Abstract
Background:
It has been shown that adding lateral extra-articular tenodesis (LET) to standard anterior cruciate ligament (ACL) reconstruction significantly decreases the loads on the ACL composite graft. To date, the possible effect of LET on ACL graft incorporation is not known.
Purpose:
To compare the incorporation in tibial bone tunnels of a standard quadrupled semitendinosus (ST4) graft to an ST4 graft plus LET at 1 year postoperatively using magnetic resonance imaging (MRI).
Study Design:
Cohort study; Level of evidence, 3.
Methods:
A total of 62 patients who underwent ACL reconstruction were enrolled prospectively: 31 received an ST4 graft, and 31 received an ST4 graft plus LET. Graft incorporation was evaluated with MRI at the 1-year follow-up visit. The following parameters were evaluated: signal-to-noise quotient (SNQ), tibial tunnel widening, graft healing, and graft maturity according to the Howell scale. The primary endpoint was the SNQ of the ST4 graft at 1 year postoperatively; this parameter was adjusted because of unequal baseline characteristics between groups. Clinical and functional outcomes as well as incorporation of the graft were analyzed as secondary endpoints.
Results:
The mean adjusted SNQ was 0.5 ± 2.1 (95% CI, 0.4-4.6) in the ST4 + LET group and 5.9 ± 3.7 (95% CI, 4.7-7.0) in the ST4 group (P = .0297). The mean tibial tunnel widening was 73.7% ± 42.2% in the ST4 + LET group versus 77.5% ± 46.7% in the ST4 group (P = .5685). Howell grade I, indicative of better graft maturity, was statistically more frequent in the ST4 + LET group (P = .0379). No statistically significant difference was seen between groups in terms of graft healing (P = .1663). The Lysholm score was statistically higher in the ST4 + LET group (P = .0058). No significant differences were found between groups in terms of the International Knee Documentation Committee subjective score (P = .2683) or Tegner score (P = .7428). The mean SNQ of the LET graft at the 1-year follow-up visit was 2.6 ± 4.9.
Conclusion:
At 1 year postoperatively, the MRI appearance of ACL grafts showed generally better incorporation and maturation when combined with LET.
Keywords: ACL reconstruction, ST4, incorporation, maturation, ligamentization, anterolateral ligament reconstruction, lateral extra-articular tenodesis, SNQ
The concept of lateral extra-articular tenodesis (LET) is not new; several authors have previously proposed performing combined anterior cruciate ligament (ACL) reconstruction and LET to better control rotational stability.26,29,31,46 Hewison et al22 showed through a meta-analysis that the rate of a positive pivot shift was significantly reduced after combined ACL reconstruction and LET. The LET procedure is most often performed with iliotibial band or gracilis grafts.22
Anatomic reconstruction of the anterolateral ligament (ALL) is a LET technique performed during ACL reconstruction that yields better results in terms of rerupture rate, medial meniscal repair, and reconstruction after a chronic ACL tear, without increasing the number of complications.21,28,43,44 Engebretsen et al14 found that adding LET to an existing standardized intra-articular reconstruction procedure significantly decreases loads on the ACL composite graft by an average of 43%. To our knowledge, no study has analyzed how LET influences the incorporation of an ACL graft.
Claes et al10 reported that many factors influence the graft integration process, especially the mechanical environment and constraints around the graft. Integration can be evaluated with magnetic resonance imaging (MRI) by several methods, including the signal-to-noise quotient (SNQ), which is a relevant imaging parameter reflecting the graft’s mechanical properties and vascularization.6,10,11
We hypothesized that the MRI parameters assessing ACL graft incorporation would be better when combined with ALL reconstruction. The primary objective of this study was to compare the incorporation of a quadrupled semitendinosus (ST4) graft alone versus an ST4 graft plus LET (ST4 + LET) based on the SNQ at 1 year postoperatively. The secondary objective was to compare clinical and functional outcomes between these 2 groups.
Methods
The study was conducted as a quasi-experimental, before-after, comparative single-center cohort study. All patients were enrolled prospectively and consecutively. In our facility, isolated ACL reconstruction with an ST4 graft used to be performed routinely. Since June 2017, patients presenting with ACL failure could undergo combined reconstruction with an ST4 graft and LET if needed. The decision was based on criteria cited below. This study was approved by our institutional review board.
Patients
During the study period, 261 patients with an ACL tear underwent ACL reconstruction with an ST4 graft by a single surgeon (E.C.) at our facility, including 56 ST4 + LET procedures.
The following study inclusion criteria were used: (1) male sex (hormonal changes can affect the graft’s incorporation during the menstrual cycle),27 (2) closed growth plates and age younger than 50 years at the time of surgery, (3) symptoms as well as clinical examination and MRI findings indicative of an ACL tear, (4) healthy contralateral knee, (5) no prior injuries in the knee undergoing surgical repair, (6) no patellofemoral pain, and (7) agreement to return for a 1-year follow-up visit.
The following exclusion criteria were used during the preoperative phase: (1) a grade >2 posterior cruciate ligament (PCL), lateral collateral ligament, or medial collateral ligament injury on both MRI and clinical examination; and (2) a stage >2 chondral injury according to the Outerbridge classification. Additional exclusion criteria were applied during the study: (3) wrong tunnel position, defined by Ayala-Mejias et al1 as an overly vertical tibial tunnel that leads to excessive widening; and (4) a retear of the ACL before the 1-year MRI scan. Tunnel placement was evaluated on MRI using 3-dimensional proton density–weighted turbo spin echo (PD-TSE) sequences.48
Of the initial 261 patients, 171 did not meet the inclusion criteria. Of the remaining 90 patients, 26 did not agree to return for the 1-year follow-up visit. Also, 2 patients in the ST4 group were excluded after enrollment: the first because of an ACL retear and the second because of incorrect tibial tunnel positioning. In the end, 62 patients were included in the study: 31 isolated ST4 graft procedures and 31 ST4 + LET procedures (Figure 1).
Figure 1.
Flowchart. ACL, anterior cruciate ligament; LET, lateral extra-articular tenodesis; ST4, quadrupled semitendinosus.
Surgical Procedure
The patients underwent ACL reconstruction using an ST4 graft technique or combined ST4 + LET. For LET, a gracilis tendon folded into 2 was used. The indication for combined reconstruction was based on ultrasound analysis.4 Ultrasonography has been shown to be a reliable modality for diagnosing ALL injuries.5,8,15 The ALL was considered injured if it was not continuous over its entire length or if it was avulsed from its tibial insertion (true Segond fracture or ultrasonographic Segond lesion).7 Dynamic testing in internal rotation was conducted to improve the ability to check ligament continuity.4 A detailed description of the reconstruction techniques and the product names for all of the fixation devices are available in the Appendix.
