by one tablet every 24 h up to 48 h after the last sexual intercourse.
We followed participants every 3 months. Participants completed self-administered questionnaires on sexual behaviour every 3 months and on recreational drug use and mental health outcomes every 12 months. At each study visit, we tested for HIV antigens and antibodies using the LIAISON XL Murex HIV Ag/Ab assay (Diasorin, Saluggia, Italy) with immunoblot confirmation (INNO_LIPA HIV I/II Score; Fujirebio, Ghent, Belgium), and for Treponema pallidum using a serological-based assay (LIAISON Treponema Screen, Diasorin) or a rapid plasma regain test if a participant had a history of syphilis infection (RPR-Nosticon II; bioMérieux, Boxtel, The Netherlands). We analysed urine, anal and pharyngeal swabs using nucleic amplification testing for Chlamydia trachomatis and Neisseria gonorrhoeae (Aptima combo 2, Hologic, San Diego, USA). Additional testing in case of STI-suspected symptoms or receiving partner notification took place in-between regular study visits.
2.3. Outcomes
We assessed PrEP regimen switches from event-driven to daily PrEP and daily PrEP to event-driven PrEP (based on self-report) and discontinuation of PrEP use for each participant at each follow-up study visit. We defined PrEP discontinuation as formally stopping study participation or having no study visit for more than 6 months and never returning to the study. We considered the latter group lost to follow-up (LTFU). Individuals who became HIV-positive during follow-up were right-censored from the date of their diagnosis.
2.4. Determinants
We evaluated several determinants from self-reported questionnaires. Demographic variables included age, ethnicity, city of residence, education level, employment, net monthly income and living situation.
Sexual behaviour variables included sexual orientation, number of anal sex partners, number of casual sex partners, having ≥1 HIV-positive sex partner, number of anal sex acts, number of sex acts with casual partners, any CAS, any CAS with casual sex partners, number of CAS acts, number of CAS acts with casual sex partners.
All participants were asked if they used any of the following drugs during sex: alcohol, amphetamine, cannabis, cocaine, erectile dysfunction drugs (EDD), γ-hydroxybutyrate (GHB)/γ-butyrolactone (GBL), ketamine, methamphetamine, mephedrone, nitrites, 3,4-methylenedioxy-N-methylamphetamine (XTC/MDMA), and other drugs. We also assessed whether drugs were injected. We defined chemsex as self-reported use of methamphetamine, GHB/GBL, and/or mephedrone during sex.
We included STI results from any visit to our clinic in this analysis. Any bacterial STI infection was defined as an incident diagnosis with syphilis, chlamydia, and/or gonorrhoea in the preceding 3 months.
We evaluated five mental health outcomes: anxiety or depressive mood disorder using the 5-item Mental Health Inventory (MHI-5), sexual compulsivity using the Sexual Compulsivity Scale (SCS), sexual satisfaction using the New Sexual Satisfaction Scale (NSSS), alcohol-use disorder using the Alcohol Use Disorder Identification Test (AUDIT), and drug-use disorder using the Drug Use Disorder Identification Test (DUDIT). A summary of assessments can be found in the Supplement. Briefly, indication of anxiety or depressive mood disorder was defined as an MHI-5 <60, sexual compulsivity as an SCS ≥ 24, alcohol-use disorder as an AUDIT ≥8, and drug-use disorder as a DUDIT ≥8. Sexual satisfaction was assessed on a continuous scale.
Time-fixed variables were ethnicity, city of residence, education level, employment, net monthly income, living situation, and sexual orientation (obtained at enrolment). All other variables were time-updated, with the recall period for behavioural factors being 3 months, except for chemsex (6 months) and injecting drug use (12 months). For questions not asked every 3 months, we imputed missing data from the subsequent visit with available data.
2.5. Statistical analysis
For this analysis, follow-up began at enrolment and ended at PrEP discontinuation, date of HIV diagnosis, or last visit before 1 April 2019, whichever occurred first. We calculated the cumulative proportion of participants who switched at least once and of participants who discontinued PrEP using Kaplan–Meier methods, overall, and by PrEP regimen at enrolment.
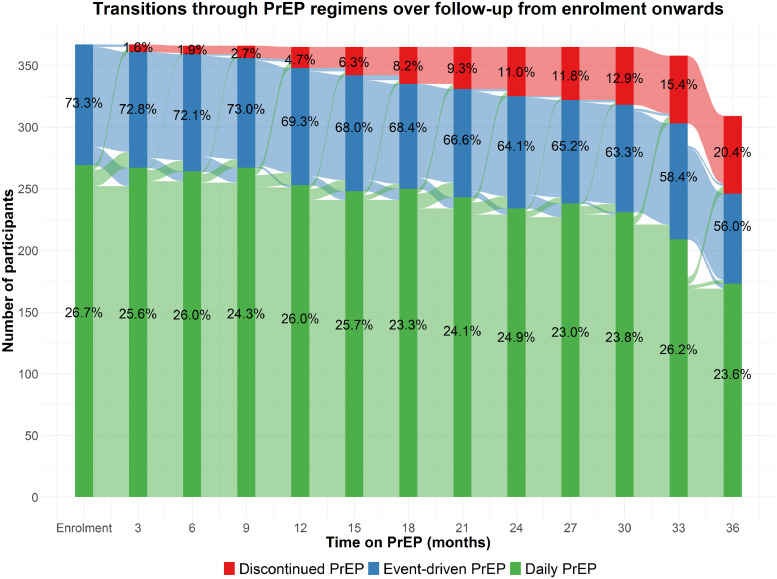
We considered three states which participants could occupy during follow-up: (1) daily PrEP, (2) event-driven PrEP, and (3) discontinued PrEP use. Using a time-homogenous, continuous-time, multi-state Markov model, we modelled transition intensities (TI), which are given as instantaneous rates of a transition occurring (i.e. incidence rates) and depend on the probability of occupying a certain state at each study visit. We simultaneously estimated the TI for transitions between PrEP regimens – from event-driven to daily PrEP and from daily PrEP to event-driven PrEP separately – and from either PrEP regimen to discontinuation (Fig. 1). PrEP discontinuation was modelled as an absorbing state, i.e. participants were considered to have permanently discontinued PrEP once entering this state.
