Abstract
Direct oral anticoagulants (DOAC) are first line treatment for pulmonary embolism (PE). Treatment of acute PE is traditionally hospital based and associated with high costs. The aims of this study were to evaluate potential cost savings with outpatient DOAC treatment compared to inpatient DOAC treatment in patients with low risk PE. A retrospective study in patients with DOAC treated low risk PE (simplified pulmonary severity index [sPESI] ≤ 1) admitted to 8 hospitals during 2013-2015. Health care costs were compared in 223(44%) patients treated as outpatients and 287(56%) treated in hospital. Total cost per patient was 8293 EUR in the inpatient group, and 2176 EUR in the outpatient group (p < 0.001). Total costs for inpatients were higher (p < 0.001) compared to outpatients in both subgroups with sPESI 0 and 1. In multivariate analysis, type of treatment (in- or outpatient, p = < 0.001) and sPESI group (0 or 1, p = < 0.001) were associated with total cost below or above median, whereas age (p = 0.565) and gender (p = 0.177) was not. Adherence to guidelines recommending outpatient treatment with DOAC in patients with low risk PE enables significant savings.
Keywords: cost savings, pulmonary embolism, direct oral anticoagulant, venous thromboembolism, outpatient, inpatient
Background
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE) affects 5% of the population during a lifetime.1 PE is associated with a wide prognostic spectrum, ranging from prompt resolution to sudden death.2
Anticoagulant (AC) therapy of acute PE1–4 has traditionally been hospital based, with a mean hospital stay of 6 days.5 Outpatient treatment of PE was suggested already in the 1990s,6 however, and outpatient treatment is recommended for selected low-risk patients in both European7 and American8 guidelines. In spite of the facts that different eligibility criteria for identification of low-risk patients suitable for outpatient treatment have been evaluated in prospective and retrospective studies,9–11 a recent prospective study has documented the safety of this approach,12 and another randomized study is ongoing,13 the proportion of patients selected for outpatient treatment is still low in most industrialized countries.14
During recent years direct oral anticoagulants (DOAC) with a favorable risk profile have been introduced as an alternative to warfarin for VTE treament,15–21 and are now considered as first line treatment of PE.8 As the need for monintoring of DOAC treatment is less than for warfarin,21,22 outpatient treatment of VTE is potentially facilitated by DOAC use. We have recently demonstrated that selected low-risk PE patients in our institution could be safely treated with DOAC on an outpatient basis.23
In the United States PE is estimated to cause annual costs ranging from 8.5 to 19.8 billion US dollars (USD).24 Dasta et al reported that the daily cost for inpatient PE treatment started at 2034 USD and was highest during the first 3 days, and that the total mean daily cost for inpatient care of PE was 1735 USD.25
Recently presented data indicate that outpatient treatment of low-risk PE (LRPE) is associated with potential savings,26,27 however, the economic burden incurred by PE is lower in patients with short length of stay (LOS). Furthermore, PE patients with short LOS also run a noticeable lower risk for hospital acquired conditions.28
The aim of this study was to evaluate whether outpatient DOAC treatment of patients with low risk PE is associated with cost savings compared to treatment in hospital.
Methods
We performed a retrospective multicenter cohort study in consecutive patients diagnosed with acute PE in the emergency departments (ED) of all 8 hospitals in Sweden´s southernmost health care region (1.3 million inhabitants) in 2013-2015, a period during which DOAC were gradually replacing warfarin as first line PE treatment. Patient data were extracted from the Swedish quality register for anticoagulation AuriculA,29 from digital patient files, and from imaging databases.