All patients participated in the same postoperative rehabilitation protocol. No hinged brace was applied except for at 6 weeks in patients who underwent meniscal repair (12 patients in each group); full weightbearing was allowed immediately after surgery. Physical therapy began the day after surgery; running was allowed in the third month. Return to pivoting and contact sports was allowed after the isokinetic testing results were satisfactory, usually around the seventh month.
To summarize, the patients in this study came from the same population pool and were operated on by the same surgeon using the same instrumentation and the same technique. The fixation methods and rehabilitation protocol were identical for both groups. The only difference between the 2 groups was the addition of the LET procedure.
Endpoints
According to Claes et al,10 ligamentization is the histological evolution of the graft. Because histological sections cannot be carried out in humans, the best way to evaluate incorporation is with MRI. The methodology used in this study has been previously validated.6,11
Several MRI criteria have been validated for evaluating graft incorporation: (1) SNQ,19,24,35,49 (2) tibial tunnel widening,17,18,23,25 (3) graft healing (signal intensity at the bone-graft interface),18 and (4) graft maturity (water content of the graft based on the Howell scale).24
At the 1-year follow-up visit, a knee MRI examination was conducted after the patient had rested for 1 hour. A 3-T MRI unit (Magnetom Skyra; Siemens) with a 15-channel volume array coil was used. The following sequences were taken: 3-dimensional PD-TSE and sagittal proton density–weighted fat suppression (PD-FS).
The SNQ for each graft was calculated with the following formula:
| 1 |
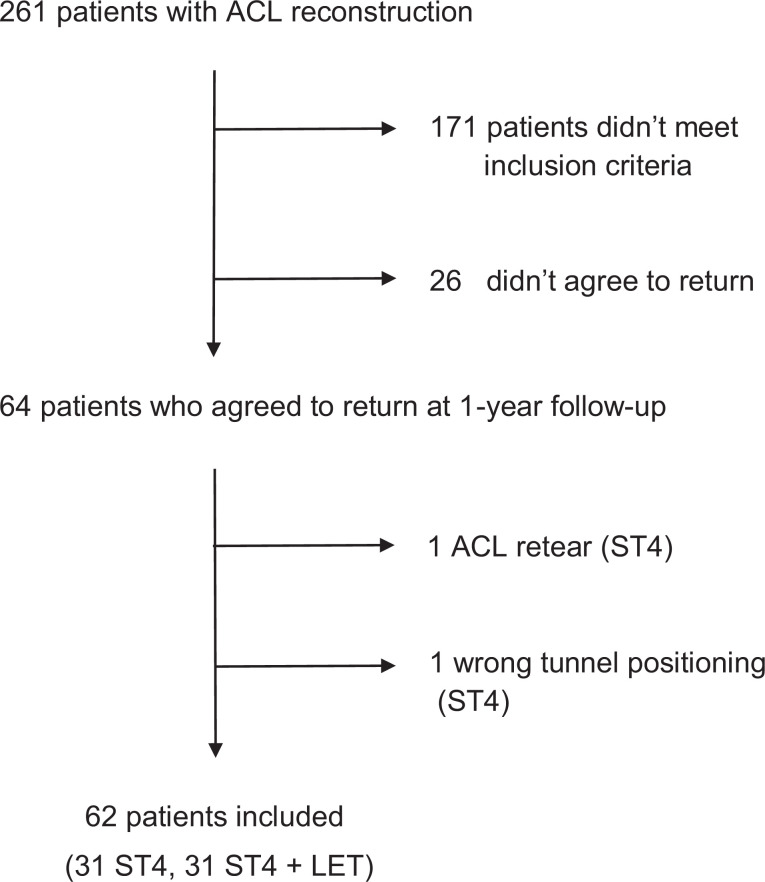
The graft signal values were averaged as described by Weiler et al.49 For MRI analysis, the signal intensity was measured in 0.05-cm2 circular regions of interest on oblique sagittal PD-FS images, tangent to the intra-articular ACL cross section. The graft signal was measured in its intra-articular portion at 3 sites (superior, middle, and inferior), and the average was calculated. The background signal was measured 2 cm anterior to the patellar tendon (Figure 2). The SNQ reflects the graft’s mechanical strength.10,19,24,35,49
Figure 2.
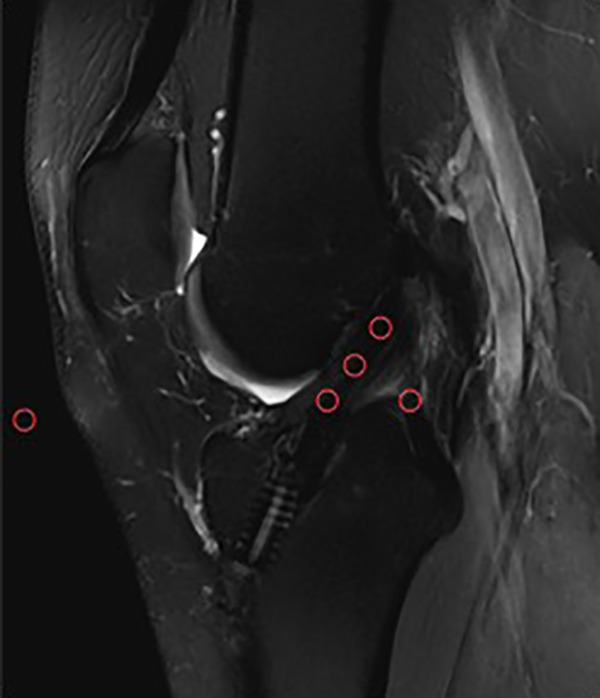
Placement of regions of interest (ROIs) used to calculate the signal-to-noise quotient. There were three 0.05-cm2 ROIs placed on the graft (superior, middle, and inferior), 1 ROI on the posterior cruciate ligament, and 1 ROI on an empty area 2 cm anterior to the patellar tendon.