Fig. 1.
Transitions (black arrows) between PrEP regimens and from each regimen to discontinuation, among 365 MSM and 2 TGP participating in the AMPrEP project, Amsterdam, the Netherlands, 2015–2019. Non-black arrows represent continuations of the chosen regimen at each visit.
To identify determinants for transitions between states, the TI between levels of factors can be modelled as a proportional hazard. From this model, we calculated univariable and multivariable hazard ratios (HR) and their 95% confidence intervals (CI) comparing levels of factors. Since determinants for switching regimens and long-term discontinuation are mostly unknown, we decided to use a predictive modelling approach. We included all factors that were associated (P <0·05) with at least one of the four transitions in multivariable analysis. A backwards selection procedure was subsequently employed to obtain a parsimonious model by sequentially removing variables that were no longer associated with at least one out of four transitions. We assessed collinearity using polychoric correlations. We did not test for effect modification between variables.
We carried out analysis using Stata version 15·0 (StataCorp, College Station, TX, USA) and the msm package [15] in the R statistical computing environment version 3·5·2 (Vienna, Australia).
2.6. Role of the funding source
The study funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The authors had full access to all data and were responsible for the decision to submit for publication.
3. STROBE guidelines
This article adheres to the STROBE guidelines for reporting of observational cohort studies.
4. Results
Of 376 enrolled participants, nine (2·4%) did not have follow-up data and were excluded. Of the 367 included in analysis, 365 were MSM and 2 transgender women (Tables 1 and S1). At enrolment, median age was 40·4 years (interquartile range [IQR] 32·6–48·5), 315 (85·8%) self-identified as white, 223 (60·8%) lived in Amsterdam, 280 (76·3%) had a college or university degree and 289 (79·0%) declared being exclusively homosexual. A total of 269 (73·3%) participants initiated daily and 98 (26·7%) participants event-driven PrEP.
Table 1.
Characteristics and behaviours of 365 MSM and 2 TGP participating in the AMPrEP project, at enrolment and at the time of discontinuing PrEP or at last visit before censoring, Amsterdam, the Netherlands, 2015–2019.
| Total (at enrolment) | Discontinued PrEP (by last visit) |
||
|---|---|---|---|
| No | Yes | ||
| N = 367 | n = 298 | n = 67 | |
| Factorsa | N (%) | n (%) | n (%) |
| Socio-demographic characteristics | |||
| Age (years), median [IQR] | 40·4 [32·6–48·5] | 44·7 [36·8–53·0] | 36·4 [29·9–44·9] |
| <35 | 121 (33·0%) | 57 (19·1%) | 29 (43·3%) |
| 35–44 | 111 (30·3%) | 96 (32·2%) | 22 (32.8%) |
| ≥45 | 135 (36·8%) | 145 (48·7%) | 16 (23·9%) |
| Gender identity | |||
| Male | 365 (99·5%) | 297 (99·7%) | 66 (98.5%) |
| Transgender woman | 2 (0·5%) | 1 (0·3%) | (1·5%) |
| Non-white ethnicity | 52 (14·2%) | 44 (14·8%) | 8 (11·9%) |
| Residency outside Amsterdam | 144 (39·2%) | 111 (37·3%) | 31 (46·3%) |
| College/university degree | 280 (76·3%) | 231 (77·5%) | 48 (71·6%) |
| Employment | |||
| Employed | 283/363 (78·0%) | 233/294 (79·3%) | 48 (71.6%) |
| Unemployed | 18/363 (5·0%) | 13/294 (4·4%) |
|
| Other | 62/363 (17·1%) | 48/294 (16·3%) | 14 (20·9%) |
| Net monthly income (Euro) | |||
| <1700 | 97/351 (27·6%) | 74/285 (26·0%) | 22/64 (34·4%) |
| 1701–2950 | 150/351 (42·7%) | 123/285 (43·2%) | 27/64 (42·2%) |
| >2950 | 104/351 (29·6%) | 88/285 (30·9%) | 15/64 (23·4%) |
| Living situation | |||
| Alone | 195 (53·1%) | 158 (53·0%) | 36 (53.%) |
| With partner | 117 (31·9%) | 102 (34·2%) | 14 (20.9%) |
| With parents/flatmates | 55 (15·0%) | 38 (12·8%) | 17 (25·4%) |
| Sexual orientation: not exclusively homosexual | 77/366 (21·0%) | 62/297 (20·9%) | 15 (22·4%) |
| Sexual behaviour | |||
| Number of anal sex partners, median [IQR]b | 12 [6–25] | 9 [4–20]e | 6 [1–17·5]f |
| Number of casual sex partners, median [IQR]c | 12 [5–23] | 8 [3–20]e | 6 [0–15]f |
| Having ≥1 HIV-positive sex partnerb | 229 (62·4%) | 141/287 (49·1%) | 21/59 (35·6%) |
| Number of anal sex acts, median [IQR]b | 22 [11–36] | 15 [6–30]e | 13 [5–40]e |
| Number of sex acts with casual sex partners, median [IQR]b | 15 [7–28] | 10 [4–24]e | 7 [0–27]e |
| Any CASb | 341 (92·9%) | 269/290 (92·8%) | 47/60 (78·3%) |
| Any CAS with casual sex partnersb | 324 (88·3%) | 253/290 (87·2%) | 37/60 (61·7%) |
| Number of CAS acts, median [IQR]b | 11 [4–23] | 15 [5–30]e | 10 [3–20]f |
| Number of CAS acts with casual partners, median [IQR]b | 6 [2–14] | 8·5 [2–20]e | 5 [0–18]f |
| Recreational drug use and mental health characteristics | |||
| Chemsex | 155/362 (42·8%) | 42/102 (41·2%) | 5/22 (22·7%) |
| Injecting drug use | 11/362 (3·0%)b | 8/163 (4·9%)d | 2/26 (7·7%)d |
| Any bacterial STIb | 72/365 (19·7%) | 77/296 (26·0%) | 10/64 (15·6%) |
| Chlamydia | 36/358 (10·1%) | 40/295 (13·6%) | 7/64 (10·9%) |
| Gonorrhoea | 35/359 (9·7%) | 46/295 (15·6%) | 4/64 (6·3%) |
| Syphilis | 5/358 (1·4%) | 9/296 (3·0%) | 1/64 (1·6%) |
| DUDIT score ≥8d | 135/365 (37·0%) | 33/104 (31·7%) | 7/23 (30·4%) |
| AUDIT score ≥8d | 100/363 (27·5%) | 25/104 (24·0%) | 7/23 (30·4%) |
| MHI-5 score <60d | 76 (20·7%) | 22/101 (21·8%) | 6/23 (26·1%) |
| SCS score ≥24d | 83/366 (22·7%) | 8/101 (7·9%) | 3/23 (13·0%) |
| NSSS score, median [IQR]d | 45 [39–48] | 47 [39–48]g | 43 [33–52]h |
| Follow-up indicators | |||
| Years of follow-up, median [IQR] | n.a. | 3·1 [2·9–3·3] | 1·7 [1·1–2·7] |
| Event-driven PrEP | 98 (26·7%) | 94 (31·5%) | 24 (35·8%) |
| Switched PrEP regimen at least once | 121 (33·0%) | 103 (34·6%) | 18 (26·9%) |
AUDIT: Alcohol Use Disorder Identification Test; CAS: condomless anal sex; chemsex: use of methamphetamine, γ-hydroxybutyric acid(GHB)/γ-butyrolactone(GBL) and/or mephedrone during sex; DUDIT: Drug Use Disorder Identification Test; HIV: human immunodeficiency virus; MHI-5: 5-item Mental Health Inventory; n.a.: not applicable; NSSS: New Sexual Satisfaction Scale; PrEP: pre-exposure prophylaxis; SCS: Sexual Compulsivity Scale; STI: sexually transmitted infection.