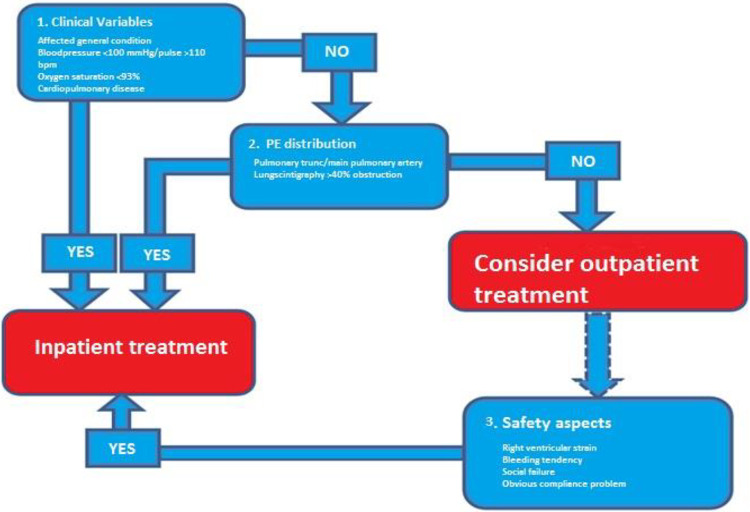
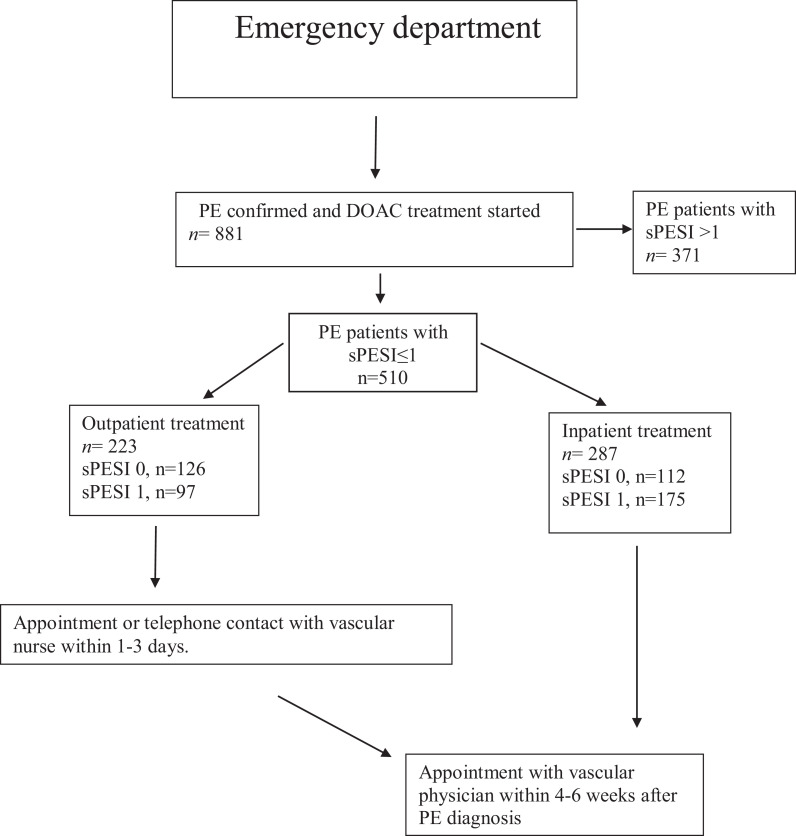
For selection of PE patients suitable for outpatient treatment, the 8 hospitals in the region use a flow chart with pragmatic Criteria for Guiding Outpatient treatment (CGO, Figure 1 30). Outpatients (ED stay ≤ 24 h) were offered an appointment or telephone appointment with a nurse in a vascular unit within 24 to 72 hours, and both outpatients and hospitalized patients were offered an appointment with a vascular physician within 4-6 weeks after diagnosis (Figure 2). A previous study has documented that outpatient treatment based on the CGO-algorithm is safe and effective, although 48% of the patients were high risk PE patients according to the most validated risk stratification tool (Simplified Pulmonary Embolism Severity Index, sPESI ≥ 1).23
Figure 1.
Selection criteria for outpatient treatment of low-risk patients with pulmonary embolism (PE) at the 8 study hospitals in in Sweden´s southernmost health care region.30
Figure 2.
Flow chart depicting treatment and follow-up of 510 patients diagnosed with low risk (simplified pulmonary embolism severity score [sPESI] scores 0-1) PE in the emergency departments of the 8 hospitals in Sweden´s southernmost healthcare region treated with direct oral anticoagulants (DOAC) during 2013-2015. DOAC treated PE patients with sPESI >1 (n = 371) are excluded.
As factors such as compliance problems and social conditions might affect decisions on hospitalization, we restricted our comparison of out- and inpatients to low risk PE patients with sPESI 0 and 1.
Digital patient files revealed that 223 (48%) of the 510 low risk (sPESI ≤ 1) PE patients had been selected for outpatient DOAC-treatment (Figure 2), i.e. The treatment had been started already during an ED visit not exceeding 24 hours,23 whereas the remaining 287 (52%) patients had been treated on a hospital basis.