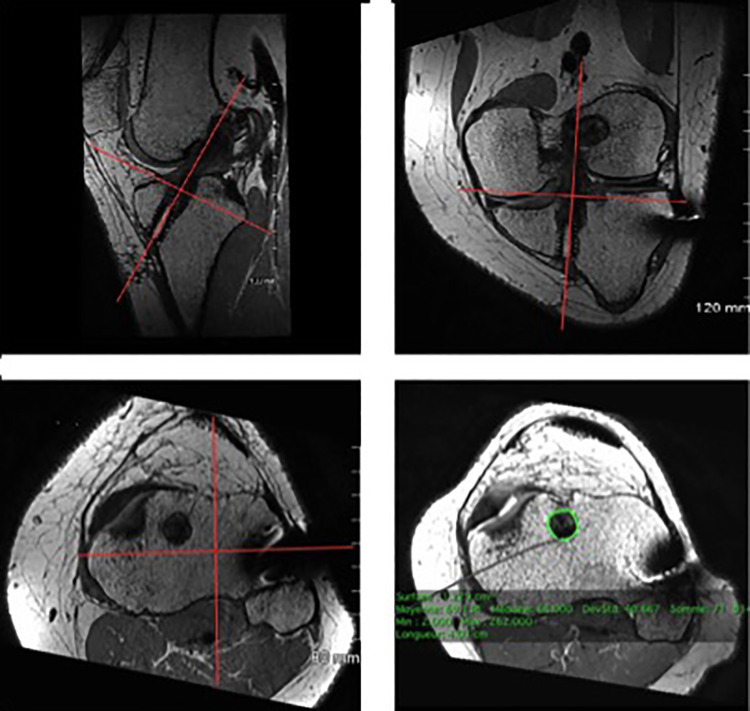
To determine tunnel widening,17 the mean area was measured at the entrance of each tibial tunnel on oblique MRI scans perpendicular to the tunnel’s cross section. The cross-sectional area (CSA; in cm2) of the superior portion of the tibial bone tunnel was measured using image postprocessing software (OsiriX) on PD-TSE sequences (Figure 3). Additionally, 3-dimensional reconstruction was used to define a perpendicular axis to the graft. Tunnel widening (in percentages) was calculated (in percentages) with the following formula:
Figure 3.
Measurement of the cross-sectional area of the tibial bone tunnel with OsiriX software using 3-dimensional reconstruction.
| 2 |
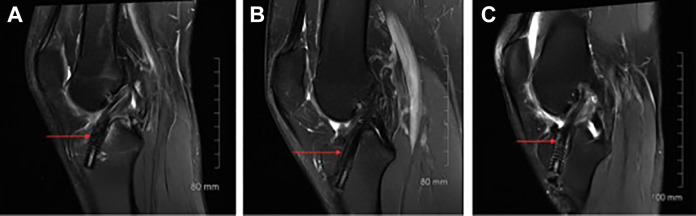
The protocol described by Ge et al18 was used to measure graft healing based on signal intensity at the bone-graft interface. Healing was evaluated on sagittal oblique images from PD-FS sequences tangent to the tibial tunnel’s cross section. Based on this information, the ST4 grafts were assigned 1 of 3 grades (Figure 4): I, low intensity, no fibrosis at the bone-graft interface, and full attachment; II, high intensity over a portion of the interface; or III, high intensity over the entire bone-graft interface and poor attachment.
Figure 4.
Examples of the 3 grades assigned to classify graft healing at the bone-graft interface: (A) grade I, (B) grade II, and (C) grade III.
Graft maturity according to the Howell scale24 was chosen to study integration of the graft within the tibial tunnel.6,11,34,36 Sagittal slices tangent to the graft inside the tunnel were obtained from PD-TSE sequences by using the same oblique axial reconstruction employed for tibial tunnel widening. Graft maturity was measured with a 4-grade system according to Howell et al24 (Figure 5): I, homogeneous, low-intensity signal indistinguishable from the PCL and patellar tendon; II, normal ligament signal over at least 50% of its volume, intermingled with portions that have increased signal intensity; III, increased signal intensity over at least 50% of its volume, intermingled with portions that have a normal ligament signal; or IV, diffuse increase in signal intensity without strands with a normal ligament appearance.
Figure 5.
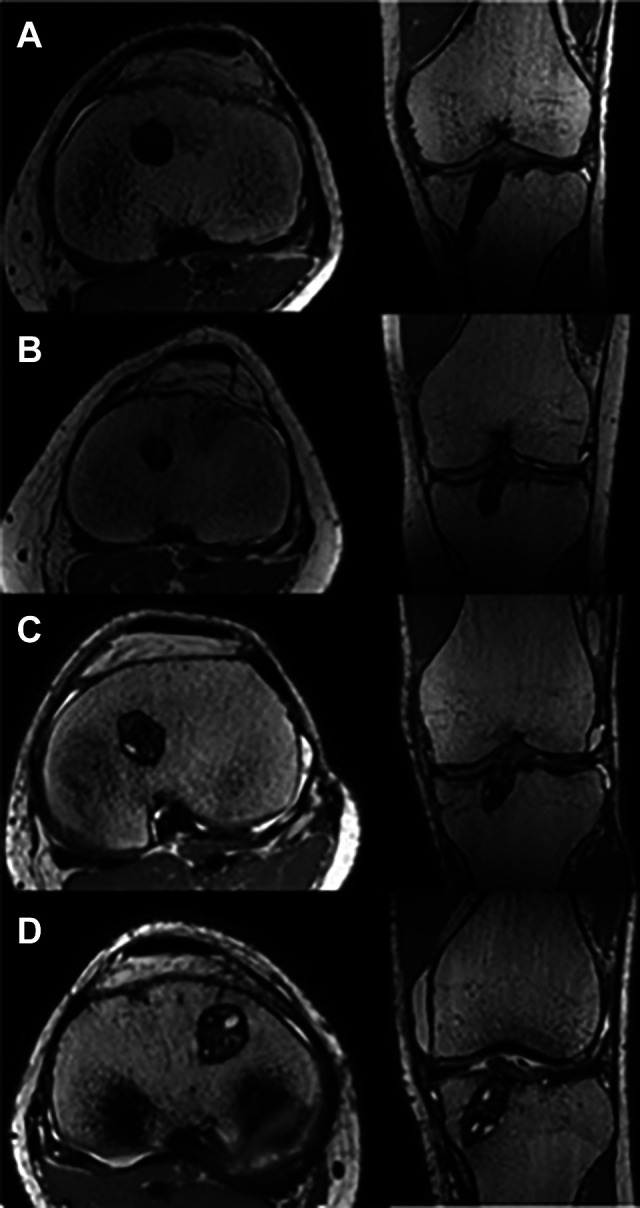
Examples of the 4 grades assigned to graft water content according to the Howell scale24: (A) grade I, (B) grade II, (C) grade III, and (D) grade IV.