All factors were time-updated except for ethnicity, city of residence, education level, employment, net monthly income, living situation and sexual orientation.
In the past 3 months.
in the past 6 months.
in the past 12 months.
8 missing.
7 missing.
197 missing.
44 missing.
Median follow-up was 3·1 years (IQR 2·9–3·2), totalling 1026·1 person-years (PY). Fig. 2 shows all transitions between states over the course of the first three years of follow-up. 121 participants switched their PrEP regimen at least once (3-year cumulative probability 34·2%, 95% CI 29·4–39·6). Of those who started daily PrEP, 75 switched to event-driven PrEP (3-year cumulative probability 28·8%, 95% CI 23·6–34·9), while the median time until first switch was 1·1 years (IQR 0·5–1·9). Of those who started event-driven PrEP, 46 switched to daily PrEP (3-year cumulative probability 49·2%, 95% CI 39·3–60·1), while the median time until first switch was 0·5 years (IQR 0·2–1·4). Among those who switched regimens, the number of switches until the end of follow-up (n = 203) was distributed as follows: once, n = 67; twice, n = 36; thrice, n = 11; four times, n = 4; five times, n = 3. The overall incidence of switching, regardless of the direction, slightly decreased over time from 0·23/PY (95% CI 0·19–0·29) in the first year on PrEP to 0·16/PY (95% CI 0·12–0·22) in the third year on PrEP. Of the 203 switches, 85 (41·9%) occurred in the first year on PrEP, 61 (30·0%) in the second year and 48 (23·6%) in the third year.
Fig. 2.
Transitions between PrEP states over the course of the first three years since PrEP initiation, among 365 MSM and 2 TGP participating in the AMPrEP project, Amsterdam, the Netherlands, 2015–2019
The total number of participants included in all three states slightly decreases towards the end of follow-up as not all participants had yet reached these visits or were diagnosed with HIV (n = 2).
Fig. 1 visualises the number of participants per PrEP state and transitions between states over follow-up. Switches from event-driven to daily PrEP occurred at a mean TI of 0·35/PY (95% CI 0·29–0·44) and daily to event-driven PrEP at a mean TI of 0·16/PY (95% CI 0·13–0·20). Switches from event-driven to daily PrEP were 2·18 times (95% CI 1·67–2·85) more likely than the reverse.
In multivariable analysis, switching from event-driven to daily PrEP was associated with younger age, not reporting sex with any HIV-positive partners, engaging in chemsex and a SCS score of ≥24, compared to staying on event-driven PrEP (Table 2). Switching from daily to event-driven PrEP was associated with younger age, engaging in chemsex, and lower NSSS score.
Table 2.
Determinants of switching to and from daily and event-driven PrEP among 365 MSM and 2 TGP participating in the AMPrEP project, Amsterdam, the Netherlands, 2015–2019.