Symptoms and signs at presentation were retrospectively retreived from files and imaging databases, and the sPESI risk stratification score23,31 was calculated. Furthermore, we retrospectively assessed all other hospitalizations and scheduled and unscheduled health care appointments from 6 months before to 6 months after the acute PE episode, together with mortality, recurrent VTE episodes, and bleeding complications.
Assessment of Costs for Health Care 6-Months Pre and Post Diagnosis
Cost data were obtained from the central economic unit of the administrative body of our Health Care Region, and are those debited to an insurance company or an external region. The figures in SEK were converted to EUR (1 EUR = 9.3 SEK, currency year 2018). The daily cost for hospitalization is 554 EUR based on the average cost for room and staff, imaging, laboratory tests, and medication. We also inlcluded costs for outpatient visits to physicians (175 EUR) and nurses (131 EUR), both during the 6 months preceding hospitalization for PE and during the 6 months after discharge. As the costs for the initial ED visit (435 EUR) and CT-examination (255 EUR) were the same in both groups, these figures were not included in the calculations. Costs for telephone appointments are not debited in our region.
Data Analysis
We performed a descriptive analysis with comparison of the in- and outpatient subgroups by Mann-Whitney tests. Results were also evaluated separately in subgroups with sPESI 0 and 1. Results are expressed as n (%), mean ± standard deviation (SD), or median and interquartile range (IQR) as indicated. Multivariate analysis was conducted to identify baseline variables significantly associated with a total cost below or above median for this variable. Analyses were performed using SPSS for Windows, version 25.0 (SPSS Inc, Chicago, IL).
Ethical Considerations and Permission
Ethical permission was obtained from the Ethics Committee in Lund, Sweden (dnr 2015/143).
Results
Among the 223 outpatients, 97 (43%) patients had sPESI score 0 and 126 (57%) sPESI score 1. Age (p = < 0.001), but not gender distribution (p = 0.806) differed significantly between out- and inpatients (Table 1).
Table 1.
Comparison of Patients With Pulmonary Embolism (PE) and Simplified Pulmonary Embolism Score Index (s-PESI) 0 and 1 Treated With Direct Acting Oral Anticoagulants as Outpatients or Inpatients During 2013-2015.
| Kolumn1 | Kolumn2 | Kolumn3 | Kolumn4 | Kolumn5 | Kolumn6 | Kolumn7 | Kolumn8 | Kolumn9 | Kolumn10 | Kolumn11 | Kolumn12 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| All patients (n = 510) | Inpatients (n = 287) | Outpatients (n = 223) |
P-value |
sPESI 0 (n = 238) | Inpatients (n = 112) | Outpatients (n =126) |
P-value |
sPESI 1 (n = 272) | Inpatients (n = 175) | Outpatients (n = 97) |
P-value |
| Age (years) | 69 (SD 17) | 65 (SD 15) | <0.001 | 60 (SD 15) | 54 (SD 18) | 0.007 | 69 (SD 15) | 65 (SD 13) | 0.011 | ||
| Male gender | 146 (51) | 111 (49) | 0.806 | 48% (male) | 45% (male) | 0.976 | 53% (male) | 50% (male) | 0.652 | ||
| LOS at PE diagnosis (days) | 7.4 (3-8) | 7.1 (3-5.8) | <0.001 | 7.6 (3-9) | <0.001 | ||||||
| LOS 6 months prior to PE diagnosis (days) | 2.4 (0 -1) | 1.3 (0 -1) | <0.002 | 1.4 (0 -1) | 1.2 (0 -1) | 0.271 | 3.1 (0-3) | 1.4 (0 -1) | 0.368 | ||
| LOS 6 months post PE diagnosis (days) | 3.5 (0 -1) | 1.3 (0 -1) | <0.001 | 1.8 (0 -1) | 1.1 (0 -1) | 0.100 | 4.5 (0-3) | 1.6 (0 -1) | 0.002 | ||
| Total LOS (days) | 13.1 (3-15) | 3.0 (0-2) | <0.001 | 10.1 (3-8) | 2.7 (0 -1) | <0.001 | 15.1 (4-19) | 3.3 (0-3) | <0.001 | ||