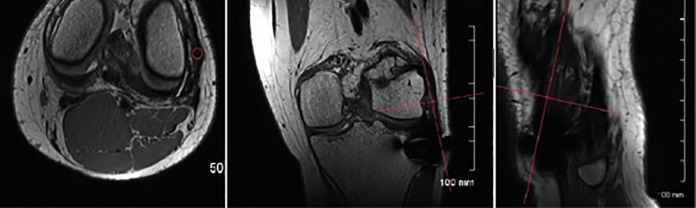
One of the secondary endpoints was incorporation of the ALL graft. Also, a subgroup analysis was conducted in the ST4 + LET group only. The SNQ of the ALL graft was calculated according to Weiler et al.49 Oblique PD-TSE sequences perpendicular to the graft were obtained after 3-dimensional reconstruction. Signal intensity was measured in 0.05-cm2 regions of interest at 3 sites (superior, middle, and inferior), and the average was calculated (Figure 6). The background signal was measured 2 cm lateral to the fibular head using the formula in Equation 1.
Figure 6.
Method used to calculate the signal-to-noise quotient of the anterolateral ligament graft. On axial slices, three 0.05-cm2 regions of interest were placed on the graft (superior, middle, and inferior) using OsiriX software and 3-dimensional reconstruction.
Analysis was performed on a PACS workstation (Horizon Rad Station; McKesson).
The MRI scans were analyzed by 2 orthopaedic surgeons (T.M., F.A.) . Each rater was blinded to the grade assigned by the other rater on the same examination. MRI endpoints were the mean of both raters. The intraclass correlation coefficient (ICC) with 95% CI was calculated to assess interobserver reproducibility. For the SNQ measurement, the reliability of the mean (between raters 1 and 2) was ICC = 0.70 (95% CI, 0.51-0.82). For signal intensity at the bone-graft interface, the reliability of the mean was ICC = 0.71 (95% CI, 0.52-0.83), and for tibial tunnel widening, it was ICC = 0.81 (95% CI, 0.68-0.89). Finally, for the ALL graft, the reliability of the mean SNQ (between raters 1 and 2) was ICC = 0.85 (95% CI, 0.68-0.93).
Knee stability was measured during the 1-year follow-up visit by a trained orthopaedic fellow (T.M.). The Lachman test results were graded as either 0 (<3 mm), 1 (3-6 mm), 2 (7-10 mm), or 3 (>10 mm).20 Anterior drawer was graded as a negative or positive test finding. Range of motion was measured passively with a manual goniometer. The pivot shift was graded as 0 (absent), 1 (glide), 2 (jerk), or 3 (subluxation).20
Functional outcomes consisted of Lysholm,3 Tegner,47 and International Knee Documentation Committee (IKDC) subjective20 scores at the 1-year follow-up visit. Patients graded their satisfaction with the outcome as very satisfied, satisfied, or dissatisfied.
Statistical Analysis
This was a superiority study. We assumed that the SNQ would be lower in the combined ST4 + LET group than in the ST4-only group. Based on a previous study,6 a sample size of 62 patients (31 in each group) would allow us to show a mean standardized difference in the SNQ between the 2 groups of ≥0.8 SD (with a 2-sided alpha rate of 5% and power >80%).
Before the statistical comparisons, missing, aberrant, or inconsistent data were identified. After corrections, the database was locked. Analysis was performed on the locked database. Descriptive statistics included the number of nonmissing observations, mean with standard deviation for continuous variables, and number of nonmissing observations with frequency (%) for categorical variables. Endpoints were compared between groups at 1 year. The Student t test was used to compare the distribution of continuous endpoints (or the Mann-Whitney test if the distribution departed from normality or if homoscedasticity was rejected). Categorical endpoints were compared between groups using the chi-square test (or the Fisher exact test when necessary). To take the unequal baseline characteristics between groups into account, the adjusted mean SNQ was assessed in each group using a linear regression model. All reported P values were 2-sided, and the significance threshold was <.05. Statistical analyses were conducted using Stata software 14.1 (StataCorp).
Results
The 2 groups were comparable (Table 1), except for age (older for the ST4 group) and time between surgery and MRI (longer for the ST4 group). The analysis of the primary endpoint (SNQ) was adjusted for these differences between groups.
Table 1.
Patient Characteristicsa
| ST4 (n = 31) | ST4 + LET (n = 31) | P Value | |
|---|---|---|---|
| Age, y | 33.1 ± 8.3 | 27.2 ± 6.7 | .0043 |
| Body mass index, kg/m2 | 24.4 ± 3.4 | 25.2 ± 4.4 | .5402 |
| Preoperative Tegner score (out of 10) | 6.9 ± 2.0 | 6.7 ± 1.7 | .6807 |
| Time between surgery and MRI, d | 405.0 ± 60.7 | 349.0 ± 39.0 | <.0001 |
| Meniscal injury, n (%) | 14 (45) | 13 (42) | .7978 |
| Graft diameter, mm | 8.9 ± 0.9 | 9.1 ± 0.7 | .4306 |
aValues are shown as mean ± SD unless otherwise indicated. LET, lateral extra-articular tenodesis; MRI, magnetic resonance imaging; ST4, quadrupled semitendinosus.
Signal-to-Noise Quotient
The mean SNQ was 0.5 (95% CI, 0.4-4.6) in the ST4 + LET group and 5.9 (95% CI, 4.8-6.9) in the ST4 group (P = .0285). After adjusting for differences in age and time between surgery and MRI, the mean SNQ was 0.5 ± 2.1 (95% CI, 0.4-4.6) in the ST4 + LET group and 5.9 ± 3.7 (95% CI, 4.7-7.0) in the ST4 group (P = .0297). The SNQ in the ST4 + LET group was statistically lower than in the ST4 group, suggesting better graft incorporation.
Secondary Endpoints
The mean tibial tunnel widening was not statistically significant, with 74% ± 42% in the ST4 + LET group versus 78% ± 47% in the ST4 group (P = .5685). In terms of graft healing, the mean signal intensity at the bone-graft interface was not statistically lower in the ST4 + LET group (1.7 ± 0.6) than in the ST4 group (2.0 ± 0.6) (P = .1663).
The Howell scale was used to assess graft maturity in the tibial tunnel (Table 2). The ST4 + LET group had a statistically significant greater number of grafts judged to be Howell grade I (P = .0379).
Table 2.
Graft Maturity According to the Howell Scalea
| ST4 (n = 31) | ST4 + LET (n = 31) | Total (N = 62) | P Value | |
|---|---|---|---|---|
| Grade I | 4 (13) | 11 (36) | 15 (24) | .0379 |
| Grade II | 14 (45) | 14 (45) | 28 (45) | |
| Grade III | 12 (39) | 6 (19) | 18 (29) | |
| Grade IV | 1 (3) | 0 (0) | 1 (2) |
aValues are shown as n (%). LET, lateral extra-articular tenodesis; ST4, quadrupled semitendinosus.