| Univariable HR |
Multivariable |
|||
|---|---|---|---|---|
| Event-driven → Daily PrEP | Daily → Event-driven PrEP | Event-driven → Daily PrEP | Daily → Event-driven PrEP | |
| Factorsa | HR (95% CI)f | HR (95% CI)f | aHR (95% CI)f | aHR (95% CI) |
| Calendar year (per year increase) | 0·63 (0·50–0·79) | 0·89 (0·85–1·71) | ||
| Socio-demographic characteristics | ||||
| Age (per 10 year increase) | 0·73 (0·61–0·88) | 0·77 (0·65–0·91) | 0·67 (0·52–0·87) | 0·69 (0·55–0·88) |
| Non-white ethnicity | 0·94 (0·51–1·74) | 1·03 (0·61–1·72) | ||
| Residency outside Amsterdam | 0·76 (0·48–1·19) | 0·72 (0·48–1·06) | ||
| College/university degree | 1·53 (0·81–2·89) | 1·70 (1·03–2·82) | ||
| Employment | ||||
| Employed | ref. | ref. | ||
| Unemployed | 0·44 (0·16–1·22) | 0·95 (0·35–2·59) | ||
| Other | 0·61 (0·32–1·15) | 0·82 (0·48–1·40) | ||
| Net monthly income >1700€ | 0·92 (0·58–1·45) | 0·61 (0·41–0·91) | ||
| Living with partner/others (vs. alone) | 0·52 (0·33–0·81) | 0·87 (0·60–1·26) | ||
| Not exclusively homosexual | 1·24 (0·75–2·04) | 1·13 (0·72–1·75) | ||
| Sexual behaviour | ||||
| Number of anal sex partnersb,c | 1·20 (0·98–1·47) | 0·73 (0·60–0·90) | ||
| Number of casual sex partnersb,c | 1·18 (0·98–1·43) | 0·73 (0·61–0·88) | 1·39 (0·84–2·30) | 0·83 (0·55–1·25) |
| Having ≥1 HIV-positive sex partnerc | 0·78 (0·51–1·19) | 0·63 (0·43–0·92) | 0·48 (0·26–0·91) | 0·72 (0·42–1·23) |
| Number of anal sex actsb,c | 1·11 (0·92–1·35) | 0·86 (0·71–1·04) | ||
| Number of sex acts with casual sex partnersb,c | 1·20 (1·00–1·44) | 0·76 (0·64–0·90) | ||
| Any CASc | 0·98 (0·49–1·95) | 1·05 (0·43–2·60) | ||
| Number of CAS actsb,c | 0·95 (0·80–1·14) | 0·86 (0·73–1·02) | ||
| Any CAS with casual sex partnersc | 1·42 (0·77–2·63) | 0·69 (0·38–1·22) | ||
| Number of CAS acts with casual partnersb,c | 1·06 (0·89–1·28) | 0·79 (0·68–0·93) | 0·88 (0·53–1·45) | 0·90 (0·64–1·28) |
| Recreational drug use and mental health characteristics | ||||
| Chemsexd | 1·70 (1·01–2·84) | 1·34 (0·86–2·09) | 2·22 (1·15–4·31) | 1·71 (1·02–2·86) |
| Injecting drug usee | 0·86 (0·27–2·74) | 0·78 (0·28–2·13) | ||
| Any bacterial STIc | 2·34 (1·52–3·60) | 0·70 (0·45–1·08) | ||
| Chlamydiac | 1·67 (0·92–3·01) | 0·48 (0·25–0·92) | ||
| Gonorrhoeac | 2·34 (1·41–3·89) | 0·97 (0·60–1·58) | ||
| Syphilisc | 2·34 (1·07–5·13) | 0·72 (0·23–2·27) | ||
| DUDIT score ≥8 | 1·99 (1·28–3·08) | 1·28 (0·86–1·92) | ||
| AUDIT score ≥8 | 0·97 (0·58–1·61) | 0·97 (0·61–1·52) | ||
| MHI-5 score <60 | 1·43 (0·87–2·32) | 1·77 (1·14–2·74) | ||
| SCS score ≥24 | 1·93 (1·11–3·36) | 1·29 (0·77–2·16) | 2·32 (1·17–4·63) | 0·76 (0·37–1·54) |
| Per extra point on NSSS | 1·00 (0·98–1·02) | 0·96 (0·94–0·98) | 1·00 (0·97–1·03) | 0·96 (0·94–0·98) |
AUDIT: Alcohol Use Disorder Identification Test; CAS: condomless anal sex; Chemsex: use of methamphetamine, γ-hydroxybutyric acid(GHB)/γ-butyrolactone(GBL) and/or mephedrone during sex; DUDIT: Drug Use Disorder Identification Test; HIV: human immunodeficiency virus; MHI-5: 5-item Mental Health Inventory; NSSS: New Sexual Satisfaction Scale; SCS: Sexual Compulsivity Scale; STI: sexually transmitted infection.
All factors were time-updated except for ethnicity, city of residence, education level, employment, net monthly income, living situation and sexual orientation.
Per ln increase.
In the past 3 months.
In the past 6 months.
In the past 12 months.
HRs <1 denote that the factor was more likely among those who transitioned versus those who continued their regimen.
A total of 67 participants discontinued PrEP (3-year cumulative probability 17·7%, 95% CI 14·1–22·2), with no difference in TI between regimens from which participants discontinued: daily PrEP (n = 44, TI 0·06, 95% CI 0·04–0·08) and event-driven PrEP (n = 23, TI 0·08, 95% CI 0·05–0·13) (Fig. S1). Two participants, one consistent and one former user of daily PrEP who both never switched regimens [3], discontinued PrEP after being diagnosed HIV-positive and were right-censored in analysis. Of the 67 participants who discontinued PrEP, 33 (49·3%) gave a reason for discontinuation (in Supplement) and 34 (50·7%) discontinued PrEP without providing a reason. Of the 67 participants who discontinued PrEP, 37/60 (61·7%) reported CAS with a casual partner in the 3 months prior to their last study visit (Table 1), with no difference between those who provided a reason for discontinuing and those who did not (results not shown). Nine out of 67 (13·4%) participants later restarted PrEP in the context of this study, of whom three had originally discontinued PrEP due to side effects. These transitions to restarting PrEP were not included in analysis.
In multivariable analysis, discontinuing daily PrEP was associated with younger age, fewer casual partners and more CAS acts with casual partners (Table 3). In univariable analysis, those who had fewer sex partners (any and casual), reported fewer sex acts (any and condomless) with casual partners, reported lower levels of any CAS (overall or with casual partners), or had lower NSSS scores were more likely to discontinue event-driven PrEP, but none of these factors were retained or significant in the multivariable model.
Table 3.
Determinants of discontinuing PrEP from event-driven and daily PrEP among 365 MSM and 2 TGP participating in the AMPrEP project, Amsterdam, the Netherlands, 2015–2019.