| Nurse appointments 6 months prior to PE diagnosis | 0.7 (0 -1) | 0.6 (0 -1) | 0.005 | 0.5 (0-0) | 0.3 (0-0) | 0.113 | 0.8 (0 -1) | 1.2 (0 -1) | 0.740 | ||
| Physician appointments 6 months prior to PE diagnosis | 1.3 (0-2) | 1.5 (0-2) | 0.001 | 1.1 (0-2) | 1.1 (0-2) | 0.601 | 1.5 (0-2) | 2.1 (0-3) | 0.055 | ||
| Nurse appointments 6 months post PE diagnosis | 1.0 (0 -1) | 1.0 (0 -1) | <0.001 | 0.9 (0 -1) | 0.5 (0 -1) | 0.025 | 1.1 (0 -1) | 1.6 (0 -1) | 0.166 | ||
| Physician appointments 6 months post PE diagnosis | 2.7 (1-4) | 2.5 (1-3) | 0.014 | 2.4 (1-4) | 2.2 (1-3) | 0.500 | 2.9 (1-5) | 3.0 (1-4) | 0.462 | ||
| Mortality | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| Health care costs 6 months prior to PE diagnosis | 1330 | 720 | <0.001 | 776 | 665 | 0.271 | 1717 | 776 | 0.368 | ||
| Health care costs at PE diagnosis | 4100 | 3933 | 4210 | ||||||||
| Health care costs 6 months after PE diagnosis | 1939 | 720 | <0.001 | 997 | 609 | 0.100 | 2493 | 886 | 0.002 | ||
| Total hospital costs | 7369 | 1440 | <0.001 | 5706 | 1274 | <0.001 | 8420 | 1662 | <0.001 | ||
| Nurse appointment costs prior to PE diagnosis | 92 | 79 | 0.005 | 66 | 39 | 0.113 | 105 | 157 | 0.740 | ||
| Physician appointment costs prior to PE diagnosis | 228 | 263 | 0.001 | 193 | 193 | 0.601 | 263 | 368 | 0.055 | ||
| Nurse appointment costs after PE diagnosis | 131 | 131 | <0.001 | 118 | 66 | 0.025 | 144 | 210 | 0.166 | ||
| Physician appointment costs after PE diagnosis | 473 | 263 | 0.014 | 420 | 385 | 0.500 | 508 | 525 | 0.462 | ||
| Total cost | 8293 | 2176 | <0.001 | 6503 | 1957 | <0.001 | 9440 | 2922 | <0.001 |
Costs in Eur per patient for hospital stay and outpatient visits during the 6 month before and after diagnosis of PE. N (%), mean (SD) or median (IQR). Los = Length of Stay.
Inpatients had a mean stay of 7.4 days for the index PE, incurring a cost of 4100 EUR. Inpatients also spent more days in hospital both during the 6 months before and after the acute PE episode, however, leading to cost differences between groups (1330 EUR in inpatients versus 720 EUR in outpatients [p = < 0.001] before the index PE and 1939 EUR versus 720 EUR [p = < 0.001] after the index PE).
The costs for outpatient health care visits during the 6 months before and 6 months after the index PE episode were also higher in the inpatient group, contributing to a significantly higher total cost in the inpatient group compared to PE patients treated on an out of hospital basis, 8293 EUR vs 2176 EUR (p = < 0.001). In multivariate analysis, type of treatment (in- or outpatient, p = < 0.001) and sPESI group (0 or 1, p = < 0.001) were both significantly associated with a total cost below or above median, whereas age (p = 0.565) or gender (p = 0.177) were not (Table 2).
Table 2.
Multivariate Analysis of Factors Influencing Whether Total Treatment Cost Was Above or Below Median in Patients With Pulmonary Embolism (PE) and Simplified Pulmonary Embolism Score Index (s-PESI) 0 and 1 Treated With Direct Acting Oral Anticoagulants as Outpatients or Inpatients During 2013-2015.
| ß | P-value | OR | 95% CI | |
|---|---|---|---|---|
| Age | 0.004 | 0.565 | 1.004 | 0.990 -1.018 |
| sPESI 0 or 1 | -0.768 | <0.001 | 0.464 | 0.301-0.715 |
| Gender | -0.283 | 0.177 | 0.753 | 0.499 -1.136 |
| In or outpatient treatment | 2.180 | <0.001 | 8.842 | 5.793-13.496 |
No mortality, reccurent VTE, or major bleeding episodes were observed during 6 months of follow-up in either group.