There was no significant difference between the 2 groups during the clinical examination: Lachman (P > .9999), anterior drawer (P > .9999), and pivot-shift (P > .9999) tests. Only 1 patient in the ST4 group had a Lachman grade 3 (>10 mm), stage 1 pivot shift, and positive anterior drawer. All 31 patients in the ST4 + LET group had a Lachman grade 0 (<3 mm), stage 0 pivot shift, and negative anterior drawer. There was no significant difference between the 2 groups regarding range of motion (P = .3032).
No significant difference between groups was found in the IKDC subjective score (P = .2683) or postoperative Tegner score (P = .7428). On the other hand, the Lysholm score was statistically higher in the ST4 + LET group (P = .0058) (Table 3).
Table 3.
Functional Outcomesa
| ST4 | ST4 + LET | P Value | |
|---|---|---|---|
| Lysholm (out of 100) | 92.0 ± 5.6 | 96.2 ± 5.7 | .0058 |
| Tegner (out of 10) | 5.7 ± 2.0 | 5.9 ± 1.8 | .7428 |
| IKDC subjective (out of 100) | 89.1 ± 9.7 | 86.8 ± 9.8 | .2683 |
aValues are shown as mean ± SD. IKDC, International Knee Documentation Committee; LET, lateral extra-articular tenodesis; ST4, quadrupled semitendinosus.
In terms of satisfaction, 22 (71%) patients were very satisfied, 9 (29%) were satisfied, and 0 (0%) were dissatisfied in the ST4 group, while 22 (71%) patients were very satisfied, 8 (26%) were satisfied, and 1 (3%) was dissatisfied in the ST4 + LET group. There was no significant difference in the satisfaction level between groups (P > .9999).
The time between surgery and return to sport was 204.4 ± 63.0 days in the ST4 group and 218.1 ± 56.3 days in the ST4 + LET group, which was not significantly different (P = .3860).
Lateral Extra-articular Tenodesis
The mean SNQ for the LET graft at the 1-year follow-up was 2.6 ± 4.9. There were no signs of impingement between the LET graft or its fixation device and the ACL femoral tunnel.
Discussion
Our main finding was that MRI indicators of ACL graft incorporation were generally better when combined with LET. This is the first study to compare the incorporation of an ACL graft with and without LET.
Many studies have described the ligamentization of an ACL graft.10,17–19,24 According to Weiler et al,49 changes in MRI signal intensity over time represent the incorporation process of the graft. The SNQ is a validated measure that reflects the graft’s mechanical properties. Even without contrast enhancement, the SNQ has a significant negative linear correlation with load to failure and tensile strength.19,49
It has been shown that adding LET to an existing standardized intra-articular reconstruction procedure significantly reduces loads on the ACL composite graft.14 In our study, the mean SNQ was lower in the ST4 + LET group. Hence, by creating a favorable mechanical environment, adding LET significantly improves ACL graft incorporation.
The SNQ values found in the literature range from 0.078 ± 0.62 for an autologous ST4 graft at 6 months11 to 5.49 ± 3.71 for an allograft after 2 years.18 We found mean SNQ values of 0.5 ± 2.1 for the ST4 + LET group and 5.9 ± 3.7 for the ST4 group at 1 year. However, precaution must be taken comparing data from the literature because SNQ values are dependent on the characteristics of the MRI unit used, number of channel volume array coils, and types of sequences. It is known that the graft’s remodeling process and therefore the MRI signal continues to change over time after 1 year, but most of the changes occur between 6 and 9 months after surgery.12 For this reason, and to be clinically relevant with rehabilitation protocols and early return to sport, we purposely chose to conduct our evaluations at the 1-year follow-up visit.
Tibial tunnel widening occurs during the first few months after ACL reconstruction. Fules et al17 showed that MRI was a good modality for evaluating tunnel widening on transverse slices. Published tibial tunnel widening values range from 57% at 6 months11 to 80% for the ST4 graft technique at 10 years.45 We found mean values of 74% ± 42% for the ST4 + LET group and 78% ± 47% for the ST4 group at 1 year postoperatively. In our opinion, tibial tunnel enlargement is a multifactorial phenomenon that goes through different phases: early widening is caused by mechanical stress during surgery, such as the thermogenic effect of drilling, resulting in bone necrosis; the second phase of widening occurring contemporary to the remodeling phase of the ACL graft and caused by inflammation and cytokines; and late widening, which can be attributed to device resorption and progression of this slow phenomenon. Main studies about this topic suggest that it occurs within the first year after ACL reconstruction up to 3 years.17,23 In our study, there was no statistical difference between groups in terms of tibial tunnel widening.
We used 2 additional parameters to describe the ACL graft’s incorporation and maturation process: graft healing, as described by Ge et al,18 representing incorporation of the graft and its attachment to the bone inside the tibial tunnel; and graft maturation, as described by Howell et al.24 Based on our study, no difference was found in terms of graft healing, but ACL graft maturation in the tibial tunnel was better at 1 year postoperatively when combined with LET. We hypothesize that adding LET may decrease translation and shear stress and improve the mechanical environment for the ACL graft.
Clinically, we found no differences between the 2 groups. Of the 62 patients who agreed to return for the 1-year follow-up visit, 1 patient suffered an ACL retear, and another had positive Lachman and pivot-shift test results . All 31 patients in the ST4 + LET group had satisfactory knee stability with negative Lachman and anterior drawer test results and a grade 0 pivot shift. We found no differences in the IKDC subjective score or postoperative Tegner score between groups, and the Lysholm score was significantly better in the ST4 + LET group (P = .0058).33 Again, and in agreement with the literature, in our assessments using the Tegner, Lysholm, and IKDC scores, we could not prove the superiority of combined ACL and ALL reconstruction over standard single ACL reconstruction in terms of functional outcomes.32,41,43,44
At 1 year postoperatively, the mean SNQ of the LET graft was 2.6 ± 4.9. Hence, the SNQ of the LET graft was low, which is evidence of good integration and mechanical properties. Because this additional subgroup analysis was experimental, we cannot compare our results with others in the literature. For LET, contrary to other previously described techniques, only a 5.5-mm graft tunnel is needed to screw the suture anchor into the femoral cortex. The graft is attached to the cortical bone both on the femur (anchor) and on the tibia (staple); this type of LET technique has minimal impact on the bone stock. As there is no graft in the bone tunnel, the question of graft integration was essential for us; the mean SNQ of 2.6 ± 4.9 indicated satisfactory graft incorporation. It has been shown that convergence of the ACL and ALL femoral tunnels can occur in 67% of cases.42 Tunnel convergence can become a major issue if a weak femoral attachment causes the reconstructed ACL to be inefficient. The femoral tunnel that we use for ACL reconstruction is a 10 mm–long blind tunnel, which has been shown to be sufficient for hamstring graft integration.6
In view of our results, faster ACL graft incorporation when combined with LET might allow quicker return to play without an increased risk of graft ruptures. This study may provide additional arguments to extend the indication of associated ACL and ALL reconstruction in a selected population of young athletes.