| Univariable HR |
Multivariable |
|||
|---|---|---|---|---|
| Event-driven PrEP → PrEP discontinuation | Daily PrEP → PrEP discontinuation | Event-driven PrEP → PrEP discontinuation | Daily PrEP → PrEP discontinuation | |
| Factorsa | HR (95% CI)f | HR (95% CI)f | aHR (95% CI)f | aHR (95% CI)f |
| Calendar year (per year increase) | 1·16 (0·72–1·87) | 1·21 (0·85–1·71) | ||
| Socio-demographic characteristics | ||||
| Age (per 10 year increase) | 0·80 (0·55–1·16) | 0·64 (0·47–0·86) | 0·97 (0·58–1·64) | 0·62 (0·43–0·89) |
| Non-white ethnicity | 0·95 (0·27–3·37) | 0·73 (0·27–1·96) | ||
| Residency outside Amsterdam | 1·67 (0·71–3·94) | 1·26 (0·68–2·34) | ||
| College/university degree | 0·74 (0·26–2·08) | 0·69 (0·36–1·33) | ||
| Employment | ||||
| Employed | ref. | ref. | ||
| Unemployed | 0·90 (0·20–4·17) | 2·35 (0·70–7·92) | ||
| Other | 0·68 (0·19–2·47) | 1·83 (0·90–3·73) | ||
| Net monthly income >1700€ | 0·90 (0·35–2·34) | 0·62 (0·32–1·21) | ||
| Living with partner/others (vs·alone) | 0·83 (0·35–1·97) | 1·05 (0·58–1·95) | ||
| Not exclusively homosexual | 1·20 (0·42–3·38) | 1·08 (0·52–2·25) | ||
| Sexual behaviour | ||||
| Number of anal sex partnersb,c | 0·57 (0·36–0·89) | 0·71 (0·51–0·99) | ||
| Number of casual sex partnersb,c | 0·54 (0·35–0·83) | 0·70 (0·52–0·95) | 1·24 (0·60–2·57) | 0·35 (0·17–0·71) |
| Having ≥1 HIV-positive sex partnerc | 0·43 (0·17–1·09) | 0·44 (0·23–0·83) | 0·77 (0·16–3·57) | 0·78 (0·34–1·80) |
| Number of anal sex actsb,c | 0·78 (0·53–1·14) | 0·70 (0·52–0·95) | ||
| Number of sex acts with casual sex partnersb,c | 0·48 (0·31–0·74) | 0·74 (0·56–0·98) | ||
| Any CASc | 0·20 (0·08–0·50) | 0·73 (0·20–2·58) | ||
| Number of CAS actsb,c | 0·77 (0·53–1·12) | 0·83 (0·63–1·09) | ||
| Any CAS with casual sex partnersc | 0·10 (0·04–0·25) | 0·69 (0··26–1·85) | ||
| Number of CAS acts with casual partnersb,c | 0·39 (0·23–0·67) | 0·86 (0·66–1·11) | 0·23 (0·05–1·11) | 2·21 (1·19–4·08) |
| Recreational drug use and mental health characteristics | ||||
| Chemsexd | 0·54 (0·18–1·60) | 0·76 (0·36–1·57) | 1·10 (0·22–5·59) | 0·77 (0·34–1·77) |
| Injecting drug usee | – | 1·58 (0·48–5·23) | ||
| Any bacterial STIc | 0·19 (0·02–1·88) | 0·75 (0·37–1·50) | ||
| Chlamydiac | 0·44 (0·05–3·93) | 0·78 (0·32–1·87) | ||
| Gonorrhoeac | – | 0·84 (0·37–1·89) | ||
| Syphilisc | 0·26 (0·00–22·2) | 0·57 (0·08–4·24) | ||
| DUDIT score ≥8 | 0·72 (0·23–2·31) | 1·62 (0·86–3·05) | ||
| AUDIT score ≥8 | 0·20 (0·02–2·10) | 1·65 (0·87–3·16) | ||
| MHI-5 score <60 | 1·56 (0·59–4·17) | 1·59 (0·77–3·27) | ||
| SCS score ≥24 | 0·68 (0·12–3·95) | 1·48 (0·69–3·18) | 0·75 (0·10–5·53) | 1·35 (0·53–3·44) |
| Per extra point on NSSS | 0·98 (0·94–1·02) | 1·00 (0·96–1·03) | 0·99 (0·95–1·04) | 0·95 (0·95–1·03) |
AUDIT: Alcohol Use Disorder Identification Test; CAS: condomless anal sex; Chemsex: use of methamphetamine, γ-hydroxybutyric acid(GHB)/γ-butyrolactone(GBL) and/or mephedrone during sex; DUDIT: Drug Use Disorder Identification Test; HIV: human immunodeficiency virus; MHI-5: 5-item Mental Health Inventory; NSSS: New Sexual Satisfaction Scale; SCS: Sexual Compulsivity Scale; STI: sexually transmitted infection.
All factors except ethnicity, city of residence, education level, employment, net monthly income, living situation and sexual orientation were time-updated.
Per ln increase.
In the past 3 months.
In the past 6 months.
In the past 12 months.
HRs <1 denote that the factor was more likely among those who transitioned versus those who continued their regimen.
5. Discussion
Based on the first three years after PrEP initiation in AMPrEP, we provide a comprehensive, longitudinal description of how individuals chose their PrEP regimen over the course of PrEP use and how frequently individuals discontinued their regimen. Switching was frequent: within a three-year time span, about one-third of individuals switched their PrEP regimen. These switches were found more frequently among younger MSM and were related to certain sexual behaviours. Switching from event-driven to daily PrEP was associated with chemsex, reporting signs of sexual compulsivity, and not reporting sex with known HIV-positive partners. Switching from daily to event-driven PrEP also was associated with chemsex, and with lower sexual satisfaction. In the same time frame, almost 20% of participants discontinued PrEP. Discontinuation, mostly from daily PrEP, was similarly most likely to occur among younger MSM and be related to sexual behaviour with casual partners. Discontinuation was not linked to the type of PrEP regimen.
In the current study, we found that participants who switched to daily PrEP were more likely to engage in chemsex, report signs of sexual compulsivity, and not have reported sex with known HIV-positive partners. This suggests that participants choose to switch to daily PrEP because of higher HIV risk. Chemsex and sexual compulsivity have both been previously implicated in HIV acquisition [16,17]. In addition, HIV risk is arguably more present during sex with a partner of unknown or uncertain HIV status, given that the vast majority of known HIV-positive individuals in these settings have undetectable viral loads [18].
Participants who switched to event-driven PrEP had lower sexual satisfaction; some former users of daily PrEP mentioned that their daily regimen facilitated more sex than preferred and/or sex perceived as less intimate and satisfying [9]. For these users, the desire to have less sex or more control over sex drove their choice to switch to event-driven PrEP. Changes from daily to event-driven PrEP and vice versa were both associated with chemsex, indicating frequent switching for these individuals. This finding is seemingly contradictory and could be explained by factors specific to chemsex, i.e. frequency of use, settings in which chemsex occurs, planning around chemsex [19].