SPESI 0
Among the 238 patients with sPESI 0, 112 (47%) patients were hospitalized and 126 (53%) were treated as outpatients. Inpatients were significantly older (p = 0.007), whereas gender distribution did not differ between groups (p = 0.976, Table 1).
Inpatients had a mean stay of 7 days in hospital for the index PE incurring a cost of 3933 EUR, whereas health care costs 6 months before or after the acute PE episode did not differ significantly between in- and outpatients (Table 1).
Total cost in the inpatient group was higher compared to for outpatients, (6503 EUR versus 1957 EUR, p = < 0.001).
SPESI 1
Among the 272 patients with sPESI 1, 175 (64%) patients were treated as inpatients and 97 (36%) as outpatients. No significant differences in age (p = 0.011) or gender distribution (p = 0.652) were noted betweent out- and inpatients (Table 1).
Inpatients had a mean stay of 7.6 days, incurring costs of 4210 EUR and also higher number of hospital days 6 months after the acute PE episode (Table 1).
No significant cost differences were observed for health care 6 months before the acute PE episode (1717 EUR and 776 EUR, p = 0.368), whereas costs both 6 months after the index PE (2493 EUR versus 886 EUR, p = 0.002) and total costs (9440 EUR versus 2922 EUR, p = < 0.001) were higher the inpatient group
Discussion
The numbers of hospitalized patients with PE, the third most common acute cardiovascular disease after myocardial infarction and stroke2,26 are increasing, as well as treatment costs.26 Traditionally, patients with PE are treated in hospital with a mean LOS of 6 days.5 As hospitalization is a major driver of total cost, any reduction in the number of inpatient days may translate into important cost savings.25
In our retrospective study comparing costs in low-risk (sPESI ≤ 1) hospitalized PE patients with costs in outpatients, total costs for the outpatient group were 6117 EUR lower than for the inpatient group. By definition, this was mainly driven by the difference in the cost for hospital stay caused by the index acute episode of PE, but inpatients also spent slightly more time in hospital before and after the acute PE. This difference was probably due to the increased prevalence of comorbidities in hospitalized patients.
As 30-day survival after PE with or without DVT might be as low as 59%,26,32 risk stratification tools such as sPESI31 or Hestia critera10 are crucial3 to guide decisions on treatment modality. Selection for outpatient treatment with DOAC treatment by using the CGO-criteria (Figure 1) was safe and efficient and associated with significant savings without mortality, reccurent VTE, or major bleeding. Importantly, these results were demonstrated in a population in which a majority had sPESI 1, a group for which many guidelines recommend against outpatient treatment. In this context, it is of interest that the CGO-criteria recommends evaluations of safety aspects not included in the sPESI classification before the decision on in- or outpatient treatment.
Furthermore, as both the in- or outpatient treatment variable and the sPESI group variable were associated with costs in multivariate analysis, it was of special interest to evaluate sPESI groups 0 and 1 separately documenting cost savings with outpatient treatment in both groups.23 Our study therefore corroborates the results of Dasta et al,25 showing that LOS is a major cost driver in PE and that any reduction in LOS may translate into relatively important cost savings.
As DOAC have a more predictable dose response than warfarin and allow fixed dosage without need for routine laboratory monitoring, DOAC treatment in itself might potentially lead to shorter hospitalization. Dobesh recently reported that such advantages could reduce the costs for the health care system by potentially preventing recurrent VTE and its complications.33 To determine whether prolonged LOS is always caused by complications, or in itself might lead to complications is not always easy, however.
Wang et al28 recently presented data from 1918 patients with LRPE, whereof 688 had a short LOS, defined as ≤2 days. They reported that patients with short LOS had both fewer hospital acquired conditions and less occurrence of pneumonia. Furthermore, costs in patients with short (≤2 days) and longer LOS total were 9056 and 12544 USD, respectively, implying that LRPE patients with short LOS had a better net clinical outcome at a lower cost than matched LRPE patients with long LOS.28
Among our patients selected for either out- or inpatient treatment, total costs were 2176 EUR and 8293 EUR, respectively. When comparing these figures with those previously published,28,34 it should be kept in mind that our patients had a higher mean age and a more balanced gender distribution compared to patients in previous studies.28 Furthermore, as sPESI scores were not presented by Wang et al,28 and as some of their patients underwent thrombolysis or placement of inferior vena caval filters, one might suspect that these patients had a more complicated course of PE than ours. Furthermore, there were no recurrent VTE or major haemorrhages in our study whereas Wang and colleagues reported 14 recurent DVTs and 5 bleeding episodes.