However, our study has several limitations. Women were excluded from this study because hormonal changes can affect the graft’s incorporation during the menstrual cycle.2,13,23 In an animal study, Kiapour et al27 showed that graft structural properties and knee laxity were worse in female than male specimens. In our screening population, women represented only 6% of our patients. Concerning the rising incidence of ACL ruptures in women, further investigation is needed to study incorporation in this specific population.
According to Muramatsu et al,35 the SNQ peaks at 6 months and then decreases until 60 months postoperatively. This means that we may have evaluated our patients too early in the follow-up period. This is consistent with studies39,40 showing that remodeling persists for up to 24 to 36 months, at which point the graft becomes quiescent. However, the meta-analysis performed by Claes et al10 found no agreement on the duration of the various stages of ligamentization. Also, according to Li et al,30 changes in terms of MRI-based graft maturity were not correlated with clinical and functional outcomes in patients at the 1-year follow-up visit. The follow-up time was too short for a clinical follow-up but suitable for the imaging follow-up as the primary endpoint.
Weiler et al49 observed that higher signal intensity on contrast-enhanced MRI corresponded to lower mechanical strength of the graft during the early remodeling phase. Hence, the SNQ is inversely proportional to the graft’s tensile strength. Several variations of the SNQ have been described, many of which do not require gadolinium injections.19,24,35 Other authors have compared the graft’s signal with the quadriceps tendon18,35 instead of the PCL, such as Weiler et al.11,49 We decided to use the same methodology as Weiler et al, who developed the SNQ measurements on MRI by comparing them with histological evaluations. Moreover, we chose not to perform a gadolinium injection because Weiler et al observed that it does not alter the signal in the graft at 1 year. To remain consistent with the literature and because it is technically easier, we chose to use the Howell scale and evaluate graft healing in the tibial tunnel only.11,17
Because we added LET to our test group, we could not perform a double-blind evaluation for both MRI and a clinical examination. On the other hand, the MRI evaluations were conducted by 2 different raters blinded to each other’s results. The endpoints were the mean of both raters, and reliability was satisfactory. Moreover, our 2 groups were not comparable in their age and time between surgery and MRI. However, the younger ST4 + LET group had a shorter time between surgery and MRI than the ST4 group, which minimized the potential bias. Further, the data were adjusted for those parameters, and the results were provided with a strong statistical correlation. Last, we chose not to base our decision for when to perform LET on the pivot-shift phenomenon because it can be multifactorial. At first glance, we could be criticized for not using it as a selection criterion between groups, but the aim was specifically to study integration in the reconstructed knee after identifying ALL injuries. In fact, an injury to the ALL has been shown to be the most important risk factor for a grade 3 pivot shift in acute ACL-injured knees.16
Conclusion
At the 1-year postoperative follow-up visit, MRI parameters evaluating ACL graft incorporation and maturation were generally better when ACL reconstruction was combined with LET compared with reconstruction alone. Graft healing was also better, but this was not a statistically significant difference.
Acknowledgment
The authors thank Johanne Archambault for the English translation of this article.
Appendix
1. Surgical Setup and Preoperative Examination
The patient was placed supine on the operating table in the standard arthroscopic position, with a lateral post proximal to the knee at the level of the tourniquet and 2 foot rolls at 90° and 120° of flexion. Bony landmarks were marked after anesthesia and setup but before draping. The Gerdy tubercle, the head of the fibula, and the lateral epicondyle were first located by palpation. An ultrasonography machine with a 12-MHz superficial probe (Sonosite; Fujifilm) was then used to confirm the position of the bony landmarks and evaluate the anterolateral ligament (ALL).4,9 This ultrasonographic analysis allowed for small percutaneous incisions to be made exactly at the desired location.
2. Graft Harvesting and Preparation
A standard vertical 2-cm incision was made medial to the anterior tibial tuberosity. The semitendinosus and gracilis tendons were harvested with an open tendon stripper and then cleaned and cut close to their tibial insertion. Hyperflexion provided better access to the most proximal vincula.38 The semitendinosus tendon, used as the ACL graft, was prepared in 4 strands on a TightRope device (Arthrex) with a No. 2 FiberWire suture (Arthrex) at the distal end.6 The gracilis tendon was not prepared. Both tendons were soaked in a vancomycin solution before implantation.37
3. ACL Reconstruction
ACL reconstruction was performed first with the 4-strand semitendinosus graft. The tibial tunnel was drilled completely from the hamstring incision with an outside-in guide. An inside-out guide was used to drill a 10 mm–long femoral tunnel.6 The graft was passed from distal to proximal, the TightRope fixation system was secured on the femoral cortex, and the graft was tightened with a BioComposite interference screw (Arthrex) on the tibial side at 30° of flexion. A screw of the same diameter as the graft was used.
4. ALL Reconstruction
After fixation of the ACL graft, 2 incisions were made: one just posterior and proximal to the lateral epicondyle and the other midway between the Gerdy tubercle and the fibular head. Starting at the proximal incision, the fascia lata was incised. A 5.5-mm suture anchor with 2 No. 2 Hi-Fi sutures (ConMed) was screwed in the femoral cortex. A Kelly clamp was introduced through the proximal incision under the fascia lata and superficial to the lateral collateral ligament toward the distal incision. The gracilis tendon was folded into 2 and pulled with the clamp from distal to proximal with the 2 free ends hanging distally. The proximal end of the graft was sutured on the femoral anchor by passing a strand of each suture through the folded tendon. With the knee in full extension and neutral rotation, the distal part of the graft was tightened and secured with a 6 × 20–mm Spiked Ligament Staple (Arthrex), which was impacted posterior to the Gerdy tubercle. The free end of the graft was cut flush to the ligament staple.
Footnotes
Final revision submitted July 9, 2020; accepted July 30, 2020.
One or more of the authors has declared the following potential conflict of interest or source of funding: E.C. and B.S.-C. are consultants for Arthrex. B.S.-C. receives royalties from Arthrex. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from Centre Hospitalier Universitaire (CHU) de Toulouse (ID No. 09-1116).