Similar factors appeared to be associated with PrEP discontinuation. Those discontinuing daily PrEP reported fewer casual partners and more CAS acts with casual partners. This observation could represent a shift towards more sex with fewer known casual partners, which carries lower HIV risk or perceived HIV risk. Albeit not significant in multivariable analysis, those who discontinued event-driven PrEP also reported fewer partners and sex acts, suggesting a lower level of sexual activity. Importantly, we did not find any associations between PrEP discontinuation and engaging in chemsex, injecting drug use, or STI diagnoses. Our data suggest it is indeed possible, in the setting of Amsterdam, to retain PrEP users with frequent use of recreational drugs and injecting drug use, which has been associated with discontinuations in an Australian study [20]. However, some data on drug use might not have been available at the visit directly prior to PrEP discontinuation, hence the lack of association might be due to missing data.
When considering the analysis on switching PrEP regimens and PrEP discontinuation together, we observe that these endpoints are substantially driven by certain patterns of sexual behaviour. More specifically, it would appear that intermittent periods of PrEP use parallel intermittent periods of risk, corresponding to what has been previously described in literature as “seasons of risk” [7] and is supported by previous qualitative research within AMPrEP [9]. PrEP care should therefore focus on time-varying individual needs and preferences. PrEP care providers should consider the PrEP user's patterns of sexual behaviour in order to facilitate suitable regimen choices. The low HIV incidence [11] and high rates of adherence to both daily and event-driven PrEP observed in AMPrEP [21,22] suggest that our participants made appropriate regimen choices during their time on PrEP. We also observed that 13% of the participants who initially discontinued PrEP later restarted it, thus blurring the line between periodic PrEP use and PrEP discontinuation. This might be concerning given that some individuals could have become habituated to CAS while on PrEP [11] and could be prone to continue CAS without PrEP and hence at-risk of HIV-acquisition [12,23,24]. As such, it is important that PrEP care providers counsel PrEP users on how to safely start and stop PrEP regimens and discuss all available risk reduction strategies comprehensively (e.g. condom use, knowing sexual partner's HIV viral load status or PrEP use) [24].
Importantly, we found that regimen switches and discontinuations were more likely to occur among younger MSM. Since HIV incidence rates have recently failed to decrease among young participants of an HIV-negative MSM cohort in Amsterdam [25], additional, intensified and tailored counselling is needed for this group. PrEP uptake and retention of younger MSM might also be increased with eHealth tools, such as mobile applications, web-based education modules and interactive websites [26].
Nevertheless, overall discontinuation rates in our study were comparatively low: other demonstration projects found similar rates over much shorter follow-up time [20,27,28] and higher discontinuation rates were observed in larger-scale clinical or primary care implementation settings [13,29]. As our project is one of the few offering a choice of PrEP regimens, PrEP discontinuation might be low when having access to an alternative, non-daily regimen. This is supported by an analysis of Australian daily PrEP users, in which interest in event-driven PrEP was particularly high among those discontinuing PrEP [8]. Another explanation could be that our study population was composed of highly-educated, white-identifying Dutch, early adopting MSM, and may reflect individuals more eager and willing to remain on PrEP and contribute to a research project. PrEP and clinical testing was provided free-of-charge and by a small, dedicated team, which could increase motivation to continue study participation. Whether having multiple regimen options indeed improves PrEP retention and adherence should be further researched.
Our study has some limitations worth addressing. First, some participants could have discontinued participation in AMPrEP yet still received PrEP elsewhere, particularly during later years of follow-up when generic PrEP became available via general practitioners. We could not distinguish these participants from those who truly stopped PrEP. Nevertheless, data from exit questionnaires from those discontinuing follow-up suggest that the main reason for discontinuation was related to changes in sexual behaviour and no longer needing PrEP. Second, we were unable to assess some reasons, which were mentioned by participants themselves as reasons for switching regimen, such as a changing relationship status, traveling, fear or experience of side effects, ability to plan sexual activity, and self-efficacy [9]. Third, we right-censored participants who discontinued PrEP, even if they restarted later in time. This applied to only a few individuals in our study and would not have likely affected our results. Still, caution should be applied when interpreting our results to settings where temporary PrEP discontinuation is more common. Fourth, since we did not consider drug levels or pill use in these analyses, we could not determine how adherence relates to PrEP switch and LTFU. Fifth, we did not include STI diagnoses done outside our clinic in our analyses. However, this was just a small proportion of all STI diagnosed [11]. Finally, our study population constituted mainly highly educated, native Dutch middle-aged early adopters of PrEP, and included few transgender persons despite recruitment efforts, and may therefore not represent all those eligible for PrEP in the Netherlands.
In conclusion, we demonstrated that switches between daily and event-driven PrEP were frequent and linked to younger age and certain sexual behaviours among AMPrEP participants. Rates of PrEP discontinuation, which might be temporary, were comparatively low and did not differ between PrEP regimens. Consultations should include a comprehensive discussion of all available risk reduction strategies, including PrEP regimens, taking in consideration patterns of sexual behaviour. It should also include education on how to safely start, switch and stop PrEP regimens, especially among young MSM, to ensure adequate protection against HIV acquisition.
Data sharing
The AMPrEP data are owned by the Public Health Service of Amsterdam. Original data can be requested by submitting a study proposal to the steering committee of AMPrEP. The proposal format can be obtained from the corresponding author (lcoyer@ggd.amsterdam.nl or amprep@ggd.amsterdam.nl). Requests for further information can also be submitted via the same email addresses. The AMPrEP steering committee will check each proposal for compatibility with general objectives, ethical approvals, and informed consent forms of the AMPrEP study, and potential overlap with ongoing work. There are no other restrictions to obtaining the data and all data requests will be processed in the same manner.