In the study by Dasta et al25 mean LOS was 5.4 days, mean age of PE patients was 60 years, and gender distribution was equal. Both LOS and mean age in their study were thus slightly lower than in our study, whereas gender distribution was comparable. The mean daily cost per patient reported by Dasta was 1735 USD, whereof room and board accounted for 38% to 59% of the total, the main cost driver in our study as well. Mean total hospitalization cost per patient in Dasta´s study was 11486 USD, which of course was higher compared to outpatients (0 EUR), but also compared to hospitalized patients (7369 EUR) in our study.
No data regarding mortality, reccurent VTE and other complications were reported by Dasta and co-workers, but in contrast to our group of patients with sPESI scores ≤1, 24% of their patients25 underwent intensive care for their acute PE. The mean total hospitalization cost for patients in this subgroup was 19901 USD, whereof surgery and supplies accounted for 1576 USD. No such costs were incurred in our study, as our patients had a sPESI score ≤1, and did not require intensive care facilities or surgical treatment.
The use of LMH injections during institution of warfarin treatment is associated with prolonged hospital stay.35 As this is not necessary for treatment with rivaroxaban or apixaban, use of these DOAC might potentially have further beneficial effects on costs. Coleman et al36 showed that rivaroxaban use was associated with a 1.36-day shorter LOS and 2304 USD reduction in total costs compared to parenteral bridging during institution of warfarin. Furthermore, this cost reduction was achieved without increasing the short-term risk of readmission for VTE or major bleeding.
Similarly, Bookhart and colleagues37 evaluated the impact of rivaroxaban on LOS among 321 hospitalized acute PE patients recruited into EINSTEIN PE in North America. In these patients, rivaroxaban use resulted in a 1.7-day mean reduction in LOS compared with enoxaparin and vitamin K antagonists, enabling a reduction of total hospital costs of 3000 USD per patient. As the use of risk stratification tools such as sPESI or Hestia criteria was not reported and patient ages were slightly lower than in our study, however, their results cannot be directly compared to ours.37
Bookhart and collegues also highlighted that the data they used were obtained before rivaroxaban was widely used, and its efficacy fully understood.37
Our patients were studied between 2013 and 2015, when DOAC were gradually introduced in Sweden. Lack of familiarity with these new agents and hesitation to follow the outpatient algorithm 30 might therefore have resulted in unnecessary long LOS, reducing potential cost savings in this study. As DOAC are now generally accepted as first line PE treatment,7,8 potential savings might now be even greater. Coleman et al estimated that up to 50% of PE patients can be treated safely in an outpatient setting,36 and Peacock and colleagues subsequently27 documented total savings of 2496 USD per patient with rivaroxaban treatment and early discharge of LRPE patients identified using Hestia criteria.10 Mean age in their patients was nearly 20 years lower than in our study, however.
Study Limitations
Our study is retrospective, not fully matched in terms of other comorbidities, and the selection of patients for in- and outpatient treatment was based upon clinical judgement guided by regional criteria30 and not randomized. It is also important that our results should not be applied to other health care organizations. Furthermore, patients were not assessed after 6 months concerning long-term complications such as chronic thromboembolic pulmonary hypertension, a condition associated with high costs.31 As this condition rarely occurs in patients with LRPE, however, this is probably not an important study limitation. Another potentially important limitation, however, is the fact that we did not have the possibility to assess whether the number of sick-leave days or potential outpatient visits out of hospital differed between groups.
In previous studies,34 outpatient visits were most frequently with internists, family physicians, or pulmonologists, whereas in our vascular unit outpatients are offered a nurse appointment within 72 hours and an appointment with an angiologist within 4-6 weeks (Figure 2). Whether this approach leads to decreased risk for VTE reccurence, better compliance, and increased cost-effecitivity remains to be investigated.
Conclusion
Calculations of costs incurred by PE in different medical systems cannot be directly compared, but there seems to be a strong correlation between the economic burden of PE and LOS. Outpatient PE treatment with DOAC after careful selection of PE patients with validated risk stratification tools and comorbidities taken into account therefore seems to be a promising strategy to decrease the economic burden to society caused by this disease.