References
- 1. Ayala-Mejias JD, Garcia-Gonzalez B, Alcocer-Perez-España L, Villafañe JH, Berjano P. Relationship between widening and position of the tunnels and clinical results of anterior cruciate ligament reconstruction to knee osteoarthritis: 30 patients at a minimum follow-up of 10 years. J Knee Surg. 2017;30(6):501–508. [DOI] [PubMed] [Google Scholar]
- 2. Blecher AM, Richmond JC. Transient laxity of an anterior cruciate ligament-reconstructed knee related to pregnancy. Arthroscopy. 1998;14(1):77–79. [DOI] [PubMed] [Google Scholar]
- 3. Briggs KK, Lysholm J, Tegner Y, Rodkey WG, Kocher MS, Steadman JR. The reliability, validity, and responsiveness of the Lysholm score and Tegner activity scale for anterior cruciate ligament injuries of the knee: 25 years later. Am J Sports Med. 2009;37(5):890–897. [DOI] [PubMed] [Google Scholar]
- 4. Cavaignac E, Faruch M, Wytrykowski K, et al. Ultrasonographic evaluation of anterolateral ligament injuries: correlation with magnetic resonance imaging and pivot-shift testing. Arthroscopy. 2017;33(7):1384–1390. [DOI] [PubMed] [Google Scholar]
- 5. Cavaignac E, Laumond G, Reina N, et al. How to test the anterolateral ligament with ultrasound. Arthrosc Tech. 2018;7(1):e29–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cavaignac E, Marot V, Faruch M, et al. Hamstring graft incorporation according to the length of the graft inside tunnels. Am J Sports Med. 2018;46(2):348–356. [DOI] [PubMed] [Google Scholar]
- 7. Cavaignac E, Saithna A, Monaco E, et al. Is treatment of Segond fracture necessary with combined anterior cruciate ligament reconstruction? Letter to the editor. Am J Sports Med. 2018;46(5):NP13–NP14. [DOI] [PubMed] [Google Scholar]
- 8. Cavaignac E, Wytrykowski K, Murgier J, Reina N, Chiron P, Faruch M. Regarding “editorial commentary: ultrasound barely beats magnetic resonance imaging in knee anterolateral ligament evaluation…but does this change the treatment of the anterior cruciate ligament-deficient knee?” Arthroscopy. 2017;33(11):1918–1919. [DOI] [PubMed] [Google Scholar]
- 9. Cavaignac E, Wytrykowski K, Reina N, et al. Ultrasonographic identification of the anterolateral ligament of the knee. Arthroscopy. 2016;32(1):120–126. [DOI] [PubMed] [Google Scholar]
- 10. Claes S, Verdonk P, Forsyth R, Bellemans J. The “ligamentization” process in anterior cruciate ligament reconstruction: what happens to the human graft? A systematic review of the literature. Am J Sports Med. 2011;39(11):2476–2483. [DOI] [PubMed] [Google Scholar]
- 11. Colombet P, Graveleau N, Jambou S. Incorporation of hamstring grafts within the tibial tunnel after anterior cruciate ligament reconstruction: magnetic resonance imaging of suspensory fixation versus interference screws. Am J Sports Med. 2016;44(11):2838–2845. [DOI] [PubMed] [Google Scholar]
- 12. Covey DC, Sandoval KE, Riffenburgh RH. Contrast-enhanced MRI evaluation of bone-patellar tendon-bone and hamstring ACL autograft healing in humans: a prospective randomized study. Orthop J Sports Med. 2018;6(10):2325967118800298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dragoo JL, Padrez K, Workman R, Lindsey DP. The effect of relaxin on the female anterior cruciate ligament: analysis of mechanical properties in an animal model. Knee. 2009;16(1):69–72. [DOI] [PubMed] [Google Scholar]
- 14. Engebretsen L, Lew WD, Lewis JL, Hunter RE. The effect of an iliotibial tenodesis on intraarticular graft forces and knee joint motion. Am J Sports Med. 1990;18(2):169–176. [DOI] [PubMed] [Google Scholar]
- 15. Faruch Bilfeld M, Cavaignac E, Wytrykowski K, et al. Anterolateral ligament injuries in knees with an anterior cruciate ligament tear: contribution of ultrasonography and MRI. Eur Radiol. 2018;28(1):58–65. [DOI] [PubMed] [Google Scholar]
- 16. Ferretti A, Monaco E, Gai E, et al. Risk factors for grade 3 pivot shift in acute ACL-injured knees: a comprehensive evaluation of the importance of osseous and soft-tissue parameters from the SANTI Study Group. Am J Sports Med. 2020;48(10):2408–2417. [DOI] [PubMed] [Google Scholar]
- 17. Fules PJ, Madhav RT, Goddard RK, Newman-Sanders A, Mowbray MAS. Evaluation of tibial bone tunnel enlargement using MRI scan cross-sectional area measurement after autologous hamstring tendon ACL replacement. Knee. 2003;10(1):87–91. [DOI] [PubMed] [Google Scholar]
- 18. Ge Y, Li H, Tao H, Hua Y, Chen J, Chen S. Comparison of tendon-bone healing between autografts and allografts after anterior cruciate ligament reconstruction using magnetic resonance imaging. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):954–960. [DOI] [PubMed] [Google Scholar]
- 19. Gohil S, Annear PO, Breidahl W. Anterior cruciate ligament reconstruction using autologous double hamstrings: a comparison of standard versus minimal debridement techniques using MRI to assess revascularisation. A randomised prospective study with a one-year follow-up. J Bone Joint Surg Br. 2007;89(9):1165–1171. [DOI] [PubMed] [Google Scholar]
- 20. Hefti F, Müller W, Jakob RP, Stäubli HU. Evaluation of knee ligament injuries with the IKDC form. Knee Surg Sports Traumatol Arthrosc. 1993;1(3-4):226–234. [DOI] [PubMed] [Google Scholar]
- 21. Helito CP, Camargo DB, Sobrado MF, et al. Combined reconstruction of the anterolateral ligament in chronic ACL injuries leads to better clinical outcomes than isolated ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2018;26(12):3652–3659. [DOI] [PubMed] [Google Scholar]
- 22. Hewison CE, Tran MN, Kaniki N, Remtulla A, Bryant D, Getgood AM. Lateral extra-articular tenodesis reduces rotational laxity when combined with anterior cruciate ligament reconstruction: a systematic review of the literature. Arthroscopy. 2015;31(10):2022–2034. [DOI] [PubMed] [Google Scholar]
- 23. Höher J, Möller HD, Fu FH. Bone tunnel enlargement after anterior cruciate ligament reconstruction: fact or fiction? Knee Surg Sports Traumatol Arthrosc. 1998;6(4):231–240. [DOI] [PubMed] [Google Scholar]
- 24. Howell SM, Clark JA, Blasier RD. Serial magnetic resonance imaging of hamstring anterior cruciate ligament autografts during the first year of implantation: a preliminary study. Am J Sports Med. 1991;19(1):42–47. [DOI] [PubMed] [Google Scholar]