Funding
The AMPrEP study received funding as part of the H-TEAM initiative from ZonMw (grant number: 522002003), the National Institute for Public Health and the Environment (RIVM), GGD research funds, Gilead Sciences (CO-US-276-1712), and the H-TEAM initiative. The study drug and an unrestricted research grant was provided by Gilead Sciences. The H-TEAM initiative is supported by the Aidsfonds Netherlands (grant number: 2013169), Stichting Amsterdam Dinner Foundation, Gilead Sciences Europe Ltd (grant number: PA-HIV-PREP-16-0024), Gilead Sciences (protocol numbers: CO-NL-276-4222), Janssen Pharmaceuticals (reference number: PHNL/JAN/0714/0005b/1912fde), M.A.C. AIDS Fund, and ViiV Healthcare (PO numbers: 3000268822, 3000747780).
Declaration of Interests
L. Coyer, M. van den Elshout, Dr. Matser, Dr. Schim van der Loeff, Dr. Davidovich, Dr. de Vries, Dr. Hoornenborg and Dr. Boyd report grants from ZonMw, the National Institute for Public Health and the Environment, GGD research funds, H-TEAM, and grants and non-financial support from Gilead Sciences during the conduct of the study. Dr. Achterbergh reports non-financial support from Gilead Sciences during the conduct of the study. Dr. Prins reports grants and speaker fees from Gilead Sciences, Roche, Abbvie, MSD, all of which were paid to her Institute, during the conduct of the study.
Acknowledgements
The authors wish to thank all AMPrEP participants. Additionally, we would like to thank the following persons for their support to this study, Marjo Broeren, Yvonne van Duijnhoven, Ertan Ersan, Princella Felipa, Arjan Hogewoning, Kees de Jong, Michelle Kroone, Myra van Leeuwen, Dominique Loomans, Ilya Peters, Martijn van Rooijen, Adriaan Tempert, and Kenneth Yap. Furthermore, we thank the members of the AMPrEP advisory board and the community engagement group, and all of those who contributed to the H-TEAM initiative (in Supplement).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100650.
Contributor Information
Liza Coyer, Email: lcoyer@ggd.amsterdam.nl.
Mark A M van den Elshout, Email: mvdelshout@ggd.amsterdam.nl.
Roel C A Achterbergh, Email: rachterbergh@ggd.amsterdam.nl.
Amy Matser, Email: amatser@ggd.amsterdam.nl.
Maarten F Schim van der Loeff, Email: mschim@ggd.amsterdam.nl.
Udi Davidovich, Email: udavidovich@ggd.amsterdam.nl.
Henry J C de Vries, Email: h.j.devries@amc.uva.nl.
Maria Prins, Email: mprins@ggd.amsterdam.nl.
Elske Hoornenborg, Email: ehoornenborg@ggd.amsterdam.nl.
Anders Boyd, Email: aboyd@ggd.amsterdam.nl.
Appendix. Supplementary materials
References
- 1.Fonner V.A., Dalglish S.L., Kennedy C.E., Baggaley R., O'Reilly K.R., Koechlin F.M. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS. 2016;30(12):1973–1983. doi: 10.1097/QAD.0000000000001145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.EACS. Guidelines for treatment of HIV-positive adults. Version 9.1: European Aids Clinical Society. 2018.
- 3.Hoornenborg E., Achterbergh R.C., van der Loeff M.F.S., Davidovich U., van der Helm J.J., Hogewoning A. Men who have sex with men more often chose daily than event-driven use of pre-exposure prophylaxis: baseline analysis of a demonstration study in Amsterdam. J Int AIDS Soc. 2018;21(3):e25105. doi: 10.1002/jia2.25105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reyniers T N.C., Laga M., De Baetselier I., Crucitti T., Wouters K., Smekens B., Buyze J., Vuylsteke B. Choosing between daily and event-driven pre-exposure prophylaxis: results of a Belgian PrEP demonstration project. J Acquir Immune Defic Syndr. 2018;79(2):186–194. doi: 10.1097/QAI.0000000000001791. [DOI] [PubMed] [Google Scholar]
- 5.Vaccher S.J., Gianacas C., Templeton D.J., Poynten I.M., Haire B.G., Ooi C. Baseline preferences for daily, event-driven, or periodic HIV pre-exposure prophylaxis among gay and bisexual men in the PRELUDE demonstration project. Front Publ Health. 2017;5:341. doi: 10.3389/fpubh.2017.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siguier M., Mera R., Pialoux G., Ohayon M., Cotte L., Valin N. First year of pre-exposure prophylaxis implementation in France with daily or on-demand tenofovir disoproxil fumarate/emtricitabine. J Antimicrob Chemother. 2019;74(9):2752–2758. doi: 10.1093/jac/dkz220. [DOI] [PubMed] [Google Scholar]
- 7.Elsesser S.A., Oldenburg C.E., Biello K.B., Mimiaga M.J., Safren S.A., Egan J.E. Seasons of risk: anticipated behavior on vacation and interest in episodic antiretroviral pre-exposure prophylaxis (PrEP) among a large national sample of U.S. men who have sex with men (MSM) AIDS Behav. 2016;20(7):1400–1407. doi: 10.1007/s10461-015-1238-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornelisse V.J., Lal L., Price B., Ryan K.E., Bell C., Owen L. Interest in switching to on-demand HIV pre-exposure prophylaxis (PrEP) among Australian users of daily PrEP: an online survey. Open Forum Infect Dis. 2019;6(7):ofz287. doi: 10.1093/ofid/ofz287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmermann H.M., Eekman S.W., Achterbergh R.C., Schim van der Loeff M.F., Prins M., de Vries H.J. Motives for choosing, switching and stopping daily or event-driven pre-exposure prophylaxis – a qualitative analysis. J Int AIDS Soc. 2019;22(10):e25389. doi: 10.1002/jia2.25389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sidebottom D., Ekstrom A.M., Stromdahl S. A systematic review of adherence to oral pre-exposure prophylaxis for HIV – how can we improve uptake and adherence? BMC Infect Dis. 2018;18(1):581. doi: 10.1186/s12879-018-3463-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoornenborg E., Coyer L., Achterbergh R.C.A., Matser A., Schim van der Loeff M.F., Boyd A. Sexual behaviour and incidence of HIV and sexually transmitted infections among men who have sex with men using daily and event-driven pre-exposure prophylaxis in AMPrEP: 2 year results from a demonstration study. Lancet HIV. 2019;6(7):e447–e455. doi: 10.1016/S2352-3018(19)30136-5. [DOI] [PubMed] [Google Scholar]