Footnotes
Authors’ Note: All authors contributed to the study concept and design. RG contributed to the acquisition of the data. RG, AG, and JE all contributed to data analysis and interpretation, and drafting. RG, AG, JE, JH, and SL contributed to critical revision of the manuscript. Statistical analysis was performed by RG. The unidentified dataset supporting the conclusions of this article is available upon request by contacting the corresponding author. All procedures performed in studies involving human participants were in accordance with the ethical standard of the institutional and/or national research committe and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants from the Ernhold Lundström Foundation, Research Funds at Skåne University Hospital and at Region Skåne, the Hulda Ahlmroth Foundation, from the Swedish Government under the LUA/ALF agreement. Auricula is funded by the Swedish Association of Local Authorities and Regions (SKL).
ORCID iD: Raein Ghazvinian  https://orcid.org/0000-0002-9363-0428
https://orcid.org/0000-0002-9363-0428
Anders Gottsäter  https://orcid.org/0000-0003-0865-0000
https://orcid.org/0000-0003-0865-0000
References
- 1. Guyatt GH, Akl EA, Crowther M, Gutterman DD. Antithrombotic therapy and prevention of thrombosis. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):7–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Donadini MP, Dentali F, Castellaneta M. et al. for the LORPELHS Study Group. Pulmonary embolism prognostic factors and length of hospital stay: a cohort study. Thromb Res. 2017;156:155–159. [DOI] [PubMed] [Google Scholar]
- 3. Konstantinides SV, Torbicki A, Agnelli G, et al. ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014:35(43):3033–3069. [DOI] [PubMed] [Google Scholar]
- 4. Yeh C, Gross P, Weitz J. Evolving use of new oral anticoagulants for treatment of venous thromboembolism. Blood. 2014;124(7):1020–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aujesky D, Stone RA, Kim S, et al. Length of hospital stay and postdischarge mortality in patients with pulmonary embolism: a statewide perspective. Arch Intern Med. 2008;168(7):706–712. [DOI] [PubMed] [Google Scholar]
- 6. Wells PS, Kovacs MJ, Bormanis J, et al. Expanding eligibility for outpatient treatment of deep vein thrombosis and pulmonary embolism with low-molecular-weight-heparin: a comparison of patient self-injection with homecare injection. Arch Intern Med. 1998;158(16):1809–1812. [DOI] [PubMed] [Google Scholar]
- 7. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guildelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Respir J. 2019;54(3):1901647. [DOI] [PubMed] [Google Scholar]
- 8. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease CHEST guideline and expert panel report. Chest. 2016;149(2):315–352. [DOI] [PubMed] [Google Scholar]
- 9. Roy PM, Moumneh T, Penaloza A, Sanchez O. Outpatient management of pulmonary embolism. Thromb Res. 2017;157:92–100. [DOI] [PubMed] [Google Scholar]
- 10. Zondag W, Mos ICM, Creemers-Schild D; et al. On Behalf of the HESTIA Study Investigators. Outpatient treatment in patients with acute pulmonary embolism: the HESTIA study. J Thromb Haemost. 2011;9(8):1500–1507. [DOI] [PubMed] [Google Scholar]
- 11. Den Exter PL, Zondag W, Klok FA, et al. Efficacy and safety of outpatient treatment based on the HESTIA clinical decision rule with or without N-terminal pro-brain natriuretic peptide testing in patients with acute pulmonary embolism, a randomized clinical trial. Am J Resp Crit Care Med. 2016;194(8):998–1006. [DOI] [PubMed] [Google Scholar]
- 12. Barco S, Schmidtmann I, Ageno W, et al. Early discharge and home treatment of patients with low-risk pulmonary embolism with the oral factor Xa inhibitor rivaroxaban: an international multicentre single-arm clinical trial. Eur Heart J. 2020;41(4):509–518. [DOI] [PubMed] [Google Scholar]
- 13. Available at: https://clinicaltrials.gov/ct2/show/NCT02811237. Accessed May 3, 2020.