- 25. Iorio R, Vadalà A, Argento G, Di Sanzo V, Ferretti A. Bone tunnel enlargement after ACL reconstruction using autologous hamstring tendons: a CT study. Int Orthop. 2007;31(1):49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ireland J, Trickey EL. Macintosh tenodesis for anterolateral instability of the knee. J Bone Joint Surg Br. 1980;62(3):340–345. [DOI] [PubMed] [Google Scholar]
- 27. Kiapour AM, Fleming BC, Proffen BL, Murray MM. Sex influences the biomechanical outcomes of anterior cruciate ligament reconstruction in a preclinical large animal model. Am J Sports Med. 2015;43(7):1623–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee DW, Kim JG, Cho SI, Kim DH. Clinical outcomes of isolated revision anterior cruciate ligament reconstruction or in combination with anatomic anterolateral ligament reconstruction. Am J Sports Med. 2019;47(2):324–333. [DOI] [PubMed] [Google Scholar]
- 29. Lemaire M, Combelles F. [Plastic repair with fascia lata for old tears of the anterior cruciate ligament (author’s transl)]. Rev Chir Orthop Reparatrice Appar Mot. 1980;66(8):523–525. [PubMed] [Google Scholar]
- 30. Li H, Chen J, Li H, Wu Z, Chen S. MRI-based ACL graft maturity does not predict clinical and functional outcomes during the first year after ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2017;25(10):3171–3178. [DOI] [PubMed] [Google Scholar]
- 31. Losee RE, Johnson TR, Southwick WO. Anterior subluxation of the lateral tibial plateau: a diagnostic test and operative repair. J Bone Joint Surg Am. 1978;60(8):1015–1030. [PubMed] [Google Scholar]
- 32. Lutz C. Role of anterolateral reconstruction in patients undergoing anterior cruciate ligament reconstruction. Orthop Traumatol Surg Res. 2018;104(1 suppl):S47–S53. [DOI] [PubMed] [Google Scholar]
- 33. Marot V, Murgier J, Carrozzo A, et al. Determination of normal KOOS and WOMAC values in a healthy population. Knee Surg Sports Traumatol Arthrosc. 2019;27(2):541–548. [DOI] [PubMed] [Google Scholar]
- 34. Murakami Y, Sumen Y, Ochi M, Fujimoto E, Deie M, Ikuta Y. Appearance of anterior cruciate ligament autografts in their tibial bone tunnels on oblique axial MRI. Magn Reson Imaging. 1999;17(5):679–687. [DOI] [PubMed] [Google Scholar]
- 35. Muramatsu K, Hachiya Y, Izawa H. Serial evaluation of human anterior cruciate ligament grafts by contrast-enhanced magnetic resonance imaging: comparison of allografts and autografts. Arthroscopy. 2008;24(9):1038–1044. [DOI] [PubMed] [Google Scholar]
- 36. Pauvert A, Robert H, Gicquel P, et al. MRI study of the ligamentization of ACL grafts in children with open growth plates. Orthop Traumatol Surg Res. 2018;104(8 suppl):S161–S167. [DOI] [PubMed] [Google Scholar]
- 37. Pérez-Prieto D, Torres-Claramunt R, Gelber PE, Shehata TMA, Pelfort X, Monllau JC. Autograft soaking in vancomycin reduces the risk of infection after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2016;24(9):2724–2728. [DOI] [PubMed] [Google Scholar]
- 38. Reina N, Abbo O, Gomez-Brouchet A, Chiron P, Moscovici J, Laffosse J-M. Anatomy of the bands of the hamstring tendon: how can we improve harvest quality? Knee. 2013;20(2):90–95. [DOI] [PubMed] [Google Scholar]
- 39. Rougraff B, Shelbourne KD, Gerth PK, Warner J. Arthroscopic and histologic analysis of human patellar tendon autografts used for anterior cruciate ligament reconstruction. Am J Sports Med. 1993;21(2):277–284. [DOI] [PubMed] [Google Scholar]
- 40. Sánchez M, Anitua E, Azofra J, Prado R, Muruzabal F, Andia I. Ligamentization of tendon grafts treated with an endogenous preparation rich in growth factors: gross morphology and histology. Arthroscopy. 2010;26(4):470–480. [DOI] [PubMed] [Google Scholar]
- 41. Schlatterer B, Jund S, Delépine F, Razafindratsiva C, de Peretti F. [Acute anterior cruciate ligament repair with combined intra- and extra-articular reconstruction using an iliotibial band with the modified MacIntosh technique: a five-year follow-up study of 50 pivoting sport athletes]. Rev Chir Orthop Reparatrice Appar Mot. 2006;92(8):778–787. [DOI] [PubMed] [Google Scholar]
- 42. Smeets K, Bellemans J, Lamers G, et al. High risk of tunnel convergence during combined anterior cruciate ligament and anterolateral ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2019;27(2):611–617. [DOI] [PubMed] [Google Scholar]
- 43. Sonnery-Cottet B, Saithna A, Blakeney WG, et al. Anterolateral ligament reconstruction protects the repaired medial meniscus: a comparative study of 383 anterior cruciate ligament reconstructions from the SANTI Study Group with a minimum follow-up of 2 years. Am J Sports Med. 2018;46(8):1819–1826. [DOI] [PubMed] [Google Scholar]
- 44. Sonnery-Cottet B, Saithna A, Cavalier M, et al. Anterolateral ligament reconstruction is associated with significantly reduced ACL graft rupture rates at a minimum follow-up of 2 years: a prospective comparative study of 502 patients from the SANTI Study Group. Am J Sports Med. 2017;45(7):1547–1557. [DOI] [PubMed] [Google Scholar]
- 45. Streich NA, Reichenbacher S, Barié A, Buchner M, Schmitt H. Long-term outcome of anterior cruciate ligament reconstruction with an autologous four-strand semitendinosus tendon autograft. Int Orthop. 2013;37(2):279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Strickler FP. A satisfactory method of repairing crucial ligaments. Ann Surg. 1937;105(6):912–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;198:43–49. [PubMed] [Google Scholar]
- 48. Tomczak RJ, Hehl G, Mergo PJ, Merkle E, Rieber A, Brambs HJ. Tunnel placement in anterior cruciate ligament reconstruction: MRI analysis as an important factor in the radiological report. Skeletal Radiol. 1997;26(7):409–413. [DOI] [PubMed] [Google Scholar]
- 49. Weiler A, Peters G, Mäurer J, Unterhauser FN, Südkamp NP. Biomechanical properties and vascularity of an anterior cruciate ligament graft can be predicted by contrast-enhanced magnetic resonance imaging: a two-year study in sheep. Am J Sports Med. 2001;29(6):751–761. [DOI] [PubMed] [Google Scholar]