- 12.Krakower D., Maloney K.M., Powell V.E., Levine K., Grasso C., Melbourne K. Patterns and clinical consequences of discontinuing HIV preexposure prophylaxis during primary care. J Int AIDS Soc. 2019;22(2):e25250. doi: 10.1002/jia2.25250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan P.A., Patel R.R., Mena L., Marshall B.D., Rose J., Sutten Coats C. Long-term retention in pre-exposure prophylaxis care among men who have sex with men and transgender women in the United States. J Int AIDS Soc. 2019;22(8):e25385. doi: 10.1002/jia2.25385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rao D.W., Carr J., Naismith K., Hood J.E., Hughes J.P., Morris M. Monitoring HIV preexposure prophylaxis use among men who have sex with men in Washington state. Sex Transm Dis. 2019;46(4):221–228. doi: 10.1097/OLQ.0000000000000965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson C.H. Multi-state models for panel data: the MSM package for R. J Stat Softw. 2011;38(8):1–29. [Google Scholar]
- 16.Pakianathan M., Whittaker W., Lee M.J., Avery J., Green S., Nathan B. Chemsex and new HIV diagnosis in gay, bisexual and other men who have sex with men attending sexual health clinics. HIV Med. 2018 doi: 10.1111/hiv.12629. [DOI] [PubMed] [Google Scholar]
- 17.Drumright L.N., Patterson T.L., Strathdee S.A. Club drugs as causal risk factors for HIV acquisition among men who have sex with men: a review. Subst Use Misuse. 2006;41(10–12):1551–1601. doi: 10.1080/10826080600847894. [DOI] [PubMed] [Google Scholar]
- 18.ECDC . Monitoring implementation of the Dublin declaration on partnership to fight HIV/AIDS in Europe and Central Asia: 2018 progress report. ECDC; Stockholm: 2018. Continuum of HIV care. [Google Scholar]
- 19.Closson E.F., Mitty J.A., Malone J., Mayer K.H., Mimiaga M.J. Exploring strategies for PrEP adherence and dosing preferences in the context of sexualized recreational drug use among MSM: a qualitative study. AIDS Care. 2018;30(2):191–198. doi: 10.1080/09540121.2017.1360992. [DOI] [PubMed] [Google Scholar]
- 20.Ryan K.E., Asselin J., Fairley C.K., Lal L., Nguyen L., Penn M. MOAD0303: results from a large Australian PrEP demonstration study: discontinuation and subsequent HIV and other sexually transmitted infection risk. Proceedings of the 10th international AIDS society conference; Mexico City, Mexico; 2019. 21-24 July. [Google Scholar]
- 21.Jongen V.W., Hoornenborg E., van den Elshout M., Coyer L., Davidovich U., de Vries H.J.C. O16: using a daily mobile application and dried blot spots to measure adherence to event-driven PrEP among participants of the AMPrEP cohort study. Proceedings of the 12th Netherlands conference on HIV pathogenesis, epidemiology, prevention and treatment (NCHIV); Amsterdam, the Netherlands; 2019. 13 November. [Google Scholar]
- 22.van den Elshout M.A.M., Hoornenborg E., Achterbergh R.C.A., Coyer L., Anderson P., Davidovich U. P05: achieving higher adherence to daily PrEP among MSM in Amsterdam by providing feedback via a mobile application: results of a randomised clinical trial. Proceedings of the 12th Netherlands conference on HIV pathogenesis, epidemiology, prevention and treatment (NCHIV); Amsterdam, the Netherlands; 2019. 13 November. [Google Scholar]
- 23.Marcus J.L., Hurley L.B., Bradley Hare C., Phuong Nguyen D., Phengrasamy T., Silverberg M.J. Preexposure prophylaxis for HIV prevention in a large integrated health care system: adherence, renal safety, and discontinuation. J Acquir Immune Defic Syndr. 2016;73(5):540–546. doi: 10.1097/QAI.0000000000001129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jonas K.J., Yaemim N. HIV prevention after discontinuing pre-exposure prophylaxis: conclusions from a case study. Front Publ Health. 2018;6:137. doi: 10.3389/fpubh.2018.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Bilsen W.P.H., Boyd A., van der Loeff M.F.S., Davidovich U., Hogewoning A., van der Hoek L. Diverging trends in incidence of HIV versus other sexually transmitted infections in HIV-negative men who have sex with men (MSM) in Amsterdam. AIDS. 2019;34(2):301–309. doi: 10.1097/QAD.0000000000002417. [DOI] [PubMed] [Google Scholar]
- 26.Schnall R., Travers J., Rojas M., Carballo-Dieguez A. eHealth interventions for HIV prevention in high-risk men who have sex with men: a systematic review. J Med Internet Res. 2014;16(5):e134. doi: 10.2196/jmir.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vuylsteke B., Reyniers T., De Baetselier I., Nöstlinger C., Crucitti T., Buyze J. Daily and event-driven pre-exposure prophylaxis for men who have sex with men in Belgium: results of a prospective cohort measuring adherence, sexual behaviour and STI incidence. J Int AIDS Soc. 2019;22(10):e25407. doi: 10.1002/jia2.25407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grinsztejn B., Hoagland B., Moreira R.I., Kallas E.G., Madruga J.V., Goulart S. Retention, engagement, and adherence to pre-exposure prophylaxis for men who have sex with men and transgender women in PrEP Brasil: 48 week results of a demonstration study. Lancet HIV. 2018;5(3):e136–ee45. doi: 10.1016/S2352-3018(18)30008-0. [DOI] [PubMed] [Google Scholar]
- 29.Scott H.M., Spinelli M., Vittinghoff E., Morehead-Gee A., Hirozawa A., James C. Racial/ethnic and HIV risk category disparities in preexposure prophylaxis discontinuation among patients in publicly funded primary care clinics. AIDS. 2019;33(14):2189–2195. doi: 10.1097/QAD.0000000000002347. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.