- 14. Zondag W, Kooiman J, Klok FA, et al. Outpatient versus inpatient treatment in patients with pulmonary embolism: a meta-analysis. Eur Resp J. 2013;42(1):134–144. [DOI] [PubMed] [Google Scholar]
- 15. Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342–2352. [DOI] [PubMed] [Google Scholar]
- 16. EINSTEIN Investigators Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363(26):2499–2510. [DOI] [PubMed] [Google Scholar]
- 17. Hokusai-VTE Investigators Buller HR, Decousus H, Grosso MA, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369(15):1406–1415. [DOI] [PubMed] [Google Scholar]
- 18. Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799–808. [DOI] [PubMed] [Google Scholar]
- 19. EINSTEIN-PE Investigators Buller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366(14):1287–1297. [DOI] [PubMed] [Google Scholar]
- 20. Van Es N, Coppens M, Schulman S, et al. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014;124(12):1968–1975. [DOI] [PubMed] [Google Scholar]
- 21. Comerota AJ, Ramacciotti E. A comprehensive overview of direct oral anticoagulants for the management of venous thromboembolism. Am J Med Sci. 2016;352(1):92–106. [DOI] [PubMed] [Google Scholar]
- 22. Piran S, Schulman S. Management of venous thromboembolism: an update. Thromb J. 2016;14(suppl 1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ghazvinian R, Gottsater A, Elf J. Efficacy and safety of outpatient treatment with direct oral anticoagulation in pulmonary embolism. J Thromb Thrombol. 2018;45(2):319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mahan CE, Borrego ME, Woersching AL, et al. Venous thromboembolism: annualised United States models for total, hospital-acquired and reventable costs utilising long-term attack rates. Thromb Haemost. 2012;108(2):291–302. [DOI] [PubMed] [Google Scholar]
- 25. Dasta JF, Pilon D, Mody SH, et al. Daily hospitalization costs in patients with deep vein thrombosis or pulmonary embolism treated with anticoagulant therapy. Thromb Res. 2015;135(2):303–310. [DOI] [PubMed] [Google Scholar]
- 26. LaMori JC, Shoheiber O, Mody SH, et al. Inpatient resource use and cost burden of deep vein thrombosis and pulmonary embolism in the United States. Clin Ther. 2015;37(1):62–70. [DOI] [PubMed] [Google Scholar]
- 27. Peacock FW, Coleman CI, Diercks DB, et al. Emergency department discharge of pulmonary embolus patients. Acad Emerg Med. 2018;25(9):995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang LI, Baser O, Wells P. Benefit of early discharge among patients with low-risk pulmonary embolism. PLOS One. 2017;12(10):e0185022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wieloch M, Själander A, Frykman V, et al. Anticoagulation control in Sweden: reports of time in therapeutic range, major bleeding, and thrombo-embolic complications from the national quality registry AuriculA. Eur Heart J. 2011;32(18):2282–2289. [DOI] [PubMed] [Google Scholar]
- 30. Elf JE, Jögi J, Bajc M. Home treatment of patients with small to medium sized acute pulmonary embolism. J Thromb Thrombolysis. 2015;39(2):166–172. [DOI] [PubMed] [Google Scholar]
- 31. RIETE Investigators Jimenez D, Aujesky D, Moores L, et al. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med. 2010;170(145):1383–1389. [DOI] [PubMed] [Google Scholar]
- 32. Go AS, Mozaffarian D, Roger VL, et al. For the American Heart Association Statistics Committe and Stroke Statistics Subcommittee. Heart disease and stroke statisics-2014 update: a report from the American Heart Association. Circulation. 2013;129(3):288–292. [Google Scholar]
- 33. Dobesh PP. Economic implications of inadequate treatment of venous thromboembolism and potential solutions. J Pharm Pract. 2014;27(2):178–186. [DOI] [PubMed] [Google Scholar]
- 34. Spyropoulus AC, Lin J. Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: an administrative claims analysis from 30 managed care organizations. J Manag Care Pharm. 2007;13(6):475–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smoyer-Tomic K, Siu K, Walker DR, et al. Anticoagulant use, the prevalence of bridgning, and relation to length of stay among hospitalized patients with non-valvular atrial fibrillation. Am J Cardiovasc Drugs. 2012;12(6):403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Coleman CI, Fermann GJ, Weeda ER, et al. Is rivaroxaban associated with shorter hospital stays and reduced costs versus parenteral bridging to warfarin among patients with pulmonary embolism? Thromb Hemost. 2017;23(7):830–837. [DOI] [PubMed] [Google Scholar]
- 37. Bookhart BK, Haskell L, Bamber L, et al. Length of stay and economic consequences with rivaroxaban vs enoxaparin/vitamin K antagonist in patients with DVT and PE: findings from the North American EINSTEIN clinicial trial program. J Med Econ. 2014;17(10):691–695. [DOI] [PubMed] [Google Scholar]